Abstract
Objective
To answer the question what is the best source or composition of omega-3 polyunsaturated fatty acids (PUFA) that will provide the most favorable and safe outcome for peripheral neuropathy (PN) in an animal model of obesity? Traditionally encapsulated fish oil is the primary source of omega-3 PUFA as a nutritional supplement. However, other sources exist that could be a better environmental, safety, and/or economic choice.
Methods
Male Sprague Dawley rats 12 weeks of age were fed a 45% kcal diet to induce obesity and model pre-diabetes. Early and late intervention protocols were used to determine the ability of omega-3 PUFA derived from menhaden (fish) oil, krill oil, algal oils, or ethyl esters to slow the progression or reverse PN associated with pre-diabetes by examining multiple endpoints of sensory nerve function, morphometry and vascular reactivity. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the primary omega-3 PUFA, and a combination exist in fish and krill oil. However, algal oils and ethyl esters are available as EPA, DHA, or EPA & DHA and each were used in this study.
Results
We report that multiple sources of omega-3 PUFA are a proactive treatment for PN that occurs with pre-diabetes including improvement in sensory nerve conduction velocity, thermal nociception and cornea sensitivity and corneal nerve fiber length. Improvement in vascular reactivity of epineurial arterioles of the sciatic nerve was observed. We also report that EPA and DHA had different outcomes for these endpoints.
Conclusion
We confirm that omega-3 PUFA are an effective treatment to prevent and reverse PN associated with obesity and pre-diabetes. Additional studies will be needed to definitively determine what would be the best and most consistent source of this important nutritional supplement from an environmental and economical viewpoint.
Keywords: omega-3 polyunsaturated fatty acids, fish oil, peripheral neuropathy, obesity
Introduction
Peripheral neuropathy (PN) has long been considered a primary complication associated with diabetes however, about 30% of obese subjects with insulin resistance also have evidence of PN.1,2 Indicating that hyperglycemia is not the only factor contributing to PN. PN has a wide range of symptoms that reduces the quality of life making finding a treatment of vital importance.3 Abnormal lipid metabolism has been shown to be a contributing factor in complications associated with metabolic syndrome and type 2 diabetes.4 We have extensively sought interventions that would prevent or slow the progression of PN in rodent models of obesity or diabetes. Recently, our studies have focused on fish oil that contain long-chain omega-3 (n-3) polyunsaturated fatty acids (PUFA), (primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) and their metabolites as a treatment for microvascular and neural complications associated with diet-induced obesity and diabetes.5–8 Over the past 10 years, there have been numerous epidemiological studies conducted reporting on the health benefits of omega-3 PUFA such as lowering blood triglycerides, cardiovascular disease, diabetes, cancer, Alzheimer’s disease, dementia, depression, visual and neurological development, and maternal and child health and development.9–16
As research continues to unveil the importance of omega-3 PUFA in healthy aging and management of diseases, issues such as sustainability, quality control, and environmental concerns raise the question whether fish oil is the best long-term source of omega-3 PUFA?17 There are multiple sources of omega-3 fatty acids that are plant, animal or pharmaceutical based. However, how these different sources can be utilized to promote a healthy omega-3 index or omega-6/omega-3 fatty acid ratio and impact metabolic health including PN in obesity is understudied.18,19 A secondary question that requires investigation is what are the health-related benefits of EPA and DHA acids individually and for the best outcome are a combination required? These are the two questions we sought to address in this study.
Materials and Methods
All laboratory supplies were obtained from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
Animals, Diets and Experimental Design
Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN) male rats 10–11 weeks of age were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI (control diet)) and water were provided ad libitum. The Iowa City VA Healthcare system Institutional Animal Care and Use Committee approved this study (ACORP 2190501). The Iowa City VA animal care facility is AAALAC accredited and Guide for the Care and Use of Laboratory Animals as well as VA Directive 1200.07 were followed. To create the early or late intervention experimental groups 12-week-old rats were fed a control or 45% kcal high fat diet for 8 weeks or 18 weeks, respectively (D12451; Research Diets, New Brunswick, NJ). The number of rats in each individual study group ranged from 10 to 14 unless stated otherwise (based on a power analysis of 12). Afterwards, the high fat fed rats (early or late intervention) were treated with diets enriched with menhaden (fish) oil (D16021504), krill oil ((Jedwards International, Braintree, MA) (D21062302)), or 3 different algal oils derived from algae that produce primarily EPA, AzAlgae EPA15+ crude extract ((Arizona Algae Products, Holbrook, AZ) (D21062303)), primarily DHA, Life’s DHA S40 – O400 (DSM Nutritional Products, Columbia, MD) (D21062304)), or the combination of EPA and DHA, Life’s Omega 45 O2412 – O100 (DSM Nutritional Products, Columbia, MD) (D21062305)). The treatment diets containing menhaden oil, krill oil or the algal oils were created by replacing ½ kcal in the 45% high fat diet with the respective oil (app. 1420 g oil per 12.5 kg diet). For the EPA, DHA or EPA & DHA ethyl ester treatment, high fat fed rats were treated with diets containing, MEG-3 ultra-high EPA EE oil (DSM Nutritional Products, Columbia, MD) (D21062306), DHA, MEG-3 0070 EE oil (DSM Nutritional Products, Columbia, MD) (D21062305), or EPA & DHA, MEG-3 5020 EE oil (DSM Nutritional Products, Columbia, MD) (D21062307). The appropriate amount of each of the ethyl ester (app. 200 g per 12.5 kg diet) compounds to incorporate into the diets was determined through a preliminary study of each compound with the goal of achieving a “healthy omega-3 index” from each of 8–12%. The omega-3 index is defined as the EPA and DHA content of erythrocytes expressed as a percent of total identified fatty acids.20 Included in this preliminary dose finding study were rats fed solely a control diet and 45% kcal high fat diet. All diets were made by Research Diets (New Brunswick, NJ) by us providing them with the appropriate amount of material for each of the individual diets. Treatment period for both early and late intervention protocols was 12 weeks. Supplemental Table 1 contains data for the fatty acid composition of the diets described above demonstrating that these different sources of omega-3 fatty acids provided diets that were enriched in either EPA, DHA or EPA and DHA.
Endpoints Related to Nerve and Vascular Reactivity
The endpoints studied were cornea sensitivity and thermal nociceptive latency of the hindpaws in un-anesthetized rats. Sensory and motor nerve conduction velocity and corneal nerve density were determined in rats anesthetized with sodium pentobarbital (50 mg/kg, i.p., Sagent Pharmaceuticals, Schaumburg, Il). Following euthanasia, tissues were harvested to evaluate intraepidermal nerve fiber density in skin from the hindpaw and microvascular reactivity of epineurial arterioles providing blood flow to the sciatic nerve to acetylcholine and calcitonin gene-related peptide (CGRP). These endpoints are common measurements for this laboratory. Refer to these references for descriptive information.5,7,8 Red blood cells, liver and serum were also collected. Serum was used to determine levels of free cholesterol (BioVision, Milpitas, CA), triglycerides, and free fatty acids using ELISA.
