Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become a global epidemic, yet effective pharmacological treatments remain limited. Secreted proteins play diverse roles in regulating glucose and lipid metabolism, and their dysregulation is implicated in the development of various metabolic diseases, including MASLD. Therefore, targeting secreted proteins and modulating associated signaling pathways represents a promising therapeutic strategy for MASLD. In this review, we summarize recent findings on the roles of emerging families of secreted proteins in MASLD and related metabolic disorders. These include the orosomucoid (ORM) family, secreted acidic cysteine rich glycoprotein (SPARC) family, neuregulin (Nrg) family, growth differentiation factor (GDF) family, interleukin (IL) family, fibroblast growth factor (FGF) family, bone morphogenic protein (BMP) family, as well as isthmin-1 (Ism1) and mesencephalic astrocyte-derived neurotrophic factor (MANF). The review highlights their impact on glucose and lipid metabolism and discusses the clinical potential of targeting these secreted proteins as a therapeutic approach for MASLD.
Keywords: secreted proteins, metabolic dysfunction-associated steatotic liver disease, metabolic dysfunction-associated steatohepatitis, hepatic lipid metabolism, clinical application
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), has become the most common liver disease worldwide (Miao et al., 2024). MASLD encompasses a spectrum of conditions, ranging from simple steatosis to a more serious form known as metabolic dysfunction-associated steatohepatitis (MASH) (Feng et al., 2024; Miao et al., 2024). While the primary cause of simple steatosis is the excessive accumulation of triglycerides (TG) in hepatocytes (Feng et al., 2024; Miao et al., 2024), MASH is characterized by chronic liver inflammation and injury. Moreover, MASH can progress to liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) (Musale et al., 2023). Alarmingly, global epidemiological data from 2010 to 2019 indicate that MASH is the fastest growing cause of HCC, particularly in the Americas (Huang et al., 2022; Loomba et al., 2021). Mechanistically, the two-hit hypothesis proposed over two decades ago (Day and James, 1998) is now considered outdated. Instead, the multiple-hit hypothesis has gained prominence, incorporating factors such as genetic susceptibility, dysregulation of lipid metabolism in hepatocytes, alterations in gut microbiota, inflammasome activation, and inter-organ interactions (Fabbrini et al., 2010; Friedman et al., 2018; Loomba et al., 2021; Zhao et al., 2023). Currently, Resmetirom, a thyroid hormone receptor β agonist, is the only approved medication for patients with MASLD/MASH (Harrison et al., 2024b; Xie et al., 2024). Although Resmetirom has shown efficacy in achieving MASH resolution, improving fibrosis, and enhancing health-related quality of life, its therapeutic benefits are limited, with only 25%–30% of patients responding to the treatment (Harrison et al., 2024a; Younossi et al., 2025). This highlights the urgent need for more effective therapeutic strategies and underscores the ongoing challenges in developing novel treatments for MASLD/MASH (Tilg et al., 2023).
Secreted proteins play pivotal roles in regulating glucose and lipid metabolism through autocrine, paracrine and endocrine signaling. Pharmacotherapies based on secreted proteins, such as insulin analogues and GLP-1-like incretin mimetics, are integral to the treatment of diabetes or obesity. Over the past few decades, a growing number of secreted proteins with metabolic functions have been identified. These include hepatokines from the liver (Jensen-Cody and Potthoff, 2021), adipokines from adipose tissue (Jung and Choi, 2014), myokines from skeletal muscle (Chen et al., 2024c), cytokines from immune cells (Ouyang and O’Garra, 2019), and secreted proteins originating from the gut (Muskiet et al., 2017). These findings provide valuable opportunities for the development of new pharmaceutical therapies for metabolic diseases, including MASLD/MASH. While the molecular effects of many secreted proteins are currently under investigation, advancing our understanding of their clinical significance is equally important. In this review, we highlight the latest progress in understanding the roles of secreted proteins in hepatic TG metabolism, liver inflammation and fibrosis, and related metabolic disorders (Fig. 1). We also discuss their preclinical and clinical applications in treating MASLD/MASH (Fig. 2). These secreted proteins include the orosomucoid (ORM) family, secreted acidic cysteine rich glycoprotein (SPARC) family, neuregulin (Nrg) family, growth differentiation factor (GDF) family, interleukin (IL) family, fibroblast growth factor (FGF) family, bone morphogenic protein (BMP) family, as well as isthmin-1 (Ism1) and mesencephalic astrocyte-derived neurotrophic factor (MANF).
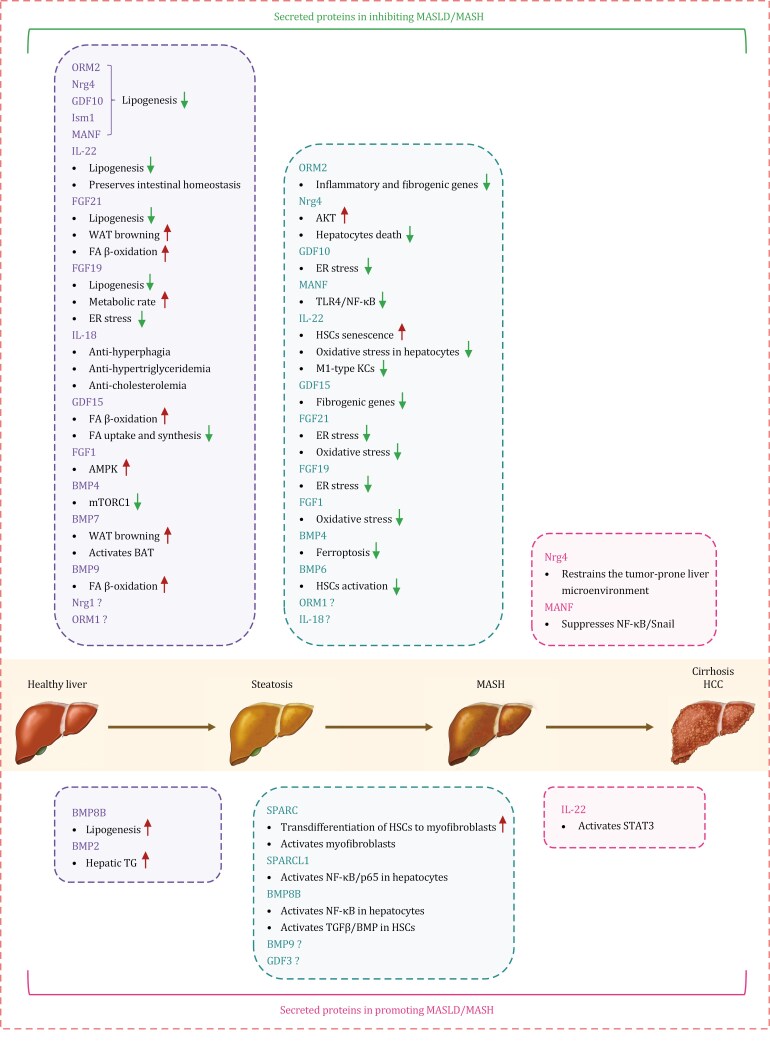
Figure 1.
Roles of secreted proteins in the development and progression of metabolic dysfunction-associated steatotic liver disease. ORM2, Nrg4, GDF10, Ism1, MANF, IL-22, FGF21, FGF19, IL-18, GDF15, FGF1, BMP4, BMP7, BMP9, Nrg1, and ORM1 can ameliorate hepatic steatosis, while BMP8B and BMP2 facilitate it. ORM2, Nrg4, GDF10, MANF, IL-22, GDF15, FGF21, FGF19, FGF1, BMP4, BMP6, ORM1, and IL-18 can alleviate MASH, whereas SPARC, SPARCL1, BMP8B, BMP9, and GDF3 aggravate it. Nrg4 suppresses the progression from MASH to hepatocellular carcinoma, whereas MANF inhibits hepatocellular carcinoma development. BAT, brown adipose tissue; ER, endoplasmic reticulum; FA, fatty acid; HSCs, hepatic stellate cells; KCs, Kupffer cells; WAT, white adipose tissue.
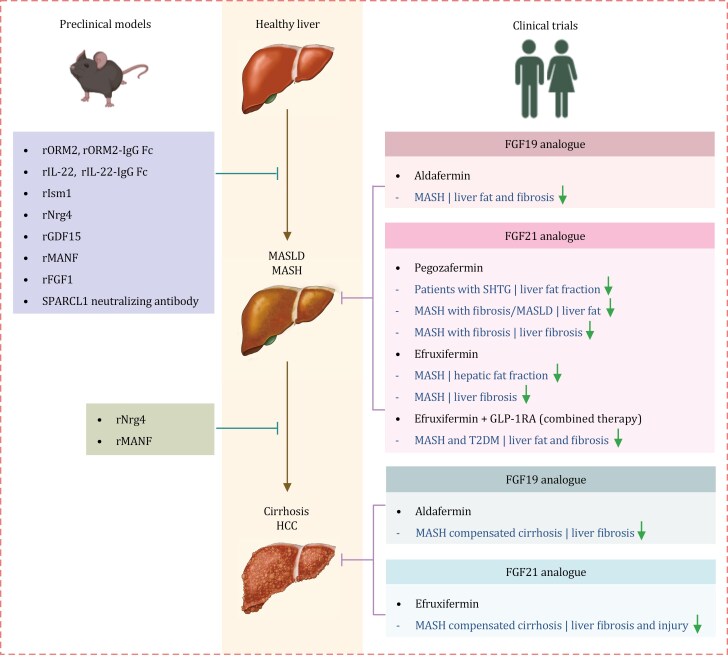
Figure 2.
Secreted proteins as therapeutic targets for treating MASLD and MASH: preclinical and clinical evidence. Preclinical studies show that recombinant proteins like ORM2, ORM2-IgG Fc, IL-22, IL-22-IgG Fc, Ism1, Nrg4, GDF15, MANF, and FGF1, can potentially relieve hepatic steatosis. Moreover, ORM2, Nrg4, GDF15, MANF, FGF1, and SPARCL1 neutralizing antibody can attenuate MASH. In addition, Nrg4 can suppress the progression from MASH to hepatocellular carcinoma, and MANF can inhibit the development of hepatocellular carcinoma. Clinical trials reveal that the FGF19 analogue aldafermin can decrease liver fat content and fibrosis in MASH patients. The FGF21 analogue pegozafermin and efruxifermin can reduce liver fat, fibrosis, and injury in patients with severe hypertriglyceridemia, MASH, and liver fibrosis. Efruxifermin combined with GLP-1 receptor agonist therapy can reduce liver fat and fibrosis in MASH patients with T2DM. Additionally, the FGF19 analogue aldafermin and the FGF21 analogue efruxifermin can improve liver fibrosis and liver injury in patients with MASH-compensated cirrhosis. GLP-1 RA, GLP-1 receptor agonist; r, recombinant; SHTG, severe hypertriglyceridemia.
Secreted proteins as therapeutic targets for treating MASLD/MASH
Orosomucoids
Orosomucoids (ORMs) exist in three isoforms (ORM1, ORM2, and ORM3) in mice and two isoforms (ORM1 and ORM2) in humans. Among these, ORM1 constitutes approximately 75% of plasma ORMs and is mainly expressed in various peripheral tissues, including the liver (Luo et al., 2015). Traditionally, ORMs have been recognized as carriers of drugs and lipids; however, recent studies have revealed their important roles in metabolic regulation (Heo et al., 2024).
ORM1
ORM1 is primarily expressed in peripheral tissues, such as adipose tissue, liver, and skeletal muscle (Luo et al., 2015). ORM1-deficient mice exhibit exacerbated high-fat diet (HFD)-induced MASLD and methionine- and choline-deficient diet (MCD)-induced MASH, suggesting that endogenous ORM1 plays a protective role, although the underlying mechanism remains unclear. Interestingly, liver-specific overexpression of ORM1 fails to mitigate MASLD (Sun et al., 2025), indicating that the liver may not be the primary target for ORM1. Moreover, mice lacking ORM1 show increased fat mass, body weight, and hyperleptinemia. Conversely, exogenous administration of ORM, likely ORM1, significantly reduces food intake and body weight in obese mice by binding to the leptin receptor and activating the JAK2-STAT3 signaling in hypothalamic tissue (Sun et al., 2016). Additionally, ORM1 suppresses inflammation in adipocytes and macrophages, thereby maintaining energy homeostasis (Lee et al., 2010). Treatment with exogenous ORM inhibits adipogenesis and lipogenesis in adipocytes by suppressing multiple early-adipogenic transcription factors and interfering with C/EBPα and PPARγ activation, thereby preventing obesity (Lee et al., 2021). ORM1 is also considered as a myokine; in mice, ORM1 levels increase in skeletal muscle during fatigue, promoting muscle glycogen storage and enhancing muscle endurance via the C-C chemokine receptor type 5 (CCR5)-activated AMPK pathway (Qin et al., 2016). Overall, these findings suggest that ORM1 may improve MASLD/MASH through extrahepatic tissues such as adipose tissue and muscle.
Dysregulation of ORM1 has been linked to MASLD-related disorders. Elevated serum ORM levels have been observed in individuals, mice, and Ossabaw pigs with obesity (Alfadda et al., 2012; Bell et al., 2010; Lee et al., 2010; Sun et al., 2016, 2025). In humans, elevated ORM levels correlate with body mass index (BMI), body fat mass, and fasting plasma glucose levels (Gomes et al., 2003; Lee et al., 2010). Urinary ORM1 is positively correlated with hepatic fat content, homeostasis model assessment of insulin resistance (HOMA-IR), and liver injury parameters such as fibrosis and alanine aminotransferase (ALT) (Liu et al., 2022). A genome-wide association study suggests that ORM1 mediates the link between obesity and MASLD and impacts MASLD independently of obesity (Liu et al., 2023b). Collectively, these findings indicate that ORM1 plays a protective role against MASLD, although further research is required to fully elucidate its mechanisms.
ORM2
Orosomucoid 2 (ORM2) is an acute-phase secretory glycoprotein primarily produced by the liver, particularly in hepatocytes, in response to systemic injury (Luo et al., 2015). Like other acute-phase proteins, ORM2 expression is upregulated in response to many conditions, including inflammation, infection, tissue injury, and tumors (Heo et al., 2024; Hochepied et al., 2003; Huang and Ung, 2013; Irmak et al., 2009; Luo et al., 2015). Recent studies have identified ORM2 as a pivotal regulator of energy homeostasis, inflammation, immune response, and oxidative stress, primarily targeting hepatocytes and other extrahepatic tissues, including adipose tissue and the intestine (Han et al., 2010; Li et al., 2023, 2024; Wan et al., 2019; Zhou et al., 2022; Zhu et al., 2024). We recently found that both hepatic and circulating ORM2 levels were downregulated in mice and patients with MASLD/MASH (Zhou et al., 2022). In vivo studies demonstrated that ORM2 deficiency exacerbates HFD-induced hepatic steatosis and high-fat high-cholesterol (HFHC) diet-induced steatohepatitis in mice. Conversely, ORM2 overexpression mitigates hepatic steatosis and steatohepatitis and improves plasma lipid profiles. These beneficial effects are mainly attributed to the suppression of de novo lipogenesis as well as fatty acid uptake in hepatocytes, independent of body weight and food intake (Li et al., 2023; Zhang et al., 2024a; Zhou et al., 2022). Mechanistically, ORM2 binds to inositol 1,4,5-trisphosphate receptor type 2 (ITPR2) to activate AMP-activated protein kinase (AMPK) signaling, thereby inhibiting the sterol regulatory element binding protein 1c (SREBP-1c)-mediated lipogenic gene program (Zhou et al., 2022).
Hepatocyte-derived ORM2 also influences peripheral tissues, such as adipose tissue. In response to metabolic interventions, including intermittent fasting, bile acid treatment, and bariatric surgery, ORM2 expression is significantly increased. This induction promotes systemic metabolic effects by inducing adipose tissue browning and reducing inflammation in mice (Li et al., 2024; Zhu et al., 2024). Furthermore, although the exact mechanisms remain unclear, ORM2 deficiency has been associated with gut microbiome disturbances and the stimulation of intestinal inflammation in diet-induced obese mice (Li et al., 2024). Beyond peripheral tissues, ORM2 also targets the central nervous system, where it regulates neuronal mitochondrial biogenesis and promotes functional recovery after stroke (Jing et al., 2024).
