Abstract
Background
Gastric cancer (GC) is the fourth most common cause of cancer death and has the fifth highest incidence of cancer worldwide. Circular RNAs (circRNAs) have emerged as essential regulators of tumorigenesis and cancer progression. However, the underlying regulatory mechanisms and their roles as diagnostic biomarkers have yet to be well elucidated in GC.
Methods
In this study, we implemented genome-wide sequencing to identify circRNAs whose expression in plasma extracellular vesicles (EVs) significantly changed. Functional experiments were performed to assess its effect on the proliferation, viability, migration and apoptosis of GC cell lines. Luciferase reporter and RNA pull-down assays were used to validate their combinations. Finally, RNA-Seq and RT-qPCR were used to identify the downstream regulatory pathway involved.
Results
We found that the circRNA of MORC family CW-type zinc finger 1 gene (circMORC1) was decreased in the plasma EVs of GC patients but increase in GC cells. In vivo, circMORC1 promoted proliferation, viability, and migration and inhibited apoptosis in AGS and SGC-7901 GC cell lines. CircMORC1 was found to bind to miR-103a-1-5p, which is essential for the regulation of phenotypes. In addition, the Wnt pathway was found to be the downstream regulatory pathway of circMORC1 through a miR-103a-1-5p sponge.
Conclusions
Our results shed light on circMORC1 promotes the proliferation of gastric cancer cells, as a RNA sponge of miR-103a-1-5p and counteracted the down-regulation of Wnt signaling function of miR-103a-1-5p. Revealed a novel mechanism by which circMORC1 regulates tumorigenesis in gastric cancer and provided a new biomarker for GC diagnosis and prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14688-7.
Keywords: circRNA, Gastric cancer, miR-103a-1-5p, Wnt pathway, EV
Background
Gastric cancer (GC) is the fourth most common cause of cancer death and has the fifth highest incidence of cancer worldwide [1]. Considerable progress has been made toward curative treatment for GC over the last few decades, and limitations remain in the early diagnosis and pathogenesis of GC. Most of the GC molecular markers currently applied in the clinic, such as serum CEA (Carcinoma Embryonic Antigen), glycol antigens and AFP (alpha-fetoprotein), are broad-spectrum tumor-related markers that lack specificity and sensitivity [2]. Therefore, identifying potential targets for GC and elucidating their role in the pathogenesis of GC development are urgently needed.
Circular RNA (circRNA) are a class of circular noncoding RNAs [3]. These RNAs exhibit greater stability and corrosion resistance than the corresponding linear RNAs due to their covalently closed-loop structures without 5’ cap structures or 3’ poly tails [4]. CircRNAs mainly exist in the cytoplasm of eukaryotic cells and are also expressed in extracellular vesicles (EVs). Due to the special structural properties and distribution of circRNAs, they have obvious advantages as biomarkers for clinical diagnosis of conditions such as cancer and autoimmune diseases, and further, they can also serve as therapeutic targets for GC [5, 6]. CircRNAs regulate multiple biological functions by regulating gene expression through acting as competitive endogenous sponges for microRNAs (miRNAs) in combination with RNA binding proteins and regulators of RNA splicing [7]. Accumulating evidence suggests that circRNAs make valuable contributions to the carcinogenesis and progression of many malignancies, including GC [8–10]. CircRNA derived from EVs in GC patient plasma not only carries disease-related information but also provides target molecules for the study of circRNA function [11, 12]. As EVs may carry circRNAs that play a key role in the regulation of cellular functions, elucidating the function of differentially expressed circRNAs in GC has research value, and it makes sense to offer new perspectives for further cancer treatment and diagnosis.
Numerous studies have reported that circRNAs act as miRNA sponges to absorb miRNAs and inhibit miRNA function, which is a common circRNA mechanism [13–15]. MicroRNAs are a class of noncoding RNAs 18–25 nucleotides in length that are widely present in the genomes of organisms but are highly conserved during evolution. It regulates gene expression by binding to the 3’ untranslated regions (3’-UTRs) of target mRNAs through complementary interactions [16]. MiRNAs are involved in cell proliferation, metastasis, differentiation, and autophagy, and their abnormal expression is involved in tumorigenesis and development [17]. In addition, miRNAs in cancer have been widely studied, and miRNAs are aberrantly expressed in various types of cancer tissues and cells and act as oncogenes and tumor suppressors [18].
In this study, we found that circMORC1 is differentially expressed in the plasma EVs of patients with GC and verified its expression relationship between GC cells and secreted EVs. Further research revealed that circMORC1 functions as a tumor-promoting factor in GC and verified that circMORC1 can specifically bind to miR-103a-1-5p. Moreover, miR-103a-1-5p has been reported to be down-regulated in GC and is closely associated with the occurrence and progression of gastric cancer [19, 20], is a once-miRNA involved in Wnt/β-catenin pathway regulation [21], reduce the inhibitory effect of miR-103a-1-5p on the TCF7, LEF1 and Wnt11 genes, and thus activate the Wnt signaling pathway to promote tumors.
