Abstract
Gastric cancer (GC) is a highly prevalent and lethal malignancy worldwide. Accumulating evidence has shown that microRNAs (miRNAs) play essential roles in the development and progression of GC. In this study, we aimed to investigate the expression, functions, and molecular mechanisms of miRNA-204-5p in GC. We found that miRNA-204-5p was significantly downregulated in GC cell lines compared to their normal counterparts. Functional experiments demonstrated that miRNA-204-5p inhibited the migration, invasion, and glycolysis of GC cells in vitro and suppressed tumor lung metastasis in vivo. Mechanistically, miRNA-204-5p exerted its tumor-suppressive effects by directly targeting RAB22A and inhibiting the PI3K/AKT signaling pathway. Overexpression of RAB22A partially reversed the inhibitory effects of miRNA-204-5p on the malignant phenotypes and the PI3K/AKT pathway activation in GC cells. Furthermore, miRNA-204-5p regulated the expression of molecules related to epithelial-mesenchymal transition, and glycolysis through the RAB22A/PI3K/AKT axis. Our findings suggest that miRNA-204-5p functions as a tumor suppressor in GC by targeting RAB22A and provide novel insights into the molecular mechanisms underlying GC progression. The miRNA-204-5p/RAB22A axis may serve as a potential diagnostic biomarker and therapeutic target for GC.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-02139-z.
Keywords: Gastric cancer, miRNA-204-5p, Migration, Invasion, Glycolysis, RAB22A, PI3K/AKT signaling pathway
Subject terms: Cancer, Cell biology, Molecular biology, Gastroenterology, Oncology
Introduction
Gastric cancer (GC) is a highly prevalent and lethal malignancy, ranking as the fifth most common cancer and the third leading cause of cancer-related deaths globally1. Despite significant advancements in diagnostic techniques and therapeutic strategies, the prognosis of GC patients remains poor, with a 5-year survival rate of less than 30%2,3. The high mortality rate of GC is primarily attributed to its late diagnosis and the lack of effective therapeutic targets4. Therefore, it is crucial to explore the molecular mechanisms underlying the development and progression of GC and identify novel biomarkers and therapeutic targets.
MicroRNAs (miRNAs) are a class of endogenous, small non-coding RNAs with a length of 18–25 nucleotides5. They regulate gene expression post-transcriptionally by binding to the 3’-untranslated region (3’-UTR) of target mRNAs, leading to mRNA degradation or translational repression6. Accumulating evidence has shown that miRNAs are aberrantly expressed in various cancers and play essential roles in tumor development and progression by regulating cell proliferation, apoptosis, migration, invasion, and metabolism6,7. In GC, several miRNAs have been reported to function as either oncogenes or tumor suppressors, such as miR-21, miR-148a-3p, miR-29c, and miR-3758–11.
miRNA-204-5p, located at the cancer-associated genomic region 9q21.12, has been reported to be dysregulated in several types of cancers and exert tumor-suppressive functions. In breast cancer, miRNA-204-5p is downregulated and inhibits cell proliferation and invasion by targeting MMP-912. Similarly, in prostate cancer, miRNA-204-5p is downregulated and promoted cell apoptosis by targeting BCL213. In hepatocellular carcinoma, miRNA-204-5p is also downregulated and inhibits cell proliferation by targeting SIRT114. These studies suggest that miRNA-204-5p may play important roles in the development and progression of various cancers. However, the expression pattern, biological functions, and underlying mechanisms of miRNA-204-5p in GC remain largely unknown.
In the present study, we aimed to investigate the expression, functions, and molecular mechanisms of miRNA-204-5p in GC. We first examined the expression levels of miRNA-204-5p in GC cell lines compared to their normal counterparts. Then, we performed functional experiments to evaluate the effects of miRNA-204-5p on the malignant phenotypes of GC cells, including migration, invasion, and glycolysis. Furthermore, we explored the potential target genes and signaling pathways regulated by miRNA-204-5p in GC cells using bioinformatics analysis, luciferase reporter assay, qRT-PCR assay and western blot assay. The findings of this study may provide new insights into the molecular pathogenesis of GC and facilitate the development of novel diagnostic and therapeutic strategies for this deadly disease. By elucidating the tumor-suppressive functions and mechanisms of miRNA-204-5p in GC, we may identify new biomarkers and therapeutic targets that can improve the diagnosis, prognosis, and treatment of GC patients. Moreover, this study may also contribute to our understanding of the complex regulatory networks between miRNAs, their target genes, and cancer-related signaling pathways in GC and other cancers.
Materials and methods
TCGA data analysis
The miRNA expression data and corresponding clinical information of GC patients were downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). The expression levels of miRNA-204-5p in GC tissues were analyzed using the EdgeR package in R software. The associations between miRNA-204-5p expression and clinicopathological features, including age, gender, tumor location, stage, and lymph node metastasis, were evaluated using the Wilcoxon rank-sum test or Kruskal-Wallis test. The Kaplan-Meier method and log-rank test were used to assess the correlation between miRNA-204-5p expression and patient survival.
