Abstract
SKAP-HOM is a cytosolic adaptor protein representing a specific substrate for the Src family protein tyrosine kinase Fyn. Previously, several groups have provided experimental evidence that SKAP-HOM (most likely in cooperation with the cytosolic adaptor protein ADAP) is involved in regulating leukocyte adhesion. To further assess the physiological role of SKAP-HOM, we investigated the immune system of SKAP-HOM-deficient mice. Our data show that T-cell responses towards a variety of stimuli are unaffected in the absence of SKAP-HOM. Similarly, B-cell receptor (BCR)-mediated total tyrosine phosphorylation and phosphorylation of Erk, p38, and JNK, as well as immunoreceptor-mediated Ca2+ responses, are normal in SKAP-HOM−/− animals. However, despite apparently normal membrane-proximal signaling events, BCR-mediated proliferation is strongly attenuated in the absence of SKAP-HOM−/−. In addition, adhesion of activated B cells to fibronectin (a ligand for β1 integrins) as well as to ICAM-1 (a ligand for β2 integrins) is strongly reduced. In vivo, the loss of SKAP-HOM results in a less severe clinical course of experimental autoimmune encephalomyelitis following immunization of mice with the encephalitogenic peptide of MOG (myelin oligodendrocyte glycoprotein). This is accompanied by strongly reduced serum levels of MOG-specific antibodies and lower MOG-specific T-cell responses. In summary, our data suggest that SKAP-HOM is required for proper activation of the immune system, likely by regulating the cross-talk between immunoreceptors and integrins.
Adaptor proteins are multifunctional signaling molecules which are capable of coupling engaged immunoreceptors (e.g., the T-cell receptor [TCR] or the B-cell receptor [BCR]) to intracellular signaling pathways and effector systems. In general, adaptor proteins do not exert enzymatic or transcriptional activities. Rather, they contain a variety of modular domains that mediate constitutive or inducible protein-protein or protein-lipid interactions after engagement of signal-transducing receptors.
Several cytosolic adaptor proteins have been identified during the last years which appear to be involved in reorganization of the cytoskeleton and/or integrin-mediated adhesion after external engagement of immunoreceptors. In T cells, these include the cytosolic adaptor proteins ADAP (adhesion and degranulation promoting adaptor protein) (27) and SKAP55 (Src-kinase-associated phosphoprotein of 55 kDa) (31).
ADAP was among the first adaptor proteins shown to translate TCR stimulation to avidity modulation of β1 and β2 integrins (a mechanism called inside-out signaling). Thus, despite almost normal proximal signaling events (global tyrosine phosphorylation, TCR-mediated increases in intracellular calcium, Erk activation, actin polymerization, and TCR clustering), TCR-mediated clustering of integrins and the adhesion of T cells to the β1 and β2 integrin ligands fibronectin and ICAM-1 were found to be strongly impaired in ADAP-deficient T cells. The failure to activate integrins via inside-out signaling leads to a defect in TCR-mediated proliferation, interleukin-2 (IL-2) production, and a strongly impaired T-cell response in vivo (9, 27).
While ADAP is expressed in T cells and myeloid cells, SKAP55 is expressed exclusively in T lymphocytes (5, 20). SKAP55 comprises a pleckstrin homology domain, a C-terminal SH3 domain, and an interdomain that carries three tyrosine-based signaling motifs (21). Overexpression experiments in Jurkat T cells suggested that SKAP55 interacts with the protein tyrosine phosphatase CD45 and possibly regulates the mitogen-activated protein kinase pathway (32, 33). More recently it was demonstrated that SKAP55 is capable of regulating integrin-mediated adhesion, conjugate formation between T cells, and antigen-presenting cell (APC)- and TCR-mediated clustering of LFA-1 in mouse T cells (15, 31). Thus, the functional effects of SKAP55 and ADAP seem to be similar.
In line with this assumption is the observation that in primary T cells and in the Jurkat T-cell line, SKAP55 tightly associates with ADAP. This interaction involves the SH3 domain of SKAP55 and a proline-rich segment in ADAP (17, 21). Biochemical analysis had further suggested that all SKAP55 molecules expressed in T lymphocytes associate with ADAP. All these data indicate that in T lymphocytes, SKAP55 and ADAP form a functional unit and that a role of this unit is to modulate T-cell adhesion after engagement of the TCR/CD3 complex. However, it is still unknown whether regulation of adhesion is the only task that is fulfilled by SKAP55 and ADAP during an ongoing immune response.
In contrast to SKAP55, the cytosolic adaptor SKAP-HOM (SKAP55 homologue) or SKAP55R (SKAP55 related) is an adaptor protein that is more widely expressed within the hematopoetic system (4, 16, 23). SKAP-HOM comprises an almost identical structure as SKAP55, except for a unique N-terminal putative coiled-coil region and only two tyrosine-based signaling motifs in the interdomain. Similar to SKAP55, SKAP-HOM has been reported to associate with ADAP via its SH3 domain and to represent a specific substrate for the Src family protein tyrosine kinase p59Fyn (17, 23).
An involvement of SKAP-HOM in integrin-mediated signaling was initially suggested by the analysis of murine bone marrow-derived macrophages (BMM) in which tyrosine phosphorylation of SKAP-HOM (and of ADAP) was induced after adhesion to fibronectin (1, 30). Moreover, in BMM from mice homozygous for the “motheaten” (me) mutation (these me/me mice lack expression of the cytosolic tyrosine phosphatase SHP-1), ADAP and SKAP-HOM were found to be expressed as constitutively tyrosine phosphorylated proteins, and this augmented phosphorylation correlates well with the known hyperadhesiveness of me/me macrophages (28, 30). Finally, enteropathogenic species of Yersinia enterocolitica exert resistance to phagocytosis by injecting the virulence factor YopH, a protein tyrosine phosphatase, into host cells. In these cells YopH apparently dephosphorylates ADAP and SKAP-HOM. Either by itself or in concert with other mechanisms, this leads to abrogation of the phagocytic process and causes cellular detachment and rounding up (1, 6). Thus, not only ADAP and SKAP55 but also SKAP-HOM seem to be involved in integrin-mediated signaling pathways. However, the function of SKAP-HOM within the immune system remains elusive.
To gain further insight into the physiologic role of SKAP-HOM, we investigated the immune system of SKAP-HOM-deficient mice. T-cell, platelet, and macrophage functions appear to be unaffected by loss of SKAP-HOM. In marked contrast, despite apparently normal membrane-proximal signaling events, both in vitro and in vivo B-cell responses are impaired in the absence of SKAP-HOM. Indeed, after antibody-mediated stimulation of the BCR, SKAP-HOM-deficient B cells show reduced in vitro proliferation and an impaired capability to adhere to either fibronectin or ICAM, two physiologic ligands for β1 and β2 integrins. Moreover, when challenged in vivo, SKAP-HOM-deficient mice display a decrease in clinical severity of experimental autoimmune encephalomyelitis (EAE) and a strongly reduced production of MOG (myelin oligodendrocyte glycoprotein)-specific immunoglobulins. Taken together, our findings indicate an important role for SKAP-HOM in regulating homeostasis of the immune system. Moreover, they suggest that SKAP-HOM might be an attractive target for therapeutic intervention in autoimmune diseases such as multiple sclerosis.
MATERIALS AND METHODS
Mice strains.
The SKAP-HOM-deficient mouse was generated by retrovirus-based gene trap technology (Lexicon Genetics Inc.) (34). Mice were backcrossed to C57BL/6JBom (Taconics) for a minimum of six generations. In all experiments, 8- to 12-week-old littermate mice were used. All procedures were conducted according to protocols approved by the local authorities. Mice were genotyped by two independent PCRs using the following primer sequences: neomycin sense, 5′GAT GCC GCC GTG TTC C; neomycin antisense, 5′GCC CCT GAT GCT CTT CGT C; SKAP-HOM sense, 5′CCT GCG GCC TTT GAT GGT G; SKAP-HOM antisense, 5′ACT GCT TTG CTG GGG GTG GTG TT.
Determination of hematological parameters.
To measure blood cell parameters, whole blood was collected by heart puncture in tubes containing heparin and analyzed on an automatic hematology counter (Cell-Dyn 1600; Abbott).
Cell preparations.
The spleens were passed through a fine mesh filter to obtain a single cell suspension. In all assays, total splenocytes were isolated, and B and T cells were purified by negative selection using mouse B-cell isolation kits or mouse T-cell isolation kits and AutoMACS (Miltenyi Biotec), respectively. CD4- and CD8-positive splenocytes were prepared by positive selection using anti-CD4 or anti-CD8 microbead-coupled antibody and AutoMACS. Purity was >90% as checked by flow cytometry. The cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Biochrom AG), and 50 μM 2-mercaptoethanol.
