Abstract
Regulation of gene transcription by the progesterone receptor (PR) in cooperation with coactivator/corepressor complexes coordinates crucial processes in female reproduction. To investigate functional relationships between PR and steroid receptor coactivators (SRCs) in distinct cell types of uterine tissue during gene transcription, we generated a new transgenic mouse model utilizing a Progesterone Receptor Activity Indicator (PRAI) system that could monitor PR activity in vivo. The PRAI system consists of a modified PR bacterial artificial chromosome (BAC) clone in which the DNA binding domain of the PR was replaced with the yeast Gal4 DNA binding domain. A humanized green fluorescent protein (hrGFP) reporter controlled by the Upstream Activating Sequences for the Gal4 gene (UASG) was inserted in tandem with the modified PR gene. Expression of hrGFP in the uterus demonstrated that the PRAI animal model faithfully replicated PR signaling under various endocrine states. Bigenic PRAI-SRC-1−/− mice revealed that SRC-1 modulates PR activity in the uterus in a cell-specific fashion and is involved in PR gene activation in stroma and myometrium of the uterus in response to estrogen and progesterone. In contrast, SRC-1 was involved in the down-regulation of PR target gene expression in the luminal and glandular epithelial compartments of the uterus after chronic progesterone treatment. Finally, we dissected the means by which SRC-1 dynamically regulates PR activity in each uterine cell compartment and demonstrated that it involves the differential ability of SRC-1 to modulate expression levels of distinct coactivators, corepressors, and PR in a cell-specific fashion.
The progesterone receptor (PR) is an essential factor in female reproduction (24). Mice in which the PR has been ablated by homologous recombination in embryonic stem cells (PR knockout mice) show defects in all aspects of female fertility (23). One of the major target organs of PR action and a major cause of the female infertility of PR knockout mice is the uterus. The uterus of PR knockout mice is unable to support embryo implantation and uterine stromal cells fail to respond to progesterone after experimental induction of the decidual response. The PR knockout uterus also shows an increase in hyperplasia of the luminal and glandular epithelial cells in response to chronic estrogen (E2) and progesterone (P4) treatment when the mitogenic action of E2 is unopposed by P4. Thus, PR plays a critical role in regulation of uterine cell proliferation and uterine function for survival of all animal species.
Transcriptional regulation of gene expression by PR is facilitated by coordinate interactions with the steroid receptor coregulators, including the SRCs/p160 coactivators (26). In the absence of ligand, PR exists in a transcriptionally inactive state and is associated with heat shock proteins and other cellular chaperones (31). After binding to P4, the receptor undergoes a conformational change, dissociates from the heat shock proteins, and then translocates to the nucleus, where it binds to progesterone response elements to induce gene expression (16, 37). PR associates with steroid receptor coactivators such as SRCs to activate transcription. Three members of the SRC/p160 family have been identified and characterized in PR-dependent transcription: SRC-1 (ERAP140/ERAP160/NcoA-1/p160) (12, 28, 29, 36), SRC-2 (TIF-2/p160/GRIP-1/NcoA-2) (7, 15, 18, 36, 38, 39), and SRC-3 (p/CIP/RAC3/AIB1/TRAM-1/ACTR) (1, 3, 20, 32, 34, 42). SRCs interact with PR and CBP/p300 to coactivate PR-dependent gene expression. Interestingly, there is a specificity of interaction between receptor and coactivator that is tightly regulated in a tissue-specific manner to activate gene transcription. For example, ligand-bound PR interacts preferentially with SRC-1 and glucocorticoid receptor interacts preferentially with SRC-2 to activate the expression of their target genes (21).
In addition to coactivators, corepressors also modulate PR activity in vivo. Nuclear receptor corepressor (NcoR) first was identified as a factor that interacts with RU486-bound PR but not agonist-bound PR (17). Silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) also binds to RU486-bound PR to antagonize its function (40). Taken together, these findings suggest that NcoR and/or SMRT complexes can be recruited to the promoter region by antagonist-bound PR to repress PR-dependent transcription.
Understanding the role of the SRC family members in regulating PR gene transcription and function in vivo has been aided by the ablation of these genes in mice (10, 43, 44). The initial analyses of phenotypes of the individual SRC genes in mice reveals that SRC-1 plays a role in modulating PR function in the uterus. Although SRC-1−/− mice were viable and fertile, further analyses revealed a significantly reduced uterine decidual response (44). These mice also showed an altered response to estrogen in the uterus as measured by the ability of estrogen treatment to increase uterine weight. Although the totality of evidence supports the fact that SRC-1 modulates the activity of both PR and estrogen receptor in the mouse uterus in vivo, no information exists to as to how SRC-1 modulates the “activity” of PR under various hormonal milieus in the multiple specific cellular compartments of the uterus. This is an especially pertinent question in terms of uterine biology since the uterus is a complex organ composed of multiple compartments, including luminal epithelium, glandular epithelium, stroma, and myometrium.
The functions of these cellular subtypes have been reported to be critically interdependent for mammalian pregnancy (9, 27, 41). PR levels change dramatically in uterine cellular compartments over the course of the menstrual cycle and in pregnancy and can be manipulated by the administration of sex steroids. Estrogen increases PR in the stroma and myometrium while decreasing PR in the luminal epithelium. Progesterone alone represses PR expression in all compartments. During implantation PR increases in the epithelium and stroma but is restricted to the stromal compartment postimplantation. Since known approaches were unsuitable to accurately assess cell-specific functions of PR in uterine compartments, we designed a genetically engineered PR activity indicator (PRAI) mouse to assay the transcriptional activity of the PR in specific cell subtypes under various hormonal conditions and in SRC-1 null mice.
The PRAI mouse model employs a bacterial artificial chromosome (BAC) containing a modified PR gene and a responsive reporter gene. A BAC approach is advantageous since it utilizes large fragments of genomic DNA with an average of 100 to 300 kb in size that can be cloned in bacterial vectors and stably propagated in bacteria. The large size of BAC clones permits faithful direct tissue-specific expression of heterologous genes in vivo in BAC-transgenic mice if the appropriate regulatory sequences of the gene are present (11, 13). Also, modification of BAC clones (i.e., single-base changes, deletions, and insertions) can be introduced easily by homologous recombination using the appropriate bacterial strain DY380 cells (8, 46).
MATERIALS AND METHODS
PR BAC clone modification.
To replace the PR DNA-binding domain (DBD) in the PR BAC clone with the Gal4 DBD (147 amino acids), the 5′ homologous region -Gal4 DBD including splicing junction -FRT-kanamycin resistance gene (Kanr) -FRT-3′ homologous region targeting cassette was constructed (Fig. 1A and 1B). The 57 bp of intron 1 and 62 bp of exon 2 region were isolated by PCR with primers PR51 (5′-AGGTACACTGCTATCTGATGAAGAAAG-3′) and PR52 (5′-GATCTTCTGAGGTAAGGA-3′) from the mouse genome to generate the 5′-end homologous region. The splicing junction region in between exon 3 and intron 3 (12 bp of exon 3 and 14 bp of intron 3 region) was attached into the C terminus of Gal4 DBD region for the splicing. The 120 bp of intron 3, closely located to the exon 3-intron 3 splicing junction region, was isolated by PCR with primers PR31 (5′-TTGCACTTTACTATAACACTGAGTC-3′) and PR32 (5′-CTTAGTCTTGCTAAGATTGGATGTCC-3′) to use as 3′-end homologous region. DY380 cells carrying the PR BAC clone (RPCI23 422 I 15) were electroporated with Gal4 DBD targeting cassette (19, 47). Electroporation was performed by using a Bio-Rad Micropluser set at 1.8 kV/cm (Ec1).
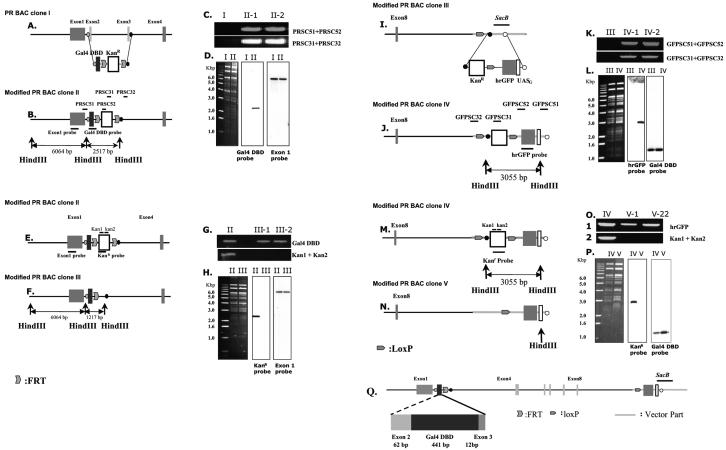
FIG. 1.
