Abstract
Umbilical cord blood (UCB) units are an alternative source of human hematopoietic stem cells (HSCs) for allogeneic stem cell transplants. A large quantity of HSCs is needed but the low number of accessible cells from UCB has been a significant limitation. Improving the ex vivo growth of HSCs while preserving their functioning is required. Here, we report that andrographolide (AP) enhanced the expansion of human UCB-derived HSCs (HSPCs) and pro-moted primitive HSCs (CD34+CD38−CD90+). AP also improved HSC functionality, evidenced by increased growth of colony-forming units and multilineage differentiation. AP upregulated genes involved in the Wnt/β-catenin and Notch signaling pathways. AP also modulated signaling pathways involved in HSC self-renewal, proliferation, survival, and differentiation, demonstrated by Nanostring analysis. The results of this study suggest that andrographolide enhances ex vivo UCB-HSC expansion while maintaining functionality and has potential for treatment of hematological diseases.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-15647-9.
Keywords: Andrographolide, Hematopoietic stem cells (HSCs), Ex vivo expansion, Umbilical cord blood (UCB), Hematologic disorders, Hematopoietic stem cell transplantation
Subject terms: Drug discovery, Stem cells
Introduction
The treatment of malignant and non-malignant hematopoietic diseases, including leukemia, lymphoma, and thalassemia, has been achieved through hematopoietic stem cell (HSC) transplantation1. However, aspirating bone marrow-derived HSCs are painful and can lead to infection and disease. For a transplant to be successful, matched samples must be obtained because optimal HSC levels are not always available2. Recently, hematopoietic stem cells (HSCs) from umbilical cord blood (UCB) units hold a great promise for applications in regenerative medicine and transplant therapy3,4. In comparison to those found in bone marrow, UCB comprises HSCs have greater rates of proliferation and immunological tolerance and UCB-HSCs are readily available with minimal risk of infection and low risk of graft-versus-host disease (GVHD)5,6. However, a major disadvantage of their use in clinical settings is the limited quantity of HSCs in each UCB unit7. This limitation leads to delayed engraftment and hematopoietic recovery, risk of graft rejection, and aberrant immune reconstitution, which are significant challenges in adult treatment8. Ex vivo expansion of UCB-HSCs could potentially enhance cord blood transplantations (UCBT) in large dosages9. The development of techniques to expand HSCs ex vivo, particularly UCB is a goal in hematology.
Recently, several cytokines and numerous promising conditions have been proposed to expand UCB-HSC numbers in culture10,11but these methods are inadequate to sustain robust, long-term HSC expansion and did not lead to clinical application12. Several factors impede the ex vivo expansion of HSCs, including differentiation, apoptosis, and senescence13. Therefore, novel protocols are needed to expand large numbers of functional HSCs which self-renew and maintain characteristics of primitive HSCs (CD34+CD38- or CD34+CD38-CD90+) following long term culture. In recent years, several novel small-molecule compounds have been reported to improve ex vivo HSC expansion by promoting self-renewal, delaying differentiation, increasing homing, and inhibiting apoptosis14. Recently, we reported the potential of andrographolide (AP), a small molecule derived from Andrographis paniculata, to promote mesenchymal stem cells in vitro via activation of Wnt/β-catenin signaling15. However, the effects and mechanisms of AP on UCB-HSC expansion have not yet been investigated. Therefore, this study aimed to investigate the effects of AP on the ex vivo expansion of human UCB-derived HSCs (UCB-CD34+ cells).
Materials and methods
Ethics approval and consent to participate
All experimental protocols and donor-informed consent for human umbilical cord blood (UCB) experiments in this study were approved by The Human Research Ethics Committee of Thammasat University (Medicine); Number of COA 100/2021; MTU-EC-DS-6-052/64, in accordance with the Declaration of Helsinki, the Belmont Report, and ICH-GCP, date of approval: 26 April 2021. Also, all methods were carried out in accordance with relevant guidelines and regulations. All donors signed a participant information sheet and informed consent before sampling.
UCB-CD34+ cell isolation and in vitro culture
Highly purified CD34+ cells from fresh whole umbilical cord blood were obtained using a straightforward, two-step procedure. First, hematopoietic progenitor cells were pre-enriched using the RosetteSep™ human cord blood CD34+ pre-enrichment cocktail (STEMCELL Technologies, Canada). Next, CD34+ cells were selected by magnetic isolation using the EasySep™ umbilical cord Human CD34 positive selection kit according to the manufacturer’s protocol (STEMCELL Technologies, Canada).
To determine the purity of the CD34+ cells, isolated cells were stained with APC-labeled anti-human CD34+ (BD; 555824) in phosphate-buffered saline (PBS) at 4 °C for 30 min. The stained cells were then washed with PBS and analyzed using a DX Flex (Beckman-Coulter) flow cytometer. The purity of UCB- CD34+ cells was determined by flow cytometry to be over 95%. For in vitro culture, isolated UCB-CD34+ cells were cultured in StemSpan™ expansion medium (STEMCELL Technologies, Canada) supplemented with 50 ng/mL stem cell factor (SCF), 50 ng/mL thrombopoietin (TPO), 20 ng/mL FLT-3 ligand (Flt-3 L), 20 ng/mL interleukin-6 (IL-6), 20 ng/mL soluble IL-6 receptor (sIL-6R) (all from Peprotech, US), and 1% (v/v) penicillin/streptomycin (P/S) (Merck Millipore, US) at 37 °C in a humidified atmosphere flushed with 5% CO2 in air.
Cell viability assay
To determine the optimal dose of AP for UCB-CD34+ cell survival, an MTS assay was performed. Briefly, cells were seeded into 96-well plates at a density of 1,000 cells/well with different concentrations of AP (Fig. 1A, Sigma-Aldrich, St. Louis, MO, USA) for 3, 5, and 7 days. Control cells receive an equal amount of medium with 0.01% of methanol. At the indicated time points, 100 µL of MTS (Promega) was added to each well and then incubated in the dark at 37 °C for 4 h. The absorbance was measured at 490 nm. All groups were normalized to the control group. All data were collected from three independent experiments.
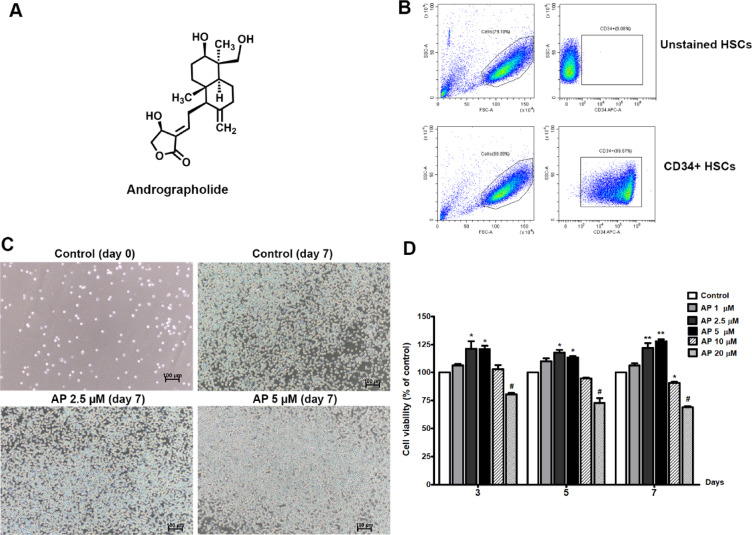
Fig. 1.
