Abstract
α-Lipoic acid (LA) is a cofactor for mitochondrial α-ketoacid dehydrogenase complexes and is one of the most potent, natural antioxidants. Reduction of oxidative stress by LA supplementation has been demonstrated in patients with diabetic neuropathy and in animal models. To determine how normal development or pathological conditions are affected by genetic alterations in the ability of mammalian cells to synthesize LA and whether dietary LA can circumvent its endogenous absence, we have generated mice deficient in lipoic acid synthase (Lias). Mice heterozygous for disruption of the Lias gene develop normally, and their plasma levels of thiobarbituric acid-reactive substances do not differ from those of wild-type mice. However, the heterozygotes have significantly reduced erythrocyte glutathione levels, indicating that their endogenous antioxidant capacity is lower than those of wild-type mice. Homozygous embryos lacking Lias appear healthy at the blastocyst stage, but their development is retarded globally by 7.5 days postcoitum (dpc), and all the null embryos die before 9.5 dpc. Supplementing the diet of heterozygous mothers with LA (1.65 g/kg of body weight) during pregnancy fails to prevent the prenatal deaths of homozygous embryos. Thus, endogenous LA synthesis is essential for developmental survival and cannot be replaced by LA in maternal tissues and blood.
R-α-Lipoic acid (6,8-thioctic acid) (LA) is a crucial cofactor that is required for the activity of the multienzyme complexes involved in the decarboxylation of α-ketoacids, important steps in energy metabolism. These complexes include pyruvate dehydrogenase complex (PDC), α-ketoglutarate dehydrogenase complex, branched chain α-ketoacid dehydrogenase complex (32), and a glycine cleavage system (11). LA is covalently attached via an amide bond to specific lysine residues of the E2 subunits of these complexes. LA is universally present in prokaryotes and eukaryotes (28), and previous investigations have shown that it is synthesized by lipoic acid synthase from octanoic acid and a sulfur source (23, 25). Mouse lipoic acid synthase is encoded by the nuclear gene Lias.
LA, one of the most potent natural antioxidants, has received considerable attention as a general dietary supplement for the past 15 years (3). Many lines of evidence have shown that both LA and its reduced form, dihydrolipoic acid (DHLA), have multifunctional antioxidant activities with the following characteristics (29). First, LA/DHLA are amphiphilic and readily cross the blood-brain barrier and cell membranes. Second, LA/DHLA possess metal-chelating activity. Third, LA is reduced to DHLA by several antioxidant enzymes that are expressed constitutively in most types of cells. In addition, because of its strong negative redox potential, DHLA can recycle other antioxidants, such as vitamin C, vitamin E, glutathione, coenzyme Q10, and ubiquinone (14, 15, 21, 29, 33).
A growing body of evidence implicates oxidative stress as an important pathogenic element in a variety of diseases, including atherosclerosis and diabetes (34, 36). Previous investigations suggest that supplements of LA reduce oxidative stress in both diabetic patients and animal models (9, 24) and that it is particularly suitable for prevention and/or treatment of diabetic complications (28). Indeed, LA has been used in Germany by patients with diabetic neuropathy for more than 30 years (44). However, treatments with LA at doses that produce a significant reduction of oxidative stress have not been clearly shown to affect the pathological processes resulting in diabetes and atherosclerosis. In addition, nothing is known about how endogenous LA synthesis is modified under pathological conditions. Furthermore, it is not known whether genetic alterations that reduce LA synthesis will modify disease development. To explore the physiological effects of reduced endogenous LA synthesis and to assess to what degree the exogenous LA can circumvent the effects of reduction in its synthesis, we have inactivated the Lias gene in mouse embryonic stem (ES) cells using homologous recombination. We show here that homozygous Lias null embryos die in utero. Heterozygotes survive but show evidence of increased oxidative stress compared to their wild-type littermates.
MATERIALS AND METHODS
Construction of a targeting vector.
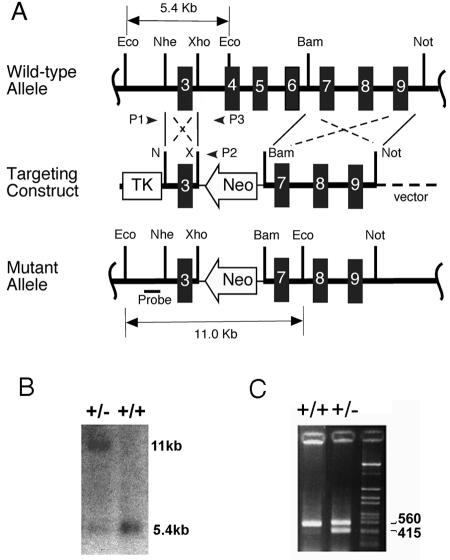
A λ phage clone containing exons 4 to 9 of the endogenous Lias gene was isolated from a mouse genomic phage library. A 1.3-kb NheI/XhoI fragment containing exon 3 and its flanking intronic sequences and an approximately 6-kb BamHI/NotI fragment containing exons 7 to 9 were isolated from the phage clone and used as 5′ and 3′ homologous arms, respectively, flanking the neomycin phosphotransferase gene (Neo [see Fig. 1]). The construct was designed so that homologous recombination between the endogenous Lias gene and the targeting construct would delete 3.7 kb of DNA, including exons 4 through 6 of the Lias gene, and replace them with the Neo gene. The targeting construct was linearized at a NotI site and electroporated into W4/129S6 cells (Taconic, Germantown, NY), which are derived from 129S6 strain mice. Colonies surviving after selection with G418 and ganciclovir were first screened by PCR with a primer, p1, from introns 2 to 3 (5′-GAC TGG TGT ACA GCG TAG TCT-3′) and a primer, p2, from the 3′ end of the Neo gene (5′-GCT TCC TCG TGC TTT ACG GTA T-3′). Targeted ES cells were identified by the presence of 1.3-kb PCR product and were confirmed by Southern blot analysis of EcoRI-digested ES cell genomic DNA with a probe specific to exon 2.