Fatty Acid Composition
Gas–liquid chromatography was used to determine the fatty acid profile of the diets, serum and liver.9 Fatty acid standards from Nu Check Prep (Elysian, MN) were used to verify the individual fatty acids and data was reported as % of total fatty acids.9 The omega-3 index of red blood cell membranes was determined according to Harris et al.21
Data Analysis
Data are presented as mean ± SEM. Statistical analysis, one-way ANOVA and Bonferroni posttest comparison, was performed using GraphPad Prism software (San Diego, CA). Responsiveness to acetylcholine and CGRP were compared applying a two-way repeated measures analysis of variance with autoregressive covariance structure using proc mixed program of SAS.5,7,8 A p value of less than 0.05 was deemed significant.
Results
Weight Change, Blood Glucose, Omega-3 Index and Fatty Acid Composition of Serum and Liver
The age and average starting weight of all rats in this study was 12 weeks and 325 ± 2 grams, respectively. At the end of the study period, control rats in the early and late intervention groups gained about 160 and 200 grams, respectively. Rats on the high fat diets in the early and late intervention groups gained an additional 80 and 120 grams, respectively or about 50–60% more than the control rats. Non-fasting blood glucose levels at the end of the two study protocols ranged from 140 to 165 mg/dl. Weight gain or blood glucose values were not changed by the dietary manipulations (data not shown for either weight change or blood glucose). Treating rats with a high fat diet caused a significant increase in triglycerides in both early and late intervention protocols that was prevented by all treatments with omega-3 PUFA. There was a significant increase in free cholesterol in the late intervention high fat fed rats that was normalized with all treatments with omega-3 PUFA except for EPA derived from algal oil. Level of serum free fatty acids were not changed in all conditions (Supplemental Tables 2–5).
Data from Table 1 present the fatty acid composition of red blood cell membranes and omega-3 index from each of the experimental conditions tested after 12 weeks on their respective diets. The omega-3 index for control and high fat fed rats had a range from 3 to 4. All dietary interventions significantly increased the omega-3 index with rats treated with diets enriched with menhaden oil, krill oil, algal oil enriched with both EPA and DHA, or ethyl ester of EPA having the greatest impact. The diet containing the ethyl ester of DHA resulted in the smallest change in the omega-3 index. However, the omega-3 index for each dietary condition can be manipulated by adjusting the amount of compound incorporated into the diet. Our goal was to achieve what is a healthy range for humans of 8–12%.20 Whether the determination of a healthy range for the omega-3 index is clinically interchangeable between humans and mammals/rodents is unknown and is used here point of reference.
Table 1.
Fatty Acid % Composition of Red Blood Cell Membranes and Omega-3 Index After 12 Weeks of Treatment
| Diet | 16:0 | 18:0 | 18:1 | 18:2 | 20:4 | 20:5 | 22:5 | 22:6 | Omega-3 Index (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 28.3 ± 2.7 | 16.2 ± 1.1 | 8.6 ± 0.6 | 7.8 ± 0.9 | 20.2 ± 1.4 | < 1 | 1.4 ± 0.3 | 1.2 ± 0.2 | 1.7 ± 0.1 |
| High Fat (HF) | 23.6 ± 1.8 | 17.6 ± 1.4 | 8.8 ± 0.6 | 8.4 ± 1.1 | 22.9 ± 1.9 | < 1 | 2.0 ± 0.2 | 1.5 ± 0.3 | 2.0 ± 0.3 |
| HF + Menhaden oil | 22.5 ± 1.1 | 16.5 ± 0.9 | 8.9 ± 0.5 | 8.5 ± 0.8 | 14.9 ± 1.1 | 5.0 ± 0.4 | 3.7 ± 0.1 | 4.1 ± 0.2 | 9.1 ± 0.6a,b |
| HF + Krill oil | 28.9 ± 1.9 | 14.2 ± 1.2 | 9.3 ± 0.6 | 9.8 ± 0.9 | 9.7 ± 0.7 | 6.4 ± 0.3 | 3.5 ± 0.2 | 4.3 ± 0.3 | 10.7 ± 0.6a,b |
| HF + Algal oil EPA | 22.5 ± 1.6 | 13.8 ± 1.4 | 6.6 ± 0.4 | 7.8 ± 0.7 | 15.4 ± 1.2 | 3.7 ± 0.3 | 5.0 ± 0.9 | < 1 | 4.2 ± 0.4a,b |
| HF + Algal oil DHA | 23.2 ± 1.5 | 13.0 ± 1.0 | 7.1 ± 0.5 | 7.3 ± 0.6 | 16.5 ± 1.3 | < 1 | 1.0 ± 0.2 | 7.0 ± 0.3 | 7.5 ± 0.3a,b |
| HF + Algal oil EPA/DHA | 25.1 ± 1.3 | 16.4 ± 1.1 | 9.3 ± 0.6 | 7.2 ± 0.5 | 11.9 ± 0.9 | 5.0 ± 0.5 | 2.4 ± 0.3 | 6.0 ± 0.4 | 11.0 ± 0.6a,b |
| HF + Ethyl Ester EPA | 21.7 ± 1.6 | 16.9 ± 1.1 | 8.2 ± 0.6 | 8.9 ± 0.7 | 16.6 ± 1.1 | 4.6 ± 0.2 | 3.0 ± 0.3 | < 1 | 5.1 ± 0.3a,b |
| HF + Ethyl Ester DHA | 26.5 ± 1.8 | 15.6 ± 1.0 | 8.7 ± 0.6 | 10.2 ± 0.8 | 16.0 ± 1.2 | < 1 | 1.6 ± 0.1 | 5.3 ± 0.3 | 5.8 ± 0.3a,b |
| HF + Ethyl Ester EPA/DHA | 22.9 ± 0.9 | 16.6 ± 0.9 | 8.4 ± 0.5 | 9.4 ± 0.7 | 20.2 ± 1.3 | 2.6 ± 0.2 | 7.0 ± 0.7 | 4.0 ± 0.4 | 8.6 ± 0.4a,b |
Notes: Data are presented as the mean of three determinations ± S.E.M. a P < 0.05 vs Control; b P < 0.05 vs High Fat.
Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Supplemental Tables 6 and 7 provide data of the fatty acid composition of serum and liver for rats treated with the different diets for 12 weeks and late intervention protocol. Similar results were obtained following 12 weeks of treatment and early intervention protocol (data not shown). The unsaturation index, a measure of the degree of unsaturation in membranes calculated as the average number of double bonds per fatty acid residue multiplied by 100, was significantly increased in serum from rats dietarily treated with menhaden oil, krill oil, and each of the diets containing the three algal oils enriched in EPA, DHA or the combination of EPA/DHA and ethyl esters of EPA, DHA, and EPA/DHA compared to serum from control or high fat fed rats. Individual analysis of serum from rats fed each of the diets reveals that EPA and DHA are increased in serum of rats treated with diets enriched only with EPA or DHA derived from algal oils or pharmaceutical derived ethyl esters, respectively. When diets were enriched with both EPA and DHA derived from menhaden oil, krill oil or algal oils or ethyl ester that contain both EPA/DHA the levels of EPA and DHA are increased with few exceptions. The corresponding fatty acid composition of the liver following feeding these enriched diets was different compared to serum. The unsaturation index was significantly increased with diets containing menhaden oil or algal oils enriched with DHA or EPA/DHA compared to control and high fat fed rats. Diets enriched primarily with EPA caused a moderate increase in EPA in the liver that was offset by decrease in other polyunsaturated fatty acids in the liver, thus the unsaturation index was unchanged. Feeding rats, a diet containing krill oil resulted in significant increase in EPA and DHA, but this was offset by decreases in arachidonic acid and no change in the unsaturation index.