Adhesion assay.
For fibronectin binding, the central area of a 35-mm diameter tissue culture dish (Falcon) was coated with 50 μl fibronectin (100 μg/ml; Roche Diagnostics) for 90 min at room temperature. For ICAM-1 binding, tissue culture dishes were precoated for 16 h at 4°C with goat anti-human immunoglobulin G (IgG) Fc fragment (7.2 μg/ml; Dianova) to allow directional coating. After washing, dishes were coated with 12.5 μg/ml recombinant murine ICAM-1 human Fc chimera (R&D Systems) for 90 min at room temperature. After coating, dishes were washed three times with phosphate-buffered saline (PBS) (without Ca2+ or Mg2+) and blocked with 1% bovine serum albumin (BSA) in PBS for an additional 2 h.
Cells (5 × 106) were washed with Hanks balanced salt solution (HBSS) and either left unstimulated or stimulated with tetradecanoyl phorbol acetate (50 ng/ml), MnCl2 (2 mM), or anti-mouse IgM F(ab′)2 (2 μg/ml) for 30 min at 37°C. Cells were plated on the dishes and incubated at 37°C for 30 min. Nonadherent cells were removed by washing the dishes three times with HBSS. Cell adhesion was measured by counting six independent fields by microscopy using an ocular counting reticule. Specific adhesion was expressed as the increase over unstimulated control samples.
Proliferation assay.
Freshly isolated mouse cells were resuspended in complete medium and seeded in quadruplicate at 1 × 105 cells in 96-well round-bottomed microtiter plates (Costar). Cells were either left unstimulated or stimulated for 72 h at 37°C with the indicated concentrations of affinity-purified goat anti-mouse IgM or the corresponding F(ab′)2 fragment (Jackson ImmunoResearch), lipopolysaccharide (LPS; 2.5 μg/ml; Sigma), IL-4 (20 ng/ml; Peprotech Inc.), anti-mouse CD40 (1 μg/ml; Pharmingen), phorbol myristate acetate (PMA) (2 × 10−8 M; Sigma), and ionomycin (500 ng/ml; Calbiochem). For the last 8 h, 0.5 μCi [3H]thymidine was added to the cultures. After harvesting, the radioactivity incorporated into the DNA was measured by liquid scintillation counting (1450 MicroBeta Trilux; PerkinElmer Wallac GmbH). The stimulation index was calculated as the ratio of counts per minute (cpm) of stimulated to unstimulated proliferation.
Measurement of bone marrow-derived macrophage proliferation in response to macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF).
BMMs from mice of the indicated genotypes were differentiated ex vivo as previously described (10) with some modification. Specifically, bone marrow cells were preplated for 48 h on tissue culture plates, and cells remaining in suspension were enumerated. Cells (1 × 106) were seeded onto 35-mm petri plates. The cells were incubated at 37°C in Dulbecco's modified Eagle medium containing 10% heat-inactivated serum supplemented with either 10 ng/ml M-CSF or 20 ng/ml of GM-CSF (Peprotech). Cells were fed every 2 days. At the indicated time, cells were rinsed with PBS, harvested in trypsin-EDTA, and counted using a Z2 Coulter Counter.
FcR and complement receptor-mediated phagocytosis.
To opsonize sheep red blood cells (RBCs) with IgG, RBCs were washed and resuspended in veronal-buffered saline (Sigma-Aldrich) containing 1:2,500 rabbit anti-RBC serum and incubated at 37°C for 1.5 to 2 h. To opsonize RBCs with complement, RBCs were incubated with anti-sheep RBC IgM for 1 h (1:5,000), followed by incubation with 10% C5-deficient serum (Sigma-Aldrich) for 20 min. Opsonized RBCs were washed with veronal-buffered saline and resuspended in DMEM at ∼5 × 107 RBCs/ml. BMMs (7 to 8 days old) were seeded in 24-well plates (3 × 105/well) for 24 h, starved for 2 h, and incubated with 0.4 ml of opsonized RBCs at 37°C for 20 min. Phagocytosis was terminated by lysing nonphagocytosed RBCs with H2O for 40 s, and BMMs were fixed in 4% paraformaldehyde. To visualize internalized RBCs, the cells were permeabilized with 0.5% Triton X-100 for 5 min, rinsed three times in PBS, pH 7.4, and incubated with rabbit anti-RBC serum (1:10,000) and rhodamine phalloidin. After washing, the cells were incubated in fluorescein isothiocyanate (FITC)-conjugated anti-rabbit Ig and washed three times prior to being photographed using a Zeiss microscope. Four-hundred cells were counted in three randomly chosen fields. The percentage of active cells (greater than 3 RBCs/cell) and the average number of RBCs per active cell were determined for each field.
Flow cytometry.
The cells were stained with the following antibodies: anti-CD3, anti-CD5, anti-CD11a, anti-CD18, anti-CD21, anti-CD23, anti-CD29, anti-CD45R/B220, anti-CD49d, anti-IgM, and anti-IgD (all from Pharmingen). Flow cytometry was performed on a FACSCalibur using CellquestPro software (Becton Dickinson).
Western blotting.
Western blotting was performed as previously described (22). For immunodetection, the following primary antibodies were used: anti-phospho-Tyr (4G10; Upstate Biotechnology), anti-phospho-Erk1/2, anti-Erk1/2, anti-phospho-JNK, anti-JNK, anti-phospho-p38, anti-p38, anti-phospho-Akt (Cell Signaling Technology, Inc.), and anti-SKAP55 (BD Biosciences). Goat polyclonal anti-ADAP sera were previously described (25). Affinity-purified rabbit polyclonal anti-SKAP-HOM antiserum was prepared as described elsewhere (23). Specific binding was detected using peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies by applying the enhanced chemiluminescence system (Amersham Biosciences) according to the manufacturer's protocol.
Calcium flux.
Purified splenic B cells were resuspended at 1 × 106 cells/ml in RPMI 1640 without phenol red, supplemented with 10% fetal calf serum, and loaded with 3.75 μM indo-1 acetoxymethyl ester (Molecular Probes) for 45 min at 37°C. Cells were washed, resuspended in the same RPMI medium, and incubated for an additional 30 min at 37°C. After establishing a stable baseline level, B cells were stimulated with 10 μg/ml affinity-purified goat anti-mouse IgM F(ab′)2 fragment (Jackson ImmunoResearch). Equal loading of samples was checked by treatment of cells with 100 nM ionomycin (Calbiochem). The ratio of indo-1 violet/blue was analyzed by flow cytometry using LSR (Becton Dickinson). Data analysis was performed using FloJo software (Tree Star).
Enzyme-linked immunosorbent assays (ELISAs).
For the determination of spontaneous levels of IgM and IgG isotypes, microtiter plates (MaxiSorb; Nunc, Wiesbaden, Germany) were coated with 3.6 μg/ml goat anti-mouse IgG plus IgM (heavy plus light chains) (Dianova, Hamburg, Germany) at 4°C overnight and blocked with 1% BSA. Sera were serially diluted in duplicate. Isotype-specific antibodies were detected with the respective alkaline phosphatase-conjugated goat anti-mouse antibodies to IgG1, IgG2a, IgG2b, IgG3, or IgM (Southern Biotechnology Associates, Inc.). The substrate p-nitrophenyl phosphate (Sigma Aldrich) was used for color development, and after 30 min the absorbance was read at 405 nm on an ELISA plate reader (Dynatech). Immunoglobulin concentrations were calculated by interpolation from a standard curve using the respective isotype controls from mouse myeloma (Sigma-Aldrich).
For the determination of specific anti-2,4-dinitrophenyl (DNP) and anti-MOG p35-55, IgG and IgM plates were coated with 3 μg/ml DNP-BSA or 10 μg/ml MOG in bicarbonate buffer, respectively, overnight at 37°C. After blocking with 1% BSA, the plates were incubated with serial dilutions of mouse serum overnight at 4°C. Specific binding was detected using alkaline phosphatase-labeled goat anti-mouse IgM (μ chain specific; Serotec) or goat anti-mouse IgG (subclasses 1 plus 2a plus 2b plus 3; Fcγ fragment specific; Dianova).
Immunization of mice.
