Abstract
Background
Malaria remains a leading cause of morbidity and mortality in sub-Saharan Africa, particularly among children under five. The introduction of the malaria vaccine presents an opportunity to reduce malaria-related deaths. However, the success of vaccination campaigns depends on community acceptance and willingness to vaccinate. This study aimed to assess the pooled acceptance and willingness to adopt the malaria vaccine in sub-Saharan Africa, with a focus on variations across regions and the impact of the COVID-19 pandemic.
Methods
A systematic review and meta-analysis were conducted following PRISMA guidelines. A comprehensive search of databases, including PubMed, ScienceDirect, Google Scholar, and African Journals Online, was performed. Studies reporting on malaria vaccine acceptance and willingness among caregivers of children under five in sub-Saharan Africa were included. Data were extracted and analysed using STATA, with heterogeneity assessed through the I2 statistic. Subgroup analyses were performed based on region and pre- and post-COVID periods. Publication bias was assessed using Egger’s test.
Results
A total of 1611 records were identified, and 34 studies met inclusion criteria after screening. Of these, 25 studies with a combined sample of 25,867 participants were included in the meta-analysis. The pooled acceptance rate for the malaria vaccine among caregivers of children under five in sub-Saharan Africa was 82% (95% CI: 73%–90%), while the pooled willingness rate was 80% (95% CI: 70%–90%). Subgroup analyses showed no statistically significant differences in acceptance or willingness by COVID-19 period or region, though the lowest acceptance (53%) was reported in the DRC. High heterogeneity was observed (I2 > 99%), and publication bias was indicated in the willingness outcome (Egger’s test, P = 0.002).
Conclusion
The findings indicate high levels of acceptance and willingness among caregivers to vaccinate children under five against malaria in sub-Saharan Africa, suggesting strong community readiness for vaccine rollout. However, the observed heterogeneity and potential publication bias highlight the need for context-specific strategies and further high-quality studies to support implementation and uptake across diverse regions.
Systematic review registration The protocol has been registered with PROSPERO registration number: CRD42023480528
Keywords: Malaria vaccine, Acceptance, Willingness, Sub-Saharan Africa, COVID-19, Vaccination
Background
Malaria remains a major global health challenge, disproportionately affecting disadvantaged populations, particularly in sub-Saharan Africa, which accounts for nearly 90% of malaria-related morbidity and mortality [1]. Among the most vulnerable are children under five years, who suffer the majority of malaria-related deaths in the region [2]. In 2022, malaria was responsible for an estimated 249 million clinical cases and 608,000 deaths worldwide, with the World Health Organization (WHO) African Region bearing 95% of cases and 96% of deaths [3]. The COVID-19 pandemic further exacerbated this issue, causing a surge in malaria cases and deaths due to disruptions in healthcare services [4]. While the WHO recommendation of widespread use of the malaria vaccine in 2021 presents a promising step toward reducing this burden, its successful implementation hinges on community readiness and acceptance, particularly in the most affected regions [5]. Without strong community engagement, the impact of this life-saving intervention may be limited, highlighting the importance of addressing local concerns and ensuring widespread vaccine uptake. Beyond its devastating health impacts, malaria imposes a substantial economic burden on both individuals and governments, with direct costs estimated at over $12 billion annually [6].
In 2015, the RTS,S malaria vaccine demonstrated a 39% reduction in clinical malaria cases among children aged 5–17 months during Phase 3 trials [7, 8]. Subsequently, pilot programs commenced in 2019 in Malawi, Ghana, and Kenya to assess the vaccine's feasibility, safety, and impact within routine immunization schedules [9]. By 2021, based on positive outcomes from these pilots, the WHO recommended the widespread use of RTS,S/AS01 for children in regions with moderate to high malaria transmission [2, 8]. The RTS,S/AS01 (RTS,S) being the first malaria vaccine approved by the WHO in 2023, provides protection against Plasmodium falciparum malaria and has the potential to greatly reduce the global malaria burden, particularly among children [10]. Additionally, R21/Matrix-M is the second malaria vaccine, approved by the WHO in 2023 [11]. It has shown high efficacy in clinical trials, presents another promising tool in the fight against malaria [12]. However, vaccine acceptance and uptake pose major challenges in low- and middle-income countries (LMICs), where immunization rates can be alarmingly low [9, 12, 13]. This initiative marks a significant advancement in the fight against malaria, which continues to be one of the leading causes of death in Africa.
Vaccine hesitancy, defined by the WHO as the “delay in acceptance or refusal of vaccines despite the availability of services [14],” is an increasing concern in LMICs [15]. Ensuring the success of the approved malaria vaccines requires a comprehensive understanding of community readiness and acceptance, particularly among caregivers of children under five, who are the primary target of the vaccination campaign [5]. Despite the availability of vaccination services, previous research has shown that many children, particularly in low- and middle-income countries, do not receive adequate immunization, with only about 1 in 10 fully vaccinated [13, 16]. Observational studies assessing caregivers’ readiness and acceptance of the malaria vaccine for children under five in African countries have produced mixed results [17–20], highlighting the need for further investigation. Therefore, this study aims to determine the pooled readiness and acceptance of the malaria vaccine in sub-Saharan Africa through a systematic review and meta-analysis. The findings of this study will offer valuable insights for policymakers and stakeholders in designing public health strategies to maximize vaccination coverage and uptake. Additionally, it will address the current gap between the development of new prevention methods and their effective implementation in sub-Saharan Africa.
Research question
What is the current state of community readiness and acceptance for the implementation of the malaria vaccine among at-risk children in sub-Saharan Africa, based on available evidence?
Methods
Design
This study is a systematic review and meta-analysis, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [15]. The review was registered with the Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023480528.
Definitions
Acceptance was defined as the expressed intention or agreement by caregivers to vaccinate their child with the malaria vaccine, often measured through direct “yes/no” questions or binary responses. Willingness was defined as the openness or likelihood of a caregiver to consider vaccinating their child, often assessed using Likert scales.
Search for literature
A comprehensive two-step search strategy was employed to identify relevant literature. First, a systematic search of electronic databases was conducted, including PubMed, ScienceDirect, Google Scholar, and African Journals Online (AJOL). The search strategy was designed using Boolean operators and database-specific thesauri, incorporating key terms such as malaria vaccine, readiness, acceptance, and Africa. The primary search string was developed for PubMed and adapted for other databases (Supplementary Table 1). The search was not restricted by language or date of publication. Secondly, reference lists of included studies and relevant systematic reviews were screened for additional studies. Unpublished data were manually searched in institutional libraries and repositories to ensure a thorough literature review.