Effects of AP on cell morphology and expansion of UCB- CD34+ cells. (A) Chemical structure of AP, (B) Purity of isolated CD34+ cells, (C) Cell morphology before and after culturing with AP at two different concentrations (2.5 and 5 µM), photos were captured on day 7 (10× objective, scale bar; 100 μm). The control was composed of cytokines and methanol, (D) Viability of UCB-CD34+ cells after treatment with AP at different concentrations for 3, 5, and 7 days. Data are shown as mean ± SEM, *p < 0.05, **p < 0.01 compared to control.
Cell proliferation and immunophenotypic analysis in expanded cells
The proliferation of UCB-CD34+ cells was evaluated by FITC BrdU Flow Kit (BD Biosciences San Jose, CA, USA)) and analyzed by flow cytometry. Briefly, BrdU was added to cell culture media at a final concentration of 10 µM and incubated at 37 °C for 2 h, collected, and centrifuged for 5 min at 1500 rpm. The harvested cells were washed once, fixed and subsequently permeabilized and resuspended in Cytoperm Permeabilization Buffer Plus. Following centrifugation at 300 g for 6 min, cells were treated with DNase and resuspended in a solution containing 300 µg/ml DNase. After the treatment of cells with DNase to expose incorporated BrdU, cells were incubated in darkness for one hour at 37 °C and then washed BD Perm/Wash Buffer. To stain BrdU with fluorescent antibodies. cells were resuspended with 50 µl of BD Perm/Wash Buffer containing diluted fluorescent anti-BrdU and were incubated in the dark for 20 min at room temperature. Wash the cells with 1mL of 1X Perm/Wash Buffer and analysis was performed by flow cytometry (DX Flex, Beckman-Coulter). The data was expressed as the percentage of BrdU-positive cells.
For phenotypic analysis, cells were collected and were stained at 4°C for 30–60 min in phosphate-buffered saline (PBS) supplemented with a combination of the following antibodies and fluorophores: APC-labeled anti-human CD34 (BD; 555824), PE-Cy7-labeled anti-human CD38 (BD; 560677), APC-H7-labeled anti-human CD45RA (BD; 560674), and BV421-labeled anti-human CD90 (BD; 562556). Running at least 104 events, cell phenotypes in expanded cells were analyzed by a DxFlex flow cytometer (Beckman Coulter, Brea, CA, USA) using CytExpert for DxFLEX software version 2.0 (https://www.beckman.com/flow-cytometry/research-flow-cytometers/cytoflex/software).
Cell cycle analysis
UCB-CD34+ cells were washed with ice-cold PBS and then fixed and permeabilized in cold 70% ethanol at -20 °C for at least 2 h. After that, the cell pellets were stained with 0.5 mL PI/RNase Staining Buffer (BD Biosciences) for 15 min at room temperature in the dark, and data analysis was performed by a DxFlex flow cytometer (Beckman Coulter, Brea, CA, USA) using CytExpert for DxFLEX software version 2.0 (https://www.beckman.com/flow-cytometry/research-flow-cytometers/cytoflex/software).
Senescence-associated β-galactosidase (SA-β-gal) staining
UCB-CD34+ cells were stained for SA-β-gal activity following the manufacturer’s protocol (Cell Signaling Technology, Danvers, MA, USA). Briefly, HSCs (106) were fixed in 4% paraformaldehyde at room temperature for 15 min. The cells were washed with PBS and incubated at 37 °C without CO2 for 16 h in β-galactosidase staining solution. The number of β-galactosidase positive cells per 400 total cells was counted under a microscope (Nikon TS100, Japan).
Colony forming unit assay
UCB-CD34+ cells were cultured in the expansion medium containing AP at 2.5µM and 5µM for 7 days. After treatment, the cells were sorted again using CD34+ micro-bead kit. Then 500 sorted cells were plated in methylcellulose-based media (Methocult H4434, Stem Cell Technologies, Canada) containing 50 ng/mL human SCF, 10 ng/mL human granulocyte-macrophage colony-stimulating factor, 10 ng/mL human IL-3, and 3 IU/mL human EPO (StemCell Technologies) with and without andrographolide. After 14 days of culture, the colonies including burst-forming unit-erythroid (BFU-E), CFU granulocyte-macrophage (CFU-GM), and CFU granulocyte-erythrocyte-macrophage-megakaryocyte (CFUs-GEMM) were counted under a microscope. Only colonies with > 100 cells were counted as positive colonies.
RNA extraction and quantitative RT-QPCR analysis
Total RNA of UCB- CD34+ cells was extracted using RNeasy Mini Kit (Qiagen, California) following the manufacturer’s instructions. The integrity and quality of RNA samples was assessed using a Nano Drop (ND-1000) spectrophotometer (Thermo Fisher Scientific, US). Then 500 nanograms of the total RNA were subjected to reverse transcription using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories Inc). iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories Inc.) was used in qRT-QPCR, which was carried out using ABI Step One Plus (Applied Biosystems, CA, USA). Data was analyzed using the comparison threshold cycle value (2−ΔΔCt) method and using StepOne Software version 2.3 (https://www.thermofisher.com/id/en/home/technical-resources/software-downloads/StepOne-and-StepOnePlus-Real-Time-PCR-System.html). The GAPDH normalized transcript data are shown as a relative gene expression. The primer sequences for qRT-qPCR are listed in Table 1.
Table 1.
Human primer sequences used for quantitative RT-qPCR analysis.