Twelve-week-old mice were immunized intraperitoneally (i.p.) with 100 μg of 2,4-dinitrophenyl-conjugated keyhole limphet hemocyanin (DNP-KLH; Calbiochem) in complete Freund's adjuvant (CFA; Sigma-Aldrich, Taufkirchen, Germany) and were boosted 21 days later. Mice were bled before and after boosting (day 21 and day 28), and hapten-specific serum antibody levels were measured by ELISA (see above). Also, mice were immunized i.p. with 100 μg of 2,4,6-trinitrophenyl (TNP)-LPS in saline. Mice were bled before and 5 and 10 days after immunization, and hapten-specific serum antibody levels were measured by ELISA.
Induction of EAE and clinical evaluation.
MOG p35-55 corresponding to a mouse sequence (MEVGWYRSPFSRVVHLYRNGK) was synthesized on a peptide synthesizer by standard 9-fluorenylmethoxycarbonyl chemistry and purified by high-performance liquid chromatography (HPLC). Active EAE was induced in 8- to 12-week-old mice by immunization with 200 μg MOG p35-55 emulsified in complete Freund's adjuvant (CFA; Sigma-Aldrich, Taufkirchen, Germany) containing 800 μg of heat-killed Mycobacterium tuberculosis (Difco Laboratories, Detroit, Mich.). The emulsion was administered subcutaneously as four 50-μl injections into the flanks of each leg. In addition, 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) dissolved in 200 μl PBS was injected i.p. on days 0 and 2. Mice were weighed daily, monitored for clinical signs of EAE, and graded on a scale of increasing severity from 0 to 5 by blinded investigators as described earlier (29). Daily clinical scores were calculated as the average of all individual disease scores of each group, including mice not developing clinical signs of EAE.
Cytokine concentration.
Levels of cytokines in culture supernatants were determined using the mouse inflammatory cytometric bead array kit (Becton Dickinson).
Conjugation assay.
Splenic B cells obtained from wild-type or knock-out littermates were cultured at a concentration of 2 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Biochrom AG), and 50 μM 2-mercaptoethanol for 16 h with (loaded) or without (unloaded) 0.2 mg/ml chicken ovalbumin 323-339 peptide (OVA323-339). Cells were then washed and set to 1 × 106 cells/ml in the same medium. T cells enriched by negative selection using a T-cell kit (Miltenyi Biotec) obtained from OT-II mice were loaded with 5 (and 6-)-carboxfluorescein diacetate succinimidyl ester (CFSE). After loading, T cells were diluted to 1 × 106 cells/ml RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Biochrom AG), and 50 μM 2-mercaptoethanol. Peptide-loaded or unloaded B cells (100 μl) were mixed with equal volume of T cells in a 96-well plate. After coincubation for 8 h, cells were fixed by adding 200 μl of 4% paraformaldehyde. After 20 min at 37°C, cells were carefully transferred into Falcon tubes and measured by a fluorescence-activated cell sorter. Events with positive CFSE staining and increased forward scatter were counted as conjugates.
Actin polymerization.
Purified splenic B cells (100 μl; 2 × 106 cells/ml in PBS) were stimulated with 10 μg/ml affinity-purified goat anti-mouse IgM F(ab′)2 fragment. At the end of the stimulation, 100 μl 4% formaldehyde, 0.2% saponin, and 100 μl FITC-phalloidin (5 μg/ml) were added. The tubes were vortexed and kept at room temperature for 15 min. After washing, cells were resuspended in 300 μl paraformaldehyde. Actin polymerization was measured by flow cytometry and expressed as an increase in mean fluorescence intensity.
Statistical analysis.
Values are expressed as the means ± standard errors of the means (SEM) of at least three independent experiments. One-way analysis of variance was used to assess the statistical significance of the differences. Probability (P) values of <0.05 (*) and <0.01 (**) were considered significant.
RESULTS
Expression of SKAP-HOM within the hematopoietic system.
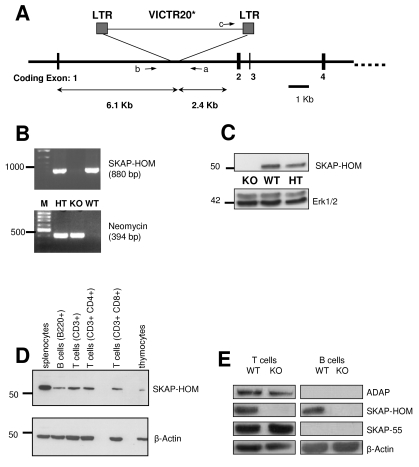
In order to asses the in vivo function of SKAP-HOM, we generated SKAP-HOM−/− mice by a retrovirus-based gene trap technology (Lexicon Genetics Inc.) (Fig. 1A). SKAP-HOM deficiency was confirmed by PCR and by anti-SKAP-HOM Western blotting (Fig. 1B and C). SKAP-HOM−/− mice were born healthy at the expected Mendelian frequency and did not show any obvious abnormalities. Moreover, the total numbers of leukocytes or of different leukocyte subsets (lymphocytes, monocytes, and granulocytes) were normal in the peripheral blood of SKAP-HOM-deficient animals (Table 1). Similarly, the numbers of splenocytes, thymocytes, and lymph node cells were unaffected in the absence of SKAP-HOM. Platelet counts were slightly (but not significantly) lower in SKAP-HOM-deficient mice compared to wild-type animals. Within the lymphatic organs of wild-type mice, SKAP-HOM was found to be expressed in T cells, B cells, CD4+ T cells, CD8+ T cells, and, to a lesser extent, thymocytes (Fig. 1D). Unfortunately, we could not analyze the expression pattern of SKAP-HOM within the T-cell compartment in more detail (e.g., whether SKAP-HOM is selectively expressed in particular T-cell subsets), because the polyclonal antiserum we used for Western blotting was not suitable for flow cytometry or for microscopy.
FIG. 1.
Scheme showing the retroviral insertion into the SKAP-HOM locus, the expression pattern of SKAP-HOM in lymphoid organs, and the loss of SKAP-HOM protein in SKAP-HOM-deficient mice. (A) The VICTR20 retroviral insertion site in the SKAP-HOM locus was determined by restriction site mapping and comparing the wild-type and retroviral-targeted alleles. PCR products generated by oligonucleotide primers (a to c; also used for genotyping) positioned as shown were sequenced to identify the precise site of retroviral integration. Only the first 4 out of 12 coding exons are shown. (B) Mice were genotyped by PCR detecting the wild-type SKAP-HOM allele and the recombinant allele containing the neomycin resistance cassette. (C) Postnuclear lysates of total splenocytes of knock-out, wild-type, and heterozygous animals were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by blotting onto nitrocellulose sheets. Immunoblots were probed with a polyclonal rabbit anti-SKAP-HOM antiserum. (D) Total splenocytes and thymocytes were prepared from wild-type animals. Splenic B cells and T cells were obtained by negative selection with microbeads. CD4+ T cells and CD8+ T cells were obtained by positive selection with microbeads. Postnuclear lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resulting blots were probed as for panel C. (E) Loss of SKAP-HOM does not affect expression of ADAP and SKAP55 in the T-cell compartment. Postnuclear lysates of purified splenic T- and B-cell populations were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by anti-SKAP-HOM, anti-ADAP, and anti-SKAP55 Western blotting. LTR, long terminal repeat; KO, knockout; WT, wild-type; HT, heterozygous.
TABLE 1.
Hematopoietic cellularity in 8- to 12-week-old wild-type and SKAP-HOM-deficient littermatesa
| Hematopoietic cellularity | Mouse
|
|
|---|---|---|
| Wild type (n = 12) | SKAP-HOM−/− (n = 10) | |
| Splenocyte number (106) | 148 ± 15 | 182 ± 30 |
| Thymocyte number (106) | 154 ± 12 | 184 ± 18 |
| Lymph node cells (106) (inguinal + axillary) | 12 ± 2 | 11 ± 2 |
| White blood cells (103 cells/μl) | 4.4 ± 0.5 | 4.5 ± 0.7 |
| Lymphocytes (103 cells/μl) | 3.6 ± 0.4 | 3.9 ± 0.5 |
| Monocytes (103 cells/μl) | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Granulocytes (103 cells/μl) | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Platelets (103 cells/μl) | 555 ± 105 | 442 ± 102 |
Note that the differences in platelet counts did not reach statistical significance as assessed by one-way analysis of variance.
