Abstract
In recent years, significant progress has been made in the development of organoids, which offer promising opportunities for developmental and translational research. With advances in cell biology and bioengineering techniques, skin models are evolving from conventional multilayered structures to appendage-bearing spheroids or 3D biomimetic models. This comprehensive review aims to provide an in-depth understanding of organoid models of the skin, covering topics such as skin development, construction strategies and key elements, types of organoid models, biomedical applications, and challenges. Embryonic skin development is briefly introduced to provide a foundational understanding of construction principles. Current engineering strategies are outlined, highlighting key elements such as cell sources, bioengineering techniques, 3D scaffolds, and crucial signaling pathways. Furthermore, recent advances in generating organoids with structural and functional parallels to native skin are meticulously summarized. These developments facilitate the utilization of organoids in diverse applications, such as modeling skin disorders, developing regenerative solutions, and understanding skin development. Finally, the challenges and prospects in the field are discussed. The integration of state-of-the-art bioengineering techniques with a deep understanding of skin biology is promoting the production and biomedical application of these organoid models.
Keywords: Organoid models of skin, Skin development, Skin appendages, Stem cells, Bioengineering methods, 3D scaffolds, Signaling pathways
Graphical Abstract
Graphical Abstract.
Highlights.
Dissection of construction strategies by integrating insights from skin developmental biology.
Comprehensive overview of key elements, including cell sources, bioengineering techniques, 3D scaffolds, and crucial signaling pathways.
Summary of recent progress in engineered skin organoids and their diverse applications in research and medicine.
Background
The skin, as the body's largest organ, is responsible for numerous essential physiological functions, including barrier protection, homeostasis, endocrine regulation, and sensation. Structurally, the skin can be divided into three distinct layers: the epidermis, dermis, and subcutaneous tissue [1, 2]. Skin appendages such as hair follicles (HFs), sebaceous glands (SeGs), and sweat glands (SGs) are embedded within the skin and perform various functions, including hair growth, thermoregulation, secretion, and metabolism [1, 2]. Additionally, the skin is a complex organ that contains various cell types from multiple embryonic origins. The epidermis, which is of ectodermal origin, is a stratified squamous epithelium containing epidermal stem cells (EpSCs), keratinocytes (KCs), melanocytes, Langerhans cells, Meckel cells, etc. [3] The dermis, which is derived primarily from a mesodermal origin, is composed mainly of fibroblasts and extracellular matrix (ECM). Appendages such as HFs, SeGs, and SGs are located in the dermis and extend into the epidermis [4]. Subcutaneous tissue contains fat and connective tissue, and it provides nutrients and support to the skin [1, 2]. Because of the complexity of the structure and function of the skin, establishing an in vitro model that accurately mimics native skin has proven to be challenging.
The organoid technique provides new approaches to recapitulate the complexity of skin. An organoid is a 3D multicellular structure derived from stem cells in vitro that mimics the organ or disease they represent. Organoids form mainly through self-organization and can better recapitulate the specific structure and function of the corresponding in vivo tissue [5, 6]. The earliest documented 3D skin model can be traced back to the 1970s, when Rheinwald and Green developed a groundbreaking technique to cultivate KCs at the air–liquid interface (ALI), leading to the differentiation and formation of stratified layers resembling epidermal tissue [7, 8]. This pioneering work laid the foundation for the development of 3D skin models, with the ALI model emerging as the dominant skin construct and undergoing extensive use in subsequent decades. The ALI model successfully achieved barrier properties and phenotypic differentiation; however, the inclusion of appendages such as HFs and SGs has yet to be realized [9]. With advances in bioengineering techniques, biomaterials and the understanding of skin development, new organoid models integrating skin appendages have been established. The donor cells include not only the skin cells but also adult stem cells in other body parts, as well as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). The bioengineering methods have significantly diversified to include spheroid culture, 3D bioprinting, and microfluidic techniques, and the utilization of 3D scaffolds has been enriched by the introduction of a wider array of natural and synthetic materials. To date, 3D skin constructs such as epidermal organoids [10–12], HF organoids [13], SeG organoids [1, 4] and SG organoids [14–20], as well as appendage-bearing multilayered skin organoids [21–28], have been established. In this review, these constructs are summarized as organoid models of skin, as they are developed from stem cells, contain multiple cell types, and exhibit distinct morphological, and functional characteristics of skin.
In this review, we provide an overview of the advances in creating organoid models of skin. We aim to provide a comprehensive understanding of the development of skin-based organoids, covering topics of in vivo skin development, construction strategies and key elements, various types of skin-based organoid models, biomedical applications, challenges, etc. Given extensive previous reviews of ALI models, we focus on recent advances in organoid models of skin developed through self-assembly or the use of cutting–edge bioengineering techniques. With ongoing advances in bioengineering technologies and an enhanced understanding of skin development, organoid models of skin hold broad prospects for application in the biomedical field.
Review
In vivo skin development
Understanding in vivo skin development is crucial for guiding the construction of skin models. Mammalian skin development is a multistage process that includes the formation of the epidermis and dermis, along with the growth of skin appendages, including HFs, SeGs, and SGs (Figure 1) [13, 29]. In mice, the development of the epidermis begins from the surface ectoderm at embryonic day (E) 9.5 [30]. The ectoderm receives signals from the mesoderm, which inhibits neural differentiation and promotes epidermal fate determination [31–33]. Prior to the key developmental milestone at E12.5, the majority of basal progenitor cells in murine skin divide symmetrically along the axis of the basement membrane. From E13.5 onward, more basal progenitor cells shift their division axis in a perpendicular orientation, undergoing asymmetric division and contributing to the formation of a stratified epidermis [34, 35]. During the initial stages of stratification, a temporary protective layer called the periderm, consisting of tightly connected squamous cells, covers the epidermis. The periderm is shed at approximately E17–18, indicating the completion of stratification [14, 15, 29].
Figure 1.
Schematic diagram of skin development and appendage formation. Starting at embryonic day 9.5 (E9.5), the single-layered epidermis undergoes proliferation and stratification, leading to the formation of a stratified epidermis (E13.5–E17/18). Close interactions between hair placodes and DCs (E13.5–E14.5) lead to the gradual formation of hair germs (E15.5–E17.5). These hair germs undergo differentiation, giving rise to HFs and subsequently SeGs, which appear at approximately E18.5 and become functional after birth. SG germ begins to form just before birth (E16.5–17.5). Progenitor cells originating from the epidermal basal layer invaginate and extend deeply into the dermis between E17.5 and postnatal day 0 (P0), subsequently differentiating into secretory coils by P1. The glands achieve full functionality by P21. ORS outer root sheath, IRS inner root sheath
Unlike the ectodermal origin of the epidermis, the embryonic origins of dermal fibroblasts in mammals are dependent on anatomical location [13, 16, 29]. In the facial and anterior head regions, dermal fibroblasts are derived from cranial neural crest (NC) cells, whereas those in the posterior head region arise from the cephalic mesoderm [17]. Fibroblasts in the dorsal and ventral regions originate from somite dermomyotomes and lateral plate mesoderm, respectively [18–20]. To date, a comprehensive molecular understanding of how these fibroblast populations develop is lacking. The dorsal trunk in mouse embryos has been extensively studied with regard to the development and specification of fibroblasts. After dermal specification, canonical wingless-related integration site (WNT) signaling drives the differentiation of dermal fibroblasts into distinct papillary and reticular subpopulations, which can be distinguished at E16.5 by using lineage tracing methods [21]. Recently, with advances in single-cell sequencing techniques, the diversification of fibroblasts into functional lineages was reported to occur as early as E12.5 [30].
The development of skin appendages depends primarily on reciprocal epithelial and mesenchymal interactions (EMIs). The morphogenetic signals HF formation arise primarily from the dermis. At E13.5–14.5, specific subsets of mesenchymal cells aggregate to form dermal condensates (DCs) that determine HF locations and induce hair placode formation [22, 23]. Crosstalk between DCs and hair placodes drives further maturation of the HF and subsequent hair growth cycles [22, 24]. At approximately E18.5, SeGs become apparent, typically coinciding with the maturation of HFs [14, 25]. At E16.5–17.5, SGs start to develop as epithelial invaginations. These invaginations undergo further maturation, forming elongated ducts that penetrate into the dermis and become fully functional and mature by postnatal day 21 [26, 27].
The development of human skin shares broad similarities with that of rodent skin. The human epithelium also originates from the embryonic ectoderm and undergoes processes of proliferation, differentiation, stratification, and homeostasis. The origin of the dermis is diverse, and the reciprocal interaction between the epidermis and dermis determines the development of skin appendages [36]. However, notable differences exist between these two species (Figure 2). For example, epidermal stratification in mice occurs during mid- to late-embryonic development, whereas in humans, it takes place from early to mid-gestation. In terms of skin appendages, the first hair growth wave in mice generally occurs postnatally, whereas in humans, mature HFs are already present at 22 weeks of gestation. Similarly, SGs in mice mature after birth, whereas in humans, they undergo differentiation and maturation by 24 weeks of gestation. Additionally, there are anatomical and distribution differences between murine and human skin [28, 37], such as differences in the thickness of the epidermis and dermis, as well as the locations of HFs and SGs [28]. The distribution of HFs is sparse in humans and dense in mice. Human skin has both eccrine and apocrine SGs, with the former being widely distributed across the body and the latter being confined to very hairy body regions. In contrast, mice possess only eccrine SGs, which are exclusively located in the paw pads ([26]). Consequently, skin organoids derived from human tissue can more precisely mimic skin development and the onset and progression of skin diseases.
Figure 2.

The timelines of murine versus human skin development. In mice, stratification of the epidermis begins at approximately embryonic day (E) 9.5 and concludes by E18, whereas in humans, epidermal stratification starts as early as 4 weeks of gestation (G4) and is completed by G20. Additionally, mice begin developing HFs at E14.5 and achieve fully mature HFs and hair growth after birth, whereas human HF development begins at G18, with hair growth beginning at G22. SG development in mice begins just prior to birth (E18), whereas in humans, SGs reach functional maturity by G24. SeG sebaceous gland
Construction strategies and key elements to develop organoid models of skin
The precise understanding of embryonic skin development provides the foundation for accurately recapitulating the complexity and functionality of skin in organoid models. By offering stem cells a suitable microenvironment that simulates in vivo skin development, scientists have developed diverse skin-based organoids that accurately recapitulate the intricate structure and function of the skin (Figure 3). Three main strategies have been employed to develop organoid models of skin in vitro. The first strategy involves the use of a single type of skin cell to generate organoids. Stem cells from the epidermis or dermis aggregate in vitro and develop into organoids with the aid of various biomaterial scaffolds and signaling molecules [10–12, 38–41]. Because of the crucial role of EMIs in skin appendage development, many researchers have adopted another approach to develop organoid models of the skin. Cells derived from both the epidermis and dermis are isolated and induced to self-organize in vitro, leading to the formation of various skin appendage organoids, particularly HFs [42–46]. The third strategy involves the directed differentiation of ESCs or iPSCs into both epithelial and mesenchymal cells, allowing the two distinct cell populations to organize in vitro, replicating the process observed in embryonic skin development. By guiding the differentiation of PSCs into the starting population of skin progenitors, researchers have generated skin organoids that mimic the skin developmental process, including appendage induction [47–50]. These strategies involve the interaction of four key elements: cell sources, bioengineering methods, 3D scaffolds, and biological signals. These elements complement each other and collectively establish the foundation for the creation of organoid models.
Figure 3.