Eligibility criteria
Studies were selected based on the Population, Exposure, Comparator, and Outcome (PECO) framework [21]. Only studies that met the following criteria were included:
Population: Children under five years of age in sub-Saharan Africa.
Exposure: Cross-sectional studies assessing the community’s readiness and acceptance of the RTS,S malaria vaccine.
Comparator: Studies comparing levels of vaccine readiness or acceptance, where applicable.
Outcome: Measures of community readiness or acceptance of the RTS,S malaria vaccine.
Studies having the following characteristics were excluded from the analysis:
Studies lacking relevant outcomes (readiness or acceptance).
Commentaries, letters to the editor, or reviews.
Studies without accessible full texts for data extraction.
Study selection
After performing the database search, articles were exported to Rayyan software in the research information system (RIS) standardized tag format for screening. A four-stage selection process including screening titles and abstracts, retrieving full articles, screening full texts, and selecting full texts was employed [22]. Initially, studies were included or excluded based on titles and abstracts. All included studies underwent full-text screening and selection, performed independently by three reviewers who were blinded. Any disagreements were resolved through dialogue or, if necessary, by the principal investigator. The entire process was documented in a flow diagram (Fig. 1) [23].
Fig. 1.
Flow chart of the study selection process using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
Data extraction from included studies
A prewritten data extraction form designed in Microsoft Excel (2013) was developed and pretested on a few studies before use. Two independent reviewers extracted the data separately. In case of disagreements, they were resolved by discussion and, if necessary, consultation with a third reviewer. For articles obtained but with missing data, the corresponding authors for those studies were contacted to provide the needed data. The data collected included the author, title, year of publication, country, sample size, sampling strategy, demographic factors, quality, region, level of willingness, level of acceptance, and associated factors, among other relevant data.
Risk of bias assessment
The risk of bias for the included studies was assessed using the Newcasttle–Ottawa Scale (NOS) adapted for cross-sectional studies [24]. However, no study was excluded based on its quality. Studies were categorized as having high or low quality based [25]. Supplementary Table 2 indicates the quality assessment for all included studies. According to the tool, the score ranged from 0 to 9, and any study with a score of 7–9 was categorized as good, and below 7 as of poor quality.
Data management and synthesis
Before data analysis, the data in the extraction sheet was cleaned and organized in a form that can be read by the analytical software. The degree of observed heterogeneity and feasibility to conduct a meaningful quantitative synthesis was assessed. Heterogeneity, the variability between included studies was first assessed through the I2 statistic. There was a high level of heterogeneity (more than 50%), and employed the random-effects model for obtaining the common effect [26]. The index of heterogeneity (I2) was calculated with uncertainty intervals to indicate its level of precision. I2 is based on the Q statistic, obtained as the sum of squared deviations of each study’s estimate from the overall estimate. A forest plot was presented to provide a summary of the individual study estimates and the pooled estimate for all the individual included studies regarding community willingness and readiness for the implementation of the malaria vaccine. The effect of each study was shown as a square box with a horizontal line as a 95% confidence interval, whereas the overall effect (pooled prevalence) was shown as a diamond, with its length symbolizing the confidence interval.
To preserve the validity of the review, publication bias was assessed. As a rule of thumb, publication bias is assessed graphically through analysis of funnel plot asymmetry when at least 10 studies are included in the meta-analysis [27]. Additionally, a non-significant p value (P > 0.05) of Egger’s test indicates the absence of publication bias. Additional subgroup analysis and sensitivity analysis was performed. Subgroup analysis was performed based on the expected sources of heterogeneity such as regions and the period the study was conducted. Where over 10 studies have been included in the analysis, leave-one-out sensitivity analysis was performed to assess the effect of inclusion of each study on the overall estimate [28].
All the statistical analysis was performed using STATA version 17 (StataCorp LLC, College Station, TX) with the metan package for pooling effect sizes and conducting overall meta-analyses.
Results
After the systematic search, a total of 1611 records were retrieved. These were screened and after removing the 209 duplicate records, 1402 records remained. These were subjected to title and abstract screening by three different reviewers who were able to include 34 articles after resolving discrepancies. For the nine articles that were excluded; eight did not report on the main study outcome, and one article could not be accessed for data extraction. The results are elaborated in Fig. 1.
Characteristics of included studies
A total of 25 studies were included in the meta-analysis, with a total sample size of 25,867 participants, ranging from 150 to 5502 participants. Out of the 25 studies, most of the studies (11) were conducted in Nigeria, four studies in Ghana, two in Ethiopia, Sierra Leone, and Tanzania, and one study from the Democratic Republic of Congo, Guinea, Kenya, and Uganda. All the studies were surveys, with seven conducted before the Covid-19 while 18 after emergence of the Covid-19 pandemic in December 2019. The earliest study was conducted between May and June 2013, and the latest between October and December 2023. The study participants were basically parents and care givers of children under five years. Supplementary Table 3 shows the details of the included studies.
Pooled acceptance rate for malaria vaccine in sub-Saharan Africa
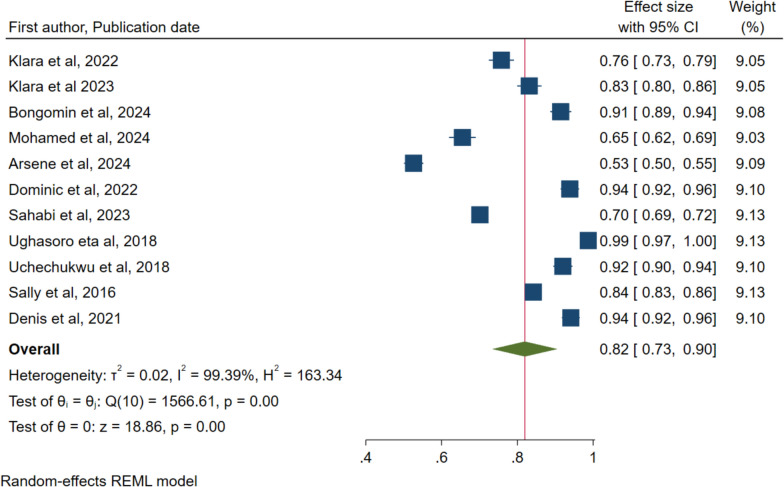
A total of 11 studies reported on acceptance of the malaria vaccine for children under five years’ acceptance rate of 82% with a 95% confidence interval of 73% to 90% (Fig. 2). The results also show a high level of heterogeneity within the included studies with I2 = 99.39% and P < 0.001.