Given its protective roles, ORM2 holds promise as a therapeutic target for MASLD/MASH and related metabolic diseases. Exogenous administration of recombinant ORM2 or long-acting ORM2-IgG Fc has been shown to attenuate hepatic steatosis, steatohepatitis, hyperlipidemia, and atherosclerosis in preclinical mouse models (Li et al., 2023; Zhou et al., 2022). Clinically, hepatic and plasma ORM2 levels are markedly reduced in obese murine models and patients with MASLD (Zhou et al., 2022). Therefore, multicenter clinical studies are necessary to solidify the connection between ORM2 expression and the development and progression of MASLD/MASH. Furthermore, future studies should assess the beneficial and potential adverse effects of ORM2 treatment in non-human primates before its use in clinic.
Secreted acidic cysteine-rich glycoprotein family
The secreted acidic cysteine-rich glycoprotein (SPARC) family, a group of matricellular proteins, plays key roles in regulating cell proliferation and migration, thereby influencing processes, such as embryogenesis, tissue remodeling, and tumorigenesis (Klingler et al., 2020; Sullivan and Sage, 2004). This family includes molecules, such as SPARC, SPARCL-like 1 (SPARCL1), thrombospondin 1 and 2, and osteopontin, all of which have been implicated in the development of obesity, insulin resistance, and chronic liver diseases (Bai et al., 2020; Icer and Gezmen-Karadag, 2018; Kos and Wilding, 2010).
Secreted acidic cysteine-rich glycoprotein
Secreted acidic cysteine-rich glycoprotein (SPARC), also known as osteonectin or basement-membrane protein 40 (BM-40) (Ryu et al., 2022), is widely expressed in various tissues and organs, including adipose tissue, liver, and skeletal muscle (Kos and Wilding, 2010). As a multifunctional extracellular matrix (ECM) binding protein, SPARC is essential for cellular adhesion, ECM organization and remodeling, cellular growth and differentiation, wound repair, and fibrosis (Bradshaw and Sage, 2001; Rosset and Bradshaw, 2016). As a secreted protein, SPARC also plays a critical role in inter-tissue communication and metabolic regulation.
Induced SPARC expression has been observed in the fibrotic livers of both rodents and humans (Atorrasagasti et al., 2013; Blazejewski et al., 1997; Camino et al., 2008; Frizell et al., 1995; Mazzolini et al., 2018). The upregulation of SPARC in the liver is associated with elevated levels of liver injury and fibrosis markers, such as collagen Iα and transforming growth factor β (TGFβ), in both rodents and humans (Mazzolini et al., 2018). In murine fibrosis models, knockdown or knockout of SPARC markedly attenuates hepatic fibrosis, partly by suppressing the trans-differentiation of hepatic stellate cells (HSCs) into a myofibroblasts-like phenotype and inhibiting myofibroblasts activation (Atorrasagasti et al., 2013; Camino et al., 2008). Knockdown or knockout of SPARC also reduces inflammatory activity, partially by decreasing CD4+ T cells infiltration in the liver (Atorrasagasti et al., 2013; Camino et al., 2008). Furthermore, SPARC knockdown in activated HSCs significantly decreases platelet-derived growth factor-BB (PDGF-BB) or TGFβ-induced migration and reduces the expression of collagen and other ECM molecules (Atorrasagasti et al., 2011). These findings strongly suggest that SPARC upregulation is involved in fibrosis and positions it as a potential therapeutic target for liver fibrosis.
SPARC is also linked to hyperglycemia and insulin resistance. Elevated plasma SPARC levels have been detected in individuals with type 2 diabetes mellitus (T2DM) (Lee et al., 2013). Sparc-knockout mice exhibit abnormal insulin-regulated glucose metabolism, increased adipose tissue deposition, and impaired glucose homeostasis (Atorrasagasti et al., 2019). SPARC has also been implicated in alterations to pancreatic β-cell function. It promotes glucose-stimulated insulin secretion in pancreatic β cells (Harries et al., 2013; Hu et al., 2020). Correspondingly, the absence of SPARC leads to a decrease in GLUT2 expression in β cells, highlighting a defect in the glucose-sensing machinery (Atorrasagasti et al., 2019). Given its effects on lipid metabolism, fibrogenesis, inflammation, and insulin secretion, SPARC presents as an intriguing molecule to study in the context of MASLD and T2DM. Although little clinical studies have yet explored its therapeutic effects, its modulation could potentially impact metabolic diseases, including MASLD/MASH.
SPARC reduction also protects against obesity. In humans, a 2-year, 14% caloric restriction (CR) inhibits SPARC expression in adipose tissue (Ryu et al., 2022). Similarly, weight loss through CR and low-protein diet feeding in mice decreases SPARC expression, while HFD-induced obesity increases SPARC expression in adipose tissue (Ryu et al., 2023). Deletion of SPARC expression in adipocytes protects mice from HFD-induced adiposity, chronic inflammation, and metabolic disorders, and also reduces inflammation and prolongs health span during aging (Ryu et al., 2023). Mechanistically, SPARC activates the NLRP3 inflammasome and JNK signaling to promote inflammation via toll-like receptor 4 (TLR4) (Ryu et al., 2023). Additionally, SPARC dampens mitochondrial respiration in macrophages, and inhibition of glycolysis abolishes interferon-stimulated gene expression through transcription factors IRF3/7 (Ryu et al., 2022).
SPARC-like 1
SPARC-like 1 (SPARCL1) expression is highly enriched in white adipose tissue (WAT) and mature adipocytes (Gagliardi et al., 2017; Liu et al., 2021; Xiao et al., 2021) but is lower in the gastrointestinal tract, skeletal muscle, liver, kidney, spleen, and testis (Klingler et al., 2020). There may also be differences in SPARCL1 expression profiles between mice and humans. For example, SPARCL1 expression is relatively low in murine liver but moderate in human livers (Klingler et al., 2020). SPARCL1 has been shown to regulate numerous physiological processes, including cell adhesion, migration, proliferation, differentiation, and muscle development (Sakai et al., 2022; Sullivan and Sage, 2004; Wang et al., 2019c) and is considered a tumor suppressor in human cancers (Gagliardi et al., 2017).
Recent studies from our group and others have revealed a previously unrecognized role for SPARCL1 in MASLD/MASH (Liu et al., 2021; Meissburger et al., 2016; Xiao et al., 2021). SPARCL1 negatively impacts preadipocyte differentiation and suppresses lipid droplet accumulation by modulating peroxisome proliferator-activated receptor-gamma (PPARγ), CCAAT/enhancer-binding protein-alpha (C/EBPα), lipoprotein lipase (LPL), and insulin-like growth factor 1 (IGF1). This effect may lead to enhanced adipocyte hypertrophy in obesity (Meissburger et al., 2016; Xiao et al., 2021), suggesting that SPARCL1 controls adipogenesis in a paracrine/autocrine manner. As an adipokine, SPARCL1 exerts its metabolic regulatory functions through crosstalk with other organs such as the liver. Chronic recombinant SPARCL1 treatment induces hepatic steatosis, an inflammatory response, fibrosis, and liver injury in mice, to a greater extent than that observed in mice fed a 28-week high-fat high-cholesterol (HFHC) diet (Liu et al., 2021). Conversely, SPARCL1 deficiency, achieved through genetic manipulation or neutralizing antibodies, protects mice from long-term HFHC diet-induced hepatic inflammation and fibrosis (Liu et al., 2021). At the molecular level, SPARCL1 promotes the expression of C-C motif chemokine ligand 2 (CCL2) in hepatocytes, partly by binding to TLR4 and activating the NF-κB/p65 signaling pathway. Supporting this, another study reported that SPARCL1 is upregulated in liver-specific Apobec1 complementation factor transgenic mice, a model for steatosis, fibrosis, and hepatocellular carcinoma (Blanc et al., 2021).
SPARCL1 also regulates systemic glucose homeostasis. Recombinant SPARCL1 protein induces fasting hyperglycemia, hyperinsulinemia, and worsens insulin sensitivity in HFHC diet-fed mice. In contrast, SPARCL1-deficient mice are partially protected against HFHC diet-induced glucose dysregulation (Liu et al., 2021). In vitro, SPARCL1 treatment suppresses phosphorylation of AKT and GSK3β in hepatocytes, indicating an insulin-resistant state. SPARCL1 treatment also stimulates adipose tissue inflammation, which may exacerbate systemic insulin resistance (Huang et al., 2024). These findings highlight the metabolic hazards associated with SPARCL1, suggesting that blocking its action, such as through the use of neutralizing antibody, could serve as a potential therapy for treating MASLD/MASH.
Clinically, plasma SPARCL1 levels are significantly higher in patients with MASH than in normal individuals or patients with simple steatosis (Liu et al., 2021), in line with its role in driving steatosis-to-MASH progression. In the cohort study, plasma SPARCL1 levels are positively correlated with liver injury markers, such as ALT, aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT) levels (Liu et al., 2021). Notably, the SPARCL1-ALT-AST model demonstrates improved accuracy compared to the ALT-AST model in identifying patients with MASH, suggesting its potential as a noninvasive diagnostic marker (Liu et al., 2021). However, prospective cohort studies are warranted to provide more evidence in humans.
Neuregulins
Neuregulins (Nrgs) are a group of secreted proteins related to the epidermal growth factor (EGF) family, which act as ligands to activate tyrosine kinase receptors. The EGF receptors include EGFR (ErbB1), ErbB2, ErbB3, and ErbB4, while the Nrg family consists of Nrg1, Nrg2, Nrg3, and Nrg4 (Burden and Yarden, 1997). Nrgs are well known for their roles in development, nervous system regulation, and inter-organ communications (Cespedes et al., 2018).
Neuregulin 1
Nrg1 is ubiquitously expressed in endothelial and mesenchymal cells and plays a role in cell proliferation, survival, migration, and differentiation (Meyer et al., 1997). Nrg1 primarily binds to ErbB3 and ErbB4 to initiate downstream signaling. In vitro studies have shown that Nrg1 alleviates palmitic acid-induced lipid accumulation through ErbB3 phosphorylation and the PI3K-AKT signaling pathway (Meng et al., 2021). Similarly, Nrg1 mediates the beneficial effects of circular RNA ribonucleotide reductase subunit M2 (CircRRM2) in alleviating hepatic steatosis in mice (Wu et al., 2024). Nrg1 also impacts glucose homeostasis. In the liver, Nrg1 treatment activates AKT signaling (Caillaud et al., 2016), thereby reducing blood glucose levels, enhancing glucose tolerance (Ennequin et al., 2015), increasing insulin sensitivity (Guma et al., 2020), and suppressing food intake in obese or diabetic mice (Zhang et al., 2018). These metabolic benefits primarily result from inhibiting hepatic gluconeogenesis, restricting caloric intake via proopiomelanocortin (POMC) neurons (Zhang et al., 2018), improving mitochondrial respiration in skeletal muscle via complex 2 (Ennequin et al., 2017), and enhancing mitochondrial oxidation and insulin sensitivity in skeletal muscle (Canto et al., 2007). Furthermore, individuals with T2DM have substantially lower serum Nrg1 levels compared to controls (Al-Zuhairi et al., 2024; Eldin et al., 2023). Serum Nrg1 levels are negatively correlated with fasting blood glucose and hemoglobin A1c (HbA1c) levels, and insulin resistance (Eldin et al., 2023). These findings provide evidence for Nrg1’s potential as a therapeutic target for T2DM. However, due to the short half-life of native Nrg1 (Liu et al., 2006), a fusion protein (Nrg1-IgG Fc) with a longer half-life may be required for therapeutic applications.
Neuregulin 4
Neuregulin 4 (Nrg4) is an adipokine predominantly secreted by brown adipose tissue (BAT) and white adipose tissue (WAT) and primarily activates the ErbB4 receptor tyrosine kinase (Geissler et al., 2020; Harari et al., 1999; Villarroya et al., 2017; Wang et al., 2014a). Nrg4 exerts its effects primarily in adipose tissue and other metabolically active organs, such as the liver, hypothalamus, pancreas, skeletal muscle, and sympathetic neurons, in autocrine, paracrine, or endocrine manners (Gavalda-Navarro et al., 2022; Zhang et al., 2023b). Compelling evidence has established that Nrg4 plays a protective role in the development and progression of MASLD/MASH (Guo et al., 2017; Wang et al., 2014a; Zhang et al., 2022). Gain- and loss-of-function studies in mice have demonstrated that Nrg4 acts on hepatocytes to alleviate diet-induced hepatic steatosis by suppressing de novo lipogenesis through inhibiting LXR/SREBP1c signaling (Wang et al., 2014a). In vitro experiments using mouse primary hepatocytes further confirmed that Nrg4 dampens de novo lipogenesis via the ErbB4/STAT5/SREBP-1c axis (Li et al., 2021). Moreover, Nrg4 was shown to attenuate liver inflammation and injury through activation of ErbB4/AKT signaling and subsequent suppression of JNK-mediated hepatocyte death (Guo et al., 2017). In addition, a recent study found that Nrg4 can be induced by exercise to alleviate MASLD/MASH, which is also dependent on AKT signaling and suppression of cGAS-STING pathway-mediated inflammation and steatosis in hepatocytes (Chen et al., 2025). Nrg4 also acts as a checkpoint to suppress the progression from MASH to HCC by restraining the tumor-prone liver microenvironment (Zhang et al., 2022). These findings suggest that Nrg4 is involved in all stages of MASLD/MASH-HCC, highlighting the potential for Nrg4-based therapy in the treatment of these diseases.
In addition to its role in lipid metabolism, Nrg4 also regulates systemic glucose metabolism. Nrg4 deficiency impairs glucose and insulin tolerance and elevates blood glucose levels. Conversely, transgenic overexpression of Nrg4 can partly correct these abnormalities. In mice, Nrg4 exerts glucose-lowering effects partly through modulating gluconeogenesis in the liver and glucose utilization in skeletal muscle (Chen et al., 2017b; Wang et al., 2014a; Zhang et al., 2019). Nrg4/ErbB4 signaling also enhances insulin secretion in pancreatic β cells and glucose uptake in adipocytes (South et al., 2013; Zeng et al., 2018). Furthermore, recombinant Nrg4 protein has been shown to ameliorate age-associated glucose metabolic disorders. In the Nrg4-treated group, glucose tolerance and insulin resistance were improved, along with lower blood glucose and insulin levels compared to controls (Chen et al., 2024a).
The role of Nrg4 in obesity and whole-body energy homeostasis is gradually becoming clearer. Nrg4-null mice exhibit greater weight gain, increased fat mass, and decreased lean mass. In contrast, Nrg4 transgenic mice on an HFD have lower body weight and adiposity than controls, mediated by modulating beige fat thermogenesis (Chen et al., 2024b; Wang et al., 2014a). ErbB4-deletion mice also exhibit increased amounts of subcutaneous and visceral fat compared with wild-type mice (Zeng et al., 2018). Mechanistically, Nrg4 acts on the central nervous system to regulate body weight. Overexpression of Nrg4 in the paraventricular nucleus (PVN) of the brain protects against HFD-induced obesity, whereas ErbB4 knockdown in oxytocin neurons accelerates obesity (Zhang et al., 2023b).