| Gene | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
|---|---|---|
| CD34 | GGAAGGATGCTGGTCCG | CTGGGGTAGCAGTACCGTTG |
| CD90 | CGCTCTCCTGCTAACAGTCTT | CAGGCTGAACTCGTACTGGA |
| CD133 | CCATTGGCATTCTCTTTGAA | TTTGGATTCATATGCCTTCTGT |
| CD117 | TGGGATTTTCTCTGCGTTCT | TGATTTTCCTGGATGGATGG |
| ALDH1 | AATGGCATGATTCAGTGAGTGGC | GAGGAGTTTGCTCTGCTGGTTTG |
| ALDH2 | CAAGATAGAGATGCCCGGCG | ACAGGGAACACTCTCCCACT |
| BMI1 | CTTTCATTGTCTTTTCCGCC | TCCACAAAGCACACACATCA |
| HOXB4 | CCTGGATGCGCAAAGTTCA | AATTCCTTCTCCAGCTCCAAGA |
| GATA2 | CACAAGATGAATGGGCAGAA | ACAATTTGCACAACAGGTGC |
| RUNX1 | CGATGGCTTCAGACAGCATA | GGCATCGTGGACGTCTCTA |
| CXCR4 | CAGCAGGTAGCAAAGTGACG | CCCATTTCCTCGGTGTAGTT |
| NOTCH1 | CCGCAGTTGTGCTCCTGAA | ACCTTGGCGGTCTCGTAGCT |
| HES1 | CCAGCCAGTGTCAACACGA | AATGCCGGGAGCTATCTTTCT |
| HES5 | TTCTCAGAGAATGTGTGTGCAGAGT | GGTCAGACACTTGGCAGAAGATG |
| HEY1 | CTGCAGATGACCGTGGATCA | CAACTTCTGCCAGGCATTCC |
| CTNNB1 | GCTTGTTCGTGCACATCAGGA | TGTGAACATCCCGAGCTAGGA |
| WNT6 | TCTGGATACTGGGCTCCCC | ATTGATACTAACCTCACCCACC |
| AXIN2 | CCTGGCTCCAGAAGATCACA | AGCATCCTCCGGTATGGAAT |
| LEF1 | GCCAGACAAGCACAAACCTCT | TGGCATCATTATGTACCCGG |
| FZD2 | GTCCTCAAGGTGCCATCCTATC | GCGTCCCTCCTGTGAGAAGAA |
| CCDN1 | GATCAAGTGTGACCCGGACTG | CCTTGGGGTCCATGTTCTGC |
| CD44 | CCAACTCTAATGTCAATCGTTC | GCCAAGATGATCAGCCATTCTG |
| c-MYC | ATGGCCCATTACAAAGCCG | TTTCTGGAGTAGCAGCTCCTAA |
| GAPDH | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
NanoString analysis
The NanoString nCounter Analysis System employs digital fluorescent barcode technology to facilitate the direct multiplexed measurement of gene expression (mRNA). We hybridized 100 ng of RNA and subsequently processed it using the NanoString prep station and nCounter (NanoString Technologies) in accordance with the manufacturer’s instructions on 770 genes in the nCounter® Stem Cells Panel. We normalized the results with 29 housekeeping genes. All heat maps were created using the advanced analysis packages in nSolver 4.0 NanoString nSolver software (https://nanostring.com/products/ncounter-analysis-system/ncounter-analysis-solutions/).
Statistical analysis
Data are expressed as means ± SEM. Comparison between groups was done using one-way ANOVA followed by Dunnett’s multiple comparisons test. Analysis was performed using GraphPad Prism 10 statistical analysis software (GraphPad Software Inc., San Diego, CA, USA, https://www.graphpad.com/features#analysis). Differences were considered statistically significant at a p-value of less than 0.05.
Results
Effects of AP on cell morphology and cell viability of UCB-CD34+ cells
After isolating CD34+ cells from umbilical cord blood, we determined their purity before employing the cells in subsequent experiments. Flow cytometry analysis showed that the purity of the CD34+ cells isolated from UBC was higher than 99% (Fig. 1B) and the cells exhibited HSC morphology (Fig. 1C).
The optimal concentration of AP for culture of CD34+ cells was determined using MTS assay. CD34+ cells were incubated with optimized expansion medium with and without AP at the indicated concentrations for 3, 5, and 7 days. The results showed that treatment with AP at concentrations of 1, 2.5, and 5 µM increased viability of CD34+ cells, however, treatment with AP at concentrations higher than 10 µM and 20 µM markedly reduced cell viability by about 10% and 30% at day 7 (Fig. 1D). Therefore, AP at 1, 2.5, and 5 µM were selected for subsequent experiments.
AP increased the expansion of UCB-CD34+ cells and inhibited the senescence of expanded cells
UCB-CD34+ cells were cultured in an expansion medium in the presence or absence of 1, 2.5, and 5 µM AP for 1, 3, 5, and 7 days. Using trypan blue exclusion assay, we observed that the total number and relative fold-expansion of cultured cells was significantly higher in the 2.5 and 5 µM AP-treated than in the control at days 3, 5, and 7 (Fig. 2A). The proliferative effect of AP was also confirmed by BrdU assay on day 7. We found that AP at 2.5 and 5 µM significantly increased the number of BrdU+ cells compared to the control (Fig. 2B). The cells increased by AP were confirmed to be CD34+ cells. As shown in Fig. 2C, AP at 1, 2.5, and 5 µM increased CD34+ cells (fold change) to 1.35 ± 0.08, 1.68 ± 0.122, and 2.26 ± 0.19, respectively, compared to the control (the percentage of CD34+ cells was shown in supplementary data, Fig. S1).
Fig. 2.
Effects of AP on the proliferation, expansion of UCB-CD34+ cells, cell cycle and senescence of expanded UCB-CD34+ cells. (A) Total viable cell number throughout 7 days of culture after treatment with AP (B) Fold change of BrdU + cells along with flow cytometric data showing the percentage of BrdU + cells (C) relative fold-expansion of CD34+ cells after treatment with AP for 7 days (D) Cell cycle analysis (E) The number of β-galactosidase positive cells (senescent cells). Data are shown as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, compared with the control.
Cell cycle assays revealed that AP treatment increased the cell population in the S phase while decreasing the cell population in the G1/G0 phase (Fig. 2D). In this study, the biomarker of cellular senescence was Senescence-Associated β-Galactosidase (SA-βgal). After 14 days of incubation, the number of the SA-βgal positive cells in the AP-treated group (5 µM) was significantly less than that of the control (P < 0.01) (Fig. 2E). These results indicated that AP increased the expansion of HSCs by promoting cell cycle progression without leading to cell apoptosis or death and by inhibiting HSC senescence.
Effect of AP on primitive HSCs expansion
The most primitive hematopoietic stem cell (HSC) population reported to date is characterized as CD34+CD38-CD90+CD45R-. We next investigated the effect of AP on phenotypically defined primitive HSC markers, including CD34 positive, CD38 negative, CD90 positive and CD45R negative populations (CD34+, CD38-, CD90+, CD45R-). UCB- CD34+ cells were cultured in expansion medium in the presence or absence of 1, 2.5, and 5 µM AP for 7 days. The results show that the fold-expansion of CD34+CD45RA- (Fig. 3A), CD34+CD90+ CD45RA- (Fig. 3B), CD34+ CD38- CD45RA- (Fig. 3C), and particularly CD34+CD38-CD90+ CD45RA- cells (Fig. 3D) significantly increased when cultures were supplemented with AP at 2.5 and 5 µM. Collectively, these results suggest that AP promoted cytokine-enhanced expansion of primitive HSCs.
Fig. 3.