Methods
Sequencing data and information of clinical samples
A total of 73 plasma samples were collected from three participant groups recruited at the Fifth Affiliated Hospital of Southern Medical University and Zhujiang Hospital of Southern Medical University, including 30 patients with gastric cancer (GC), 27 with chronic gastritis, and 16 healthy individuals. Among these, 15 GC, 10 chronic gastritis, and 5 healthy samples were subjected to high-throughput sequencing, while 15 GC, 17 chronic gastritis, and 11 healthy samples were analyzed using RT-qPCR assays. All plasma samples from cancer patients were obtained prior to the initiation of cancer treatment. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The collection and use of plasma samples were conducted under protocols approved by the Ethics Committees of the Fifth Affiliated Hospital of Southern Medical University and Zhujiang Hospital of Southern Medical University. The detailed clinical characteristics of the participants are described in Supplementary Table S1.
RNA extraction and sequencing of plasma EVs
RNA from plasma EVs was extracted from approximately 5 ml of plasma with an exoRNeasy Serum/Plasma Maxi Kit (QIAGEN, # 77064) following the manufacturer’s instructions. In brief, 5 mL of plasma was prefiltered, then was mixed with 2x binding buffer. The mixture is added to the exoEasy membrane affinity to bind the EVs to the membrane. After centrifugation, the wash buffer was added to wash off non-specific material in the column. After enriching EVs, QIAZOL was added to the column to lyse the vesicles and chloroform was added to the lysate collected after centrifugation. After the aqueous phase is recovered and mixed with ethanol, and the sample-ethanol mixture is added to the RNeasy MinElute spin column and centrifuged. Washing the column with buffer RWT, then wash twice with buffer RPE. And finally elute RNA in water. The RNA-sequencing libraries were constructed with a TruSeq Stranded Total RNA Library Prep Kit (Illumina, # RS-122-2501). Subsequently, the quality and quantification of the libraries were assessed using the BioAnalyzer 2100 system. Finally, 10 pM libraries were denatured as single-stranded DNA molecules, captured on Illumina flow cells, amplified in situ as clusters, and finally subjected to 150 cycles on an Illumina HiSeq 4000 sequencer following the manufacturer’s instructions.
Processing of high-throughput RNA sequencing data
The 3’ adapter of the raw read was trimmed, and the low-quality read was removed by using Cutadapt software (v1.9.3). First, the reads were aligned to the reference genome (hg19) and transcriptome with STAR software (v2.5.1b) [22]. Then, the circRNAs were detected and identified by DCC software (v0.4.4) [23]. Normalized expression values of circRNAs were calculated by using edgeR software (v3.16.5) [24]. The differentially expressed circRNAs were identified by the edgeR package of R software with a cutoff threshold of a |log2-fold change| ≥ 2 and a P value < 0.05 (Supplemental Table 2). Principal component analysis (PCA) and visualization of the results generated by the rgl package (v0.1). The enrichment of GO functions was implemented and visualized by using Metascape [25].
Cell culture
RPMI 1640 medium (Gibco, #11875-093) and F12K medium (Gibco, 21127-022) were used for the GC cell lines SGC-7901 (RRID: CVCL_0520) and AGS (RRID: CVCL_0139). All basal media were supplemented with 10% FBS (Gibco, #10091-148) and 1% penicillin-streptomycin (Gibco, #15140-122). The cell lines were all cultured in a humidified incubator at 37 °C with 5% CO2.
Transfection
The circMORC1 small interfering RNA (siRNA of circMORC1), miR-103a-1-5p mimic/inhibitor, and their corresponding negative controls (si-NC, miR-NC, and anti-miR-NC) were designed (GenePharma, #B02003) and transfected into AGS or SGC-7901 cells. All sequences of siRNA and RNA mimic are shown in Supplementary Table S2. All transient transfections were performed using Lipofectamine 2000 (Invitrogen, #11668-019), and RT-qPCR was used to assess the transfection efficiency 48 h after transfection. The circMORC1 overexpression plasmid was constructed by inserting the circMORC1 sequence into the circRNA expression vector pLC5-ciR (RRID: Addgene_169139, Geneseed Biotech, # GS0108). To construct stable circMORC1-overexpressing cell lines, a lentiviral packaging vector, a pLC5-circMORC1 overexpression plasmid and the corresponding negative control pLC5-ciRNC were transfected into AGS and SGC-7901 cells, after which colonies with stable expression were selected with 2 mg/ml puromycin (Gibco, # A1113803) for 3 generations.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cultured cells was extracted with TRIzol (Invitrogen, #15596026CN). Total RNA was reverse transcribed with a PrimeScript™ RT reagent kit (TaKaRa, #RR037A) following the manufacturer’s guidelines. For the analysis of circMORC1 and mRNA, Oligo dT Primer and Random 6-mers were used for reverse transcription, whereas the specific stem-loop RT primer was used for reverse transcription of miRNA. U6 was used as an internal control in the experiments. qPCR was performed on an Applied Biosystems 7500 Real-Time PCR System with TB Green™ Premix Ex Taq™ II reagent(TaKaRa, #RR820A). The 2−ΔΔCt method was used for data analysis. All sequences of primers used for RT-qPCR are shown in Supplementary Table S2.
Cell viability analysis
The cell viability was determined by a WST-8 assay using a Cell Counting Kit-8 (CCK-8). (Dojindo Laboratories, #CK04) Transfected cells at a concentration of 3,000 cells per well were seeded into 96-well plates. At 0, 24, 48, and 72 h after transfection, the activity was measured by the addition of 10 µl of WST-8 reagent to 100 µl of medium in each well. After incubating for 1 h at 37 °C, and 5% CO2, a microplate reader was used to detect the absorbance at a wavelength of 450 nm.