Fig. 2.
Forest plot of the pooled proportion of malaria vaccine acceptance by caretakers of children under five years in Sub-Saharan Africa
Sub-group analysis and sensitivity analysis
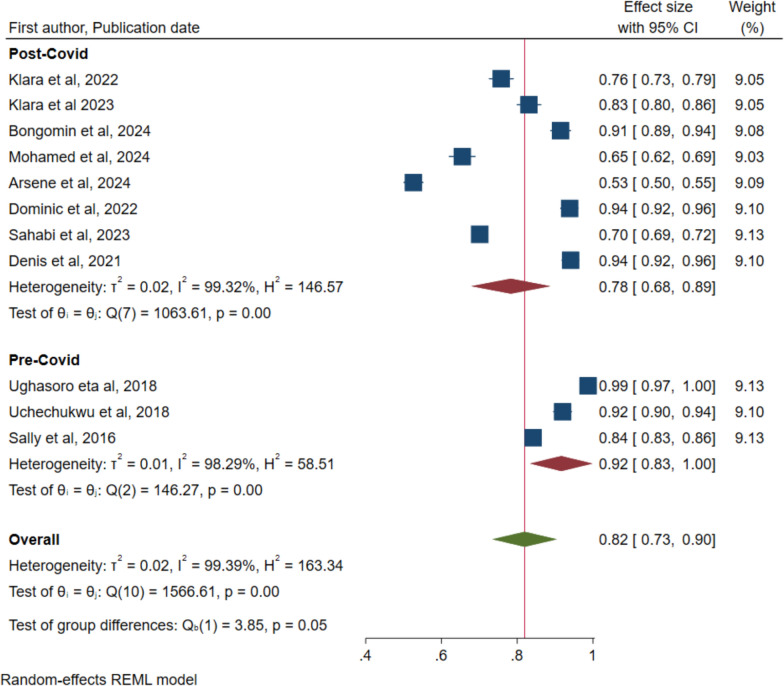
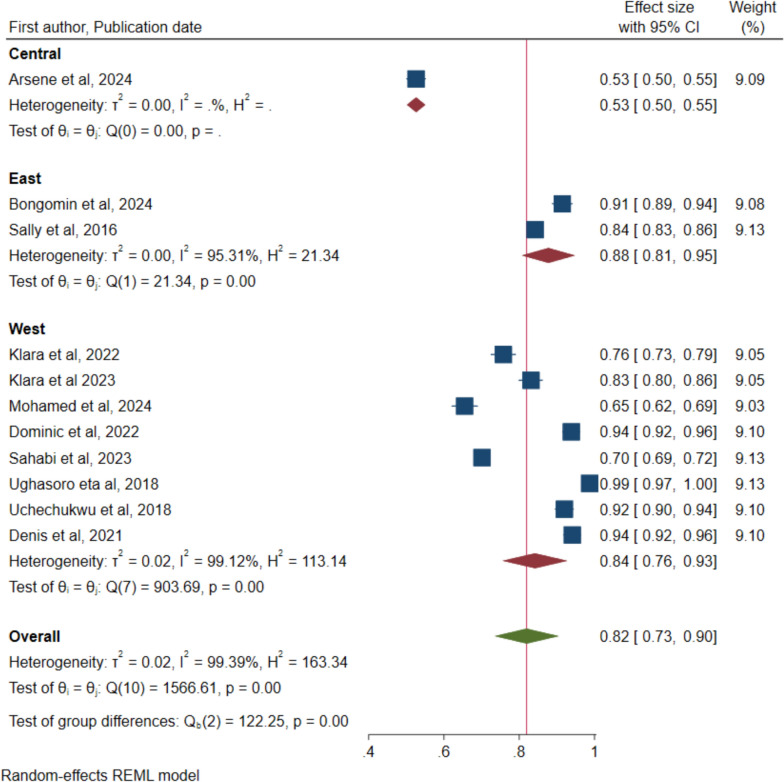
The data was also analysed to detect any differences in the uptake acceptance of the malaria vaccine acceptance by the COVID-19 period and region where the study was undertaken respectively. Figure 3 indicates 92% (95% CI: 0.83 to 1.00) for pre-COID and 78% (95% CI: 0.68 to 0.89) for post-COVID, however, the overlap in the confidence intervals indicates no statistical significance. Figure 4 for variation in regions also indicates no significant difference with overlapping confidence intervals: Eastern region—88% (95% CI: 81–95), and Western region—84% (95% CI: 76–93). The central region had only one study conducted in the Democratic Republic of Congo (DRC) with a rate of 53% (95% CI: 50–55). Leave-one out sensitivity was performed to assess the significant impact of single studies on the overall rate and ranged from 80% (95% CI: 72–90) to 85% (95% CI: 78–92) (Supplementary Fig. 1). Therefore, no single study had a significant impact on the overall rate of acceptance.
Fig. 3.
Forest plot of pooled rate of malaria vaccine acceptance for children under five years in sub-Saharan Africa by COVID period
Fig. 4.
Forest plot of pooled rate of malaria vaccine acceptance for children under five years in sub-Saharan Africa by region
Publication bias assessment
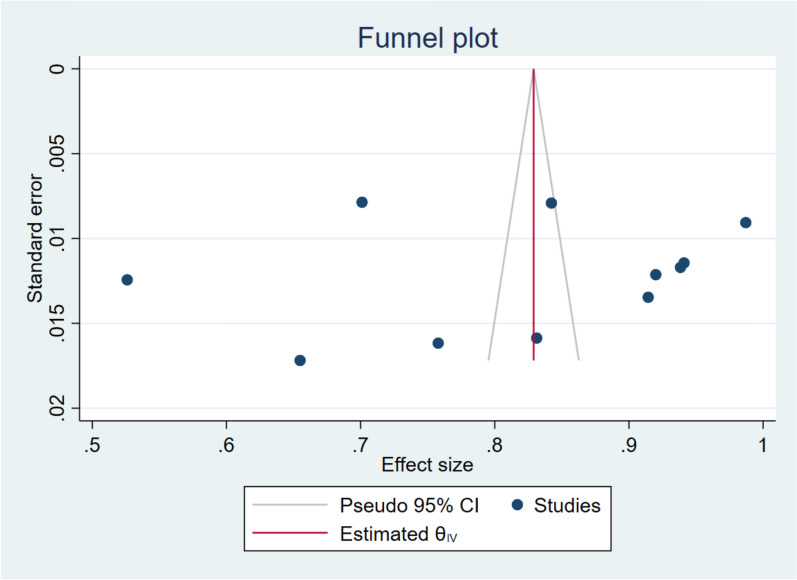
Figures 5, 6, 7 shows a funnel plot visual assessment of publication bias for the overall estimate, and for the different subgroup analyses, by study period and by region where the study was conducted. Additionally, assessment through the Eggers test (P = 0.408) revealed absence of publication bias from small study effects.