Preclinical evidence shows that in obese mice, both Nrg4 expression in adipose tissue and hypothalamic ErbB4 phosphorylation are decreased (Wang et al., 2014a; Zhang et al., 2023b). Similarly, in individuals with high BMI, the mRNA expression levels of hepatic ERBB4 and NRG4 are reduced (Bograya et al., 2024), suggesting a protective role for Nrg4-ErbB4 signaling against obesity. Besides, circulating Nrg4 levels are lower in individuals with obesity (Cai et al., 2016; Guo et al., 2021), MASLD (Dai et al., 2015; Tutunchi et al., 2021; Wang et al., 2019a), and T2DM (Zhang et al., 2017), compared to healthy controls. There is also an inverse association between Nrg4 levels and metabolic syndrome indices, including BMI, waist circumference, fasting plasma glucose, TGs, HOMA-IR, and high-sensitivity C-reactive protein (Ziqubu et al., 2024). However, some studies have shown that Nrg4 levels are elevated in obese individuals (Martinez et al., 2022), which may reflect a compensatory response or resistance to its receptor (Wang et al., 2019b). Most of the current clinical evidence is cross-sectional or observational; therefore, large-sample, long-term prospective cohort studies are necessary to establish causality. Importantly, genetic studies have linked ErbB4 with T2DM and obesity (Boger and Sedor, 2012; Locke et al., 2015; Maeda et al., 2013; Sandholm et al., 2012). In addition, we recently identified two human Nrg4 variants, R44H and E47Q, in severely obese individuals. Nrg4 E47Q can activate ErbB4 phosphorylation and inhibit de novo lipogenesis via the ErbB4/STAT5/SREBP-1c pathway. In contrast, Nrg4 R44H has lost this function, thereby promoting lipogenesis and metabolic disorders (Li et al., 2021).
Growth differentiation factor family
The growth differentiation factor (GDF) family is part of the TGFβ superfamily and encompasses several members, such as GDF15 and GDF10 (Herpin et al., 2004). These proteins interact with distinct type I and type II serine/threonine kinase receptors (Rochette et al., 2020) and are involved in a variety of pathophysiological processes.
GDF15
Growth differentiation factor 15 (GDF15), a member of the TGFβ superfamily (Hsiao et al., 2000), is expressed in most body cell types and is induced under stress conditions (Sigvardsen et al., 2024). GDF15 functions via the orphan GFRα family member GFRAL and signals through the Ret coreceptor (Emmerson et al., 2017; Mullican et al., 2017).
Initially, GDF15 was shown to regulate appetite control and body weight through central actions (Tsai et al., 2014). Activation of the GDF15/GFRAL axis induces anorexia and weight loss (Emmerson et al., 2017; Hsu et al., 2017; Mullican et al., 2017; Yang et al., 2017). Transgenic Gdf15-overexpressing mice exhibit reduced food intake, a lean phenotype, and resistance to obesity, metabolic inflammation, and glucose intolerance (Chrysovergis et al., 2014; Macia et al., 2012; Wang et al., 2014b, 2014c). Similarly, ablation of Gdf15 or Gfral leads to increased fat depots and body weight in HFD-induced obese mice (Hsu et al., 2017; Tran et al., 2018). Recombinant GDF15 limits food intake and body weight in mice, but these effects are absent in Gfral-deficient mice (Emmerson et al., 2017; Hsu et al., 2017; Yang et al., 2017). Besides, recent discoveries found that this signaling is also required for ketogenic diet (KD)-induced weight loss (Breit et al., 2023; Lu et al., 2023).
Beyond its central actions, GDF15 also directly targets peripheral tissues such as the liver and skeletal muscle, enhancing oxidative metabolism and lipid mobilization (Chung et al., 2017), increasing thermogenic and lipolytic gene expression in adipose tissue (Chrysovergis et al., 2014), and ameliorating proinflammatory cytokine-induced pancreatic β cell apoptosis (Nakayasu et al., 2020). GDF15 also enhances insulin action in the liver and adipose tissue via a β adrenergic receptor-mediated mechanism (Sjoberg et al., 2023). In patients with MASLD/MASH, GDF15 levels are elevated and increase with disease progression, suggesting that GDF15 may serve as a novel biomarker (Koo et al., 2018). The endogenous induction of GDF15 may represent an adaptive and compensatory response to mitigate MASH progression (Breit et al., 2021; Kim et al., 2018). In diet-induced MASH mice, GDF15 ablation exacerbates hepatic steatosis, inflammation, fibrosis, and liver injury, while transgenic GDF15 expression alleviates these phenotypes and metabolic deterioration (Kim et al., 2018; Wang et al., 2022b). Consistently, recombinant GDF15 treatment significantly reduces hepatic steatosis in mice (Chung et al., 2017). Besides, upon KD feeding, Gdf15 mRNA levels increase in hepatocytes, elevating circulating GDF15 levels, with all MASLD-related changes occurring without altering food intake. Additionally, Gdf15 or Gfral ablation abolishes the beneficial effects of the KD on reducing hepatic lipids and plasma ALT/AST levels in mice (Lu et al., 2023). The anti-lipogenic, anti-fibrogenic, and anti-inflammatory effects of GDF15 are attributed to enhanced hepatic fatty acid β-oxidation (Kim et al., 2018; Li et al., 2018), suppression of fibrosis-related gene expression in HSCs (Kim et al., 2018; Wang et al., 2022b), inhibition of fatty acid uptake and synthesis, and dampening of AIM2 inflammasome activation (Wang et al., 2022b). These findings suggest that GDF15 directly affects the liver to mitigate MASLD/MASH.
GDF15 holds promise as a therapeutic target for metabolic disorders, with encouraging preclinical results. However, in the Phase 1 study of LY3463251 (a long-acting GDF15 analog), there was only a modest impact on weight loss in obese individuals, although it significantly reduced food intake and appetite (Benichou et al., 2023). Similarly, in the Phase 2 study of MBL949 (a recombinant human GDF15 dimer with an extended half-life), biweekly dosing for 14 weeks resulted in minimal weight loss. Both clinical studies suggest that the robust weight loss effects observed in nonclinical models did not translate into significant weight loss efficacy in humans (Smith et al., 2024). Thus, more clinical trials are required to fully assess its therapeutic potential in humans. Additionally, combining GDF15 with GLP-1 treatment may represent a promising strategy for weight loss, as synergistic effects of these two proteins have been observed in mice and non-human primates (Zhang et al., 2023a). However, it remains to be determined if this combination is effective in humans.
GDF10
Growth differentiation factor 10 (GDF10), also known as BMP-3b, is an atypical member of the TGFβ superfamily (Matsumoto et al., 2012). In diet-induced hepatic steatosis, circulating GDF10 levels are elevated (Platko et al., 2019). GDF10 prevents excessive lipid accumulation in hepatocytes by reducing nuclear PPARγ activity. When GDF10 is ablated in mice, liver TG deposition increases, and an obese phenotype develops even on a normal diet (Platko et al., 2019). In response to an HFD, GDF10-ablated mice exhibit increased steatosis, ER stress, fibrosis, and liver injury compared to controls (Platko et al., 2019). These findings highlight GDF10’s hepatoprotective role, as it inhibits de novo lipogenesis and protects against liver injury. GDF10 is also required for maintaining glucose metabolism homeostasis during aging. GDF10 knockout mice develop glucose intolerance and insulin resistance, along with elevated plasma cholesterol and TG levels (Marti-Pamies et al., 2020).
GDF3
In the liver, we found that GDF3 was predominantly expressed in Kupffer cells and macrophages (Xiang et al., 2022). Hepatic GDF3 expression and plasma GDF3 concentrations were specifically upregulated in MASH mice, while they remained unchanged in mice with simple steatosis (Xiang et al., 2022). Consistently, plasma GDF3 levels are significantly elevated and correlated with hepatic pathological features in patients with MASH, offering high diagnostic accuracy for MASH with good sensitivity and specificity (Xiang et al., 2022). In addition, GDF3 expression has been associated with obesity and diabetes (Bu et al., 2018; Izumi, 2023), and its ectopic expression can induce insulin resistance in lean and healthy mice (Hall et al., 2020). Further research is needed to investigate the mechanism by which GDF3 contributes to MASH pathogenesis.
Interleukins
Interleukins (IL) are produced by various immune cells in both the innate and adaptive immune systems. They are crucial messenger molecules that regulate biological functions of target cells through autocrine or paracrine mechanisms (Ouyang and O’Garra, 2019).
Interleukin-22
Interleukin-22 (IL-22), a member of the IL-10 cytokine family, is primarily produced by cells of the adaptive immune system and innate lymphoid cells (Ouyang et al., 2011). Its functional receptor, IL-22RA1, is expressed in various organs and tissues, including the liver, pancreas, kidney, intestine, adipose tissue, and skin (Dudakov et al., 2015). IL-22RA1 pairs with IL-10RB to mediate IL-22 signaling, primarily through signal transducer and activator of transcription (STAT) pathway (Dudakov et al., 2015). Initially recognized for its roles in tissue protection, immune regulation, and tissue regeneration (Dudakov et al., 2015), emerging evidence now highlights IL-22’s versatile functions in metabolic regulation and its potential for improving MASLD/MASH.
Through multiple mouse models, IL-22 has been shown to alleviate hepatic steatosis and injury by inhibiting lipogenesis (Gaudino et al., 2024; Huang et al., 2025; Sajiir et al., 2024a; Wang et al., 2014d; Yang et al., 2010; Zhang et al., 2024b). In contrast, hepatocyte-specific IL-22RA1 knockout mice exhibited diet-induced hepatic steatosis and liver injury, primarily driven by elevated lipogenesis and hepatic oxysterol accumulation (Huang et al., 2025). Mechanistically, we found that hepatic IL-22RA1 deficiency impairs STAT3 signaling, which leads to the upregulation of activating transcription factor 3 (ATF3). Subsequently, ATF3 inhibits oxysterol 7 alpha-hydroxylase (CYP7B1) expression, causing oxysterol accumulation to activate LXRα, resulting in the transactivation of SREBP-1c and lipogenic genes (Huang et al., 2025). IL-22 also mitigates hepatic fibrosis by targeting HSCs (Hwang et al., 2020; Kong et al., 2012; Zhang et al., 2024b), Kupffer cells (KCs), and monocyte-derived macrophages (Zhang et al., 2024b). It reduces liver inflammation and fibrosis induced by carbon tetrachloride (CCl4) (Kong et al., 2012), neutrophil-driven inflammation (Hwang et al., 2020), and diet-induced liver injury (Hwang et al., 2020; Zhang et al., 2024b), and aids in the resolution of liver fibrosis during recovery (Kong et al., 2012). In agreement, hepatocyte-specific IL-22RA1 deficiency exacerbates diet-induced inflammation and fibrosis in mice (Huang et al., 2025). IL-22 attenuates fibrosis through multiple ways, including blocking hepatic oxidative stress and related stress kinases (Hwang et al., 2020), inducing HSC senescence via p53 and p21-dependent pathways (Kong et al., 2012), reducing HSC activation and expansion, and decreasing M1-type Kupffer cells and monocyte-derived macrophages (Zhang et al., 2024b). In addition, IL-22 mitigates the inflammatory functions of hepatocyte-derived, mitochondrial DNA-enriched extracellular vesicles, thereby suppressing liver inflammation in MASH (Hwang et al., 2020). IL-22 also helps maintain intestinal homeostasis to alleviate MASLD. Obesogenic diets suppress IL-22 expression in small intestine innate lymphoid cells, inhibiting STAT3 signaling in intestinal epithelial cells (IECs) and expanding the absorptive enterocyte compartment. In IECs, exogenous IL-22 binds to its receptor, activates STAT3 and inhibits WNT/β-catenin signaling, thus exerting therapeutic effects (Zhang et al., 2024b). Furthermore, IL-22RA1 signaling deficiency in IECs promotes hepatocyte ballooning and lipid droplet deposition in a microbiota-dependent manner, suggesting that IECs might be required for the anti-steatotic roles of IL-22 (Gaudino et al., 2024). Notably, a recent study showed that the effects of IL-22 appear to be sex-dependent. In MASLD, females exhibit higher hepatic IL-22 expression, and the absence of IL-22RA1 signaling worsens hepatic pathology more severely in females (Abdelnabi et al., 2022).
In extrahepatic tissues, IL-22 enhances insulin secretion by reducing β cell oxidative and ER stress (Hasnain et al., 2014; Sajiir et al., 2024b), improving mitochondrial function, and maintaining β cell identity (Yu et al., 2024). IL-22 signaling suppresses inflammation and promotes high-quality insulin secretion, thereby restoring glucose homeostasis in diabetic obese models (Hasnain et al., 2014). Consistently, blocking IL-22RA1 in healthy mouse islets leads to increased ER stress and reduced glucose-stimulated insulin secretion (GSIS). In mice with pancreatic β cell IL-22RA1 deficiency, hyperglycemia, defective insulin secretion, impaired glucose tolerance, and decreased insulin quality are observed (Sajiir et al., 2024b; Yu et al., 2024). Similar to its effects on lipid metabolism, the effects of IL-22 on glucose regulation seem to be sex-dependent, although some conflicting findings exist (Sajiir et al., 2024b; Yu et al., 2024). Beyond insulin secretion, IL-22 also improves insulin sensitivity in muscle and adipose tissues upon insulin challenge (Wang et al., 2014d). Likewise, IL-22 treatment enhances glucose tolerance and insulin sensitivity in both diet- and genetically induced obese mice (Huang et al., 2025; Wang et al., 2014d). Animal models with global, hepatocyte-, or IEC-specific IL-22RA1 knockout show worsened obesity-associated insulin resistance and/or glucose intolerance (Gaudino et al., 2024; Huang et al., 2025; Wang et al., 2014d; Zhang et al., 2024b). Nevertheless, WAT-specific IL-22RA1 deficiency does not influence metabolic disorders (Gaudino et al., 2024; Wang et al., 2014d), suggesting that IL-22 exerts its metabolic effects in a context-dependent manner.
Given its pleiotropic functions, including anti-steatotic, anti-inflammatory, and anti-fibrotic effects within the liver, IL-22 is a promising candidate for treating MASLD/MASH (Huang et al., 2025). Multiple preclinical studies show that recombinant IL-22 or IL-22Fc can reduce hepatic steatosis, fibrosis, and inflammation, and attenuate weight gain in diet-induced obese mice (Hwang et al., 2020; Wang et al., 2014d; Yang et al., 2010; Zhang et al., 2024b). Furthermore, recombinant IL-22 can reverse glucose intolerance, hyperglycemia, and insulin resistance in obese mice (Hasnain et al., 2014; Wang et al., 2014d). Liver-targeted IL-22 gene delivery via nanoparticles also alleviates HFD-induced hepatic steatosis, hyperglycemia, and insulin resistance (Zai et al., 2019). IL-22-bispecific biologics targeting the liver and pancreas can reduce hepatic steatosis, inflammation, and fibrosis, and restore glycemic control in MASH mice (Sajiir et al., 2024a). Clinical studies support these findings, as newly diagnosed T2DM patients show lower circulating IL-22 levels (Asadikaram et al., 2018), suggesting that lower IL-22 levels may be associated with higher T2DM risk. However, patients with established metabolic syndrome, cardiometabolic disorders, and/or T2DM exhibit elevated circulating IL-22 levels, potentially as a compensatory response (Asadikaram et al., 2018; Gong et al., 2016; Gu et al., 2022).
F-652, a recombinant fusion protein of human IL-22 and IgG2 Fc, has undergone clinical trials, demonstrating safety and minimal adverse effects in Phase I trials (Tang et al., 2019). Phase IIa trials showed safety and efficacy in severe alcoholic hepatitis, reducing inflammation and injury markers, and promoting hepatic regeneration (Arab et al., 2020). However, gastrointestinal and dermatological side effects were noted, likely due to IL-22’s proliferative effects on epithelial tissues (Arshad et al., 2020; Moniruzzaman et al., 2019). The understanding of IL-22/IL-22RA1 signaling in metabolic health is advancing, highlighting its therapeutic potential. IL-22 exhibits both protective and pathogenic effects (Dudakov et al., 2015) and regulates tissue regeneration (Dudakov et al., 2015; Lindemans et al., 2015). Paradoxically, supraphysiological IL-22 levels have been shown to increase susceptibility to tumorigenesis (Jiang et al., 2011), while another study reports no spontaneous development of liver tumors (Park et al., 2011). Therefore, targeted delivery, structure-based design, precise modulation strategies, and combination therapies could help improve efficacy and minimize off-target effects, thereby unlocking IL-22’s therapeutic potential for treating MASLD/MASH.