Modification of PR BAC clone. (A) The structure of PR BAC clone I. (B) Recombinant PR BAC clone II. PR DBD region in PR BAC clone I was replaced with Gal4 DBD-FRT-kanamycin resistance gene (Kanr) -FRT cassette by using bacterial recombination system in the DY380 strain. (C) Colony PCR results showing the integration. Colonies carrying recombinant PR BAC clone II (II-1 and II-2) were PCR amplified with primers that identified integration at either 5′-end site (PRSC51 and PRSC52) or 3′-end site (PRSC31 and PRSC32). (D) Southern blot analysis with HindIII-digested BAC clones showed the fidelity of recombination processing. (E) The structure of recombinant PR BAC II. (F) The Kanr gene in recombinant PR BAC clone II was excised by Flp recombination in a 294-FLP strain. (G) Colony PCR results showing the Kanr gene excision. Colonies carrying recombinant PR BAC clone III (III-1 and III-2) were PCR amplified with primers for Gal4 DBD region but not with primers for Kanr gene (Kan1 and Kan 2). (H) Southern blot analysis with HindIII-digested BAC clones showed the Kanr gene excision. (I) Recombinant PR BAC III. (J) The hrGFP reporter cassette, Kanr gene-loxP-hrGFP-minimal promoter-five copies of UASG, was inserted into the sacB gene region in recombinant PR BAC clone III by using bacterial recombination in DY380. (K) Colony PCR results showing the integration. Colonies carrying recombinant PR BAC clone IV (IV-1 and IV-2) were PCR amplified with primers that identified integration either 5′-end site (GFPSC51 and GFPSC52) or 3′-end site (GFPSC31 and PRSC32). (L) Southern blot analysis with HindIII-digested BAC clones showed the fidelity of recombination processing. (M) Recombinant PR BAC IV. (N) The Kanr gene in recombinant PR BAC clone IV was excised by Cre recombination in the 294-Cre strain. (O) Colony PCR results showing the Kanr gene excision. Colonies carrying recombinant PR BAC clone V (V-1 and V-2) were PCR amplified with primers for hrGFP region but not with primers for Kanr gene (Kan1 and Kan 2). (P) Southern blot analysis with HindIII-digested BAC clones showed the Kanr gene excision. (Q) The final modified PR BAC clone containing Gal4 DBD and hrGFP reporter system.
After electroporation, cells were incubated in 1 ml of SOC medium for 1 h and spread onto LB agar plates containing chloramphenicol (12.5 μg/ml), tetracycline (10 μg/ml), and kanamycin (10 μg/ml). Colonies were allowed to form over 24 h at 30°C to isolate chloramphenicol/tetracycline/kanamycin-resistant colonies. PCR was performed by using primers designated to generate products verifying the homologous recombination.
PRSC51 (5′-TCCATATCAAAGCCTGGCCTTGAAC-3′) binds the intron 1 region located outside of targeting region and PRSC52 (5′-CGATACAGTCAACTGTCTTTGA-3′) binds Gal4 DBD region; they were used to verify the homologous recombination at the 5′-region (Fig. 1C). The primers PRSC31 (5′-CAGTTCATTCAGGGCACCGGACAGGTCGGTC-3′), which binds in the Kanr gene, and PRSC32 (5′-GTCTCTGACTCTGCCATTGTATTCC-3′), which binds in the intron 3 region (located outside of targeting region), were used to verify homologous recombination at the 3′ region (Fig. 1C).
To confirm insertion of Gal4 DBD, the HindIII-digested BAC DNA was examined by Southern analysis. A radiolabeled probe specific to Gal4 DBD and exon 1 were hybridized to the Southern blot (Fig. 1D). To remove the Kanr gene by Flp recombination, the modified BAC clone was electroporated into 294-FLP (5, 19) (Fig. 1E and 1F). After incubating in SOC medium for 1 h, cells were spread onto LB/agar plates containing chloramphenicol and tetracycline. Colonies were allowed to form over 24 h at 30°C to isolate chloramphenicol/tetracycline resistance colonies. PCR was performed using two Kanr primers, kan1 (5′-CAGTTCATTCAGGGCACCGGACAGGTCGGTC-3′) and kan2 (5′-ACTGTCAGACCAAG-3′), were used to verify excision of the Kanr gene (Fig. 1G). To confirm excision of Kanr gene, the HindIII-digested BAC DNA was examined by Southern analysis. A radiolabeled probe specific to Kanr gene and exon1 were hybridized to the Southern blot (Fig. 1H).
The humanized green fluorescent protein (hrGFP) reporter cassette, 5′ homologous region-hrGFP-minimal promoter-five copies of the upstream activation sequence (UAS) for the Gal4 gene (UASG)-loxP-Kanr gene-3′ homologous region, was constructed (Fig. 1I and 1J). The 200 bp of sacB gene which is in the BAC plasmid was isolated by PCR with primers sacb51 (5′-AGGAGCGACTCAAGCCTTCGCGAA-3′) and scab52 (5′-CGGCCAGCTGTCCCACACATC-3′) to generate the 5′-homologous recombination region; the other 200 bp of sacB gene was isolated by PCR with primers sacb31 (5′-TGTGCTGCAAATGGGTCTTGA-3′) and scab32 (5′-GCCAAGCTTCTTAATGAACATCA-3′) to generate the 3′-homologous recombination region.
DY380 cells carrying a modified Gal4-PR BAC clone were electroporated with hrGFP reporter targeting cassette (19, 47). After electroporation, cells were incubated in 1 ml of SOC medium for 1 h and spread onto LB/agar plates containing chloramphenicol, tetracycline, and kanamycin. Colonies were allowed to form over 24 h at 30°C to isolate chloramphenicol/tetracycline/kanamycin-resistant colonies. PCR was performed using primers designated to generate products verifying the homologous recombination. Both GFPSC51 (5′-GACTGCACTTCTGGCAGGAGG-3′), which binds outside of the 5′-homologous region of the sacB gene, and GFPSC52 (5′-ACTGTTGGTAAAGCCACCATGGTGAGCAAGCAGATCCTG-3′) which binds in the hrGFP gene were used to verify the homologous recombination in the 5′-region (Fig. 1K). Other primers, GFPSC31 (5′-CTGCTCTGATGCCGCCGTGTTCCGGCTGTC-3′), which binds in the Kanr gene, and GFPSC32 (5′-CAGCTGTCCTTGCTCCAGGAT-3′), which binds outside of the 3′-homologous region of the sacB gene, were used to verify the homologous recombination at 3′ region (Fig. 1K).
To confirm insertion of hrGFP reporter, the HindIII-digested BAC DNA was examined by Southern analysis. A radiolabeled probe specific to hrGFP and Gal4 DBD was hybridized to the Southern blot (Fig. 1L). To remove the Kanr gene by Cre recombination, the modified BAC clone was electroporated into a 294-CRE strain (5, 19) (Fig. 1M and N). After incubating in SOC medium for 1 h, cells were spread onto LB/agar plates containing chloramphenicol and tetracycline. Colonies were allowed to form over 24 h at 30°C to isolate chloramphenicol/tetracycline resistance colonies. PCR was performed using the two primers, kan1 and kan2 to verify excision of Kanr gene (Fig. 1O). To confirm excision of Kanr gene, the HindIII-digested BAC DNA was examined by Southern analysis. A radiolabeled probe specific to Kanr gene and Gal4 DBD were hybridized to the Southern blot (Fig. 1P).
Generation of progesterone receptor activity indicator transgenic mice.
The final modified PR BAC clone was purified by using ClonTech BAC purification kit. After digestion with PI-SceI restriction enzyme at 37°C overnight, the linearized BAC DNA was dialyzed against microinjection buffer (10 mM Tris-Cl, pH 7.5, 0.1 mM EDTA, and 100 mM NaCl) at 4°C for 4 h. The linearized BAC fragment was injected at a concentration of 1 ng/μl into fertilized mouse oocytes. Oocytes that survived injection were transferred into both oviducts of pseudo-pregnant mothers.
Screening of transgenic founder mice by using PCR and Southern blot analysis.
Transgenic mice were identified both by PCR and southern blot analysis with genomic DNA isolated from tail biopsies. To screen transgenic founder mice the following sets of primer were used: 5′-ATGAAGCTACTGTCTTCTATCG-3′ and 5′-CGATACAGTCAACTGTCTTTGA-3′ (amplified a 440-bp PCR fragment in gal4DBD region); 5′-AATAGGCTGTCCCCAGTGCAAGT-3′ and 5′-ATGGTCTTCTTCATCACGGGGC-3′(amplified a 707-bp PCR fragment in hrGFP region). For the Southern blot analyses, 10 μg of genomic DNA was isolated from tail biopsies digested with SacI, and then size-fractionated in a 1% agarose gel in Tris-acetate-EDTA buffer. The Gal4 DBD region was used as a hybridization probe to identify transgene in founder mice (Fig. 2A).
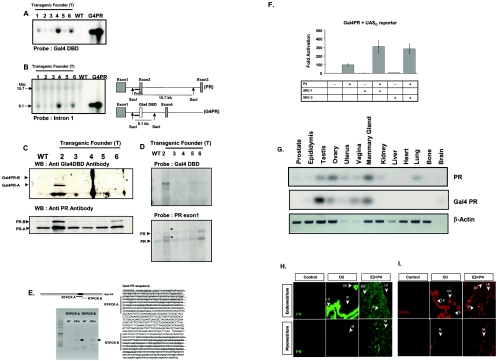
FIG. 2.