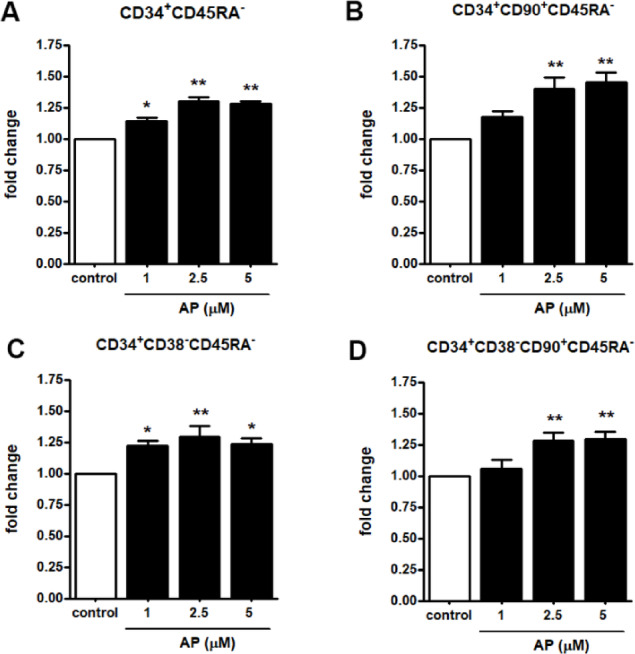
Effect of AP on ex vivo expansion of defined primitive HSCs. Representative FACs analysis plots of CD34+CD38-CD90+CD45RA- cells stained with CD34, CD38, CD90 and CD45RA antibodies at 7 days of culture. (A) CD34+CD45RA- (B) CD34+CD90+CD45RA- (C) CD34+CD38-CD45RA- (D) CD34+CD38-CD90+ CD45RA-. Data are represented as mean ± SEM from three independent experiments, * P < 0.05, * * P < 0.01 compared with the control.
Ability of AP to induce HSC expansion while maintaining their stemness in vitro
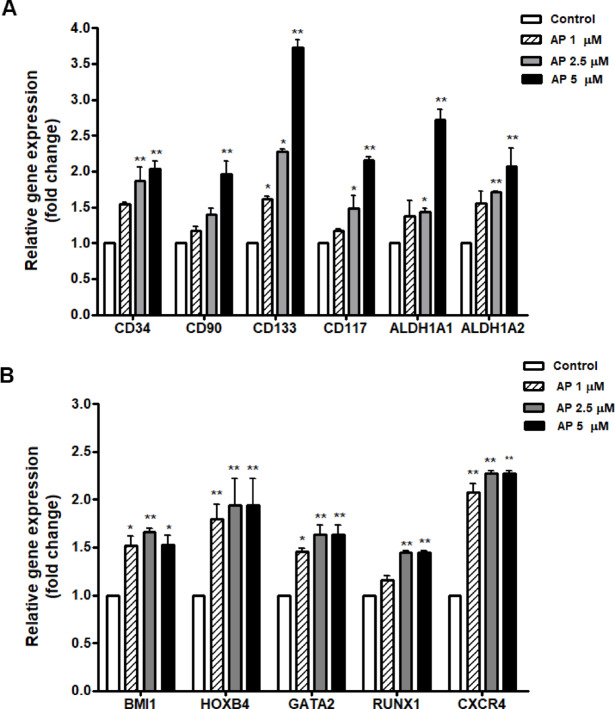
The expression of characteristic genes related to HSC stemness was then assessed. Based on the RT-qPCR results, HSC-specific markers and functionally important genes (CD34, CD90, CD133, CD117, ALDH1) were upregulated in the AP treated cells in a dose-dependent manner (1, 2.5, and 5 µM) (Fig. 4A). In addition, HSC-relevant genes and transcriptional factors including BMI1, HOXB4, GATA-2, RUNX1, and CXCR4 were also upregulated in the AP treated cells compared to the control (Fig. 4B).
Fig. 4.
Effect of AP on the expression of characteristic genes related to HSC stemness. Relative gene expressions as fold change at 7 days of culture with AP; (A) HSC-specific markers and functionally important genes (CD34, CD90, CD133, CD117, ALDH1, ALDH2) and (B) HSC-relevant genes and transcriptional factors (BMI1, HOXB4, GATA-2, RUNX1, and CXCR4). Data is represented as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the control.
AP sustained multipotency of HSC in vitro
The loss of multipotency is one of the major limitations of ex vivo HSC expansion. We therefore further investigated whether cells treated with AP have multi-lineage potential. We performed CFC assay after 7 days of culture; 500 cells were transferred to a semisolid differentiation medium for another 14 days to allow colony formation. Representative morphological images of different types of colonies are shown in Fig. 5A. AP-treated cells showed prominent colony-forming potential of granulocytes and macrophages (GM) compared to the control. A comparable increase was also observed in granulocyte–erythrocyte-macrophage–megakaryocyte (GEMM) colonies, which reflected the function of multipotent progenitors (MPPs), when treated with AP. As for the erythroid colonies, the number of BFU-E and CFU-E were also significantly increased (Fig. 5B). These results indicated that AP retained the capacity of hematopoietic stem and progenitor cells (HSPCs) to differentiate into multilineages in cultures.
Fig. 5.
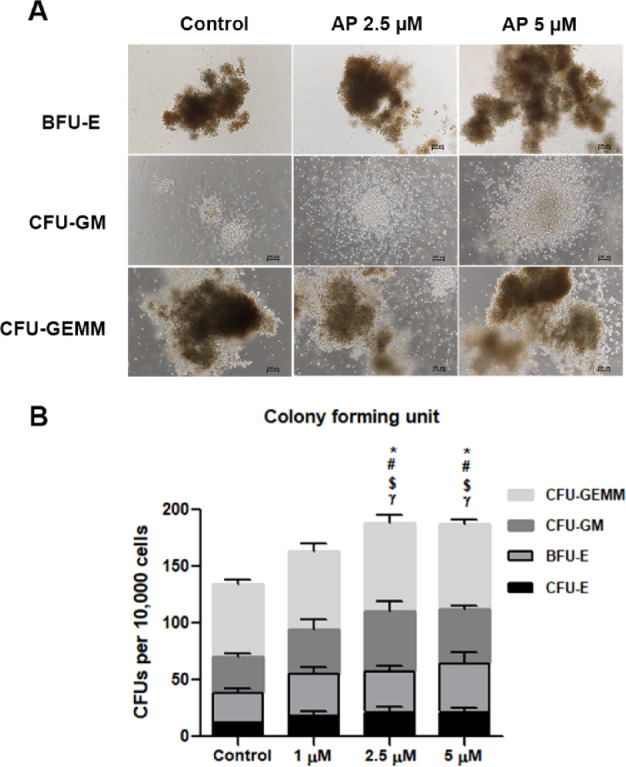
The colony-forming potential of AP-expanded CD34+ cells. (A) Representative images of colony formation (BFU-E, CFU-GM and CFU-GEMM) in Methocult® media following 14 days of culture. (B) The colony-forming potential of expanded CD34+ cells after culture with cytokines alone or 1, 2.5 or 5 µM AP. Colonies were counted according to colony size and cellular composition. Abbreviations: BFU-E, erythroid burst forming units; CFU-GM, granulocyte/macrophage common precursors. Comparing each colony between group: * represents CFU-E colony, # represents BFU-E colony, $ represents CFU-GM colony and γ represents CFU-GEMM colony). Data is represented as mean ± SEM from three independent experiments. Data is represented as mean ± SEM from three independent experiments. *P < 0.05, #P < 0.05, $P < 0.05 and γP < 0.05, compared with control group for each day.