Effect on Motor and Sensory Nerve Conduction Velocity, Thermal and Cornea Sensitivity, Intraepidermal and Corneal Nerve Fiber Density
Tables 2 and 3 provide data of the effect of treating obese/pre-diabetic rats with diets enriched in menhaden oil, krill oil, and algal oils containing primarily EPA, DHA or EPA/DHA on PN-related endpoints following early and late intervention, respectively. As we have previously reported sensory nerve conduction velocity is significantly decreased in obese rats but not motor nerve conduction velocity.8 With early intervention all treatments significantly improved sensory nerve conduction velocity compared to untreated obese rats. With late intervention treatment, diet containing algal oil EPA improved sensory nerve conduction velocity but not significantly compared to untreated obese rats. Intraepidermal nerve fiber density and thermal nociception are two reliable endpoints for PN.22 Thermal latency was significantly increased in untreated obese rats in both protocols compared to control rats. All treatments significantly improved thermal sensitivity with dietary treatment with algal oil EPA having the best response. Intraepidermal nerve fiber density was significantly decreased in obese rats in both protocols compared to control rats. Treatment with menhaden oil enriched diet was the only treatment that provided a significant improvement, but it also remained significantly decreased compared to control rats. Cornea nerve fiber length and cornea sensitivity have been promoted as being early markers for PN and has been detected in humans with insulin resistance.23 Cornea nerve fiber length was significantly decreased in obese rats in both protocols compared to control rats. All dietary treatments except algal oil EPA significantly improved cornea nerve fiber length. Cornea sensitivity was measured by two independent methods. The method of Crochet Bonnet revealed a significant impairment in cornea sensitivity in obese rats after each study protocol compared to control rats. Treatment with omega-3 polyunsaturated fatty acids independent of the source significantly improved cornea sensitivity compared to untreated obese rats except for algal oil EPA in the early intervention. Using the hyperosmolarity method we developed for examining cornea sensitivity demonstrated significant decrease in sensitivity in obese rats compared to control rats.24 With early intervention dietary treatment with menhaden oil and algal oil containing both EPA/DHA significantly improved cornea sensitivity compared to untreated obese rats. Krill oil was equally effective but did not reach significance to untreated obese rats. Treatment with algal oils enriched in EPA or DHA were less effective. With late intervention treatment menhaden oil and to a lesser extent algal oil containing both EPA/DHA were effective but did not reach significance compared to untreated obese rats. Other treatments were less effective.
Table 2.
Effect of Early Intervention of Omega-3 Polyunsaturated Fatty Acid Derived from Menhaden Oil, Krill Oil, or Algal Oils Enriched in EPA, DHA or EPA/DHA on Peripheral Neuropathy-Related Endpoints in Obese Sprague Dawley Rats
| Conditions | MNCV (m/sec) | SNCV (m/sec) | Thermal Nociception (sec) | IENF (Profiles/mm) | Cornea Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 53.1 ± 1.5 | 39.2 ± 0.7 | 11.6 ± 0.7 | 26.2 ± 0.2 | 7.5 ± 0.4 | 5.94 ± 0.04 | 27 ± 9 |
| Obese | 50.9 ± 1.7 | 33.4 ± 0.7a | 20.4 ± 1.3a | 19.3 ± 0.3a | 3.4 ± 0.2a | 5.17 ± 0.12a | 120 ± 12a |
| Obese + MO | 58.2 ± 1.8b | 38.9 ± 0.9b | 12.9 ± 0.6b | 22.2 ± 0.3a,b | 6.9 ± 0.5b | 5.78 ± 0.08b | 71 ± 7b |
| Obese + KO | 54.6 ± 2.1 | 38.5 ± 0.9b | 14.2 ± 0.5b | 20.6 ± 0.4a | 6.4 ± 0.3b | 5.94 ± 0.04b | 76 ± 15 |
| Obese + EPA | 56.5 ± 1.6 | 37.5 ± 0.9b | 10.6 ± 0.9b | 18.7 ± 0.3a | 4.7 ± 0.4a,c | 5.66 ± 0.09 | 106 ± 15a |
| Obese + DHA | 56.8 ± 1.5 | 39.4 ± 0.8b | 12.6 ± 0.7b | 20.8 ± 0.2a | 6.4 ± 0.5b | 5.75 ± 0.07b | 89 ± 10a |
| Obese + EPA/DHA | 55.0 ± 2.6 | 38.8 ± 1.0b | 11.9 ± 0.6b | 19.4 ± 0.3a | 6.3 ± 0.4b | 5.81 ± 0.08b | 68 ± 12b |
Notes: Data are presented as the mean ± S.E.M. n = 10–14. a: P < 0.05 vs control; b: P < 0.05 vs Obese; c: P < 0.05 vs Obese + MO, Obese + KO, Obese + DHA and Obese + EPA/DHA.
Abbreviations: IENF, intraepidermal nerve fiber; MO, menhaden oil; KO, krill oil; AO, algal oil; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Table 3.
Effect of Late Intervention of Omega-3 Polyunsaturated Fatty Acid Derived from Menhaden Oil, Krill Oil, or Algal Oils Enriched in EPA, DHA or EPA/DHA on Peripheral Neuropathy-Related Endpoints in Obese Sprague Dawley Rats
| Conditions | MNCV (m/sec) | SNCV (m/sec) | Thermal Nociception (sec) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 56.9 ± 1.8 | 39.0 ± 0.4 | 12.2 ± 0.7 | 26.0 ± 0.3 | 7.5 ± 0.3 | 5.88 ± 0.06 | 54 ± 4 |
| Obese | 53.2 ± 1.2 | 33.0 ± 1.0a | 20.3 ± 1.1a | 19.7 ± 0.2a | 3.5 ± 0.3a | 5.03 ± 0.14a | 143 ± 12a |
| Obese + MO | 54.8 ± 2.1 | 37.6 ± 0.8b | 13.5 ± 0.6b | 22.4 ± 0.3a,b | 6.2 ± 0.3b | 5.81 ± 0.07b | 52 ± 15 |
| Obese + KO | 54.4 ± 1.6 | 37.7 ± 1.0b | 14.0 ± 0.6b | 20.7 ± 0.3a | 6.4 ± 0.6b | 5.67 ± 0.08b | 103 ± 17 |
| Obese + EPA | 50.8 ± 1.5 | 36.7 ± 1.2 | 10.8 ± 0.5b | 20.5 ± 0.3a | 4.2 ± 0.5a,c | 5.61 ± 0.10b | 102 ± 12 |
| Obese + DHA | 52.6 ± 1.8 | 38.4 ± 0.5b | 14.3 ± 0.6b | 20.6 ± 0.2a | 5.2 ± 0.5b | 5.75 ± 0.06b | 110 ± 11 |
| Obese + EPA/DHA | 53.3 ± 1.6 | 38.7 ± 0.9b | 13.4 ± 0.8b | 20.6 ± 0.3a | 6.5 ± 0.3b | 5.81 ± 0.10b | 78 ± 12 |
Notes: Data are presented as the mean ± S.E.M. n = 10–14. aP < 0.05 vs control; bP < 0.05 vs Obese; cP < 0.05 vs Obese + MO, Obese + KO, Obese + DHA and Obese + EPA/DHA.