Cell culture
Four human GC cell lines (AGS, MKN7, MKN28, and MKN45) and a normal gastric epithelial cell line (GES-1) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HEK-293T cells were obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China). All cells were cultured in RPMI-1640 medium (GES-1, AGS, MKN7, MKN28, and MKN45) or DMEM (HEK-293T) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The concentration and purity of RNA were measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). For miRNA-204-5p detection, cDNA was synthesized using the miRNA 1st Strand cDNA Synthesis Kit (by stem-loop) (Vazyme, China). For mRNA detection, cDNA was synthesized using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan). qRT-PCR was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara, Japan) on a Real-Time PCR System (Yarui, china). U6 and β-actin were used as internal controls for miRNA and mRNA, respectively. The primer sequences are listed in Table 1. The relative expression levels were calculated using the 2−ΔΔCt method.
Table 1.
Primer sequences for qRT-PCR.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| U6 | CTCGCTTCGGCAGCACA | / |
| miRNA-204-5p | TTCCCTTTGTCATCCTATG | / |
| β-actin | CTCCATCCTGGCCTCGCTGT | GCTGTCACCTTCACCGTTCC |
| E-cadherin | GTGCCTGAGAACGAGGCTAA | CTGCATCTTGCCAGGTCCTT |
| N-cadherin | AGCTCCACCATATGACTCCCT | TCATAGTCCTGCTCACCACC |
| LDHA | GGAGATTCCAGTGTGCCTGT | GCCCAGGATGTGTAGCCTTT |
| HK2 | GATTGCCTCGCATCTGCTTG | GCTCCAAGCCCTTTCTCCAT |
Cell transfection
The miRNA-204-5p mimic, inhibitor, and their corresponding negative controls (NC mimic and NC inhibitor) were purchased from GenePharma (Shanghai, China). Cells were transfected with mimic (100 pmol) or inhibitor (150 pmol) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. The transfection efficiency was verified by qRT-PCR. For stable transfection, lentiviruses carrying miRNA-204-5p overexpression sequence (OE-miRNA-204-5p) or negative control sequence (OE-NC) were constructed by GeneChem (Shanghai, China). MKN45 cells were infected with lentiviruses at a multiplicity of infection (MOI) of 50 and selected with puromycin (5 µg/mL) to establish stable cell lines.
Wound healing assay
Cell migration ability was evaluated by wound healing assay. Transfected cells were seeded into 6-well plates and cultured until reaching 90% confluence. A straight scratch was made using a 200 µL pipette tip, and the cells were washed with PBS to remove debris. The wound closure was photographed at 0, 24 h using an inverted microscope (Nikon, Japan). The wound healing rate was calculated using ImageJ software (NIH, USA).
Transwell invasion assay
Cell invasion ability was assessed using Transwell chambers (8 μm pore size; Corning, USA) precoated with Matrigel (BD Biosciences, USA). Transfected cells (5 × 104 cells for AGS and 2 × 105 cells for MKN45) in serum-free medium were seeded into the upper chamber, and complete medium containing 10% FBS was added to the lower chamber. After incubation for 48 h, the invaded cells were fixed with 4% paraformaldehyde, stained with crystal violet, and counted under a microscope.
Glycolysis assays
The Glucose uptake, lactate production, and ATP levels were determined using the Glucose Uptake Assay Kit (Biovision, USA), Lactate Assay Kit (Sigma-Aldrich, USA), and ATP Assay Kit (Beyotime, China), respectively, following the manufacturers’ protocols.
Western blot analysis
Total protein was extracted from cells using RIPA lysis buffer (Beyotime, China) containing protease and phosphatase inhibitors. The protein concentration was determined using the BCA Protein Assay Kit (Beyotime, China). Equal amounts of protein (30 µg) were separated by SDS-PAGE and transferred onto PVDF membranes (Millipore, USA). The membranes were blocked with 5% non-fat milk for 1 h at room temperature and then incubated with primary antibodies overnight at 4℃. The following primary antibodies were used: RAB22A (1:500; Proteintech, China), p-PI3K (1:1000; Cell Signaling Technology, USA), PI3K (1:1000; Cell Signaling Technology, USA), p-AKT (1:1000; Cell Signaling Technology, USA), AKT (1:1000; Cell Signaling Technology, USA), E-cadherin (1:5000; Proteintech, China), N-cadherin (1:5000; Proteintech, China), LDHA (1:5000; Proteintech, China), HK2 (1:1000; Proteintech, China) and β-actin (1:5000; Proteintech, China). After washing with TBST, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. The protein bands were visualized using an ECL detection system (Millipore, USA) and quantified by ImageJ software.
Dual-luciferase reporter assay
The wild-type (WT) and mutant (MUT) 3’-UTR sequences of RAB22A containing the predicted miRNA-204-5p binding sites were synthesized and cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA). HEK-293T cells were co-transfected with the luciferase reporter plasmids and miRNA-204-5p mimic or NC mimic using Lipofectamine 2000. After 48 h, the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, USA) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to Renilla luciferase activity.
Animals and treatments
All animal experiments were performed in strict accordance with the guidelines and regulations approved by the Animal Ethics Committee of our institution (Approval No. 2023-41). Sixty female BALB/c nude mice (4–5 weeks old, weighing 18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were housed under specific pathogen-free conditions with controlled temperature (22–24℃), humidity (45–50%), and a 12-hour light/dark cycle. The animals had ad libitum access to food and water. Following a one-week acclimation period, for the lung metastasis model, MKN45 cells stably overexpressing miRNA-204-5p or negative control were injected into the tail vein of the mice at a density of 1 × 106 cells per mouse (n = 5 per group). Six weeks post-injection, the mice were sacrificed, and the lungs were harvested, photographed, and subjected to histological examination to assess the presence of metastatic lesions.