FIG. 1.
Targeted modification of the mouse Lias gene. (A) Schematic representation of the endogenous mouse Lias allele, targeting vector, and the mutant allele. The thick horizontal black line indicates genomic DNA containing exons 3 to 9. The NheI/XhoI and BamHI/NotI fragments flank the Neo gene in the targeting construct. Small arrowheads labeled P1 and P2 represent the locations of the P1 and P2 PCR primers used to identify the targeted allele in embryonic stem cells. The probe used for Southern blot analysis is shown below the mutant allele. Eco, EcoRI; Nhe, NheI; Xho, XhoI; Bam, BamHI; Not, NotI. (B) Confirmation of the targeted allele by Southern blot analysis. Genomic DNA from Lias+/− (+/−) or Lias+/+ (+/+) mice was digested with EcoRI and hybridized with the probe shown in panel A. The probe detects an 11.0-kb targeted fragment in addition to a 5.4-kb endogenous fragment in the correctly modified ES cells. (C) Detection of mouse genotype by PCR. Genomic DNA from F1 offspring of chimeric mice was amplified with the primers described in Materials and Methods. A 415-bp fragment indicates the presence of the targeted locus, while a 560-bp fragment indicates the endogenous locus.
Lias-deficient mice.
Two ES cell lines with a correctly targeted Lias locus were identified and injected into C57BL/6J recipient blastocysts, which were transferred to the uteri of CD1 pseudopregnant females. The chimeric male mice were bred with C57BL/6J females (The Jackson Laboratory, Bar Harbor, ME) to produce F1 progeny carrying the mutant allele. F2 offspring were obtained by crossing the F1 heterozygotes. Transmitting chimeras were also mated with 129S6 females (Taconic, Germantown, NY) to establish an inbred line of mutants. Mice were maintained according to the Guide for the Care and Use of Laboratory Animals (26a), and all experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Mice were on regular chow except that the lipoic acid-supplemented diet contained 45% (wt/wt) fat, 0.05% cholesterol, 20% sucrose, and 1.65 g of LA (Research Diets, Inc., New Brunswick, NJ) per kg of body weight.
Genotyping.
Genotypes of mice were determined by PCR of tail DNA with three primers, a primer (p2) from Neo, p3 (5′-GGA GAC ATA GGC AGT GGA TA-3′) from 5′ to the XhoI site in intron 3, and p4 (5′-GAA GCA GAA GCA GGC AGA TA-3′) from 3′ to the XhoI site in intron 3. A 560-bp fragment produced with p2 and p3 detects the endogenous locus, and a 415-bp fragment generated with p3 and p4 detects the targeted locus, allowing homozygous, heterozygous, and wild-type mice to be distinguished.
Laser capture microdissection.
The genotype of 7.5 days postcoitum (dpc) embryos was determined as follows. Decidual swellings were dissected at 7.5 dpc from heterozygous mothers, fixed in 4% formaldehyde in phosphate-buffered saline overnight, and embedded in paraffin. The embryos with their decidua were serially sectioned and deparaffinized through xylene and graded alcohols, stained with hematoxylin and eosin, dehydrated in graded ethanol, and cleared in xylene. Embryonic tissues were captured under direct microscopic visualization, collected by laser capture microdissection (Leica Microsystems, Wetzlar, Germany) following the manufacturer's protocol, and lysed in 10 mM Tris-HCl (pH 8.0) containing 0.1% Tween 20. After treatment with proteinase K (100 ng/ml) at 55°C for 4 h followed by inactivation of proteinase K at 95°C for 5 min, DNA was subjected to 40 cycles of PCR.
mRNA analysis.
Tissue RNA was extracted with TRIzol reagent (Carlsbad, CA) according to the manufacturer's protocol. The amount of Lias mRNA was determined by real-time quantitative reverse transcription-PCR, with β-actin as the reference gene in each reaction mixture (20). Primers for Lias gene amplification were 5′-CCC GTC GGC CAC ATC ATC TCG AT-3′ and 5′-GGG TCT GGA TTA TGT TGT CC-3′. The probe for Lias detection was 5′-FAM-ATC GAG ATC ATG TGG CCG ACG GG-TAMRA-3′ (FAM, 6-carboxyfluorescine; TAMRA, 6-carboxytetramethylrhodamine). The primers for β-actin detection were 5′-GAA GCG GCA TCC ATT GCT-3′ and 5′-GAA GCG GCA TCC ATT GCT-3′, and the probe was 5′-tetrachloro-fluorescein phosphoramidite-CAC TAT TGG CAA CGA GCG GTT CCG-TAMRA-3′.