Cell proliferation analysis
We performed 5-ethynyl-2’-deoxyuridine (EdU) assays to assess cell proliferation following the instructions of the Cell-Light EdU Apollo488 In Vitro Kit (RiBoBio, #C10310-3). The blue fluorescent DNA stain 4′,6-diamidino-2-phenylindole (DAPI) (RiBoBio, #C0060) was used for nuclear staining. The cells were photographed and imaged by fluorescence microscopy, and the proliferation rate was calculated as (the number of EdU-positive cells/the number of Hoechst-stained cells) × 100%.
Colony formation assay
For the colony formation assay, cells were suspended and seeded into 6-well plates after transfection for 48 h. Each well was filled with 2 ml of cell suspension containing 300 cells. After culturing for 2 weeks, the colonies were fixed with 4% paraformaldehyde and stained with 1% crystal violet solution (Sigma-Aldrich, #C0775) for 20 min. After rinsing, the colonies were cleaned and dried, digital images were captured, and visible colonies were counted.
Cell scratch assay
The migration ability of cells was detected by a scratch assay. In the scratch experiment, the cells were inoculated in 24-well plates. When the degree of fusion reached more than 90%, the cells were scratched with a clear tip, and the scratch area was photographed under a microscope at 0 h and 48 h. The relative mobility was calculated according to the change in the scratch area.
Flow cytometry (FCM)
A flow cytometry assay was performed to detect cell apoptosis. A commercial Annexin V-FITC/PI apoptosis kit (Multi Sciences, #AP101) was used to analyze cell apoptosis. Forty-eight hours after transfection, the cells were washed with PBS and collected with 0.25% trypsin (Gibco, #15050-057). The fluorescence signal was detected by flow cytometry, and the percentage of apoptotic cells was determined by FlowJo software (FlowJo, RRID: SCR_008520).
RNA-RNA binding site prediction
The sequences of circMORC1 and miR-103a-1-5p were downloaded from UCSC and miRBase, respectively. The binding sites between circMORC1 and miR-103a-1-5p were predicted using miRanda (v3.3a) with the default settings (Alignment score ≥ 140, Alignments Energy ≤ -1 kcal/mol).
Dual-luciferase reporter assay
circMORC1 wild-type and mutant sequences containing the miR-103a-1-5p binding sites were synthesized and inserted into pGL3 luciferase reporter vector (RRID: Addgene_44264, Promega, #E1771) to construct wild-type and mutant circMORC1. HEK-293T cells (RRID: CVCL_0063) plated in 96-well plates were cotransfected with the following plasmids: circMORC1-wt or circMORC1-mut luciferase reporter plasmid, miR-103a-1-5p or NC mimic, and pRL-TK Renilla luciferase control reporter vector (Promega, #E2231) using Lipofectamine 2000. After transfection for 48 h, a Dual-Lumi™ Luciferase Reporter Gene Assay Kit (Beyotime, #RG028) was used to detect the activities of firefly luciferase (FL) and Renilla luciferase (RL) according to the manufacturer’s instructions. The relative ratio of FL/RL was used to reveal the interactions between miR-103a-1-5p and circMORC1.
RNA-RNA pull-down assay
The probe for miR-103a-1-5p and its negative control were labeled with a Pierce™ RNA 3’ End Desthiobiotinylation Kit (Thermo, #20163). Over 1 × 107 cell lysate was incubated with probe-conjugated beads using the Pierce™ Magnetic Pull-down Kit (Thermo, #20164) following the manufacturer’s protocol. After pulldown, the quantity of the circMORC1-bound RNA complex was measured by qRT-PCR.
RNA-seq and bioinformatics analysis
The sequence libraries were constructed following a previous study, and they were sequenced on an Illumina NovaSeq platform with 150 bp paired-end reads. Paired-end clean reads were aligned to the reference genome using HISAT2 (ver. 2.0.5). The feature counts (v1.5.0-p3) were used to count the number of reads mapped to each gene. Differential expression analysis was performed using the DESeq2 R package (DESeq, RRID: SCR_000154, ver. 1.20.0). Genes with an adjusted P value < = 0.05 according to DESeq2 were considered to be differentially expressed. GO enrichment analysis of differentially expressed genes was performed by Panther (ver18.0).
Western blot
The electrophoretic transfer system (Bio-Rad, # 1658033) were used for western blot, cyclin D1 (abcam, #ab134175), Smad 2 + 3 (abcam, # ab217553), TCF7 (CST, #2203) and β-catenin (Protein-tech, #66379-1-Ig) were detected by Clarity™ Western ECL Substrate (Bio-Rad, #1705061) after Goat Anti-Rabbit/Mouse IgG H&L (HRP) (abcam, # ab6721/ ab6789) incubation.
Statistical analysis
All experiments were carried out at least three times, and the data are presented as the mean ± SD. The statistical analyses of the gene data were carried out via SPSS 22.0 (SPSS, RRID: SCR_002865) statistical software, and the data were graphed with Prism software (GraphPad Prism, RRID: SCR_002798, version 8.1.1). Comparisons between two groups were evaluated by Student’s t test. Wilcoxon rank-sum test was used to compare the clinical data between the cancer and non-cancer groups. The significance was represented by *P < 0.05, **P < 0.01, ***P < 0.001 versus the vector or control groups.
