ABSTRACT
Background
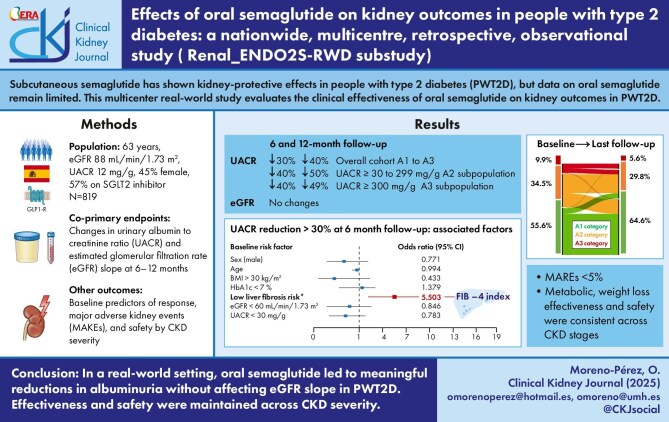
Subcutaneous semaglutide has shown kidney-protective effects in people with type 2 diabetes (PWT2D), but data on oral semaglutide remain limited. This multicentre real-world study evaluates the clinical effectiveness of oral semaglutide on kidney outcomes in PWT2D.
Methods
We included PW2TD ≥18 years of age who initiated oral semaglutide in routine practice between 2021 and 2022 in the Spanish National Health System, with at least one report of clinical follow-up (FU) data at 3 months. Co-primary endpoints were changes in urine albumin:creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) slope at 6–12 months. We also assessed baseline predictors of response, drug persistence and safety by CKD severity.
Results
Data were available for 819 PWT2D (median age 63 years, eGFR 88.1 ml/min/1.73 m2, UACR 12 mg/g, 45.8% female, median FU 8.96 months). Oral semaglutide decreased UACR by 30.3% and 40.0% in the overall cohort, by 40.2% and 50.7% in those with a UACR ≥30 mg/g and by 40.6% and 49.9% in those with a UACR ≥300 mg/g at 6 and 12 months of FU, respectively. PWT2D with a low age-adjusted risk of liver fibrosis by the Fibrosis-4 index had increased odds of achieving a >30% reduction in UACR [adjusted odds ratio 5.50 (95% confidence interval 1.6–18.7)] regardless of baseline background. Metabolic and weight loss effectiveness, safety and persistence of oral semaglutide were consistent across CKD severities.
Conclusions
In a real-world setting, oral semaglutide treatment for up to 52 weeks resulted in clinically meaningful reductions in albuminuria without changes in the eGFR slope in PWT2D. Effectiveness, safety and tolerability were not influenced by CKD severity.
Keywords: glucagon-like peptide-1 receptor agonist, kidney function, oral semaglutide, real-world data, type 2 diabetes mellitus
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Subcutaneous semaglutide reduces the urine albumin:creatinine ratio (UACR) in patients with type 2 diabetes and chronic kidney disease (CKD).
Evidence for oral semaglutide is limited to pooled results, which suggest a trend towards UACR reduction.
Recently, the SOUL trial (NCT03914326) confirmed the major adverse cardiovascular events benefit of oral semaglutide, failed on major renal events and did not provide UACR data.
This study adds
In a large real-world cohort, oral semaglutide significantly reduced UACR in the medium term, with a stable estimated glomerular filtration rate slope, even in a sodium–glucose co-transporter 2 inhibitor background.
The low baseline risk of liver fibrosis was independently associated with the UACR benefit.
Effectiveness, safety and tolerability were not influenced by CKD severity.
Potential impact:
The evidence here filled existing precision medicine approach knowledge gaps.
Oral semaglutide seems a valid therapeutic option, regardless of CKD stage, to join ongoing efforts to implement rapid sequence up-titration for the disease.
Dedicated studies are warranted to explore the hepatic–kidney crosstalk of the renal benefits of glucagon-like peptide-1 receptor agonists.
INTRODUCTION
Chronic kidney disease (CKD) is a common complication of type 2 diabetes (T2D) [1]. Dysfunctional adipose tissue and metabolic dysfunction–associated fatty liver disease are linked to an increased risk of CKD in people with T2D (PWT2D) [2–4]. Changes in liver fibrosis, as measured by the Fibrosis-4 (FIB-4) index, are positively correlated with changes in albuminuria and proteinuria in this population [5]. However, the potential of liver fibrosis risk as a biomarker for the response to nephroprotective drugs remains to be evaluated.
The breakthrough in highly effective weight loss drugs means that an integrated therapeutic framework using weight loss as a disease-modifying intervention could simplify the approach to preventing or potentially remitting CKD [2]. Evidence has emerged regarding the kidney-protective effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in PWT2D or adiposity-based chronic disease (ABCD), classically known as obesity [2, 6, 7]. Thus, in the FLOW study (NCT03819153), subcutaneous semaglutide reduced the risk of kidney and cardiovascular outcomes and death in PWT2D and CKD [6], while in the SMART study (NCT04889183), subcutaneous semaglutide provided a clinically meaningful reduction in albuminuria in patients with ABCD and non-diabetic CKD [7]. This evidence supports an important therapeutic role for subcutaneous semaglutide in this population.
