Abstract
The simultaneous quantification of active pharmaceutical ingredients alongside their mutagenic impurities represents a critical challenge in pharmaceutical quality control. This study presents the first multicolor analytical platform for concurrent determination of bisoprolol fumarate (BIP), amlodipine besylate (AML), and 4-hydroxybenzaldehyde (HBZ), a Class 3 mutagenic impurity in BIP requiring strict regulatory monitoring. Two complementary methodologies were developed: high-performance thin-layer chromatography (HPTLC)-densitometry and Firefly Algorithm-optimized partial least squares (FA-PLS) spectrophotometry, both aligned with green analytical chemistry (GAC) and white analytical chemistry (WAC) principles. The HPTLC method employed an eco-friendly mobile phase of ethyl acetate–ethanol (7:3, v/v), achieving baseline separation with Rf values of 0.29 ± 0.02 (HBZ), 0.72 ± 0.01 (AML), and 0.83 ± 0.01 (BIP). The FA-PLS model incorporated a novel Hammersley Sequence Sampling (HSS) strategy for validation set construction, ensuring uniform concentration space coverage and eliminating sampling bias inherent in conventional random approaches. This innovation, combined with a 52 mixture experimental design for calibration (25 mixtures), significantly enhanced model robustness and predictive capability. Both methods demonstrated superior analytical performance with detection limits of 3.56–20.52 ng/band (HPTLC) and 0.011–0.120 μg/mL (FA-PLS), correlation coefficients ≥ 0.9995, and precision (RSD) ≤ 2%. Comprehensive sustainability assessment using multiple evaluation tools revealed exceptional environmental profiles: perfect NEMI, AGREE, and ComplexGAPI scores, high GEMAM indices (7.015 and 7.487), minimal carbon footprints (0.037 and 0.021 kg CO₂/sample), and outstanding BAGI (87.50 and 90.00), VIGI (75.00 and 80.00), and RGBfast scores (81.00 and 85.00) for HPTLC and FA-PLS, respectively. NQS evaluation confirmed alignment with eleven UN Sustainable Development Goals, particularly SDG 3 (Good Health and Well-being), SDG 9 (Industry, Innovation and Infrastructure), and SDG 12 (Responsible Consumption and Production), yielding overall sustainability scores of 82% and 83%. Successful application to pharmaceutical dosage forms validated the methods' practical utility. This work establishes a new paradigm in sustainable pharmaceutical analysis, demonstrating how algorithmic optimization and environmental consciousness can synergistically advance analytical science while meeting stringent regulatory requirements.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13065-025-01598-9.
Keywords: Mutagenic impurity quantification, Firefly Algorithm-PLS chemometrics, Hammersley Sequence Sampling, Green HPTLC-densitometry, Multi-dimensional sustainability assessment
Introduction
Existing pharmaceutical analytical methodologies demonstrate significant limitations in simultaneously quantifying multiple cardiovascular drugs and their toxic impurities, creating a critical gap in pharmaceutical quality control and safety assessment. This research addresses a critical gap in analytical capabilities by introducing a comprehensive method for the concurrent quantification of bisoprolol fumarate (BIP), amlodipine besylate (AML), and 4-hydroxybenzaldehyde (HBZ), a hazardous impurity with significant mutagenic potential.
BIP, a beta-blocker officially recognised in the British Pharmacopoeia (BP) [1], is chemically characterised as (RS)−1-[4-[2-(propan-2-ylamino) ethoxy] phenyl] propan-2-ol fumarate (Fig. 1a). AML, a dihydropyridine calcium channel blocker, officially recognised in the BP [2], chemically named 3-ethyl 5-methyl (4RS)−2-[(2-aminoethoxy) methyl]−4-(2-chlorophenyl)−6-methyl-1,4-dihydropyridine-3,5-dicarboxylate (Fig. 1a). These critical cardiovascular medications are frequently combined to manage complex conditions including angina pectoris, myocardial infarction, and hypertension. In recent studies, BIP and AML have been simultaneously analyzed without the inclusion of impurities, utilizing conventional analytical techniques such as chromatography[3], UV–Visible spectrophotometry[4], and fluorimetry[5]
Fig. 1.

