Abstract
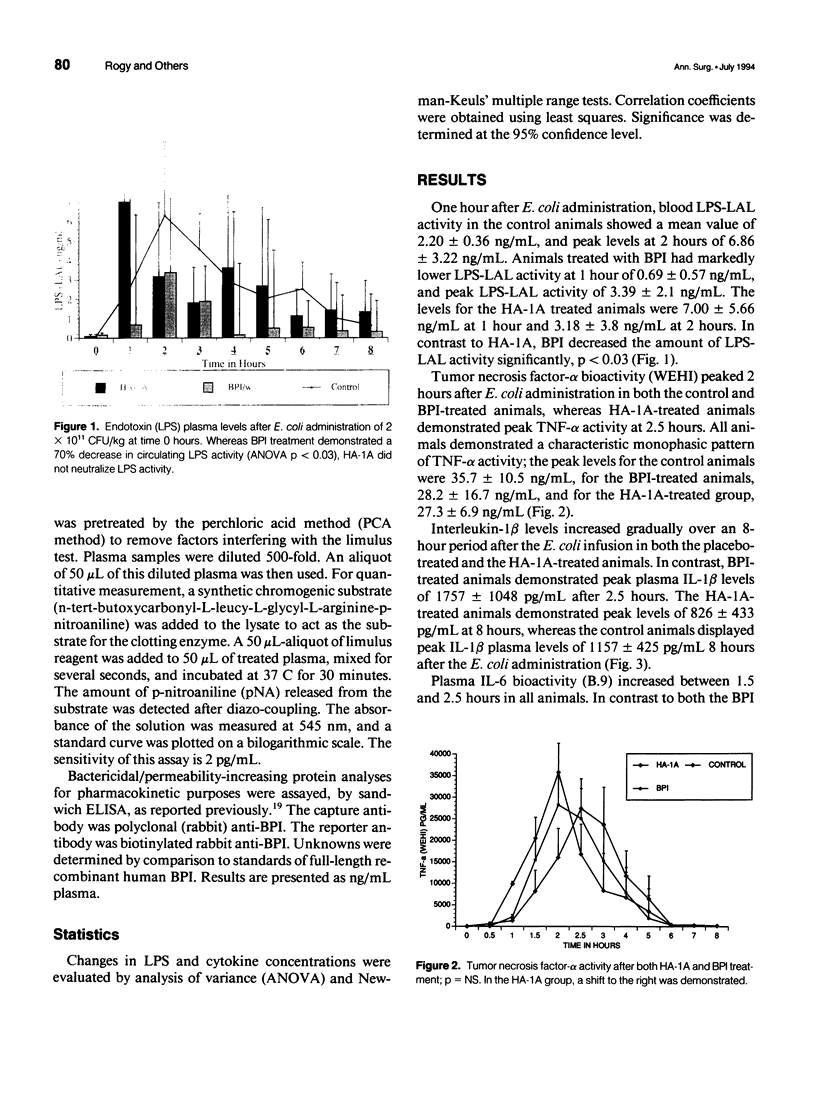
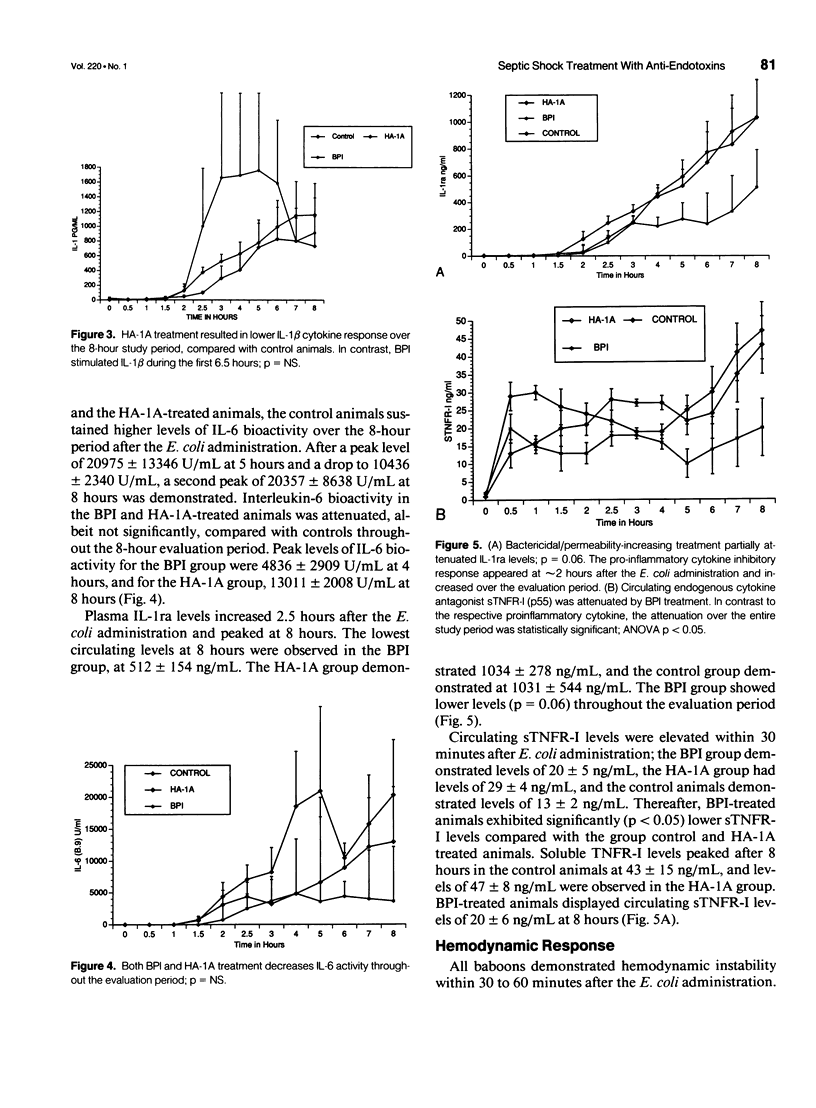
OBJECTIVE: The in vivo neutralizing activities of an anti-lipopolysaccharide (LPS) antibody HA-1A (Centoxin [Centocor, Malvern, PA]), a human immunoglobulin M monoclonal antibody, and of bactericidal/permeability-increasing protein (BPI), an endogenously produced human LPS-neutralizing protein, were studied in a primate model of lethal Escherichia coli bacteremia. SUMMARY BACKGROUND DATA: HA-1A has been used with variable success against LPS activity in some animal models and in a recently reported clinical trial. However, no data assessing the efficacy of this agent in subhuman primates is available. Bactericidal/permeability-increasing protein is a product of polymorphomononuclear cells (PMNs) that is stored in azurophilic granules and exhibits LPS-neutralizing activity in vitro and in some in vivo models. METHODS: Immediately after E. coli infusion and in a blinded fashion, three baboons were treated with BPI (5 mg/kg bolus infusion and 95 micrograms/kg/min infusion over 4 hr). Three animals received 3 mg/kg BW of HA-1A, whereas another three baboons received a placebo treatment. RESULTS: The BPI-treated animals demonstrated significantly (p < 0.03) lower circulating LPS-limulus amoebocyte lysate (LAL) activity compared with the control animals, but this reduction in LPS-LAL activity was not associated with improved survival. HA-1A treatment did not reduce LPS-LAL activity. However, both BPI and HA-1A treatment did attenuate the pro-inflammatory cytokine response. CONCLUSION: The current data suggests that incomplete neutralization of endotoxin activity does not alter mortality from severe bacteremia. Given the diversity of mediator production under such circumstances, a strategy of combination therapy in the form of anti-lipopolysaccharide and anticytokine treatment may be necessary to achieve optimal survival.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D. Immunotherapy with antibodies to core lipopolysaccharide: a critical appraisal. Infect Dis Clin North Am. 1991 Dec;5(4):915–927. [PubMed] [Google Scholar]
- Bogard W. C., Jr, Siegel S. A., Leone A. O., Damiano E., Shealy D. J., Ely T. M., Frederick B., Mascelli M. A., Siegel R. C., Machielse B. Human monoclonal antibody HA-1A binds to endotoxin via an epitope in the lipid A domain of lipopolysaccharide. J Immunol. 1993 May 15;150(10):4438–4449. [PubMed] [Google Scholar]
- Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989 Feb;159(2):195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Dripps D. J., Brandhuber B. J., Thompson R. C., Eisenberg S. P. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem. 1991 Jun 5;266(16):10331–10336. [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Efficacy of type-specific and cross-reactive murine monoclonal antibodies directed against endotoxin during experimental sepsis. Surgery. 1985 Aug;98(2):283–290. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fischer E., Marano M. A., Van Zee K. J., Rock C. S., Hawes A. S., Thompson W. A., DeForge L., Kenney J. S., Remick D. G., Bloedow D. C. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992 May;89(5):1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. J., Jr, Opal S. M., Dhainaut J. F., Stephens S., Zimmerman J. L., Nightingale P., Harris S. J., Schein R. M., Panacek E. A., Vincent J. L. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. The CB0006 Sepsis Syndrome Study Group. Crit Care Med. 1993 Mar;21(3):318–327. doi: 10.1097/00003246-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P. Mediators of septic shock: new approaches for interrupting the endogenous inflammatory cascade. Crit Care Med. 1993 May;21(5):780–789. [PubMed] [Google Scholar]
- Greenman R. L., Schein R. M., Martin M. A., Wenzel R. P., MacIntyre N. R., Emmanuel G., Chmel H., Kohler R. B., McCarthy M., Plouffe J. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991 Aug 28;266(8):1097–1102. [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Ikeda T., Hirata K., Tabuchi K., Tamura H., Tanaka S. Quantitative measurement of endotoxin in canine plasma using the new endotoxin-specific chromogenic test. Circ Shock. 1987;23(4):263–269. [PubMed] [Google Scholar]
- Kelly C. J., Cech A. C., Argenteanu M., Gallagher H., Shou J., Minnard E., Daly J. M. Role of bactericidal permeability-increasing protein in the treatment of gram-negative pneumonia. Surgery. 1993 Aug;114(2):140–146. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., Henson P. M. Mediators of inflammation. Annu Rev Immunol. 1983;1:335–359. doi: 10.1146/annurev.iy.01.040183.002003. [DOI] [PubMed] [Google Scholar]
- Luce J. M. Introduction of new technology into critical care practice: a history of HA-1A human monoclonal antibody against endotoxin. Crit Care Med. 1993 Aug;21(8):1233–1241. doi: 10.1097/00003246-199308000-00026. [DOI] [PubMed] [Google Scholar]
- Lynn W. A., Golenbock D. T. Lipopolysaccharide antagonists. Immunol Today. 1992 Jul;13(7):271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- Mannion B. A., Weiss J., Elsbach P. Separation of sublethal and lethal effects of polymorphonuclear leukocytes on Escherichia coli. J Clin Invest. 1990 Aug;86(2):631–641. doi: 10.1172/JCI114755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Collins M. S., Snable J. L., Thornton M. B., Scott R. W. The role of bactericidal/permeability-increasing protein as a natural inhibitor of bacterial endotoxin. J Immunol. 1992 Jan 15;148(2):532–537. [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Griffith J. E., Snable J. L., Scott R. W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990 Jan 15;144(2):662–666. [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Doerfler M. E., Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55-60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991 Sep 1;174(3):649–655. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S. M., Cross A. S., Sadoff J. C., Collins H. H., Kelly N. M., Victor G. H., Palardy J. E., Bodmer M. W. Efficacy of antilipopolysaccharide and anti-tumor necrosis factor monoclonal antibodies in a neutropenic rat model of Pseudomonas sepsis. J Clin Invest. 1991 Sep;88(3):885–890. doi: 10.1172/JCI115390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezado Z. M., Natanson C., Alling D. W., Banks S. M., Koev C. A., Elin R. J., Hosseini J. M., Bacher J. D., Danner R. L., Hoffman W. D. A controlled trial of HA-1A in a canine model of gram-negative septic shock. JAMA. 1993 May 5;269(17):2221–2227. [PubMed] [Google Scholar]