Recently it had been demonstrated that loss of ADAP in the T-cell line Jurkat induces a concomitant loss of its binding partner SKAP55, likely because SKAP55 is subjected to degradation when not complexed with ADAP (13). Since SKAP-HOM is also expressed in T-cells and, similar to SKAP55, is capable of associating with ADAP, we had to formally exclude the possibility that loss of SKAP-HOM−/− in T and/or B cells influences the expression levels of either ADAP or SKAP55. Figure 1E shows that this is not the case. Indeed, T-cells of SKAP-HOM-deficient mice express normal amounts of both ADAP and SKAP55. While these data exclude the possibility that the expression of ADAP or SKAP55 is dependent on SKAP-HOM, they also indicate that loss of SKAP-HOM within the T-cell compartment could be compensated for by the ADAP/SKAP55 complex. In contrast to T cells (and as reported previously), B lymphocytes express SKAP-HOM but not ADAP or SKAP55 (5, 20, 23).
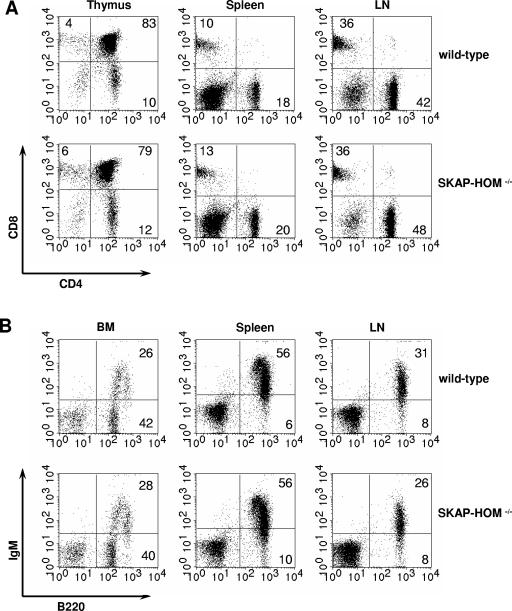
Lymphocyte populations of spleen, lymph nodes, and bone marrow of SKAP-HOM-deficient mice were further analyzed by flow cytometry with a panel of lineage- and stage-specific monoclonal antibodies. The number and distribution of T cells in the various immunological organs (thymus, spleen, lymph nodes, and bone marrow) as well as the CD4/CD8 ratio was normal in SKAP-HOM−/− mice. Also, developmental and maturation markers of B cells (including marginal zone B cells and B1 cells) showed no abnormalities in bone marrow, spleen, lymph nodes, and the peritoneal cavity (Fig. 2 and data not shown). Thus, although SKAP-HOM is ubiquitously expressed within the hematopoietic system, its absence is either dispensable for normal hematopoietic cell development or can be compensated for by other molecules.
FIG. 2.
Normal T- and B-lymphocyte differentiation in SKAP-HOM-deficient mice. (A) Thymic T-cell precursors, splenic T cells, and lymph node T cells were analyzed by flow cytometry after staining with CD4-FITC and CD8-phycoerythrin. (B) Bone marrow B-cell precursors, splenic B cells, and lymph node B cells were analyzed after staining with B220-FITC and IgM-phycoerythrin. The percentages of cells within each lymphocyte population are indicated. BM, bone marrow; LN, lymph node.
Impaired B-cell responses in SKAP-HOM−/− mice.
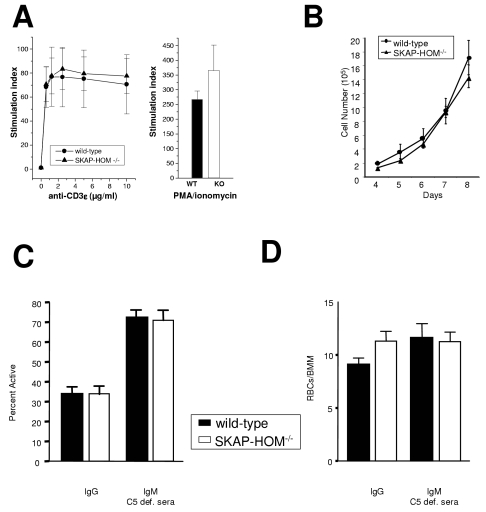
We next assessed the consequences of SKAP-HOM deficiency in various hematopoietic cell populations known to express SKAP-HOM. These experiments revealed that T-cell proliferation (as judged by [3H]thymidine incorporation) towards a variety of different stimuli (anti-βTCR, anti-CD3ɛ, PMA plus ionomycin) was comparable between SKAP-HOM-deficient mice and control animals (Fig. 3A). Note that the apparent hyperproliferation of PMA-ionomycin-stimulated T cells is due to variability within the different samples and did not reach significance. There was also no difference in the production of tumor necrosis factor α, IL-4, IL-5, gamma interferon, and IL-2 by stimulated T cells between knock-out and wild-type mice (data not shown). This strongly suggests that loss of SKAP-HOM does not alter in vitro T-cell functions, possibly because its loss is compensated for by the ADAP/SKAP55 complex (see above).
FIG. 3.
Normal T-cell and BMM proliferation and normal Fc- and complement receptor-mediated phagocytosis in SKAP-HOM-deficient mice. (A) Purified splenic T cells were stimulated with increasing concentrations of CD3ɛ MAbs or with a combination of PMA plus ionomycin. Data of quadruplicate cultures are shown as means ± SEM; n = 5. (B) BMMs isolated from wild-type or knockout mice were cultured for 2 days and then plated in replicates in the presence of 10 ng/ml M-CSF. Cells were harvested at the indicated times and counted. (C) BMMs were incubated with sheep red blood cells opsonized with either IgG or IgM and C5-deficient (def.) sera. BMMs were scored for the percent of phagocytically active cells and for the ability of active cells to phagocytize RBCs (D). Results are representative of three separate experiments.
Similar to T cells, stimulation of SKAP-HOM deficient platelets with collagen-related peptide (a ligand for the ITAM-coupled receptor glycoprotein VI [GPVI]) or thrombin (which activates the G protein-coupled receptors protease-activated receptors 3 and 4 [PAR3 and PAR4]) exhibited normal aggregation and α-granule secretion. Platelet spreading on collagen and fibrinogen was also normal in SKAP-HOM-deficient mice. Likewise, tyrosine phosphorylation of LAT, SLP-76, and PLGγ2 after stimulation of GPVI with collagen-related peptide was unaffected (data not shown). These findings suggest that SKAP-HOM is also dispensable for platelet function.
SKAP-HOM is expressed in macrophages, and previous overexpression studies had suggested that in myeloid cells it negatively regulates cytokine-mediated growth (2, 4). To assess whether loss of SKAP-HOM alters growth of ex vivo-isolated myeloid cells, we measured M-CSF-induced proliferation of BMM prepared from wild-type, heterozygous, and SKAP-HOM-deficient mice. As shown in Fig. 3B, BMMs of SKAP-HOM−/− mice and wild-type mice showed almost identical growth rates after stimulation with M-CSF and GM-CSF (data not shown). Thus, SKAP-HOM does not seem to play a major role in regulating cytokine-mediated growth of BMMs. Moreover, analysis of SKAP-HOM-deficient BMMs did not reveal significant differences in Fc- or complement receptor-mediated phagocytosis of IgG- or IgM-coated sheep red blood cells (Fig. 3C and D).
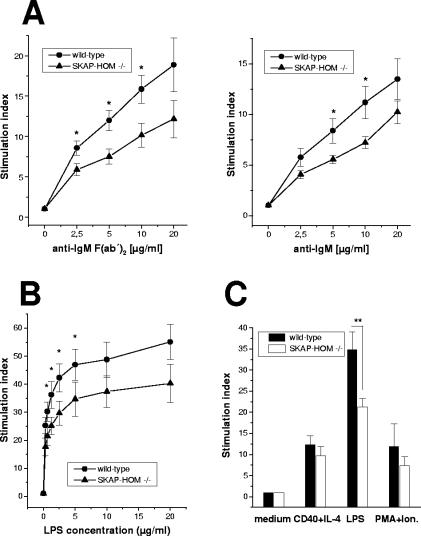
In marked contrast to T cells, platelets, and macrophages, the proliferative responses of anti-IgM stimulated B lymphocytes were significantly decreased in SKAP-HOM−/− mice (Fig. 4A). Similarly, the response to LPS, which is mediated via toll-like receptor 4 (TLR4), was strongly impaired (Fig. 4B). In contrast, the same cells responded normally after stimulation with PMA-ionomycin or a combination of CD40 monoclonal antibody (MAb) plus IL-4, ruling out a general defect in the capability of B cells to become activated (Fig. 4C). These results were confirmed with CFSE staining, excluding the possibility that the decreased proliferation observed was due to increased apoptosis (data not shown). Rather, the data suggest that SKAP-HOM is involved in the regulation of BCR- and TLR4-mediated activation of B lymphocytes.