Interleukin-18
Interleukin-18 (IL-18) is produced by various cell types, including Kupffer cells, hematopoietic cells, and non-hematopoietic cells such as intestinal epithelial cells, keratinocytes, and endothelial cells (Arend et al., 2008; Kaplanski, 2018). IL-18 binds to its specific receptor, which comprises the IL-18Rα and IL-18Rβ chains, triggering intracellular signaling pathways similar to those of IL-1 and activating NF-κB and inflammatory processes (Robinson et al., 1997). In the liver, IL-18 is primarily produced by macrophages (Belkaya et al., 2019; Lebel-Binay et al., 2000) and mediates the liver’s defense against bacterial, parasitic, viral infections, and drug-induced injuries (Imaeda et al., 2009; Maltez et al., 2015; Serti et al., 2014). Recent studies have implicated IL-18 signaling in hepatic steatosis and fibrosis. IL-18 is induced upon NLRP1 activation and decreased upon NLRP1 loss (Murphy et al., 2016), and its production could prevent hepatic steatosis (Murphy et al., 2016). Consequently, the loss or gain of NLRP1 function recapitulates obesity-related phenotypes in mice lacking or overexpressing IL-18 (Murphy et al., 2016). Mice with constitutively activated NLRP1, having elevated IL-18 levels, do not develop liver lipid accumulation, and IL-18 depletion reverses this protective effect (Murphy et al., 2016; Netea and Joosten, 2016). Exogenous IL-18 administration has been shown to counteract steatohepatitis in mice (Murphy et al., 2016), whereas IL-18-deficient mice exhibit hepatic steatosis and steatohepatitis (Lana et al., 2016; Netea et al., 2006; Yamanishi et al., 2016). Furthermore, elevating IL-18 levels by disrupting macrophage phosphatase SHP2 protects mice from HFD-induced hepatic steatosis (Liu et al., 2020b). The inhibitory effects of IL-18 on liver lipid storage and fibrosis may arise from its direct regulation of lipid homeostasis, including anti-hypercholesterolemia and anti-hypertriglyceridemia effects (Murphy et al., 2016), as well as from indirect effects, such as anti-hyperphagia and resulting anti-obesity effects (Netea et al., 2006) and modulation of gut microbiota composition (Henao-Mejia et al., 2012).
Conversely, other studies suggest that IL-18 can enhance hepatic fibrosis and injury. Recombinant IL-18 protein accelerates the trans-differentiation of primary murine HSCs into myofibroblasts, promoting ECM deposition and contributing to liver fibrosis (Knorr et al., 2023). In vivo, IL-18 receptor-deficient mice show reduced liver fibrosis in an HSC-specific NLRP3 overactivation-induced fibrosis model (Knorr et al., 2023). Similarly, IL-18R-deficient mice, but not IL-1R-deficient mice, are protected from early liver damage (Hohenester et al., 2020). These findings highlight the complex role of IL-18 in the liver, demonstrating a balance between its protective effects and potential fibrogenic risks. IL-18 also plays a role in glucose homeostasis. IL-18-deficient mice exhibit hyperinsulinemia, consistent with insulin resistance related to hyperglycemia, primarily due to enhanced liver gluconeogenesis and defective STAT3 phosphorylation (Netea et al., 2006). Additionally, IL-18 mediates the positive effects of the Nlrp1b inflammasome on glucose tolerance and insulin sensitivity (Salazar-Leon et al., 2019).
Clinically, circulating IL-18 levels are linked to increased liver injury markers (AST, ALT) and portal fibrosis (Lopez-Bermejo et al., 2005; Mehta et al., 2014). Patients with MASLD and chronic liver disease have higher plasma IL-18 levels (Li et al., 2010; Ludwiczek et al., 2002). IL-18 polymorphisms may contribute to susceptibility to certain types of chronic liver diseases (Zhang et al., 2020). Many clinical studies report upregulated IL-18 circulating levels in patients with T2DM (Moriwaki et al., 2003; Zaharieva et al., 2018; Zhuang et al., 2019). Prediabetic patients have higher IL-18 levels than obese normoglycemic controls (Gateva et al., 2020). Additionally, circulating IL-18 levels are positively correlated with the HOMA-IR index (Fischer et al., 2005). Conversely, a decrease in IL-18 has been identified as an independent factor for the improvement of β cell function in T2DM (Kim et al., 2007). An IL-18 gene polymorphism associated with higher circulating IL-18 levels is linked to impaired insulin sensitivity (Presta et al., 2009). High blood IL-18 levels in T2DM may indicate IL-18 resistance, akin to insulin resistance.
Fibroblast growth factors
The fibroblast growth factor (FGF) family consists of 22 structurally related proteins that play diverse roles in cell proliferation, differentiation, embryonic development, wound repair, and angiogenesis (Beenken and Mohammadi, 2009). Among its members, two endocrine FGFs (FGF21 and FGF19) and the canonical FGF1 are important regulators of glucose and lipid metabolism, as well as energy homeostasis.
Fibroblast growth factor 21
Fibroblast growth factor 21 (FGF21) was initially identified as a prototypical hepatokine released primarily by the liver. Subsequent studies showed that circulating FGF21 levels are derived from both the liver and adipose tissue in rodents (Markan et al., 2014) and humans (Hansen et al., 2015). A variety of nutritional factors (e.g. fasting, high-carbohydrate, low-protein/amino acid levels) (Flippo and Potthoff, 2021; Potthoff, 2017) and stress signals (e.g. cold exposure, alcohol consumption) (BonDurant and Potthoff, 2018; Fisher and Maratos-Flier, 2016; Jensen-Cody and Potthoff, 2021; Soberg et al., 2018) strongly induce FGF21 expression. FGF21 elicits its biological effects by binding to and activating a receptor complex consisting of the co-receptor β-Klotho (KLB) and fibroblast growth factor receptor 1c (FGFR1c). KLB expression is primarily restricted to specific metabolic tissues, such as the liver, pancreas, and adipose tissue, determining the specificity of FGF21 signaling (Kurosu et al., 2007; Suzuki et al., 2008). FGF21 influences glucose and lipid metabolism and energy homeostasis by targeting diverse tissues, including the liver, pancreas, adipocytes, skeletal muscle, and brain (Bookout et al., 2013; Jensen-Cody et al., 2020; Liang et al., 2014). The most prominent and consistent metabolic effect of FGF21 across species is its impact on lipid metabolism (Markan and Potthoff, 2016). The liver is both the primary source and target of FGF21. In obese rodents, FGF21 exerts potent TG-lowering effects in the liver and plasma (Ahuja et al., 2023; Badman et al., 2007; Byun et al., 2020; Fisher et al., 2014; Geng et al., 2020,Li et al., 2014; Xu et al., 2009a; Zarei et al., 2018). It achieves this mainly by increasing hepatic fatty acid oxidation, inhibiting lipogenesis, promoting the browning of WAT, and enhancing whole-body energy expenditure. Additionally, FGF21 reduces hepatic ER stress and oxidative stress (Fisher et al., 2014; Kim et al., 2015; Ye et al., 2014; Zarei et al., 2018), restricts liver inflammation and injury (Feingold et al., 2012; Fisher et al., 2014), and mitigates fibrosis in mice (Fisher et al., 2014; Ji et al., 2024; Liu et al., 2023a). Moreover, treatment with LY2405319, an engineered FGF21 analogue, significantly decreases MASH scores, serum liver fibrosis markers, injury markers, and pro-inflammatory markers in mice with MASH (Lee et al., 2016). These anti-lipogenic, anti-inflammatory, and anti-fibrotic effects are attributed to targeting both hepatocytes and HSCs in the liver (Harrison et al., 2024b). In hepatocytes, recent findings have identified serine and threonine phosphatase PPP6C as a direct target of FGF21. Mechanistically, PPP6C is sufficient to bind with the coreceptor βKlotho upon FGF21 treatment, directly dephosphorylating tuberous sclerosis complex 2 (TSC2) at Ser939 and Thr1462, thereby inhibiting mTORC1 activation (Liu et al., 2025). Thus, these results clearly demonstrate a fundamental and critical mechanism FGF21 action in hepatocytes to ameliorate MASLD/MASH. Furthermore, FGF21 also regulates lipid metabolism in adipose tissues by lowering plasma TGs through facilitating lipoprotein catabolism in WAT and BAT (Schlein et al., 2016).
Beyond lipid regulation, FGF21 is a potent acute insulin-sensitizer and a potential modulator of glucose metabolism. Mice lacking FGF21 exhibit elevated sugar intake, while FGF21 overexpression inhibits sugar intake (von Holstein-Rathlou et al., 2016). A single FGF21 injection can reduce plasma glucose by more than 50% in obese animal models (BonDurant et al., 2017; Xu et al., 2009b). Chronic FGF21 administration improves insulin sensitivity and glycemic control in obese animal models (Berglund et al., 2009; Coskun et al., 2008; Kharitonenkov et al., 2005; Xu et al., 2009a). Plasma glucose reduction primarily occurs through peripheral glucose disposal (BonDurant et al., 2017; Feingold et al., 2012; Xu et al., 2009b), particularly in adipose tissues (BonDurant et al., 2017; Lan et al., 2017). The anti-hyperglycemia effects of FGF21 have been replicated in diabetic monkey models (Adams et al., 2013; Kharitonenkov et al., 2007; Stanislaus et al., 2017; Talukdar et al., 2016), without inducing mitogenesis or hypoglycemia in rodents or monkeys. FGF21 also improves insulin sensitivity in various tissues, including the liver, adipose tissues, and skeletal muscle, thereby enhancing glucose homeostasis (Geng et al., 2020).
Prolonged administration of FGF21 or its analogues results in significant weight loss in rodents and non-human primates (BonDurant and Potthoff, 2018; Coskun et al., 2008; Kharitonenkov et al., 2005, 2007; Talukdar et al., 2016; Xu et al., 2009a). PF-05231023, a long-acting FGF21 analogue, leads to notable weight loss in obese monkeys and humans (Talukdar et al., 2016). The weight-loss effects of FGF21 or its analogue are mediated through direct actions on the central nervous system to facilitate energy expenditure (Ameka et al., 2019; Flippo et al., 2020; Hill et al., 2019; Lan et al., 2017; Owen et al., 2014), rather than through direct actions on adipose tissues (BonDurant et al., 2017; Chen et al., 2017a; Lan et al., 2017), although the underlying mechanism remains incompletely understood.
FGF21 analogues are under investigation for metabolic diseases, such as obesity, dyslipidemia, T2DM, and MASLD/MASH (Gaich et al., 2013; Harrison et al., 2021b, 2023a, 2023b; Loomba et al., 2023b; Rader et al., 2022; Sanyal et al., 2019). While their efficacy in glycemic control and weight loss is mild and inconsistent (Chui et al., 2024), benefits in dyslipidemia, hepatic steatosis, and MASH resolution have been observed. Efruxifermin (Harrison et al., 2025) and pegozafermin (Bhatt et al., 2023) met Phase IIb endpoints in patients with MASH. Efruxifermin reduces liver fat fraction (Harrison et al., 2021b), improves liver histology (Harrison et al., 2023a) and fibrosis (Harrison et al., 2023b), enhances lipid profiles and glycemic control, and may aid in weight loss (Harrison et al., 2023a). It also improves fibrosis and liver injury biomarkers in patients with compensated MASH cirrhosis; 57% of treated patients showed fibrosis improvement or resolution of MASH, compared to 0% in the placebo group (Harrison et al., 2023b). Pegozafermin, a long-acting glycol-pegylated recombinant FGF21 analogue, effectively reduces liver fat and fibrosis in patients with severe hypertriglyceridemia, MASH, and liver fibrosis. (Bhatt et al., 2023; Loomba et al., 2023a, 2023b). In addition to monotherapy, combination therapy shows strong strength. In a Phase II clinical trial, the combination of efruxifermin and GLP-1 receptor agonist (GLP-1RA) (Harrison et al., 2025) achieved strong hepatic fat reduction in patients with MASH, fibrosis, and T2DM. Efruxifermin improves noninvasive markers of liver injury, fibrosis, glucose, and lipid metabolism, maintains GLP-1RA-mediated weight loss, and is safe and well-tolerated. During clinical studies, no significant changes in bone density (Loomba et al., 2023b, 2024) or increased fracture incidence were reported (Abdelmalek et al., 2024). Phase III programs for efruxifermin and pegozafermin are underway. Thus, FGF21 analogues show promise for MASH treatment due to their pleiotropic effects in hepatocytes, anti-fibrotic properties, and systemic benefits.
FGF19
Fibroblast growth factor 19 (FGF19, known as FGF15 in rodents), primarily expressed in ileal enterocytes (Somm and Jornayvaz, 2018), is released into the enterohepatic circulation postprandially in response to bile acids via activation of the farnesoid X receptor (FXR) (Walters, 2014). FGF19’s involvement in hepatic fat metabolism was first recognized in 2002. Transgenic mice overexpressing FGF19 show reduced hepatic fat accumulation, fat mass, and body weight (Tomlinson et al., 2002). Similarly, FGF15-deficient mice exhibit exacerbated hepatic steatosis, whereas FGF19 treatment reverses this condition (Alvarez-Sola et al., 2017). Recombinant FGF19 or its analogue can resolve steatohepatitis and fibrosis in mice (Zhou et al., 2017). Mechanistically, FGF19 regulates hepatic fat metabolism via multiple pathways. It represses hepatic de novo lipogenesis (Bhatnagar et al., 2009; Fu et al., 2004; Tomlinson et al., 2002), increases the metabolic rate (Fu et al., 2004), inhibits ER stress (Alvarez-Sola et al., 2017), and reduces lipotoxicity (Alvarez-Sola et al., 2017; Zhou et al., 2017). These processes primarily involve the activation of signal transducer and activator of transcription 3 (STAT3) and the inhibition of peroxisome proliferator-activated receptor coactivator-1β (PGC-1β) and sterol regulatory element-binding protein 1c (SREBP-1c) (Sciarrillo et al., 2021). Furthermore, FGF19 may influence fat metabolism in tissues other than the liver (Potthoff et al., 2012). In addition to regulating lipid homeostasis, FGF15/19 also plays a role in carbohydrate metabolism. Fgf15-knockout mice exhibit impaired serum glucose regulation, reduced hepatic glycogen content, and glucose intolerance, while FGF19 administration reverses these metabolic impairments (Kir et al., 2011). Mechanistically, FGF15/19 promotes hepatic glycogen synthesis via extracellular-signal-regulated kinase (ERK) activation and glycogen synthase kinase-3 (GSK3) phosphorylation/inactivation, independent of insulin (Kir et al., 2011). FGF19 also inhibits gluconeogenesis by dephosphorylating and inactivating cyclic adenosine monophosphate (cAMP) regulatory element-binding protein (CREB) (Kir et al., 2011; Potthoff et al., 2011).
In healthy individuals, circulating FGF19 levels exhibit diurnal fluctuations closely associated with postprandial serum bile acids (Lundasen et al., 2006). Reduced fasting plasma levels of FGF19 have been observed in subjects with obesity, T2DM, and MASLD (Alisi et al., 2013; Eren et al., 2012; Mraz et al., 2011). Conversely, hepatic KLB and FGFR4 expression are increased in patients with MASLD/MASH, suggesting potential disruption in the FGF19/KLB/FGFR4 signaling pathway (DePaoli et al., 2019). Aldafermin (also known as M70 and NGM282), an engineered FGF19 analogue, is undergoing extensive clinical trials for treating metabolic liver diseases (Zhou et al., 2014). Aldafermin treatment leads to rapid and sustained reductions in liver fat content, liver injury and fibrosis markers, and improved liver histology in patients with MASH (Harrison et al., 2018, 2020, 2021a). However, a Phase 2b study (ALPINE 2/3, NCT03912532) involving patients with fibrosis stage 2 or 3 reported that aldafermin did not reach pre-specified significance, although significant improvements in steatosis, inflammation, liver injury, and fibrosis were achieved (Harrison et al., 2022). In a recently reported Phase 2b trial (NCT04210245) involving patients with compensated MASH cirrhosis, aldafermin treatment led to a significant reduction in enhanced liver fibrosis (Rinella et al., 2024). Additionally, aldafermin fails to reduce hyperglycemia in patients with T2DM (DePaoli et al., 2019). Thus, more clinical trials are needed to further validate its therapeutic effects.