Hematoxylin and Eosin (H&E) staining
To assess the presence of metastatic lesions in the lungs, harvested lung tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. The sections were then deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). The stained sections were examined and photographed under a microscope to evaluate the extent of metastatic tumor cell infiltration.
Statistical analysis
All data are presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, USA). Student’s t-test was used for comparisons between two groups, and one-way ANOVA followed by Tukey’s post hoc test was used for comparisons among multiple groups. P < 0.05 was considered statistically significant.
Results
miRNA-204-5p is downregulated in cell lines and is associated with lymph node metastasis and poor prognosis
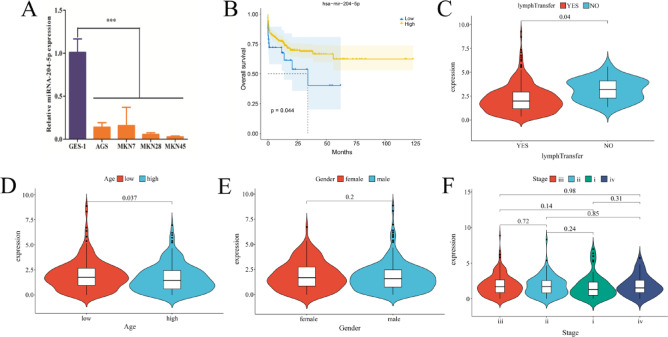
To investigate the expression pattern of miRNA-204-5p in GC, we analyzed expression of miRNA-204-5p in four GC cell lines (AGS, MKN7, MKN28, and MKN45) and a normal gastric epithelial cell line (GES-1). Results showed miRNA-204-5p expression was markedly decreased in all four GC cell lines compared to GES-1 cells (P < 0.05, Fig. 1A).
Fig. 1.
miRNA-204-5p is downregulated in gastric cancer and is associated with clinicopathological features. (A) Relative expression of miRNA-204-5p in four gastric cancer cell lines (AGS, MKN7, MKN28, and MKN45) compared to a normal gastric epithelial cell line (GES-1) determined by qRT-PCR. ***P < 0.001 vs. GES-1. (B) Kaplan-Meier survival analysis of gastric cancer patients stratified by miRNA-204-5p expression level (n = 436). P-value determined by log-rank test. (C) Comparison of miRNA-204-5p expression between GC patients with and without lymph node metastasis. (D) Comparison of miRNA-204-5p expression between younger and older GC patients. (E) Comparison of miRNA-204-5p expression between male and female GC patients. (F) Comparison of miRNA-204-5p expression among different tumor stages (i, ii, iii, and iv) in GC patients.
To further explore the clinical relevance of miRNA-204-5p in GC, we analyzed the miRNA expression data and clinical information of 436 GC patients from TCGA database. For survival analysis, patients were stratified into high and low miRNA-204-5p expression groups using an optimal cutoff value of 0.5346015, which was determined using the maxstat R package (version 0.7–25) based on maximally selected rank statistics. Kaplan-Meier survival analysis demonstrated that patients with high miRNA-204-5p expression exhibited significantly prolonged overall survival compared to those with low expression (P < 0.05, Fig. 1B). Furthermore, comparative analysis revealed that miRNA-204-5p expression was significantly lower in patients with lymph node metastasis compared to those without (P < 0.05, Fig. 1C), while expression levels were significantly higher in older patients (≥ 65 years) compared to younger patients (< 65 years) (P < 0.05, Fig. 1D). However, no significant correlations were observed between miRNA-204-5p expression and gender, or stage (Fig. 1E,F). These findings suggest that the downregulation of miRNA-204-5p may contribute to the development, progression, and lymph node metastasis of GC. The aberrant expression of miRNA-204-5p in cell lines, as well as its association with clinical features, highlights the need for further investigation into its biological functions and molecular mechanisms in GC.
miRNA-204-5p inhibits the migration, and invasion of GC cells
To elucidate the biological functions of miRNA-204-5p in GC, we performed gain- and loss-of-function experiments by transfecting AGS and MKN45 cells with miRNA-204-5p mimic or inhibitor, respectively. The transfection efficiency was validated by qRT-PCR (P < 0.05, Fig. 2A). Wound healing assay demonstrated that miRNA-204-5p overexpression suppressed the migration of AGS and MKN45 cells at 24 h, whereas miRNA-204-5p knockdown enhanced cell migration at these time points (P < 0.05, Fig. 2B,E). Similarly, Transwell invasion assay revealed that the invasive ability of AGS and MKN45 cells was significantly reduced by miRNA-204-5p overexpression and increased by miRNA-204-5p knockdown (P < 0.05, Fig. 2F,G). Collectively, these data suggest that miRNA-204-5p plays a tumor-suppressive role in GC by inhibiting cell migration, and invasion.
Fig. 2.
miRNA-204-5p inhibits the migration and invasion of GC cells. (A) Validation of transfection efficiency of miRNA-204-5p mimic and inhibitor in AGS and MKN45 cells by qRT-PCR. (B, C) Wound healing assay showing the effect of miRNA-204-5p overexpression on the migration of AGS (B) and MKN45 (C) cells. (D, E) Wound healing assay showing the effect of miRNA-204-5p knockdown on the migration of AGS (D) and MKN45 (E) cells. (F, G) Transwell invasion assay showing the effect of miRNA-204-5p overexpression (F) and knockdown (G) on the invasive ability of AGS and MKN45 cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
miRNA-204-5p suppresses the Glycolysis of GC cells
Given the importance of glycolysis in cancer cell metabolism and progression, we investigated whether miRNA-204-5p regulates the glycolytic phenotype of GC cells. As shown in Fig. 3A,B, overexpression of miRNA-204-5p significantly reduced glucose uptake, lactate production, and ATP levels in AGS and MKN45 cells, whereas miRNA-204-5p knockdown enhanced these glycolytic parameters (P < 0.05, Fig. 3A–D). These findings suggest that miRNA-204-5p suppresses the glycolysis of GC cells.