FIG. 4.
Impaired B-cell proliferation in SKAP-HOM-deficient mice. (A) Purified splenic B cells from wild-type or SKAP-HOM-deficient mice were stimulated with the indicated concentration of soluble anti-IgM F(ab′)2 or soluble anti-IgM; n = 10; results are means ± SEM. The mean counts per minute of maximally IgM F(ab′)2-stimulated wild-type B cells was 7,045 ± 893 cpm versus 407 ± 69 cpm of unstimulated cells. For SKAP-HOM-deficient B cells, the mean counts per minute was 6,417 ± 663 versus 628 ± 60, respectively. (B) Purified splenic B cells were stimulated with the indicated concentration of LPS (0.31 to 20 μg/ml). (C) Purified splenic B cells were activated with either anti-CD40+IL-4, LPS (2.5 μg/ml), or PMA plus ionomycin (Ion.). The stimulation index was calculated as the ratio of counts per minute (cpm) of stimulated to unstimulated proliferation. Nonstimulated B cells are shown as controls; n = 8; shown are means ± SEM. *, P < 0.05; **, P < 0.01.
In summary, the data shown in Fig. 3 and 4 indicate that loss of SKAP-HOM primarily alters B-cell functions.
Reduced levels of serum immunoglobulins in SKAP-HOM−/− mice.
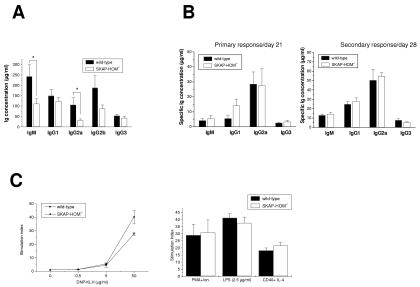
The impaired proliferative in vitro response of SKAP-HOM-deficient B cells prompted us to assess the spontaneous and induced production of immunoglobulins in wild-type and SKAP-HOM-deficient animals. As shown in Fig. 5, considerably lower concentrations of constitutive serum immunoglobulins were found in SKAP-HOM−/− mice compared to wild-type littermates. This was in particular true for the IgM, IgG2a, and IgG2b isotypes (Fig. 5A).
FIG. 5.
Constitutive serum immunoglobulin levels and humoral immune responses in SKAP-HOM-deficient mice. (A) Constitutive serum immunoglobulin levels in nonimmunized wild-type and SKAP-HOM-deficient animals; n = 11; shown are means ± SEM. (B) Humoral immune response after immunization with the T-dependent antigen DNP-KLH. Twelve-week-old mice were injected at days 0 and day 21 intraperitoneally with DNP-KLH. Mice were bled before boostering (on day 21, corresponding to primary response) and after boostering (on day 28, corresponding to secondary response). Anti-DNP-specific levels of IgM, IgG1, IgG2a, and IgG3 were measured by ELISA. Four animals were investigated for each group. (C) Proliferative response of splenocytes after the secondary immunization to DNP-KLH. Lymphocytes isolated from the spleen at day 28 (after boostering) were stimulated with the indicated concentrations of DNP-KLH for 72 h. Results are given as the mean stimulation index of three independent experiments (means ± SEM). Ion., ionomycin.
To investigate the humoral response after in vivo immunization, wild-type and SKAP-HOM-deficient animals were immunized with a T-dependent (DNP-KLH) or a T-independent model antigen (TNP-LPS). In the former case, the primary immune response was measured 21 days after the first immunization. On the same day the mice received a second immunization (boostering), and the secondary immune response was assessed 7 days later. As shown in Fig. 5B, the magnitude of DNP-KLH-specific IgM, IgG1, IgG2a, and IgG3 was similar in wild-type and SKAP-HOM−/− animals with regard to both the primary and the secondary immune response. Moreover, DNP-KLH and mitogen-induced in vitro proliferation of splenocytes after the second immunization was unaffected in SKAP-HOM-deficient animals (Fig. 5C).
Similar to the response after immunization with DNP-KLH, SKAP-HOM-deficient mice responded normally after immunization with the T-independent antigen TNP-LPS (data not shown). Collectively, these data indicate that loss of SKAP-HOM affects basal immunoglobulin production, whereas SKAP-HOM-deficient mice are able to mount normal nitrophenyl-specific primary and secondary responses to all immunoglobulin isotypes.
Normal proximal signaling in SKAP-HOM−/− B cells.
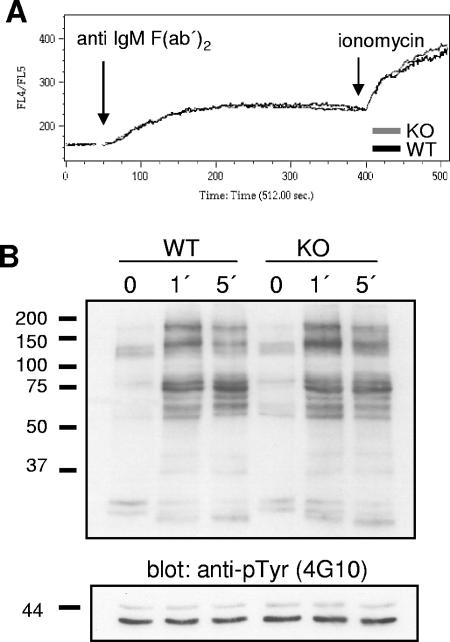
Despite the apparently normal B-cell response after immunization with T-dependent and T-independent antigens, the impaired B-cell proliferation following anti-IgM and LPS stimulation suggested that loss of SKAP-HOM results in a subtle failure(s) of the BCR to be coupled to intracellular signaling pathways. To further elucidate the molecular mechanism underlying the functional alterations of SKAP-HOM-deficient B cells, we analyzed membrane-proximal signaling events downstream of the BCR. First, we assessed the ability of SKAP-HOM−/− B cells to elicit a calcium response upon stimulation with an anti-IgM MAb. As shown in Fig. 6A, at an antibody concentration at which impaired B-cell proliferation of SKAP-HOM deficient B-cells was clearly evident, no differences in BCR-mediated calcium flux were discernible between splenic B cells of wild-type and SKAP-HOM−/− mice. Similarly, BCR stimulation did not reveal any significant differences in global tyrosine phosphorylation (Fig. 6B) as well as in the phosphorylation of Erk1/2, JNK, p38, and PKB/Akt after short-time (up to 10 min) as well as after long-time stimulation (up to 60 min; data not shown). Thus, loss of SKAP-HOM does not affect the major membrane-proximal signaling events downstream of the BCR.
FIG. 6.
Normal proximal BCR-mediated signaling in the absence of SKAP-HOM. (A) Freshly isolated, indo-1-loaded splenic B cells were stimulated with anti-IgM F(ab′)2 (10 μg/ml) or, as a positive control, with ionomycin (100 nM). Intracellular calcium levels were measured by flow cytometry. (B) Purified splenic B cells were left unstimulated or treated with soluble anti-IgM F(ab′)2 (10 μg/ml) for the indicated minutes. Cellular lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blots were probed with anti-phosphotyrosine (anti-pTyr) MAb 4G10. The expression levels of total Erk1 and Erk2 are shown as loading controls in the lower panel (additional data not shown). KO, knockout; WT, wild type.
Impaired adhesion of SKAP-HOM−/− mice B cells.
A strongly reduced proliferation after immunoreceptor engagement in combination with almost normal membrane-proximal signaling events is reminiscent of the situation that has been described for T cells lacking the cytosolic adaptor protein ADAP (9, 27). ADAP−/− T cells are severely compromised in their ability to adhere to ligands of β1 and β2 integrins, such as ICAM-1 and fibronectin. Similarly, downregulation of SKAP55 (e.g., by RNA interference) has the same effect (15 and S. Kliche and B. Schraven, unpublished data).