Results
CircMORC1 expression decreased in the plasma EVs of GC patients and intracellular enrichment in overexpressed cell lines
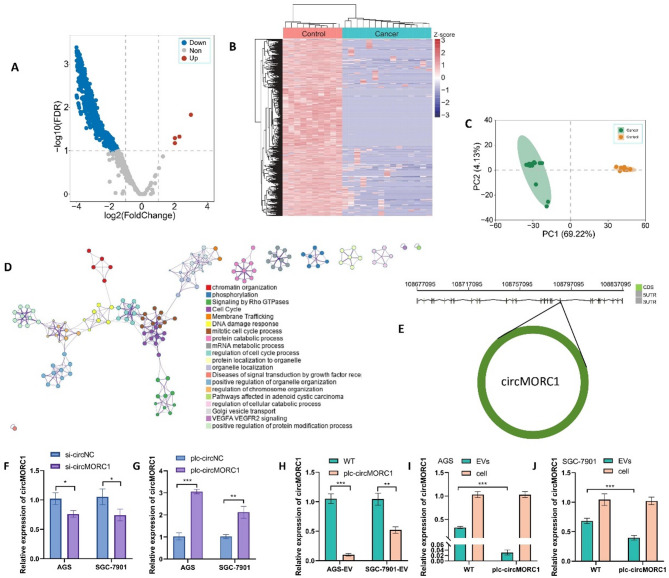
By comparing the RNA profiles of plasma EVs from 15 with GC, 10 with chronic gastritis, and 5 healthy, we identified 1,577 differentially expressed circRNAs, including 4 with increased expression and 1,573 with decreased expression (Fig. 1A and Supplementary Table S3). A heatmap of these circRNA results showed that the expression patterns differed between gastric cancer patients and healthy individuals (Fig. 1B). In addition, the principal component analysis (PCA) results showed that the gastric cancer patients and healthy individuals clustered into two different groups (Fig. 1C). The unique biochemical characteristics of circRNAs make them promising biomarkers for cancer diagnosis. Gene functional analysis revealed that these circRNAs are closely related to the functions of the cell cycle, phosphorylation and some tumor-related signaling pathways (Fig. 1D). According to the fold change in expression levels, we selected circMORC1, which exhibited the greatest decrease in expression, for further functional study (Supplementary Table S3). circMORC1, also known as hsa_circ_0141931 in circBase, is approximately 641 nucleotides in length and originates from the MORC1 gene located on human chromosome chr3:108773574–108,788,604 (Fig. 1E). The circMORC1 expression levels in 43 clinical samples were also measured by RT-qPCR, and receiver operator characteristic curve (ROC curves) (Supplementary Figure S1) were generated according to group assignment (15 with GC, 17 with chronic gastritis, and 11 healthy).
Fig. 1.
circMORC1 expression is decreased in EVs from the plasma of gastric cancer patients and the relative expression is down-regulated in EVs secreted by gastric cancer overexpressed cell lines. (A) Volcano plot of differentially expressed plasma EV circRNAs between gastric cancer patients and healthy individuals. (B) Heatmap of differentially expressed circRNAs. (C) PCA of the differentially expressed circRNAs in gastric cancer patients and healthy individuals. (D) Gene function enrichment analysis of the differentially expressed circRNAs. (E) The gene structure of circMORC1. (F, G) The knockdown and overexpression efficiency of circMORC1 in gastric cancer cells measured by RT-qPCR. (H) Comparison of circMORC1 expression in EVs between circMORC1-overexpressing and wild-type cell lines. (I, J) The expression of circMORC1 in cells and EVs from circMORC1-overexpressing and wild-type gastric cancer cells was measured by RT-qPCR. Statistical significance in two-group experiments was evaluated using a two-sided Student’s t test. Data are represented as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 and -, no significance
To investigate the effects of circMORC1 on gastric cancer cells, we transfected siRNAs into cells to knock down the expression of circMORC1 and constructed stable circMORC1-overexpressing cell lines. RT-qPCR was used to verify the knockdown and overexpression efficiency. The results revealed that the expression level of circMORC1 greatly increased in the stable circMORC1-overexpressing cell lines but decreased in the cells transfected with si-circMORC1. (Figure 1F and G). The expression levels of circMORC1 in EVs from stable circMORC1-overexpressing GC cells and wild-type cells were compared by RT-qPCR. The results revealed that the expression level of circMORC1 in EVs from stable circMORC1-overexpressing GC cells was lower than that in EVs from wild-type cells (Fig. 1H). After analyzing the expression levels of circMORC1 in EVs and cells from two cell lines subjected to different treatments, we observed that the abundance of circMORC1 in EVs decreased after circMORC1 was overexpressed in gastric cancer cells. Compared with those in the wild-type strain, more circMORC1 molecules were retained in the cells (Fig. 1I and J). U6 was used as the control gene. All the data are expressed as the means ± SDs of at least three independent experiments.