Fig. 5.
Funnel plot assessment of publication bias
Fig. 6.
Funnel plot assessment of publication bias by survey period
Fig. 7.
Funnel plot assessment of publication bias by survey period
Pooled rate of willingness for malaria vaccine in sub-Saharan Africa
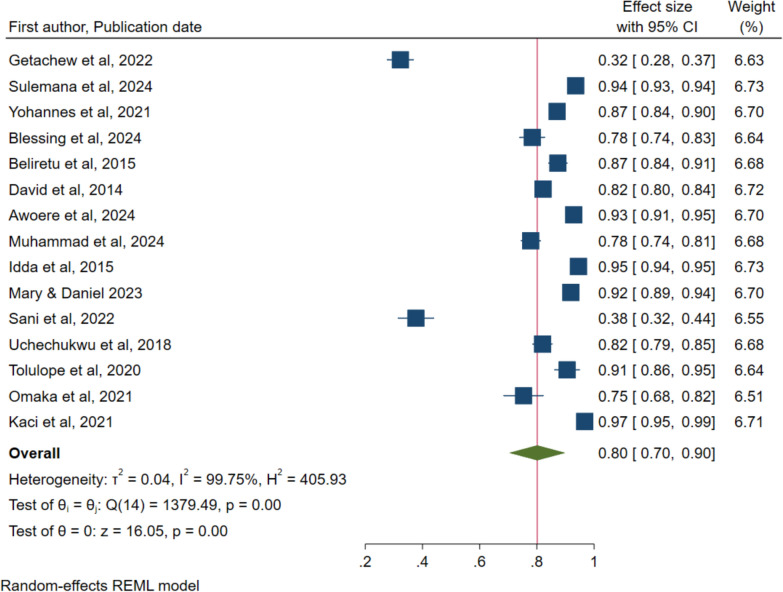
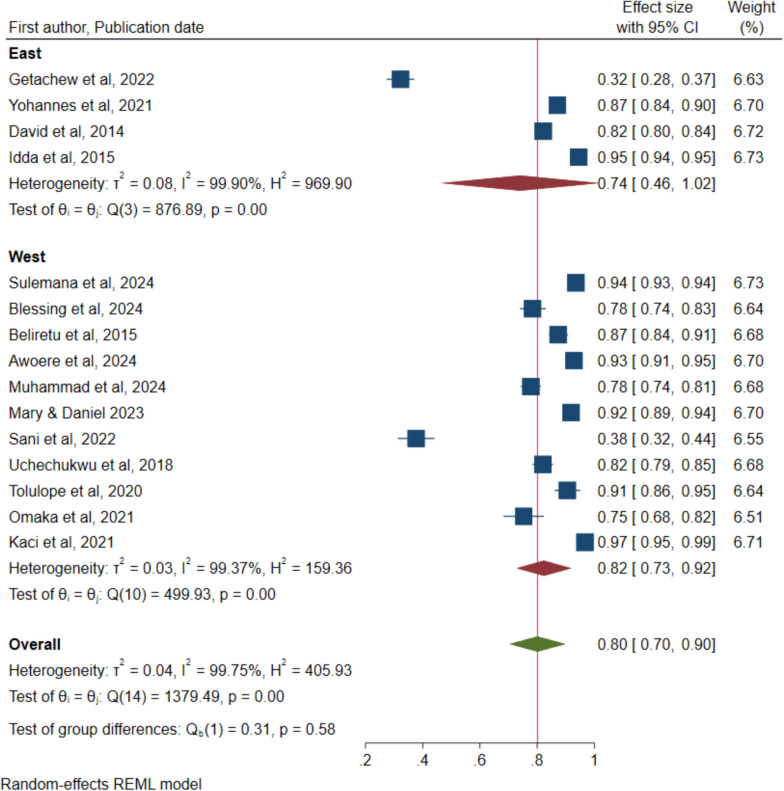
Figure 8 shows that a total of 15 studies reported on willingness to accept the malaria vaccine for children under five years and a pooled rate of 80%, 95% CI of 70% to 90%. The figure also shows that a high heterogeneity of 99.75% with a P value of < 0.001 were reported.
Fig. 8.
Forest plot of the pooled proportion of willingness to accept the malaria vaccine for children under five years in Sub-Saharan Africa
Sub-group analysis and sensitivity analysis
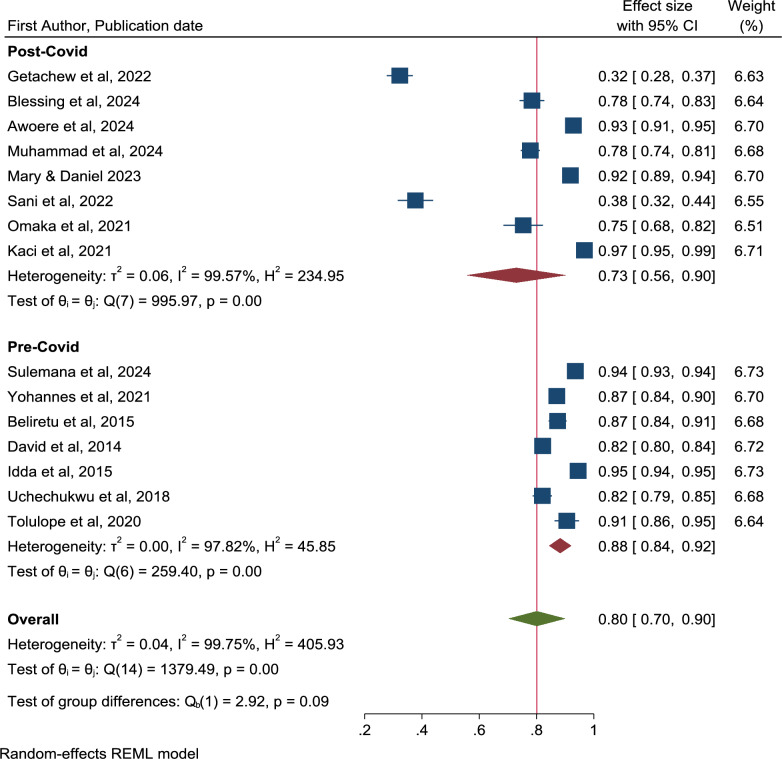
Similarly, sub-group analysis was conducted based on period when the study was conducted and the region of SSA and reported no significant differences based on the overlapping confidence intervals. Figure 9 shows that the rate of willingness to accept the malaria vaccine before the COVID was 88% (95% CI: 84–92) compared to 73% (95% CI: 56–90) after the emergence of the COVID pandemic. Figure 10 indicates that the level of willingness for the Western region was 82% (95% CI: 73–92) versus 74% (95% CI: 46–100) for the Eastern region. As demonstrated by the leave-one-out sensitivity analysis, no single study affected the total estimations (Supplementary Fig. 2).