However, data on oral semaglutide and kidney outcomes are scarce. In this regard, a pooled analysis of the SUSTAIN 6 (NCT01720446)/PIONEER 6 (NCT02692716) studies in PWT2D suggests that semaglutide may reduce the rate of estimated glomerular filtration rate (eGFR) decline [2], and, in the PIONEER 5 study (NCT02827708), oral semaglutide demonstrated renal safety, as eGFR remained stable and the geometric mean urine albumin:creatinine ratio (UACR) tended to decrease throughout the study [8]. More recently, the SOUL trial (NCT03914326) showed that oral semaglutide reduced major adverse kidney events (MAKE) by 14%, regardless of concomitant sodium–glucose co-transporter 2 (SGLT2) inhibitor treatment (26.9% of participants were receiving it at baseline), with no differences in major renal events [9, 10]. Unfortunately, this trial did not collect UACR data.
Since clinical trials may not provide adequate estimates of efficacy or safety that reflect real clinical settings [11], insights into the real-world use of oral semaglutide are needed [12]. To date, only two 6-month retrospective studies have been published evaluating the effects of oral semaglutide on kidney parameters in PWT2D. One study compared patients treated with oral semaglutide or subcutaneous semaglutide was too small to have the necessary capacity to overcome the variability of albuminuria [13] and another study compared subjects treated with dapagliflozin alone and dapagliflozin plus oral semaglutide, with the combination showing a smaller reduction in albuminuria [14].
Previously, in the ENDOcrinology Oral Semaglutide Real-World Data (ENDO2S-RWD) study, we reported the effectiveness, safety and tolerability of oral semaglutide in a large cohort of PWT2D in Spain [15]. Here, we report a prespecified analysis of the clinical effectiveness of oral semaglutide on kidney outcomes in the largest multicentre real-world study to date of PWT2D treated with oral semaglutide in routine clinical practice [15, 16]. Factors associated with kidney outcomes, including the FIB-4 index, achievement of treatment goals and safety by CKD severity, were also evaluated.
MATERIALS AND METHODS
Study design and participants
The ENDO2S-RWD was a real-world, nationwide, multicentre, retrospective, observational study of the effectiveness and safety of oral semaglutide in PWT2D. The design, baseline characteristics and primary results have been published elsewhere [15]. Briefly, the main inclusion criteria were people ≥18 years of age with T2D who started treatment with oral semaglutide in routine clinical practice between November 2021 and November 2022 in 12 health centres of the Spanish National Health System, with at least one report of clinical follow-up (FU) data at 3 months. The source of recruitment was the drug prescription registry of each health centre.
The study was endorsed by the Spanish Society of Endocrinology and Nutrition and approved by the Ethics Committee of the Dr Balmis General University Hospital, Alicante, Spain (2022-0386).
For this substudy, we selected PWT2D who had undergone kidney function testing from 3 months before starting oral semaglutide and who had at least one record of FU at 3 months and eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula with or without UACR (first morning void, determined by turbidimetry or direct colorimetry). We also calculated the FIB-4 index at baseline according to Sterling et al. [17]; a FIB-4 index <1.3 or 2.0 was considered as a ‘low probability of hepatic fibrosis’ in people <65 or >65 years of age, respectively [18].
We recorded clinical outcomes in two periods: at 3–6 months (6-month FU visit) and 6–12 months (12-month FU visit).
The co-primary endpoints of this study were the change in UACR and eGFR. A ≥30% reduction in the UACR was defined as clinically relevant [19]. Secondary endpoints were factors associated with these changes and the occurrence of MAKE or ≥30% eGFR decrease at FU. MAKE were defined as progression to end-stage renal disease (dialysis, kidney transplantation or confirmed eGFR <15 ml/min/1.73 m2), a sustained decrease in eGFR of at least 50% from baseline, a de novo diagnosis of CKD [20] or death from renal causes. Co-primary outcomes and associated factors were only assessed for PWT2D undergoing treatment with oral semaglutide, while safety data and MAKE were analysed for the whole cohort.
Metabolic treatment objectives [weight loss percentage (WLP), haemoglobin A1c (HbA1c) change and a combined endpoint defined as a WLP ≥5% from baseline and an HbA1c reduction ≥1%], treatment persistence and safety by CKD severity were also assessed.
Statistical analysis
Quantitative variables were summarized as median and interquartile range (IQR) and qualitative variables as absolute and relative frequencies. Comparative analyses of continuous variables were performed using Mann–Whitney and Wilcoxon signed-rank tests for independent and paired samples, respectively. The chi-squared test was used to evaluate differences in categorical variables.
Missing data were handled using a complete-case analysis approach, given the retrospective nature of the study and the proportion of missing values being relatively low. Variables with unrecoverable data from the electronic medical records were assessed and no systematic bias was identified. As the data were assumed to be missing at random, we proceeded without imputation to avoid introducing additional model assumptions, ensuring transparency and preserving the internal validity of the analysis.
Multiple logistic regression models were constructed to explore the association between baseline data and short- and mid-term clinical outcomes in PWT2D, considering both primary (UACR reduction) and co-primary (MAKE) outcomes. Results were presented as adjusted odds ratios (aORs) and 95% confidence intervals (CIs). Variables with >30% missing data were excluded from the multiple logistic regression models in order to minimise bias and preserve model validity.