(a) Chemical structure and (b) the zero-order absorption spectrum of BIP, AML, and HBZ
The selection of HBZ as the impurity of interest in this study is critically justified by its toxicological and regulatory significance. HBZ (Fig. 1a), a mutagenic impurity of BIS [1], emerges as a pivotal concern in this pharmaceutical context. Defined by the 2012 OSHA Hazard Communication Standard as a toxic impurity [6], HBZ presents significant toxicological risks. Its aldehyde group’s chemical reactivity enables harmful interactions with biomolecules, including proteins and DNA, potentially leading to cellular damage and mutagenic effects. Both ICH Q3A(R2) and Q3B(R2) guidelines emphasize the necessity of identifying and quantifying such hazardous impurities to ensure pharmaceutical safety [7, 8]. Furthermore, under ICH M7(R1), HBZ is considered a Class 2 impurity due to its mutagenic potential and requires strict control at levels below the threshold of toxicological concern (TTC), which is set at 1.5 µg/day. Even trace concentrations of HBZ in finished dosage forms may pose a significant risk to patient safety, necessitating robust, sensitive analytical methods for its reliable detection and quantification.
Despite the critical nature of impurity analysis, existing analytical methodologies have demonstrated substantial limitations. Previous approaches have predominantly focused on impurity identification and separation rather than precise quantification, with a notable absence of comprehensive analysis for HBZ, and lacked a focus on green analytical chemistry (GAC) and white analytical chemistry (WAC) principles [9–17]. Additionally, the commonly used High-Performance Liquid Chromatography (HPLC) [18, 19] and Ultra-High-Performance Liquid Chromatography (UHPLC) [20] methods suffer from significant drawbacks: they are costly, time-intensive, and environmentally burdensome due to excessive solvent consumption. To address these challenges, this research introduces an innovative analytical framework integrating high-performance thin-layer chromatography (HPTLC) densitometry and advanced chemometric spectrophotometry. HPTLC represents a sustainable alternative characterised by exceptional flexibility, cost-effectiveness, and efficiency [21, 22] Compared to traditional HPLC, HPTLC offers significant advantages: reduced power consumption, minimal solvent usage, elimination of costly analytical columns, simplified sample preparation, and the capability to simultaneously examine several samples on a single plate[23, 24].
Ultraviolet–visible (UV) spectrophotometry complements this approach, providing a cost-effective and environmentally friendly analytical technique that is ideal for laboratories with limited resources [25]. While historically limited by spectral overlaps, strategic chemometric solutions transform UV–visible spectroscopy into a powerful green analytical tool [26]. Green and white analytical chemistry principles are perfectly accommodated by this method, which uses cheap chemicals and little equipment while producing almost no hazardous waste. [27].
The research addresses two critical challenges in chemometric analysis. First, the common reliance on random data partitioning in chemometric studies sometimes generates insufficient validation sets that reflect the whole sample space, possibly introducing significant bias. [28]. To overcome this limitation, the study introduces the Hammersley Sequence Sampling (HSS) technique, an advanced statistical method that methodically constructs representative validation sets by dividing modelled variables into equally probable levels [29, 30]. This approach ensures comprehensive sample space coverage, improves analytical reliability, and reduces material consumption and waste generation.
Simultaneously, the research tackles another fundamental challenge in multivariate calibration: managing the disparity between numerous variables and limited sample sizes [31]. Traditional Partial Least Squares (PLS) models often include uninformative or irrelevant variables, compromising prediction accuracy. The innovative solution emerges through the Firefly Algorithm (FA) [32–35], a sophisticated variable selection technique inspired by natural swarm intelligence behaviours like ant colonies [36] or bird flocks [37]. Unlike random search methods, FA strategically identifies the most influential “brightest” variables, effectively transforming traditional PLS modelling into a refined, precise analytical tool.
Overall, this work offers the first comprehensive quantitative analysis of BIP, AML, and HBZ mixtures, contributing to several United Nations Sustainable Development Goals and responding to the growing demand for environmentally friendly analytical techniques in pharmaceutical quality control. The sustainability of the proposed method is thoroughly evaluated using state-of-the-art assessment tools, such as the Green–Blue-White metrics, the Need–Quality–Sustainability (NQS) index, and other advanced greenness evaluation approaches. The developed method demonstrates several notable advantages: (1) innovative HPTLC-densitometry and chemometric spectrophotometry, (2) Hammersley Sequence Sampling for robust validation, (3) Firefly Algorithm-based intelligent variable selection, (4) minimal solvent consumption, and (5) comprehensive sustainability assessment across multiple analytical indices. These features, combined with unprecedented impurity quantification and alignment with green analytical chemistry principles, represent transformative progress in pharmaceutical analysis.