Biochemical analysis.
Blood was collected from the retro-orbital sinus under anesthesia and was anticoagulated with heparin or EDTA. Oxidative stress in plasma was assessed by measuring thiobarbituric acid-reactive substances (TBARS), a common indicator of oxidative stress (22). In erythrocytes, reduced levels of erythrocyte glutathione (GSH), an endogenous antioxidant marker, were determined using a commercially available kit (Cayman, Ann Arbor, MI). Plasma glucose, total cholesterol, and triglycerides were measured using the respective kits (Sigma, St. Louis, MO) following the manufacturers' protocols. Lactic acid concentrations in tissues were determined as described previously (41). Protein content was determined by the Bio-Rad protein assay kit using bovine serum albumin as a standard. PDC activity in liver was determined by spectrophotometric assay as described previously (35)
Histological analysis.
Embryos (7.5 dpc) from timed mating between heterozygotes were removed from uteri and fixed together with their surrounding deciduas in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4), dehydrated, and embedded in paraffin. Five-micron sections were cut, mounted on slides, and stained with hematoxylin and eosin.
RESULTS
Generation of Lias-deficient mice.
Homologous recombination between the endogenous Lias locus and the targeting DNA construct deleted DNA containing exons 4 through 6 and replaced it with the Neo gene (Fig. 1A). This deletion resulted in a null allele because these exons encode the functionally conserved Cys motifs of the enzyme that are important for inserting a sulfur atom into the hydrocarbon chain of octanoic acid (23). Two correctly targeted ES cells carrying a disrupted Lias gene were identified by PCR and confirmed by Southern blot analysis, which showed an 11.0-kb band from the disrupted Lias gene, together with a 5.4-kb fragment band from the unmodified Lias gene (Fig. 1B).
The Lias gene is essential for the development of mouse embryos.
Fifteen chimeric mice were obtained from the two targeted ES cell lines. Six transmitted the modified Lias gene to the next generation. F1 Lias+/− mice showed no overt differences from their Lias+/+ littermates in gross appearance, behavior, growth curve, and fertility. However, intercrossing the Lias+/− F1 mice failed to produce any homozygous Lias−/− offspring (Table 1). The numbers of wild-type and heterozygous pups, genotyped by PCR (Fig. 1C), were as expected. This suggests that the complete lack of Lias is prenatally lethal.
TABLE 1.
Genotypes of embryos from Lias+/− × Lias+/− mating
| Age at dissection | No. with the following genotype:
|
No. resorbed | P valuea | |||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | Total | |||
| Live-born mice | 52 | 112 | 0 | 164 | <0.001 | |
| E12.5b | 14 | 40 | 0 | 54 | 16 | <0.001 |
| E10.5 | 7 | 18 | 3 | 28 | 5 | NS |
| E9.5 | 6 | 14 | 3 | 23 | 4 | NS |
| E7.5 | 6 | 4 | 2 | 12 | NS | |
| E3.5 | 1 | 6 | 2 | 9 | NS | |
P value by chi-square test. NS, not significant.
E12.5, embryonic day 12.5.
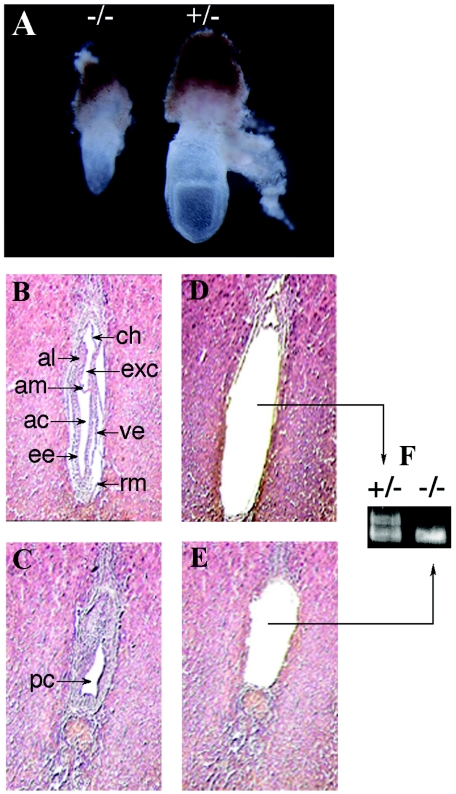
In order to determine the stage of embryonic death, embryos at different dpc were collected from the uteri of pregnant dams from Lias+/− × Lias+/− mating. As shown in Table 1, homozygous Lias−/− embryos were present at the 3.5 dpc blastocyst stage. They were indistinguishable in appearance from Lias+/+ and Lias+/− blastocysts. However, after 9.5 dpc Lias−/− embryos were not recovered. Three out of the four resorbed embryos from pregnancies at 9.5 dpc were genotyped as Lias−/−; the other was wild type. The last is not unexpected, since a small percentage of wild-type embryos are naturally resorbed. Lias−/− embryos were recovered at 7.5 dpc but were noticeably smaller than Lias+/+ or Lias+/− embryos (Fig. 2). The genotypes of the embryos were determined using laser capture microdissection (Fig. 2F). Histological examinations revealed that the homozygous 7.5 dpc embryos had no ectoplacental cavities and were developmentally retarded (Fig. 2C). This suggests that the Lias−/− embryos do not reach the early primitive streak stage or late streak stage; they appear to stop growing at the egg cylinder stage (6.5 dpc). In addition, hemorrhage surrounding the homozygous 7.5 dpc embryos suggests that they were being resorbed. These results indicate that the Lias−/− embryos die at an early implantation stage between 7.5 dpc and 9.5 dpc and that LA in maternal blood and tissues cannot support their development.