Fig. 3.
miRNA-204-5p suppresses the glycolysis of GC cells. (A, B) Effect of miRNA-204-5p overexpression on ATP levels, glucose uptake, and lactate production in AGS (A) and MKN45 (B) cells. (C, D) Effect of miRNA-204-5p knockdown on ATP levels, glucose uptake, and lactate production in AGS (C) and MKN45 (D) cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of miRNA-204-5p on GC tumor metastasis in vivo
To further investigate the tumor-suppressive role of miRNA-204-5p in vivo, the lung metastasis model was used to analysed, MKN45 cells stably overexpressing miRNA-204-5p (OE-miRNA-204-5p) or negative control (OE-NC) were injected into nude mice via the tail vein. At the end of the experiment, the lungs were harvested for macroscopic observation and H&E staining (Fig. 4A–C). The OE-NC group exhibited visible metastatic nodules on the lung surface and extensive tumor cell infiltration in the lung tissue. In contrast, the OE-miRNA-204-5p group showed significantly fewer metastatic nodules (P < 0.05) and minimal tumor cell infiltration. These results demonstrate that miRNA-204-5p suppresses GC cell lung metastasis in vivo.
Fig. 4.
miRNA-204-5p suppresses GC tumor metastasis in vivo. (A) Macroscopic observation comparing lung metastatic nodules between mice injected with MKN45 cells stably overexpressing miRNA-204-5p (OE-miRNA-204-5p) and negative control (OE-NC). (B) H&E staining showing tumor cell infiltration in lung tissues from both groups. (C) Quantification of tumor cell infiltration in lung tissues by H&E staining. Data are presented as mean ± SD. **P < 0.01.
Collectively, these in vivo experiments provide strong evidence that miRNA-204-5p functions as a tumor suppressor in GC by inhibiting tumor metastasis. The inhibition of lung metastasis by miRNA-204-5p further highlights its potential as a therapeutic target for preventing GC progression and metastasis.
miRNA-204-5p inhibits the PI3K/AKT pathway and the expression of EMT-, and glycolysis-related molecules in GC cells
To investigate the molecular mechanisms underlying the tumor-suppressive effects of miRNA-204-5p, we examined the expression of PI3K/AKT pathway proteins, and molecules related to EMT, and glycolysis in AGS and MKN45 cells transfected with miRNA-204-5p mimic or inhibitor. The results are presented in Figs. 5 and 6. Western blot analysis revealed that miRNA-204-5p overexpression significantly decreased the protein levels of p-PI3K, and p-AKT (P < 0.05), while miRNA-204-5p knockdown had the opposite effects (P < 0.05). Furthermore, qRT-PCR and Western blot analyses showed that miRNA-204-5p overexpression markedly downregulated the mRNA and protein expression of EMT markers (N-cadherin), and glycolysis-related molecules (LDHA and HK2) but upregulated the expression of E-cadherin (P < 0.05). In contrast, miRNA-204-5p knockdown significantly increased the expression of these molecules but decreased E-cadherin expression (P < 0.05). These data suggest that miRNA-204-5p exerts its tumor-suppressive effects by regulating the PI3K/AKT pathway and molecules related to EMT, and glycolysis in GC cells.
Fig. 5.
miRNA-204-5p inhibits the PI3K/AKT pathway and the expression of EMT and glycolysis-related molecules in gastric cancer cells. (A, B) Representative Western blot images showing the effect of miRNA-204-5p mimic on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in AGS (A) and MKN45 (B) cells. (C, D) Quantitative analysis of the effect of miRNA-204-5p mimic on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in AGS (C) and MKN45 (D) cells. (E, F) Representative Western blot images showing the effect of miRNA-204-5p inhibitor on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in AGS (E) and MKN45 (F) cells. (G, H) Quantitative analysis of the effect of miRNA-204-5p inhibitor on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in AGS (G) and MKN45 (H) cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Fig. 6.
miRNA-204-5p regulates the mRNA expression of glycolysis and EMT-related markers in gastric cancer cells. (A, B) qRT-PCR analysis of the effect of miRNA-204-5p mimic on the mRNA expression of glycolysis and EMT markers in AGS (A) and MKN45 (B) cells. (C, D) qRT-PCR analysis of the effect of miRNA-204-5p inhibitor on the mRNA expression of glycolysis and EMT markers in AGS (C) and MKN45 (D) cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
RAB22A is a direct target of miRNA-204-5p in GC cells
To elucidate the molecular mechanisms underlying the tumor-suppressive functions of miRNA-204-5p, we used TargetScan to predict its potential target genes. Among the candidates, RAB22A, a member of the RAS oncogene family, caught our attention because it was reported to be upregulated and promote tumor progression in several cancers16–18. As shown in Fig. 7A, the 3’UTR of RAB22A contains two putative binding sites for miRNA-204-5p. To verify whether miRNA-204-5p could directly target RAB22A, we performed luciferase reporter assays by co-transfecting GC cells with wild-type or mutant RAB22A 3’UTR reporter plasmids and miRNA-204-5p mimics or negative controls. The results showed that miRNA-204-5p overexpression significantly reduced the luciferase activity of the wild-type RAB22A 3’UTR reporter but had no effect on the mutant reporter (P < 0.05, Fig. 7B), suggesting that miRNA-204-5p can directly bind to the 3’UTR of RAB22A and Inhibit its expression.