Generation of progesterone receptor activity indicator mice. (A) Genomic DNA was isolated from tail biopsies of six transgenic founders (T1, T2, T3, T4, T5, and T6) and nontransgenic (WT) mice. Genomic DNA (10 μg) and 10 or 50 ng of final modified Gal4 PR BAC clone V (G4PR, Fig. 1Q) were digested with SacI. Digested DNA was size-fractionated on a 1% agarose gel and then hybridized with the Gal4 DBD probe. (B) To determine the copy number of the BAC transgene in mice, Southern blot analyses was done with an intron 1 probe; 15.7 kb of hybridized signal was endogenous PR locus and 6.1 kb of hybridized signal was modified PR BAC locus in PRAI mice. (C) The protein level of Gal4 PR and endogenous PR in the uterus of PRAI mice were determined by Western blot analysis with antibody against Gal4 DBD and PR, respectively. (D) The mRNA level of Gal4 PR and endogenous PR in the uterus was also detected by Northern blot analysis with Gal4 DBD and PR exon 1 probe. Additional hybridized signals marked with * in the bottom panel are mRNA of Gal4 PR. (E) Sequence of Gal4 PR. To investigate the in-frame fusion of Gal4 DBD to the PR transcript, RT-PCR was carried out from uterine total RNA of wild-type and PRAI mice with two primer sets designed to amplify 5′ (RT-PCR A) and 3′ (RT-CPR B) ends of Gal4 DBD fusion to the PR transcript. Only specific RT-PCR products marked with arrows were detected from PRAI mice. PR sequence was written as lowercase and Gal4 DBD sequence was written as uppercase in the Gal4 PR cDNA sequence shown. Each RT-PCR product sequence is underlined. (F) HeLa cells were transfected by Lipofectamine according to the manufacturer's protocol (Life Technologies, Inc., Gaithersburg, MD) with UASG reporter plasmid, Gal4 PR expression vector (0.1 μg/ml), the vector PCR3.1-SRC-1 (0.3 μg/ml), and the vector PCR3.1-SRC-3 (0.3 μg/ml). After transfection, medium containing 10−7 M progesterone (P4) or vehicle was added. Cells were harvested after 48 h and assayed for luciferase activity. Luciferase activities were corrected for protein content and determined using the protein assay according to the manufacturer's protocol (Bio-Rad Laboratories, Inc., Hercules, CA). (G) To determine the tissue-specific expression both of PR and Gal4 PR in PRAI mice, total RNA was isolated from various tissues of PRAI mice. The mRNA level of each PR was amplified by low cycle (20 cycles) of RT-PCR and amplified signals were determined by Southern blot analysis with each probe. The mRNA level of each PR in various tissues was normalized by β-actin. (H and I) Two weeks after ovariectomy, daily doses of oil or estrogen (13 pmol/kg) plus progesterone (12 μmol/kg) were given from Day 1 to 3. (H) PR expression in the uterus was visualized under the fluorescein isothiocyanate excitation. The rabbit immunoglobulin was used in control experiments instead of anti-PR antibody. (I) Gal4 PR (G4PR) expression was visualized under Texas Red excitation. Uterine sections from nontransgenic mice were used as control groups to confirm specificity of the anti-Gal4 DBD antibody.
RT-PCR.
The first-strand cDNA was synthesized from 5 μg of total RNA by using SuperScriptsII reverse transcription RT kit (Invitrogen) and 250 ng of random primer according to its protocol. The PCR primer pairs were as followed: 5′-GTCCCGCCACTCATCAACCT-3′ and 5′-GGGCAACTGGGCAGCAATAACT-3′ (amplified a 705-bp fragment from PR); 5′-TAAAGATGCCGTCACAGATAGATT-3′ and 5′-ACCCCAGGCCAAACACCAT-3′ (amplified a 530-bp fragment from Gal4 PR); 5′-GCTTGGCATTCCGGTACTGT-3′ and 5′-GGTTGCCGAACAGGATGTTG-3′ (amplified a 120-bp fragment from hrGFP); 5′-TATTACGAGTCCAAGGCCCA-3′ and 5′-TAAGCACATCACTGAAGGTGG-3′ (amplified a 199-bp fragment from Indian Hedgehog); 5′-TGAACCAGTTTGCAGCAAATG-3′ and 5′-TGTTTCCCATTGTTCCTTGC-3′ (amplified 648-bp fragment from SRC-1); 5′-GCATGGGTCGGGACAAGAAGA-3′ and 5′-CTCCAGCAGGGGGCACCACT-3′ (amplified a 599-bp fragment from beta-actin). The PCR products were analyzed on a 1.5% agarose gel in Tris-acetate-EDTA buffer.
Immunofluorescence.
Mice were anesthetized with Avertin and perfused through the heart with 4% paraformaldehyde in phosphate-buffered saline. The uterus was removed and kept in the same fixative for 16 h at 4°C. Samples were dehydrated in ethanol series, cleared in xylene, and embedded in paraffin. Sections were cut at 7 μm. For immunostaining, sections were dewaxed, rehydrated, and boiled for 10 min in 10 mM citrate buffer, pH 6.0. To reduce nonspecific binding of antibodies, sections were washed in phosphate-buffered saline again and preincubated with 500 μg/ml goat IgG and 5% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature. Anti-hrGFP antibody (1:300; Stratagene), anti-PR antibody (1:300; Santa Cruz), anti-Gal4 DBD antibody (1:50; Santa Cruz), anti- Indian hedgehog (IHH) antibody (1:100; Santa Cruz), anti-SRC-1 antibody (1:300; Santa Cruz), anti-SRC-2 antibody (1:500; gifted from Jun Qin), anti-SRC-3 antibody (1:300; Santa Cruz), anti-NcoR antibody (1:500 gifted from Jiemin Wong), and anti-SMRT antibody (1:500 gifted from Jiemin Wong) were used to detect expression of each protein in tissues. Sections then were incubated sequentially with biotinylated horse anti-rabbit immunoglobulin G (1:500), and fluorescein isothiocyanate- or Texas Red-conjugated streptavidin (1:1000; Vector Laboratories).
To measure fluorescence intensity in each compartment of the uterus, capture levels were first adjusted so as to avoid saturation and then kept constant throughout experiments. For each compartment of the uterus examined, a box was drawn through each compartment in a random orientation. The pixel intensity value of each box (in arbitrary units) was measured and a mean value was obtained. Values shown in all figures are a mean value of intensity (n = 3) ± standard deviation. To normalize fluorescence intensity of each compartment we used fluorescence intensity from normal serum. Relative Fluorescence Intensity (RFI) means the ratio of specific antibody fluorescence intensity to normal serum fluorescence intensity in each compartment of the uterus.
In situ hybridization.
The protocol for in situ hybridization was essentially as described (2). PCR primers were designed and synthesized on the basis of reported mouse cDNA sequences. IL-13ra2 primers were 5′-GCTTGGATCAT GCCTTACAGTG-3′ and 5′-TAATACGACTCACTATAGGGTCGCTCCAAATTCC ATCATCT −3′. Mig-6 primers were 5′-GGCCTCCAGAATGAAAATAGATA-3′ and 5′-TAATACGACTCACTATAGGGCTGTTTTGGGGTCTCGCTGTTCAT-3′. [35S]UTP riboprobes were generated by in vitro transcription of amplified DNA products containing the T7 polymerase promoter sequence flanking the desired nucleotide primer sequence. 35S-labeled cRNA probes were generated for Mig-6 and IL13ra2 with T7 polymerase.
RESULTS
Generation and characterization of a PR activity indicator mouse model.
Gal4 PR, containing the DNA binding domain of the yeast Gal4 transcription factor instead of its own DBD, activated UASG reporter expression in a P4-dependent manner and responded to SRC-1 or SRC-3 similar to endogenous PR (Fig. 2F). These data support our contention that Gal4 PR is functionally equivalent to endogenous PR. In the PRAI model, the DBD of the PR gene is exchanged with the DBD of the yeast Gal4 transcription factor thus generating a mutant PR that should only activate GAL4 responsive genes upon activation by P4. The BAC transgenic mice contained a Gal4 responsive reporter gene in tandem with the mutant PR. The reporter gene consisted of the humanized green fluorescent protein, hrGFP, under the control of a minimal promoter with the enhancer elements for the upstream activating sequences for the Gal4 UASG. This reporter gene was inserted into the BAC clone using the bacterial recombination system and activity of the transgenic PR was monitored by assaying the expression of the hrGFP reporter gene.
We first determined the fidelity of the PRAI model to mimic PR activity in vivo under various endocrine states and prior to investigating the involvement of SRC-1 in functional modulation of PR activity in vivo. We used a RPCI-23-422 I 15 PR BAC clone containing 62 kb of genomic DNA flanking 5′ to exon 1 and 70 kb of genomic DNA flanking 3′ to exon 8 of the PR gene. Using bacterial recombination, the PR DBD located from exon2 to exon 3 in the PR gene was replaced with the Gal4 DBD generating the mutant PR, Gal4-PR. A second modification of this BAC clone included the incorporation of a reporter system consisting of five copies of UASG binding sites, a minimal promoter, and an hrGFP reporter gene (Fig. 1).
Transgenic mice were generated from linear 200-kb fragments of the modified PR BAC clone V (Fig. 1Q). Both Northern and Western blot analyses showed that Gal4 PR was expressed only in line 2 (Fig. 2C and 2D). The cDNA sequences of the 5′ and 3′ ends of Gal4 DBD fusions to the PR transcripts in Gal4 PR showed that Gal4 DBD was fused in frame to the PR transcript in line 2 (Fig. 2E). Southern blot analysis with an intron 1 probe showed that ∼2 copies of BAC transgene are present in our mice (line 2) (Fig. 2B). We used heterozygous transgenic mice (Tg/+) of line 2 as PRAI mice in our study.