CircMORC1 promoted GC cell proliferation, viability, and migration
To verify the functional roles of circMORC1 in gastric cancer progression, we assessed the effects of circMORC1 on cell proliferation, viability, migration. Both CCK-8 and colony formation assays revealed that cell viability and colony formation ability were increased in the circMORC1-overexpressing AGS and SGC-7901 gastric cancer cell lines (Fig. 2A and B). Moreover, the viability and colony formation ability of AGS and SGC-7901 cells decreased after the inhibition of circMORC1 (Fig. 1F and G). The results of the EdU assay suggested that knockdown of circMORC1 obviously retarded the proliferation of gastric cancer cells, while overexpression of circMORC1 resulted in increased cell viability (Fig. 2C). Scratch assay results showed that knockdown of circMORC1 significantly decreased the migration ability of AGS cells, whereas upregulation of circMORC1 had the opposite effect (Fig. 2D). Flow cytometry analysis show that over expression of circMORC1 can improve cell survival, and suppression circMORC1 lead to worse cell survival (Q1: Annexin V-, PI+; Q2: Annexin V+, PI+; Q3: Annexin V+, PI-;Q4: Annexin V-, PI-; Q2 + Q3 = Cells undergoing apoptosis; Q4 = normal cells) (Fig. 2E). In addition, Western blot analysis showed that the expression levels of cyclinD1 and Smad 2 + 3 in AGS and SGC-7901 were significantly decreased after siRNA-circMORC1 treatment (Supplementary Fig. 2). These data suggested that circMORC1 promoted gastric cancer cell proliferation, viability, and migration.
Fig. 2.
The functions of circMORC1. (A) CCK-8 assays were performed to analyze the proliferative capacity of AGS and SGC-7901 cells after circMORC1 overexpression or knockdown. (B) Colony formation assays were designed to analyze the colony-forming ability of AGS cells after circMORC1 overexpression or knockdown (n = 3). (C) EdU assays showing the viability of AGS and SGC-7901 cells with circMORC1 overexpression or knockdown (n = 3, scale bar, 200 μm). (D) A scratch assay was performed to determine the migration abilities of AGS cells after circMORC1 overexpression or knockdown (n = 3). (E) Flow cytometry assessment of apoptosis in AGS and SGC-7901 cells with circMORC1 overexpression or knockdown (n = 3). Data are represented as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 and -, no significance
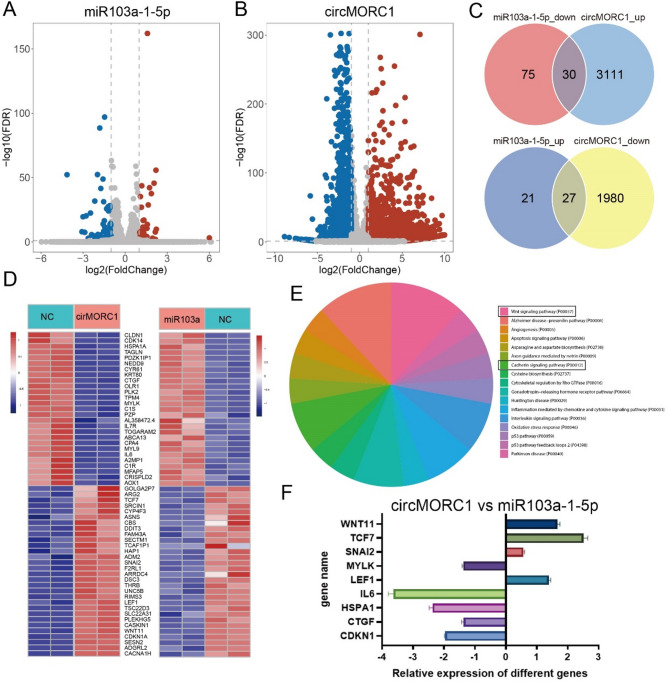
CircMORC1 functions as a sponge for miR-103a-1-5p
The RNA-RNA interaction results showed that miR-103a-1-5p could bind to two predicted binding sites on circMORC1 (Fig. 3A and B). Then, a dual-luciferase reporter assay was used to validate the targeting relationship between circMORC1 and miR-103a-1-5p for the two binding sites. The results showed that the luciferase activity at both sites markedly decreased in the miR-103a-1-5p and circMORC1-WT cotransfection group but had no effect on the luciferase activity of the circMORC1-MUT group (Fig. 3C and D). An RNA-RNA pull-down assay was performed using HEK293T cell lysates to further confirm the interactions between circMORC1 and miR-103a-1-5p. As shown in Fig. 4E, circMORC1 was captured by biotin-labeled miR-103a-1-5p but not by the negative control. Further RT-qPCR revealed that miR-103a-1-5p levels decreased in response to the overexpression of circMORC1 in gastric cancer cells (Fig. 3F). In summary, the above results showed that circMORC1 could bind to miR-103a-1-5p and act as a sponge for miR-103a-1-5p.
Fig. 3.
circMORC1 functions as a sponge for miR-103a-1-5p. (A) Schematic of dual-luciferase reporter assay and the two predicted binding sites and mutation sites to verify the relationship between circMORC1 and miR-103a-1-5p. The gray part in sequence shows the mutated sites of circMORC1. (B) Schematic of RNA pull-down assay to verify the relationship between circMORC1 and miR-103a-1-5p. (C, D) The result of dual-luciferase reporter assay (n = 3). (E) The result of RNA pull-down assay (n = 3). (F) Expression of miR-103a-1-5p in circMORC1-overexpressing cell lines was determined by RT-qPCR (n = 3). Data are represented as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 and -, no significance
Fig. 4.