Fig. 7.
RAB22A is a direct target of miRNA-204-5p in gastric cancer cells. (A) Predicted binding sites of miRNA-204-5p in the 3’UTR of RAB22A. (B) Dual-luciferase reporter assay validating the binding interaction between miRNA-204-5p and RAB22A. Data are presented as mean ± SD. **P < 0.01.
Our findings indicate that RAB22A is a direct target of miRNA-204-5p in GC cells. These results suggest that the miRNA-204-5p/RAB22A axis may be involved in the development and progression of GC.
Overexpression of RAB22A attenuates the inhibitory effects of miRNA-204-5p on GC cell EMT, and Glycolysis
To further confirm whether miRNA-204-5p exerts its functions through RAB22A, we co-transfected AGS and MKN45 cells with miRNA-204-5p mimic and RAB22A overexpression plasmid (OE-RAB22A) and evaluated the changes in cell migration, invasion, and glycolysis. Wound healing assay demonstrated that RAB22A overexpression markedly reversed the suppressive effects of miRNA-204-5p on GC cell migration at 24 h (P < 0.05, Fig. 8A,B). Transwell invasion assay also revealed that RAB22A overexpression significantly counteracted the inhibitory effects of miRNA-204-5p on GC cell invasion (P < 0.05, Fig. 8C). Regarding glycolysis, RAB22A overexpression significantly enhanced glucose uptake, ATP production, and lactate production (P < 0.05, Fig. 9A,B) in miRNA-204-5p-overexpressing cells, indicating that RAB22A overexpression attenuates the inhibitory effects of miRNA-204-5p on GC cell glycolysis.
Fig. 8.
RAB22A overexpression attenuates the inhibitory effects of miRNA-204-5p on gastric cancer cell migration and invasion. (A, B) Wound healing assay assessing the effect of RAB22A overexpression on the migration of miRNA-204-5p-overexpressing AGS (A) and MKN45 (B) cells. (C) Transwell invasion assay evaluating the effect of RAB22A overexpression on the invasion of miRNA-204-5p-overexpressing AGS and MKN45 cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Fig. 9.
RAB22A overexpression reverses the inhibitory effects of miRNA-204-5p on gastric cancer cell glycolysis. (A, B) ATP production, glucose uptake, and lactate production in miRNA-204-5p-overexpressing AGS (A) and MKN45 (B) cells with RAB22A overexpression. Data are presented as mean ± SD. **P < 0.01.
Overexpression of RAB22A reverses the regulatory effects of miRNA-204-5p on RAB22A, p-PI3K, p-AKT, and molecules related to proliferation, EMT, and Glycolysis
We further investigated whether RAB22A overexpression could affect the regulatory effects of miRNA-204-5p on the PI3K/AKT pathway and molecules related to EMT, and glycolysis. The results are presented in Figs. 10 and 11. Western blot analysis showed that RAB22A overexpression significantly reversed the inhibitory effects of miRNA-204-5p on the protein levels of RAB22A, p-PI3K, and p-AKT (P < 0.05), confirming that miRNA-204-5p regulates the PI3K/AKT pathway through RAB22A. Moreover, qRT-PCR and Western blot analyses revealed that RAB22A overexpression markedly attenuated the regulatory effects of miRNA-204-5p on the expression of EMT markers (E-cadherin, and aN-cadherin), and glycolysis-related molecules (HK2, and LDHA) at both mRNA and protein levels (P < 0.05). These findings suggest that miRNA-204-5p regulates the expression of these molecules through the RAB22A/PI3K/AKT/mTOR axis, thereby inhibiting GC progression.
Fig. 10.
RAB22A overexpression reverses the regulatory effects of miRNA-204-5p on RAB22A, p-PI3K, p-AKT, and molecules related to EMT and glycolysis. (A, B) Representative Western blot images showing the effect of RAB22A overexpression on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in miRNA-204-5p-overexpressing AGS (A) and MKN45 (B) cells. (C, D) Quantitative analysis of the effect of RAB22A overexpression on the expression of PI3K/AKT pathway proteins, glycolysis-related proteins, and EMT markers in miRNA-204-5p-overexpressing AGS (C) and MKN45 (D) cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Fig. 11.
RAB22A overexpression reverses the regulatory effects of miRNA-204-5p on the mRNA expression of glycolysis and EMT markers. (A, B) qRT-PCR analysis of the effect of RAB22A overexpression on the mRNA expression of glycolysis and EMT markers in miRNA-204-5p-overexpressing AGS (A) and MKN45 (B) cells. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Discussion and conclusion
Gastric cancer (GC) is a highly prevalent and lethal malignancy worldwide, with a poor prognosis due to its late diagnosis and the lack of effective therapeutic targets1–4. Accumulating evidence has shown that microRNAs (miRNAs) play essential roles in the development and progression of GC by regulating various cancer-related processes6,7. In this study, we aimed to investigate the expression, functions, and molecular mechanisms of miRNA-204-5p in GC.