The fidelity of the tissue-specific expression of the Gal4-PR transgene compared to the endogenous PR gene was determined by semiquantitative RT-PCR analysis of total RNA isolated from various tissues of transgenic mice using probes specific for the Gal4 DBD and PR DBD. The expression profile of Gal4-PR of a PRAI mouse was similar to the endogenous PR with the exception of the lungs and kidneys, in which low expression of the endogenous PR could be detected but not that of the Gal4-PR (Fig. 2G). This indicates that 62 kb of 5′- and 70 kb of 3′-flanking regions in the modified PR BAC clone were sufficient to promote tissue-specific expression Gal4-PR in male and female reproductive organs.
The uterus is a multicompartmental organ consisting of the endometrium and the myometrium. The endometrium can be further subdivided into the luminal epithelium, glandular epithelium, and stroma. PR expression in each compartment is dynamic and is thought to change in response to physiologic state and endocrine status (6, 35). In order to ensure that the compartment-specific expression of the Gal4-PR faithfully reflected the expression pattern of the endogenous PR, immunohistochemical analysis was conducted. Nontransgenic and PRAI mice were ovariectomized and treated with oil or estrogen plus progesterone (E2+P4). Histological sections of the uterus were immunostained with antibodies specific to PR and to Gal4 DBD. The results are shown in Fig. 2H and 2I.
Endogenous PR was expressed only in luminal epithelium and glandular epithelium uterine compartments of untreated animals (Fig. 2H). However, PR expression in each compartment of the uterus was observed upon treatment with E2+P4. PR expression was reduced in both luminal epithelium and glandular epithelium compartments but increased in the stroma and myometrium compartments by E2+P4 treatment (Fig. 2H). In concert with the expression of the endogenous PR gene in nontransgenic mice, the Gal4 PR also was expressed only in luminal epithelium and glandular epithelium compartments in the uteri of ovariectomized mice treated with oil, and its expression was enhanced in the stroma compartment but reduced in both luminal epithelium and glandular epithelium compartments following E2+P4 treatment (Fig. 2I). Therefore, Gal4 PR expression reflected the expression pattern of the endogenous PR in each uterine compartment under the endocrine states tested. Taken together, our results indicate that tissue specific and compartment specific expression of Gal4-PR faithfully replicates endogenous PR in the mouse uterus.
Differential PR activity in the uteri of PRAI mice occurs in response to acute versus chronic P4 treatment.
The ability of Gal4 PR to activate the hrGFP reporter expression in response to acute and chronic P4 treatment in the uterus of PRAI transgenic mice was investigated. Ovariectomized mice were injected with P4 (12 μmol/kg) daily and sacrificed 6 h after the first injection (acute treatment) or 6 h after the third injection (chronic treatment). The uteri were removed from these mice and assayed for hrGFP expression by RT-PCR and immunohistochemical analyses. As shown in Fig. 3A (middle panel), acute treatment with P4 induced hrGFP expression in the uterus within 6 h. Chronic P4 treatment revealed a reduced uterine hrGFP expression compared with the acute progesterone treatment (Fig. 3A). To confirm that the P4-mediated hrGFP expression in the transgenic PRAI mimicked endogenous PR activity, we measured the expression of a known P4 target gene, Indian hedgehog (25, 33). Acute P4 treatment induced hrGFP expression to a greater extent than that of the chronic P4 treatment (Fig. 3A), mimicking the changes in IHH expression, again substantiating that the PRAI model is a faithful indicator of PR activity in the mouse uterus.
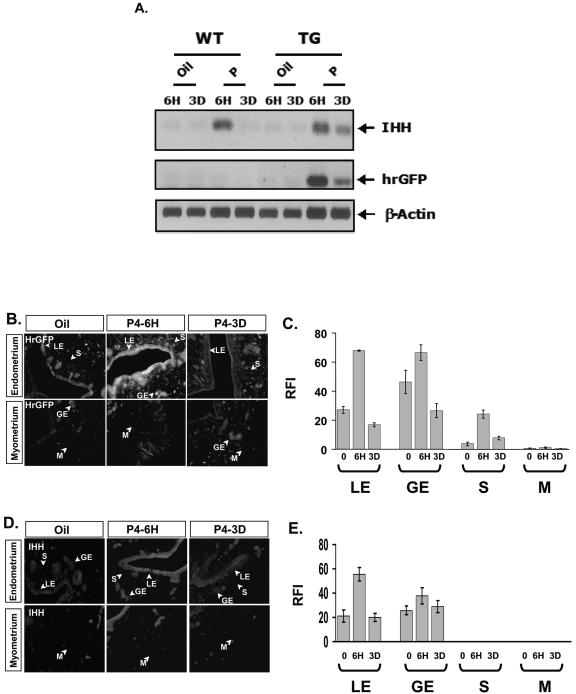
FIG. 3.
PR activities change dynamically in the uterus in response to P4. Two weeks after ovariectomy, daily dose of oil or P4 (12 μmol/kg) were given to wild-type (WT) and PRAI mice (TG) from days 1 to 3. To determine the response to acute progesterone effect on PR activity in the uterus mice were killed 6 h (6H) after injection to harvest the uterus. For the chronic progesterone effect on PR activity mice were killed 6 h after the last injection (3D). (A) Total uterine RNA was pooled from three mice used for each group. The mRNA level of hrGPF or Indian hedgehog (IHH) was determined by RT-PCR. The β-actin signal was used for normalization. (B) hrGFP protein expression in the uterus in response to P4 treatment was examined by immunofluorescence. (C) Graph showing the RFI value of hrGFP in each compartment of the uterus, described in Materials and Methods. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium. (D) IHH expression in each compartment of the uterus in response to P4 treatment was examined by immunofluorescence. (E) Graph showing the RFI value of IHH in each compartment of the uterus.
The above RT-PCR analyses demonstrated a progesterone-induced change in total tissue mRNA. In order to investigate transcriptional PR activity within the specific uterine compartments in response to P4, transgenic hrGFP expression was assessed by immunofluorescence analysis using anti-hrGFP antibody. Acute P4 treatment induced hrGFP expression in luminal epithelium, glandular epithelium, and subepithelial stroma compartments of the uterus but not in the myometrium compartment (Fig. 3B and 3C). However, chronic P4 (3 days) treatment did not result in hrGFP expression in luminal epithelium, glandular epithelium, and stroma compartments of the uterus compared with acute P4 treatment (Fig. 3B and 3C). These immunofluorescence analyses for the hrGFP closely corresponded with total uterine hrGFP mRNA expression determined by RT-PCR (Fig. 3A).
Endogenous IHH expression in luminal epithelium and glandular epithelium compartments of the uterus also was induced by P4 within 6 h, but IHH expression was repressed to control levels with chronic P4 treatment (Fig. 3D and 3E). IHH was not expressed in stroma compartment of the uterus in response to acute P4 treatment (33). However, IL13ra2 and Mig-6, recently identified PR target genes in the uterus, were induced by acute P4 treatment in stroma compartment of the uterus (J. Jeong and F. J. DeMayo, unpublished data). Thus, compartment-specific changes in hrGFP reporter expression mediated by P4 were coincident with changes in endogenous target genes of PR (IHH, IL13ra2, and Mig-6) in all cellular compartments.
Chronic progesterone treatment regulates PR levels and coregulator expression to modulate PR activity.
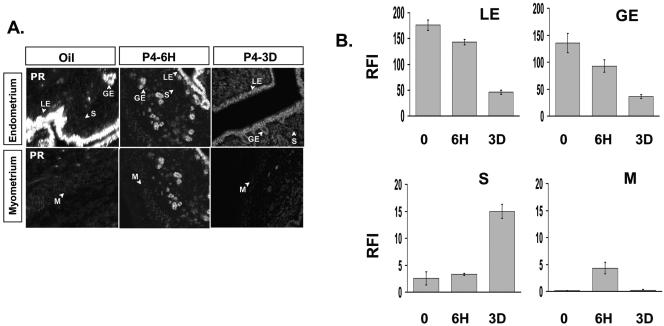
In order to investigate how chronic P4 treatment reduces PR activity compared to acute P4 treatment, we investigated the expression of PR in the uterus in response to P4 treatment by immunohistochemical analysis. The results of this analysis are shown in Fig. 4. PR expression in luminal epithelium and glandular epithelium compartments was reduced by chronic P4 treatment compared with oil. PR expression in the stroma compartment of the uterus was elevated (Fig. 4A and 4B). Thus, reduction of PR activity in luminal epithelium and glandular epithelium in response to chronic P4 treatment could be explained by reduced PR expression. However, although PR expression in the stroma was greatly enhanced, chronic P4 treatment resulted in very little increase in PR transcriptional activity in this compartment.
FIG. 4.