Rescue experiments. (A) RT-qPCR was used to examine the transfection efficiency of the miR-103a-1-5p mimic (n = 3). (B, C, D) Cell viability, proliferation (B)(C) and apoptosis (D) were detected by the CCK-8 assay, colony formation assay and flow cytometry, respectively (n = 3). Data are represented as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 and -, no significance
Upregulation of miR-103a-1-5p reversed the effects of circMORC1 overexpression
To further verify that miR-103a-1-5p is the target molecule of circMORC1, miR-103a-1-5p and the NC mimic were transfected into circMORC1-overexpressing cancer cell lines RT-qPCR demonstrated the high transfection efficiency of the miR-103a-1-5p mimic (Fig. 4A). The results of the CCK-8 and colony formation assays showed that cell viability and colony formation, which were obviously enhanced by overexpression of circMORC1, were decreased after the addition of the miR-103a-1-5p mimic (Fig. 4B and C). Compared to the overexpression of circMORC1, transfection with miR-103a-1-5p resulted in a significant reduction in cell viability (Supplementary Figure S4). The expression level of endogenous miR-103a-1-5p in GC cells was significantly lower than that in normal cells. Flow cytometry analysis suggested that in the circMORC1-overexpressing cell lines, cell apoptosis increased after transfection with the miR-103a-1-5p mimic (Q1: Annexin V-, PI+; Q2: Annexin V+, PI+; Q3: Annexin V+, PI-;Q4: Annexin V-, PI-; Q2 + Q3 = Cells undergoing apoptosis; Q4 = normal cells) (Fig. 4D). Taken together, the rescue experiments confirmed that circMORC1 can affect gastric cancer cell proliferation, migration, and invasion by regulating miR-103a-1-5p.
CircMORC1 affected the activation level of Wnt pathway through miR-103a-1-5p
To explore the downstream regulatory pathways of circMORC1, we performed RNA-Seq after AGS cells were transfected with circMORC1 and miR-103a-1-5p. A total of 5148 and 151 differentially expressed genes were found in the circMORC1- and miR-103a-1-5p-overexpressing cell lines, respectively (Fig. 5A, B; Supplementary Table S4). There were 57 genes whose expression tended to reverse between these two treatments (Fig. 5D). The functional annotation of these genes revealed that they were enriched in Wnt pathways (Fig. 5E). According to the gene function annotation, 10 cancer-related genes were selected for further validation. We further validated the RNA-Seq results using RT-qPCR. The results are shown in Fig. 5F. In addition, the expression levels of Wnt pathway key proteins TCF7 and β-catenin in MORC1 overexpressing AGS and SGC7901 cell line and their control groups were detected by Western blot, the expression levels were significantly down-regulated after miR-103a-1-5p overexpression (Supplementary Fig. 3).
Fig. 5.
Downstream regulatory pathways of circMORC1 through miR-103a-1-5p. (A, B) Differentially expressed genes after the AGS cell line was separately transfected with circMORC1 and miR-103a-1-5p (n = 4). (C) The number of genes with opposite expression trends. (D) Heatmaps of differentially expressed genes between groups. (E) The pathways regulated by circMORC1. (F) RT-qPCR of the selected genes (n = 3). The relative expressions of different genes were compared between overexpress cell lines of circMORC1 and miR-103a-1-5p. The positive value of the gene means the gene high express in circMORC1 overexpress cell line and vice versa
Discussion
The high morbidity and mortality of gastric cancer have made it a research hotspot in modern medicine [26]. Limitations in early diagnosis, incomplete treatment, and metastasis and recurrence of tumors contribute to the low survival rate of gastric cancer patients [27, 28]. Therefore, studying the underlying mechanism of tumorigenesis could be of great benefit to GC patients. In this study, we identified a novel gastric cancer-associated circRNA, circMORC1, whose expression is downregulated in the plasma EVs of gastric cancer patients. circMORC1 is located at chromosome (chr) 3:108773575–108,788,604, whose coding gene is MORC1 [29]. We also found that circMORC1 was downregulated in EVs but increased in gastric cancer cell lines. Functionally, we found that silencing circMORC1 markedly inhibited the proliferation and migration and accelerated the apoptosis of gastric cancer cells. Finally, we found that miR-103a-1-5p adsorbs and inhibits the function of circMORC1, activating the Wnt pathway to promote gastric cancer through miR-103a-1-5p. Therefore, our study revealed a novel functional mechanism of circRNAs in gastric cancer and revealed that understanding the relationship between circRNA expression levels in body fluids and tissues is highly important for further diagnosis and treatment.
The significance of the differential expression of circMORC1 in plasma EVs in GC is still unclear. Our study compared circRNA expression in plasma EVs between GC patients and healthy individuals. These two groups showed different patterns, which indicated the roles of circRNAs as diagnostic and prognostic biomarkers. Among them, circMORC1 in plasma-derived EVs was significantly different between the two groups. In addition, since circMORC1 has opposite expression trends in GC cells and cell-secreted EVs and the intracellular enrichment of circMORC1 is closely related to tumor development, the detection of circMORC1 expression in plasma-derived EVs can indirectly reflect the expression of circMORC1 in tissue, thus providing support for auxiliary tumor detection in clinical diagnosis.