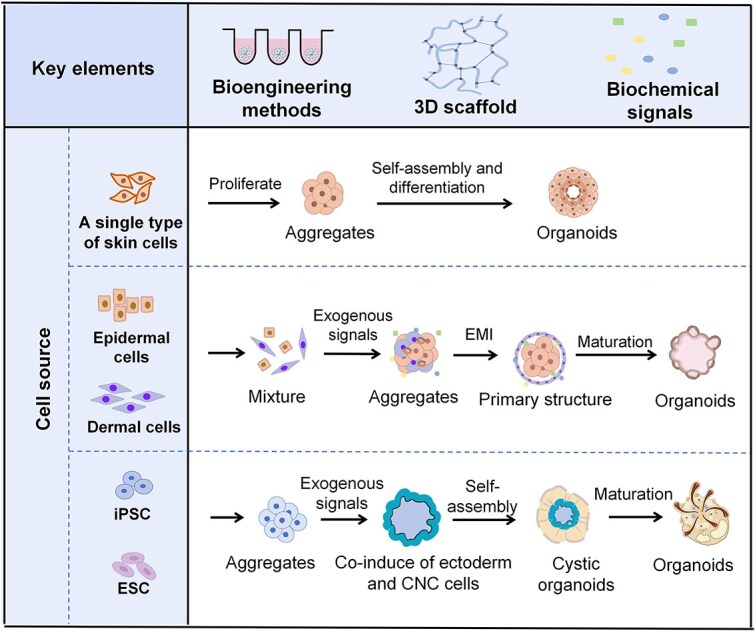
Schematic illustration of skin-based organoid generation. Several key elements are required to generate organoid models of skin. These include the following: (i) Suitable cell sources include single or multiple types of primary skin cells derived from biopsies, as well as pluripotent embryonic stem cells (ESCs) and iPSCs. (ii) selection of proper bioengineering techniques to support the construction of organoids. (iii) 3D scaffolds provide support for the attachment and organization of cells. (iv) Manipulation of proper biochemical signals. Growth factors and supplements were added to regulate specific signaling pathways, mimicking the environment needed for proper cell behavior and development. Organoid models have been constructed by integrating these key elements via different strategies. The first approach involves aggregating a single type of skin stem cell to form organoids, such as epidermal organoids or SeG organoids. The second strategy employs epidermal and dermal-derived cells, following the principle of EMIs to self-organize into organoids, such as HF organoids. The third strategy involves the directed differentiation of multipotent ESCs or iPSCs into both epithelial and mesenchymal cells, which subsequently develop into skin organoids through self-assembly. CNC, cranial neural crest. Created with BioRender.com
Cell sources
Stem cells, which include adult stem or progenitor cells and PSCs, are essential for organoid formation and growth [6, 51]. Adult stem or progenitor cells are typically found in specific areas of the body and have limited self-renewal and differentiation ability [52–54]. The creation of skin-based organoids involves the use of cells from various layers of the skin, including EpSCs [11, 55], KCs [10, 12, 56], dermal fibroblasts [46, 57], HF stem cells [58], dermal papilla cells (DPCs) [59–63], and SG cells [40, 41]. These organoids can also be produced from adult stem cells from different body parts, such as mesenchymal cells from the bone marrow [64, 65] and adipose tissue [66].
Additionally, skin organoids can be generated from PSCs through stepwise differentiation and self-assembly [47, 48, 50, 67–69]. PSCs can be ESCs derived from embryos or iPSCs [70] generated through induced dedifferentiation from somatic cells via cell reprogramming. PSCs can undergo indefinite self-renewal and give rise to all cell types in the body, but this versatility also increases the risk of off-target induction [48, 71].
Bioengineering methods
In general, there are several main methods for constructing organoids (Figure 4), including the ALI method, hanging–drop method, spherical aggregation method, 3D printing technique, and microfluidic method. As described above, the ALI method is a well-established technique for constructing epithelial models, such as skin and airway models [72–74]. In this method, apical, and basolateral compartments are separated by a permeable membrane. After being seeded on the membrane, the cells were grown to confluence in the culture medium. The medium on the apical side was subsequently removed to expose the cells to air, initiating the process of differentiation. This epidermal analog included stratified epithelial layers expressing structural proteins such as keratin 10 and tight junction proteins such as claudin and occludin [75]. Alternatively, a full-thickness human skin equivalent can be prepared by combining the epidermis with fibroblast-seeded collagen or a fibrin matrix [46, 76, 77]. Although ALI skin equivalents have been widely used to evaluate damage to the skin barrier, it should be noted that such skin-like structures lack skin appendages and have a limited lifespan (~2 weeks) [9, 78].
Figure 4.

Different bioengineering methods for constructing organoid models of skin. (a) In the classical ALI model, cells are plated on a membrane and allowed to reach confluence in culture medium. They are then exposed to air, prompting their development into stratified skin substitutes. (b) The hanging-drop method involves the aggregation and maturation of cells at the liquid–air interface of hanging droplets. (c) In spheroid culture, stem cells are plated in biomaterials and cultured in ULA plates to self-assemble into spherical aggregates. (d) Organoid construction via 3D printing. (i) To simulate the microenvironment of HFs, special 3D molds are printed, followed by sequential assembly of extramold fibroblasts and collagen, as well as intramold DPCs and keratinocytes. (ii) To prepare HF microgels, two tiny adjacent collagen droplets containing dermal and epidermal cells are bioprinted on a matrix, and subsequent spontaneous contraction significantly enhances EMIs during HF formation. These bioprinting techniques can be scaled up using an automated spotter. (iii) Computer-assisted 3D bioprinting techniques facilitate the automated and simultaneous spatial arrangement of cells and biomaterials; for example, these strategies have been used in the construction of SG organoids. (e) Microfluidic technology combined with skin models. (i) The microfluidic chip comprises a network of grooves or microchannels etched into various substrates, which allows for the designed assembly of skin models and other types of organoids or cells. Microfluidic technology is a computer-aided technique that provides precise control and analysis of physicochemical reactions inside a chip. (ii) Microfluidic technology is employed for the one-step generation of biomimetic microspheres. These double-layer microspheres entrap double-layered cells and bioactive molecules, which act as a bionic organoid model to induce hair formation
The hanging drop method is a special form of the ALI technique that depends on cell aggregation at the liquid–air interface to form spheroids [62, 79]. Some disadvantages of this method include the inability to hold droplets >50 μl and to change the medium without disturbing the spheroids [62, 80].
One of the most widely adopted techniques in organoid research was developed by the Hans Clevers team. In this method, stem cells are plated in Matrigel or another ECM matrix and cultured in low-adhesion plates to self-assemble into spherical aggregates at the bottom [81]. Most of the organoid models of skin mentioned in the review were developed by using this method. This ECM scaffold method is rather simple to perform, allows high throughput, and has the potential for the fusion and coculture of multiple organoids.
Recently, 3D printing has gained considerable attention in organoid fabrication. This technique enables the precise and versatile printing of living cells and multiple biomaterials, facilitating the construction of complex structures [76, 82]. For example, researchers have utilized 3D printing technology to create specialized molds resembling human HF structures, enabling physiological 3D cell organization within a suitable microenvironment [44]. Bioprinting also enables the fabrication of tiny objects with high accuracy, allowing scalable automated biomanufacturing [42, 83]. As an example, hair microgels can be prepared by bioprinting two tiny adjacent collagen droplets, one with mesenchymal cells and the other with epithelial cells. During culture, these microgel pairs contract due to cell and collagen traction forces, resulting in >10-fold enrichment in cell density. Scaling up this technique using an automated spotter enables the preparation of large numbers of hair microgels, which is crucial for clinical applications [42]. Moreover, Nanmo et al. successfully printed hair microgels onto surgical suture guides, leading to significant improvement in hair-shaft sprouting through the skin when these guide-inserted hair microgels were used [42, 84]. 3D bioprinting strategies also enable the simultaneous spatial patterning of cells and biomaterials. For example, mesenchymal stem cells (MSCs) can be printed in a specific bioink that provides biochemical and architectural cues for the conversion of MSCs into SG cells, ultimately leading to the formation of SG organoids [64, 65].
As organoid technology continues to advance, microfluidic organoid-on-chip technology has been developed on the basis of various organoid construction methods [85]. The reported skin-on-a-chip models integrate an ALI skin model within a microfluidically controlled microenvironment, closely mimicking the mechanical forces and biochemical cues encountered by natural human skin. [9, 86, 87] Such microfluidic skin models have demonstrated significant potential, as they enhance nutrient perfusion, prevent necrosis, and enable the accurate replication of cell–cell contacts, matrix properties, mechanical and biochemical cues and stimuli [9, 87–89]. It is foreseeable that an increasing number of skin or appendage organoids will be coupled with microfluidic techniques to produce powerful models for biomedical research. Microfluidic technology might also be introduced in the generation of bioengineered organoids. In a recent report, microfluidic technology enabled the one-step generation of core–shell biomimetic microspheres. These microspheres consist of double aqueous microdroplets that encapsulate double-layer cells and growth factors, creating a highly mimetic environment for hair regeneration. This innovative technique enables the rapid and scalable preparation of double-layer cell spheres specifically designed for hair regeneration [66].
Three-dimensional scaffolds for microenvironment simulation
Bioengineering techniques are often supplemented by the utilization of biomaterials to mimic the intricate structure and components of human skin [90]. Hydrogels are widely used to provide 3D scaffolds for organoid construction because of their advantages of biocompatibility, controllability, and delivery capability [91, 92]. Hydrogels include natural hydrogels, such as Matrigel, decellularized ECM (dECM), collagen, keratin, hyaluronic acid (HA), alginate and chitosan, and synthetic hydrogels (Figure 5). [91, 92].
Figure 5.
Overview of the hydrogels commonly used in the generation of organoids, especially organoid models of skin
Matrigel, derived primarily from Engelbreth–Holm–Swarm mouse sarcoma, is a solubilized basement membrane extract containing various ECM proteins and growth factors [93]. It has been widely used to culture all types of organoids, including skin organoids [10, 40, 48, 94]. Although Matrigel provides a favorable microenvironment for skin organoid development, its rigidity upon solidification is considerably lower than that of skin tissue [95]. This disparity can potentially affect the determination of the fate of skin stem cells [96]. In addition, its ill-defined composition, batch-to-batch variability and animal-derived nature lead to experimental uncertainty and variable outcomes in organoid protocols [97].
Like Matrigel, dECM is a heterogeneous extract derived from various tissues. The tissue-specific dECM modulates cell morphology and behavior by providing inherent instructive signals that guide tissue morphogenesis [98]. For example, Zhang et al. successfully restored the hair-inducing properties of high-generation DPCs by culturing them in dECM from human placental cells [99]. Yao et al. used a specific matrix from the mouse plantar region dermis to guide the fate conversion of MSCs to SG cells [64]. In addition, functional chemicals and proteins are frequently used in combination with dECM to increase performance [100].
With the development of organoid culture technology, proteins or polysaccharides from natural tissues with well-defined biochemical compositions and mechanical properties have been employed to construct organoid models of skin [2, 101]. Collagen and keratin are the most abundant ECM proteins in the dermis and epidermis, respectively [102]. For example, Abaci et al. used collagen and fibroblasts to mimic the dermis and spatially assigned DPCs and KCs to such dermal analogs, creating an array of HF organoids by using 3D printing technology [44]. Kageyama et al. achieved the formation of HF grafts (HFGs) by encapsulating mouse embryonic MSCs or human DPCs along with epithelial cells in collagen microgels, facilitating EMIs within contracted collagen gels [45]. Similarly, keratin, a key component of the epidermis, has been used in the construction of the epidermis [103] and other skin appendages, such as DPC spheroids [61].