All tests were two-tailed and statistical significance was set at P < .05. SPSS Statistics 25 (IBM, Armonk, NY, USA) was used for the analyses.
RESULTS
From the initial cohort of 1018 PWT2D who started oral semaglutide treatment in routine clinical practice during the study period, baseline and FU information on eGFR and/or UACR was available for 819 individuals. These individuals were ultimately included in the study (Table 1). Importantly, age, sex, comorbidities, body mass index (BMI), baseline T2D therapy or degree of renal impairment or proteinuria did not differ between the total cohort and the included patients, with the exception of baseline HbA1c [median 7.9% (IQR 6.9–8.8) versus 7.6% (6.6–8.5), P = .02].
Table 1:
Baseline characteristics (N = 819).
| Characteristics | Values |
|---|---|
| Age (years), median (IQR) | 63 (56–70.7) |
| Sex, % | |
| Female | 45.8 |
| Male | 54.2 |
| Ischaemic cardiopathy, % | 19.2 |
| Heart failure, % | 9.2 |
| Stroke, % | 6.6 |
| Peripheric arteriopathy, % | 8.4 |
| Diabetic kidney disease, % | 25.4 |
| Diabetic retinopathy, % | 13.8 |
| Diabetic polyneuropathy, % | 6.7 |
| GLP-1RAs, % | 14.2 |
| Insulin, % | 27.9 |
| SGLT2 inhibitor, % | 57.1 |
| Metformin, % | 79.8 |
| Statin, % | 68.5 |
| Change from DPP-4 inhibitor, % | 33.2 |
| Antihypertensive drug, % | 67.3 |
| ACE inhibitors/ARBs, % | 57.9 |
| Semaglutide starting dose (mg), % | |
| 3 | 90.9 |
| 7 | 4.5 |
| 14 | 4.6 |
| HbA1c (%), median (IQR) | 7.9 (6.9–8.8) |
| ≥8, % | 45.4 |
| ≥9, % | 21.2 |
| eGFR (ml/min/1.73 m2), median (IQR) | 88.1 (67.6–90.0) |
| G1, % | 46.7 |
| G2, % | 34.3 |
| G3, % | 17 |
| G4, % | 2 |
| CKD, % | 47.2 |
| LDL (mg/dl), median (IQR) | 87 (65–111) |
| Triglycerides (mg/dl), median (IQR) | 162 (114–233) |
| UACR (mg/g), median (IQR) | 12 (4–45) |
| A1: <30 mg/g | 66.43 |
| A2: 30–299 mg/g | 28.31 |
| A3: ≥300 mg/g | 5.17 |
| Platelets (/μl), median (IQR) | 244 (195–280) |
| AST (U/l), median (IQR) | 21 (17–31) |
| ALT (U/l), median (IQR) | 23 (16.75–37) |
| FIB-4, median (IQR) | 1.21 (0.9–1.7) |
| At risk of liver fibrosis adjusted by age, % | 30.1 |
| FIB-4 ≥2.0 and age >65 years | 10.6 |
| FIB-4 ≥1.3 and age ≤65 years | 19.5 |
| Height (m), median (IQR) | 1.65 (1.58–1.72) |
| Weight (kg), median (IQR) | 94 (84–107.7) |
| BMI (kg/m2), median (IQR) | 33.8 (31.2–38.7) |
| <30, % | 7.8 |
| 30–35, % | 50.1 |
| 35–40, % | 21.7 |
| >40, % | 20.4 |
| Systolic blood pressure (mmHg), median (IQR) | 137 (125.8–148) |
| Diastolic blood pressure (mmHg), median (IQR) | 81 (74–90) |
DPP-4: dipeptidyl peptidase-4; ARB: angiotensin receptor blocker; ACE: angiotensin-converting enzyme.
Discontinuation of oral semaglutide was observed in 13.9% and 19.1% of the subjects at 6 and 12 months of FU, respectively. Therefore, data on oral semaglutide was available for 705 and 663 subjects in the observation period, with a median FU time of 8.96 months (IQR 6–11.6).
CKD was present in 47.2% (95% CI 43.3–51.2) of the cohort. The median eGFR was 88.1 ml/min/1.73 m2 (IQR 67.6–90.0), the median UACR was 12 mg/g (IQR 4–45; n = 561), 22.8% had a UACR below the laboratory technique's detection limit and 33.5% of PWT2D had a UACR ≥30 mg/g. The median FIB-4 was 1.21 (IQR 0.9–1.7; n = 576), with 30.1% at risk of liver fibrosis adjusted for age. The mean oral semaglutide dose was 9.3 and 11.6 mg/day at 6 and 12 months of FU. The median changes in HbA1c, weight and WLP at the 6-month visit were −0.8%, −4.2 kg and −4.6%, respectively (P < .001 for all). At the 12-month FU visit, these changes were −1.0%, −6.5 kg and −7.2%, respectively (P < .001 for all).