The function of circMORC1 is still unclear. Our study first explored its potential functions in vivo. In vivo experiments showed that it could regulate cell proliferation, viability, migration and apoptosis in gastric cells (Fig. 2). In addition, we further explored its potential regulatory mechanisms. Using dual luciferase reporter assays, RNA-RNA pull-down assays and rescue experiments, we found that circMORC1 functions as a miRNA sponge of miR-103a-1-5p (Figs. 3 and 4). Previous studies have reported that miR-103a-1-5p is down-regulated in GC and is associated with the progression of GC. Our study revealed that in GC cells, the up-regulated circMORC1 can adsorb miR-103a-1-5p, leading to a reduction in its intracellular expression and subsequently promoting tumor development. Using RNA-Seq and RT-qPCR, we found that miR-103a-1-5p adsorbs and inhibits the function of circMORC1, activating the Wnt pathway to promote gastric cancer (Fig. 5).
However, there are also some limitations in our study. For example, as a target miRNA of circMORC1, miR-103a-1-5p has been reported to induce apoptosis, but its role in gastric cancer has not been elucidated extensively [30, 31]. Due to limited access to matched clinical tissue samples, differences in the expression levels of circMORC1 in tumor tissues have not yet resulted in the current study, future, the studies incorporating paired tumor and adjacent normal tissues is necessary for further validate. After comparing the expression of miR-16 and U6 in clinical samples, U6 with more stable expression in samples was selected as the endogenous control for the detection of RNA molecules in blood EVs, but the evaluation of a larger sample is still needed to verify the stability of U6 as the endogenous control. In this study, the molecules that act directly on miR-103a-1-5p were not further verified. Only the pathways enriched in genes whose expression tended to reverse after overexpression of circMORC1 or miR-103a-1-5p were investigated and the effects on the Wnt signaling pathway were verified. Meanwhile, our molecular mechanism study model was limited to gastric cancer cells, and in vivo experiments were not performed to verify the functional effect of this mechanism in vivo. Moreover, further investigation of the other potential mechanism by which circMORC1 affects gastric cancer, such as protein binding, is needed.
Conclusion
This study showed that circMORC1 is more likely to be enriched intracellularly than secreted by EVs in gastric cancer cells. Intracellular circMORC1 could promote gastric cancer cell proliferation, migration, and invasion. The upregulated circMORC1 functions as a miR-103a-1-5p sponge and downregulates miR-103a-1-5p to increase the expression levels of Wnt signaling pathway-related genes such as TCF7 and Wnt11, which contributes to the progression of gastric cancer. This study revealed a novel mechanism by which circMORC1 regulates tumorigenesis in gastric cancer and provided a new biomarker for GC diagnosis and prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by project grants from the National Natural Science Foundation of China [81872416; 82173001]. We thank Prof. Zhou Li from General surgery Department of Zhujiang Hospital of Southern Medical University (The Second Clinical Medical College) for the clinical sample collection.
Abbreviations
- GC
Gastric cancer
- EVs
Extracellular vesicles
- RT-qPCR
Real-Time Quantitative Polymerase Chain Reaction
- circRNA
Circular noncoding RNA
- miRNA
MicroRNA
- ROC curve
Receiver operator characteristic curve
- PCA
Principal component analysis
- MORC1
MORC family CW-type zinc finger 1
- U6
U6 small nuclear RNA
- MUT
Mutator
- Wnt pathway
Wingless/Integrated pathway
- TCF7
Transcription factor 7
- Wnt11
Wnt family member 11
- LEF1
Lymphoid enhancer binding factor 1
- HSPA1A
Heat shock protein family A (Hsp70) member 1A
- MYLK
Myosin light chain kinase
- IL6
Interleukin 6
- CDKN1A
Cyclin dependent kinase inhibitor 1A
- CTGF
Connective tissue growth factor
- SNAI2
Snail family transcriptional repressor 2
Author contributions
Z-w G and Y-s W designed and supervised the study. X-m Z, Y-q Y and Z-w G analyzed and interpreted the data and prepared the manuscript. X-m Z, Y-q Y and Z-w G analyzed and interpreted the data. L L provided suggestions for manuscript revision. All authors vouched for the respective data and analysis, reviewed and approved the final version and agreed to publish the manuscript.
Funding
This work was supported by project grants from the National Natural Science Foundation of China [81872416; 82173001].
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The raw data of sequence has been uploaded to National Genomics Data Center (NGDC), [PRJCA025617], address is ‘https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA025617’.
Declarations
Ethics approval and consent to participate
The study was approved by the Zhujiang Hospital of Southern Medical University, and written informed consent was obtained from each patient. The Use Committees of the hospital approved the use of the clinical information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed to equally: Xiang-Ming Zhai and Yi-Qi Yang.
Contributor Information
Zhi-Wei Guo, Email: gzw188@126.com.