Abbreviations: IENF, intraepidermal nerve fiber; MO, menhaden oil; KO, krill oil; AO, algal oil; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Tables 4 and 5 present data for the effect of early and late intervention treatment with diets containing ethyl esters of EPA, DHA or EPA/DHA of obese rats on PN-related endpoints. As a positive control treatment with menhaden oil was included. The decrease in sensory nerve conduction velocity in obese rats was significantly improved with menhaden oil and ethyl esters of DHA and EPA/DHA following either protocol. Treatment with EPA ethyl ester improved sensory nerve conduction velocity, but the difference did not reach significance. The decrease in thermal response latency observed in obese rats was significantly improved with all treatments. Thermal sensitivity improved even though intraepidermal nerve fiber density remained significantly decreased compared to control rats for untreated and omega-3 PUFA treated obese rats. There was moderate improvement when obese rats were treated with menhaden oil as observed in the study of algal oils. Cornea sensitivity as determined using Crochet Bonnet was decreased in obese rats and significantly improved with dietary treatment with menhaden oil, or ethyl esters of DHA and EPA/DHA in both early and late intervention protocols. It was also improved with treatment with EPA ethyl ester, but the difference did not reach significance for the early intervention. Early intervention treatment of obese rats with menhaden oil and the ethyl ester of EPA/DHA significantly improved cornea sensitivity as determined using a hyperosmotic solution. Improvement was also observed with ethyl ester treatment of EPA or DHA but did not reach significance. For the late intervention treatment protocol all the treatments improved the decrease in cornea sensitivity compared to untreated obese rats but did not reach significance. The decrease in cornea nerve fiber length in obese rats was significantly improved following treatment with menhaden oil and ethyl esters of DHA and EPA/DHA in both protocols. Following treatment with EPA ethyl ester cornea nerve fiber length remained significantly decreased compared to control with marginal improvement compared to untreated obese rats with early intervention.
Table 4.
Effect of Early Intervention of Omega-3 Polyunsaturated Fatty Acid Derived from Menhaden Oil, or Ethyl Esters of EPA, DHA or EPA/DHA on Peripheral Neuropathy-Related Endpoints in Obese Sprague Dawley Rats
| Conditions | MNCV (m/sec) | SNCV (m/sec) | Thermal Nociception (sec) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 56.1 ± 2.4 | 38.8 ± 0.6 | 12.3 ± 0.3 | 26.3 ± 0.2 | 7.5 ± 0.3 | 5.86 ± 0.05 | 35 ± 5 |
| Obese | 52.4 ± 1.6 | 33.6 ± 0.7a | 19.3 ± 1.1a | 19.2 ± 0.3a | 3.7 ± 0.2a | 5.36 ± 0.11a | 114 ± 10a |
| Obese + MO | 53.8 ± 1.6 | 38.8 ± 0.8b | 13.4 ± 0.5b | 22.5 ± 0.4a,b | 6.6 ± 0.2b | 5.81 ± 0.07b | 41 ± 10b |
| Obese + EPA | 54.7 ± 2.2 | 36.0 ± 0.7 | 14.4 ± 1.7b | 20.3 ± 0.4a | 5.5 ± 0.7a | 5.75 ± 0.08 | 77 ± 17 |
| Obese + DHA | 54.6 ± 1.7 | 38.6 ± 1.0b | 14.7 ± 0.9b | 21.1 ± 0.4a | 7.0 ± 0.3b | 5.80 ± 0.08b | 81 ± 13 |
| Obese + EPA/DHA | 55.3 ± 2.1 | 38.2 ± 0.2b | 13.0 ± 0.9b | 20.8 ± 0.4a | 7.3 ± 0.4b | 5.82 ± 0.08b | 49 ± 10b |
Notes: Data are presented as the mean ± S.E.M. n = 10–14. aP < 0.05 vs control; bP < 0.05 vs Obese.
Abbreviations: IENF, intraepidermal nerve fiber; MO, menhaden oil, or Ethyl Esters; EPA, eicosapentaenoic acid, DHA, docosahexaenoic acid.
Table 5.
Effect of Late Intervention of Omega-3 Polyunsaturated Fatty Acid Derived from Menhaden Oil, or Ethyl Esters of EPA, DHA or EPA/DHA on Peripheral Neuropathy-Related Endpoints in Obese Sprague Dawley Rats
| Conditions | MNCV (m/sec) | SNCV (m/sec) | Thermal Nociception (sec) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 55.2 ± 1.8 | 40.0 ± 1.0 | 12.2 ± 0.4 | 25.9 ± 0.1 | 8.5 ± 0.4 | 5.88 ± 0.06 | 47 ± 9 |
| Obese | 52.0 ± 1.7 | 32.8 ± 1.4a | 19.6 ± 1.7a | 19.5 ± 0.3a | 4.3 ± 0.2a | 5.43 ± 0.15a | 136 ± 7a |
| Obese + MO | 56.1 ± 1.4 | 38.7 ± 0.8b | 13.0 ± 0.8b | 22.3 ± 0.3a,b | 7.0 ± 0.4b | 5.84 ± 0.10b | 62 ± 18 |
| Obese + EPA | 49.4 ± 1.2 | 35.6 ± 1.0 | 12.3 ± 1.3b | 19.6 ± 0.5a | 4.9 ± 0.3a | 5.81 ± 0.10b | 80 ± 16 |
| Obese + DHA | 53.8 ± 0.9 | 39.5 ± 0.9b | 11.3 ± 0.9b | 19.4 ± 0.6a | 6.8 ± 0.3b | 5.81 ± 0.09b | 85 ± 17 |
| Obese + EPA/DHA | 52.5 ± 1.2 | 38.2 ± 1.0b | 12.9 ± 0.9b | 19.7 ± 0.6a | 7.6 ± 0.8b | 5.81 ± 0.06b | 78 ± 16 |
Notes: Data are presented as the mean ± S.E.M. n = 10–14. aP < 0.05 vs control; bP < 0.05 vs Obese.