Fig. 9.
Forest plot of pooled rate of willingness to accept the malaria vaccine for children under five years in sub-Saharan Africa by COVID period
Fig. 10.
Forest plot of pooled rate of willingness to accept the malaria vaccine for children under five years in sub-Saharan Africa by region of SSA
Publication bias assessment
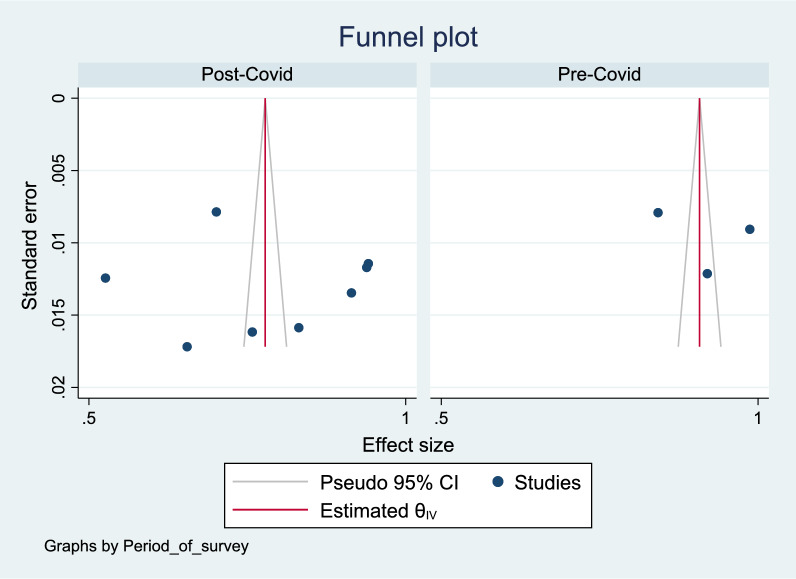
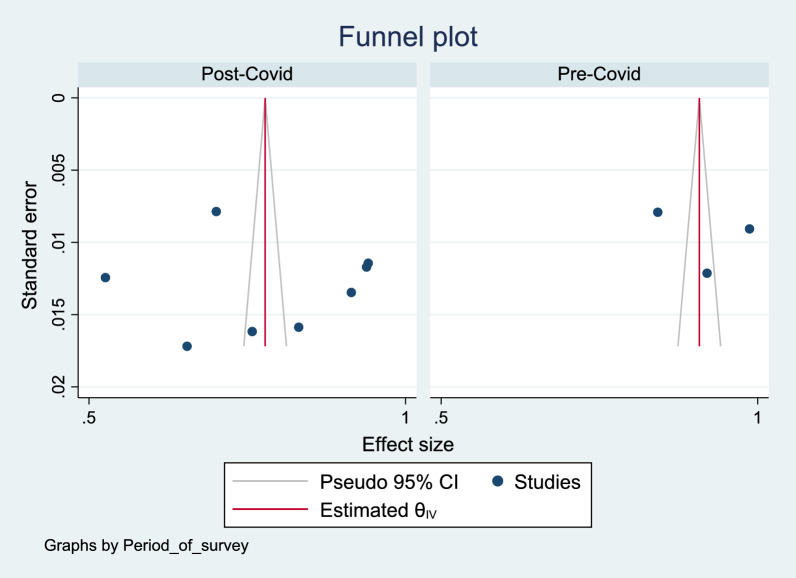
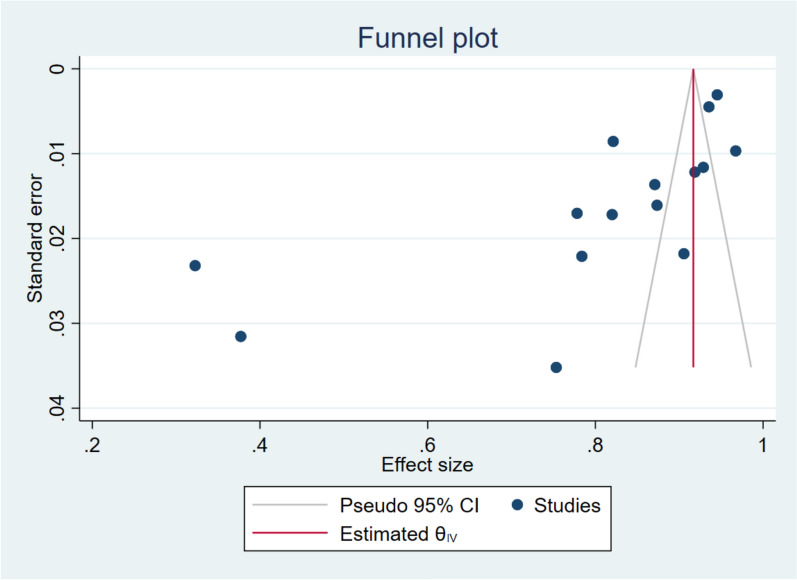
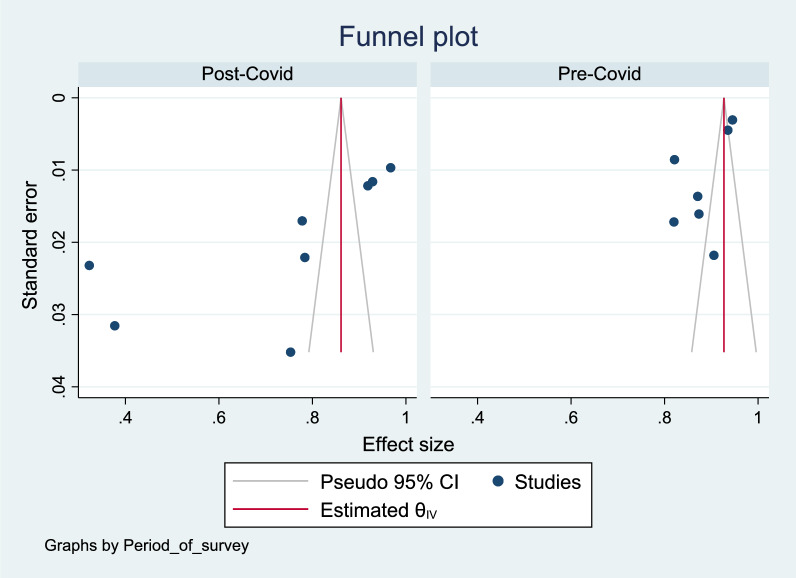
Figures 11, 12, 13 show a funnel plot visual assessment of publication bias, and for subgroup analyses of period of survey and region where the study was conducted. The overall plot indicates asymmetry of the studies, therefore potential publication bias. The differences in the symmetry of the subgroup analyses funnel plots are indicative of the potential cause of the high heterogeneity. Additionally, running the Eggers test (P = 0.002) revealed possible presence of publication bias from small study effects.