Ability of AP to modulate the Notch and Wnt/β-catenin signaling pathways
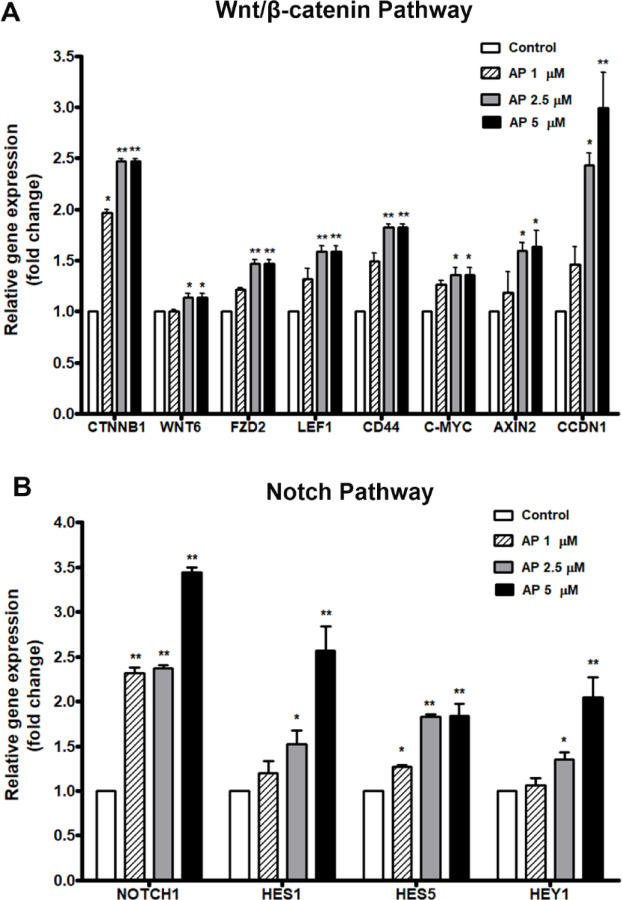
As AP preserved the HSC phenotype with inhibition of differentiation rather than promotion of the expansion of total mononuclear cells, we then investigated the effect of AP on the expression of genes in two major signaling pathways related to HSC self-renewal and differentiation: the canonical Wnt/β-catenin and Notch signaling pathways. The results showed that the relative expression of β-catenin gene (CTNNB1), which is a key gene in the canonical Wnt signaling pathway, was significantly increased in AP treated cells compared to the control. Likewise, the β-catenin target genes including AXIN2, LEF1, FZD2, CCDN1, CD44, and c-MYC were also significantly enhanced in the AP treated cells compared with the control (Fig. 6A). Moreover, the key gene in the Notch signaling pathway, NOTCH1 was elevated in AP-treated cells compared to the control, and the Notch target genes HES1, HEY1, and HES5 were also significantly upregulated by AP (Fig. 6B). Taking these results together, we suggest that AP may affect signaling of the cytokines in ways that activate the Notch and Wnt/β-catenin pathway for the preservation of HSC stemness, while preventing the effect of cytokines on cell differentiation, ultimately resulting in enhanced CD34+ cell expansion.
Fig. 6.
Effect of AP on gene expression of UCB-CD34+ cells determined by RT-qPCR. (A) key genes and target genes in Wnt/β-catenin pathway (B) key gene and target genes in Notch pathway. Bars represent the mean fold-changes of gene expression in the AP-treated cells relative to the control. *P < 0.05, **P < 0.01 compared to the control.
Pathway analysis by nanostring
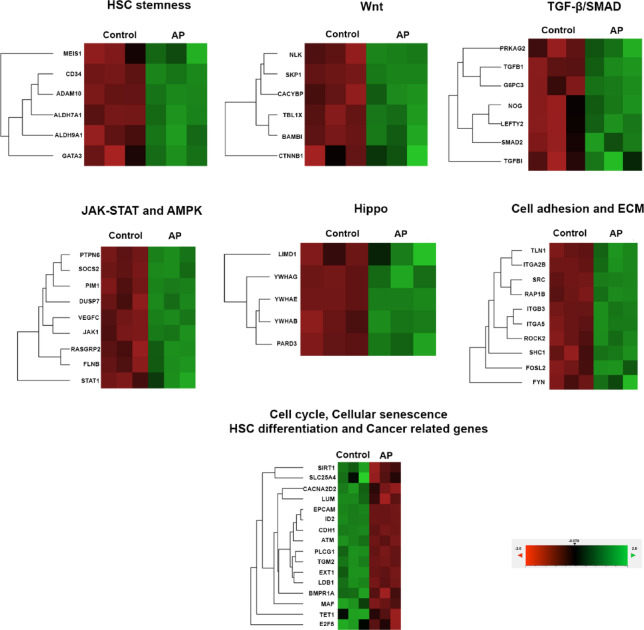
To clarify the mechanism by which AP enhances the proliferation and expansion of UCB-CD34+ cells, genes in stem cell pathways were analyzed and determined by the NanoString nCounter Analysis as in Fig. 7; Table 2. The modulation of AP mostly affected the expression of genes that are involved in the self-renewal, maintenance, differentiation, survival, and senescence of HSCs. In comparison with the control, we found that AP significantly upregulated the expression of Wnt/β-catenin signaling related genes such as BAMBI, CACYBP, CTBP1, CTNNB1, NLK, SKP1 and TBL1X which are important for HSC renewal and expansion. In addition, cell growth promoting and survival genes in the signaling pathway of TGF-β, SMAD, Hippo, JAK-STAT and AMPK are upregulated by AP. These pathways are not only important for cell growth but also for controlling pluripotency of stem cells and inhibiting cellular senescence. Moreover, numerous important genes for maintaining primitive HSCs such as ADAM10, ALDH7A1, ALDH9A1, CD34, and GATA3 were upregulated by AP. Apart from signaling pathways involved in cell proliferation and stemness, AP also affected signaling molecules involved in cell adhesion and extracellular matrix remodeling which in turn regulate genes for proliferation and differentiation of HSCs such as FYN, ITGA5, RAP1B, ROCK2, SHC1, SRC, TLN1, FOSL2, ITGA2B and ITGB3.
Fig. 7.
Stem cell pathway analysis in expanded CD34 + cells treated with AP (5µM) determined by Nanostring analysis system. P < 0.05, three independent experiments.
Table 2.