Abbreviations: IENF, intraepidermal nerve fiber; MO, menhaden oil, or Ethyl Esters; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Effect on Vascular Reactivity to Acetylcholine and CGRP by Epineurial Arterioles Providing Blood Flow to the Sciatic Nerve
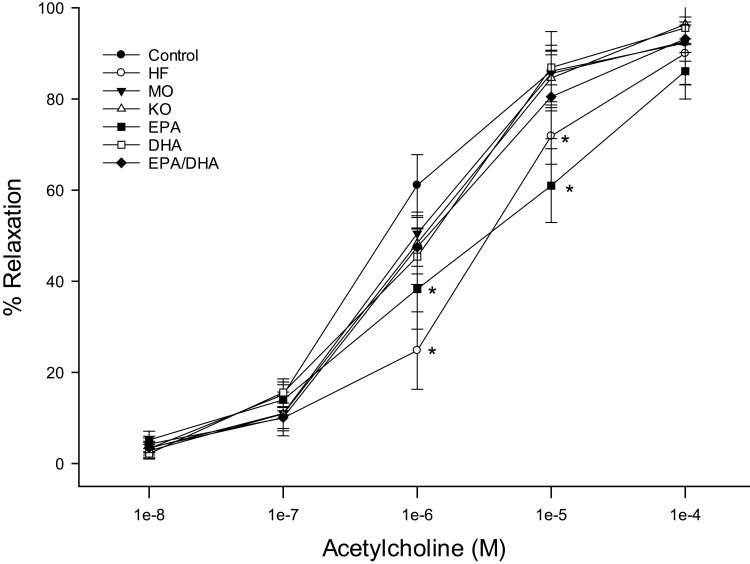
The data reported in Figures 1–4 are from the late intervention protocol. Similar results were observed in the early prevention protocol (data not reported). Data from Figures 1 and 2 are for vessels treated with acetylcholine from rats treated with menhaden oil, krill oil or algal oils of EPA, DHA or EPA/DHA (Figure 1) or menhaden oil or ethyl esters of EPA, DHA or EPA/DHA (Figure 2). Vascular relaxation to acetylcholine was significantly decreased in untreated obese rats (Figures 1 and 2). Treating obese rats with algal oil enriched in EPA (Figure 1) or ethyl ester of EPA (Figure 2) did not improve vascular relaxation. In contrast, treating obese rats with menhaden oil, krill oil or algal oils or ethyl esters of DHA or EPA/DHA improved vascular relaxation to acetylcholine to different degrees, but all were not statistically different from control rats. The other vasodilator we have investigated extensively in epineurial arterioles is calcitonin gene-related peptide (CGRP).25 In Figures 3 and 4 the results were like what was observed with acetylcholine. Vascular relaxation to CGRP was significantly decreased in obese rats compared to control rats. Treatment with menhaden oil, krill oil and algal oils and ethyl esters other than EPA improved vascular relaxation to CGRP.
Figure 1.
Effect of dietary intervention with menhaden oil, krill oil, and oils from algae that produce EPA, DHA or the combination of EPA/DHA on vascular relaxation by acetylcholine in epineurial arterioles of the sciatic nerve in diet-induced obese/pre-diabetic Sprague-Dawley rats. Pressurized arterioles (40 mm Hg and ranging from 60–100 µm luminal diameters) were constricted with phenylephrine (30–50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. The number of rats in each group ranged from 10–14. Data are presented as the mean of % relaxation ± S.E.M. *p < 0.05 compared to control rats.
Figure 2.
Effect of dietary intervention with menhaden oil, and ethyl esters of EPA, DHA or the combination of EPA/DHA on vascular relaxation by acetylcholine in epineurial arterioles of the sciatic nerve in diet-induced obese/pre-diabetic Sprague-Dawley rats. Pressurized arterioles (40 mm Hg and ranging from 60–100 µm luminal diameters) were constricted with phenylephrine (30–50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. The number of rats in each group ranged from 10–14. Data are presented as the mean of % relaxation ± S.E.M. *p < 0.05 compared to control rats.
Figure 3.
Effect of dietary intervention with menhaden oil, krill oil, and oils from algae that produce EPA, DHA or the combination of EPA/DHA on vascular relaxation by CGRP in epineurial arterioles of the sciatic nerve in diet-induced obese/pre-diabetic Sprague-Dawley rats. Pressurized arterioles (40 mm Hg and ranging from 60–100 µm luminal diameters) were constricted with phenylephrine (30–50%) and incremental doses of CGRP were added to the bathing solution while recording steady state vessel diameter. The number of rats in each group ranged from 10–14. Data are presented as the mean of % relaxation ± S.E.M. *p < 0.05 compared to control rats.
Figure 4.
Effect of dietary intervention with menhaden oil, and ethyl esters of EPA, DHA or the combination of EPA/DHA on vascular relaxation by CGRP in epineurial arterioles of the sciatic nerve in diet-induced obese/pre-diabetic Sprague-Dawley rats. Pressurized arterioles (40 mm Hg and ranging from 60–100 µm luminal diameters) were constricted with phenylephrine (30–50%) and incremental doses of CGRP were added to the bathing solution while recording steady state vessel diameter. The number of rats in each group ranged from 10–14. Data are presented as the mean of % relaxation ± S.E.M. *p < 0.05 compared to control rats.
Discussion
There is an expanding interest in the potential benefits of omega-3 PUFA in age-related diseases.14,26,27 It has been shown that long-term intake of omega-3 PUFA is generally safe and has been shown to be beneficial for cardiovascular disease, cognitive function, diabetes, insulin resistance, dyslipidemia, muscle atrophy, cancer, and fetal development.14,26–28 If omega-3 PUFA supplementation is to become an important nutrient enrichment or treatment for metabolic related disease an important question to address is capsulated fish oil the best source of omega-3 PUFA? In the United States, the Food and Drug Administration (FDA) regulates prescription fish oil supplements but not capsules over-the counter (OTC). That means some OTC supplement products may not contain what the label says. Fish oil is derived from the tissues of oily fish, such as mackerel, herring, tuna, and salmon. Many of these fish are farm raised and the environmental condition in that they are raised and diet provided, impacts the quantity and quality of omega-3 PUFA harvested. Lastly, depending on the size of the fish being harvested toxic elements such as mercury need to be considered. Can similar health benefits be realized from a more environmentally friendly, reliable, and safe source of omega-3 PUFA?