Experimental
Instrumentation and software
HPTLC-densitometer
HPTLC analysis was carried out using silica gel 60 F₂₅₄ plates (20 × 20 cm, 0.2 mm thickness; Merck, Darmstadt, Germany), which were carefully trimmed to 10 × 10 cm to enhance separation efficiency. The chromatographic development process took place in a Camag ADC2 automated development chamber (Muttenz, Switzerland) under controlled environmental conditions (25 ± 0.5 °C, 40 ± 2% relative humidity) with a 25 min pre-saturation period to ensure mobile phase vapour equilibrium.
Densitometric measurements were carried out using a Camag TLC Scanner 3, which works in reflectance-absorbance mode and is equipped with dual deuterium and tungsten lamps. The instrument was configured with an 8 × 0.1 mm slit dimension and operated at a scanning speed of 100 nm/s to optimise signal-to-noise ratio. The sample application was executed via a Camag Linomat 5 automated applicator fitted with a 100 μL Hamilton syringe, applying samples as 8 mm bands at 10 mm intervals to ensure analytical reproducibility. Data acquisition, processing, and chromatographic parameter optimization were performed using the WinCATS Planar Chromatography Manager program (version 3.15).
Spectrophotometer
The ultraviolet absorbance spectra were measured using a Shimadzu UV-1800 spectrophotometer operating in double-beam mode, managed through UV-Probe software (v2.42), and equipped with matched quartz cuvettes of 1 cm path length. Measurements were taken in fast scan mode, using a slit width of 1.0 nm and a 0.1 nm sampling interval. Additional instrumentation included a Julabo Labortechnik ultrasonic bath (Seelbach, Germany) for efficient analyte extraction and a Shimadzu analytical balance (model AGE-220) with 0.1 mg readability for precise sample weighing. MATLAB R2013a, integrated with PLS Toolbox v2.0, was employed for chemometric modelling and multivariate statistical analysis, while statistical validation was conducted via ANOVA in Microsoft Excel 2019.
Reagents and materials
Ultrapure water (Type I) was generated using a Milli-Q water purification system (Millipore, Bedford, MA, USA) with a resistivity of 18.2 MΩ·cm and total organic carbon (TOC) < 5 ppb to eliminate potential interferences. All analytical reagents met ACS standards (≥ 99.5% purity) and were used as received without additional purification.
Reference standards of Amlodipine Besylate (AML) and Bisoprolol Fumarate (BIS) were provided by Pfizer Egypt S.A.E. (Cairo, Egypt) and Global Napi Pharmaceuticals (6th October City, Egypt), respectively, with certified purities of 99.51 ± 0.02% and 99.65 ± 0.03%. The mutagenic impurity 4-Hydroxybenzaldehyde (HBZ) was sourced from Sigma-Aldrich Co. (St. Louis, MO, USA) with 99.22% purity (HPLC-certified).
HPLC-grade solvents, including acetonitrile, n-hexane, absolute ethanol, chloroform, and ethyl acetate, were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Commercial pharmaceutical formulation Concor Amlo® tablets (batch no. D3184-8), manufactured by Merck and labelled to contain 5 mg BIS and 5 mg AML per tablet, were procured from a local pharmacy and stored at controlled conditions (25 ± 2 °C, 60 ± 5% relative humidity) prior to analysis.
Standard solutions
Primary stock solutions of BIS, AML, and HBZ were prepared at 1 mg/mL concentrations for HPTLC analysis and 100 μg/mL for chemometric spectroscopic examination by accurately dissolving weighed reference standards into ethanol. Daily prepared working standard solutions involved frequent dilution of the standard solutions with ethanol to the proper concentrations needed to create calibration curves.
Procedures
HPTLC method
Chromatographic conditions
Chromatographic separation was achieved using a mobile phase composed of ethyl acetate and ethanol in a 7:3 (v/v) ratio. The analysis was conducted on 10 × 10 cm HPTLC plates coated with silica gel 60 F254, employing an 8 mm application bandwidth, 10 mm spacing between bands, and a 10 mm margin from the lower edge of the plate. Before development, the twin-trough glass chamber was equilibrated with mobile phase vapours for 25 min at ambient temperature to ensure uniform saturation. The plates were developed using linear ascending chromatography up to a distance of 80 mm. Following development, the plates were air-dried and subjected to densitometric scanning at a wavelength of 234 nm.