Fig. 11.
Funnel plot assessment of publication bias
Fig. 12.
Funnel plot assessment of publication bias by survey period
Fig. 13.
Funnel plot assessment of publication bias by region
Discussion
This systematic review and meta-analysis aimed to estimate the pooled acceptance and perceived usefulness of the malaria vaccine among various populations across sub-Saharan Africa. The pooled willingness to accept rate was 80% (95% CI: 70% to 90%), with a higher rate observed pre-COVID-19 (89%) compared to post-COVID-19 (77%) suggesting a generally favourable public outlook towards the malaria vaccine [4]. However, interpretation of these results must consider several important contextual and methodological distinctions. These results highlight the broader impact of the COVID-19 pandemic on health behaviours and attitudes, underscoring the pandemic’s role in altering vaccine perceptions and priorities [29]. Interestingly, regional variations were evident in willingness as well, with the Western region reporting higher levels of willingness (82%) than the Eastern region (74%). This suggests that while overall acceptance and willingness are high, there are still underlying variations that could affect the success of a mass vaccination program if not addressed.
Firstly, the included studies varied significantly in terms of timing, geographic scope, and how key outcomes such as acceptance and usefulness were assessed. Definitions ranged from hypothetical willingness to vaccinate to acceptance of a recommended vaccine, with variation in survey tools and populations targeted [30, 31]. This definitional inconsistency likely contributed to the high statistical heterogeneity observed (I2 = 99.66% for acceptance and 97.88% for willingness), emphasizing the need for standardized tools in future research to ensure comparability. In contrast, studies conducted during or after the Malaria Vaccine Implementation Programme (2019–2023) occurred in countries such as Ghana, Kenya, and Malawi, where the malaria vaccine had been piloted [9]. In these contexts, public awareness of the vaccine’s availability and partial efficacy may have influenced participant responses, making acceptance more grounded in experience than speculation [9]. Furthermore, the temporal division of sub-group analyses into pre- and during-COVID-19 periods, though initially conceived to explore the potential influence of pandemic-related vaccine hesitancy, also coincides with the pre- and post-implementation phases of the malaria vaccine in MVIP countries.
The timing of studies on malaria vaccine acceptance has significantly influenced their outcomes, particularly in the context of the COVID-19 pandemic. Research conducted before 2020 primarily focused on hypothetical acceptance of the RTS,S malaria vaccine, as it had not yet been widely implemented. In contrast, studies post-2020, especially in Kenya, Malawi, and Ghana, coincided with the Malaria Vaccine Implementation Programme and the pandemic, revealing a decline in willingness to accept the vaccine from 89 to 77% [9, 32]. This shift underscores the pandemic's broader impact on health behaviours and vaccine perceptions.
The willingness to accept malaria vaccines varies significantly across different regions in Africa, influenced by factors such as cultural beliefs, trust in healthcare systems, and prior vaccination experiences. The acceptance of malaria vaccines in East and West Africa is notably high, with various studies indicating a strong willingness among caregivers and the general population to vaccinate against malaria [33, 34]. This acceptance is crucial for the successful implementation of vaccination programs aimed at reducing malaria morbidity and mortality in the region. In contrast, Central Africa, particularly the Democratic Republic of Congo (DRC), reports the lowest acceptance at 53%, primarily due to political instability and challenges in healthcare access [35, 36]. These regional disparities underscore the importance of contextual factors in shaping vaccine attitudes. These regional findings align with prior literature that reported context-specific influences on vaccine acceptance [37].
Several key factors influence these acceptance rates. Cultural beliefs play a significant role; variations in cultural perceptions can greatly impact vaccine acceptance [38]. Trust in healthcare systems is another critical factor, with higher trust levels correlating with increased willingness to accept vaccines [34]. Additionally, prior experiences with vaccination campaigns shape community attitudes toward new vaccines [39]. Despite these challenges, there is optimism that overall vaccine acceptance in Africa is improving, driven by increased awareness and education efforts, which may help mitigate regional disparities in the future [40].
Funnel plots are essential tools for assessing heterogeneity and potential publication bias in vaccine acceptance studies. In a systematic review of malaria vaccine acceptance, only two out of eleven studies fell within the 95% confidence interval, indicating substantial variability in acceptance rates across different populations [4]. Similarly, a meta-analysis of COVID-19 vaccine acceptance revealed a pooled acceptance rate of 60.2%, with significant heterogeneity (I2 = 100%) among studies [41]. These findings suggest high heterogeneity and potential small-study effects in vaccine acceptance research.