FIG. 2.
(A) Developmental retardation of homozygous embryos. Embryos (7.5 dpc) from timed Lias+/− × Lias+/− mating were dissected from the uteri with the removal of deciduas, along with extraembryonic membrane and ectoplacental cone. This panel was photographed at an original objective lens magnification of ×15. Lias−/− null embryos (−/−) are smaller than heterozygous (+/−) or wild-type embryo littermates. Embryos (7.5 dpc) were subjected to histological analysis and genotyping by microdissection. Sections were stained with hematoxylin and eosin and photographed at an original objective lens magnification of ×10. (B) A Lias+/− embryo shows postgastrulation with distinct germ layers, ectoderm, and visceral endoderm. ve, visceral endoderm; ee, embryonic ectoderm; al, allantois; ac, amniotic cavity; exc, exocoelomic cavity; am, amnion; ch, chorion; rm, Reichert's membrane. (C) A Lias−/− embryo shows obvious lack of organization. pc, proamniotic canal. (D and E) Embryonic tissues were collected by laser capture microdissection, and the remaining sections are shown in panels D and E. The genotypes of the embryos were determined by PCR of the captured tissues (F). The 415-bp band indicates the null allele, and the 560-bp band indicates the wild-type alleles.
Supplementing the diet of Lias+/− mothers with LA fails to rescue Lias−/− embryos.
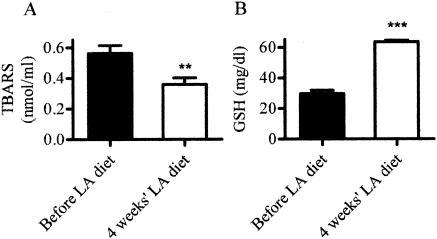
We attempted to rescue the Lias−/− embryos by providing their heterozygous mothers with extra lipoic acid (1.65 g/kg) in their diet. This dose is commonly used in rodents for therapeutic purposes, and a 50% effective dose of LA for correcting blood flow deficits in diabetic rats has been estimated at approximately 38 mg kg−1 day−1 (5). Supplementation of LA in the diet for 1 month increased GSH in erythrocytes and decreased plasma TBARS significantly (Fig. 3), which demonstrates that dietary LA reaches maternal circulation. However, no live-born Lias−/− mice were found among 34 pups of LA-supplemented dams, and the supplementation did not affect the average litter size. Thus, high-dose LA supplementation, which sufficiently affects the levels of TBARS and GSH in the maternal blood, failed to rescue pups that completely lack endogenous production of LA.
FIG. 3.
Plasma TBARS and erythrocyte GSH levels before (black bars) and after (open bars) Lias−/− mothers were fed a diet containing LA for 4 weeks. Data are the means ± standard errors of the means (error bars) for seven mice. Values that are significantly different from the values of mice before they were fed a diet containing LA are indicated (P < 0.01 [**]; P < 0.001 [***]).
The Lias+/− mice have reduced Lias mRNA expression.
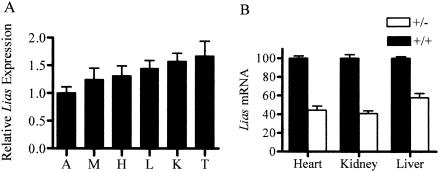
Quantitative reverse transcription-PCR revealed that the Lias gene is highly expressed in the testes, kidney, liver, heart, skeletal muscle, and aorta of wild-type C57BL/6J mice (Fig. 4A). Northern blot analysis showed similar results (data not shown). The levels of expression of Lias mRNA in the kidney, liver, and heart of the heterozygous Lias+/− mice are 45 to 60% of the levels of their wild-type littermates (Fig. 4B), which does not differ significantly from the 50% level expected in a heterozygote for a gene that is not subject to homeostatic compensation.
FIG. 4.
(A). Expression of the Lias gene. (A) The mRNA expression levels of the Lias gene in aorta (A), skeletal muscle (M), heart (H), liver (L), kidney (K), and testis (t) in eight wild-type C57BL/6J mice. Lias mRNA levels relative to β-actin mRNA in each tissue were determined by the real-time quantitative PCR as described in Materials and Methods. Expression levels in tissues are presented relative to the level in the aorta, which was set at 1.0. Data are shown as means ± standard errors of the means (error bars). (B) Lias mRNA expression in the heterozygotes. The amounts of Lias+/− mRNA in the heart, kidney, and liver are expressed relative to those in Lias+/+ mice. Values are the means ± standard errors of the means (error bars) of nine animals in each group of Lias+/+ or Lias−/− mice.