Polysaccharides such as HA are among the major components of the ECM that is widely distributed in the dermis [104]. Kalabusheva et al. demonstrated that HA promotes the proliferation of KCs and DPCs, leading to increased organoid size in cultures consisting of KCs and DPCs [105]. HA offers advantages such as a stable composition and fully controlled physical and chemical properties [92, 106]. However, it does not support cell attachment [107] and is often combined with protein-based materials to enhance cellular adhesion. Other non-skin-specific proteins or polysaccharides, such as silk proteins [63, 108], alginate [82], and chitosan [65], have also been used in the construction of HF or SG organoids in combination with other biomaterials.
To address the limitations of natural hydrogels, synthetic hydrogels have emerged as promising alternatives for organoid construction. These synthetic hydrogels have good biocompatibility and tunable mechanical properties, allowing for controlled differentiation and recapitulation of in vitro tissue morphogenesis [101]. GelMA is a gelatin-modified light-curable hydrogel that retains the Arg-Gly-Asp (RGD) sequence and temperature sensitivity of gelatin and has been widely used in cell culture, 3D bioprinting, and drug delivery because of its good biocompatibility, excellent molding properties, and tunable physicochemical properties [92, 106, 109]. GelMA has been reported to load MSCs, DPCs or SKP cells, and efficiently regenerate SGs or HFs in vivo. [110–112]
Polyethylene glycol (PEG) is another commonly used synthetic polymer in organoid cultures [101]. It is bioinert and is often functionalized with reactive end groups such as acrylates, thiols, and N-hydroxyl succinimide (NHS) esters to incorporate key ECM biofunctions [113–115]. There are increasing reports on the construction of organoids using PEG mixed with HA [116], adhesion peptides [117], and GelMA [118]. Although the utilization of these chemically defined hydrogels in the construction of skin-based organoids is relatively rare, there is ongoing research focused on developing organoids using these chemically defined hydrogels.
Each biomaterial has advantages and limitations. As summarized in Figure 5, natural materials closely resemble the ECM of original tissues, resulting in excellent biocompatibility and biological activity, which promote cell proliferation and migration. However, natural materials exhibit complex and variable compositions, and their physical or chemical properties are not stable or controllable. In contrast, synthetic materials are highly processable, easily modifiable, and can be prepared under well-defined and controlled conditions [95]. However, hydrogels prepared from synthetic materials are bioinert, which inevitably affects cell adhesion and growth. There is a growing tendency to combine natural and synthetic materials in the fabrication of ECMs for organoid culture, aiming to leverage the advantages of both while mitigating their respective drawbacks.
Critical signaling molecules
Bioengineering techniques and 3D scaffolds help stem cells self-organize or distribute in a controlled manner; nevertheless, they are insufficient to direct cells to commit oriented differentiation and produce functional organoids. Therefore, the modulation of signaling molecules in the culture system to mimic in vivo skin morphogenesis is another critical determinant for the generation of organoid models.
The interplay of the fibroblast growth factor (FGF), bone morphogenetic protein (BMP), WNT, and transforming growth factor beta (TGF-β) pathways plays a crucial role in the development of the skin epidermis and dermis [13, 32, 119–121], and the precise regulation of one or more of these pathways enables the production of specific skin cells or skin organoids from PSCs (Figure 6) [48, 56, 57, 67, 94].
Figure 6.

The signaling pathways involved in skin cell differentiation and appendage formation. The inhibition of the BMP and TGF-β signaling pathways (dSMADi) promotes the neural fate of PSCs. BMPs are generally accepted as epidermal inducers. BMP activation promotes epidermal fate when WNT is present, as WNT inhibits FGF signaling to prevent neural differentiation. NC cells are the main contributors to the development of the craniofacial dermis. BMP and FGF signaling are essential for NC induction, and the levels of these signaling molecules determine the cell fate. Low BMP expression and moderate FGF activity induce early induction of NC, and high levels of BMP and FGF promote epidermal and neural fates, respectively. The dorsal and ventral dermis have different developmental origins. The BMP and FGF signaling pathways are involved in the spatially and temporally specific development of the dermis. For example, BMP functions as an inducer of lateral mesoderm (contributing to the ventral dermis) specification, and BMP inhibition is required for paraxial mesoderm (contributing to the dorsal dermis) formation and differentiation. After the primary skin structure is established, EMI is critical for the formation of various skin appendages. During epithelial development, dispersed cells in the epidermal layer respond to elevated WNT signaling (WNThigh) and aggregate to form placodes. These placodes subsequently SHH, which recruits adjacent mesenchymal cells to form DCs and produces BMP inhibitors. Repeated EMI drives the formation of diverse HF cell lineages and the maturation of HF structures. DC signals are temporally regulated during skin morphogenesis. If DCs produce BMP and FGF signals that suppress epithelial-derived SHH production, the placode will develop into SGs
BMP4 is a generally accepted epidermal inducer. In chick embryos, BMP signaling directs epidermal fate in the presence of WNT; conversely, a lack of exposure to BMP and WNT permits FGF to induce a neural fate [122, 123]. By regulating FGF, BMP, WNT, and TGF-β signaling activity, all major ectodermal lineages, including surface ectoderm and NC cells, have been induced from human PSCs [32]. For example, high levels of BMP promote epidermal induction, and reduced BMP signaling favors the early induction of the NC cells [32, 124]. In Lee’s skin organoid model, treatment of embryoid bodies with BMP4 induced surface ectoderm, giving rise to the epidermis, and a subsequent morphogen cocktail with BMP inhibition and bFGF activation promoted the appearance of NC cells, giving rise to the craniofacial dermis [48]. Compared with the well-known surface ectodermal origin of the epidermis, the dermis develops from various germ layers according to its anatomical location. The understanding of dermal development remains limited, despite previous studies indicating the involvement of the TGF-β, BMP, and FGF pathways in the development of the dermis from the mesoderm [125]. To date, dermal organoids derived from the mesoderm have rarely been reported.
After the basic epidermal and dermal layers have developed, epithelial–mesenchymal cross-talk is critical for the generation and patterning of skin appendages, including HFs and SGs [13, 126]. During epithelial development, dispersed epidermal cells react to elevated WNT signaling and aggregate to form placodes [127, 128]. Placodes release secrete sonic hedgehog (SHH) signals to recruit adjacent mesenchymal cells, leading to the formation of DCs, which are precursors to DPCs. If the underlying DCs produce signals of BMP inhibitors, e.g. Noggin, the placodes will form HFs [128, 129]. When the underlying mesenchyme produces robust BMP signaling, the placodes differentiate into SGs [52, 126].
Researchers have employed a variety of genetic or chemical techniques to manipulate these pathways to regenerate skin appendages or establish corresponding organoids. WNT signaling plays a decisive role in the generation of HFs. Subcutaneous injection of WNT into the bald scalp can reactivate hair growth [130], and pretreatment of collagen-encapsulated iPSCs with WNT10b promoted the generation of bioengineered HFs [67]. Lymphoid enhancer-binding factor 1 (Lef1), a downstream transcription factor of the WNT pathway [131], markedly augmented the hair-inducing capability of engineered HFs when introduced into DPCs [44]. Moreover, other molecules, including CHIR99021 (a GSK3β inhibitor that promotes canonical WNT activation) [44, 50, 94], purmorphamine (SHH activator) [94], and Noggin (competitive BMP inhibitor) [58], have been shown to promote HF generation. Conversely, XAV-939, an inhibitor of WNT [132], inhibits the formation of HF-like structures and subsequent HF budding when added to the hair germ [43].
In contrast, the development of SGs primarily depends on factors such as ectodysplasin A (EDA), BMPs, and FGFs. These factors inhibit SHH signals within the epithelial region, promoting increased BMP signaling activity and facilitating the formation of SGs [120, 126, 133]. Both the WNT and EDA pathways seem to participate in promoting placode formation and enhancing SG formation [26, 120, 134]. Moreover, EDA has been reported to direct KC conversion into SG cells [40, 56, 134].
In addition to molecules that regulate fate determination, other substances have also been used to optimize skin organoid culture. Inhibitors of the TGF-β signaling pathway, such as A83-01 or SB431542, increased the colony formation efficiency of iPSCs and were essential for epidermal induction [40, 50, 68, 135]. Activators of the cyclic adenosine monophosphate (cAMP) pathway, e.g. forskolin, are often used to promote cell proliferation in culture [10, 11]. Additionally, selective inhibitors of Rho-associated kinases, such as Y27632, reduce stem cell apoptosis and modulate the spatial distribution of epithelial and mesenchymal cells, thereby increasing the rate of HF budding [136].
Various types of organoid models
Through the integration of stem cells, bioengineering methods, 3D scaffolds, and signaling molecules, a range of skin models that closely mimic the structure and function of the skin have been developed. A summary of the preparation methods and applications of these organoid models of skin is provided in Table 1.
Table 1.