- Riedo F. X., Munford R. S., Campbell W. B., Reisch J. S., Chien K. R., Gerard R. D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990 May 1;144(9):3506–3512. [PubMed] [Google Scholar]
- Rogy M. A., Coyle S. M., Oldenburg H. S., Rock C. S., Barie P. S., Van Zee K. J., Smith C. G., Moldawer L. L., Lowry S. F. Persistently elevated soluble tumor necrosis factor receptor and interleukin-1 receptor antagonist levels in critically ill patients. J Am Coll Surg. 1994 Feb;178(2):132–138. [PubMed] [Google Scholar]
- Rogy M. A., Oldenburg H. S., Calvano S. E., Montegut W. J., Stackpole S. A., Van Zee K. J., Marra M. N., Scott R. W., Seilhammer J. J., Moldawer L. L. The role of bactericidal/permeability-increasing protein in the treatment of primate bacteremia and septic shock. J Clin Immunol. 1994 Mar;14(2):120–133. doi: 10.1007/BF01541345. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Spinas G. A., Bloesch D., Kaufmann M. T., Keller U., Dayer J. M. Induction of plasma inhibitors of interleukin 1 and TNF-alpha activity by endotoxin administration to normal humans. Am J Physiol. 1990 Nov;259(5 Pt 2):R993–R997. doi: 10.1152/ajpregu.1990.259.5.R993. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Van Zee K. J., Kohno T., Fischer E., Rock C. S., Moldawer L. L., Lowry S. F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Amato S. F., Fitting C., Black K. M., Loiselle P. M., Pasternack M. S., Cavaillon J. M. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993 Jan 1;177(1):89–97. doi: 10.1084/jem.177.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersink A. J., van Kessel K. P., van den Tol M. E., van Strijp J. A., Torensma R., Verhoef J., Elsbach P., Weiss J. Human granulocytes express a 55-kDa lipopolysaccharide-binding protein on the cell surface that is identical to the bactericidal/permeability-increasing protein. J Immunol. 1993 Jan 1;150(1):253–263. [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Shu C., Castillo J., Grinna L., Horwitz A., Theofan G. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Invest. 1992 Sep;90(3):1122–1130. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Young L. S., Gascon R., Alam S., Bermudez L. E. Monoclonal antibodies for treatment of gram-negative infections. Rev Infect Dis. 1989 Nov-Dec;11 (Suppl 7):S1564–S1571. doi: 10.1093/clinids/11.supplement_7.s1564. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Jansen J., van Leenen D., von der Möhlen M., Levi M., ten Cate H., Gallati H., ten Cate J. W., van Deventer S. J. Release of soluble receptors for tumor necrosis factor in clinical sepsis and experimental endotoxemia. J Infect Dis. 1993 Oct;168(4):955–960. doi: 10.1093/infdis/168.4.955. [DOI] [PubMed] [Google Scholar]