Metabolic status of Lias+/− mice.
To test whether the 50% reduction in the level of expression of Lias alters the metabolic and antioxidant status of the heterozygous mice, we assayed a series of biochemical parameters (Table 2). The Lias+/− mice had normal levels of plasma glucose, triglyceride, and cholesterol. They exhibited normal glucose tolerance (data not shown). Lactic acid in the liver was higher, and the activity of liver pyruvate dehydrogenase complex was lower in Lias+/− mice than in wild-type mice, although neither reached significance (P = 0.08 and 0.09, respectively). The level of GSH in the erythrocytes of Lias+/− mice was significantly lower than that of wild-type mice, although the plasma TBARS levels in the heterozygotes were not different from the levels in wild-type mice. Thus, these data show that half the normal level of Lias mRNA is not sufficient to maintain the normal antioxidant capacity of mice even under the unstressed condition of this experiment.
TABLE 2.
Comparison of biochemical parameters in Lias+/+ and Lias+/− micea
| Biochemical parameter | Value for mice (mean ± SEM)
|
P valueb | |
|---|---|---|---|
| Lias+/+ | Lias+/− | ||
| Plasma glucose (mg/dl) | 186 ± 9.3 | 206 ± 17.2 | NS |
| Plasma triglyceride (mg/dl) | 38.1 ± 5.5 | 33.5 ± 2.3 | NS |
| Plasma cholesterol (mg/dl) | 56.6 ± 1.7 | 55.9 ± 2.2 | NS |
| Liver lactate (mmol/mg protein) | 1.16 ± 0.10 | 1.47 ± 0.13 | 0.09 |
| Liver PDHc activity (μmol/min/mg protein) | 2.16 ± 0.04 | 2.04 ± 0.05 | 0.08 |
| Plasma TBARS (nmol/ml) | 18.3 ± 2.1 | 20.1 ± 3.8 | NS |
| Erythrocyte GSH (mg/ml) | 32.3 ± 1.8 | 28.1 ± 1.7 | 0.005 |
Data were obtained from two independent assays of nine male mice (12 to 16 weeks old) for each genotype. Data for GSH assay were acquired from 21 Lias+/+ and 19 Lias+/− mice.
P value by analysis of variance. NS, not significant.
PDH, pyruvate dehydrogenase.
DISCUSSION
We have demonstrated that the absence of Lias gene expression leads to early embryonic death and that this absence cannot be rescued by maternal LA or by supplementing the diet of the mothers with LA. Heterozygotes expressing Lias mRNA at 50% of the normal levels showed metabolic disturbances indicating a significant reduction in erythrocyte GSH levels, even under unstressed conditions. Nevertheless, they develop normally and show no overt abnormalities in 1 year.
The death of the Lias−/− embryos shortly after implantation coincides with the time when oocyte-derived proteins are no longer able to support the rapidly growing embryos (42). While further studies are necessary to determine whether the absence of LA synthesis damages preimplantation embryos, no visual abnormalities in the Lias−/− blastocysts were observed. Published studies have shown that the mouse embryo switches from oxidative metabolism to glycolytic metabolism shortly prior to implantation but reverts to oxidative metabolism soon after implantation (6, 39). Thus, dihydrolipoamide dehydrogenase (E3)-deficient embryos fail gastrulation and die before 7.5 dpc, and pyruvate dehydrogenase (E1)-deficient mice die around 9.5 dpc (18, 19). The similarities in timing of death of these and the Lias−/− embryos indicates that LA synthesized by Lias, E1, and E3 are all essential components of the PDC enzyme complex so that inactivation of E1 or E3 activity and total loss of Lias would all lead to impairment of glucose oxidative metabolism. Dihydrolipoamide acyltransferase (E2) has a central catalytic and structural role in the enzyme complex, because it provides a scaffold for the binding and positioning of E1 and E3. The carboxyl group of LA forms an amide bond with a lysine residue in E2 and generates a “swinging arm” that permits the thiol/disulfide moieties of the lipoyl arm to rotate between the active sites of E1, E2, and E3 and facilitates electron transfer between them (31). Since E2 is shared in the multienzyme complexes that produce essential amino acids, pyrimidines, and porphyrins and ATP via the citric acid cycle, cellular metabolism is likely to be compromised more broadly by the total absence of LA than it is by the absence of individual E1 or E3.
Nevertheless, we were surprised to find that LA cannot be supplied through maternal tissues, despite the fact that it is a small amphiphilic molecule, readily able to cross the blood-brain barrier and cell membranes (27, 28). Supplementing the maternal diet with LA failed to rescue Lias−/− mice from embryonic lethality, although it is likely that LA can cross the placenta efficiently, since Al Ghafli et al. have demonstrated that LA supplementation (20 mg kg−1, given intraperitoneally) improved the survival of embryos in rats with diabetes induced by streptozotocin during the pregnancy (1).