FGF1
FGF1 is an autocrine and paracrine regulator that binds to heparan sulfate proteoglycans, preventing its secretion into circulation (Beenken and Mohammadi, 2009). Although FGF1 is involved in processes, such as embryonic development, wound healing, angiogenesis, and neurogenesis, global Fgf1 knockout mice show no significant deficiencies in these processes (Miller et al., 2000). Recently, FGF1 has been found to exert an unexpected metabolic role in regulating lipid metabolism and glucose homeostasis in obesity, MASLD, and diabetes (Jonker et al., 2012; Suh et al., 2014). Chronic FGF1 treatment alleviates hepatic steatosis, inflammation, apoptosis, and oxidative stress in ob/ob, db/db, and high-fat/high-sucrose diet-induced MASLD model mice (Lin et al., 2021; Suh et al., 2014; Xu et al., 2020). FGF1 also reverses high-fat/high-sucrose diet-induced steatohepatitis and fibrosis in apolipoprotein E knockout mice and choline-deficient diet-fed mice through FGFR4-mediated AMP-activated kinase α (AMPKα) signaling (Lin et al., 2021; Liu et al., 2016), highlighting FGF1’s importance in maintaining hepatic lipid homeostasis.
FGF1 also plays a unique role in glucose homeostasis. A single-dose parenteral delivery of recombinant FGF1 normalizes blood glucose in obese mice without causing hypoglycemia (Suh et al., 2014). Chronic recombinant FGF1 treatment sustains glucose-lowering effects by promoting insulin-dependent glucose uptake in skeletal muscle and suppressing hepatic glucose production (HGP), thereby achieving insulin sensitization (Suh et al., 2014). The glucose-lowering and insulin-sensitizing effects of recombinant FGF1 are partially mediated by FGFR1 in adipose tissue (Suh et al., 2014). Mechanistically, FGF1 inhibits lipolysis in adipose tissue and suppresses HGP (Sancar et al., 2022). FGF1 also exerts central actions, as it crosses the blood–brain barrier (Lou et al., 2012) and is involved in feeding-suppression regulation (Suzuki et al., 2001). A single intracerebroventricular injection of FGF1 at low doses sustains remission of hyperglycemia in obese mice and diabetic rats (Scarlett et al., 2016). FGF1’s central effect in lowering hyperglycemia occurs through the induction of hepatic glucokinase, which increases hepatic glucose uptake (Scarlett et al., 2019). However, the specific neuronal types and brain regions involved in FGF1’s central anti-hyperglycemia actions remain unidentified.
Although FGF1 has antidiabetic effects, the tumorigenic risks associated with chronic FGF1 administration have raised concerns about its use as a pharmacotherapy for diabetes (Jiao et al., 2015; Kwabi-Addo et al., 2004). However, the mitogenic and antidiabetic activities of FGF1 appear to be separable. FGF1 induces cell proliferation mainly via FGFR3 and FGFR4, while its metabolic activity is predominantly mediated by FGFR1 (Degirolamo et al., 2016; Suh et al., 2014; Wu et al., 2011). Engineered FGF1 variants, such as FGF1ΔHBS (which abolishes heparan sulfate-assisted FGFR dimerization/activation) and FGF1ΔNT (with 24 N-terminal amino acids deleted), have been developed to reduce its mitogenic properties while retaining metabolic activity (Huang et al., 2017; Suh et al., 2014).
Bone morphogenic proteins
Bone morphogenic proteins (BMPs), a subset of the TGFβ superfamily of cell-regulatory proteins, are crucial for osteogenic and chondrogenic differentiation. They play pleiotropic roles in cellular differentiation, proliferation, and survival (Xiao et al., 2007). Emerging evidence suggests that BMPs have diverse functions in the development and progression of MASLD/MASH.
BMP2
Treatment of hepatoma cells with BMP2 induces the expression and activity of DGAT2 (the gene involved in triglyceride synthesis) through intracellular SMAD signaling, suggesting a potential role for BMP2 in hepatic lipid metabolism (Thayer et al., 2020). Palmitic acid upregulates BMP2 expression and secretion in human hepatocytes. BMP2 expression is abnormally elevated in the livers of patients with MASLD compared with normal liver tissues, as reflected in higher serum BMP2 levels. An algorithm based on serum BMP2 levels and clinically relevant MASLD variables can distinguish MASH (Maranon et al., 2022), indicating that abnormal BMP2 expression is linked to MASLD/MASH.
BMP4
BMP4 has been shown to inhibit HFD-induced hepatic steatosis in mice by regulating genes involved in lipid metabolism and mTORC1 signaling in hepatocytes (Peng et al., 2019). Studies using spheroids composed of human stellate cells and hepatocytes demonstrate that BMP4 exhibits anti-senescent, anti-steatotic, anti-inflammatory, and anti-fibrotic effects (Baboota et al., 2022). BMP4 suppresses markers of hepatic steatosis, inflammation, and liver injury by upregulating glutathione peroxidase 4 (GPX4), thereby reducing ferroptosis (Wang et al., 2022a). Moreover, BMP4 expression is upregulated in both patients and mice with MASLD (Peng et al., 2019; Wang et al., 2022a).
BMP6
Similar to BMP4, BMP6 appears to protect against MASLD/MASH. BMP6 is upregulated in MASLD and is correlated with hepatic steatosis but not with liver inflammation or injury (Arndt et al., 2015). Interestingly, BMP6 is solely elevated in MASLD but not in other murine liver injury models or diseased human livers (Arndt et al., 2015). Compared with wild-type mice, BMP6-deficient mice exhibit increased hepatic inflammation and fibrosis following methionine-choline-deficient (MCD) and HFD feeding (Arndt et al., 2015). Recombinant BMP6 inhibits HSCs activation and reduces proinflammatory and profibrogenic gene expression in activated HSCs (Arndt et al., 2015). Thus, we speculate that upregulation of BMP6 in MASLD may be hepatoprotective, providing a promising anti-steatotic and anti-fibrogenic strategy.
BMP7
BMP7 has been demonstrated to exert therapeutic potential for MASLD. Treating HFD-induced obese mice and ob/ob mice with liver-directed adeno-associated virus (AAV)-BMP7 vectors increases circulating BMP7 levels, induces the browning of WAT, activates BAT, alleviates hepatic steatosis, and normalizes body weight and insulin resistance (Casana et al., 2022), highlights the potential of AAV-BMP7-mediated gene therapy for the treatment of MASLD/MASH.
BMP8B
In contrast, BMP8B seems to be positively associated with MASLD/MASH progression. BMP8B has been shown to promote fatty acid uptake, de novo lipogenesis, NF-κB activation, and proinflammatory gene expression in hepatocytes (Mahli et al., 2019). BMP8B also promotes the proinflammatory phenotype in HSCs through both the SMAD2/3 and SMAD1/5/9 branches of the TGFβ/BMP pathway (Vacca et al., 2020). In vivo, the absence of BMP8B prevents HSC activation, reduces inflammation, and limits the progression of MASH (Vacca et al., 2020). Fatty acids induce BMP8B expression in a dose-dependent manner in primary human hepatocytes (Mahli et al., 2019). Furthermore, hepatic BMP8B expression is significantly increased in a murine MASLD model (Mahli et al., 2019). Notably, human data show that circulating BMP8B levels are elevated in patients with MASH compared with simple steatosis and healthy individuals. Elevated BMP8B levels are positively correlated with AST, ALT, the platelet ratio index (APRI), and the fibrosis-4 index (FIB-4) in patients with MASH (Mounika et al., 2023).
BMP9
The role of BMP9 in MASLD remains contentious. Some studies demonstrate its hepatoprotective activity (Yang et al., 2024). For example, BMP9-deficient mice develop hepatic steatosis due to downregulation of peroxisome proliferator-activated receptor α (PPARα) expression and reduced fatty acid oxidation. Furthermore, recombinant BMP9 treatment reduces TG accumulation in Hepa 1-6 cells (Yang et al., 2020). In vivo studies showed that showed that Cers6, Cidea, Fabp4 involved in lipid and glucose metabolism and Fos, Ccl2, Tlr1 involved in inflammatory response were significantly downregulated after BMP9 treatment in HFD mouse liver (Sun et al., 2020). Additionally, administration of MB109, a recombinant derivative of human BMP9, in obese mice enhances FGF21 expression, alleviates HFD-induced obesity, and reduces liver lipid droplets (Kim et al., 2016). In contrast, other studies have shown that BMP9 overexpression increases M1 macrophage gene expression (CD86, IL1β, IL6, MCP-1, TNFα) and the number of M1 macrophages in the liver, thereby promoting MCD diet-induced steatohepatitis in mice (Jiang et al., 2021a). Besides, Breitkopf-Heinlein et al. identified quiescent and activated HSCs as the major BMP9 producing liver cell type (Breitkopf-Heinlein et al., 2017). Upon HSC activation, endogenous BMP-9 levels increase, causing enhanced damage upon acute or chronic injury. The in vitro experiments showed that treatment of cultured hepatocytes with BMP9 inhibited proliferation, epithelial to mesenchymal transition and preserved expression of metabolic enzymes like cytochrome P450. As a result, in vivo application of BMP9 after partial hepatectomy significantly enhanced liver damage and disturbed the proliferative response (Breitkopf-Heinlein et al., 2017). Therefore, these conflicting findings suggest that further efforts should be made to investigate the function and mechanism of BMP9 in the liver.
Emerging secreted proteins associated with MASLD
Some other secreted proteins, such as Isthmin-1 (Ism1) and mesencephalic astrocyte-derived neurotrophic factor (MANF), have been identified as regulators of MASLD/MASH. Ism1 was originally discovered in 2002 (Pera et al., 2002). As an adipokine, Ism1 plays dual roles: it enhances adipocyte glucose uptake while suppressing hepatic lipid synthesis, thereby improving hyperglycemia and reducing lipid accumulation in mouse models through the activation of PI3K-AKT signaling pathway. Notably, recombinant Ism1 alleviates hepatic steatosis by suppressing SREBP-1c in a diet-induced fatty liver mouse model and ameliorates diabetes in diet-induced obese mice. Ism1 shares glucoregulatory functions with insulin and counters liver lipid accumulation by shifting hepatocytes from a lipogenic state to a protein-synthesis state (Jiang et al., 2021b). Overall, Ism1 emerges as an intriguing secreted factor that regulates glycolipid metabolism. Future research should focus on identifying the receptors modulated by Ism1 that mediate its regulatory functions on glucose and lipid homeostasis.
MANF is an endoplasmic reticulum-resident protein first identified in 2003 (Petrova et al., 2003). In vitro and in vivo studies have demonstrated that MANF plays a role in suppressing lipogenesis by modulating SREBP-1 expression (He et al., 2020; Yan et al., 2022). Liver-specific MANF knockout mice develop hepatic steatosis, whereas MANF overexpression reduces hepatic lipid accumulation (Wu et al., 2021; Yan et al., 2022). Decreased MANF expression results in liver damage, steatosis, and fibrosis, while MANF supplementation improves diet-induced steatosis and age-related metabolic dysfunction (Sousa-Victor et al., 2019). Recent data suggest that MANF deficiency in hepatocytes or hepatic monocyte/macrophages exacerbates hepatic fibrosis. Notably, systemic administration of recombinant human MANF markedly alleviates CCl4-induced hepatic fibrosis in both wild-type and hepatocyte-specific MANF knockout mice by targeting TLR4/NF-κB signaling (Hou et al., 2023). Additionally, MANF inhibits HCC through suppressing NF-κB/Snail signaling (Liu et al., 2020a). Thus, MANF has shown beneficial effects in MASLD/MASH and related chronic liver diseases.
Conclusions and future perspectives
The secretome holds significant potential for regulating liver metabolism and systemic homeostasis. Current research in this area is important for the development of novel and potentially effective treatments for MASLD/MASH. Thus, understanding the role of secreted proteins, such as hepatokines and adipokines, will enhance our understanding of hepatic steatosis, inflammation, fibrosis, cirrhosis, and HCC. For diagnostic purposes, some secreted proteins have emerged as noninvasive diagnostic markers, such as SPARCL1 and GDF3. For example, we found that the SPARCL1-ALT-AST model outperforms the ALT-AST model in identifying patients with MASH, demonstrating its value in assessing liver function. In terms of treatment, optimizing the beneficial effects of secreted proteins while minimizing risks is crucial. For instance, enhancing IL-22’s metabolic protective effects while preventing its pro-regeneration and pro-inflammation impacts on specific organs and tissues is essential. In the near future, with the development of multi-omics and more comprehensive models, such as organoid models or humanized mice, identification of secreted proteins and investigation of their roles and mechanisms will become easier, offering exciting potential as therapeutic targets for MASLD/MASH. However, further research is necessary to fully understand their functions, interactions, and to overcome the challenges of translating this knowledge into pharmacological therapies. In addition, to ensure the clinical application of secreted proteins, more issues need to be considered:
Physiological functions and heterogeneity
Though usually neglected, understanding the physiological functions of secreted proteins is also important, including their regulatory roles in normal hepatocytes, macrophages, HSCs, as well as their expression patterns and rhythmic regulation. In addition, MASLD/MASH is a heterogeneous disease, and different subtypes may exhibit distinct patterns of dysregulation in secreted protein. Ethnicity, age, gender, and comorbidities may influence the dynamics of secreted proteins in MASLD/MASH. Future studies should investigate how these factors, along with disease severity and histological features, impact the regulation of secreted proteins.
Upstream regulatory mechanisms
The upstream regulatory factors and mechanisms governing the expression and secretion of secreted proteins are yet to be fully elucidated. Whether and how nutrients and diets, calorie restriction, temperature, exercise, mood, air pollution, and other factors could regulate secreted proteins warrants further study.
Systemic and adverse effects
Some secreted proteins may have systemic or even adverse effects. For instance, FGF21 has been shown to induce severe bone loss, osteoporosis, and affect fertility in mice (Liu et al., 2024; Moeckli et al., 2022; Wei et al., 2012). Thus, hepatocytes-specific delivery strategies, such as utilizing lipid nanoparticle (LNP) or N-Acetylgalactosamine (GalNAC), could be employed to specifically target secreted protein in treating MASLD/MASH while minimizing side effects.
Translational challenges
Differences in metabolism and physiology between animal models and humans often result in divergent responses to therapies. For instance, while GDF15 treatment shows significant weight loss effects in preclinical studies, its efficacy in humans is limited. To address this challenge, it is essential to prioritize the development of more clinically relevant animal models. Advanced technologies, such as humanized animal models, 3D organoids, and microphysiological systems, can better replicate human diseases and enhance the predictability of therapeutic outcomes.
Acknowledgements
We acknowledge the contributions of many important studies. However, due to space limitations, we are unable to cite all of these studies in this review.
Contributor Information
Yeping Huang, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Clinical Centre for Diabetes, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200233, China.
Bin Liu, Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening, College of Pharmacy, Jiangsu Ocean University, Lianyungang 222005, China.
Cheng Hu, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Clinical Centre for Diabetes, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200233, China; Institute for Metabolic Disease, Fengxian Central Hospital Affiliated to Southern Medical University, Shanghai 201499, China.
Yan Lu, Institute of Metabolism and Regenerative Medicine, Digestive Endoscopic Center, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200233, China.
Author contributions
Y. H. and Y. L. conceived and wrote the manuscript. B. L. and C. H. contributed to discussion and revised the manuscript. All the authors validated and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study is supported by the National Natural Science Foundation of China (92268109, 82325010, 82273167, 82400937).