Changes in UACR by persistent albuminuria categories
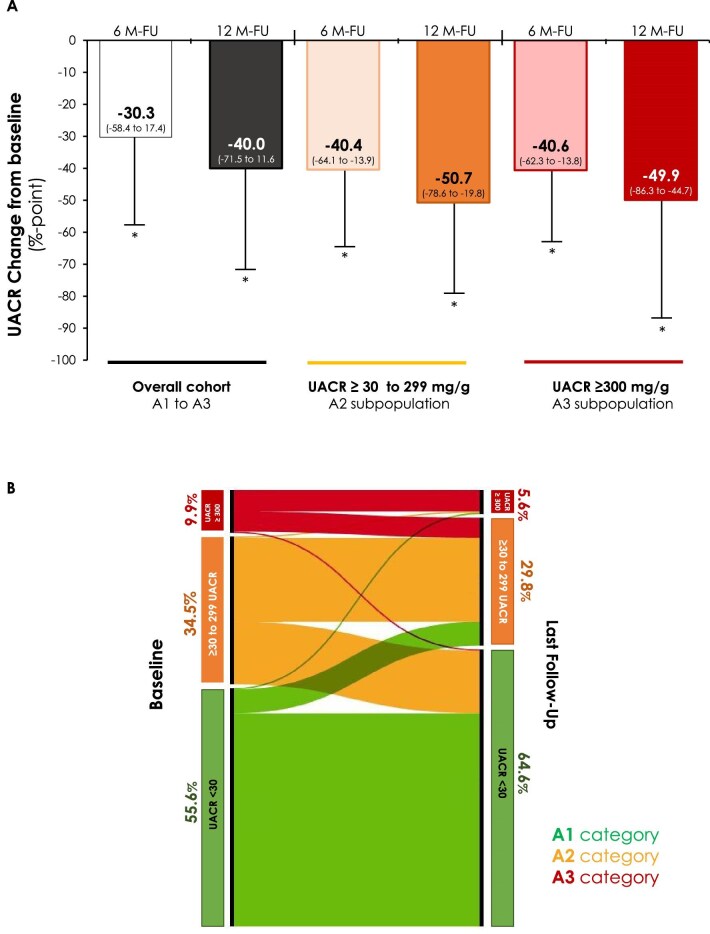
The median percentages of UACR reduction according to baseline albuminuria, as well as the changes in persistent albuminuria categories in response to oral semaglutide, are shown in Fig. 1. The UACR decreased by 30.3%, 40.0% and 40.6% at 6 months and by 40.0%, 50.7% and 49.9% at 12 months (overall cohort, category A2 and category A3, respectively).
Figure 1:
Changes in UACR from baseline at 6 and 12 months of follow-up in PWT2D undergoing treatment with oral semaglutide. (A) Median percentage change in UACR from baseline in the overall cohort and in subjects with persistent albuminuria categories A2 (30–299 mg/g) and A3 (>300 mg/g) at baseline. (B) Alluvial plot of percentage change in persistent albuminuria categories [A1 UACR<30 mg/g (green), A2 (orange) and A3 (red)] in response to oral semaglutide, considering only subjects with UACR data registered at the end of follow-up and UACR above the detection limit at baseline. *P < .05 by Mann–Whitney U test versus baseline. M: month.
In PWT2D undergoing treatment with oral semaglutide in the FU and naïve for SGLT2 inhibitor at baseline, an SGLT2 inhibitor was initiated and maintained in 92 and 82 PWT2D at 6 and 12 months of FU, respectively. Although PWT2D and UACR >30 mg/g, in whom SGLT2 was initiated and maintained, showed a greater quantitative decrease in their UACR, no significant differences in the changes in UACR were observed when the drugs were used together.
Changes in eGFR slope by GFR categories
There were no significant or clinically relevant changes in eGFR at FU, regardless of the degree of albuminuria or CKD stage by the Kidney Disease: Improving Global Outcomes criteria [20], with a median eGFR of 88.1 ml/min/1.73 m2 (IQR 68–90) and 87 ml/min/1.73 m2 (IQR 66–90) at 6 and 12 months (P = .5 for all). Baseline use of an SGLT2 inhibitor had no significant effect.
Albuminuria reduction, eGFR decrease, MAKE and associated factors
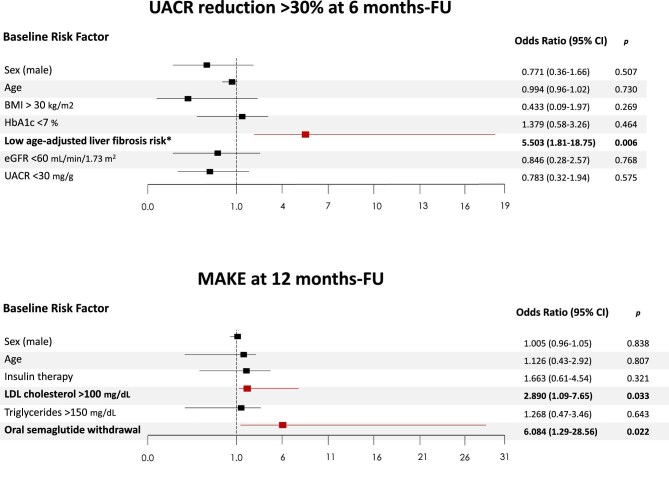
A ≥30% reduction in the UACR was achieved in 17.3% (95% CI 13.4–22.0) of PWT2D treated with oral semaglutide at the 6-month FU. Neither changes in HbA1c nor weight loss were associated with the UACR endpoint. In the multivariable analysis, PWT2D with low age-adjusted liver fibrosis risk had increased odds of achieving this outcome [aOR 5.50 (95% CI 1.6–18.7), P = .006], independent of sex, age, BMI, metabolic control or CKD (Fig. 2A). This association was not influenced by the inclusion of SGLT2 inhibitor use in the model nor at baseline [aOR 5.54 (95% CI 1.6–8.9)] or initiated during the study [aOR 5.56 (95% CI 1.6–19.0)].