The covalent attachment of exogenous LA to target apoenzymes occurs in two steps. The first step is the formation of lipoyl-nucleotide monophosphate, catalyzed by a lipoate-activating enzyme. The second step is the transfer of the lipoyl moiety to the apoproteins, catalyzed by a lipoyl transferase. In Escherichia coli, a single enzyme, LipA, catalyzes both of these steps (26). In mammals, a gene encoding a protein with the lipoate-activating enzyme activity has been cloned in cows (13); its nucleotide sequence suggests that it is a homolog of medium-chain acyl coenzyme A synthase. A different gene encoding lipoyl transferase activity has also been cloned and characterized in cows and humans (12, 13). Their presence suggests that mammalian cells may also be capable of utilizing exogenous LA to provide the LA moiety of the multienzyme complexes. However, the death of Lias−/− embryos at an early implantation stage shows that the transfer of exogenous LA to apoenzyme, even if it occurs in vivo, is not sufficient to supplant the need for endogenous synthesis at least during mammalian development.
The pathway for endogenous LA synthesis in mitochondria begins in Saccharomyces cerevisiae with the transfer of octanoic acid, linked by a thioester bond to the 4′-phosphopantetheine sulfhydryl group of the acyl carrier protein (ACP), to a specific ɛ-amino group of a target apoenzyme with the formation of an amide bond (4, 43). This transfer is mediated by lipoyl (octanoyl)-transferase, encoded by yeast LIP2. Subsequently, two sulfur atoms are inserted into the C-6 and C-8 positions of octanoic acid to form a lipoic acid moiety in situ on the enzyme. Lipoyl synthase, a metalloenzyme encoded by LIP5 in yeast, and the substrate S-adenosylmethionine are required for this reaction. In yeasts, the absence of endogenous LA synthesis in mutants lacking either ACP1 or LIP5 cannot be complemented by LA in the medium (4, 38). The early embryonic death of Lias−/− mice that we observe suggests that free LA cannot be used to form the lipoic acid moiety of E2-containing enzyme complexes or that LA cannot be transported into mitochondria in mice even though it can probably pass through the placenta.
Mammalian cells have numerous antioxidant systems that play complex roles in the cellular antioxidant system. These antioxidant genes are expressed early in embryonic development but reach peak levels only during the neonatal period (2). Low levels of antioxidants predispose embryos to the risk of oxidative damage (10), and the absence of some antioxidant gene products, such as phospholipid hydroperoxide glutathione peroxidase, manganese-dependent superoxide dismutase (MnSOD), and γ-glutamylcysteine synthetase, cause early embryonic death (17, 37, 40). On the other hand, other endogenous antioxidants are not essential for embryonic survival. Thus, the absence of catalase (16), glutathione peroxidase-1 (7), or copper/zinc-dependent SOD (CuZnSOD) (30) does not affect embryonic survival. The corresponding null embryos show no pathological changes under normal physiological conditions, although they exhibit a pronounced susceptibility to oxidative stress. The death of the Lias−/− embryos at 7.5 dpc, because it is so sudden, is unlikely to be due to the lack of LA as an antioxidant but is readily understood if it is caused by the inability of the developing embryos to obtain energy from oxidative metabolism. Nevertheless, this does not exclude the possibilities that antioxidant activity of endogenously produced LA could be crucial for controlling reactive oxidative species production in mitochondria. Such a failure to balance the increased generation of reactive oxygen species that accompanies the embryo's switch to oxidative metabolism may further delay embryonic development (8).
In conclusion, our study shows that the endogenous production of LA is essential for early embryonic development, mainly we suggest because of its role as an enzyme cofactor but partly because it is an antioxidant. These functions cannot be replaced by exogenous LA synthesized by the Lias+/− mother or given as a dietary supplement. We also show that the heterozygous Lias+/− mice have reduced antioxidant capacities, and these mice may therefore provide a useful model for studying the role of mitochondrial antioxidant function in the pathogenesis of various metabolic disorders and for defining the role of antioxidant enzymes in cellular defense against oxidant-mediated tissue injury.
Acknowledgments
This work was supported by grants (HL42630 and HL70523) from the National Institutes of Health.
We thank Oliver Smithies, Kathleen Caron, Kathleen Sulik, Hyung-Suk Kim, Seigo Hatada, and Nobuyuki Takahashi for valuable advice. We also thank Sylvia Hiller, Angie Ponguta, John Hagaman, Jennifer Wilder, and John Cowhig for expert technical assistance and Raymond Givens and Lance Johnson for helpful comments on the manuscript.