Consent for publication
All the authors listed have approved the manuscript.
Data availability
Not applicable.
References
- Abdelmalek MF, Sanyal AJ, Nakajima A. et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and compensated cirrhosis (FALCON 2): a randomized phase 2b study. Clin Gastroenterol Hepatol 2024;22:113–123.e9. [DOI] [PubMed] [Google Scholar]
- Abdelnabi MN, Flores Molina M, Soucy G. et al. Sex-dependent hepatoprotective role of IL-22 receptor signaling in non-alcoholic fatty liver disease-related fibrosis. Cell Mol Gastroenterol Hepatol 2022;14:1269–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Halstead CA, Hansen BC. et al. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One 2013;8:e65763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja P, Bi X, Ng CF. et al. Src homology 3 domain binding kinase 1 protects against hepatic steatosis and insulin resistance through the Nur77-FGF21 pathway. Hepatology 2023;77:213–229. [DOI] [PubMed] [Google Scholar]
- Alfadda AA, Fatma S, Chishti MA. et al. Orosomucoid serum concentrations and fat depot-specific mRNA and protein expression in humans. Mol Cells 2012;33:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisi A, Ceccarelli S, Panera N. et al. Association between serum atypical fibroblast growth factors 21 and 19 and pediatric nonalcoholic fatty liver disease. PLoS One 2013;8:e67160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Sola G, Uriarte I, Latasa MU. et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017;66:1818–1828. [DOI] [PubMed] [Google Scholar]
- Al-Zuhairi WS, Sadeghi L, Hassan EA.. The link between serum neuregulin-1 and atherogenic index in type 2 diabetes mellitus. Ir J Med Sci 2024;193:2209–2216. [DOI] [PubMed] [Google Scholar]
- Ameka M, Markan KR, Morgan DA. et al. Liver derived FGF21 maintains core body temperature during acute cold exposure. Sci Rep 2019;9:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab JP, Sehrawat TS, Simonetto DA. et al. An open-label, dose-escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcohol-associated hepatitis. Hepatology 2020;72:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C.. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 2008;223:20–38. [DOI] [PubMed] [Google Scholar]
- Arndt S, Wacker E, Dorn C. et al. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut 2015;64:973–981. [DOI] [PubMed] [Google Scholar]
- Arshad T, Mansur F, Palek R. et al. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front Immunol 2020;11:2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadikaram G, Akbari H, Safi Z. et al. Downregulation of IL-22 can be considered as a risk factor for onset of type 2 diabetes. J Cell Biochem 2018;119:9254–9260. [DOI] [PubMed] [Google Scholar]
- Atorrasagasti C, Aquino JB, Hofman L. et al. SPARC downregulation attenuates the profibrogenic response of hepatic stellate cells induced by TGF-beta1 and PDGF. Am J Physiol Gastrointest Liver Physiol 2011;300:G739–G748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atorrasagasti C, Onorato A, Gimeno ML. et al. SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin Sci 2019;133:351–365. [DOI] [PubMed] [Google Scholar]
- Atorrasagasti C, Peixoto E, Aquino JB. et al. Lack of the matricellular protein SPARC (secreted protein, acidic and rich in cysteine) attenuates liver fibrogenesis in mice. PLoS One 2013;8:e54962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboota RK, Rawshani A, Bonnet L. et al. BMP4 and Gremlin 1 regulate hepatic cell senescence during clinical progression of NAFLD/NASH. Nat Metab 2022;4:1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR. et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–437. [DOI] [PubMed] [Google Scholar]
- Bai J, Xia M, Xue Y. et al. Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans. EBioMedicine 2020;57:102849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M.. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009;8:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaya S, Michailidis E, Korol CB. et al. Inherited IL-18BP deficiency in human fulminant viral hepatitis. J Exp Med 2019;216:1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LN, Lee L, Saxena R. et al. Serum proteomic analysis of diet-induced steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Am J Physiol Gastrointest Liver Physiol 2010;298:G746–G754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou O, Coskun T, Gonciarz MD. et al. Discovery, development, and clinical proof of mechanism of LY3463251, a long-acting GDF15 receptor agonist. Cell Metab 2023;35:274–286.e10. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Bina HA. et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009;150:4084–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Damron HA, Hillgartner FB.. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem 2009;284:10023–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Bays HE, Miller M. et al. ; ENTRIGUE Principal Investigators. The FGF21 analog pegozafermin in severe hypertriglyceridemia: a randomized phase 2 trial. Nat Med 2023;29:1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V, Riordan JD, Soleymanjahi S. et al. Apobec1 complementation factor overexpression promotes hepatic steatosis, fibrosis, and hepatocellular cancer. J Clin Invest 2021;131:e138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewski S, Le Bail B, Boussarie L. et al. Osteonectin (SPARC) expression in human liver and in cultured human liver myofibroblasts. Am J Pathol 1997;151:651–657. [PMC free article] [PubMed] [Google Scholar]
- Boger CA, Sedor JR.. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet 2012;8:e1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bograya M, Vulf M, Minchenko A. et al. The putative antilipogenic role of NRG4 and ERBB4: first expression study on human liver samples. Front Biosci 2024;29:414. [DOI] [PubMed] [Google Scholar]
- BonDurant LD, Ameka M, Naber MC. et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab 2017;25:935–944.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, Potthoff MJ.. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu Rev Nutr 2018;38:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 2013;19:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH.. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 2001;107:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit SN, Brown DA, Tsai VW.. The GDF15-GFRAL pathway in health and metabolic disease: friend or foe? Annu Rev Physiol 2021;83:127–151. [DOI] [PubMed] [Google Scholar]
- Breit SN, Manandhar R, Zhang HP. et al. GDF15 enhances body weight and adiposity reduction in obese mice by leveraging the leptin pathway. Cell Metab 2023;35:1341–1355.e3. [DOI] [PubMed] [Google Scholar]
- Breitkopf-Heinlein K, Meyer C, Konig C. et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 2017;66:939–954. [DOI] [PubMed] [Google Scholar]
- Bu Y, Okunishi K, Yogosawa S. et al. Insulin regulates lipolysis and fat mass by upregulating growth/differentiation factor 3 in adipose tissue macrophages. Diabetes 2018;67:1761–1772. [DOI] [PubMed] [Google Scholar]
- Burden S, Yarden Y.. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 1997;18:847–855. [DOI] [PubMed] [Google Scholar]
- Byun S, Seok S, Kim YC. et al. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun 2020;11:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Lin M, Xu Y. et al. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med 2016;14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud K, Boisseau N, Ennequin G. et al. Neuregulin 1 improves glucose tolerance in adult and old rats. Diabetes Metab 2016;42:96–104. [DOI] [PubMed] [Google Scholar]
- Camino AM, Atorrasagasti C, Maccio D. et al. Adenovirus-mediated inhibition of SPARC attenuates liver fibrosis in rats. J Gene Med 2008;10:993–1004. [DOI] [PubMed] [Google Scholar]
- Canto C, Pich S, Paz JC. et al. Neuregulins increase mitochondrial oxidative capacity and insulin sensitivity in skeletal muscle cells. Diabetes 2007;56:2185–2193. [DOI] [PubMed] [Google Scholar]
- Casana E, Jimenez V, Jambrina C. et al. AAV-mediated BMP7 gene therapy counteracts insulin resistance and obesity. Mol Ther Methods Clin Dev 2022;25:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespedes JC, Liu M, Harbuzariu A. et al. Neuregulin in health and disease. Int J Brain Disord Treat 2018;4:024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MZ, Chang JC, Zavala-Solorio J. et al. FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/βKlotho complex in non-adipocytes. Mol Metab 2017a;6:1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang GX, Ma SL. et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab 2017b;6:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xuan Y, Zhu Y. et al. Adipocyte secreted NRG4 ameliorates age-associated metabolic dysfunction. Biochem Pharmacol 2024a;225:116327. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang P, Liu T. et al. Neuregulin 4 mediates the metabolic benefits of mild cold exposure by promoting beige fat thermogenesis. JCI Insight 2024b;9:e172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZT, Weng ZX, Lin JD. et al. Myokines: metabolic regulation in obesity and type 2 diabetes. Life Metab. 2024c;3:loae006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li Y, Zhu JY. et al. Exercise-induced adipokine Nrg4 alleviates MASLD by disrupting hepatic cGAS-STING signaling. Cell Rep 2025;44:115251. [DOI] [PubMed] [Google Scholar]
- Chrysovergis K, Wang X, Kosak J. et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes 2014;38:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui ZSW, Shen Q, Xu A.. Current status and future perspectives of FGF21 analogues in clinical trials. Trends Endocrinol Metab 2024;35:371–384. [DOI] [PubMed] [Google Scholar]
- Chung HK, Ryu D, Kim KS. et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol 2017;216:149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–6027. [DOI] [PubMed] [Google Scholar]
- Dai YN, Zhu JZ, Fang ZY. et al. A case-control study: association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism 2015;64:1667–1673. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF.. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–845. [DOI] [PubMed] [Google Scholar]
- Degirolamo C, Sabba C, Moschetta A.. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016;15:51–69. [DOI] [PubMed] [Google Scholar]
- DePaoli AM, Zhou M, Kaplan DD. et al. FGF19 analog as a surgical factor mimetic that contributes to metabolic effects beyond glucose homeostasis. Diabetes 2019;68:1315–1328. [DOI] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, van den Brink MR.. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015;33:747–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin AS, Fawzy O, Mahmoud E. et al. Serum neuregulin 1 in relation to ventricular function and subclinical atherosclerosis in type 2 diabetes patients. Endocrinol Diabetes Nutr (Engl Ed) 2023;70:619–627. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Wang F, Du Y. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 2017;23:1215–1219. [DOI] [PubMed] [Google Scholar]
- Ennequin G, Boisseau N, Caillaud K. et al. Neuregulin 1 improves glucose tolerance in db/db mice. PLoS One 2015;10:e0130568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennequin G, Capel F, Caillaud K. et al. Neuregulin 1 improves complex 2-mediated mitochondrial respiration in skeletal muscle of healthy and diabetic mice. Sci Rep 2017;7:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren F, Kurt R, Ermis F. et al. Preliminary evidence of a reduced serum level of fibroblast growth factor 19 in patients with biopsy-proven nonalcoholic fatty liver disease. Clin Biochem 2012;45:655–658. [DOI] [PubMed] [Google Scholar]
- Fabbrini E, Sullivan S, Klein S.. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR, Grunfeld C, Heuer JG. et al. FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology 2012;153:2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Valenti L, Wong VW. et al. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2024;21:46–56. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Perstrup LB, Berntsen A. et al. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 2005;117:152–160. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Nasser IA. et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 2014;147:1073–83.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Maratos-Flier E.. Understanding the physiology of FGF21. Annu Rev Physiol 2016;78:223–241. [DOI] [PubMed] [Google Scholar]
- Flippo KH, Jensen-Cody SO, Claflin KE. et al. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci Rep 2020;10:19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo KH, Potthoff MJ.. Metabolic messengers: FGF21. Nat Metab 2021;3:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Neuschwander-Tetri BA, Rinella M. et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizell E, Liu SL, Abraham A. et al. Expression of SPARC in normal and fibrotic livers. Hepatology 1995;21:847–854. [PubMed] [Google Scholar]
- Fu L, John LM, Adams SH. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004;145:2594–2603. [DOI] [PubMed] [Google Scholar]
- Gagliardi F, Narayanan A, Mortini P.. SPARCL1 a novel player in cancer biology. Crit Rev Oncol Hematol 2017;109:63–68. [DOI] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18:333–340. [DOI] [PubMed] [Google Scholar]
- Gateva A, Kamenov Z, Karamfilova V. et al. Higher levels of IL-18 in patients with prediabetes compared to obese normoglycaemic controls. Arch Physiol Biochem 2020;126:449–452. [DOI] [PubMed] [Google Scholar]
- Gaudino SJ, Singh A, Huang H. et al. Intestinal IL-22RA1 signaling regulates intrinsic and systemic lipid and glucose metabolism to alleviate obesity-associated disorders. Nat Commun 2024;15:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalda-Navarro A, Villarroya J, Cereijo R. et al. The endocrine role of brown adipose tissue: an update on actors and actions. Rev Endocr Metab Disord 2022;23:31–41. [DOI] [PubMed] [Google Scholar]
- Geissler A, Ryzhov S, Sawyer DB.. Neuregulins: protective and reparative growth factors in multiple forms of cardiovascular disease. Clin Sci 2020;134:2623–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Lam KSL, Xu A.. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol 2020;16:654–667. [DOI] [PubMed] [Google Scholar]
- Gomes MB, Piccirillo LJ, Nogueira VG. et al. Acute-phase proteins among patients with type 1 diabetes. Diabetes Metab 2003;29:405–411. [DOI] [PubMed] [Google Scholar]
- Gong F, Wu J, Zhou P. et al. Interleukin-22 might act as a double-edged sword in type 2 diabetes and coronary artery disease. Mediators Inflamm 2016;2016:8254797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Zhou P, Liu Y. et al. Down-regulating Interleukin-22/Interleukin-22 binding protein axis promotes inflammation and aggravates diet-induced metabolic disorders. Mol Cell Endocrinol 2022;557:111776. [DOI] [PubMed] [Google Scholar]
- Guma A, Diaz-Saez F, Camps M. et al. Neuregulin, an effector on mitochondria metabolism that preserves insulin sensitivity. Front Physiol 2020;11:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Liu J, Zhang P. et al. Adiposity measurements and metabolic syndrome are linked through circulating neuregulin 4 and adipsin levels in obese adults. Front Physiol 2021;12:667330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhang P, Chen Z. et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J Clin Invest 2017;127:4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Ramachandran D, Roh HC. et al. Obesity-linked PPARgamma S273 phosphorylation promotes insulin resistance through growth differentiation factor 3. Cell Metab 2020;32:665–675.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lone MA, Schneiter R. et al. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc Natl Acad Sci U S A 2010;107:5851–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JS, Clemmesen JO, Secher NH. et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 2015;4:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari D, Tzahar E, Romano J. et al. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene 1999;18:2681–2689. [DOI] [PubMed] [Google Scholar]
- Harries LW, McCulloch LJ, Holley JE. et al. A role for SPARC in the moderation of human insulin secretion. PLoS One 2013;8:e68253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Abdelmalek MF, Neff G. et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol 2022;7:603–616. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Bedossa P, Guy CD. et al. ; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med 2024a;390:497–509. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Frias JP, Lucas KJ. et al. Safety and efficacy of efruxifermin in combination with a GLP-1 receptor agonist in patients with NASH/MASH and Type 2 diabetes in a randomized phase 2 study. Clin Gastroenterol Hepatol 2025;23:103–113. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Frias JP, Neff G. et al. ; HARMONY Study Group. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol 2023a;8:1080–1093. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Neff G, Guy CD. et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology 2021a;160:219–231.e1. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Rinella ME, Abdelmalek MF. et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174–1185. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Rolph T, Knott M. et al. FGF21 agonists: an emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J Hepatol 2024b;81:562–576. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Rossi SJ, Paredes AH. et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 2020;71:1198–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Ruane PJ, Freilich BL. et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med 2021b;27:1262–1271. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Ruane PJ, Freilich B. et al. A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep 2023b;5:100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Borg DJ, Harcourt BE. et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 2014;20:1417–1426. [DOI] [PubMed] [Google Scholar]
- He M, Wang C, Long XH. et al. Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism. World J Gastroenterol 2020;26:1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo MJ, Cheon I, Kim KH.. More than carriers, orosomucoids are key metabolic modulators. Trends Endocrinol Metab 2024:S1043-2760(24)00322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A, Lelong C, Favrel P.. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol 2004;28:461–485. [DOI] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Dehner M. et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep 2019;27:2934–2947.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochepied T, Berger FG, Baumann H. et al. α1-Acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev 2003;14:25–34. [DOI] [PubMed] [Google Scholar]