Overview of organoid models of skin
| Organoid identity | Species | Cell source | Biomaterials | Bioengineering methods | Application |
|---|---|---|---|---|---|
| Epidermal organoids | Rodent | Epidermal KCs | Matrigel | Spherical aggregates | Organoids expand over 6 months, maintaining the structure of the epidermis [10]. Modeling of skin infections caused by Staphylococcus aureus [12]. |
| Human | Primary skin epidermal cells | Matrigel | Spherical aggregates | Contributing to epidermal regeneration at wound sites [55]. Modeling of dermatophyte infections caused by T. rubrum [11]. | |
| HFs (dermal cells) | Rodent | DPCs and ASCs | Chitosan | Sequential assembly of DPCs and ASCs to develop into core-shell spheres | Reconstruct cellular arrangements and microenvironmental niches for hair formation [59]. |
| DPCs | Gelatin/alginate [60], keratin [61] | Layer-by-layer self-assembly [60], spherical aggregates [61] | New HF regeneration following DP spheroid implantation [60, 61]. | ||
| Human | hDPCs | NA [62, 137], silk-gelatin hydrogel [63]. | Hanging drop [62], spherical aggregates [63, 137] | DP aggregates induce HF neogenesis in vivo [62, 137]. Modeling for drug screening of androgenic alopecia [63]. | |
| hDPCs, matrix cells, and DS cup cells | Matrigel | Spherical aggregates | A microengineered system for scalable production of hDPC spheroids and HF regeneration [58]. | ||
| HFs (containing epidermal and dermal cells) | Rodent | Embryonic epithelial and mesenchymal cells | Microwell-array PDMS chip [45], Matrigel [43] | Self-assembly [43, 45], large-scale production on the chip [45] | Establishment of in vitro HF model [43, 45], enabling the large-scale production of HF germs on PDMS chip. [45] |
| MSCs and epidermal cells | GelMA cores and HA shells | Microfluidic-assisted technology | One-step generation of biomimetic microspheres for hair regeneration [66]. | ||
|
Rodent/ Human |
Mouse epidermal cells, human DP cells [83, 138] or HUVECs [138] | Collagen type I, [83, 138] microwell-array PDMS chip [138] |
Self-assembly in contracted collagen gels, large-scale production using automated spotter | Efficient generation of HF upon transplantation [83, 138]. HF containing HUVEC produced higher hair inductivity [138]. | |
| Human | Fetal/adult epithelial and mesenchymal cells | Matrigel or collagen | Self-assembly into spherical aggregates | In vitro models of human HF-like structure, which are valuable for drug screening [139]. | |
| Primary or N/TERT-1 KCs, hDPCs and fibroblasts | Collagen | 3D installation | Generation and integration of HF-primed spheroids in bioengineered skin constructs [46]. | ||
| SeG | Rodent | Sorted Blimp1+ cells from mouse epidermis | Matrigel | Spherical aggregates | Models for the mechanical exploration of acne vulgaris [39]. |
| SG | Rodent | Epithelial progenitors [82], MSCs [64] | Gelatin/alginate scaffold with dECM of plantar dermis | Cells were 3D bioprinted with matrix biomaterials | SG–like matrix contributed to the conversion of epithelial progenitors [82] or MSC [64] into functional SGs and promoted SG recovery in vivo. |
| SG cells from adult paw pads | Matrigel | Spherical aggregates (ULA) | Regeneration of epidermis and SG when SG organoids were transplanted in vivo [40]. | ||
| MSCs | Collagen/chitosan porous scaffold | Scaffold loaded with Lipofectamine 2000/pDNA-EGF | Accelerate wound healing process and induce regeneration with SG-like structures [65]. | ||
| Human | Epithelial cells from eccrine SG | Matrigel | Spherical aggregates(ULA) | Models of human eccrine SG [140]. | |
| Primary SG cells | NA | Spherical aggregates (hanging drop) | Models of human eccrine SG in response to the stimulation [41]. | ||
| Forced expression of ectodysplasin-A in KCs | Matrigel | Conversion of KC into SG cells, assisted with microfluidic technology | SG organoids exhibit features of native SGs and develop into fully functioning SGs after transplantation into mouse models [56]. | ||
| Skin organoids | Rodent | ESC or iPSCs | Matrigel | Spherical aggregates (ULA) | In vitro skin organoids containing epidermis and dermis, as well as HF. [50] |
| Human | Endothelial cells, fibroblasts and KCs | NA | Spherical aggregates (ULA) | Organoids exhibited specific structure with surface-anchored KCs enveloping a central stromal core [57] | |
| hESCs and hiPSCs | Matrigel | Spherical aggregates | Hair-bearing human skin models generated entirely from PSCs [48, 49] | ||
| hiPSCs | Matrigel, Collagen I | Self-assembly, followed by ALI | Modeling atopic dermatitis by bacterial colonization and infection [47]. | ||
| hiPSCs | Matrigel | Spherical aggregates(ULA) | Skin organoid models of SARS-CoV-2 infection [69] and EB [141]. | ||
| Skin-on-a-chip | Human | HaCaT cells, HS27 fibroblasts, HUVECs | PET membranes coated with fibronectin, PDMS channel | Microfluidic-assisted technology | Modeling of skin inflammation and edema; evaluating the efficacy of therapeutic drug [87]. |
| Fibroblasts and hKCs | PDMS chips | ALI and Microfluidic-assisted technology | A wrinkled skin-on-a-chip model was developed using cyclic stretching. This model can be used to study skin aging and evaluate anti-wrinkle cosmetics and medications [88]. | ||
| HaCaT and leukemic lymphoma cell line (U937) | Assembly of PMMA, PS and PDMS sheet. | Microfluidic-assisted technology | Development of an immune competent in vitro model of human skin. This model has been used to evaluate drug efficiency and toxicity [89]. |
Abbreviations: not applicable (NA), ectodysplasin-A (EDA), gelatin methacryloyl (GelMA), human adult keratinocyte cell line (HaCaT), poly(methyl methacrylate) (PMMA), polystyrene (PS), polydimethylsiloxane (PDMS), polyethylene terephthalate (PET), ultralow attachment (ULA)
Epidermal organoids
Although 2D murine/human EpSC cultures were established long ago [73], a feeder- and serum-free murine epidermal organoid culture system was only recently established, enabling the long-term expansion (>6 months) of adult EpSCs [10]. The culture medium combines high calcium concentrations; the activation of cAMP, FGF, and R-spondin signaling; and the inhibition of BMP signaling. The epidermal organoids exhibited layered structures; the dividing epithelial cells expressing proliferation markers were located in the outermost layer, and the differentiated KCs expressing Keratin 6A, Keratin 1 and Loricrin formed toward the center of the organoids. A similar epidermal organoid culture system derived from human skin was also established by using chemically defined medium containing activators of cAMP, epidermal growth factor (EGF), and WNT3a and inhibitors of the TGF-β pathway. These human epidermal organoids replicate the morphological, molecular, and functional characteristics of the human epidermis and maintain their viability for up to 6 weeks [11]. The discrepancy in in vitro expansion time between human and murine epidermal organoid systems might be attributed to species differences, and further optimization of the culture system of human epidermal organoids remains challenging.
Organoids or aggregates mimicking skin appendages
Skin appendages are highly complex structures. Dynamic interactions of various chemical signals between the epidermis and dermis are necessary to promote the induction and maturation of skin appendages during fetal development [13, 142]. Consequently, it is challenging to create organoids that mimic skin appendages solely through the self-assembly of a single type of stem cell. Instead, a common approach involves the assembly of cells from different skin layers in specific spatial arrangements within a bioengineered scaffold. Various terms, such as organoids, aggregates, and 3D spheroids, have been utilized to describe the 3D multicellular structures that replicate the distinctive structure and function of different skin appendages.
Human follicle organoids
The HF consists of cylindrically multilayered KCs [including HF stem cells (HFSCs)] and mesenchymal hair-inductive DPCs located at its base [13, 84]. DPCs play crucial roles in generating dermal cell populations, including dermal sheath (DS) cells, which are located within the connective tissue sheath surrounding the HF [143, 144]. HFs not only contribute to hair growth but also play a crucial role in skin regeneration after injury [145–147]. Therefore, numerous attempts have been made to regenerate HFs both in vivo and in vitro [43, 58, 79, 139, 148–150].
Transplantation experiments conducted in nude mice have demonstrated that the crosstalk between HFSCs and DPCs is responsible for HF generation [84, 151–153]. Furthermore, DPCs play an essential role in the hair-inducing process, but HFSCs can be replaced by other epidermal cells [44, 46, 94]. However, human DPCs rapidly lose their hair-inductive potential in conventional culture [79, 154]. To overcome this limitation, 3D DPC cultures were established and showed improved efficacy in HF neogenesis [38, 60, 61, 63, 137]. By imitating the in vivo microenvironment of DPCs, coculturing DPCs with hair matrix cells [58], DS cells [58], KC cells [44, 46, 57], or adipose-derived stem cells (ASCs) [59] can enhance hair regeneration and HF maturation. Additionally, various bioengineering methods have been employed to assemble these cells within biomaterials, aiming to mimic the specific structure of HFs. Specifically, Abaci et al. induced hair generation by implanting aggregates of DPC and KC mixtures into a 3D-printed dermis composed of fibroblasts and type I collagen gel. The encapsulation of human umbilical vein endothelial cells (HUVECs) in the dermis promoted capillary formation and allowed efficient hair growth [44]. Using the 3D bioprinting technique, Kageyama successfully prepared hair microgels by incorporating DPCs and KCs in collagen gels [83]. Upon implantation into the back skin of murine models, these engineered microgels efficiently induced HF and shaft regeneration. In a recent study conducted by Huang et al., unsorted MSCs and epidermal cells from newborn mice were encapsulated in GelMA and photocurable catechol-grafted materials, respectively, and these core–shell biomimetic microspheres significantly enhanced hair regeneration [66]. Notably, however, all the above DPC or 3D cell cultures have to be transplanted into animal models to exert HF-inducing effects.
In recent years, significant progress has been made in generating hair in vitro. Kageyama et al. generated HFs in vitro by coculturing unsorted epithelial cells and mesenchymal cells from the skin of mouse embryos in Matrigel. This EMIs produced cell aggregates with mesenchymal cells in the outer layer and epithelial cells in the inner layer, leading to nearly 100% efficiency of HF generation in each aggregate [43]. Later, the same research group demonstrated that in vitro spherical aggregation and subsequent hair PEG-like sprouting can also be achieved by coculturing human fetal/adult epithelial and mesenchymal cells in Matrigel or collagen I [139]. A common feature observed in these studies is the involvement of dermal DPCs (or unsorted mesenchymal cells) and epithelial components in the formation of HFs, further indicating that EMI is essential for the artificial generation of HFs [84]. Notably, the hair-inducing capacity of mesenchymal cells may decline with age. In an epithelial–mesenchymal coculture model [155], mesenchymal cells derived from neonatal mice generated significantly more HFs than those from older (>2 months) mice.
Alternative approaches have also been developed to induce HF generation without relying solely on DPCs. For example, SKPs, a subset of specialized dermal cells, also show hair-inducing properties. SKPs originate from the NC and are multipotent stem cells that form self-renewing spheroids in vitro and can differentiate into various cell lineages [156, 157]. The combined transplantation of cultured EpSCs and SKPs successfully reconstituted functional HFs in nude mice [158]. In addition, DPCs can be reprogrammed from human dermal fibroblasts [159] or iPSC-derived mesenchymal cells [94].
Sebaceous gland organoids
The SeG is another important epidermal appendage that originates mainly from an HF [25] and forms the pilosebaceous unit together with the HF [90]. It is a unilobular or multilobular structure that connects to the HF through a duct lined by epithelial cells. In addition to HF-associated SeGs, independent free SeGs exist at mucosal margins and areas of modified skin [25, 39, 160, 161]. Research on SeG organoids is relatively rare, probably because of their close association with mature HFs [162]. Feldman et al. demonstrated that individual mouse Blimp1+ cells possess the capacity to form SeG organoids in vitro. These organoids express known SeG markers and display a lipidomic profile comparable to that of native SeGs in vivo [39].
Sweat gland organoids
The SG consists of a secretory coil, comprising myoepithelial, secretory clear (serous) and dark (mucous) cells, and a connecting duct that opens directly onto the skin surface [163]. The isolated SG cells rapidly differentiate into KCs and lose their specific phenotypic characteristics in 2D culture; [40, 164, 165] therefore, various methods, such as hanging drop cultivation or Matrigel encapsulation, have been used to construct 3D SG cultures. Klara et al. reported that human SG cells grown via the hanging drop cultivation method retain most of their markers and respond to cholinergic stimulation [41]. In a murine SG 3D culture, epithelial cells isolated from SGs were embedded in Matrigel and cultured in medium supplemented with EGF, basic FGF (bFGF), EDA, A83–01, forskolin, and BMP4, leading to the formation of SG organoids. These organoids retained significant stem cell properties and exhibited the ability to differentiate into either SG cells or epidermal cells [40].
SGs are very tiny and scattered throughout the dermis layer, which makes them difficult to acquire. Other cell sources, such as EpSCs, KCs, MSCs, ESCs, and amniotic fluid-derived stem cells, have demonstrated the potential to transdifferentiate into SG cells by genetic or chemical manipulation [64, 166, 167]. Considering the enormous impact of the niche on cell fate, 3D printing techniques have also been used to provide a biomimetic microenvironment to drive SG differentiation. For example, MSCs were bioprinted into a specific matrix from the mouse plantar region dermis and directed toward fate conversion into functional SG cells, leading to functional regeneration of SGs in burn mouse models [64]. Moreover, by culturing human epidermal KCs that overexpress EDA in specialized SG culture medium, KCs were efficiently converted into induced SG cells. These cells were further embedded in Matrigel and developed into SG organoids that closely mimicked the structural and biological features of native SGs. These organoids were successfully passaged and maintained for up to 3 months, and they demonstrated the ability to differentiate into functional SGs following in vivo transplantation [56]. This research underscores the significant translational potential of SG regeneration in the clinic.