Accumulating evidence has shown that miRNAs are dysregulated in various cancers and play crucial roles in tumor development and progression by regulating multiple biological processes, such as cell proliferation, apoptosis, migration, invasion, and metabolism6,7. In GC, several miRNAs have been reported to function as either oncogenes or tumor suppressors. For example, miR-21 and miR-27a are upregulated in GC and promote cell proliferation and invasion by targeting PTEN and prohibitin, respectively15,16. In contrast, miR-148a and miR-34a are downregulated in GC and inhibit cell growth and metastasis by targeting ROCK1 and Snail, respectively17,18. These findings highlight the importance of miRNAs in the pathogenesis of GC and their potential as diagnostic biomarkers and therapeutic targets. In this study, we focused on miRNA-204-5p, which has been reported to be downregulated and function as a tumor suppressor in several cancers, such as breast cancer12, prostate cancer13, and hepatocellular carcinoma14. However, the expression pattern, biological functions, and molecular mechanisms of miRNA-204-5p in GC remain largely unknown.
Our results showed that miRNA-204-5p was significantly downregulated in cell lines, which is consistent with its expression patterns in other cancers. The downregulation of miRNA-204-5p was associated with lymph node metastasis and poor prognosis in GC patients, suggesting its potential as a prognostic biomarker. Functionally, we found that miRNA-204-5p inhibited the migration, invasion, and glycolysis of GC cells. These findings indicate that miRNA-204-5p plays a tumor-suppressive role in GC by regulating multiple malignant behaviors. The in vivo experiments further confirmed the anti-tumor effects of miRNA-204-5p, as overexpression of miRNA-204-5p suppressed tumor lung metastasis in nude mice. These results are in line with the tumor-suppressive functions of miRNA-204-5p reported in other cancers. For instance, in breast cancer, overexpression of miRNA-204-5p inhibited cell proliferation and invasion by targeting MMP-912. In prostate cancer, miRNA-204-5p promoted cell apoptosis by targeting BCL213. Our study expands the understanding of the biological functions of miRNA-204-5p in cancer and highlights its role as a tumor suppressor in GC.
To further elucidate the molecular mechanisms underlying the tumor-suppressive effects of miRNA-204-5p in GC, we performed bioinformatics analysis and identified RAB22A as a potential target of miRNA-204-5p. RAB22A is a member of the RAB family of small GTPases, which are key regulators of intracellular membrane trafficking19. Recent studies have shown that RAB22A is overexpressed in several cancers, such as breast cancer20, melanoma21, lung adenocarcinoma22, and papillary thyroid carcinoma23, and promotes tumor growth and metastasis by activating oncogenic signaling pathways, such as PI3K/AKT. However, the role of RAB22A in GC and its regulation by miRNAs remain unclear. In this study, we confirmed that RAB22A is a direct target of miRNA-204-5p using luciferase reporter assay. Overexpression of miRNA-204-5p significantly reduced the protein level of RAB22A. Rescue experiments showed that overexpression of RAB22A partially reversed the inhibitory effects of miRNA-204-5p on the malignant phenotypes of GC cells, suggesting that miRNA-204-5p exerts its tumor-suppressive functions, at least in part, by targeting RAB22A.
To further investigate the downstream signaling pathways regulated by the miRNA-204-5p/RAB22A axis, we focused on the PI3K/AKT pathway, which is a well-known oncogenic pathway that regulates cell proliferation, survival, metabolism, and motility24. Previous studies have shown that the PI3K/AKT/mTOR pathway is frequently activated in GC and contributes to tumor progression and chemoresistance25,26. Interestingly, we found that overexpression of miRNA-204-5p inhibited the activation of the PI3K/AKT pathway, as evidenced by the decreased phosphorylation levels of PI3K, and AKT. In contrast, knockdown of miRNA-204-5p activated this pathway. Moreover, overexpression of RAB22A reversed the inhibitory effects of miRNA-204-5p on the PI3K/AKT pathway. These results suggest that miRNA-204-5p inhibits the PI3K/AKT signaling pathway by targeting RAB22A in GC cells. Our findings are consistent with previous studies showing that RAB22A activates the PI3K/AKT pathway in other cancers22,23,27. To the best of our knowledge, this is the first report demonstrating the regulation of the RAB22A/PI3K/AKT axis by miRNA-204-5p in GC.