Progesterone regulation of PR expression in a cell compartment specific manner. (A) PR expression in each compartment of the uterus in response of vehicle (oil), acute P4 treatment (6 hours), or chronic P4 treatment (3 days) was examined by immunofluorescence. (B) Graph showing the RFI value of PR in each compartment of the uterus. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
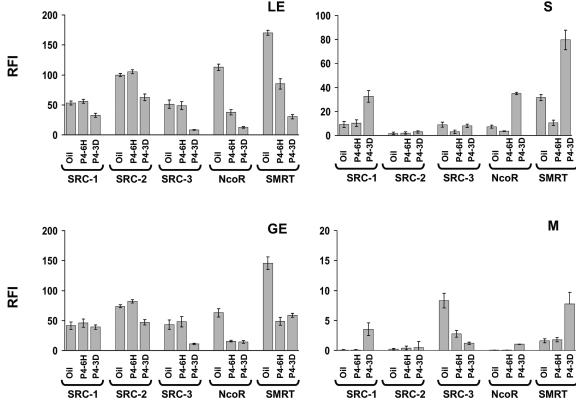
In order to investigate this apparent paradox, the expression of the primary PR coactivators: SRC-1, SRC-2, and SRC-3 as well as its corepressors (NcoR and SMRT) were measured by immunofluorescence. The results of these analyses are summarized in Fig. 5. In the stroma compartment, chronic P4 treatment significantly increased NcoR and SMRT levels compared with coactivator expression (Fig. 5). Consequently, significantly increased corepressor expression could counteract the increased PR expression occurring in the stroma to prevent enhancement of PR activity under chronic P4 conditions. In addition, decreased coactivator expression in luminal epithelium and glandular epithelium is likely to contribute to the lack of PR activity in these compartments during chronic P4 treatment.
FIG. 5.
Progesterone regulation of the expression levels of PR and coregulators. Expression of PR coregulators (SRC-1, SRC-2, SRC-3, NcoR, and SMRT) in each compartment of the uterus in response to acute (6 hours) or chronic (3 days) P4 treatment was examined by immunofluorescence. RFI values of PR coregulators in each compartment of the uterus are shown in the graphs. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
Modulation of PR activity in the uterus of PRAI mice in response to chronic E2 and E2+P4 treatment.
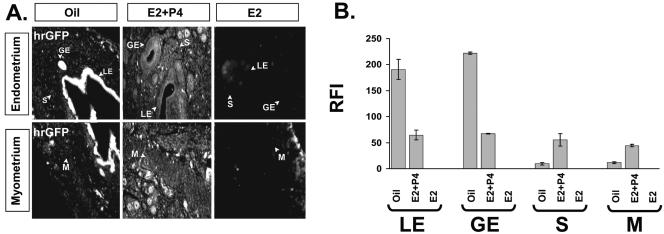
The PR expression pattern in uterine cellular compartments can be regulated by E2 (35). In order to investigate how PR activity was modulated in different compartments of the uterus in response to its physiologic steroid hormones, ovariectomized mice were treated with E2 (13 pmol/kg) or a combined treatment of E2 (13 pmol/kg) plus P4 (12 μmol/kg) for 3 days and PR activity was assayed by immunohistochemical analysis of hrGFP as described above. In the presence of only E2, hrGFP reporter expression was undetectable in the uterus (Fig. 6A and 6B), demonstrating the progestin-dependent specificity of the PRAI mouse transgenic reporter. However, in the presence of E2+P4, hrGFP expression was observed. Interestingly, the PR activity pattern in the uterus after E2+P4 treatment (Fig. 6) was quite different from PR activity induced by chronic P4 treatment (Fig. 3). PR activity was reduced in the luminal epithelium and glandular epithelium compartments compared with mice treated with oil (Fig. 6A and 6B). In contrast, PR activities in the stroma and myometrium compartments were increased (Fig. 6A and 6B). These data clearly demonstrate that E2+P4 treatment regulates PR target gene activity in a compartmental-specific fashion in the uterus.
FIG. 6.
Chronic estrogen plus progesterone treatment induced PR activity in stroma and myometrium compartments of the uterus. Two weeks after ovariectomy, daily doses of oil, E2 (13 pmol/kg), or E2 (13 pmol/kg) plus P4 (12 μmol/kg) were given to PRAI mice for 3 days. For determining the chronic E2 or E2+P4 effect on PR activity, mice were killed 6 h after the last injection to harvest the uterus. (A) hrGFP protein expression was examined by immunofluorescence. (B) Graph showing the RFI value of hrGFP in each compartment of the uterus under different hormone states. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
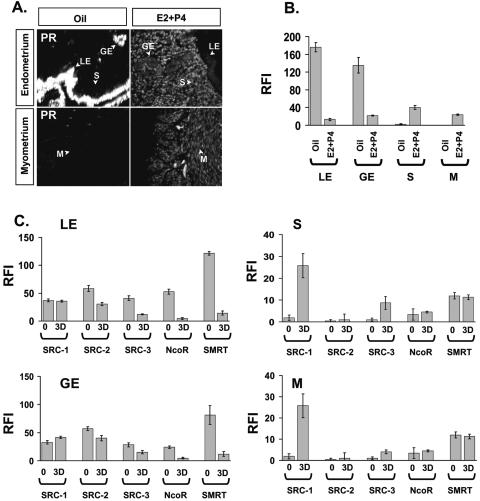
The PRAI mouse was useful to quantify compartmental changes in PR activity in the uterus in response to chronic E2+P4 treatment. We wished to measure the relative expressions of PR, coactivators, and corepressors in the luminal epithelium and glandular epithelium compartments of E2+P4 uteri in order to understand the mechanism of the reduced PR activity in these compartments (Fig. 7). Chronic E2+P4 treatment drastically reduced the expression of PR. Thus, reduction of PR activity in the luminal epithelium mediated by E2+P4 may be due to suppression of PR levels (Fig. 7A and 7B). In contrast to the reduction of PR expression in the luminal epithelium compartment, PR, SRC-1 and SCR-3 levels in the stroma and myometrium compartments were increased following chronic E2+P4 treatment (Fig. 7C, right panels).
FIG. 7.
PR and coregulator expressions in the uterus in response to E2 plus P4. Two weeks after ovariectomy, daily doses of oil or E2 (13 pmol/kg) plus P4 (12 μmol/kg) were given to PRAI mice for 3 days (3D). For the chronic E2+P4 treatment effect on PR activity mice were killed 6 h after the last injection to harvest the uterus. (A) PR expression in each compartment of the uterus in response to chronic E2+P4 treatment was examined by immunofluorescence. (B) RFI value of PR in each compartment is shown in a graph. (C) RFI values of all PR coregulators (SRC-1, SRC-2, SRC-3, NcoR, and SMRT) in each compartment of the uterus in response to chronic E2+P4 treatment examined by immunofluorescence. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
SRC-1 mediates suppression of PR activity in luminal epithelium and glandular epithelium compartments of the uterus in response to chronic P4 treatment.
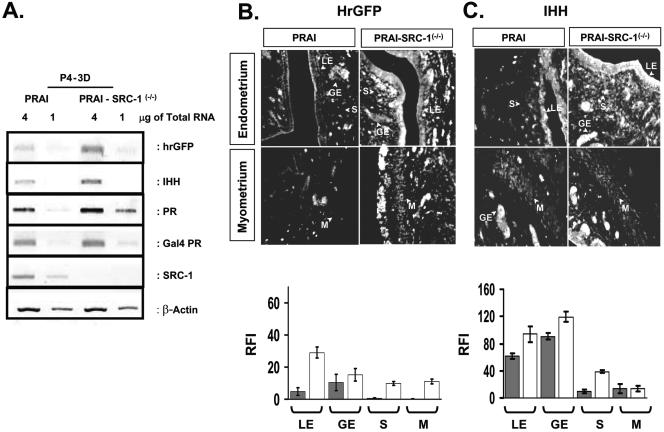
In order to investigate the role of SRC-1 in the modulation of uterine PR dependent gene regulation in more detail, PRAI transgenic mice were crossed with SRC-1−/− mice to generate bigenic PRAI-SRC-1−/− mice. The bigenic mice were treated with acute and chronic P4 treatments as well as with E2+P4 and then PR activity in each compartment of the uterus was assayed by comparing hrGFP expression in wild-type mice with SRC-1−/− mice. SRC-1 ablation had only a small negative effect on PR activation after acute P4 treatment (data not shown). However, the differential role of SRC-1 in regulating uterine gene expression in response to P4 was revealed after chronic P4 treatment (Fig. 8). When comparing PR activities in luminal epithelium and glandular epithelium of wild-type versus SRC-1−/− mice administered chronic P4 treatment, an increase in the PR activities in the SRC-1−/− mouse uteri were noted (Fig. 8A and 8B). In order to confirm that this inductive effect also occurred on endogenous genes, IHH expression was assayed in the uteri of wild-type and SRC-1−/− mice in response to chronic P4. IHH expressions in luminal epithelium and glandular epithelium of SRC-1−/− mice increased with chronic P4 treatment in concert with the hrGFP expression (Fig. 8C). Our data suggest that the SRC-1 coactivator mediates, at least in part, the down-regulation of PR activity in response to chronic P4 in the uterine luminal epithelium and glandular epithelium.
FIG. 8.