From pluripotent stem cells to skin organoids
As described previously, the skin is a complex, stratified organ that harbors multiple appendages. Constructing appendage-bearing skin in culture is a great biomedical challenge, and skin organoids that nearly mimic complete skin have only recently been established entirely from PSCs. By modulating the TGF, FGF, and BMP pathways, Lee et al. produced mouse skin organoids from ESCs and iPSCs during an incubation period of 20–30 days. Mouse PSCs were treated with SB4315432 and BMP in 3D culture to induce surface ectoderm, followed by treatment with FGF and LDN-193189 to induce placodal epithelium. Next, the aggregates were cultured into skin organoids containing self-organized skin layers and skin appendages, including HFs, SeGs and adipocytes. [50] Using a similar induction system, Lee et al. prepared human skin organoids that mimic the complex skin structure from human iPSCs. By sequentially modulating the TGF-β and FGF signaling pathways to co-induce nonneural ectoderm and NC cells within cell aggregates, cyst-like skin organoids consisting of stratified epidermis, fat-rich dermis and pigmented HFs with SeGs were induced within a 4–5-month incubation period. Notably, these organoids formed a sensory neuron and Schwann cell network, which in turn formed nerve-like bundles that targeted Merkel cells within HFs [48, 49]. Despite significant advances in the construction of human skin organoids, several challenges still persist. For example, there is a lack of SG formation [48, 49] and an absence of type VII collagen in the structure of epidermal–dermal junctions (EDJ) in skin organoids [141], and such limitations should be considered when modeling certain diseases. Moreover, off-target differentiation of hyaline cartilage in the tail region of skin organoids can be observed [48, 49], although this has reportedly been improved by additional stimulation via the WNT signaling pathway during the induction period. WNT activation also increased the size of human skin organoids, and such organoids could be further developed into skin analogs via the ALI culture method [47].
The application of organoid models in biomedical research
Traditional ALI skin models have been widely introduced in disease modeling and drug tests, such as the study of bacterial infections, wound healing, inflammatory cutaneous diseases, screening of antimicrobial peptides, and evaluation of cosmetics [9, 168–170]. Based on ALI models, microfluidic skin-on-a-chip platforms, such as cocultures of monocytes with KC-based epidermis in a dual-channel microfluidic setting [89], have been introduced to closely mimic natural human skin and help determine drug efficiency and toxicity.
The generation of organoid models of skin, especially skin appendage-bearing organoids, has offered novel opportunities for skin disease modeling, regenerative medicine, and developmental research (Figure 7). These organoids demonstrate structural, transcriptomic, and proteomic similarity to their tissue of origin [10, 11, 48, 49, 57], highlighting their exceptional potential in many aspects of skin research.
Figure 7.

Biomedical applications of organoid models of skin. Skin-based organoids have a wide range of applications, encompassing disease modeling, regenerative medicine, and developmental research. These organoids serve as invaluable tools for mimicking and studying various skin diseases in a controlled in vitro setting. In addition, they offer a platform for screening potential drugs and cosmetics for their efficacy and safety. From a regenerative medicine perspective, organoid models of skin hold promise for tissue engineering and transplantation purposes. Furthermore, these organoids provide a platform for investigating the complex processes involved in human skin development and provide a better understanding of embryonic and postnatal skin development. Created with BioRender.com
Disease modeling
Epidermal organoids present stratified histological and morphological characteristics of the epidermis. Xie et al. conducted a study using mouse primary epidermal organoids to investigate their susceptibility to methicillin-resistant Staphylococcus aureus (MRSA) USA300 infection and to explore the mechanisms of antimicrobial drug action [12]. The findings revealed that epidermal organoids support the colonization and invasion of MRSA USA300, as evidenced by the presence of swollen epithelial cells with nuclear necrosis and the secretion of inflammatory factors. Similarly, Wang et al. established human epidermal organoids to investigate the phenotype and underlying mechanism of dermatophyte infections caused by Trichophyton rubrum [11]. T. rubrum infections of human epidermal organoids accurately reflect many aspects of known clinical pathological conditions. Additionally, the repression of IL-1 signaling may contribute to the development of chronic infections with T. rubrum in human skin.
hiPSC-derived skin organoids recapitulate the complexity and function of full-thickness human skin. Jung used such a model to study atopic dermatitis caused by S. aureus (SA) colonization and infection. The findings revealed disruption of the skin barrier and upregulation of inflammatory cytokines originating from both the epidermis and dermis. Furthermore, the therapeutic effects of pretreatment with Cutibacterium acnes on SA-infected models were investigated [47]. Ma et al. studied the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to infect hiPSC-derived skin organoids containing HFs and neural cells. They reported that Keratin 17+ HFs and different types of neural cells can be infected by SARS-CoV-2, providing evidence for the association between SARS-CoV-2 infection and hair loss [69].
Skin organoids are also promising tools for studying inherited diseases and skin tumors. For example, human-induced PSCs (hiPSC)-derived hair-bearing skin organoids represent a novel approach for modeling diseases such as epidermolysis bullosa (EB). This inherited disease is characterized by abnormalities in the expression or structure of components in the EDJ. In organoids, basal KCs attach to the basement membrane through EDJ-like structures, although the lack of type VII collagen needs to be addressed [141]. Similar to other types of organoids, the utilization of patient-derived or gene-edited donor hiPSCs allows the simulation of a wide range of inherited skin diseases and facilitates drug screening experiments. Additionally, recent studies have utilized organ models to explore the development, progression, and therapy resistance of skin cancer, such as melanoma, providing valuable insights into this type of skin cancer [171].
Regenerative medicine
Skin-based organoids may serve as viable cell sources for reconstructing damaged skin and enhancing wound healing efficiency. The transplantation of epidermal organoids [55], HF organoids [42, 60, 172], SG organoids [40, 56], and iPSC-derived skin organoids [48, 67] into wound models has been shown to promote skin regeneration and the reconstruction of corresponding skin appendages. For example, human epidermal organoids were delivered to the sites of severe skin wounds with the aid of a micro-atomization device, where they facilitated skin reconstitution and wound healing [55].
Extensive evidence supports the crucial role of HFs in the processes of wound healing and skin remodeling. Clinical studies have demonstrated that the transplantation of autologous HFGs into chronic ulcers significantly accelerates wound healing [146, 173, 174]. However, the use of HF organoid technology is currently limited because of its complexity, small-scale production, and high cost. Consequently, HF organoid transplantation research has focused mainly on regenerating HFs in patients with alopecia. Several studies have successfully shown that HF organoid transplantation effectively regenerates HFs when they are transplanted into mouse models [43–45, 83]. This novel approach holds immense promise for the treatment of alopecia.
The restoration of SGs is a significant challenge for individuals with extensive skin defects. However, encouraging progress has been made in this field. By transplanting SG organoids derived from reprogrammed epidermal KCs or SG epithelial cells into a mouse wound model, researchers have successfully restored the crucial functions of SGs during wound healing [40, 56]. This advancement highlights the significant potential for SG regeneration in patients with large skin defects.
With respect to iPSC-derived skin organoids, the transplantation of these organoids into nude mice revealed remarkable integration of the organoid-derived epidermis with the host epidermis. Notably, hair growth was observed in ~55% of the xenografts. Additionally, the epidermis of the xenografts presented cornified layers and retro-ridge-like structures comparable to those observed in adult facial skin [48, 49]. With careful control of safety and reproducibility, these skin appendage-bearing organoids can contribute to the morphological and functional recovery of skin in patients with severe burns or wounds. Moreover, it is worth noting that iPSC-derived organoid models containing both epithelial and mesenchymal tissue significantly reduce the degree of skin fibrosis and increase the activity of EpSCs in patients with localized scleroderma [68]. This study highlights potential applications of organoids in addition to regeneration.
Developmental research
A precise understanding and translation of embryogenesis is the foundation of the in vitro generation of organoids. By leveraging insights into how embryonic development leads to organ formation, PSCs were differentiated to generate skin organoids with multiple lineages. Thus, the organoid technique has opened a new avenue for advancing our comprehension of skin development [175]. On the basis of scRNA-seq and immunostaining data, hiPSC-derived skin organoids (~150 days of culture) have been shown to represent human full-thickness skin tissues during the second-trimester stage of fetal development [48, 49]. Therefore, tracking and analyzing gene expression and signaling pathways during the formation of skin organoids would provide valuable insights into human skin development. Recently, Gopee et al. revealed that the crosstalk between nonimmune and immune cells is pivotal in human HF formation and vascular network remodeling via the use of skin organoids [176], thereby advancing our understanding of human skin morphogenesis. Manipulating the signaling pathways that govern skin development could also have a profound impact on the generation of skin organoids. For example, previous studies have shown that the activation of WNT signaling through the use of the GSK-3β inhibitor CHIR99021 can promote the growth of skin organoids and prevent off-target cartilage differentiation [47]. In addition, the skin organoid model is an excellent platform for studying skin innervation mechanisms, as it allows the incorporation of nerves into existing skin equivalent cultures [48, 49, 177].
Conclusions
Organoid models of skin have emerged as an effective platform for deepening the understanding of skin development, exploring skin-based diseases, and identifying new strategies for skin function reconstruction. This review has provided a comprehensive overview of their construction strategies and recent advances, with a particular emphasis on the importance of appendage-bearing models, which offer research systems with greater physiological relevance. However, the journey toward creating idealized skin organoid models is far from complete. The field continues to grapple with universal challenges inherent to organoid science, including morphological and functional heterogeneity, batch-to-batch variability that complicates reproducibility, and a reliance on labor-intensive protocols that hinder scalability for high-throughput screening and clinical application. Beyond these general hurdles, skin organoids face specific limitations. For example, hPSC-derived skin organoids form a sphere-like cyst where hair shafts sprout inward, with hair bulbs protruding outward from the surface of the cysts. Moreover, the cornified epidermis accumulated in the core, which is opposite to the structure of normal human skin. Such unique inside-out structures could also be observed in epidermal organoids, HF organoids formed by epithelial-mesenchymal interaction etc. Additionally, skin organoids are still lack of some critical cell populations of normal human skin, such as SGs, blood vessels and immune cells. Consequently, future research may continue to focus on the following areas. Must be strategically directed toward several key frontiers. First, Achieving a higher fidelity of skin's complexity and functionality. Developing skin organoids that replicate the diverse cell types, specialized microenvironments, and intricate architecture of the skin is crucial. Additionally, these organoids should demonstrate the physiological functions of native skin, including barrier function, immune responses, and wound healing capabilities. Second, vascularization, innervation and integration of skin appendages are crucial. The incorporation of a functional vascular network is essential for nutrients and oxygen supply, moreover, nerve innervation and integration of skin appendages like HF, SGs, and SeGs into the organoids are crucial for developing comprehensive skin models that replicate natural skin features. Third, enhancing model stability, scalability, and reproducibility is essential for translational success. This will require a concerted push towards chemically defined and xeno-free culture conditions, as well as the adoption of cutting-edge bioengineering techniques and automated bio-fabrication to overcome current limitations. Through ingenious design and continued technological breakthroughs, the organoid platforms are poised to deliver transformative new strategies for disease modeling, drug discovery, and regenerative medicine.
Contributor Information
Dongao Zeng, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China; School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China.
Shikai Li, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China; School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China.