Figure 2:
Independent risk factors associated with achieving a UACR reduction and MAKE. Multiple logistic regression models constructed to explore independent risk factors associated with (A) a ≥30% reduction in the UACR on oral semaglutide at the 6-month FU and (B) MAKE at the 1-year FU. The 95% CIs of the ORs have been adjusted for multiple testing. In bold, independent predictors associated with the outcomes. Those variables that reached statistical significance in univariate analysis were included in the multivariate approach. Additionally, variables such as sex or age were used in every model for adjustment since they are considered essential from a clinician’s point of view. *Liver fibrosis risk was defined by the FIB-4 index at baseline: <1.3 or 2.0 was considered as a ‘low probability of hepatic fibrosis’ in people <65 and >65 years of age, respectively.
After 12 months of FU, 19.7% (95% CI 15.0–25.4) achieved a ≥30% reduction in UACR. Multiple logistic regression models were not constructed due to missing UACR data in 25% of the sample.
In the overall cohort, 2.8% (95% CI 1.8–4.2) of PWT2D showed an eGFR decline ≥30% at 6 months and a further 1.2% (95% CI 0.5–2.7) at 12 months, while 2.6% (95% CI 1.6–4.4) and 4.7% (95% CI 2.9–7.4) experienced the first MAKE at 6 and 12 months of FU, respectively. After adjustment for the main confounders, oral semaglutide withdrawal [aOR 6.08 (95% CI 1.2–28.5)] and a baseline low-density lipoprotein (LDL) cholesterol >100 mg/dl [aOR 2.89 (95% CI 1.1–7.6)] were factors associated with an increased MAKE risk (Fig. 2B). Again, exposure to SGLT2 inhibitors did not modify these results.
Changes in HbA1c, weight loss, persistence and safety by CKD (stage 3–4 versus 1–2)
The presence of stage 3–4 CKD did not confer differences in response to oral semaglutide regarding metabolic control or weight loss.
There was no difference in the persistence of oral semaglutide in relation to more advanced stages of CKD (83.6% versus 86.6% at 6 months of FU, P = .342; 78.1% versus 81.5% at 12 months of FU, P = .322) or adverse events. Two patients died (because of a traffic accident and an unknown cause).
DISCUSSION
In this large, multicentre, real-world, nationwide retrospective cohort study in PWT2D, oral semaglutide up to a dose of 14 mg significantly reduced UACR in the medium term, with reductions of >40% in people with albuminuria, without clinically relevant changes in the eGFR slope at FU.
We also observed that, after adjustment for confounders, a >30% reduction in UACR was associated with the baseline low risk of liver fibrosis. The metabolic and weight loss effectiveness, safety and persistence of oral semaglutide were not influenced by CKD severity. Fewer than 5% of PWT2D experienced a MAKE at FU, with semaglutide withdrawal and higher LDL cholesterol linked to an increased MAKE risk.
Real-world evidence can validly complement essential evidence on medications that we gain from randomized controlled trials (RCTs) [21] by filling existing knowledge gaps on their effectiveness and safety in clinical practice [22]. The inclusion of participants with normoalbuminuria (category A1), underrepresented in the RCTs, may be questionable. However, evidence shows that even elevated UACR within the normal range is linked to higher all-cause and cardiovascular mortality across most subgroups, including those without comorbidities [23], with stronger associations in adults with high cardiovascular risk like PWT2D and ABCD [24, 25]. Therefore, assessing oral semaglutide's effect on UACR as a continuous variable across albuminuria categories is crucial in real-world populations.
The effect of subcutaneous semaglutide on UACR has been demonstrated in people with CKD and ABCD, with or without T2D. In the FLOW study, the UACR at 104 weeks was 32% lower in the subcutaneous semaglutide group than in the placebo group [6], while in the SMART study, the difference at 24 weeks was 52.1% [7].
Although it is difficult to compare our results with these studies due to significant baseline differences between them, the reduction of >30% from baseline, regardless of UACR categories, and subsequently maintained, has been associated with improved kidney and cardiovascular outcomes and is considered a valid surrogate for kidney benefit [19]. While oral semaglutide tended to reduce UACR throughout the PIONEER 5 study [8], this is the first time that the benefit of oral semaglutide on UACR has been demonstrated as a primary endpoint in routine clinical practice.
Previously, Marques et al. [13] compared oral and subcutaneous semaglutide in PWT2D with CKD, observing UACR reductions of 14% and 13.2%, respectively, but without statistical significance—likely due to the small sample size. Similarly, Lunati et al. [14] assessed dapagliflozin alone versus in combination with oral semaglutide in routine care. Both groups showed UACR improvement, with a slightly greater, though non-significant, reduction in the dapagliflozin monotherapy group.