We further explored the downstream effectors regulated by the miRNA-204-5p/RAB22A/PI3K/AKT axis in GC cells. Our results demonstrated that overexpression of miRNA-204-5p downregulated the expression of mesenchymal markers (N-cadherin), and glycolytic enzymes (LDHA, and HK2), while upregulating the expression of the epithelial marker E-cadherin. Conversely, these effects were reversed by RAB22A overexpression. Epithelial-mesenchymal transition (EMT) is a complex biological process characterized by the loss of epithelial characteristics and the acquisition of a mesenchymal phenotype, which enhances cancer cell motility, invasiveness, and metastatic potential28. During EMT, epithelial markers such as E-cadherin are downregulated, while mesenchymal markers such as N-cadherin and Vimentin are upregulated29. In our study, overexpression of miRNA-204-5p significantly increased the expression of E-cadherin and decreased the expression of N-cadherin in GC cells, indicating its suppressive effect on EMT. Conversely, knockdown of miRNA-204-5p promoted EMT, as evidenced by the downregulation of E-cadherin and upregulation of N-cadherin. These findings suggest that miRNA-204-5p inhibits GC metastasis, at least in part, by suppressing EMT. The PI3K/AKT pathway has been reported to play a critical role in the regulation of EMT in various cancers, including GC30,31. Activation of this pathway can induce EMT by modulating the expression of EMT-related transcription factors, such as Snail, Slug, and Twist32. In our study, we found that miRNA-204-5p inhibited the activation of the PI3K/AKT pathway by targeting RAB22A, which subsequently led to the suppression of EMT in GC cells. Overexpression of RAB22A reversed the inhibitory effects of miRNA-204-5p on EMT, further confirming that miRNA-204-5p regulates EMT through the RAB22A/PI3K/AKT axis. Altered energy metabolism, particularly enhanced glycolysis, is another hallmark of cancer cells33. Even in the presence of oxygen, cancer cells preferentially utilize glycolysis for energy production, a phenomenon known as the Warburg effect. This metabolic reprogramming provides cancer cells with sufficient energy and metabolic intermediates for rapid proliferation and adaptation to the tumor microenvironment34,35. In our study, we found that overexpression of miRNA-204-5p significantly downregulated the expression of key glycolytic enzymes, including lactate dehydrogenase A (LDHA), and hexokinase 2 (HK2), in GC cells. LDHA catalyzes the conversion of pyruvate to lactate, which is essential for maintaining a high glycolytic rate36. HK2 catalyzes the first rate-limiting step of glycolysis by phosphorylating glucose to glucose-6-phosphate37. The downregulation of these glycolytic enzymes by miRNA-204-5p suggests its inhibitory effect on glycolysis in GC cells. The PI3K/AKT signaling cascade orchestrates glycolytic flux through transcriptional and post-translational regulation of key metabolic enzymes, including GLUT1 and LDH38. In our study, we found that miRNA-204-5p inhibited the PI3K/AKT pathway by targeting RAB22A, which subsequently led to the downregulation of glycolytic enzymes in GC cells. Overexpression of RAB22A reversed the inhibitory effects of miRNA-204-5p on glycolysis, indicating that miRNA-204-5p regulates glycolysis through the RAB22A/PI3K/AKT axis.
In conclusion, our study demonstrates that miRNA-204-5p functions as a tumor suppressor in GC by inhibiting cell migration, invasion, and glycolysis. The decreased expression of miRNA-204-5p is associated with lymph node metastasis and poor prognosis in GC patients, suggesting its potential as a prognostic biomarker. Mechanistically, miRNA-204-5p exerts its anti-tumor effects by directly targeting RAB22A and inhibiting the RAB22A/PI3K/AKT signaling pathway. The miRNA-204-5p/RAB22A axis regulates the expression of key molecules related to EMT, and glycolysis in GC cells. Our findings provide novel insights into the molecular mechanisms underlying the pathogenesis of GC and highlight the potential of miRNA-204-5p as a diagnostic biomarker and therapeutic target for this deadly disease. However, further studies are needed to validate the clinical significance of miRNA-204-5p in larger cohorts of GC patients and to develop effective strategies for its delivery and application in GC treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thank you to the anonymous reviewers and editors who helped improve the paper.
Author contributions
W. P. and Y. T. Data curation, W. P. , Y. T. , Xh. Ch. , L. Z. and Yt. Lv. , Formal analysis, Jl. Y. Funding acquisition, Jl. Y. Investigation, W. P. , Yt. Lv and Jl. Y. Methodology, W. P. , Y. T. , and Yt. Lv. Project administration, JJl. Y. ; Resources, Xh. Ch. , and L. Z. Software, Y. T., L.Z. and Yt. Lv. Supervision, Xh. Ch. Validation, Y. T. Writing – original draft, W. P., Y. T. Xh. Ch. and L. Z. Writing – review & editing, Jl. Y. .
Funding
This work was supported by the Local projects based on central guidance of China (XZ202301YD0031C).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed following the standard of International Coordinating Committee on Global Partnerships and the revised edition of the Declaration of Helsinki. This study was approved by the Ethics Committee of Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (202341). This study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org) to ensure full and transparent reporting of the methodology and results.