Fangzhou Du, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China.
Yuchen Xia, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China; School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China.
Jingzhong Zhang, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China; School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China; Xuzhou Medical University, No. 209 Tongshan Road, Xuzhou, Jiangsu Province 221004, China.
Shuang Yu, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, No. 88, Keling Road, Suzhou New District, Suzhou, Jiangsu Province 215163, China; School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China; Xuzhou Medical University, No. 209 Tongshan Road, Xuzhou, Jiangsu Province 221004, China.
Jianhua Qin, School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine, University of Science and Technology of China, No.96 Jinzhai Road, Hefei, Anhui Province 230026, China; Division of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, No. 457 Zhongshan Road, Dalian, Liaoning Province 116023, China.
Author contributions
Dongao Zeng (Conceptualization [lead]), Shikai Li (Investigation [equal], Validation [equal]), Fangzhou Du (Investigation [equal]), Yuchen Xia (Data curation [equal]), Jingzhong Zhang (Funding acquisition [equal], Project administration [equal]), Shuang Yu (Conceptualization [equal], Funding acquisition [equal], Project administration [equal], Supervision [equal]), and Jianhua Qin (Supervision [equal]).
Ethic approval
As this article is a review of existing literature, it did not involve any new studies requiring ethical approval for research on human participants or animals.
Conflict of interest: Authors declare no competing interests.
Funding
This research was funded by the National Key Research and Development Program of China (grant/award number: 2021YFA1101100; 2022YFA1104800), National Natural Science Foundation of China (grant/award number: 82271522), and Basic Research Priorities Program of Jiangsu Province (grant/award number: BK20243003).
References
- 1. Zaidi Z, Lanigan SW. Skin: Structure and Function. London: Springer London, 2010. 1–15. 10.1007/978-1-84882-862-9_1. [DOI] [Google Scholar]
- 2. Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science 2014;346:937–40. 10.1126/science.1253734. [DOI] [PubMed] [Google Scholar]
- 3. Belokhvostova D, Berzanskyte I, Cujba A-M, Jowett G, Marshall L, Prueller J. et al. Homeostasis, regeneration and tumour formation in the mammalian epidermis. Int J Dev Biol 2018;62:571–82. 10.1387/ijdb.170341fw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. J Clin Invest 2018;128:26–35. 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L. et al. Organoids. Nat Rev Methods Primers 2022;2:1–21. 10.1038/s43586-022-00174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet 2018;19:671–87. 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 7. Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell 1975;6:317–30. 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- 8. Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977;265:421–4. 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 9. Sutterby E, Thurgood P, Baratchi S, Khoshmanesh K, Pirogova E. Microfluidic skin-on-a-chip models: toward biomimetic artificial skin. Small 2020;16:e2002515. 10.1002/smll.202002515. [DOI] [PubMed] [Google Scholar]
- 10. Boonekamp KE, Kretzschmar K, Wiener DJ, Asra P, Derakhshan S, Puschhof J. et al. Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc Natl Acad Sci USA 2019;116:14630–8. 10.1073/pnas.1715272116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Wang S, Guo B, Guo B, Guo B, Su Y. et al. Human primary epidermal organoids enable modeling of dermatophyte infections. Cell Death Dis 2021;12:35. 10.1038/s41419-020-03330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie X, Tong X, Li Z, Cheng Q, Wang X, Long Y. et al. Use of mouse primary epidermal organoids for usa300 infection modeling and drug screening. Cell Death Dis 2023;14:1–13. 10.1038/s41419-022-05525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuchs E. Scratching the surface of skin development. Nature 2007;445:834–42. 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu MS, Borrelli MR, Hong WX, Malhotra S, Cheung ATM, Ransom RC. et al. Embryonic skin development and repair. Organ 2018;14:46–63. 10.1080/15476278.2017.1421882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu S, Zhang H, Duan E. Epidermal development in mammals: key regulators, signals from beneath, and stem cells. Int J Mol Sci 2013;14:10869–95. 10.3390/ijms140610869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. WIREs Dev Biol 2018;7:e307. 10.1002/wdev.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol 2002;241:106–16. 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 18. Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL. et al. Β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006;296:164–76. 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 19. Myung P, Andl T, Atit R. The origins of skin diversity: lessons from dermal fibroblasts. Development 2022;149:dev200298. 10.1242/dev.200298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohtola J, Myers J, Akhtar-Zaidi B, Zuzindlak D, Sandesara P, Yeh K. et al. Β-catenin has sequential roles in the survival and specification of ventral dermis. Development 2008;135:2321–9. 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504:277–81. 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 2006;22:339–73. 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardy MH. The secret life of the hair follicle. Trends Genet 1992;8:55–61. 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 24. Park S. Hair follicle morphogenesis during embryogenesis, neogenesis, and organogenesis. Front Cell Dev Biol 2022;10:933370. 10.3389/fcell.2022.933370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 2012;23:928–36. 10.1016/j.semcdb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu C, Fuchs E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med 2014;4:a015222. 10.1101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N. et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 2012;150:136–50. 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerber PA, Buhren BA, Schrumpf H, Homey B, Zlotnik A, Hevezi P. The top skin-associated genes: a comparative analysis of human and mouse skin transcriptomes. Biol Chem 2014;395:577–91. 10.1515/hsz-2013-0279. [DOI] [PubMed] [Google Scholar]
- 29. Forni MF, Trombetta-Lima M, Sogayar MC. Stem cells in embryonic skin development. Biol Res 2012;45:215–22. 10.4067/S0716-97602012000300003. [DOI] [PubMed] [Google Scholar]
- 30. Jacob T, Annusver K, Czarnewski P, Dalessandri T, Kalk C, Levra Levron C. et al. Molecular and spatial landmarks of early mouse skin development. Dev Cell 2023;58:2140–62.e5. 10.1016/j.devcel.2023.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stern CD. Neural induction: old problem, new findings, yet more questions. Development 2005;132:2007–21. 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 32. Tchieu J, Zimmer B, Fattahi F, Amin S, Zeltner N, Chen S. et al. A modular platform for differentiation of human pscs into all major ectodermal lineages. Cell Stem Cell 2017;21:399–410.e7. 10.1016/j.stem.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinstein DC, Honoré E, Hemmati-Brivanlou A. Epidermal induction and inhibition of neural fate by translation initiation factor 4AIII. Development 1997;124:4235–42. 10.1242/dev.124.21.4235. [DOI] [PubMed] [Google Scholar]
- 34. Smart IH. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol 1970;82:276–82. 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- 35. Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005;437:275–80. 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ellenbogen E. Embryology of the skin. Cham: Springer International Publishing, 2021. 3–9. [Google Scholar]
- 37. Salgado G, Ng YZ, Koh LF, Goh CSM, Common JE. Human reconstructed skin xenografts on mice to model skin physiology. Differentiation 2017;98:14–24. 10.1016/j.diff.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Wang J, Guo L, Wang X, Chen H, Wang X. et al. Self-assembling peptide hydrogel scaffolds support stem cell-based hair follicle regeneration. Nanomedicine 2016;12:2115–25. 10.1016/j.nano.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 39. Feldman A, Mukha D, Maor II, Sedov E, Koren E, Yosefzon Y. et al. Blimp1+ cells generate functional mouse sebaceous gland organoids in vitro. Nat Commun 2019;10:2348. 10.1038/s41467-019-10261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diao J, Liu J, Wang S, Chang M, Wang X, Guo B. et al. Sweat gland organoids contribute to cutaneous wound healing and sweat gland regeneration. Cell Death Dis 2019;10:238. 10.1038/s41419-019-1485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klaka P, Grüdl S, Banowski B, Giesen M, Sättler A, Proksch P. et al. A novel organotypic 3D sweat gland model with physiological functionality. PLoS One 2017;12:e0182752. 10.1371/journal.pone.0182752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nanmo A, Yan L, Asaba T, Wan L, Kageyama T, Fukuda J. Bioprinting of hair follicle germs for hair regenerative medicine. Acta Biomater 2023;165:50–9. 10.1016/j.actbio.2022.06.021. [DOI] [PubMed] [Google Scholar]
- 43. Kageyama T, Shimizu A, Anakama R, Nakajima R, Suzuki K, Okubo Y. et al. Reprogramming of three-dimensional microenvironments for in vitro hair follicle induction. Sci Adv 2022;8:eadd4603. 10.1126/sciadv.add4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abaci HE, Coffman A, Doucet Y, Chen J, Jackow J, Wang E. et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 2018;9:5301. 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kageyama T, Yoshimura C, Myasnikova D, Kataoka K, Nittami T, Maruo S. et al. Spontaneous hair follicle germ (hfg) formation in vitro, enabling the large-scale production of hfgs for regenerative medicine. Biomaterials 2018;154:291–300. 10.1016/j.biomaterials.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 46. Tan CT, Leo ZY, Lim CY. Generation and integration of hair follicle-primed spheroids in bioengineered skin constructs. Biomed Mater 2022;17:061001. 10.1088/1748-605X/ac99c6. [DOI] [PubMed] [Google Scholar]
- 47. Jung S-y, You HJ, Kim M-J, Ko G, Lee S, Kang K-S. Wnt-activating human skin organoid model of atopic dermatitis induced by staphylococcus aureus and its protective effects by cutibacterium acnes. iScience 2022;25:105150. 10.1016/j.isci.2022.105150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE. et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020;582:399–404. 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee J, van der Valk WH, Serdy SA, Deakin C, Kim J, Le AP. et al. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat Protoc 2022;17:1266–305. 10.1038/s41596-022-00681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee J, Bӧscke R, Tang PC, Hartman BH, Heller S, Koehler KR. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep 2018;22:242–54. 10.1016/j.celrep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering stem cell organoids. Cell Stem Cell 2016;18:25–38. 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzales KAU, Fuchs E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell 2017;43:387–401. 10.1016/j.devcel.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu Y-C, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med 2014;20:847–56. 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu Y-C, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol 2012;13:103–14. 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang M, Liu J, Guo B, Fang X, Wang Y, Wang S. et al. Auto micro atomization delivery of human epidermal organoids improves therapeutic effects for skin wound healing. Front Bioeng Biotechnol 2020;8:110. 10.3389/fbioe.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun X, Xiang J, Chen R, Geng Z, Wang L, Liu Y. et al. Sweat gland organoids originating from reprogrammed epidermal keratinocytes functionally recapitulated damaged skin. Adv Sci 2021;8:e2103079. 10.1002/advs.202103079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ebner-Peking P, Krisch L, Wolf M, Hochmann S, Hoog A, Vári B. et al. Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration. Theranostics 2021;11:8430–47. 10.7150/thno.59661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Huang J, Kang D, Zhou Y, Du L, Qu Q. et al. Microenvironmental reprogramming of human dermal papilla cells for hair follicle tissue engineering. Acta Biomater 2023;165:31–49. 10.1016/j.actbio.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 59. Huang CF, Chang YJ, Hsueh YY, Huang CW, Wang DH, Huang TC. et al. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci Rep 2016;6:26436. 10.1038/srep26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang J, Miao Y, Huang Y, Lin B, Liu X, Xiao S. et al. Bottom-up nanoencapsulation from single cells to tunable and scalable cellular spheroids for hair follicle regeneration. Adv Healthc Mater 2018;7:1700447. 10.1002/adhm.201700447. [DOI] [PubMed] [Google Scholar]