Publication bias further complicates the interpretation of vaccine acceptance studies. In the COVID-19 vaccine acceptance studies, Egger’s test indicated significant publication bias (P = 0.003), while visual asymmetry in funnel plots suggested that bias could not be entirely ruled out [41]. The malaria vaccine acceptance study also employed funnel plots and Egger’s test to assess publication bias, reinforcing the need for careful interpretation of results due to potential biases [4]. To address these challenges, future research should incorporate subgroup-specific funnel plots to clarify patterns of vaccine acceptance and mitigate observed heterogeneity [4, 40]. Additionally, further studies are necessary to explore the determinants of vaccine acceptance and to improve public health strategies for vaccination campaigns. These findings underscore the importance of targeted research to enhance understanding and effectively address public health concerns related to vaccine acceptance.
The current literature on vaccine acceptance in sub-Saharan Africa is predominantly centred on Nigeria, which may not accurately represent the diverse contexts of other countries in the region. This concentration underscores the need for research in underrepresented areas, such as Central and Southern Africa, to achieve a more comprehensive understanding of vaccine acceptance dynamics [5, 34]. Moreover, reported willingness to accept vaccines does not always translate into actual uptake, often due to social desirability bias influencing survey responses [38]. Real-world barriers, notably missed opportunities for vaccination, significantly impede vaccine uptake.
A systematic review and meta-analysis revealed that approximately 27.3% of children in Africa experienced MOV, highlighting a critical gap in vaccination coverage [42]. Addressing these missed opportunities is essential, as studies have shown that a substantial percentage of eligible children miss vaccinations during healthcare visits [43]. Recommendations to mitigate this issue include enhancing community awareness and improving accessibility to vaccination services, particularly in rural areas [43]. While current literature provides valuable insights into vaccine acceptance, it is imperative to recognize that reported willingness does not equate to actual vaccination rates. Further research is needed to explore the complexities of vaccine uptake and the persistent barriers across various contexts in sub-Saharan Africa.
Strengths and limitations of the study
This study offers a broad and systematic review of community readiness and acceptance of the malaria vaccine across sub-Saharan Africa, providing valuable insights into regional variations and overall acceptance rates. By pooling data from multiple studies, the meta-analysis strengthens the reliability of findings and enhances the generalizability of results, making it more robust than individual studies. The study focuses on at-risk children, particularly those under five, who represent the most vulnerable group to malaria, thus addressing critical public health concern. Additionally, the analysis of vaccine acceptance before and after the COVID-19 pandemic adds a unique perspective on how global health crises influence public health initiatives and vaccine uptake.
However, the study has limitations. Some regions within sub-Saharan Africa, such as Central Africa, had limited data, potentially skewed the overall findings and made it difficult to draw definitive conclusions for these areas. The included studies may have had variations in methodology, sample size, and reporting, which could affect the consistency and comparability of the findings. While the study highlights vaccine acceptance, it does not fully address the gap between acceptance and actual vaccine uptake, which is critical for understanding the real-world impact of vaccination programs. Moreover, as the study is based on published data, there is a risk of publication bias, where studies with favorable outcomes are more likely to be included, potentially inflating acceptance rates.
Conclusion and recommendation
The systematic review and meta-analysis revealed a pooled malaria vaccine acceptance rate of 95.3% (95% CI: 93.0%–97.2%) among caregivers in low- and middle-income countries, with significant heterogeneity across studies. Notably, acceptance rates varied by country, with Nigeria at 97.6%, Ghana at 94.6%, and Tanzania at 92.5%. However, the predominance of studies from Nigeria may limit the generalizability of these findings to other sub-Saharan African regions. Moreover, a systematic review and meta-analysis reported that approximately 27.3% of children in Africa experienced missed opportunities for vaccination, highlighting a gap between willingness and actual vaccine uptake. Factors contributing to missed opportunities include healthcare access issues and socioeconomic barriers. To enhance vaccine coverage, it is recommended to conduct more research in underrepresented areas, address barriers to vaccine uptake, and implement targeted public health strategies to improve vaccination rates across diverse contexts in sub-Saharan Africa.
Acknowledgements
We gratefully acknowledge the invaluable contribution of the World Health Organization in the collective endeavours to alleviate the impact of malaria, particularly in their role in approving the malaria vaccine.
Author contributions
EK, SSP, AK, SIA, FO, MSO, and ME, contributed to the conceptualization, design, and development of the study. EK and SSP were responsible for data analysis and interpretation. EK, SSP, AK, SIA, FO, MSO, and ME participated in drafting, critical revision, and editing of the manuscript. All authors read and approved the manuscript for publication.
Funding
No author of this work received any funding to conduct the study.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have approved the submission of this work.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oladipo HJ, Tajudeen YA, Oladunjoye IO, Yusuff SI, Yusuf RO, Oluwaseyi EM, et al. Increasing challenges of malaria control in sub-Saharan Africa: priorities for public health research and policymakers. Ann Med Surg. 2022;81: 104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 18 million doses of first-ever malaria vaccine allocated to 12 African countries [Internet]. Geneva, World Health Organization, 2023 [cited 2023 Nov 2]. Available from: https://www.un.org/africarenewal/magazine/july-2023/18-million-doses-first-ever-malaria-vaccine-allocated-12-african-countries.
- 3.WHO. World Malaria Report 2022 [Internet]. Geneva, World Health Organization, 2022 [cited 2024 Sep 29]. Available from: https://www.who.int/data/gho/data/themes/malaria.
- 4.Sulaiman SK, Musa MS, Tsiga-Ahmed FI, Dayyab FM, Sulaiman AK, Bako AT. A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLoS ONE. 2022;17: e0278224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kigongo E, Kabunga A, Opollo MS, Tumwesigye R, Musinguzi M, Akello AR, et al. Community readiness and acceptance for the implementation of a novel malaria vaccine among at-risk children in sub-saharan Africa: a systematic review protocol. Malar J. 2024;23:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Prevention CC for DC and. CDC—Malaria—Malaria Worldwide—Impact of Malaria. P. 2021.
- 7.Vandoolaeghe P, Schuerman L. The RTS, S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Expert Rev Vaccines. 2016;15:1481–93. [DOI] [PubMed] [Google Scholar]
- 8.RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. [DOI] [PMC free article] [PubMed]
- 9.Asante KP, Mathanga DP, Milligan P, Akech S, Oduro A, Mwapasa V, et al. Feasibility, safety, and impact of the RTS, S/AS01E malaria vaccine when implemented through national immunisation programmes: evaluation of cluster-randomised introduction of the vaccine in Ghana, Kenya, and Malawi. Lancet. 2024;403:1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO recommends groundbreaking malaria vaccine for children at risk [Internet]. Geneva, World Health Organization, 2021 [cited 2023 Nov 2]. Available from: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk.