Consent to publish
All authors have given consent to publish.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang, W. et al. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol.29 (16), 2452–2468 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng, S. & Leong, T. Role of radiation therapy in gastric Cancer. Ann. Surg. Oncol.28 (8), 4151–4157 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Güç, Z. et al. Predicting pathological response and overall survival in locally advanced gastric cancer patients undergoing neoadjuvant chemotherapy: the role of PET/computed tomography. Nucl. Med. Commun.43 (5), 560–567 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Christodoulidis, G., Koumarelas, K. & Kouliou, M. Revolutionizing gastric cancer treatment: the potential of immunotherapy. World J. Gastroenterol.30 (4), 286–289 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang, H., Chang, H. & Baek, D. Determinants of functional MicroRNA targeting. Mol. Cells. 46 (1), 21–32 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali Syeda, Z. et al. Regulatory mechanism of MicroRNA expression in cancer. Int. J. Mol. Sci.21 (5), (2020). [DOI] [PMC free article] [PubMed]
- 7.Hill, M. & Tran, N. MiRNA interplay: mechanisms and consequences in cancer. Dis. Models Mech.14 (4), (2021). [DOI] [PMC free article] [PubMed]
- 8.Tse, J. et al. Onco-miR-21 promotes Stat3-dependent gastric cancer progression. Cancers14 (2), (2022). [DOI] [PMC free article] [PubMed]
- 9.Song, M. et al. MiR-148a-3p targets CEMIP to suppress the genesis of gastric cancer cells. Biochem. Biophys. Res. Commun.575, 42–49 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Yu, B. et al. miR-29c inhibits metastasis of gastric cancer cells by targeting VEGFA. J. Cancer. 13 (14), 3566–3574 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni, H. et al. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res. Ther.12 (1), 325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhana, A. et al. Gold nanoparticles inhibit PMA-Induced MMP-9 expression via microRNA-204-5p upregulation and deactivation of NF-κBp65 in breast cancer cells. Biology12 (6), (2023). [DOI] [PMC free article] [PubMed]
- 13.Lin, Y. et al. Tumor suppressor miRNA-204-5p promotes apoptosis by targeting BCL2 in prostate cancer cells. Asian J. Surg.40 (5), 396–406 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Jiang, G. et al. miR-204-5p targeting SIRT1 regulates hepatocellular carcinoma progression. Cell Biochem. Funct.34 (7), 505–510 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Hao, Z. et al. Targeting MicroRNA-21 suppresses gastric Cancer cell proliferation and migration via PTEN/Akt signaling Axis. Cell. Transpl.28 (3), (2019). [DOI] [PMC free article] [PubMed]
- 16.Tao, L. et al. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett.273 (2), (2008). [DOI] [PubMed]
- 17.Biqiang, Z. et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin. Cancer Res.17 (24), (2011). [DOI] [PubMed]
- 18.Yangmei, Z. et al. SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer. Cell. Cycle19 (1), (2019). [DOI] [PMC free article] [PubMed]
- 19.Lingjie, K. et al. Crucial roles of Rab22a in endosomal cargo recycling. Traffic24 (9), (2023). [DOI] [PubMed]
- 20.Miao, H. et al. Rab22a is a novel prognostic marker for cell progression in breast cancer. Int. J. Mol. Med.45 (4), (2020). [DOI] [PMC free article] [PubMed]
- 21.Qidi, H. et al. miR-204 suppresses uveal melanoma cell migration and invasion through negative regulation of RAB22A. Funct. Integr. Genomics23 (1), (2023). [DOI] [PubMed]
- 22.Jinping, W. et al. Rab22a promotes the proliferation, migration, and invasion of lung adenocarcinoma via up-regulating PI3K/Akt/mTOR signaling pathway. Exp. Cell. Res.416 (2), (2022). [DOI] [PubMed]
- 23.Xue, L. et al. Rab22a promotes Epithelial-mesenchymal transition in papillary thyroid carcinoma by activating PI3K/AKT/mTOR signaling pathway. Biomed. Res. Int.2022 (0), (2022). [DOI] [PMC free article] [PubMed]
- 24.Antonino, G. et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer22 (1), (2023). [DOI] [PMC free article] [PubMed]
- 25.Diana-Theodora, M. et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int. J. Mol. Sci.25 (3), (2024). [DOI] [PMC free article] [PubMed]
- 26.Zhiwei, X. et al. Inhibition of PI3K/Akt/mTOR signaling pathway suppresses 5-fluorouracil resistance in gastric cancer. Mol. Biotechnol. (0), (2023). [DOI] [PubMed]
- 27.Zhiwei, K., Chunming, Z. & Hui, H. Exosomal LncRNA LINC02191 promotes laryngeal squamous cell carcinoma progression by targeting miR-204-5p/RAB22A Axis and regulating PI3K/Akt/mTOR pathway. Biochem. Genet.62 (3), (2023). [DOI] [PubMed]
- 28.Fontana, R., Mestre-Farrera, A. & Yang, J. Update on Epithelial-Mesenchymal plasticity in Cancer progression. Annu. Rev. Pathol.19, 133–156 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzaei, S. et al. NF-κB as a regulator of cancer metastasis and therapy response: A focus on epithelial-mesenchymal transition. J. Cell. Physiol.237 (7), 2770–2795 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Jin, Y. et al. Super-enhancer-associated EEPD1 facilitates EMT-mediated metastasis by regulating the PI3K/AKT/mTOR pathway in gastric cancer. Biochem. Biophys. Res. Commun.689, 149188 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Peng, Y. et al. PI3K/Akt/mTOR pathway and its role in Cancer therapeutics: are we making headway?? Front. Oncol.12, 819128 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ang, H. et al. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev.43 (4), 1141–1200 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Kubik, J. et al. Targeting energy metabolism in cancer treatment. Int. J. Mol. Sci.23 (10), (2022). [DOI] [PMC free article] [PubMed]
- 34.Wang, Y. & Patti, G. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol.33 (12), 1014–1020 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberghina, L. The Warburg effect explained: integration of enhanced glycolysis with heterogeneous mitochondria to promote cancer cell proliferation. Int. J. Mol. Sci.24 (21), (2023). [DOI] [PMC free article] [PubMed]
- 36.Sharma, D., Singh, M. & Rani, R. Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Sem. Cancer Biol.87, 184–195 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Bao, C. et al. HK2: a potential regulator of osteoarthritis via glycolytic and non-glycolytic pathways. Cell. Communication Signaling: CCS. 20 (1), 132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bo, J. et al. Rhodiolin inhibits the PI3K/AKT/mTOR signaling pathway via the glycolytic enzyme GPI in human papillary thyroid cancer. Phytomedicine: Int. J. Phytotherapy Phytopharmacology. 132, 155804 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files.