SRC-1 is required for down-regulation of PR activity in the uterus in response to chronic P4 treatment. The ovariectomized PRAI and bigenic PRAI-SRC-1−/− mice were given daily doses of P4 (12 μmol/kg) for 3 days (3D) and then killed 6 h after the last injection to harvest the uterus. (A) Total uterine RNA pooled from three mice was used for each group. The cDNA was made from 1 μg or 4 μg of total RNA of each group. The mRNA level of hrGPF, Indian hedgehog (IHH), PR, Gal4 PR, and SRC-1 was determined by RT-PCR. The β-actin signal was used for normalization of RT-PCR signal of each gene. The hrGFP (B) and IHH (C) expression in each compartment of the uterus of PRAI or PRAI-SRC-1−/− mice in response to chronic P4 treatment was examined by immunofluorescence. The RFI value of hrGFP and IHH in each compartment of PRAI (solid box) or PRAI-SRC-1−/− (open box) is shown in the bottom panel. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
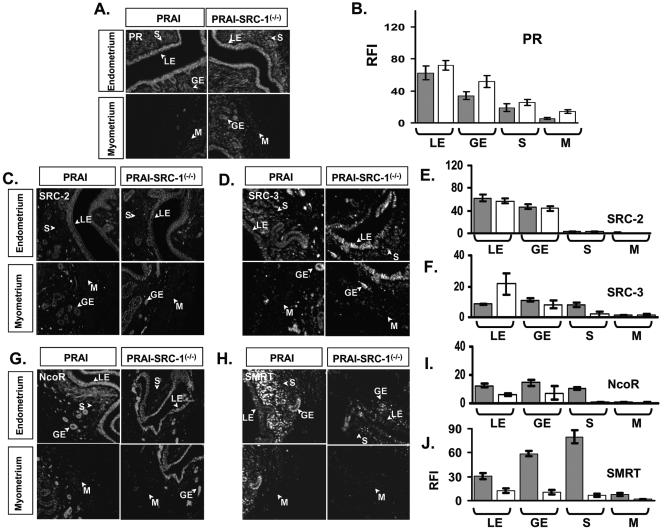
The above observation raises the question as to how SRC-1 is involved in this P4-induced suppression of PR activity. To address this question, we investigated the levels of PR, coactivator, and corepressor expression in the uteri of wild-type and SRC-1−/− mice during the chronic P4 treatment. As shown above in Fig. 4, chronic P4 treatment represses PR expression in luminal epithelium and glandular epithelium compartments of wild-type mice to reduce PR activity in these two compartments. However, in SRC-1−/− mice, PR expression was similar or slightly increased compared to that of wild-type controls (Fig. 9A and 9B). Therefore, the increased PR activity in luminal epithelium and glandular epithelium of SRC-1−/− mice cannot be explained by an effect on PR levels.
FIG. 9.
PR and coregulator expressions in the uterus of PRAI and PRAI-SRC-1−/− mice in response to chronic P4 treatment. The ovariectomized PRAI and bigenic PRAI-SRC-1−/− mice were injected with daily doses of P4 (12 μmol/kg) for 3 days and then killed 6 h after the last injection to harvest the uterus. (A) PR expression in each compartment of the uterus of PRAI and PRAI-SRC-1−/− mice was examined by immunofluorescence. (B) RFI values of PR in each compartment of the uterus of PRAI (solid box) and PRAI-SRC-1−/− mice (open box) are shown in the graph. PR regulator expression [SRC-2 (C), SRC-3 (D), NcoR (G), and SMRT (H)] in the uterus of PRAI and PRAI-SRC-1−/− mice was examined by immunofluorescence. The RFI value of PR coregulators [SRC-2 (E), SRC-3 (F), NcoR (I), and SMRT (J)] in each compartment of the uterus of PRAI (solid box) and PRAI-SRC-1−/− mice (open box) are shown in the graph. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
We then examined the levels of PR coregulators in the SRC-1−/− uterus. SRC-2 is expressed only in luminal epithelium and glandular epithelium and chronic PR treatment did not change SRC-2 expression in these compartments of wild-type or SRC-1−/− mice (Fig. 9C and 9E). In contrast to SRC-2, SRC-3 was expressed in the luminal epithelium, glandular epithelium, and stroma compartments of the uterus; in response to chronic P4 treatment, SRC-3 expression was increased specifically in the luminal epithelium of the uterus of SRC-1−/− mice compared to the wild type (Fig. 9D and 9F). Thus, increased SRC-3 expression in the luminal epithelium compartment of SRC-1−/− mice may contribute, at least in part, to the enhanced PR activity seen in these mice after chronic P4 treatment.
Previous cell culture experiments have implicated NcoR and SMRT in repression of PR activity. Thus, we investigated the expression of these corepressors in the uterus of wild-type and SRC-1−/− mice in response to chronic P4 treatment. NcoR (Fig. 9G and 9I) and SMRT (Fig. 9H and 9J) expression in luminal epithelium and glandular epithelium of the uterus was reduced in SRC-1−/− mice compared with wild-type mice after chronic P4 treatment. Thus, the reduction of the expression of these corepressors in the SRC-1−/− mice should contribute to an increase in PR activity after chronic P4 treatment in the absence of SRC-1. Taken together, increases in SRC-3 expression and decreases in the expression of NcoR and especially SMRT in luminal epithelium and glandular epithelium of the uterus in the absence of SRC-1 are likely major factors in the increase in the PR transcriptional activity seen in these cellular compartments in response to chronic P4 treatment.
SRC-1 is required for E2+P4-mediated PR activation in uterine stroma and myometrium compartments.
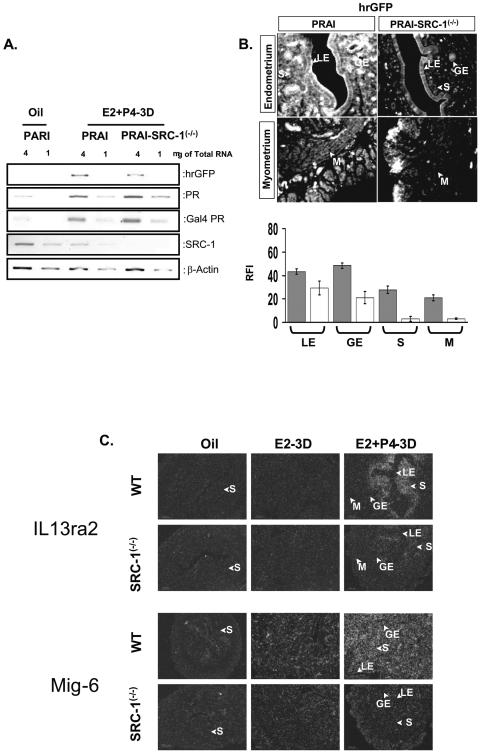
The role of SRC-1 in PR regulation of gene expression in the stroma and myometrium in response to E2+P4 was investigated by assaying hrGFP in the uterus of PRAI and PRAI-SRC-1−/− mice treated with E2+P4. The results are shown in Fig. 10. In contrast to PR activity in stroma and myometrium of wild-type uterus, PR activity in these compartments in SRC-1−/− mice was greatly reduced (Fig. 10A and 10B). The results indicate that SRC-1 plays a significant role in activating PR activity in the uterine stroma and myometrium in response to chronic E2+P4 treatment.
FIG. 10.
SRC-1 is required for PR activation in the stroma and myometrium compartments of the uterus in response to chronic E2 and P4 treatment. The ovariectomized PRAI and bigenic PRAI-SRC-1−/− mice were injected with daily doses of E2 (13 pmol/kg) plus P4 (12 μmol/kg) for 3 days (3D) and then killed 6 h after the last injection to harvest the uterus. (A) Total uterine RNA pooled from three mice was used for each group. The cDNA was made from 1 μg or 4 μg of total RNA of each group. The mRNA level of hrGPF, PR, Gal4 PR, and SRC-1 was determined by RT-PCR. The β-actin signal was used for normalization of RT-PCR signal of each gene expression. (B) The hrGFP expression in each compartment of the uterus of PRAI and PRAI-SRC-1−/− mice was examined by immunofluorescence. RFI value of hrGFP in each compartment of PRAI (solid box) or PRAI-SRC-1−/− (open box) was shown in bottom panel graph. (C) The ovariectomized wild-type and SRC-1−/− mice were given by daily doses of oil, E2 (13 pmol/kg), or E2 (13 pmol/kg) plus P4 (12 μmol/kg) for 3 days and then killed 6 h after the last injection to harvest the uterus. The mRNA levels of both IL13ra2 and Mig-6 in the uterus of wild-type and SRC-1−/− mice were examined by in situ hybridization. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
To validate this finding, in situ hybridization analysis was used to determine the expression of endogenous IL13ra2 and Mig-6, recently identified PR target genes, in wild-type and SRC-1−/− mice (J. Jeong and F. J. DeMayo, unpublished data). Results are shown in Fig. 10C. ILR13ra2 and Mig-6 expression was induced in the endometrial stroma in response to chronic E2+P4 treatment. As predicted, however, this induction was greatly reduced in the SRC-1−/− uteri. The reduction pattern of the hrGFP reporter for PR activity in each compartment of SRC-1−/− mice is coincident with changes in endogenous PR target gene expression (Fig. 10C). Therefore, in contrast with the role of SRC-1 to suppress PR activity in luminal epithelium and glandular epithelium in response to chronic P4 treatment, SRC-1 plays an opposite role to activate PR in stroma and myometrium compartments in response to chronic E2+P4 treatment.