Ying-Song Wu, Email: wg@smu.edu.cn.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Wang J, Xie Z, Tian X, Hou J, Wang D, Qian H, Shen H, Xu W. CircUSP1 as a novel marker promotes gastric cancer progression via stabilizing HuR to upregulate USP1 and Vimentin. Oncogene. 2024;43(14):1033–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29(3):481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristensen LS, Andersen MS, Stagsted L, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Huang S. Circular RNA: an emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Lett. 2018;418:41–50. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Cai GX, Zhai XM, Yang XX, Li M, Li K, Zhou CL, Liu TC, Han BW, Liu ZJ, et al. Plasma-derived extracellular vesicles circular RNAs serve as biomarkers for breast cancer diagnosis. Front Oncol. 2021;11:752651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–8. [DOI] [PubMed] [Google Scholar]
- 8.Lei M, Zheng G, Ning Q, Zheng J, Dong D. Translation and functional roles of circular RNAs in human cancer. Mol Cancer. 2020;19(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang Q, Yang Z, Jia R, Ge S. The novel roles of circrnas in human cancer. Mol Cancer. 2019;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao M, Zheng M, Xu Y, Ma S, Zhang W, Ju S. CircRNAs and their regulatory roles in cancers. Mol Med. 2021;27(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Xia J, Peng C, Cai W. Expression of plasma exosomal circLPAR1 in patients with gastric cancer and its clinical application value. Am J Cancer Res. 2023;13(9):4269–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lin YL, Shao JK, Wu XJ, Li X, Yao H, Shi FL, Li LS, Zhang WG, Chang ZY, et al. Plasma exosomal hsa_circ_0079439 as a novel biomarker for early detection of gastric cancer. World J Gastroenterol. 2023;29(22):3482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Zhong Y, Wang X, Shen J, An W. Advances in circular RNA and its applications. Int J Med Sci. 2022;19(6):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CX, Chen LL. Circular RNAs: characterization, cellular roles, and applications. Cell. 2022;185(12):2016–34. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Liu B, Xu J, Zhang B, Wang Y, Shi L, Cai X. Circular RNAs: characteristics, biogenesis, mechanisms and functions in liver cancer. J Hematol Oncol. 2021;14(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, Xie H, Peng X, Yin W, Tao Y, et al. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci. 2020;16(14):2628–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevskaya KV, Pershina AG, Hmelevskaya ES, Efimova LV, Ibragimova MK, Dolgasheva DS, Tsydenova IA, Ufandeev AA, Buyko EE, Perina EA, et al. Prevention of metastasis by suppression of stemness genes using a combination of microRNAs. J Med Chem. 2024;67(7):5591–602. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhan L, Jiang X, Tang X. Comprehensive review for non-coding RNAs: from mechanisms to therapeutic applications. Biochem Pharmacol. 2024;Jun;224:116218. 10.1016/j.bcp.2024.116218 [DOI] [PubMed]
- 19.Liang J, Liu X, Xue H, Qiu B, Wei B, Sun K. MicroRNA-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-Myb. Cell Proliferat. 2015;48(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X, Wei H, Zhang S, Zhang F. Predictive and prognostic value of an microRNA signature for gastric carcinoma undergoing adjuvant chemotherapy. DNA Cell Biol. 2021;40(11):1428–44. [DOI] [PubMed] [Google Scholar]
- 21.Saberinia A, Alinezhad A, Jafari F, Soltany S, Akhavan SR. Oncogenic miRNAs and target therapies in colorectal cancer. Clin Chim Acta. 2020;508:77–91. [DOI] [PubMed] [Google Scholar]
- 22.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, Metge F, Dieterich C. Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics. 2016;32(7):1094–6. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadhwa V, Patel N, Grover D, Ali FS, Thosani N. Interventional gastroenterology in oncology. CA Cancer J Clin. 2023;73(3):286–319. [DOI] [PubMed] [Google Scholar]
- 27.Feng T, Jie M, Deng K, Yang J, Jiang H. Targeted plasma proteomic analysis uncovers a high-performance biomarker panel for early diagnosis of gastric cancer. Clin Chim Acta. 2024;May 15;558:119675. 10.1016/j.cca.2024.119675 [DOI] [PubMed]
- 28.Cai GX, Lin L, Zhai XM, Guo ZW, Wu YS, Ye GL, Liu Q, Chen LS, Xing GY, Zhao QH, et al. A plasma-derived extracellular vesicle mRNA classifier for the detection of breast cancer. Gland Surg. 2021;10(6):2002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maass PG, Glazar P, Memczak S, Dittmar G, Hollfinger I, Schreyer L, Sauer AV, Toka O, Aiuti A, Luft FC, et al. A map of human circular RNAs in clinically relevant tissues. J Mol Med (Berl). 2017;95(11):1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie PL, Franklin DA, Liu Y, Zhang Y. p53 regulates the expression of LRP1 and apoptosis through a stress intensity-dependent microRNA feedback loop. Cell Rep. 2018;24(6):1484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay D, Cocco P, Orru S, Cherchi R, De Matteis S. The role of MicroRNAs as early biomarkers of asbestos-related lung cancer: a systematic review and meta-analysis. Pulmonology. 2024.Dec 31;31(1):2416792. 10.1016/j.pulmoe.2024.02.002 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The raw data of sequence has been uploaded to National Genomics Data Center (NGDC), [PRJCA025617], address is ‘https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA025617’.