For personal consumption, a potential source of omega-3 PUFA other than fish oil are oils or supplements from walnuts, flaxseed, chia seeds, hemp seeds, edamame, and kidney beans that are enriched in α-linolenic acid a precursor for EPA and DHA. For instance, a recent study demonstrated that daily consumption of 30g/day of ground flaxseed significantly improved several cardiometabolic risk factors, including body weight, body mass index, lipid levels, blood pressure, glycemic measures, markers of inflammation (eg, C-reactive protein and interleukin-6), oxidative stress, and liver enzymes.29 However, for larger scale production direct sources for EPA and DHA are krill oil, algae, yeast, and pharmaceutically derived compounds.30 Krill oil is a sustainable popular alternative to fish oil as a source of omega-3 fatty acids although more expensive. Krill oil is made from krill, a small crustacean found in the ocean. The omega-3 PUFA from fish oil are triglyceride based while from krill oil they are phospholipid based, which could have an impact on bioavailability and absorption.31,32 A recent meta-analysis of clinical trials using krill oil supplementation demonstrated significant improvement in lipid metabolism by decreasing the total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels.33 Algae are the primary source of omega-3 PUFA in the food chain for marine fish and mammals. Certain species of algae exist that produce primarily EPA or DHA or the combination of EPA/DHA with the oil from some algae having EPA and DHA levels similar to native fish oil.34,35 Clinical trials using algal DHA-rich oil demonstrated improvement of some markers of cardiometabolic risk and decreased plasma triacylglycerol.36 In these studies, the treatment was well tolerated without adverse events such as fishy taste and/or eructation.36 Prescription omega-3-acid ethyl esters have also provided promise as an effective delivery of omega-3 PUFA. These highly purified drugs have been found to be safe with adverse events reported to be like placebo.37–39 The recent REDUCE-IT and JELIS trials have shown that icosapent ethyl (ethyl ester of EPA) to have a statistical benefit on reducing ischemic events in subjects with hypertriglyceridemia.40,41
Fish oil, krill oil, algal oil Life’s Omega 45 O2412 – O100, or ethyl ester MEG-3 5020 EE oil contain both EPA/DHA as represented by the fatty acid composition of the diets prepared from these different omega-3 PUFA sources. In contrast, diets prepared from algal oils or ethyl esters containing either EPA or DHA, the EPA and DHA composition of the diets were represented by the omega-3 fatty acid content of the algal oils or ethyl esters used. In this study, we were careful to standardize the diets. The diets containing fish oil, krill oil or any of the three algal oils were all made by replacing 50% of the kcal in the high fat diet with the appropriate oil. Likewise, the three diets containing omega-3 PUFA as ethyl esters were made using the same amount of their oil. Each of these diets significantly increased the omega-3 index to a healthy range and the omega-3 PUFA composition of the serum and liver as expected.20 There was some variability that was probably due to differences in absorption. This could have been addressed by selectively increasing the amount of oil used for each diet. However, we choose to standardize the algal oil and ethyl ester diets for a better comparison of outcome for PN associated with obesity/pre-diabetes.
Fish oil, krill oil and algal oil and ethyl esters containing both EPA/DHA in early and late intervention protocols delayed the progression and stimulated regeneration of sensory nerve conduction velocity, thermal nociception, cornea nerve fiber length, and cornea sensitivity, respectively. The neuropathic endpoint that was less responsive to these treatments was intraepidermal nerve fiber density. The presence of skin nerve fibers was significantly improved with treatment of fish oil compared to untreated high fat fed rats but remained significantly impaired compared to control rats. Treatment with krill oil, or algal or ethyl esters containing both EPA/DHA did not improve intraepidermal nerve fiber density compared to control rats. These treatments improved vascular reactivity of epineurial arterioles to acetylcholine and CGRP. When obese/pre-diabetic rats were treated with algal oil or ethyl ester having only DHA, the effect on PN-related endpoints and vascular reactivity were like that of combined EPA/DHA treatments. In contrast, treating obese/pre-diabetes rats with algal oil or ethyl ester having only EPA was less effective in improving PN-related endpoints and provided no benefit for impaired vascular reactivity. This was unexpected since in studies using type 2 diabetic mice, we showed that daily treatment with resolvin D1 (a docosahexaenoic acid metabolite) or resolvin E1 (a eicosapentaenoic metabolite) was as effective as fish oil improving PN.42,43 We attributed the effect of these metabolites to their anti-inflammatory properties. It is likely that the beneficial effects of DHA from sources other than fish oil on PN once consumed and incorporated provide an efficient origin to produce its metabolites, whereas EPA is not as effectively utilized. A recent study by Torres-Vanegas et al reported that diet supplementation with marine omega-3 PUFA in subjects with obesity increased resolvin D1 levels and reduced inflammatory markers.44 Dzupina et al reported that treating patients with metabolic syndrome with omega-3 PUFA improved endothelial dysfunction.45
We had previously reported that treatment of obese rats with fish oil significantly improved but not fully restore intraepidermal nerve fiber density.8 The reason for the poor responsiveness of this endpoint to the other EPA/DHA combined treatments is unknown given the favorable response with these treatments to regeneration of corneal nerves. It has been previously reported that skin biopsy and corneal nerve microscopy detects PN in obese subjects with impaired glucose tolerance.46 Indicating that both can detect small-fiber damage. We can only speculate that the poor regeneration of sensory nerves in the skin by the other sources of omega-3 PUFA is a localized event and that omega-3 PUFA derived from fish oil are more beneficial due to better localization in the skin.
An important result from these studies was that treatment of obese/pre-diabetic rats with EPA/DHA, derived from fish oil, krill oil or combination of EPA/DHA from algal oil or as an ethyl ester, or DHA, derived from algal oil or as an ethyl ester, alone was better protecting vascular relaxation of epineurial arterioles to acetylcholine and CGRP than treatment with EPA alone whether EPA was derived from algal oil or as an ethyl ester. Likewise, treatment with EPA alone was also less effective in protecting/restoring sensory nerve conduction velocity and corneal nerve fiber length.
In the REDUCE-IT study, patients with elevated triglyceride levels despite the use of statins, the risk of ischemic events, including cardiovascular death, was significantly lower among those who received 2 g of icosapent ethyl twice daily than among those who received placebo.40 Further study suggested that EPA improves endothelial function during inflammation that may contribute to icosapent ethyl distinct cardiovascular benefits.41 However, these studies failed to examine the effect of the ethyl ester of DHA. In contrast, other studies using a combination of EPA/DHA failed to show any cardiovascular benefit.47 However, comparison of these study results is challenging because of dosing and other study differences. Van et al demonstrated that esterification of DHA enhanced its transport to the brain.48 This study did not examine whether esterification of EPA was beneficial to transport. A study by Joardar and Chakraborty concluded that EPA and DHA have differential effects on membrane dynamics.49 A review article by Michaeloudes et al reported a wide range of variability in the clinical effects of EPA and DHA in cardiovascular disease including individual characteristics.20
A limitation of the study results is that the algal oil EPA was from a different supplier. This compound is not readily available or as pure as the oils from DSM Nutritional Products. Nonetheless, it did modify the fatty acid composition of serum, red blood cell membranes, and liver as expected.