- 11.WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization [Internet]. Geneva, World Health Organization, 2023 [cited 2025 Apr 10]. Available from: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization.
- 12.Genton B. R21/Matrix-MTM malaria vaccine: a new tool to achieve WHO’s goal to eliminate malaria in 30 countries by 2030? J Travel Med. 2023;30:taad140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobo FT, Asante A, Woldie M, Dawson A, Hayen A. Child vaccination in sub-Saharan Africa: increasing coverage addresses inequalities. Vaccine. 2022;40:141–50. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4. [DOI] [PubMed] [Google Scholar]
- 15.Simas C, Larson HJ. Overcoming vaccine hesitancy in low-income and middle-income regions. Nat Rev Dis Primers. 2021;7:41. [DOI] [PubMed] [Google Scholar]
- 16.Adeloye D, Agarwal D, Barnes P, Bonay M, van Boven J, Bryant J, et al. Research priorities to address the global burden of chronic obstructive pulmonary disease (COPD) in the next decade. J Glob Health. 2021;11:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdulkadir BI, Ajayi IO. Willingness to accept malaria vaccine among caregivers of under-5 children in Ibadan North Local Government Area. Nigeria MalariaWorld J. 2015;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asmare G. Willingness to accept malaria vaccine among caregivers of under-5 children in Southwest Ethiopia: a community based cross-sectional study. Malar J. 2022;21:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingham A, Gaspar F, Lancaster K, Conjera J, Collymore Y, Ba-Nguz A. Community perceptions of malaria and vaccines in two districts of Mozambique. Malar J. 2012;11:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabiri D, Ouédraogo JCRP, Nortey PA. Factors associated with malaria vaccine uptake in Sunyani Municipality, Ghana. Malar J. 2021;20:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Science Open. 2015. Available from: https://www.scienceopen.com/document?vid=54b48470-4655-4081-b5d4-e8ebe8d1792e.
- 25.Ma L-L, Wang Y-Y, Yang Z-H, Huang D, Weng H, Zeng X-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343: d4002. [DOI] [PubMed] [Google Scholar]
- 28.Bown MJ, Sutton AJ. Quality control in systematic reviews and meta-analyses. Eur J Vasc Endovasc Surg. 2010;40:669–77. [DOI] [PubMed] [Google Scholar]
- 29.Puleh SS, Kigongo E, Opio IO, Akech SI, Opollo MS, Achan E, et al. Parents’ readiness to vaccinate their children aged 5 to 17 years against Covid-19 and its associated factors in Lira District, Uganda. Pediatric Health Med Ther. 2023;14:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyda A, King C, Dey A, Leask J, Dunn AG. A systematic review of studies that measure parental vaccine attitudes and beliefs in childhood vaccination. BMC Public Health. 2020;20:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darbandi A, Koupaei M, Kiani P, Ghanavati R, Najafi P, Hosseini J, et al. Acceptance-hesitancy of COVID-19 vaccination and factors affecting it in adults: systematic review study. Immun Inflamm Dis. 2024;12: e70076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price J, Gurley N, Gyapong M, Ansah EK, Awusabo-Asare K, Gyasi SF, et al. Acceptance of and adherence to a four-dose RTS, S/AS01 schedule: findings from a longitudinal qualitative evaluation study for the malaria vaccine implementation programme. Vaccines (Basel). 2023;11:1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Röbl K, Fischer H-T, Delamou A, Mbawah AK, Geurts B, Feddern L, et al. Caregiver acceptance of malaria vaccination for children under 5 years of age and associated factors: cross-sectional household survey, Guinea and Sierra Leone, 2022. Malar J. 2023;22:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansar F, Azzam A, Rauf MS, Ajmal Z, Asad Ullah G, Rauf S, et al. Global analysis of RTS, S/AS01 malaria vaccine acceptance rates and influencing factors: a systematic review. Cureus. 2024;16: e60678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyalundja AD, Bugeme PM, Guillaume AS, Ntaboba AB, Hatu’m VU, Tamuzi JL, et al. Socio-demographic factors influencing malaria vaccine acceptance for under-five children in a malaria-endemic region: a community-based study in the Democratic Republic of Congo. Vaccines (Basel). 2024;12:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malembaka EB, Karemere H, Bisimwa Balaluka G, Altare C, Odikro MA, Lwamushi SM, et al. Are people most in need utilising health facilities in post-conflict settings? A cross-sectional study from South Kivu, eastern DR Congo. Glob Health Action. 2020;13:1740419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alie MS, Abebe GF, Negesse Y, Adugna A, Girma D. Vaccine hesitancy in context of COVID-19 in East Africa: systematic review and meta-analysis. BMC Public Health. 2024;24:2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chutiyami M, Saravanakumar P, Bello UM, Salihu D, Adeleye K, Kolo MA, et al. Malaria vaccine efficacy, safety, and community perception in Africa: a scoping review of recent empirical studies. Infection. 2024;52:2007–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ateke AN, Dinga JN, Tchokfe S, Amani A, Madaina I, Ticha MNS, et al. Malaria vaccine acceptance and associated factors in Cameroon [Internet]. Rochester, NY: Social Science Research Network; 2024 [cited 2025 Apr 12]. Available from: https://papers.ssrn.com/abstract=4712743.
- 40.Ayenigbara IO, Adegboro JS, Ayenigbara GO, Adeleke OR, Olofintuyi OO. The challenges to a successful COVID-19 vaccination programme in Africa. Germs. 2021;11:427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alemayehu A. Biology and epidemiology of Plasmodium falciparum and Plasmodium vivax gametocyte carriage: implication for malaria control and elimination. Parasite Epidemiol Control. 2023;21: e00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamu AA, Jalo RI, Masresha BG, Ndwandwe D, Wiysonge CS. Mapping the implementation determinants of second dose measles vaccination in the World Health Organization African Region: a rapid review. Vaccines (Basel). 2024;12:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jejaw M, Tafere TZ, Tiruneh MG, Hagos A, Teshale G, Tilahun MM, et al. Three in four children age 12–23 months missed opportunities for vaccination in Sub-Saharan African countries: a multilevel mixed effect analysis of demographic health and surveys 2016–2023. BMC Public Health. 2025;25:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.