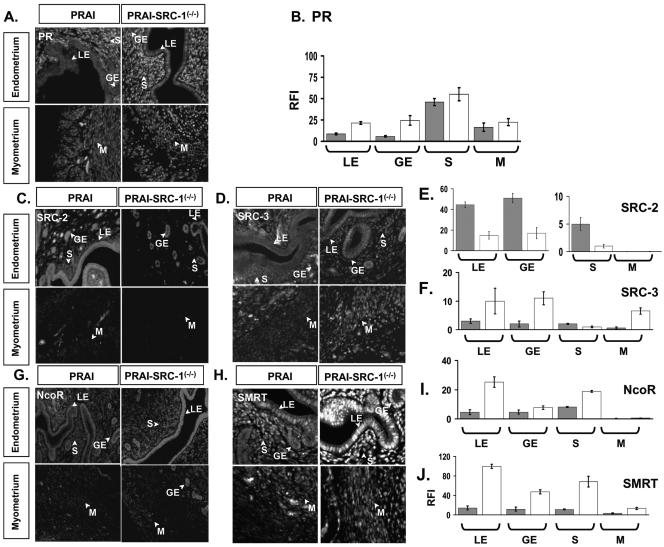
In order to further investigate the mechanism by which ablation of SRC-1 reduces PR activity in the stroma and myometrium of the uterus in response to chronic E2+P4 treatment, the expression of PR, coactivators and corepressors was assayed in wild-type and SRC-1−/− mice in response to E2+P4. The results are shown in Fig. 11. In response to chronic E2+P4 treatment, PR expressions in both stroma and myometrium were not changed in the uteri of SRC-1−/− mice compared with wild type (Fig. 11A and 11B). However, analysis of the coregulator expression in stroma and myometrium of the uterus demonstrated that SRC-2 expression was reduced (Fig. 11C and 11E). SRC-3 expression was not detected in the stroma compartment but was increased in the myometrium with E2+P4 treatment in SRC-1−/− mice compared to wild-type mice (Fig. 11D and 11F).
FIG. 11.
PR and coregulator expressions in the uterus of PRAI and PRAI-SRC-1−/− mice in response to chronic E2 plus P4 treatment. The ovariectomized PRAI and bigenic PRAI-SRC-1−/− mice were injected with daily doses of E2 (13 pmol/kg) plus P4 (12 μmol/kg) for 3 days and then killed 6 h after the last injection to harvest the uterus. (A) PR expression in each compartment of the uterus of PRAI and PRAI-SRC-1−/− mice was examined by immunofluorescence. (B) RFI values of PR in each compartment of the uterus of PRAI (solid box) and PRAI-SRC-1−/− mice (open box) are shown in the graph. PR coregulator expressions [SRC-2 (C), SRC-3 (D), NcoR (G), and SMRT (H)] in the uterus of PRAI and PRAI-SRC-1−/− mice was examined by immunofluorescence. RFI values of PR coregulators [SRC-2 (E), SRC-3 (F), NcoR (I), and SMRT (J)] in the uterus of PRAI (solid box) and PRAI-SRC-1−/− mice (open box) are shown in the graph. LE, luminal epithelium; GE, glandular epithelium; S, stroma; and M, myometrium.
Corepressor expression was also changed in a cell type-specific manner in the uteri of SRC-1−/− mice treated with E2+P4. NcoR expression in the stroma compartment was induced in SRC-1−/− mice (Fig. 11G and 11I) and SMRT expression was increased in all cellular compartments in SRC-1−/− mice compared to wild-type mice (Fig. 11H and 11J). These results indicate that decreased PR activity and PR target gene expression in response to E2+P4 treatment in SRC-1 null mutants can be influenced by a decreased expression of the SRC-2 coactivator but, perhaps most importantly, to increased expression of NcoR and SMRT corepressors.
DISCUSSION
PRAI mouse model faithfully replicates cell type-specific endogenous PR activity in vivo.
Previous studies investigating the molecular mechanisms regulating the activity of the PR utilized transient cell transfection assays as the major investigative tool. Albeit valuable, the limitation of the transient transfection assay is that it relies on overexpression of the individual components in cell culture and it does not generally mimic the stoichiometry or the appropriate cellular milieu of intact animal tissues. In order to overcome this problem, we generated a PRAI BAC transgenic mouse activator and reporter model system. This approach was chosen because previous investigators have shown that BAC transgenic systems have the potential to replicate the expression of endogenous genes when they contain sufficient genomic DNA surrounding the mouse target gene (11, 13, 14, 45).
As was anticipated, the tissue-specific Gal4-PR expression pattern in PRAI mice closely resembled the endogenous PR expression in the major reproductive sites where the PR gene is expressed. Also, the cell compartment-specific expression of the Gal4-PR in response to different hormonal treatments mimicked the endogenous PR expression in the uterus. For example, chronic E2+P4 treatment resulted in a complete loss of Gal4-PR expression in the luminal epithelium and glandular epithelium compartments of the uterus, but induced intense Gal4-PR staining in the stroma and myometrium compartments of the uterus, similar to a prior assessment of endogenous PR levels (35). Since the expression pattern of the Gal4 PR corresponded to the expression pattern of the endogenous PR, we conclude that the modified PR BAC clone in the uterus of transgenic PRAI mice largely recapitulates endogenous PR expression in vivo.
Both acute P4 treatment and chronic E2+P4 treatment were shown to enhance uterine PR functional activity when a conventional method, RT-PCR, was used to analyze the overall uterine hormone responsiveness. However, uterine function is dependent upon multiple interdependent cell types and the PR activation within precise cell types cannot be determined from tissue assays. Alternative approaches to investigate changes in cell type-specific PR activation in tissues are immunostaining and in situ hybridization. Unfortunately, few specific PR-regulated target genes have been identified in the uterus and specific antibodies suitable for histochemistry are rare. The PRAI mice serve as a model to easily and accurately determine cell type-specific PR activity in the uterus by quantifying hrGFP expression under differential hormonal conditions.
We then employed regimens of uterotropic steroids to investigate PR activity. We found that hrGFP expression was induced in the uterine luminal epithelium and glandular epithelium but not in the myometrium upon acute P4 treatment. The induction of PR activity in specific cell types confirmed previous results in which Indian hedgehog mRNA and protein levels were shown to be induced in the luminal epithelium and glandular epithelium compartments of the endometrium after a single administration of P4 to ovariectomized mice (33).
In contrast with acute P4, chronic E2+P4 treatment required for blastocyst implantation induced PR activity in both stroma and myometrium, but inhibited it in luminal epithelium and glandular epithelium compartments of the uterus. Similar to hrGFP regulation, the mRNA levels of two endogenous PR target genes (IL13ra2 and Mig-6) were induced by chronic E2+P4 treatment and the induced mRNAs were localized to the stroma compartment of the uterus. Thus, induction of PR-regulated target gene expression in specific cell types mediated by different hormone treatments correlated well with cell type-specific hrGFP expression in our PRAI mice. Such information could not be obtained by DNA microarray analysis of total RNAs prepared from intact uteri. Also, if transgenic progesterone response element (PRE)-lacZ reporter mice were constructed, they would have responded to androgens and glucocorticoids due to DNA response element cross-reaction.
The ability to determine cell type-specific PR activity in vivo under various hormone treatments permits the generation of an atlas documenting PR activity in each cell type of the uterus or other tissues. Such an atlas would be valuable for determining the impact on implantation and pregnancy of coregulators and PR antagonists, PR agonists, and specific PR modulators and specific estrogen receptor modulators on PR function in a cell type-specific manner in vivo.
Cell-specific function of SRC-1 on PR activity.
In this report we investigated the impact of the ablation of the coactivator SRC-1 in the uterus of the mouse. SRC-1 is a primary uterine coactivator for PR and gene ablation experiments revealed a decreased uterine decidual response (44). Based on these results and multiple cell transfection analyses (21), it was reasonable to hypothesize that PR utilizes SRC-1 as a primary coactivator to modulate uterine biology. Employing the PRAI mouse model system in the SRC-1 null mutant background, we provided evidence that SRC-1 indeed modulates uterine function by modulating PR activity. Surprisingly, the PRAI mouse revealed that SRC-1 did not play a significant role in the regulation of PR gene expression following acute P4 treatment alone. In contrast, SRC-1 did play a major role in determining PR activity following E2+P4 treatment.
A potential explanation for this phenomenon is that during development, PR expression in the mouse uterus is limited to the epithelium and stroma. Expression of PR occurs during postpubertal development due to estrogen stimulation of endometrial stroma. Thus, postdevelopmental expression of PR may leave little time for developmental compensation by other coregulators. Therefore, SRC-1 plays an essential role in PR activity in the stroma in response to E2+P4 treatment. This role is critical for the estrogen- and progesterone-mediated implantation of the fertilized blastocyst during the initial stages of pregnancy. Our findings support the notion that the reduced decidual response phenotype detected in the uterus of SRC-1−/− mice results from the lack of this functional interaction between PR and SRC-1 in the stroma, substantiating SRC-1 as a critical partner for PR in the physiological endocrine response of the uterus.
SRC-1 creates a compartment-specific cellular environment to dynamically modulate PR activity in the uterus.
SRC-1 modulates expression of other coregulators in a cell compartment-specific fashion to influence PR activity in response to various reproductive hormonal challenges. Previous work already showed that expression of another family member (SRC-2) can be increased in certain tissues of SRC-1−/− mice to compensate in part for its loss (44). In the present study, we showed that SRC-1 controls the expression levels of other uterine coregulators to create a compartment-specific cellular environment for PR function. For example, the SRC-1 coactivator is required to maintain a high expression ratio of corepressors to coactivators for “repression” of PR activity in all compartments of the uterus in response to chronic P4 treatment. In contrast, SRC-1 permits a reduction of that ratio in the stroma and myometrium of the uterus so as to activate PR activity when E2+P4 is administered. Consequently, SRC-1 influences the compartment specific corepressor-to-coactivator ratio to modulate PR activity.