- 61. Hu S, Li Z, Lutz H, Huang K, Su T, Cores J. et al. Dermal exosomes containing mir-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv 2020;6:eaba1685. 10.1126/sciadv.aba1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin B, Miao Y, Wang J, Fan Z, Du L, Su Y. et al. Surface tension guided hanging-drop: producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl Mater Interfaces 2016;8:5906–16. 10.1021/acsami.6b00202. [DOI] [PubMed] [Google Scholar]
- 63. Gupta A, Chawla S, Hegde A, Singh D, Bandyopadhyay B, Lakshmanan CC. et al. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J Cell Physiol 2018;233:9015–30. 10.1002/jcp.26853. [DOI] [PubMed] [Google Scholar]
- 64. Yao B, Wang R, Wang Y, Zhang Y, Hu T, Song W. et al. Biochemical and structural cues of 3D-printed matrix synergistically direct msc differentiation for functional sweat gland regeneration. Sci Adv 2020;6:eaaz1094. 10.1126/sciadv.aaz1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kolakshyapati P, Li X, Chen C, Zhang M, Tan W, Ma L. et al. Gene-activated matrix/bone marrow-derived mesenchymal stem cells constructs regenerate sweat glands-like structure in vivo. Sci Rep 2017;7:17630. 10.1038/s41598-017-17967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang J, Fu D, Wu X, Li Y, Zheng B, Liu Z. et al. One-step generation of core–shell biomimetic microspheres encapsulating double-layer cells using microfluidics for hair regeneration. Biofabrication 2023;15:025007. 10.1088/1758-5090/acb107. [DOI] [PubMed] [Google Scholar]
- 67. Takagi R, Ishimaru J, Sugawara A, Toyoshima KE, Ishida K, Ogawa M. et al. Bioengineering a 3D integumentary organ system from ips cells using an in vivo transplantation model. Sci Adv 2016;2:e1500887. 10.1126/sciadv.1500887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ma J, Li W, Cao R, Gao D, Zhang Q, Li X. et al. Application of an ipsc-derived organoid model for localized scleroderma therapy. Adv Sci 2022;9:2106075. 10.1002/advs.202106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma J, Liu J, Gao D, Li X, Zhang Q, Lv L. et al. Establishment of human pluripotent stem cell-derived skin organoids enabled pathophysiological model of sars-cov-2 infection. Adv Sci 2022;9:2104192. 10.1002/advs.202104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 71. Sharma A, Sharma A, Sances S, Workman M, Svendsen CN. Multi-lineage human ipsc-derived platforms for disease modeling and drug discovery. Cell Stem Cell 2020;26:309–29. 10.1016/j.stem.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choi KG, Wu BC, Lee AH, Baquir B, Hancock REW. Utilizing organoid and air–liquid interface models as a screening method in the development of new host defense peptides. Front Cell Infect Microbiol 2020;10:228. 10.3389/fcimb.2020.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rheinwatd JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 74. Sun J, Ahmed I, Brown J, Khosrotehrani K, Shafiee A. The empowering influence of air–liquid interface culture on skin organoid hair follicle development. Burns Trauma 2025;13:tkae070. 10.1093/burnst/tkae070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Basler K, Galliano MF, Bergmann S, Rohde H, Wladykowski E, Vidal YSS. et al. Biphasic influence of staphylococcus aureus on human epidermal tight junctions. Ann N Y Acad Sci 2017;1405:53–70. 10.1111/nyas.13418. [DOI] [PubMed] [Google Scholar]
- 76. Ramasamy S, Davoodi P, Vijayavenkataraman S, Teoh JH, Thamizhchelvan AM, Robinson KS. et al. Optimized construction of a full thickness human skin equivalent using 3D bioprinting and a pcl/collagen dermal scaffold. Bioprinting 2021;21:e00123. 10.1016/j.bprint.2020.e00123. [DOI] [Google Scholar]
- 77. Kim Y, Park N, Rim YA, Nam Y, Jung H, Lee K. et al. Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Res Ther 2018;9:217. 10.1186/s13287-018-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ikuta S, Sekino N, Hara T, Saito Y, Chida K. Mouse epidermal keratinocytes in three-dimensional organotypic coculture with dermal fibroblasts form a stratified sheet resembling skin. Biosci Biotechnol Biochem 2006;70:2669–75. 10.1271/bbb.60266. [DOI] [PubMed] [Google Scholar]
- 79. Higgins CA, Chen JC, Cerise JE, Jahoda CAB, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci USA 2013;110:19679–88. 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurosawa H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J Biosci Bioeng 2007;103:389–98. 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 81. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE. et al. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–5. 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 82. Huang S, Yao B, Xie J, Fu X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater 2016;32:170–7. 10.1016/j.actbio.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 83. Kageyama T, Yan L, Shimizu A, Maruo S, Fukuda J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials 2019;212:55–63. 10.1016/j.biomaterials.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 84. Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T. et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun 2012;3:784. 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Science 2019;364:960–5. 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang L, Li Z, Xu C, Qin J. Bioinspired engineering of organ-on-chip devices. Adv Exp Med Biol 2019;1174:401–40. 10.1007/978-981-13-9791-2_13. [DOI] [PubMed] [Google Scholar]
- 87. Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH. et al. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep 2016;6:37471. 10.1038/srep37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lim HY, Kim J, Song HJ, Kim K, Choi KC, Park S. et al. Development of wrinkled skin-on-a-chip (wsoc) by cyclic uniaxial stretching. J Ind Eng Chem 2018;68:238–45. 10.1016/j.jiec.2018.07.050. [DOI] [Google Scholar]
- 89. Ramadan Q, Ting FCW. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 2016;16:1899–908. 10.1039/c6lc00229c. [DOI] [PubMed] [Google Scholar]
- 90. Hosseini M, Koehler KR, Shafiee A. Biofabrication of human skin with its appendages. Adv Healthc Mater 2022;11:e2201626. 10.1002/adhm.202201626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vedadghavami A, Minooei F, Mohammadi MH, Khetani S, Kolahchi AR, Mashayekhan S. et al. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater 2017;62:42–63. 10.1016/j.actbio.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 92. Liu HT, Wang YQ, Cui KL, Guo YQ, Zhang X, Qin JH. Advances in hydrogels in organoids and organs-on-a-chip. Adv Mater 2019;31:e1902042. 10.1002/adma.201902042. [DOI] [PubMed] [Google Scholar]
- 93. Aisenbrey EA, Murphy WL. Synthetic alternatives to matrigel. Nat Rev Mater 2020;5:539–51. 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fukuyama M, Tsukashima A, Kimishima M, Yamazaki Y, Okano H, Ohyama M. Human ips cell-derived cell aggregates exhibited dermal papilla cell properties in in vitro three-dimensional assemblage mimicking hair follicle structures. Front Cell Dev Biol 2021;9:590333. 10.3389/fcell.2021.590333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hosseini M, Shafiee A. Engineering bioactive scaffolds for skin regeneration. Small 2021;17:2101384. 10.1002/smll.202101384. [DOI] [PubMed] [Google Scholar]
- 96. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–89. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 97. Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater 2021;6:402–20. 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shakouri-Motlagh A, O'Connor AJ, Brennecke SP, Kalionis B, Heath DE. Native and solubilized decellularized extracellular matrix: a critical assessment of their potential for improving the expansion of mesenchymal stem cells. Acta Biomater 2017;55:1–12. 10.1016/j.actbio.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 99. Zhang X, Xiao S, Liu B, Miao Y, Hu Z. Use of extracellular matrix hydrogel from human placenta to restore hair-inductive potential of dermal papilla cells. Regen Med 2019;14:741–51. 10.2217/rme-2018-0112. [DOI] [PubMed] [Google Scholar]
- 100. Zhu LY, Yuhan J, Yu H, Zhang BY, Huang KL, Zhu LJ. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment. Small 2023;19:e2207752. 10.1002/smll.202207752. [DOI] [PubMed] [Google Scholar]
- 101. Jeon EY, Sorrells L, Abaci HE. Biomaterials and bioengineering to guide tissue morphogenesis in epithelial organoids. Front Bioeng Biotechnol 2022;10:1038277. 10.3389/fbioe.2022.1038277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pfisterer K, Shaw LE, Symmank D, Weninger W. The extracellular matrix in skin inflammation and infection. Front Cell Dev Biol 2021;9:682414. 10.3389/fcell.2021.682414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim JW, Kim MJ, Ki CS, Kim HJ, Park YH. Fabrication of bi-layer scaffold of keratin nanofiber and gelatin-methacrylate hydrogel: implications for skin graft. Int J Biol Macromol 2017;105:541–8. 10.1016/j.ijbiomac.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 104. Graça MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid-based wound dressings: a review. Carbohydr Polym 2020;241:116364. 10.1016/j.carbpol.2020.116364. [DOI] [PubMed] [Google Scholar]
- 105. Kalabusheva E, Terskikh V, Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: impact of hyaluronic acid. Stem Cells Int 2017;2017:9271869. 10.1155/2017/9271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Luo L, Liu L, Ding Y, Dong Y, Ma M. Advances in biomimetic hydrogels for organoid culture. Chem Commun 2023;59:9675–86. 10.1039/d3cc01274c. [DOI] [PubMed] [Google Scholar]
- 107. Yamanlar S, Sant S, Boudou T, Picart C, Khademhosseini A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011;32:5590–9. 10.1016/j.biomaterials.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dam P, Altuntas S, Mondal R, Vega-Baudrit J, Kati A, Ghorai S. et al. Silk-based nano-biocomposite scaffolds for skin organogenesis. Mater Lett 2022;327:133024. 10.1016/j.matlet.2022.133024. [DOI] [Google Scholar]
- 109. Li S, Li Z, Yang J, Ha Y, Zhou X, He C. Inhibition of sympathetic activation by delivering calcium channel blockers from a 3D printed scaffold to promote bone defect repair. Adv Healthc Mater 2022;11:e2200785. 10.1002/adhm.202200785. [DOI] [PubMed] [Google Scholar]
- 110. Jirigala E, Yao B, Li Z, Zhang YJ, Zhang C, Liang LT. et al. In situ forming injectable msc-loaded gelma hydrogels combined with pd for vascularized sweat gland regeneration. Mil Med Res 2023;10:17. 10.1186/s40779-023-00456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen H, Ma X, Gao T, Zhao W, Xu T, Liu Z. Robot-assisted in situ bioprinting of gelatin methacrylate hydrogels with stem cells induces hair follicle-inclusive skin regeneration. Biomed Pharmacother 2023;158:114140. 10.1016/j.biopha.2022.114140. [DOI] [PubMed] [Google Scholar]
- 112. Zhang Y, Yin P, Huang J, Yang L, Liu Z, Fu D. et al. Scalable and high-throughput production of an injectable platelet-rich plasma (prp)/cell-laden microcarrier/hydrogel composite system for hair follicle tissue engineering. J Nanobiotechnol 2022;20:465. 10.1186/s12951-022-01671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005;23:47–55. 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 114. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 2009;103:655–63. 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol 2002;13:377–83. 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 116. Vallmajo-Martin Q, Broguiere N, Millan C, Zenobi-Wong M, Ehrbar M. Peg/ha hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv Funct Mater 2020;30:1910282. 10.1002/adfm.201910282. [DOI] [Google Scholar]