Conclusion
This study demonstrated that multiple sources of omega-3 PUFA can increase the omega-3 fatty acid composition of serum, red blood cells and tissue. The use of alternative sources of EPA and DHA may be economically more favorable and reduce environmental and safety risks that are associated with excessive consumption of fish.50 In a recent study using flaxseed oil as a source of α-linolenic acid the omega-3 index was not increased in dogs compared to supplementation of fish or krill oil.51 Suggesting that α-linolenic acid as a precursor to EPA and DHA is not sufficiently bio-converted to change the omega-3 index.50,52 If metabolites of EPA and DHA are important mediators of omega-3 PUFA treatment sources providing α-linolenic acid may be less beneficial. We show that multiple sources of omega-3 PUFA are capable as serving as treatment for obesity and pre-diabetes related PN and vascular dysfunction of epineurial arterioles that provide circulation to the sciatic nerve with sources of DHA alone or the combination of EPA/DHA being more effective than EPA alone. These studies were done using male rats. We have repeated these studies using 4–6 diet-induced obese female rats in each group (not reported here), and the results were unchanged from male rats.53 Thus, we conclude that the effects of omega-3 PUFA on PN and vascular dysfunction are not gender specific.
Additional studies are needed to determine the best sustainable source of omega-3 PUFA and the direct benefits these fatty acids provide for human health and disease.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development (RX003826; MY), Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development, Iowa City VA Center of Excellence for the Prevention and Treatment of Visual Loss: C9251-C and National Institutes of Health DK 126837. The content of this manuscript is new and solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies. The authors are grateful to the generosity of DSM Nutritional Products for providing the ethyl ester fatty acids for this study.
Abbreviations
CGRP, calcitonin gene-related peptide; DHA, docosahexaenoic acid; EE, ethyl ester; EPA, eicosapentaenoic acid; FDA, Food and Drug Administration; KO, krill oil; HF, high fat; MO, menhaden oil, OIG, Office of Inspector General; OTC, over-the counter; PN, peripheral neuropathy; PUFA, polyunsaturated fatty acids.
Date Sharing Statement
The datasets generated for this study will be made available on request to the corresponding author and authorization by the office of the OIG since this study was supported by Veterans Affairs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cortez M, Singleton JR, Smith AG. Glucose intolerance, metabolic syndrome, and neuropathy impaired glucose tolerance and neuropathy. Handbook of Clin Neurol. 2014;126:109–122. doi: 10.1016/B978-0-444-53480-4.00009-6 [DOI] [PubMed] [Google Scholar]
- 2.Elafros MA, Reynolds EL, Callaghan BC. Obesity-related neuropathy: the new epidemic. Curr Opin Neurol. 2024;37(5):467–477. doi: 10.1097/WCO0000000000001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring MJ, Davalos L. Peripheral neuropathy. Primary Care Clin Office Pract. 2024;51(2):327–344. doi: 10.1016/j.pop.2023.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Piccolo N, Wiggers A, Koubek EJ, Feldman EL. Neuropathy and the metabolic syndrome. eNeurologicalSci. 2024;38:100542. doi: 10.1016/j.ensci.2024.100542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppey L, Davidson E, Shevalye H, Obrosov A, Yorek M. Effect of early and late interventions with dietary oils on vascular and neural complications in a type 2 diabetic rat model. J Diabetes Res. 2019;2019:5020465. doi: 10.1155/2019/5020465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yorek MA. The potential role of fatty acids in treating diabetic neuropathy. Curr Diab Rep. 2018;18(10):86. doi: 10.1007/s11892-018-1046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA. Effect of dietary content of menhaden oil with or without salsalate on neuropathic endpoints in high-fat-fed/low-dose streptozotocin-treated Sprague Dawley rats. J Diabetes Res. 2018;2018:2967127. doi: 10.1155/2018/2967127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppey L, Davidson E, Shevalye H, Torres ME, Yorek MA. Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab Syndr Obes. 2018;11:117–127. doi: 10.2147/DMSO.S159071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation. 2017;135(15):e867–e884. doi: 10.1161/CIR.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia. Circulation. 2019;140(12):e673–e691. doi: 10.1161/CIR.0000000000000709 [DOI] [PubMed] [Google Scholar]
- 11.Welty FK. Omega-3 fatty acids and cognitive function. Curr Opin Lipidol. 2023;34(1):12–21. doi: 10.1097/MOL.0000000000000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei BZ, Li L, Dong CW, et al. The relationship of omega-3 fatty acids with dementia and cognitive decline: evidence from prospective cohort studies of supplementation, dietary intake, and blood markers. Am J Clin Nutr. 2023;117(6):1096–1109. doi: 10.1016/j.ajcnut.2023.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Wang L, Wang D, et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes cardiovascular disease, cancer, and mortality. Clin Nutr. 2022;41(8):1798–1807. doi: 10.1016/j.clnu.2022.06.034 [DOI] [PubMed] [Google Scholar]
- 14.Troesch B, Eggersdorfer M, Laviano A, et al. Expert opinion of benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. 2020;12(9):2555. doi: 10.3390/nu12092555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liput KP, Lepczynski A, Ogluszka M, et al. Effects of dietary n-3 and n-6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int J Mol Sci. 2021;22(13):6965. doi: 10.3390/ijms22136965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva Alves B, Schimith LE, da Cunha AB, Dora CL, Hort MA. Omega-3 polyunsaturated fatty acids and Parkinson’s disease: a systematic review of animal studies. J Neurochem. 2024;168(8):1655–1683. doi: 10.1111/jnc.16154 [DOI] [PubMed] [Google Scholar]
- 17.Qin J, Kurt E, LBassi T, Sa L, Xie D. Biotechnological production of omega-3 fatty acids: current status and future perspectives. Front Microbiol. 2023;14:1280296. doi: 10.3389/fmicb.2023.1280296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker EJ. Alternative sources of bioactive omega-3 fatty acids: what are the options? Curr Opin Clin Nutr Metab Care. 2024;27(2):106–115. doi: 10.1097/MCO.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 19.Shadi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9(1):345–381. doi: 10.1146/annurev-food-111319-095850 [DOI] [PubMed] [Google Scholar]
- 20.Michaeloudes C, Christodoulides S, Christodoulou P, Kyriakou TC, Patrikios I, Stephanou A. Variability in the clinical effects of the omega-3 polyunsaturated fatty acids DHA and EPA in cardiovascular disease – possible causes and future considerations. Nutrients. 2023;15(22):4830. doi: 10.103390/nu15224830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorek MA, Bohnker RR, Dudley DD, Spector AA. Comparative utilization of n-3 polyunsaturated fatty acids by cultured human Y-79 retinoblastoma cells. Biochim Biophys Acta. 1984;795(2):277–285. doi: 10.1016/0005-2760(84)90076-6 [DOI] [PubMed] [Google Scholar]