The importance of NcoR and SMRT for repression of steroid hormone action has been substantiated previously by experiments showing that overexpression of NcoR and SMRT represses the partial agonist activity of both tamoxifen-bound estrogen receptor and RU486-bound PR (22). Also, using a chromatin-based cell-free transcription system, we showed that the ability of RU486 to activate transcription was modulated directly by the coactivator-to-corepressor ratio (22). Therefore, it is reasonable to postulate that alterations in the expression ratio of NcoR and SMRT, in addition to SRC/p160 coactivators, modulate PR activity in the uterus in vivo in a tissue- and cell compartment-specific manner (30). This information now presents an atlas for understanding the complex molecular relationships inherent to the function of a crucial multicompartmental reproductive/endocrine organ such as the mammalian uterus.
Acknowledgments
We are grateful to Donald L. Court for providing the bacterial recombination strain (DY380) and A. Francis Stewart for providing the 294-Flp and 294-Cre bacterial strains. We also thank Jiemin Wong and Jun Qin for their gifts of antibody.
This work was supported by grants P01 DK59280 (B.W.O. and M.J.J.) and HD0785 7 (B.W.O.).
REFERENCES
- 1.Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. [DOI] [PubMed] [Google Scholar]
- 2.Biffo, S. 1992. In situ hybridization: optimization of the techniques for collecting and fixing the specimens. Liver 12:227-229. [DOI] [PubMed] [Google Scholar]
- 3.Borud, B., T. Hoang, M. Bakke, A. L. Jacob, J. Lund, and G. Mellgren. 2002. The nuclear receptor coactivators p300/CBP/Cointegrator-Associated Protein (p/CIP) and Transcription Intermediary Factor 2 (TIF2) differentially regulate PKA-stimulated transcriptional activity of steroidogenic factor 1. Mol. Endocrinol. 16:757-773. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz, F., P. Angrand, and A. Stewart. 1996. A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res. 24:3118-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchholz, F., P. O. Angrand, and A. F. Stewart. 1996. A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res. 24:3118-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das, S. K., J. Tan, D. C. Johnson, and S. K. Dey. 1998. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology 139:2905-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, H., R. J. O'Brien, E. T. Fung, A. A. Lanahan, P. F. Worley, and R. L. Huganir. 1997. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386:279-284. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, H. M., D. Yu, T. DiTizio, and D. L. Court. 2001. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl. Acad. Sci. USA 98:6742-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn, C. A., and A. M. Lawn. 1967. Specialized junctions between decidual cells in the uterus of the pregnant mouse. J. Ultrastruct. Res. 20:321-327. [DOI] [PubMed] [Google Scholar]
- 10.Gehin, M., M. Mark, C. Dennefeld, A. Dierich, H. Gronemeyer, and P. Chambon. 2002. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 22:5923-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong, S., C. Zheng, M. L. Doughty, K. Losos, N. Didkovsky, U. B. Schambra, N. J. Nowak, A. Joyner, G. Leblanc, M. E. Hatten, and N. Heintz. 2003. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917-925. [DOI] [PubMed] [Google Scholar]
- 12.Halachmi, S., E. Marden, G. Martin, H. MacKay, C. Abbondanza, and M. Brown. 1994. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455-1458. [DOI] [PubMed] [Google Scholar]
- 13.Heintz, N. 2000. Analysis of mammalian central nervous system gene expression and function using bacterial artificial chromosome-mediated transgenesis. Hum. Mol. Genet. 9:937-943. [DOI] [PubMed] [Google Scholar]
- 14.Heintz, N. 2001. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat. Rev. Neurosci. 2:861-870. [DOI] [PubMed] [Google Scholar]
- 15.Hong, H., K. Kohli, M. J. Garabedian, and M. R. Stallcup. 1997. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 17:2735-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ing, N. H., J. M. Beekman, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1992. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J. Biol. Chem. 267:17617-17623. [PubMed] [Google Scholar]
- 17.Jackson, T. A., J. K. Richer, D. L. Bain, G. S. Takimoto, L. Tung, and K. B. Horwitz. 1997. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol. Endocrinol. 11:693-705. [DOI] [PubMed] [Google Scholar]
- 18.Kershah, S. M., M. M. Desouki, K. L. Koterba, and B. G. Rowan. 2004. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol. Oncol. 92:304-313. [DOI] [PubMed] [Google Scholar]
- 19.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56-65. [DOI] [PubMed] [Google Scholar]
- 20.Li, H., P. J. Gomes, and J. D. Chen. 1997. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 94:8479-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, X., J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2003. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol. Cell. Biol. 23:3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, C. Q., Z. X. Wang, and Y. Yuan. 2002. Effect of mifepristone on uterine receptivity in guinea pigs. Acta. Pharmacol. Sin. 23:177-182. [PubMed] [Google Scholar]
- 23.Lydon, J. P., F. J. DeMayo, O. M. Conneely, and B. W. O'Malley. 1996. Reproductive phenotpes of the progesterone receptor null mutant mouse. J. Steroid Biochem. Mol. Biol. 56:67-77. [DOI] [PubMed] [Google Scholar]
- 24.Lydon, J. P., F. J. DeMayo, C. R. Funk, S. K. Mani, A. R. Hughes, C. A. Montgomery, Jr., G. Shyamala, O. M. Conneely, and B. W. O'Malley. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9:2266-2278. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto, H., X. Zhao, S. K. Das, B. L. Hogan, and S. K. Dey. 2002. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 245:280-290. [DOI] [PubMed] [Google Scholar]
- 26.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 27.Noda, N., H. Minoura, R. Nishiura, N. Toyoda, K. Imanaka-Yoshida, T. Sakakura, and T. Yoshida. 2000. Expression of tenascin-c in stromal cells of the murine uterus during early pregnancy: induction by interleukin-1{alpha}, prostaglandin E2, and prostaglandin F2{alpha}. Biol. Reprod. 63:1713-1720. [DOI] [PubMed] [Google Scholar]
- 28.Onate, S. A., S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354-1357. [DOI] [PubMed] [Google Scholar]
- 29.Shao, W., S. Halachmi, and M. Brown. 2002. ERAP140, a conserved tissue-specific nuclear receptor coactivator. Mol. Cell. Biol. 22:3358-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, C. L., Z. Nawaz, and B. W. O'Malley. 1997. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 11:657-666. [DOI] [PubMed] [Google Scholar]
- 31.Smith, D. F., and D. O. Toft. 1992. Composition, assembly and activation of the avian progesterone receptor. J. Steroid Biochem. Mol. Biol. 41:201-207. [DOI] [PubMed] [Google Scholar]
- 32.Suen, C. S., T. J. Berrodin, R. Mastroeni, B. J. Cheskis, C. R. Lyttle, and D. E. Frail. 1998. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J. Biol. Chem. 273:27645-27653. [DOI] [PubMed] [Google Scholar]
- 33.Takamoto, N., B. Zhao, S. Y. Tsai, and F. J. DeMayo. 2002. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol. Endocrinol. 16:2338-2348. [DOI] [PubMed] [Google Scholar]
- 34.Takeshita, A., G. R. Cardona, N. Koibuchi, C. S. Suen, and W. W. Chin. 1997. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 272:27629-27634. [DOI] [PubMed] [Google Scholar]
- 35.Tibbetts, T. A., M. Mendoza-Meneses, B. W. O'Malley, and O. M. Conneely. 1998. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol. Reprod. 59:1143-1152. [DOI] [PubMed] [Google Scholar]
- 36.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 37.Tsai, M., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 38.Voegel, J., M. Heine, C. Zechel, P. Chambon, and H. Gronemeyer. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15:3667-3675. [PMC free article] [PubMed] [Google Scholar]
- 39.Voegel, J. J., M. J. Heine, C. Zechel, P. Chambon, and H. Gronemeyer. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15:3667-3675. [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner, B. L., J. D. Norris, T. A. Knotts, N. L. Weigel, and D. P. McDonnell. 1998. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol. Cell. Biol. 18:1369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wewer, U. M., A. Damjanov, J. Weiss, L. A. Liotta, and I. Damjanov. 1986. Mouse endometrial stromal cells produce basement-membrane components. Differentiation 32:49-58. [DOI] [PubMed] [Google Scholar]
- 42.Xie, W., H. Hong, N. N. Yang, R. J. Lin, C. M. Simon, M. R. Stallcup, and R. M. Evans. 1999. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol. Endocrinol. 13:2151-2162. [DOI] [PubMed] [Google Scholar]
- 43.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, J., Y. Qiu, F. J. DeMayo, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922-1925. [DOI] [PubMed] [Google Scholar]
- 45.Yang, X. W., C. Wynder, M. L. Doughty, and N. Heintz. 1999. BAC-mediated gene-dosage analysis reveals a role for Zipro1 (Ru49/Zfp38) in progenitor cell proliferation in cerebellum and skin. Nat. Genet. 22:327-335. [DOI] [PubMed] [Google Scholar]
- 46.Yu, D., and D. L. Court. 1998. A new system to place single copies of genes, sites and lacZ fusions on the Escherichia coli chromosome. Gene 223:77-81. [DOI] [PubMed] [Google Scholar]
- 47.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]