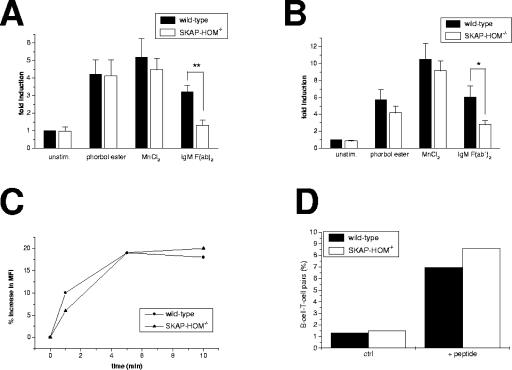
To assess whether loss of SKAP-HOM alters B-cell adhesion, purified splenic B lymphocytes were stimulated via the BCR and then allowed to adhere to fibronectin (a ligand of the β1 integrin VLA-4) or to ICAM-1 (a ligand of the β2 integrin LFA-1). As shown in Fig. 7A and B, BCR-induced adhesion to both fibronectin and ICAM-1 was strongly attenuated in SKAP-HOM−/− B cells. In contrast, treatment of the same cells with manganese chloride (which induces a conformational change of the extracellular domain of integrins, thereby switching them to the high-affinity state) or phorbol ester (which induces the high-affinity state of the integrins via inside-out signaling by bypassing membrane-proximal signaling events) induced adhesion comparable to that of wild-type cells. The latter finding excludes the possibility that loss of SKAP-HOM generates an intrinsic functional defect of either β1 or β2 integrins. Moreover, flow cytometry analysis did not reveal alterations in cell surface expression of CD11a/CD18 (LFA-1) and CD49d/CD29 (VLA-4) in SKAP-HOM−/− B cells (data not shown), which rules out the possibility that loss of adhesiveness of SKAP-HOM-deficient B cells is caused by blunted expression of integrins. Rather, it appeared that, similar to ADAP/SKAP55 in T cells, SKAP-HOM is involved in coupling the BCR to integrin activation.
FIG. 7.
Attenuated BCR-mediated adhesion but normal F-actin polymerization and conjugate formation of SKAP-HOM-deficient B cells. (A) Purified splenic B cells were stimulated with anti-IgM F(ab′)2 and then allowed to adhere to tissue cell culture dishes coated with either fibronectin (A) or recombinant murine ICAM-1 (B). Adherent cell numbers were determined by microscopy. Results are expressed as means ± SEM of six independently performed experiments. (C) Purified B cells were treated with anti-IgM F(ab′)2, permeabilized with saponin, and then stained with FITC-phalloidin. The cellular F-actin polymerization was assayed by flow cytometry after 1, 5, and 10 min. Results are expressed as percent increase of mean fluorescence intensity (MFI). The shown graph is representative of five independently performed experiments. (D) Purified B cells were loaded with OVA peptide overnight. T cells of OT-II TCR-transgenic mice were loaded with CFSE and coincubated with the peptide-pulsed B cells for 8 h. Conjugate formation was subsequently assessed by flow cytometry. Results are expressed as percentages of loaded B cells conjugated with T cells. The shown graph is representative of four independently performed experiments. unstim., unstimulated.
To investigate whether the adhesion defect of SKAP-HOM−/− B cells could be attributed to impaired dynamics of the actin cytoskeleton, we compared BCR-induced actin polymerization in wild-type and SKAP-HOM-deficient B cells. To this end, activated B cells were permeabilized with saponin and subsequently stained with phalloidin-tetramethyl rhodamine isocyanate, a probe specifically reacting with polymerized F-actin. The amount of polymerized F-actin within the cells was subsequently determined by flow cytometry. Figure 7C depicts that actin polymerization occurs normally in the absence of SKAP-HOM, which suggests that SKAP-HOM is dispensable for BCR-mediated reorganization of the cytoskeleton.
The β2 integrin LFA-1 has been shown to be involved in the formation of the immunological synapse in T cells and in conjugate formation between B and T cells at the onset of an immune response (12). To assess whether loss of SKAP-HOM impairs conjugate formation, we loaded freshly isolated splenic B cells with the model peptide OVA323-339 and then incubated the peptide-loaded B cells with T cells of OT-II TCR-transgenic mice. Subsequently, conjugate formation between T and B cells was evaluated by flow cytometry. Figure 7D shows that conjugate formation is not impaired in SKAP-HOM-deficient B lymphocytes. In addition, the formation of the B-cell synapse and the clustering of LFA-1 after MAb-mediated activation of the BCR also seem to occur normally in the absence of SKAP-HOM (F. Batista, personal communication). Thus, SKAP-HOM seems to be dispensable for conjugate formation between T cells and APCs and for formation of the immunological synapse after antibody-mediated triggering of the BCR.
Decreased severity of EAE in SKAP-HOM−/− mice.
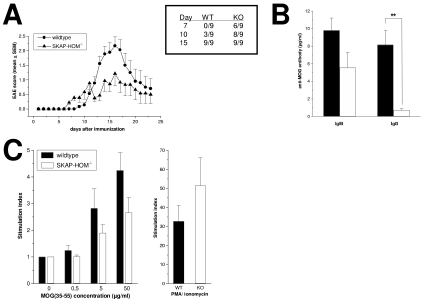
Although EAE is considered to primarily represent a Th1-mediated autoimmune disease, a number of studies have suggested a role for B cells and autoantibodies in EAE (7, 8, 14). To asses the consequences of SKAP-HOM deficiency in this autoimmune model, wild-type mice and SKAP-HOM−/− mice were immunized with the encephalitogenic peptide MOG 35-55. Figure 8A demonstrates that both groups of mice showed maximal disease scores at days 15 to 17 after immunization, which was followed by a remission phase. However, despite the fact that the first clinical signs of disease appeared somewhat earlier in SKAP-HOM−/− animals (Fig. 8A and the inset table), the mean clinical score of EAE was clearly more severe in wild-type animals (mean score of 2.2 in wild-type animals versus 1.2 in SKAP-HOM−/− animals at day 17, respectively; P < 0.05). Thus, SKAP-HOM−/− mice are not only impaired in their ability to activate B lymphocytes in vitro but also show a blunted immune response after immunization with the encephalitogenic peptide MOG in vivo.
FIG. 8.
SKAP-HOM-deficient mice show reduced severity of EAE. (A) EAE was induced following immunization with MOG 35-55 (200 μg MOG in CFA). The severity of EAE is presented as mean clinical scores (means ± SEM; n = 9). The accompanying table in the box shows the incidence of EAE within the experimental groups at days 7, 10, and 15, respectively. The shown graph is representative of three independently performed experiments. The difference in the mean clinical score was significant at days 7, 8, 13, 15, and 17 (P < 0.05). (B) Serum levels of anti-MOG-specific antibodies in sera collected at day 14 after immunization of mice with MOG 35-55. Concentration of specific IgM and IgG were measured by ELISA. (C) Proliferative response to MOG 35-55 of lymphocytes isolated from draining lymph nodes at day 14 following immunization with MOG 35-55 (n = 5). Lymph node cells were cultured with or without MOG 35-55 for 72 h. The mean counts per minute of maximally MOG-stimulated wild-type lymph node cells was 4,431 ± 856 cpm versus 1,059 ± 211 cpm of unstimulated cells. For SKAP-HOM−/− lymph node cells, the mean cpm was 1,969 ± 860 versus 610 ± 191, respectively. WT, wild type; KO, knockout.
To further elucidate the mechanisms underlying the altered response of SKAP-HOM-deficient mice in the EAE model, we measured the in vivo levels of the MOG-specific autoantibodies at day 15 after immunization. Figure 8B shows that the levels of MOG-specifc IgG and, to a lesser extent, MOG-specifc IgM antibodies in the serum were much lower in SKAP-HOM−/− mice compared to wild-type animals. In addition, a decreased MOG-specific proliferation of lymph node cells was observed (Fig. 8C). These findings suggest that loss of SKAP-HOM within the hematopoietic system leads to an attenuated clinical course of EAE which is accompanied by reduced production of MOG-specific immunoglobulins as well as a reduced MOG-induced T-cell response.
DISCUSSION
In this study we investigated the immune system of mice deficient in expression of the cytosolic adaptor protein SKAP-HOM. SKAP-HOM represents the ubiquitously expressed homologue of the T-cell-specific adaptor protein SKAP55, a physiological binding partner of the integrin-regulating adaptor ADAP (17, 21). Mice lacking SKAP-HOM−/− are viable and fertile and exhibit no obvious abnormalities. Therefore, it is likely that SKAP-HOM−/− is not required during development of the growing organism or the hematopoietic system.
Although no anatomic abnormalities were observed in the primary and secondary lymphoid organs, SKAP-HOM−/− mice showed reduced levels of spontaneous immunoglobulins in the serum. The constitutive defect in the production of IgM, IgG2a, and IgG2b apparently does not result from loss of a particular B-cell population but rather is due to a reduced ability of SKAP-HOM-deficient B cells to proliferate after stimulation of the BCR or TLR4.