The list of genes which were downregulated or upregulated by AP treatment that shows statistical significance (P < 0.05) compared to differentiation control performed by NanoString analysis.
| Gene symbol | Gene name | Fold change | P value | Gene ID |
|---|---|---|---|---|
| HSC stemness | ||||
| ADAM10 | Human ADAM metallopeptidase domain | 1.27 | 0.00003 | NM_001110.2 |
| ALDH7A1 | Aldehyde dehydrogenase 7 family, member A1 | 1.52 | 0.00017 | NM_001202404.1 |
| ALDH9A1 | Aldehyde dehydrogenase 9 family member A1 | 1.23 | 0.00193 | NM_000696.3 |
| CD34 | CD34 | 2.06 | 0.00002 | NM_001025109.1 |
| GATA3 | GATA binding protein 3 | 2.03 | 0.02395 | NM_001002295.1 |
| MEIS1 | Meis homeobox 1 | 1.28 | 0.01317 | NM_002398.2 |
| Wnt signaling pathway | ||||
| BAMBI | ETS variant 1 | 1.34 | 0.00458 | NM_012342.2 |
| CACYBP | Leukemia inhibitory factor | 1.15 | 0.00146 | NM_014412.2 |
| CTBP1 | AMP responsive element binding protein 5 | 1.12 | 0.03374 | NM_001328.2 |
| CTNNB1 | Transcription factor 7-like 1 | 1.15 | 0.00955 | NM_001098210.1 |
| NLK | Kruppel-like factor 4 | 1.21 | 0.00667 | NM_016231.4 |
| SKP1 | cAMP responsive element binding protein 3-like 1 | 1.17 | 0.00001 | NM_006930.3 |
| TBL1X | Latent transforming growth factor beta binding protein 1 | 1.31 | 0.00029 | NM_005647.4 |
| TGFB and SMAD signaling pathway | ||||
| LEFTY2 | Phosphoinositide-3-kinase, regulatory subunit 2 | 1.46 | 0.02675 | NM_003240.2 |
| NOG | FA complementation group L | 1.48 | 0.03037 | NM_005450.4 |
| SMAD2 | Protein kinase C alpha | 1.11 | 0.01511 | NM_001003652.3 |
| TGFB1 | Fas (TNF receptor superfamily, member 6) | 1.32 | 0.00059 | NM_000660.3 |
| TGFBI | Phosphoinositide-3-kinase, catalytic, beta polypeptide | 1.58 | 0.02251 | NM_000358.2 |
| G6PC3 | Phosphoinositide-3-kinase regulatory subunit 3 | 1.27 | 0.00660 | NM_138387.3 |
| PRKAG2 | Human protein kinase, AMP-activated, gamma 2 non-catalytic subunit | 1.36 | 0.00358 | NM_016203.3 |
| JAK-STAT and AMPK signaling pathway | ||||
| JAK1 | Janus kinase 1 | 1.19 | 0.00071 | NM_002227.1 |
| PTPN6 | Protein tyrosine phosphatase, non-receptor type 6 | 1.38 | 0.00003 | NM_002831.5 |
| SOCS2 | Suppressor of cytokine signaling 2 | 1.58 | 0.00021 | NM_003877.3 |
| STAT1 | Signal transducer and activator of transcription 1 | 1.26 | 0.00907 | NM_007315.3 |
| DUSP7 | Dual specificity phosphatase 7 | 1.36 | 0.00240 | NM_001947.2 |
| FLNB | Filamin B | 1.24 | 0.00080 | NM_001164317.1 |
| RASGRP2 | RAS guanyl releasing protein 2 | 1.34 | 0.00204 | NM_001098671.1 |
| VEGFC | Vascular endothelial growth factor C | 1.53 | 0.00077 | NM_005429.2 |
| Hippo signaling pathway | ||||
| LIMD1 | LIM domains containing 1 | 1.12 | 0.02612 | NM_014240.2 |
| PARD3 | Par-3 partitioning defective 3 homolog | 1.23 | 0.00138 | NM_019619.2 |
| YWHAB | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein beta | 1.1 | 0.00334 | NM_139323.3 |
| YWHAE | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein epsilon | 1.1 | 0.00002 | NM_006761.4 |
| YWHAG | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein gamma | 1.25 | 0.00445 | NM_012479.4 |
| Cell adhesion and ECM | ||||
| FYN | FYN related to SRC, FGR, YES | 1.18 | 0.00998 | NM_002037.3 |
| ITGA5 | Integrin, alpha 5 | 1.48 | 0.00007 | NM_002205.2 |
| RAP1B | RAP1B, member of RAS family | 1.32 | 0.00008 | NM_015646.5 |
| ROCK2 | Rho associated coiled-coil containing protein kinase 2 | 1.15 | 0.00045 | NM_004850.3 |
| SHC1 | SHC adaptor protein 1 | 1.22 | 0.00476 | NM_001130040.1 |
| SRC | Schmidt-Ruppin A-2 | 1.77 | 0.00007 | NM_005417.3 |
| TLN1 | Talin 1 | 1.16 | 0.00064 | NM_006289.3 |
| FOSL2 | FOS like 2 | 1.35 | 0.00094 | NM_005253.3 |
| ITGA2B | Integrin subunit alpha 2b | 1.29 | 0.00130 | NM_000419.3 |
| ITGB3 | Integrin subunit beta 3 | 1.27 | 0.00015 | NM_000212.2 |
| Cell cycle, cellular senescence, HSC differentiation and cancer related genes | ||||
| ATM | ATM | − 1.4 | 0.00005 | NM_000051.3 |
| SIRT1 | Sirtuin 1 | − 1.13 | 0.00278 | NM_012238.4 |
| SLC25A4 | Solute carrier family 25, member 4 | − 1.21 | 0.04906 | NM_001151.4 |
| CACNA2D2 | Calcium channel, voltage-dependent, alpha 2/delta subunit 2 | − 1.65 | 0.00692 | NM_001005505.1 |
| E2F5 | E2F transcription factor 5 | − 1.2 | 0.04059 | NM_001083588.1 |
| PLCG1 | Phospholipase C gamma 1 | − 1.63 | 0.00110 | NM_002660.2 |
| CDH1 | Cadherin 1, type 1, E-cadherin | − 3.23 | 0.00012 | NM_004360.2 |
| EPCAM | Epithelial cell adhesion molecule | − 2.14 | 0.00000 | NM_002354.2 |
| EXT1 | Exostosin glycosyltransferase 1 | − 1.86 | 0.00366 | NM_000127.2 |
| LUM | Lumican | − 2.02 | 0.00963 | NM_002345.3 |
| ID2 | Inhibitor of DNA binding 2 | − 1.78 | 0.00003 | NM_002166.4 |
| BMPR1A | Bone morphogenetic protein receptor type 1 A | − 3 | 0.00383 | NM_004329.2 |
| LDB1 | LIM domain binding 1 | − 1.47 | 0.00005 | NM_001113407.1 |
| TGM2 | Transglutaminase 2 | − 2.75 | 0.00007 | NM_004613.4 |
| TET1 | Tet methylcytosine dioxygenase 1 | − 1.48 | 0.03371 | NM_030625.2 |
| MAF | MAF bZIP transcription factor | − 1.45 | 0.00935 | NM_005360.4 |
In contrast, AP significantly downregulated several groups of genes which were associated with cell cycle arrest and cellular senescence (ATM, SIRT1, SLC25A4), HSC activation and differentiation (ID2, BMPR1A, LDB1, TGM2, TET1, MAF, FOSL2), tumorigenesis and target for cancer (PLCG1, CACNA2D2, CDH1) as well as ECM and proteoglycan in cancer (EXT1, LUM, EPCAM). These modulations might imply that AP did not stimulate expression of genes involved in cell differentiation and cancer development in UCB-CD34+ cells. These results indicate that AP affects multiple pathways necessary for maintaining the self-renewal and multi-lineage differentiation capacity of hematopoietic stem cells (HSC). However, the direct effects of AP on these pathways must be demonstrated using specific inhibitors for each pathway.