The benefits of oral semaglutide in reducing albuminuria were independent of concomitant SGLT2 inhibitor use, consistent with the evidence for subcutaneous semaglutide and other GLP-1RAs [26, 27]. However, the high proportion of PWT2D with background SGLT2 inhibitor use, 57% compared with 15% in the FLOW and AMPLITUDE-O (NCT03496298) trials, underlines the relevance and clinical translation of our findings.
It is important to note the association between PWT2D at low risk of liver fibrosis by the FIB-4 index and UACR response. A complex combination of metabolic and haemodynamic changes, lipid nephrotoxicity and genetic predisposition may drive these putative mechanisms linking metabolic dysfunction–associated steatotic liver disease to kidney disease [4]. We hypothesise that the risk of liver fibrosis could be a biomarker to identify individuals with a worse profile of systemic metabolic risk factors that contributes to kidney oxidative stress and immune cell–driven inflammation. In this advanced context, oral semaglutide's action on GLP-1 receptors in renal and immune cells may be less effective in downregulating pro-inflammatory and pro-fibrotic mediators, potentially explaining the differing clinical responses [28].
Recently, in the ESSENCE study (NCT04822181) in people with non-cirrhotic metabolic steatohepatitis (MASH), 2.4 mg/week of subcutaneous semaglutide showed benefits in the histological resolution of MASH and improvement in liver fibrosis [29], but kidney data were not reported. Dedicated studies are warranted to assess and quantify in detail the impact of the baseline risk of liver fibrosis and the hepatic–kidney crosstalk on the renal benefits of GLP-1RAs.
The absence of other predictors of albuminuria response is in line with available exploratory mediation analyses of kidney protection with semaglutide and tirzepatide [30, 31]. Our findings on the risk factors associated with the occurrence of MAKE are consistent with the available data [32], while the increased likelihood of events associated with oral semaglutide discontinuation provides evidence of the kidney protection of this drug across the spectrum of T2DM in daily clinical practice.
This study has limitations, including UACR variability, its retrospective design without a control group, lack of adherence data and a short follow-up that may underestimate kidney events. Selection bias remains possible despite confounder adjustments. Strengths include real-world data from the largest cohort of PWT2D starting oral semaglutide in public care, with 45.8% women, and detailed analysis of kidney outcomes and MAKE. Kidney benefits were seen even with the high use of kidney–cardiovascular protective drugs like SGLT2 inhibitors. Our findings support the ongoing efforts within nephrology to implement rapid sequence up-titration of guideline-directed medical therapy for diabetic kidney disease [33].
In conclusion, the Renal_ENDO2S-RWD substudy is the largest multicentre, real-world study of oral semaglutide-treated PWT2D evaluating kidney outcomes in routine clinical practice. Treatment with oral semaglutide decreased albuminuria by >40%, without a change in the eGFR slope, regardless of background, including use of SGLT2 inhibitors. These benefits reflect the important clinical effects of oral semaglutide on kidney outcomes among moderate- and high-risk PWT2D, particularly given the reassuring safety findings, and support a therapeutic role for oral semaglutide in this population.
While the effects of oral semaglutide on kidney outcomes could not be clarified in the SOUL trial [10], our data strongly suggest the renal benefits of oral semaglutide in a broad population of PWT2D. Given the global supply chain issues for subcutaneous GLP-1RAs, the results of this study may be helpful in supporting clinical decision-making.
Supplementary Material
Contributor Information
Oscar Moreno-Pérez, Department of Endocrinology and Nutrition, General University Hospital Dr Balmis of Alicante, Institute of Health and Biomedical Research of Alicante Alicante, Spain; Department of Clinical Medicine, Miguel Hernández University, Alicante, Spain.
Rebeca Reyes-Garcia, Endocrinology Unit, University Hospital of Torrecárdenas, Almería, Spain, CIBER de Fragilidad y Envejecimiento Saludable “CIBERFES”, Instituto de Salud Carlos III, Madrid, Spain.
Cristina Guillen-Morote, Department of Endocrinology and Nutrition, General University Hospital Dr Balmis of Alicante, Institute of Health and Biomedical Research of Alicante Alicante, Spain.
Viyey Kishore Doulatram-Gamgaram, Department of Endocrinology and Nutrition, Regional University Hospital of Malaga, Biomedical Research Institute of Malaga (IBIMA), Faculty of Medicine, University of Malaga, Malaga, Spain.
Carlos Casado Cases, Department of Endocrinology and Nutrition, University Hospital Fundación Jiménez Díaz, Madrid, Spain.
Nieves Arias Mendoza, Department of Endocrinology and Nutrition, General University Hospital of Elda, Alicante, Spain.
Cristina Tejera-Pérez, Department of Endocrinology and Nutrition, University Hospital Complex of Ferrol, A Coruña, Spain.
Jersy Cárdenas-Salas, Department of Endocrinology and Nutrition, University Hospital Fundación Jiménez Díaz, Madrid, Spain.
Sandra Martínez-Fuster, Department of Endocrinology and Nutrition, General University Hospital of Elda, Alicante, Spain.
Beatriz Lardiés-Sánchez, Department of Endocrinology and Nutrition, Obispo Polanco Hospital, Teruel, Spain.