Our attempts to identify the signaling defect(s) underlying the reduced ability of SKAP-HOM-deficient B cells to respond to external stimuli did not reveal significant alterations of BCR-mediated global tyrosine phosphorylation, calcium flux, or activation of the mitogen-activated protein kinase pathways. These data collectively suggest that the major membrane-proximal signaling events regulating B-cell activation are not impaired in the absence of SKAP-HOM. However, we found that the lack of SKAP-HOM results in a severely compromised ability to couple the BCR to regulation of β1 and β2 integrins, thereby causing reduced adhesion of B cells to fibronectin and to ICAM-1. It is tempting to speculate that these adhesion defects are the major reason for the observed alterations of both the in vivo and in vitro B-cell responses that we see in the SKAP-HOM−/− mice.
Both SKAP-HOM and SKAP55 are reported to form a complex with the cytosolic adaptor protein ADAP (17, 21). In T cells, the association between SKAP55 and ADAP has been shown to be mediated via the SH3 domain of SKAP55 and the proline-rich domain of ADAP (17, 21). Moreover, loss of ADAP in T cells results in a failure of integrin-mediated adhesion as well as impaired T-cell proliferation after TCR stimulation (9, 27). Preliminary data obtained by RNA interference approaches in the T-cell line suggest a similar adhesion-regulating function for SKAP55 (15 and S. Kliche and B. Schraven, unpublished). Moreover, a recent report had demonstrated that overexpression of SKAP55 augments both T-cell adhesion to fibronectin and ICAM-1 as well as conjugate formation between T cells and APCs (31). Thus, it appears as if in T cells ADAP and SKAP55 form a functional unit that couples the TCR to integrin activation, thereby regulating T-cell adhesion. Our finding that B-cell adhesion is impaired in SKAP-HOM-deficient mice strongly suggests that SKAP-HOM fulfils a similar function in B lymphocytes as SKAP55 and ADAP in T-cells. One of the major challenges for the future will be to assess whether a B-cell homologue of ADAP (ADAP-HOM) exists that constitutively interacts with SKAP-HOM in B lymphocytes and couples the BCR to integrin activation.
It is, however, important to note that the adhesion defect caused by loss of SKAP-HOM is apparently less severe than the T-cell adhesion defect that is observed in ADAP-knockout mice. This could indicate that ADAP regulates adhesion not only via SKAP55 but also through another, as yet unidentified signaling molecule(s). Alternatively, the possibility has to be considered that loss of SKAP-HOM in B cells is partially compensated for by another protein(s) that regulate(s) B-cell adhesion.
Although additional studies are required to solve this puzzle, it appears as if the impaired adhesion of B cells in vitro does not affect the ability of B cells to populate the different lymphoid organs in vivo. This could be explained by the fact that the regulated induction of adhesion and migration in vivo results from complex interactions of different signaling pathways and that in the orchestra of events regulating, e.g., homing of lymphocytes, the absence of one player can be compensated for by others. In line with this hypothesis is the finding that also in ADAP−/− mice (in which T cells show a strongly impaired adhesion to ICAM-1 and ICAM-2, VCAM-1, and fibronectin after TCR engagement) the colonization of the lymphoid organs appears to be almost normal (9, 27).
The unaltered response of the SKAP-HOM-deficient animals after immunization with DNP-KLH versus the clearly reduced in vitro response of SKAP-HOM-deficient B lymphocytes and the lower levels of anti-MOG antibodies after immunization of the animals with MOG-peptide seem to be contradictory. However, it is important to note that the DNP-KLH immunization for inducing a humoral immune response and, e.g., the immunization with MOG peptide that is used for induction of EAE are not directly comparable. The different nature of antigen (hapten plus carrier versus peptide), the different routes of application (intraperitoneal versus subcutaneous), and the different immunization protocols (booster dose versus single application) may explain these diverging results.
In this regard, an elegant recent in vitro study showed that integrin (e.g., LFA-1)-mediated adhesion is primarily important for B-cell activation under suboptimal conditions of stimulation (e.g., when either low-affinity antigens are used for stimulation or when the concentration of the activating peptide is very low) (3). Possibly, in terms of the B-cell response, subcutaneous immunization with MOG peptide represents a “suboptimal” condition in which the contribution of integrin-mediated adhesion is important to allow appropriate B-cell activation. In contrast, i.p. immunization with optimal doses of high-affinity T-dependent or T-independent antigens might reflect conditions of stimulation in which the contribution of integrin-mediated adhesion is less important. The elucidation of these possibilities requires immunization of the animals with different doses of T-dependent or T-independent antigens and subsequent monitoring of the B-cell response. Additionally, or alternatively, SKAP-HOM-deficient mice could be crossed with mice carrying a transgenic BCR, as described previously (3). Ex vivo-isolated B cells from such double transgenic animals could be stimulated with different concentrations of peptide or with altered peptides, followed by analysis of B-cell functions (microscopic analysis of the B-cell synapse and/or functional readouts such as CD86 expression). We are currently preparing to assess these possibilities.
Nevertheless, the impaired in vitro response of SKAP-HOM-deficient B lymphocytes, the lower constitutive concentrations of serum immunoglobulins, and the strongly reduced antibody response in the EAE model suggest that SKAP-HOM plays an important role in the generation of an appropriate immune response after challenge with particular antigens (see also below).
Loss of SKAP-HOM attenuates the clinical course of EAE. This is accompanied by a decreased synthesis of MOG-specific antibodies and a reduced secondary MOG-specific proliferation of lymph node cells. The apparently normal in vitro functions of SKAP-HOM-deficient T cells might suggest that the attenuated course of EAE is mainly due to an altered B-cell response. However, EAE is primarily considered to represent a Th1-mediated autoimmune disease, and the pathophysiolgic role of B cells and autoantibodies is controversial. For example, B-cell-deficient mice immunized with rodent MOG 35-55 develop a course of EAE that is indistinguishable from that of wild-type mice. This suggests that B cells do not play a major role in EAE (11). By contrast, passive transfer of activated B cells or serum from wild-type mice immunized with MOG restores the susceptibility of B-cell-deficient mice to develop EAE. This indicates that MOG-specific B cells and anti-MOG antibodies seem to contribute to the pathology of EAE (18, 19, 26). In line with the latter assumption is a recent report by Filatreau et al. which clearly showed a role of B cells in regulating the clinical course of EAE (7). Thus, further studies are required to assess whether impaired B-cell responses alone are responsible for the altered outcome of EAE in SKAP-HOM-deficient mice.
Indeed, it might well be that not only blunted B-cell functions but also an altered cooperation between T and B cells results in an incomplete T-cell activation leading to an attenuated MOG-specific immune response in SKAP-HOM-deficient mice. In addition (or alternatively), the possibility cannot be excluded that the altered course of EAE in the SKAP-HOM−/− mice is in part (if not primarily) due to an impaired cooperation between dendritic cells (DC), macrophages, and T cells. Although further experiments are required to precisely assess the functions of SKAP-HOM−/− DCs and macrophages, two preliminary pieces of evidence argue against a severe functional alteration of antigen-presenting cells in the absence of SKAP-HOM. First, we did not find significant impairments of Fc- or complement receptor-mediated phagocytosis in SKAP-HOM-deficient macrophages, which suggests that antigen uptake (at least via these receptors) is not dependent on SKAP-HOM. Moreover, conjugate formation between OVA323-339-loaded DCs and the corresponding OT-II TCR transgenic T cells is not affected in the absence of SKAP-HOM. However, whether it also applies for DCs loaded with MOG or other (suboptimal) peptides requires further analysis.
Finally, despite apparently normal functions in vitro (which might be due to the fact that loss of SKAP-HOM is compensated for by the presence of SKAP55, whose expression is not affected in T cells of SKAP-HOM-deficient mice), the possibility cannot be excluded that SKAP-HOM-deficient T cells are also slightly impaired in their ability to respond to specific antigens (such as MOG) in vivo. In summary, further studies (including the analysis of microglia cells which express SKAP-HOM) are required to answer the question of how loss of SKAP-HOM attenuates the clinical course of EAE. In addition, it will be necessary to assess the question of whether loss of SKAP-HOM exclusively alters the clinical outcome of EAE or whether it also affects the course of other model diseases (e.g., infection with Yersinia enterocolitica). Experiments are under way to address these questions.