REFERENCES
- 1.Al Ghafli, M. H., R. Padmanabhan, H. H. Kataya, and B. Berg. 2004. Effects of alpha-lipoic acid supplementation on maternal diabetes-induced growth retardation and congenital anomalies in rat fetuses. Mol. Cell. Biochem. 261:123-135. [DOI] [PubMed] [Google Scholar]
- 2.Allen, R., and A. Balin. 1989. Oxidative influence on development and differentiation. An overview of a free radical theory of development. Free Radic. Biol. Med. 6:631-639. [DOI] [PubMed] [Google Scholar]
- 3.Bast, A., and G. R. Haenen. 1988. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim. Biophys. Acta 963:558-561. [DOI] [PubMed] [Google Scholar]
- 4.Brody, S., C. Oh, U. Hoja, and E. Schweizer. 1997. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 408:217-220. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, N. E., M. A. Cotter, D. H. Horrobin, and H. J. Tritschler. 1998. Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia 41:390-399. [DOI] [PubMed] [Google Scholar]
- 6.Clough, J. R. 1985. Energy metabolism during mammalian embryogenesis. Biochem. Soc. Trans. 13:77-79. [DOI] [PubMed] [Google Scholar]
- 7.de Haan, J. B., C. Bladier, P. Griffiths, M. Kelner, R. D. O'Shea, N. S. Cheung, R. T. Bronson, M. J. Silvestro, S. Wild, S. S. Zheng, P. M. Beart, P. J. Hertzog, and I. Kola. 1998. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J. Biol. Chem. 273:22528-22536. [DOI] [PubMed] [Google Scholar]
- 8.Dennery, P. A. 2004. Role of redox in fetal development and neonatal diseases. Antioxid. Redox Signal. 6:147-153. [DOI] [PubMed] [Google Scholar]
- 9.Evans, J. L., and I. D. Goldfine. 2000. Alpha-lipoic acid: a multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Ther. 2:401-413. [DOI] [PubMed] [Google Scholar]
- 10.Fantell, A., C. Barber, and B. Meckels. 1992. Ischemia reperfusion: a new hypothesis for the developmental toxicity of cocaine. Teratology 46:285-292. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, K., K. Okamura, and Y. Motokawa. 1979. Hydrogen carrier protein from chicken liver: purification, characterization, and role of its prosthetic group, lipolic acid, in the glycine cleavage reaction. Arch. Biochem. Biophys. 197:454-462. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara, K., K. Okamura-Ikeda, and Y. Motokawa. 1994. Purification and characterization of lipoyl-AMP:N epsilon-lysine lipoyltransferase from bovine liver mitochondria. J. Biol. Chem. 269:16605-16609. [PubMed] [Google Scholar]
- 13.Fujiwara, K., M. Suzuki, Y. Okumachi, K. Okamura-Ikeda, T. Fujiwara, E. Takahashi, and Y. Motokawa. 1999. Molecular cloning, structural characterization and chromosomal localization of human lipoyltransferase gene. Eur. J. Biochem. 260:761-767. [DOI] [PubMed] [Google Scholar]
- 14.Gotz, M. E., A. Dirr, R. Burger, B. Janetzky, M. Weinmuller, W. W. Chan, S. C. Chen, H. Reichmann, W. D. Rausch, and P. Riederer. 1994. Effect of lipoic acid on redox state of coenzyme Q in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and diethyldithiocarbamate. Eur. J. Pharmacol. 266:291-300. [DOI] [PubMed] [Google Scholar]
- 15.Han, D., G. Handelman, L. Marcocci, C. K. Sen, S. Roy, H. Kobuchi, H. J. Tritschler, L. Flohe, and L. Packer. 1997. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors 6:321-338. [DOI] [PubMed] [Google Scholar]
- 16.Ho, Y. S., Y. Xiong, W. Ma, A. Spector, and D. S. Ho. 2004. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem. 279:32804-32812. [DOI] [PubMed] [Google Scholar]
- 17.Imai, H., F. Hirao, T. Sakamoto, K. Sekine, Y. Mizukura, M. Saito, T. Kitamoto, M. Hayasaka, K. Hanaoka, and Y. Nakagawa. 2003. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 305:278-286. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, M. T., S. Mahmood, S. L. Hyatt, H. S. Yang, P. D. Soloway, R. W. Hanson, and M. S. Patel. 2001. Inactivation of the murine pyruvate dehydrogenase (Pdha1) gene and its effect on early embryonic development. Mol. Genet. Metab. 74:293-302. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, M. T., H. S. Yang, T. Magnuson, and M. S. Patel. 1997. Targeted disruption of the murine dihydrolipoamide dehydrogenase gene (Dld) results in perigastrulation lethality. Proc. Natl. Acad. Sci. USA 94:14512-14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, H. S., G. Lee, S. W. John, N. Maeda, and O. Smithies. 2002. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc. Natl. Acad. Sci. USA 99:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozlov, A. V., L. Gille, K. Staniek, and H. Nohl. 1999. Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubiquinone and one-electron reduction of ubisemiquinone. Arch. Biochem. Biophys. 363:148-154. [DOI] [PubMed] [Google Scholar]
- 22.Lapenna, D., G. Ciofani, S. D. Pierdomenico, M. A. Giamberardino, and F. Cuccurullo. 2001. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic. Biol. Med. 31:331-335. [DOI] [PubMed] [Google Scholar]
- 23.Marquet, A., B. T. Bui, and D. Florentin. 2001. Biosynthesis of biotin and lipoic acid. Vitam. Horm. 61:51-101. [DOI] [PubMed] [Google Scholar]
- 24.Melhem, M. F., P. A. Craven, J. Liachenko, and F. R. DeRubertis. 2002. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J. Am. Soc. Nephrol. 13:108-116. [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. R., R. W. Busby, S. W. Jordan, J. Cheek, T. F. Henshaw, G. W. Ashley, J. B. Broderick, J. E. Cronan, Jr., and M. A. Marletta. 2000. Escherichia coli LipA is a lipoyl synthase: in vitro biosynthesis of lipoylated pyruvate dehydrogenase complex from octanoyl-acyl carrier protein. Biochemistry 39:15166-15178. [DOI] [PubMed] [Google Scholar]
- 26.Morris, T. W., K. E. Reed, and J. E. Cronan, Jr. 1994. Identification of the gene encoding lipoate-protein ligase A of Escherichia coli. Molecular cloning and characterization of the lplA gene and gene product. J. Biol. Chem. 269:16091-16100. [PubMed] [Google Scholar]
- 26a.National Research Council. 1996. Guide for the care and use of laboratory animals. The National Academies Press, Washington, D.C.