- 117. Cruz-Acuña R, Quirós M, Huang S, Siuda D, Spence JR, Nusrat A. et al. Peg-4mal hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc 2018;13:2102–19. 10.1038/s41596-018-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bock N, Forouz F, Hipwood L, Clegg J, Jeffery P, Gough M. et al. Gelma, click-chemistry gelatin and bioprinted polyethylene glycol-based hydrogels as 3D ex vivo drug testing platforms for patient-derived breast cancer organoids. Pharmaceutics 2023;15:261. 10.3390/pharmaceutics15010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li X, Xie R, Luo Y, Shi R, Ling Y, Zhao X. et al. Cooperation of TGF-β and FGF signalling pathways in skin development. Cell Prolif 2023;56:e13489. 10.1111/cpr.13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cui CY, Yin M, Sima J, Childress V, Michel M, Piao Y. et al. Involvement of wnt, eda and shh at defined stages of sweat gland development. Development 2014;141:3752–60. 10.1242/dev.109231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Biggs LC, Mikkola ML. Early inductive events in ectodermal appendage morphogenesis. Semin Cell Dev Biol 2014;25-26:11–21. 10.1016/j.semcdb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 122. Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci 2001;4:1161–8. 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- 123. Zhu X-J, Liu Y, Dai Z-M, Zhang X, Yang X, Li Y. et al. BMP-FGF signaling axis mediates wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 2014;10:e1004687. 10.1371/journal.pgen.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Alkobtawi M, Pla P, Monsoro-Burq AH. BMP signaling is enhanced intracellularly by fhl3 controlling wnt-dependent spatiotemporal emergence of the neural crest. Cell Rep 2021;35:109289. 10.1016/j.celrep.2021.109289. [DOI] [PubMed] [Google Scholar]
- 125. Oceguera-Yanez F, Avila-Robinson A, Woltjen K. Differentiation of pluripotent stem cells for modeling human skin development and potential applications. Front Cell Dev Biol 2022;10:1030339. 10.3389/fcell.2022.1030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lu CP, Polak L, Keyes BE, Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 2016;354:aah6102. 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ahtiainen L, Lefebvre S, Lindfors PH, Renvoisé E, Shirokova V, Vartiainen MK. et al. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev Cell 2014;28:588–602. 10.1016/j.devcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 128. Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 2003;422:317–22. 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 1998;125:3775–87. 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 130. Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L. et al. Coordinated activation of wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011;145:941–55. 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein lef1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res 2017;7:1389–406. [PMC free article] [PubMed] [Google Scholar]
- 132. Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA. et al. Tankyrase inhibition stabilizes axin and antagonizes WNT signalling. Nature 2009;461:614–20. 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 133. Chen R, Zhu Z, Ji S, Geng Z, Hou Q, Sun X. et al. Sweat gland regeneration: current strategies and future opportunities. Biomaterials 2020;255:120201. 10.1016/j.biomaterials.2020.120201. [DOI] [PubMed] [Google Scholar]
- 134. Yao B, Xie J, Liu N, Hu T, Song W, Huang S. et al. Direct reprogramming of epidermal cells toward sweat gland-like cells by defined factors. Cell Death Dis 2019;10:272. 10.1038/s41419-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nhu MTQ, Batjargal U, Song H, Kim J-M, Kwak I-S, Moon B-S. Generation of human-induced pluripotent stem cell-derived adherent 3-dimensional skin hair-follicle organoids. Organ 2022;2:e23. 10.51335/organoid.2022.2.e23. [DOI] [Google Scholar]
- 136. Kageyama T, Anakama R, Togashi H, Fukuda J. Impacts of manipulating cell sorting on in vitro hair follicle regeneration. J Biosci Bioeng 2022;134:534–40. 10.1016/j.jbiosc.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 137. Kim H, Choi N, Kim DY, Kim SY, Song SY, Sung JH. Tgf-beta2 and collagen play pivotal roles in the spheroid formation and anti-aging of human dermal papilla cells. Aging (Milano) 2021;13:19978–95. 10.18632/aging.203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kageyama T, Chun Y-S, Fukuda J. Hair follicle germs containing vascular endothelial cells for hair regenerative medicine. Sci Rep 2021;11:624. 10.1038/s41598-020-79722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kageyama T, Miyata H, Seo J, Nanmo A, Fukuda J. In vitro hair follicle growth model for drug testing. Sci Rep 2023;13:4847. 10.1038/s41598-023-31842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li H, Chen L, Zhang M, Tang S, Fu X. Three-dimensional culture and identification of human eccrine sweat glands in matrigel basement membrane matrix. Cell Tissue Res 2013;354:897–902. 10.1007/s00441-013-1718-3. [DOI] [PubMed] [Google Scholar]
- 141. Ramovs V, Janssen H, Fuentes I, Pitaval A, Rachidi W, Lopes SMCS. et al. Characterization of the epidermal-dermal junction in hipsc-derived skin organoids. Stem Cell Rep 2022;17:1279–88. 10.1016/j.stemcr.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Dong J, Hu Y, Fan X, Wu X, Mao YJ, Mao Y. et al. Single-cell rna-seq analysis unveils a prevalent epithelial/mesenchymal hybrid state during mouse organogenesis. Genome Biol 2018;19:31. 10.1186/s13059-018-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang C-C, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci 2010;57:2–11. 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci 2011;124:1179–82. 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 2008;128:1311–8. 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 146. Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the "hair growth-wound healing connection": Anagen phase promotes wound re-epithelialization. J Invest Dermatol 2011;131:518–28. 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- 147. Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med 2015;5:a023267. 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol 2015;135:1244–52. 10.1038/jid.2015.28. [DOI] [PubMed] [Google Scholar]
- 149. Reynolds AJ, Jahoda CA. Hair matrix germinative epidermal cells confer follicle-inducing capabilities on dermal sheath and high passage papilla cells. Development 1996;122:3085–94. 10.1242/dev.122.10.3085. [DOI] [PubMed] [Google Scholar]
- 150. Ohyama M, Kobayashi T, Sasaki T, Shimizu A, Amagai M. Restoration of the intrinsic properties of human dermal papilla in vitro. J Cell Sci 2012;125:4114–25. 10.1242/jcs.105700. [DOI] [PubMed] [Google Scholar]
- 151. Wu X, Scott L, Washenik K, Stenn K. Full-thickness skin with mature hair follicles generated from tissue culture expanded human cells. Tissue Eng Part A 2014;20:3314–21. 10.1089/ten.tea.2013.0759. [DOI] [PubMed] [Google Scholar]
- 152. Chen P, Miao Y, Zhang F, Huang J, Chen Y, Fan Z. et al. Nanoscale microenvironment engineering based on layer-by-layer self-assembly to regulate hair follicle stem cell fate for regenerative medicine. Theranostics 2020;10:11673–89. 10.7150/thno.48723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhang K, Bai X, Yuan Z, Cao X, Jiao X, Qin Y. et al. Cellular nanofiber structure with secretory activity-promoting characteristics for multicellular spheroid formation and hair follicle regeneration. ACS Appl Mater Interfaces 2020;12:7931–41. 10.1021/acsami.9b21125. [DOI] [PubMed] [Google Scholar]
- 154. Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH. et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol 1993;100:229–36. 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 155. Lei M, Schumacher L, Lai Y, Juan W, Yeh C, Wu P. et al. Self-organization process in newborn skin organoid formation inspires strategy to restore hair regeneration of adult cells. Proc Natl Acad Sci USA 2017;114:E7101–10. 10.1073/pnas.1700475114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hunt DPJ, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C. et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells 2008;26:163–72. 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- 157. Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L. et al. Skps derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 2009;5:610–23. 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang X, Wang X, Liu J, Cai T, Guo L, Wang S. et al. Hair follicle and sebaceous gland de novo regeneration with cultured epidermal stem cells and skin-derived precursors. Stem Cells Transl Med 2016;5:1695–706. 10.5966/sctm.2015-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhao Q, Li N, Zhang H, Lei X, Cao Y, Xia G. et al. Chemically induced transformation of human dermal fibroblasts to hair-inducing dermal papilla-like cells. Cell Prolif 2019;52:e12652. 10.1111/cpr.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tsatsou F, Zouboulis CC. Anatomy of the sebaceous gland. Berlin, Heidelberg: Springer, 2014. 27–31. 10.1007/978-3-540-69375-8_4. [DOI] [Google Scholar]
- 161. Liu Y, Gao H, Chen H, Ji S, Wu L, Zhang H. et al. Sebaceous gland organoid engineering. Burns. Trauma 2024;12:tkae003. 10.1093/burnst/tkae003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol 2012;363:138–46. 10.1016/j.ydbio.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 163. Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol 2015;24:644–50. 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Pontiggia L, Biedermann T, Böttcher-Haberzeth S, Oliveira C, Braziulis E, Klar AS. et al. De novo epidermal regeneration using human eccrine sweat gland cells: higher competence of secretory over absorptive cells. J Invest Dermatol 2014;134:1735–42. 10.1038/jid.2014.30. [DOI] [PubMed] [Google Scholar]
- 165. Rittié L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol 2013;182:163–71. 10.1016/j.ajpath.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang C, Chen Y, Fu X. Sweat gland regeneration after burn injury: is stem cell therapy a new hope? Cytotherapy 2015;17:526–35. 10.1016/j.jcyt.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 167. Sun S, Xiao J, Huo J, Geng Z, Ma K, Sun X. et al. Targeting ectodysplasin promotor by crispr/dcas9-effector effectively induces the reprogramming of human bone marrow-derived mesenchymal stem cells into sweat gland-like cells. Stem Cell Res Ther 2018;9:8. 10.1186/s13287-017-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bi H, Jin Y. Current progress of skin tissue engineering: seed cells, bioscaffolds, and construction strategies. Burns Trauma 2013;1:63–72. 10.4103/2321-3868.118928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Guerrero-Aspizua S, Conti CJ, Zapatero-Solana E, Larcher F, Del Río M. Chapter 8- current applications for bioengineered skin. Boston: Academic Press, 2016. 107–20 10.1016/B978-0-12-800548-4.00008-5. [DOI] [Google Scholar]
- 170. Sarkiri M, Fox SC, Fratila-Apachitei LE, Zadpoor AA. Bioengineered skin intended for skin disease modeling. Int J Mol Sci 2019;20:1407. 10.3390/ijms20061407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rebecca VW, Somasundaram R, Herlyn M. Pre-clinical modeling of cutaneous melanoma. Nat Commun 2020;11:2858. 10.1038/s41467-020-15546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Guo L, Wang X, Yuan J, Zhu M, Fu X, Xu RH. et al. Tsa restores hair follicle-inductive capacity of skin-derived precursors. Sci Rep 2019;9:2867. 10.1038/s41598-019-39394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kiani MT, Higgins CA, Almquist BD. The hair follicle: an underutilized source of cells and materials for regenerative medicine. ACS Biomater Sci Eng 2018;4:1193–207. 10.1021/acsbiomaterials.7b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Plotczyk M, Jimenez F. Hair follicles in wound healing and skin remodelling. Cham: Springer International Publishing, 2022. 291–304 10.1007/978-3-030-98331-4_14. [DOI] [Google Scholar]
- 175. Sahu S, Sharan SK. Translating embryogenesis to generate organoids: novel approaches to personalized medicine. iScience 2020;23:101485. 10.1016/j.isci.2020.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gopee NH, Winheim E, Olabi B, Admane C, Foster AR, Huang N. et al. A prenatal skin atlas reveals immune regulation of human skin morphogenesis. Nature 2024;635:679–89. 10.1038/s41586-024-08002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lee J, Koehler KR. Skin organoids: a new human model for developmental and translational research. Exp Dermatol 2021;30:613–20. 10.1111/exd.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]