- Hohenester S, Kanitz V, Schiergens T. et al. IL-18 but Not IL-1 signaling is pivotal for the initiation of liver injury in murine non-alcoholic fatty liver disease. Int J Mol Sci 2020;21:8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Wang D, Zhao M. et al. MANF brakes TLR4 signaling by competitively binding S100A8 with S100A9 to regulate macrophage phenotypes in hepatic fibrosis. Acta Pharm Sin B 2023;13:4234–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EC, Koniaris LG, Zimmers-Koniaris T. et al. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol 2000;20:3742–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Crawley S, Chen M. et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017;550:255–259. [DOI] [PubMed] [Google Scholar]
- Hu L, He F, Huang M. et al. SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Sci Rep 2020;10:17581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Ung T.. Effect of alpha-1-acid glycoprotein binding on pharmacokinetics and pharmacodynamics. Curr Drug Metab 2013;14:226–238. [PubMed] [Google Scholar]
- Huang DQ, Singal AG, Kono Y. et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969–977.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Gao P, Young LH. et al. Targeting white adipose tissue to combat insulin resistance. Trends Pharmacol Sci 2024;45:868–871. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yu F, Ding Y. et al. Hepatic IL22RA1 deficiency promotes hepatic steatosis by modulating oxysterol in the liver. Hepatology 2025;81:1564–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, He Y, Xiang X. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 2020;72:412–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icer MA, Gezmen-Karadag M.. The multiple functions and mechanisms of osteopontin. Clin Biochem 2018;59:17–24. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest 2009;119:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmak S, Oliveira-Ferrer L, Singer BB. et al. Pro-angiogenic properties of orosomucoid (ORM). Exp Cell Res 2009;315:3201–3209. [DOI] [PubMed] [Google Scholar]
- Izumi T. The GDF3-ALK7 signaling axis in adipose tissue: a possible therapeutic target for obesity and associated diabetes? Endocr J 2023;70:761–770. [DOI] [PubMed] [Google Scholar]
- Jensen-Cody SO, Flippo KH, Claflin KE. et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab 2020;32:273–286.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Cody SO, Potthoff MJ.. Hepatokines and metabolism: deciphering communication from the liver. Mol Metab 2021;44:101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Duan Y, Li Y. et al. A long-acting FGF21 attenuates metabolic dysfunction-associated steatohepatitis-related fibrosis by modulating NR4A1-mediated Ly6C phenotypic switch in macrophages. Br J Pharmacol 2024;181:2923–2946. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li Q, Liu B. et al. BMP9 promotes methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in non-obese mice by enhancing NF-κB dependent macrophage polarization. Int Immunopharmacol 2021a;96:107591. [DOI] [PubMed] [Google Scholar]
- Jiang R, Tan Z, Deng L. et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011;54:900–909. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zhao M, Voilquin L. et al. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and hepatic steatosis. Cell Metab 2021b;33:1836–1852.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Zhao X, Liang Y. et al. FGF1-FGFR1 axis promotes tongue squamous cell carcinoma (TSCC) metastasis through epithelial-mesenchymal transition (EMT). Biochem Biophys Res Commun 2015;466:327–332. [DOI] [PubMed] [Google Scholar]
- Jing K, Gu R, Chen F. et al. Orosomucoid 2 is an endogenous regulator of neuronal mitochondrial biogenesis and promotes functional recovery post-stroke. Pharmacol Res 2024;209:107422. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Suh JM, Atkins AR. et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 2012;485:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung UJ, Choi MS.. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 2018;281:138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A. et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A. et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007;148:774–781. [DOI] [PubMed] [Google Scholar]
- Kim S, Choe S, Lee DK.. BMP-9 enhances fibroblast growth factor 21 expression and suppresses obesity. Biochim Biophys Acta 2016;1862:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kang ES, Kim DJ. et al. Effects of rosiglitazone and metformin on inflammatory markers and adipokines: decrease in interleukin-18 is an independent factor for the improvement of homeostasis model assessment-beta in type 2 diabetes mellitus. Clin Endocrinol 2007;66:282–289. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kim SH, Han DH. et al. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep 2018;8:6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim KH, Kim HK. et al. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia 2015;58:809–818. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011;331:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler A, Regensburger D, Tenkerian C. et al. Species-, organ- and cell-type-dependent expression of SPARCL1 in human and mouse tissues. PLoS One 2020;15:e0233422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr J, Kaufmann B, Inzaugarat ME. et al. Interleukin-18 signaling promotes activation of hepatic stellate cells in mouse liver fibrosis. Hepatology 2023;77:1968–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Feng D, Wang H. et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Um SH, Seo DS. et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int 2018;38:695–705. [DOI] [PubMed] [Google Scholar]
- Kos K, Wilding JP.. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol 2010;6:225–235. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Choi M, Ogawa Y. et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007;282:26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwabi-Addo B, Ozen M, Ittmann M.. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer 2004;11:709–724. [DOI] [PubMed] [Google Scholar]
- Lan T, Morgan DA, Rahmouni K. et al. FGF19, FGF21, and an FGFR1/beta-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab 2017;26:709–718.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana JP, Martins LB, Oliveira MC. et al. TNF and IL-18 cytokines may regulate liver fat storage under homeostasis conditions. Appl Physiol Nutr Metab 2016;41:1295–1302. [DOI] [PubMed] [Google Scholar]
- Lebel-Binay S, Berger A, Zinzindohoue F. et al. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw 2000;11:15–26. [PubMed] [Google Scholar]
- Lee YS, Choi JW, Hwang I. et al. Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J Biol Chem 2010;285:22174–22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Choi JM, Jung SY. et al. The bile acid induced hepatokine orosomucoid suppresses adipocyte differentiation. Biochem Biophys Res Commun 2021;534:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kang YE, Chang JY. et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res 2016;8:4750–4763. [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee JA, Park HS. et al. Associations among SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolic parameters in Korean women. Obesity 2013;21:2296–2302. [DOI] [PubMed] [Google Scholar]
- Li L, Chen J, Sun H. et al. Orm2 deficiency aggravates high-fat diet-induced obesity through gut microbial dysbiosis and intestinal inflammation. Mol Nutr Food Res 2024;68:e2300236. [DOI] [PubMed] [Google Scholar]
- Li Y, Jin L, Jiang F. et al. Mutations of NRG4 contribute to the pathogenesis of nonalcoholic fatty liver disease and related metabolic disorders. Diabetes 2021;70:2213–2224. [DOI] [PubMed] [Google Scholar]
- Li Y, Li-Li Z, Qin L. et al. Plasma interleukin-18/interleukin-18 binding protein ratio in Chinese with NAFLD. Hepatogastroenterology 2010;57:103–106. [PubMed] [Google Scholar]
- Li L, Sun H, Chen J. et al. Mitigation of non-alcoholic steatohepatitis via recombinant Orosomucoid 2, an acute phase protein modulating the Erk1/2-PPARgamma-Cd36 pathway. Cell Rep 2023;42:112697. [DOI] [PubMed] [Google Scholar]
- Li Y, Wong K, Giles A. et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 2014;146:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhang H, Zhong Y.. Hepatic GDF15 is regulated by CHOP of the unfolded protein response and alleviates NAFLD progression in obese mice. Biochem Biophys Res Commun 2018;498:388–394. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J. et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014;63:4064–4075. [DOI] [PubMed] [Google Scholar]
- Lin Q, Huang Z, Cai G. et al. Activating adenosine monophosphate-activated protein kinase mediates fibroblast growth factor 1 protection from nonalcoholic fatty liver disease in mice. Hepatology 2021;73:2206–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM. et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015;528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gu X, Li Z. et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 2006;48:1438–1447. [DOI] [PubMed] [Google Scholar]
- Liu W, Struik D, Nies VJ. et al. Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2016;113:2288–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu Z, Han D. et al. Mesencephalic astrocyte-derived neurotrophic factor inhibits liver cancer through small ubiquitin-related modifier (SUMO)ylation-related suppression of NF-κB/Snail signaling pathway and epithelial-mesenchymal transition. Hepatology 2020a;71:1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yin Y, Wang M. et al. Disrupting phosphatase SHP2 in macrophages protects mice from high-fat diet-induced hepatic steatosis and insulin resistance by elevating IL-18 levels. J Biol Chem 2020b;295:10842–10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Xiang L, Ji J. et al. Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2. J Clin Invest 2021;131:e144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Zheng S, Wang S. et al. Urine proteome in distinguishing hepatic steatosis in patients with metabolic-associated fatty liver disease. Diagnostics 2022;12:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Schönke M, Spoorenberg B. et al. FGF21 protects against hepatic lipotoxicity and macrophage activation to attenuate fibrogenesis in nonalcoholic steatohepatitis. Elife 2023a;12:e83075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu S, Chen L. et al. Profiling the genome and proteome of metabolic dysfunction-associated steatotic liver disease identifies potential therapeutic targets. medRxiv 2023b;2023.11.30.23299247. [Google Scholar]
- Liu J, Jiang J, Li Y. et al. Effects of FGF21 overexpression in osteoporosis and bone mineral density: a two-sample, mediating Mendelian analysis. Front Endocrinol 2024;15:1439255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wei S, Jiang Y. et al. Protein phosphatase 6 regulates metabolic dysfunction-associated steatohepatitis via the mTORC1 pathway. J Hepatol 2025;S0168-8278(25)00079-0. [DOI] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI. et al. ; LifeLines Cohort Study. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Friedman SL, Shulman GI.. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184:2537–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Lawitz EJ, Frias JP. et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol Hepatol 2023a;8:120–132. [DOI] [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ, Kowdley KV. et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med 2023b;389:998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ, Nakajima A. et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and stage 3 fibrosis (FALCON 1): a randomized phase 2b study. Clin Gastroenterol Hepatol 2024;22:102–112.e9. [DOI] [PubMed] [Google Scholar]
- Lopez-Bermejo A, Bosch M, Recasens M. et al. Potential role of interleukin-18 in liver disease associated with insulin resistance. Obes Res 2005;13:1925–1931. [DOI] [PubMed] [Google Scholar]
- Lou G, Zhang Q, Xiao F. et al. Intranasal administration of TAT-haFGF14–154 attenuates disease progression in a mouse model of Alzheimer’s disease. Neuroscience 2012;223:225–237. [DOI] [PubMed] [Google Scholar]
- Lu JF, Zhu MQ, Xia B. et al. GDF15 is a major determinant of ketogenic diet-induced weight loss. Cell Metab 2023;35:2165–2182.e7. [DOI] [PubMed] [Google Scholar]
- Ludwiczek O, Kaser A, Novick D. et al. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J Clin Immunol 2002;22:331–337. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Galman C, Angelin B. et al. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 2006;260:530–536. [DOI] [PubMed] [Google Scholar]
- Luo Z, Lei H, Sun Y. et al. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem 2015;71:329–340. [DOI] [PubMed] [Google Scholar]
- Macia L, Tsai VW, Nguyen AD. et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One 2012;7:e34868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Imamura M, Kurashige M. et al. Replication study for the association of 3 SNP loci identified in a genome-wide association study for diabetic nephropathy in European type 1 diabetes with diabetic nephropathy in Japanese patients with type 2 diabetes. Clin Exp Nephrol 2013;17:866–871. [DOI] [PubMed] [Google Scholar]
- Mahli A, Seitz T, Beckroge T. et al. Bone morphogenetic protein-8B expression is induced in steatotic hepatocytes and promotes hepatic steatosis and inflammation in vitro. Cells 2019;8:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltez VI, Tubbs AL, Cook KD. et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 2015;43:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranon P, Fernandez-Garcia CE, Isaza SC. et al. Bone morphogenetic protein 2 is a new molecular target linked to non-alcoholic fatty liver disease with potential value as non-invasive screening tool. Biomark Res 2022;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK. et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014;63:4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Potthoff MJ.. Metabolic fibroblast growth factors (FGFs): mediators of energy homeostasis. Semin Cell Dev Biol 2016;53:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Latorre J, Ortega F. et al. Serum neuregulin 4 is negatively correlated with insulin sensitivity in humans and impairs mitochondrial respiration in HepG2 cells. Front Physiol 2022;13:950791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Pamies I, Thoonen R, Seale P. et al. Deficiency of bone morphogenetic protein-3b induces metabolic syndrome and increases adipogenesis. Am J Physiol Endocrinol Metab 2020;319:E363–E375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Otsuka F, Hino J. et al. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol Cell Endocrinol 2012;350:78–86. [DOI] [PubMed] [Google Scholar]
- Mazzolini G, Atorrasagasti C, Onorato A. et al. SPARC expression is associated with hepatic injury in rodents and humans with non-alcoholic fatty liver disease. Sci Rep 2018;8:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Neupane A, Wang L. et al. Expression of NALPs in adipose and the fibrotic progression of non-alcoholic fatty liver disease in obese subjects. BMC Gastroenterol 2014;14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissburger B, Perdikari A, Moest H. et al. Regulation of adipogenesis by paracrine factors from adipose stromal-vascular fraction - a link to fat depot-specific differences. Biochim Biophys Acta 2016;1861:1121–1131. [DOI] [PubMed] [Google Scholar]
- Meng D, Pan H, Chen Y. et al. Roles and mechanisms of NRG1 in modulating the pathogenesis of NAFLD through ErbB3 signaling in hepatocytes (NRG1 modulates NAFLD through ErbB3 signaling). Obes Res Clin Pract 2021;15:145–151. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A. et al. Isoform-specific expression and function of neuregulin. Development 1997;124:3575–3586. [DOI] [PubMed] [Google Scholar]
- Miao L, Targher G, Byrne CD. et al. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab 2024;35:697–707. [DOI] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O. et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol 2000;20:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeckli B, Pham TV, Slits F. et al. FGF21 negatively affects long-term female fertility in mice. Heliyon 2022;8:e11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M, Wang R, Jeet V. et al. Interleukin (IL)-22 from IL-20 subfamily of cytokines induces colonic epithelial cell proliferation predominantly through ERK1/2 pathway. Int J Mol Sci 2019;20:3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki Y, Yamamoto T, Shibutani Y. et al. Elevated levels of interleukin-18 and tumor necrosis factor-α in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 2003;52:605–608. [DOI] [PubMed] [Google Scholar]
- Mounika N, Yadav A, Kamboj P. et al. Circulatory bone morphogenetic protein (BMP) 8B is a non-invasive predictive biomarker for the diagnosis of non-alcoholic steatohepatitis (NASH). PLoS One 2023;18:e0295839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M, Lacinova Z, Kavalkova P. et al. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-alpha agonist treatment. Physiol Res 2011;60:627–636. [DOI] [PubMed] [Google Scholar]
- Mullican SE, Lin-Schmidt X, Chin CN. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 2017;23:1150–1157. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Kraakman MJ, Kammoun HL. et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab 2016;23:155–164. [DOI] [PubMed] [Google Scholar]
- Musale V, Wasserman DH, Kang L.. Extracellular matrix remodelling in obesity and metabolic disorders. Life Metab. 2023;2:load021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskiet MHA, Tonneijck L, Smits MM. et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–628. [DOI] [PubMed] [Google Scholar]
- Nakayasu ES, Syed F, Tersey SA. et al. Comprehensive proteomics analysis of stressed human islets identifies GDF15 as a target for type 1 diabetes intervention. Cell Metab 2020;31:363–374.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA.. The NLRP1-IL18 connection: a stab in the back of obesity-induced inflammation. Cell Metab 2016;23:6–7. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Lewis E. et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 2006;12:650–656. [DOI] [PubMed] [Google Scholar]
- Ouyang W, O’Garra A.. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 2019;50:871–891. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK. et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109. [DOI] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA. et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 2014;20:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park O, Wang H, Weng H. et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology 2011;54:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Chen B, Wang H. et al. Bone morphogenetic protein 4 (BMP4) alleviates hepatic steatosis by increasing hepatic lipid turnover and inhibiting the mTORC1 signaling axis in hepatocytes. Aging 2019;11:11520–11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Kim JI, Martinez SL. et al. Isthmin is a novel secreted protein expressed as part of the Fgf-8 synexpression group in the Xenopus midbrain-hindbrain organizer. Mech Dev 2002;116:169–172. [DOI] [PubMed] [Google Scholar]