Rosa Márquez-Pardo, Department of Endocrinology and Nutrition, Hospital Juan Ramón Jiménez, Huelva, Spain.
Pedro Pinés, Department of Endocrinology and Nutrition, Albacete University Hospital Complex, Albacete, Spain.
Antonio Tejera-Muñoz, Department of Endocrinology and Nutrition, General University Hospital Dr Balmis of Alicante, Institute of Health and Biomedical Research of Alicante Alicante, Spain; Health Sciente Faculty-HM Hospitals, Camilo José Cela University, Madrid, Spain.
José Carlos Fernández-García, Department of Endocrinology and Nutrition, Regional University Hospital of Malaga, Biomedical Research Institute of Malaga (IBIMA), Faculty of Medicine, University of Malaga, Malaga, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
Inés Modrego-Pardo, Department of Endocrinology and Nutrition, Marina Baixa Hospital, Villajoyosa, Spain.
FUNDING
This publication received a grant from NOVO NORDISK to subsidise the publication costs. NOVO NORDISK has had no influence on the content of the publication and has not been involved in the design or layout. Medical writing and editorial assistance was provided by the authors and the publisher and financed by NOVO NORDISK. The authors are fully responsible for the content and conclusions reflected in the manuscript. NOVO NORDISK has not influenced the content of this publication or its interpretation. JCF-G is supported by an intensification research program \(INT24/00051, ISCIII, Spain; co-funded by the Fondo Europeo de Desarrollo Regional-FEDER\). The funding organization played no role in the design of the study, review and interpretation of the data, or final approval of the manuscript.
AUTHORS’ CONTRIBUTIONS
O.M.-P. and R.R.-G. contributed to the study design. All the authors recruited patients and collected data. O.M.-P., R.R.-G., J.C.F.-G. and A.T.-M. contributed to data analysis and interpretation. O.M.-P., R.R.-G., J.C.F.-G. and I.M.-P. wrote the manuscript. All the authors reviewed and commented on the manuscript. O.M.-P. and R.R.-G. are the guarantors of this work and take responsibility for the integrity of the data and the accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
The anonymised individual participant data will be shared upon request 3 months following article publication for researchers whose proposed use of the data has been approved by an independent review committee. Requests for data should be submitted to the corresponding author.
CONFLICT OF INTEREST STATEMENT
O.M.-P. has received honoraria as a speaker and consulting fees from Lilly, Boehringer Ingelheim, AstraZeneca and Novo Nordisk. R.R.-G. has received honoraria as a speaker and consulting fees from Lilly, AstraZeneca, Dexcom and Novo Nordisk. C.T.-P. has received honoraria as a speaker and consulting fees from Novo Nordisk, AstraZeneca, Lilly, Boehringer Ingelheim, Esteve, MSD and Abbott. J.C.F.-G. has received speaker honoraria from Novo Nordisk, Lilly, Boehringer Ingelheim, Esteve, Menarini and AstraZeneca. C.S.-J. has received speaker honoraria from Novo Nordisk, Lilly, Boehringer Ingelheim, Sanofi Aventis and AstraZeneca. L.S.-B. has received honoraria as a speaker from Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Lilly, Merck Sharp & Dohme, Sanofi and Mundipharma. P.-P. has received honoraria as a speaker from AstraZeneca, Boehringer Ingelheim, Dexcom, Lilly, Novo Nordisk and Sanofi. I.M.-P. has received speaker honoraria from Novo Nordisk, Nestle and AstraZeneca. The remaining authors declare no conflicts of interest.
REFERENCES
- 1. Koye DN, Magliano DJ, Nelson RG et al. The Global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 2018;25:121–32. 10.1053/j.ackd.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreno-Pérez O, Reyes-García R, Modrego-Pardo I et al. Are we ready for an adipocentric approach in people living with type 2 diabetes and chronic kidney disease? Clin Kidney J 2024;17:sfae039. 10.1093/ckj/sfae039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei S, Song J, Xie Y et al. Metabolic dysfunction-associated fatty liver disease can significantly increase the risk of chronic kidney disease in adults with type 2 diabetes. Diabetes Res Clin Pract 2023;197:110563. 10.1016/j.diabres.2023.110563 [DOI] [PubMed] [Google Scholar]
- 4. Bilson J, Mantovani A, Byrne CD et al. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab 2024;50:101506. [DOI] [PubMed] [Google Scholar]
- 5. Terasaka Y, Takahashi H, Amano K et al. Change in liver fibrosis associates with progress of diabetic nephropathy in patients with nonalcoholic fatty liver disease. Nutrients 2023;15:3248. 10.3390/nu15143248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perkovic V, Tuttle KR, Rossing P et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024;391:109–21. 10.1056/NEJMoa2403347 [DOI] [PubMed] [Google Scholar]
- 7. Apperloo EM, Gorriz JL, Soler MJ et al. Semaglutide in patients with overweight or obesity and chronic kidney disease without diabetes: a randomized double-blind placebo-controlled clinical trial. Nat Med 2025;31:278–85. 10.1038/s41591-024-03327-6 [DOI] [PubMed] [Google Scholar]
- 8. Mosenzon O, Blicher TM, Rosenlund S et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019;7:515–27. [DOI] [PubMed] [Google Scholar]