Discussion
The expansion or maintenance of the numbers of functional human HSCs following ex vivo culture can be utilized as a platform to improve the outcomes of HSC-based therapies including transplantation and gene modification16,17. Several strategies have been extensively used to optimize culture conditions to expand and retain pluripotency of HSCs ex vivo. Bioactive compounds from medicinal plants hold great promise for ex vivo expansion of HSC as they not only enhance self-renewal of HSCs but also retain their biological properties18. Here, we revealed for the first time that treatment with AP enhanced the number of phenotypically defined primitive HSCs (CD34+CD38−CD90+ cells). AP also preserved biological properties and functions of the expanded cells, including self-renewal and multilineage differentiation. The effects of AP were mediated through the simultaneous modulation of signaling pathways involving cell proliferation and differentiation and those required for maintenance of HSC pluripotency and stemness including Wnt-β-catenin and Notch signaling pathways.
Our study demonstrated that the numbers of human primitive HSCs were greater in the AP-cultured condition compared to the control. AP activated the Wnt/β-catenin and Notch signaling pathways to preserve HSC stemness and self-renewal ability while preventing the effect of cytokines on inducing cell differentiation. The Wnt/β-catenin signaling pathway plays a significant role in the maintenance of adult stem cell self-renewal and the process of fetal hematopoiesis19. The potential role of Notch in stem cell self-renewal and ex vivo expansion involves the modulation of the expression of proteins that can inhibit cell differentiation20,21. The upregulation of key genes in Notch, such as GATA-2 and HES-1, by AP is consistent with prior reports that have indicated the expression of these genes are well-established inhibitors of multiple differentiation22. Likewise, the Wnt/β-catenin signaling pathway not only plays a critical role in the development of embryonic stem cells, but also in the proliferation and differentiation of adult stem cells including HSCs23. The major effects of Wnt are exerted through β-catenin, which has the ability to enhance the self-renewal and proliferation of HSCs19. The findings of our study demonstrated the activation of β-catenin and its associated genes which correlate with the promotion of UC-HSC expansion by AP. This finding is in line with previous studies showing that AP increases in vitro proliferation of mesenchymal stem cells (MSC) derived from human placentas with the activation of Wnt/β-catenin signaling15 .
Previous studies have shown that the activation or inhibition of cell signaling pathways can modulate the growth of HSCs, thereby controlling HSC engraftment and in vitro HSC expansion24. To gain molecular insight into how AP promotes ex vivo expansion of UC-HSCs, Nanostring RNA analysis was performed. Based on the analysis data, AP modulated signaling pathways are involved in HSC self-renewal, proliferation, maintenance, survival, and anti-senescence. These pathways include the Wnt/β-catenin, Forkhead O (FoxO), AMPK, JAK-STAT, TGF-β and Hippo signaling pathways. The activation of these signaling pathways in HSCs facilitated the retention of cells in the proliferative stage, thereby preventing them from entering the cell cycle and from becoming senescent25. Andrographolide significantly increases the human HSC expansion in culture and these expanded HSCs maintain their undifferentiated gene expression signature and differentiation capacity for various hematopoietic lineages under appropriate culture conditions. Consistent with these results, the expanded HSCs also expressed lower levels of genes related to senescence and expressed higher levels of several genes associated with WNT/β-catenin and NOTCH signaling, which are known to play an important role in the maintenance of HSC stemness. Taken together, our results suggest that andrographolide increases the expansion of human HSCs without compromising their stemness property.
Interestingly, AP also modulated genes in the Hippo signaling pathway. Recently, the role of the Hippo signaling pathway in control cell proliferation and differentiation has been widely investigated. The activation of Hippo signaling in HSCs has been reported to be related to the increased number of HSC (CD34+) and retained pluripotency of HSC26. Hippo signaling pathway has recently been explored more regarding its role in HSC regulation27. The major downstream effectors of the Hippo signaling pathway are the YAP/TAZ transcription co-activators, which are inhibited by the Hippo pathway kinase LATS1/228. In our study, AP upregulated PARD3 expression which has been reported to inhibit LATS1 activity, thereby activating the Hippo signaling pathway29. A recent study suggests that TAZ interacts with Wnt/β-catenin signaling and YAP/TAZ works with the nuclear location of β-catenin to activate genes that are responsible for cell proliferation30. If AP has the ability to activate β-catenin and the YAP/TAZ transcription co-activators, the enhancement effect of AP on HSCs expansion may be related to the crosstalk of Wnt/β-catenin and Hippo signaling pathways.
We also found that the expression of genes implicated in cellular senescence was reduced by AP, which is consistent with the observed phenotype that AP reduced the number of senescent cells in the culture. Apart from signaling pathways involved in cell proliferation, AP also affects genes involved in cell adhesion and extracellular matrix (ECM) remodeling. ECM interacts with cells to regulate diverse functions, including proliferation, migration, differentiation, and survival of HSCs. It is possible that AP promotes ECM remodeling that allows the cell to grow and undergo cell division. Collectively, AP suppressed several groups of genes which were associated with cell cycle and cellular senescence, HSC activation and differentiation and tumorigenesis and targets for cancer, suggesting a potential of AP in promoting HSCs expansion and preventing cellular differentiation while maintaining HSCs stemness. However, our gene screening data only suggests the possible molecules and pathways by which AP affects ex vivo expansion and maintenance of UCB-HSCS. To confirm the multitargeted effect of AP, its direct effect on the above-mentioned signaling pathways should be further investigated by genetic knockdown and pharmacologic inhibition.
Conclusion
We revealed that AP markedly increased the numbers of primitive HSCs (CD34+CD38−CD90+) and preserved their biological properties. Moreover, we illustrated that the effect of AP is likely mediated through multiple pathways that help maintain HSC properties. To our knowledge, this is the first study to report the action of AP on the expansion of UBC-HSCs. These findings provide a scientific rationale for the use of AP as a novel molecule to increase numbers of HSCs before hematopoietic stem cell transplantation (HSCT).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Thammasat University Hospital, the delivery room staff, and all volunteers for their kind donation of the tissues for this research. We also thank Michael Jan Everts, from the Clinical Research Centre, Faculty of Medicine, Thammasat University, for editorial assistance in improving the English in this manuscript.
Abbreviations
- AP
Andrographolide
- UCB
Umbilical cord blood
- HSCs
Hematopoietic stem cells
- CD34+CD38− or CD34+CD38−CD90+
Primitive hematopoietic stem cells
- HSCT
Hematopoietic stem cell transplantation
- G-CSF
Granulocyte colony-stimulating factor
- CFUs
Colony forming units
- BFU-E
Burst-forming unit-erythroid
- CFU-GM
Colony-forming unit-granulocyte
- CFUs-GEMM
CFU granulocyte-erythrocyte-macrophage-megakaryocyte
- GVHD
Graft-versus-host disease
- SA-β-gal
Senescence-associated β-galactosidase
Author contributions
D.T. contributed to conceptualization, study design, resources, supervision, data analysis and interpretation, drafted and revised manuscript. N.S. conducted the experiments, interpreted data, drafted and revised the manuscript. L.R contributed to conduct the experiments, data analysis and interpretation. K.B. and C.T. contributed to resources and supervision. H.S. conducted the experiments. P.K., S.M. and C.T. contributed to visualization. All authors read and agreed on the submitted version of the manuscript.