- Petrova P, Raibekas A, Pevsner J. et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 2003;20:173–188. [DOI] [PubMed] [Google Scholar]
- Platko K, Lebeau PF, Byun JH. et al. GDF10 blocks hepatic PPARgamma activation to protect against diet-induced liver injury. Mol Metab 2019;27:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ. FGF21 and metabolic disease in 2016: a new frontier in FGF21 biology. Nat Rev Endocrinol 2017;13:74–76. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M. et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab 2011;13:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ.. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012;26:312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta I, Andreozzi F, Succurro E. et al. IL-18 gene polymorphism and metabolic syndrome. Nutr Metab Cardiovasc Dis 2009;19:e5–e6. [DOI] [PubMed] [Google Scholar]
- Qin Z, Wan JJ, Sun Y. et al. ORM promotes skeletal muscle glycogen accumulation via CCR5-activated AMPK pathway in mice. Front Pharmacol 2016;7:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ, Maratos-Flier E, Nguyen A. et al. ; CLLF580X2102 Study Team. LLF580, an FGF21 analog, reduces triglycerides and hepatic fat in obese adults with modest hypertriglyceridemia. J Clin Endocrinol Metab 2022;107:e57–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella ME, Lieu HD, Kowdley KV. et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology 2024;79:674–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Shibuya K, Mui A. et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 1997;7:571–581. [DOI] [PubMed] [Google Scholar]
- Rochette L, Zeller M, Cottin Y. et al. Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol Metab 2020;31:939–951. [DOI] [PubMed] [Google Scholar]
- Rosset EM, Bradshaw AD.. SPARC/osteonectin in mineralized tissue. Matrix Biol 2016;52–54:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Sidorov S, Ravussin E. et al. The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity 2022;55:1609–1626.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Spadaro O, Sidorov S. et al. Reduction of SPARC protects mice against NLRP3 inflammasome activation and obesity. J Clin Invest 2023;133:e169173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajiir H, Keshvari S, Wong KY. et al. Liver and pancreatic-targeted interleukin-22 as a therapeutic for metabolic dysfunction-associated steatohepatitis. Nat Commun 2024a;15:4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajiir H, Wong KY, Muller A. et al. Pancreatic beta-cell IL-22 receptor deficiency induces age-dependent dysregulation of insulin biosynthesis and systemic glucose homeostasis. Nat Commun 2024b;15:4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Suzuki Y, Miyauchi Y. et al. Downregulation of Sparc-like protein 1 during cisplatin-induced inhibition of myogenic differentiation of C2C12 myoblasts. Biochem Pharmacol 2022;204:115234. [DOI] [PubMed] [Google Scholar]
- Salazar-Leon J, Valdez-Hernandez AL, Garcia-Jimenez S. et al. Nlrp1b1 negatively modulates obesity-induced inflammation by promoting IL-18 production. Sci Rep 2019;9:13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G, Liu S, Gasser E. et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab 2022;34:171–183.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm N, Salem RM, McKnight AJ. et al. ; DCCT/EDIC Research Group. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 2012;8:e1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Charles ED, Neuschwander-Tetri BA. et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019;392:2705–2717. [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Muta K, Brown JM. et al. Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes 2019;68:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett JM, Rojas JM, Matsen ME. et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 2016;22:800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein C, Talukdar S, Heine M. et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab 2016;23:441–453. [DOI] [PubMed] [Google Scholar]
- Sciarrillo CM, Keirns BH, Koemel NA. et al. Fibroblast Growth Factor 19: potential modulation of hepatic metabolism for the treatment of non-alcoholic fatty liver disease. Liver Int 2021;41:894–904. [DOI] [PubMed] [Google Scholar]
- Serti E, Werner JM, Chattergoon M. et al. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology 2014;147:209–220.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigvardsen CM, Richter MM, Engelbeen S. et al. GDF15 is still a mystery hormone. Trends Endocrinol Metab 2024:S1043-2760(24)00254-6. [DOI] [PubMed] [Google Scholar]
- Sjoberg KA, Sigvardsen CM, Alvarado-Diaz A. et al. GDF15 increases insulin action in the liver and adipose tissue via a beta-adrenergic receptor-mediated mechanism. Cell Metab 2023;35:1327–1340.e5. [DOI] [PubMed] [Google Scholar]
- Smith WB, Nguyen D, Clough T. et al. A growth-differentiation factor 15 receptor agonist in randomized placebo-controlled trials in healthy or obese persons. J Clin Endocrinol Metab 2024;110:771–786. [DOI] [PubMed] [Google Scholar]
- Soberg S, Andersen ES, Dalsgaard NB. et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab 2018;11:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, Jornayvaz FR.. Fibroblast Growth Factor 15/19: from basic functions to therapeutic perspectives. Endocr Rev 2018;39:960–989. [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P, Neves J, Cedron-Craft W. et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab 2019;1:276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South JC, Blackburn E, Brown IR. et al. The neuregulin system of ligands and their receptors in rat islets of langerhans. Endocrinology 2013;154:2385–2392. [DOI] [PubMed] [Google Scholar]
- Stanislaus S, Hecht R, Yie J. et al. A novel Fc-FGF21 with improved resistance to proteolysis, increased affinity toward beta-Klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology 2017;158:1314–1327. [DOI] [PubMed] [Google Scholar]
- Suh JM, Jonker JW, Ahmadian M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 2014;513:436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MM, Sage EH.. Hevin/SC1, a matricellular glycoprotein and potential tumor-suppressor of the SPARC/BM-40/Osteonectin family. Int J Biochem Cell Biol 2004;36:991–996. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Cai LY, Jian J. et al. The role of bone morphogenetic protein 9 in nonalcoholic fatty liver disease in mice. Front Pharmacol 2020;11:605967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dai X, Yang J. et al. Deficiency of hepatokine orosomucoid1 aggravates NAFLD progression in mice. Biochim Biophys Acta Mol Basis Dis 2025;1871:167654. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yang Y, Qin Z. et al. The acute-phase protein orosomucoid regulates food intake and energy homeostasis via leptin receptor signaling pathway. Diabetes 2016;65:1630–1641. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Li AJ, Ishisaki A. et al. Feeding suppression by fibroblast growth factor-1 is accompanied by selective induction of heat shock protein 27 in hypothalamic astrocytes. Eur J Neurosci 2001;13:2299–2308. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K. et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 2008;22:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D. et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 2016;23:427–440. [DOI] [PubMed] [Google Scholar]
- Tang KY, Lickliter J, Huang ZH. et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol 2019;16:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer TE, Lino Cardenas CL, Martyn T. et al. The role of bone morphogenetic protein signaling in non-alcoholic fatty liver disease. Sci Rep 2020;10:9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Byrne CD, Targher G.. NASH drug treatment development: challenges and lessons. Lancet Gastroenterol Hepatol 2023;8:943–954. [DOI] [PubMed] [Google Scholar]
- Tomlinson E, Fu L, John L. et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002;143:1741–1747. [DOI] [PubMed] [Google Scholar]
- Tran T, Yang J, Gardner J. et al. GDF15 deficiency promotes high fat diet-induced obesity in mice. PLoS One 2018;13:e0201584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai VW, Manandhar R, Jorgensen SB. et al. The anorectic actions of the TGFbeta cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS One 2014;9:e100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunchi H, Mobasseri M, Aghamohammadzadeh N. et al. Serum neuregulin 4 (NRG-4) level and non-alcoholic fatty liver disease (NAFLD): a case-control study. Int J Clin Pract 2021;75:e14555. [DOI] [PubMed] [Google Scholar]
- Vacca M, Leslie J, Virtue S. et al. Bone morphogenetic protein 8B promotes the progression of non-alcoholic steatohepatitis. Nat Metab 2020;2:514–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Cereijo R, Villarroya J. et al. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 2017;13:26–35. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L. et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 2016;23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 2014;11:426–434. [DOI] [PubMed] [Google Scholar]
- Wan JJ, Wang PY, Zhang Y. et al. Role of acute-phase protein ORM in a mice model of ischemic stroke. J Cell Physiol 2019;234:20533–20545. [DOI] [PubMed] [Google Scholar]
- Wang GX, Zhao XY, Meng ZX. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 2014a;20:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chrysovergis K, Kosak J. et al. Lower NLRP3 inflammasome activity in NAG-1 transgenic mice is linked to a resistance to obesity and increased insulin sensitivity. Obesity 2014b;22:1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chrysovergis K, Kosak J. et al. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging 2014c;6:690–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ota N, Manzanillo P. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014d;514:237–241. [DOI] [PubMed] [Google Scholar]
- Wang R, Yang F, Qing L. et al. Decreased serum neuregulin 4 levels associated with non-alcoholic fatty liver disease in children with obesity. Clin Obes 2019a;9:e12289. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Yu P.. Association between circulating neuregulin4 levels and diabetes mellitus: a meta-analysis of observational studies. PLoS One 2019b;14:e0225705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu S, Yan Y. et al. SPARCL1 promotes C2C12 cell differentiation via BMP7-mediated BMP/TGF-beta cell signaling pathway. Cell Death Dis 2019c;10:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma B, Wen X. et al. Bone morphogenetic protein 4 alleviates nonalcoholic steatohepatitis by inhibiting hepatic ferroptosis. Cell Death Discov 2022a;8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen C, Chen J. et al. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol 2022b;52:102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A 2012;109:3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Kolumam G, Stawicki S. et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med 2011;3:113ra126. [DOI] [PubMed] [Google Scholar]
- Wu T, Liu Q, Li Y. et al. Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J Exp Med 2021;218:e20201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LF, Zhou ZJ, Zeng YH. et al. Circular RNA RRM2 alleviates metabolic dysfunction-associated steatotic liver disease by targeting miR-142-5p to increase NRG1 expression. Am J Physiol Gastrointest Liver Physiol 2024;327:G485–G498. [DOI] [PubMed] [Google Scholar]
- Xiang L, Li X, Luo Y. et al. A multi-omic landscape of steatosis-to-NASH progression. Life Metab. 2022;1:242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Jin HG, Zhang LC. et al. Effects of SPARCL1 on the proliferation and differentiation of sheep preadipocytes. Adipocyte 2021;10:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YT, Xiang LX, Shao JZ.. Bone morphogenetic protein. Biochem Biophys Res Commun 2007;362:550–553. [DOI] [PubMed] [Google Scholar]
- Xie Z, Li Y, Cheng L. et al. Potential therapeutic strategies for MASH: from preclinical to clinical development. Life Metab 2024;3:loae029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009a;58:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Stanislaus S, Chinookoswong N. et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 2009b;297:E1105–E1114. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wu Y, Wang F. et al. Fibroblast growth factor 1 ameliorates diabetes-induced liver injury by reducing cellular stress and restoring autophagy. Front Pharmacol 2020;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K, Maeda S, Kuwahara-Otani S. et al. Interleukin-18-deficient mice develop dyslipidemia resulting in nonalcoholic fatty liver disease and steatohepatitis. Transl Res 2016;173:101–114.e7. [DOI] [PubMed] [Google Scholar]
- Yan J, Liu Q, Tang Q. et al. Mesencephalic astrocyte-derived neurotrophic factor alleviates non-alcoholic steatohepatitis induced by Western diet in mice. FASEB J 2022;36:e22349. [DOI] [PubMed] [Google Scholar]
- Yang L, Chang CC, Sun Z. et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 2017;23:1158–1166. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Wang L. et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol 2010;53:339–347. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu M, Wang W. et al. Circulating Bone morphogenetic protein 9 (BMP9) as a new biomarker for noninvasive stratification of nonalcoholic fatty liver disease and metabolic syndrome. Clin Exp Med 2024;24:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li P, Shang Q. et al. CRISPR-mediated BMP9 ablation promotes liver steatosis via the down-regulation of PPARα expression. Sci Adv 2020;6:eabc5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Wang Y, Li H. et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 2014;60:977–989. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Racila A. et al. Health-related quality of life (HRQL) assessments in a 52-week, double-blind, randomized, placebo-controlled phase III study of resmetirom (MGL-3196) in patients with metabolic dysfunction-associated steatohepatitis (MASH) and fibrosis. Hepatology 2025;81:1318–1327. [DOI] [PubMed] [Google Scholar]
- Yu F, Xie S, Wang T. et al. Pancreatic β cell interleukin-22 receptor subunit alpha 1 deficiency impairs β cell function in type 2 diabetes via cytochrome b5 reductase 3. Cell Rep 2024;43:115057. [DOI] [PubMed] [Google Scholar]
- Zaharieva E, Kamenov Z, Velikova T. et al. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr Connect 2018;7:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai W, Chen W, Wu Z. et al. Targeted interleukin-22 gene delivery in the liver by polymetformin and penetratin-based hybrid nanoparticles to treat nonalcoholic fatty liver disease. ACS Appl Mater Interfaces 2019;11:4842–4857. [DOI] [PubMed] [Google Scholar]
- Zarei M, Barroso E, Palomer X. et al. Hepatic regulation of VLDL receptor by PPARbeta/delta and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab 2018;8:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Wang Y, Kloepfer LA. et al. ErbB4 deletion predisposes to development of metabolic syndrome in mice. Am J Physiol Endocrinol Metab 2018;315:E583–E593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bai M, Tang H. et al. Role of hepatic neuregulin 4 in the regulation of gluconeogenesis in mice. Life Sci 2019;217:185–192. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chen Z, Kuang H. et al. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab 2022;34:1359–1376.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fu Y, Zhou N. et al. Circulating neuregulin 4 concentrations in patients with newly diagnosed type 2 diabetes: a cross-sectional study. Endocrine 2017;57:535–538. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kuang H, He Y. et al. NRG1-Fc improves metabolic health via dual hepatic and central action. JCI Insight 2018;3:e98522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lei S, Zhuo H. et al. TRIM24 up-regulates ORM2 to alleviate abnormal lipid metabolism, inflammation, and oxidative stress in mice with obstructive sleep apnea syndrome and metabolic dysfunction-associated steatotic liver disease. Am J Pathol 2024a;194:2091–2105. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu J, Lee A. et al. IL-22 resolves MASLD via enterocyte STAT3 restoration of diet-perturbed intestinal homeostasis. Cell Metab 2024b;36:2341–2354.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yang X, Wang W.. Associations of genetic polymorphisms in CTLA-4 and IL-18 with chronic liver diseases: evidence from a meta-analysis. Genomics 2020;112:1889–1896. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao X, Dong X. et al. Activity-balanced GLP-1/GDF15 dual agonist reduces body weight and metabolic disorder in mice and non-human primates. Cell Metab 2023a;35:287–298.e4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu Y, Wang J. et al. Neuregulin4 acts on hypothalamic ErBb4 to excite oxytocin neurons and preserve metabolic homeostasis. Adv Sci 2023b;10:e2204824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wu J, Ding Y. et al. Gut microbiota, immunity, and bile acid metabolism: decoding metabolic disease interactions. Life Metab. 2023;2:load032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Learned RM, Rossi SJ. et al. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:1024–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Luo Y, Ji N. et al. Orosomucoid 2 maintains hepatic lipid homeostasis through suppression of de novo lipogenesis. Nat Metab 2022;4:1185–1201. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang X, Phung V. et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306–3316. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Wang J. et al. Intermittent fasting-induced Orm2 promotes adipose browning via the GP130/IL23R-p38 cascade. Adv Sci 2024;11:e2407789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H, Han J, Cheng L. et al. A positive causal influence of IL-18 levels on the risk of T2DM: a Mendelian randomization study. Front Genet 2019;10:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziqubu K, Dludla PV, Mthembu SXH. et al. Low circulating levels of neuregulin 4 as a potential biomarker associated with the severity and prognosis of obesity-related metabolic diseases: a systematic review. Adipocyte 2024;13:2390833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.