- 22.Petropoulos IN, Bitirgen G, Ferdousi M, et al. Corneal confocal microscopy to image small nerve fiber degeneration: ophthalmology meets neurology. Front Pain Res. 2021;2:725363. doi: 10.3389/fpain.2021.725363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahidi AM, Sampson GP, Pritchard N, et al. Exploring retinal and functional markers of diabetic neuropathy. Clin Exp Optom. 2010;93(5):309–323. doi: 10.1111/j.1444-0938.2010.00491.x [DOI] [PubMed] [Google Scholar]
- 24.Yorek MS, Davidson EP, Poolman P, et al. Corneal sensitivity to hyperosmolar eye drops: a novel behavioral assay to assess diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2016;57(6):2412–2419. doi: 10.1167/iovs.16-19435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yorek MA, Coppey LJ, Gellett JS, Davidson EP. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: effect of streptozotocin-induced diabetes. Exp Diabesity Res. 2004;5(3):187–193. doi: 10.1080/15438600490486732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3(1):1–7. doi: 10.3945/an.111.000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gareri P. Omega-3 long-chain polyunsaturated fatty acids in the elderly: a review. OBM Geriatric. 2022;6(2):2. doi: 10.21926/obm.geriatr.2202198 [DOI] [Google Scholar]
- 28.Weintraub H. Update on marine omega-3 fatty acids: management of dyslipidemia and current omega-3 treatment options. Atherosclerosis. 2013;230(2):381–389. doi: 10.1016/j.atherosclerosis.2013.07.041 [DOI] [PubMed] [Google Scholar]
- 29.Kunutsor SK, Jassal DS, Ravandi A, Lehoczki A. Dietary flaxseed: cardiometabolic benefits and its role in promoting healthy aging. GeroScience. 2025;47(3):2895–2923. doi: 10.1007/s11357-025-01512-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari P, Sharma J, Khare P. Recent advancements and strategies for omega-3 fatty acid production in yeast. J Basic Microbiol. 2025;12:e2400491. doi: 10.1002/jobm.202400491 [DOI] [PubMed] [Google Scholar]
- 31.Colletti A, Cravotto G, Citi V, Martelli A, Testai L, Cicero AFG. Advances in technologies for highly active omega-3 fatty acids from krill oil: clinical applications. Mar Drugs. 2021;19(6):306. doi: 10.3390/md19060306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulven SM, Holven KB. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc Health Risk Manag. 2015;11:511–524. doi: 10.2147/VHRM.585165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Liao D, He B, Zhou G, Cui Y. Clinical effectiveness of krill oil supplementation on cardiovascular health in humans: an updated systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2023;17(12):102909. doi: 10.1016/j.dsx.2023.102909 [DOI] [PubMed] [Google Scholar]
- 34.Ma X-N, Chen T-P, Yang B, Liu J, Chen F Lipid production from Nannochloropsis. Mar Drugs. 2016;14:61. doi: 10.3390/md14040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Fan K-W, Wong RT, Chen F Fatty acid composition and squalene of the marine microalgae schizochytrium mangrovei. J Agric Food Chem. 2004;52:1196–1200. doi: 10.1021/jf035004c [DOI] [PubMed] [Google Scholar]
- 36.Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega-3 fatty acids: public health risks and benefits. Brit J Nutr. 2012;107(S2):S23–S52. doi: 10.1017/S0007114512001456 [DOI] [PubMed] [Google Scholar]
- 37.Brunton S, Collins N. Differentiating prescription omega-3 ethyl esters (P-OM3) from dietary-supplement omega-3 fatty acids. Curr Med Res Opin. 2007;23(5):1139–1145. doi: 10.1185/030079907X188017 [DOI] [PubMed] [Google Scholar]
- 38.Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med. 2014;126(7):7–18. doi: 10.3810/pgm.2014.11.2828 [DOI] [PubMed] [Google Scholar]
- 39.Laksmanan S, Budoff MJ. The evolving role of omega-3 fatty acids in cardiovascular disease: is icosapent ethyl the answer? Heart Int. 2021;15(1):7–13. doi: 10.17925/HI.2021.15.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt DL, Steg PG, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791–2802. doi: 10.1016/j.jacc.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 41.Sherratt SCR, Libby P, Dawoud H, Bhatt DL, Mason RP. Eicosapentaenoic acid improves nitric oxide bioavailability via changes in protein expression during inflammation. J Am Heart Assoc. 2024;13(e034076). doi: 10.1161/JAHA.123.034076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yorek MS, Coppey LJ, Shevalye H, Obrosov A, Kardon RH, Yorek MA. Effect of treatment with salsalate, menhaden oil, combination of salsalate and menhaden oil, or resolvin D1 of C57Bl/6J type 1 diabetic mouse on neuropathic endpoints. J Nutr Metab. 2016;2016:5905891. doi: 10.1155/2016/5905891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obrosov A, Coppey LJ, Shevalye H, Yorek MA. Effect of fish oil vs. resolvin D1, E1, methyl esters of resolvins D1 or D2 on diabetic peripheral neuropathy. J Neurol Neurophysiol. 2017;8(06):453. doi: 10.4172/2155-9562.1000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Vanegas J, Rodriguez-Echevarria R, Campos-Perez W, et al. Effect of a diet supplemented with marine omega-3 fatty acids on inflammatory markers in subjects with obesity: a randomized active placebo-controlled trial. Healthcare. 2025;13(2):103. doi: 10.3990/healthcare13020103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dzupina A, Pella D, Zenuch P, et al. The role of omega-3 polyunsaturated fatty acids in patients with metabolic syndrome and endothelial dysfunction. Medicina. 2025;61(1):43. doi: 10.3390/medicina61010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37(9):2643–2646. doi: 10.2337/dc14-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney TE, Gaine SP, Michos ED. Eicosapentaenoic acid vs. docosahexaenoic acid for the prevention of cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2022;30(2):87–93. doi: 10.1097/MED.0000000000000796 [DOI] [PubMed] [Google Scholar]
- 48.Lo Van A, Bernoud-Hubac N, Lagarde M. Esterification of docosahexaenoic acid enhances its transport to the brain and its potential therapeutic use in brain disease. Nutrients. 2022;14(21):4550. doi: 10.3390/nu14214550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joardar A, Chakraborty H. Differential behavior of eicosapentaenoic and docosahexaenoic acids on the organization, dynamics, and fusion of homogenous and heterogeneous membranes. Langmuir. 2023;39(12):4439–4449. doi: 10.1021/acs.langmuir.3c00119 [DOI] [PubMed] [Google Scholar]
- 50.Venegas-Caleron M, Napier JA. New alternative sources of omega-3 fish oil. Adv Food Nutr Res. 2023;105:343–398. doi: 10.1016/bs.afnr.2023.01.001 [DOI] [PubMed] [Google Scholar]
- 51.Lindqvist H, Dominguez T, Dragoy R, Ding Y, Burri L. Comparison of fish, krill and flaxseed as omega-3 sources to increase the omega-3 index in dogs. Vet Sci. 2023;10(2):162. doi: 10.3390/vetsci10020162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos HO, Price JC, Bueno AA. Beyond fish oil supplementation: the effect of alternative plant sources of omega-3 polyunsaturated fatty acids upon lipid indexes and cardiometabolic biomarkers – an overview. Nutrients. 2020;12(10):3159. doi: 10.3390/nu12103159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppey LJ, Shevalye H, Obrosov A, Davidson EP, Yorek MA. Determination of peripheral neuropathy in high-fat diet fed low-dose streptozotocin-treated female C57Bl/6J mice and Sprague-Dawley rats. J Diabetes Investig. 2018;9(5):1033–1040. doi: 10.1111/jdi.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]