Funding
The authors gratefully acknowledge funding from Thailand Science Research and Innovation Fundamental Fund (TUFF35/2565 and TUFF58/2566) and Thammasat University Research Fund under the TU Research Scholar (TUFT-FF 32/2565), Thammasat University Postdoctoral Fellowship Fund and Center of Excellence in Stem Cell Research and Innovation, Thammasat University. The funding body played no role in the design of the study and the collection, analysis, and interpretation of the data, nor in the writing of the manuscript.
Data availability
The data generated or analyzed during this study are included in this article, or if absent are available from the corresponding author upon reasonable request. The sequence data that support the findings of this study have been provided within the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nareerat Sutjarit and Laongthip Ruknarong contributed equally to this work.
References
- 1.Alexander, T. & Greco, R. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the autoimmune diseases working party (ADWP) of the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant.57, 1055–1062. 10.1038/s41409-022-01702-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czechowicz, A. & Weissman, I. L. Purified hematopoietic stem cell transplantation: the next generation of blood and immune replacement. Hematol. Oncol. Clin. N. Am.25, 75–87. 10.1016/j.hoc.2010.11.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz, J. et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med.3, 1435–1443. 10.5966/sctm.2014-0151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao, Y., Geyer, M. B., Yang, A. J. & Cairo, M. S. Cord blood transplantation and stem cell regenerative potential. Exp. Hematol.39, 393–412. 10.1016/j.exphem.2011.01.002 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Ballen, K. Umbilical cord blood transplantation: challenges and future directions. Stem Cells Transl Med.6, 1312–1315. 10.1002/sctm.17-0069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineault, N. & Abu-Khader, A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp. Hematol.43, 498–513. 10.1016/j.exphem.2015.04.011 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Gupta, A. O. & Wagner, J. E. Umbilical cord blood transplants: current status and evolving therapies. 8, 10.3389/fped.2020.570282 (2020). [DOI] [PMC free article] [PubMed]
- 8.Fraint, E., Ulloa, B. A., Feliz Norberto, M., Potts, K. S. & Bowman, T. V. Advances in preclinical hematopoietic stem cell models and possible implications for improving therapeutic transplantation. Stem Cells Transl Med.10, 337–345. 10.1002/sctm.20-0294 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meaker, G. A. & Wilkinson, A. C. Ex vivo hematopoietic stem cell expansion technologies: recent progress, applications, and open questions. Exp. Hematol.130, 104136. 10.1016/j.exphem.2023.12.001 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai, M., Ishitsuka, K., Becker, H. J. & Yamazaki, S. Ex vivo expansion of human hematopoietic stem cells and clinical applications. Cancer Sci.115, 698–705. 10.1111/cas.16066 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staversky, R. J. et al. The chemokine CCL3 regulates myeloid differentiation and hematopoietic stem cell numbers. Sci. Rep.8, 14691. 10.1038/s41598-018-32978-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, Y. & Gao, Y. Novel chemical attempts at ex vivo hematopoietic stem cell expansion. Int. J. Hematol.103, 519–529. 10.1007/s12185-016-1962-x (2016). [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson, A. C., Igarashi, K. J. & Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet.21, 541–554. 10.1038/s41576-020-0241-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghafouri-Fard, S., Niazi, V., Taheri, M. & Basiri, A. Effect of small molecule on ex vivo expansion of cord blood hematopoietic stem cells: A concise review. 910.3389/fcell.2021.649115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phunikom, N. et al. Andrographolide promotes proliferative and osteogenic potentials of human placenta-derived mesenchymal stem cells through the activation of Wnt/β-catenin signaling. Stem Cell. Res. Ther.12, 241. 10.1186/s13287-021-02312-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milsom, M. D. Ex vivo expansion of functional hematopoietic stem cells, facilitating transplantation in the absence of conditioning. HemaSphere. 3, e306 10.1097/hs9.0000000000000306 (2019). [DOI] [PMC free article] [PubMed]
- 17.Zimran, E., Papa, L. & Hoffman, R. Ex vivo expansion of hematopoietic stem cells: finally transitioning from the lab to the clinic. Blood Rev.50, 100853. 10.1016/j.blre.2021.100853 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Baron, F., Ruggeri, A. & Nagler, A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev. Hematol.9, 297–314. 10.1586/17474086.2016.1128321 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Carpenter, K. A., Thurlow, K. E., Craig, S. E. L. & Grainger, S. Wnt regulation of hematopoietic stem cell development and disease. Curr. Top. Dev. Biol.153, 255–279. 10.1016/bs.ctdb.2022.12.001 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing, W. et al. The role of the Notch signaling pathway in the differentiation of human umbilical cord-derived mesenchymal stem cells. Front. Bioscience (Landmark edition). 29, 74. 10.31083/j.fbl2902074 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Kumano, K. et al. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood98, 3283–3289. 10.1182/blood.v98.12.3283 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Varnum-Finney, B., Brashem-Stein, C. & Bernstein, I. D. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood101, 1784–1789. 10.1182/blood-2002-06-1862 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Ring, A., Kim, Y. M. & Kahn, M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell. Reviews Rep.10, 512–525. 10.1007/s12015-014-9515-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, A. K., Althoff, M. J. & Cancelas, J. A. Signaling pathways regulating hematopoietic stem cell and progenitor aging. Curr. Stem Cell Rep.4, 166–181. 10.1007/s40778-018-0128-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louria-Hayon, I. Signal, transduction, and the hematopoietic stem cell. Rambam Maimonides Med. J.5, e0033. 10.5041/rmmj.10167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanhuad, N. et al. Ex vivo expansion and functional activity preservation of adult hematopoietic stem cells by a diarylheptanoid from Curcuma comosa. Biomed. pharmacotherapy = Biomedecine Pharmacotherapie. 143, 112102. 10.1016/j.biopha.2021.112102 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Kim, I., Park, T., Noh, J. Y. & Kim, W. Emerging role of Hippo pathway in the regulation of hematopoiesis. BMB Rep.56, 417–425. 10.5483/BMBRep.2023-0094 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu, M. et al. The Hippo signalling pathway and its implications in human health and diseases. Signal. Transduct. Target. Therapy. 7, 376. 10.1038/s41392-022-01191-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv, X. B. et al. PARD3 induces TAZ activation and cell growth by promoting LATS1 and PP1 interaction. EMBO Rep.16, 975–985. 10.15252/embr.201439951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sileo, P., Simonin, C., Melnyk, P., Chartier-Harlin, M. C. & Cotelle, P. Crosstalk between the hippo pathway and the Wnt Pathway in Huntington’s disease and other neurodegenerative disorders. Cells1110.3390/cells11223631 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are included in this article, or if absent are available from the corresponding author upon reasonable request. The sequence data that support the findings of this study have been provided within the manuscript.