- 27.Packer, L., H. J. Tritschler, and K. Wessel. 1997. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic. Biol. Med. 22:359-378. [DOI] [PubMed] [Google Scholar]
- 28.Packer, L., S. U. Weber, and G. Rimbach. 2001. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 131:369S-373S. [DOI] [PubMed] [Google Scholar]
- 29.Packer, L., E. H. Witt, and H. J. Tritschler. 1995. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 19:227-250. [DOI] [PubMed] [Google Scholar]
- 30.Reaume, A. G., J. L. Elliott, E. K. Hoffman, N. W. Kowall, R. J. Ferrante, D. F. Siwek, H. M. Wilcox, D. G. Flood, M. F. Beal, R. H. Brown, Jr., R. W. Scott, and W. D. Snider. 1996. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 13:43-47. [DOI] [PubMed] [Google Scholar]
- 31.Reed, L. J., B. G. DeBusk, I. C. Gunsalus, and C. S. Hornberger, Jr. 1951. Crystalline alpha-lipoic acid: a catalytic agent associated with pyruvate dehydrogenase. Science 114:93-94. [DOI] [PubMed] [Google Scholar]
- 32.Reed, L. J., and M. L. Hackert. 1990. Structure-function relationships in dihydrolipoamide acyltransferases. J. Biol. Chem. 265:8971-8974. [PubMed] [Google Scholar]
- 33.Scholich, H., M. E. Murphy, and H. Sies. 1989. Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on alpha-tocopherol. Biochim. Biophys. Acta 1001:256-261. [DOI] [PubMed] [Google Scholar]
- 34.Schulze, P. C., and R. T. Lee. 2005. Oxidative stress and atherosclerosis. Curr. Atheroscler. Rep. 7:242-248. [DOI] [PubMed] [Google Scholar]
- 35.Scislowski, P. W., and E. J. Davis. 1986. A sensitive spectrophotometric assay of pyruvate dehydrogenase activity. Anal. Biochem. 155:400-404. [DOI] [PubMed] [Google Scholar]
- 36.Scott, J. A., and G. L. King. 2004. Oxidative stress and antioxidant treatment in diabetes. Ann. N. Y. Acad. Sci. 1031:204-213. [DOI] [PubMed] [Google Scholar]
- 37.Shi, Z. Z., J. Osei-Frimpong, G. Kala, S. V. Kala, R. J. Barrios, G. M. Habib, D. J. Lukin, C. M. Danney, M. M. Matzuk, and M. W. Lieberman. 2000. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA 97:5101-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulo, P., and N. C. Martin. 1993. Isolation and characterization of LIP5. A lipoate biosynthetic locus of Saccharomyces cerevisiae. J. Biol. Chem. 268:17634-17639. [PubMed] [Google Scholar]
- 39.Thompson, J. G., A. C. Simpson, P. A. Pugh, R. W. Wright, Jr., and H. R. Tervit. 1991. Glucose utilization by sheep embryos derived in vivo and in vitro. Reprod. Fertil. Dev. 3:571-576. [DOI] [PubMed] [Google Scholar]
- 40.Tsan, M. F., J. E. White, B. Caska, C. J. Epstein, and C. Y. Lee. 1998. Susceptibility of heterozygous MnSOD gene-knockout mice to oxygen toxicity. Am. J. Respir. Cell Mol. Biol. 19:114-120. [DOI] [PubMed] [Google Scholar]
- 41.Valero, E., and F. Garcia-Carmona. 1996. Optimizing enzymatic cycling assays: spectrophotometric determination of low levels of pyruvate and L-lactate. Anal. Biochem. 239:47-52. [DOI] [PubMed] [Google Scholar]
- 42.West, J. D., R. Leask, and J. F. Green. 1986. Quantification of the transition from oocyte-coded to embryo-coded glucose phosphate isomerase in mouse embryos. J. Embryol. Exp. Morphol. 97:225-237. [PubMed] [Google Scholar]
- 43.Zhao, X., J. R. Miller, Y. Jiang, M. A. Marletta, and J. E. Cronan. 2003. Assembly of the covalent linkage between lipoic acid and its cognate enzymes. Chem. Biol. 10:1293-1302. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler, D., M. Reljanovic, H. Mehnert, and F. A. Gries. 1999. Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 107:421-430. [DOI] [PubMed] [Google Scholar]