- 9. Marx N, Deanfield JE, Mann JFE et al. Oral semaglutide and cardiovascular outcomes in persons with type 2 diabetes, according to SGLT2i use: prespecified analyses of the SOUL randomized trial. Circulation 2025;151:1639–50. 10.1161/CIRCULATIONAHA.125.074545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGuire DK, Marx N, Mulvagh SL et al. Oral semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N Engl J Med 2025;392:2001–12. 10.1056/NEJMoa2501006 [DOI] [PubMed] [Google Scholar]
- 11. Zannad F, Berwanger O, Corda S et al. How to make cardiology clinical trials more inclusive. Nat Med 2024;30:2745–55. 10.1038/s41591-024-03273-3 [DOI] [PubMed] [Google Scholar]
- 12. Blonde L, Khunti K, Harris SB et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 2018;35:1763–74. 10.1007/s12325-018-0805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marques Vidas M, López-Sánchez P, Sánchez-Briales P et al. Efficacy and safety in a real-world study of the new oral formulation of semaglutide in patients with chronic kidney disease and type 2 diabetes mellitus. J Clin Med 2024;13:5166. 10.3390/jcm13175166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lunati ME, Cimino V, Bernasconi D et al. Type 2 diabetes mellitus pharmacological remission with dapagliflozin plus oral semaglutide. Pharmacol Res 2024;199:107040. 10.1016/j.phrs.2023.107040 [DOI] [PubMed] [Google Scholar]
- 15. Moreno-Pérez O, Reyes-Garcia R, Modrego-Pardo I et al. Real-world effectiveness and safety of oral semaglutide in people living with type 2 diabetes: a nationwide multicentre retrospective observational study (ENDO2 S-RWD). Diabetes Obes Metab 2024;26:1519–23. [DOI] [PubMed] [Google Scholar]
- 16. Marassi M, Fadini GP. Real-world evidence on oral semaglutide for the management of type 2 diabetes. A narrative review for clinical practice. Clin Ther 2025;47:102–10. 10.1016/j.clinthera.2024.11.005 [DOI] [PubMed] [Google Scholar]
- 17. Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 18. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): executive summary. Diabetologia 2024;67:2375–92. 10.1007/s00125-024-06196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Gansevoort RT, Coresh J et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104. 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024;105(4 Suppl):S117–314. 10.1016/j.kint.2023.10.018 [DOI] [PubMed] [Google Scholar]
- 21. Concato J, Corrigan-Curay J. Real-world evidence—where are we now? N Engl J Med 2022;386:1680–2. 10.1056/NEJMp2200089 [DOI] [PubMed] [Google Scholar]
- 22. Patorno E. Well-conducted real-world evidence studies can complement essential evidence from clinical trials. Circulation 2024;150:1412–5. 10.1161/CIRCULATIONAHA.124.069220 [DOI] [PubMed] [Google Scholar]
- 23. Inoue K, Streja E, Tsujimoto T et al. Urinary albumin-to-creatinine ratio within normal range and all-cause or cardiovascular mortality among U.S. adults enrolled in the NHANES during 1999–2015. Ann Epidemiol 2021;55:15–23. 10.1016/j.annepidem.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahemuti N, Zou J, Liu C et al. Urinary albumin-to-creatinine ratio in normal range, cardiovascular health, and all-cause mortality. JAMA Netw Open 2023;6:e2348333. 10.1001/jamanetworkopen.2023.48333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Claudel SE, Waikar SS, Schmidt IM et al. The relationship between low levels of albuminuria and mortality among adults without major cardiovascular risk factors. Eur J Prev Cardiol 2024;31:2046–55. 10.1093/eurjpc/zwae189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mann JFE, Rossing P, Bakris G et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med 2024;30:2849–56. 10.1038/s41591-024-03133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lam CSP, Ramasundarahettige C, Branch KRH et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose cotransporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation 2022;145:565–74. 10.1161/CIRCULATIONAHA.121.057934 [DOI] [PubMed] [Google Scholar]
- 28. Alicic RZ, Neumiller JJ, Tuttle KR. Mechanisms and clinical applications of incretin therapies for diabetes and chronic kidney disease. Curr Opin Nephrol Hypertens 2023;32:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanyal AJ, Newsome PN, Kliers I et al. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med 2025;392:2089–99. 10.1056/NEJMoa2413258 [DOI] [PubMed] [Google Scholar]
- 30. Mann JFE, Buse JB, Idorn T et al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes Metab 2021;23:2058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heerspink HJL, Sattar N, Pavo I et al. Effects of tirzepatide versus insulin glargine on cystatin C-based kidney function: a SURPASS-4 post hoc analysis. Diabetes Care 2023;46:1501–6. 10.2337/dc23-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Diabetes Association Professional Practice Committee . 11. Chronic kidney disease and risk management: standards of care in diabetes-2025. Diabetes Care 2025;48(Suppl 1):S239–51. 10.2337/dc25-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claudel SE, Verma A. Albuminuria in cardiovascular, kidney, and metabolic disorders: a state-of-the-art review. Circulation 2025;151:716–32. 10.1161/CIRCULATIONAHA.124.071079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymised individual participant data will be shared upon request 3 months following article publication for researchers whose proposed use of the data has been approved by an independent review committee. Requests for data should be submitted to the corresponding author.