Nevertheless, we have shown here that loss of SKAP-HOM attenuates B-cell functions and that it impairs the cross-talk between the BCR and integrins. These findings might be important in light of recent data showing that the α4 integrin (VLA-4) antagonistic monoclonal antibody Natalizumab reduces the development of brain lesions in murine EAE as well as in controlled clinical trials with patients suffering from multiple sclerosis (24). VLA-4 is expressed on activated T cells, B cells, and monocytes. Our data demonstrating loss of adhesion to the VLA-4 ligand fibronectin in SKAP-HOM-deficient B lymphocytes and attenuated EAE in SKAP-HOM-deficient mice might suggest that the beneficial effect of Natalizumab is partially due to an inhibition of B-cell invasion into the central nervous system. Although further histopathological studies are required to analyze the nature and the numbers of infiltrating immunocompetent cells in the central nervous system of wild-type and SKAP-HOM-deficient mice during EAE, our data indicate that interference with SKAP-HOM functions might be an attractive new avenue for pharmaceutical intervention in autoimmune diseases such as multiple sclerosis.
Acknowledgments
The studies were supported by Deutsche Forschungsgemeinschaft DFG grants Schr 533/5-1 and Schr 533/5-2 and by NIH grants P01 DK50654 and R01 DK50693 to B.G.N. A.C.P. is funded by the Wellcome Trust.
REFERENCES
- 1.Black, D. S., A. Marie-Cardine, B. Schraven, and J. B. Bliska. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2:401-414. [DOI] [PubMed] [Google Scholar]
- 2.Bourette, R. P., J. Therier, and G. Mouchiroud. 2005. Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell Signal. 17:941-949. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco, Y. R., S. J. Fleire, T. Cameron, M. L. Dustin, and F. D. Batista. 2004. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 20:589-599. [DOI] [PubMed] [Google Scholar]
- 4.Curtis, D. J., S. M. Jane, D. J. Hilton, L. Dougherty, D. M. Bodine, and C. G. Begley. 2000. Adaptor protein SKAP55R is associated with myeloid differentiation and growth arrest. Exp. Hematol. 28:1250-1259. [DOI] [PubMed] [Google Scholar]
- 5.Da Silva, A. J., Z. Li, C. de Vera, E. Canto, P. Findell, and C. E. Rudd. 1997. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA 94:7493-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallman, M., F. Deleuil, and K. McGee. 2002. Resistance to phagocytosis by Yersinia. Int. J. Med. Microbiol. 291:501-509. [DOI] [PubMed] [Google Scholar]
- 7.Fillatreau, S., C. H. Sweenie, M. J. McGeachy, D. Gray, and S. M. Anderton. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944-950. [DOI] [PubMed] [Google Scholar]
- 8.Genain, C. P., B. Cannella, S. L. Hauser, and C. S. Raine. 1999. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 5:170-175. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths, E. K., C. Krawczyk, Y. Y. Kong, M. Raab, S. J. Hyduk, D. Bouchard, V. S. Chan, I. Kozieradzki, A. J. Oliveira-Dos-Santos, A. Wakeham, P. S. Ohashi, M. I. Cybulsky, C. E. Rudd, and J. M. Penninger. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260-2263. [DOI] [PubMed] [Google Scholar]
- 10.Gu, H., R. J. Botelho, M. Yu, S. Grinstein, and B. G. Neel. 2003. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J. Cell Biol. 161:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hjelmstrom, P., A. E. Juedes, J. Fjell, and N. H. Ruddle. 1998. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J. Immunol. 161:4480-4483. [PubMed] [Google Scholar]
- 12.Hogg, N., M. Laschinger, K. Giles, and A. McDowall. 2003. T-cell integrins: more than just sticking points. J. Cell Sci. 116:4695-4705. [DOI] [PubMed] [Google Scholar]
- 13.Huang, Y., D. D. Norton, P. Precht, J. L. Martindale, J. K. Burkhardt, and R. L. Wange. 2005. Deficiency of ADAP/FYB/SLAP130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280:23576-23583. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias, A., J. Bauer, T. Litzenburger, A. Schubart, and C. Linington. 2001. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 36:220-234. [DOI] [PubMed] [Google Scholar]
- 15.Jo, E. K., H. Wang, and C. E. Rudd. 2005. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 201:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouroku, Y., A. Soyama, E. Fujita, K. Urase, T. Tsukahara, and T. Momoi. 1998. RA70 is a src kinase-associated protein expressed ubiquitously. Biochem. Biophys. Res. Commun. 252:738-742. [DOI] [PubMed] [Google Scholar]
- 17.Liu, J., H. Kang, M. Raab, A. J. Da Silva, S. K. Kraeft, and C. E. Rudd. 1998. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. USA 95:8779-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons, J. A., M. J. Ramsbottom, and A. H. Cross. 2002. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 32:1905-1913. [DOI] [PubMed] [Google Scholar]
- 19.Lyons, J. A., M. San, M. P. Happ, and A. H. Cross. 1999. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur. J. Immunol. 29:3432-3439. [DOI] [PubMed] [Google Scholar]
- 20.Marie-Cardine, A., E. Bruyns, C. Eckerskorn, H. Kirchgessner, S. C. Meuer, and B. Schraven. 1997. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 272:16077-16080. [DOI] [PubMed] [Google Scholar]
- 21.Marie-Cardine, A., L. R. Hendricks-Taylor, N. J. Boerth, H. Zhao, B. Schraven, and G. A. Koretzky. 1998. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 273:25789-25795. [DOI] [PubMed] [Google Scholar]
- 22.Marie-Cardine, A., H. Kirchgessner, C. Eckerskorn, S. C. Meuer, and B. Schraven. 1995. Human T lymphocyte activation induces tyrosine phosphorylation of alpha-tubulin and its association with the SH2 domain of the p59fyn protein tyrosine kinase. Eur. J. Immunol. 25:3290-3297. [DOI] [PubMed] [Google Scholar]
- 23.Marie-Cardine, A., A. M. Verhagen, C. Eckerskorn, and B. Schraven. 1998. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 435:55-60. [DOI] [PubMed] [Google Scholar]
- 24.Miller, D. H., O. A. Khan, W. A. Sheremata, L. D. Blumhardt, G. P. Rice, M. A. Libonati, A. J. Willmer-Hulme, C. M. Dalton, K. A. Miszkiel, and P. W. O'Connor. 2003. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348:15-23. [DOI] [PubMed] [Google Scholar]
- 25.Musci, M. A., L. R. Hendricks-Taylor, D. G. Motto, M. Paskind, J. Kamens, C. W. Turck, and G. A. Koretzky. 1997. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 272:11674-11677. [DOI] [PubMed] [Google Scholar]
- 26.Oliver, A. R., G. M. Lyon, and N. H. Ruddle. 2003. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J. Immunol. 171:462-468. [DOI] [PubMed] [Google Scholar]
- 27.Peterson, E. J., M. L. Woods, S. A. Dmowski, G. Derimanov, M. S. Jordan, J. N. Wu, P. S. Myung, Q. H. Liu, J. T. Pribila, B. D. Freedman, Y. Shimizu, and G. A. Koretzky. 2001. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293:2263-2265. [DOI] [PubMed] [Google Scholar]
- 28.Roach, T. I., S. E. Slater, L. S. White, X. Zhang, P. W. Majerus, E. J. Brown, and M. L. Thomas. 1998. The protein tyrosine phosphatase SHP-1 regulates integrin-mediated adhesion of macrophages. Curr. Biol. 8:1035-1038. [DOI] [PubMed] [Google Scholar]
- 29.Steinbrecher, A., D. Reinhold, L. Quigley, A. Gado, N. Tresser, L. Izikson, I. Born, J. Faust, K. Neubert, R. Martin, S. Ansorge, and S. Brocke. 2001. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J. Immunol. 166:2041-2048. [DOI] [PubMed] [Google Scholar]
- 30.Timms, J. F., K. D. Swanson, A. Marie-Cardine, M. Raab, C. E. Rudd, B. Schraven, and B. G. Neel. 1999. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9:927-930. [DOI] [PubMed] [Google Scholar]
- 31.Wang, H., E. Y. Moon, A. Azouz, X. Wu, A. Smith, H. Schneider, N. Hogg, and C. E. Rudd. 2003. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat. Immunol. 4:366-374. [DOI] [PubMed] [Google Scholar]
- 32.Wu, L., J. Fu, and S. H. Shen. 2002. SKAP55 coupled with CD45 positively regulates T-cell receptor-mediated gene transcription. Mol. Cell. Biol. 22:2673-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, L., Z. Yu, and S. H. Shen. 2002. SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T cell receptor activation. J. Biol. Chem. 277:40420-40427. [DOI] [PubMed] [Google Scholar]
- 34.Zambrowicz, B. P., G. A. Friedrich, E. C. Buxton, S. L. Lilleberg, C. Person, and A. T. Sands. 1998. Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature 392:608-611. [DOI] [PubMed] [Google Scholar]