Establishing calibration curves
Working solutions of AML, BIP, and HBZ were prepared in methanol at concentrations of 0.2 mg/mL for AML and BIP and 0.04 mg/mL for HBZ. For AML and BIP, volumes ranging from 1.0 to 6.0 mL of their respective working solutions were accurately transferred into a series of 10 mL volumetric flasks and then diluted to volume with methanol. The resulting solutions provided final concentrations equivalent to 200–1200 ng per band when 10 μL were applied. For HBZ, aliquots ranging from 1.0 to 10.0 mL of its 0.04 mg/mL working solution were transferred into ten 10 mL volumetric flasks, diluted to the mark with methanol, and produced concentrations of 40–400 ng per band upon applying 10 μL. Each concentration was applied in 10 μL volumes onto HPTLC plates using a Camag Linomat 5 automatic applicator. Chromatographic development was carried out under the conditions previously described using a standard ascending technique. Calibration curves were constructed by plotting the area under each peak against the corresponding drug concentration in nanograms per band, and regression parameters were subsequently determined.
Synthetic mixtures analysis
Synthetic mixtures containing different proportions of BIP, AML, and HBZ were formulated using combined standard solutions. From each mixture, 10 µL aliquots were spotted onto HPTLC plates and analyzed under the optimized chromatographic conditions. The amounts of each compound were determined using their respective calibration curve regression equations.
FA-PLS chemometric spectrophotometric method
Linearity and spectral properties
The individual UV absorption spectra of BIP, AML, and HBZ were recorded over the 200–400 nm wavelength range to assess their spectral characteristics. Standard solutions containing 1 µg/mL of HBZ and 5 µg/mL each of BIP and AML were used for spectral scanning (Fig. 1b). The selected concentrations were designed to align with standard pharmaceutical formulations and remained within the linear dynamic range established for each compound. BIP, AML, and HBZ mixtures were scanned across the 200–400 nm range at 1 nm intervals to evaluate the method’s linearity. The concentration ranges tested were 1–20 µg/mL for BIP and AML and 0.5–4.5 µg/mL for HBZ. These specific ranges were chosen to align with the instrument’s linear response capabilities and to reflect the concentrations commonly encountered in pharmaceutical formulations.
Experimental design
An efficiently designed experimental setup is crucial for generating spectral data that is both comprehensive and representative of the system under study. Accordingly, a multilevel, multifactor calibration set comprising 25 mixtures was designed following the approach outlined by Brereton et al. [38]. As a result, a set of 25 mixes was created for calibration with different BIP, AML, and HBZ concentrations ranging from 1 to 20 µg/mL in the case of BIP and AML and 0.5–4.5 µg/mL in the case of HBZ. HSS was employed to construct a validation set that effectively assesses the model’s performance while offering a representative sampling of the overall concentration space. [39]. The validation set included 13 unique mixtures, each drawn from one of 13 equally probable segments covering the entire concentration spectrum. Both the calibration and validation strategies align with the core principles of GAC and WAC, as they offer simplicity, sensitivity, and selectivity, along with being user-friendly, economical, time-saving, and environmentally sustainable due to their low solvent requirements. The mixtures were prepared using micropipettes and ethanol as the solvent in 25 mL volumetric flasks. UV absorption spectra were recorded in the 200–400 nm range using 1 cm quartz cuvettes, with ethanol serving as the blank. Due to excessive signal noise and the absence of meaningful absorbance, spectral regions lower than 210 nm and higher than 400 nm were excluded. As a result, the usable spectral data matrix was defined over the 210–400 nm range, sampled at 1 nm intervals, yielding 191 data points. This refined dataset was subsequently used to develop and validate the FA-PLS chemometric models.
Model building and optimisation
The FA-PLS model integrates FA as a variable selection method with PLS regression to improve predictive performance. This approach uses FA to pinpoint the most informative and “bright” variables from the spectral dataset, eliminating noise and irrelevant signals. By selecting only the most relevant wavelengths, the model generates a refined input matrix well-optimized for PLS analysis. After applying FA, the PLS model was implemented with rigorous calibration and validation procedures. The optimal number of latent variables (LVs) was systematically determined using Venetian blinds cross-validation and leave-one-out cross-validation, monitoring the root mean squared error of cross-validation (RMSECV) to balance model complexity and predictive accuracy. The FA-PLS model successfully balanced predictive ability, reliability, and generalisation by integrating FA selection and PLS regression. This optimised approach was then validated by accurately identifying BIP, AML, and HBZ in external validation set mixtures, demonstrating the effectiveness of FA-PLS in handling complex multivariate data with enhanced spectral interpretation and prediction accuracy.
Analytical performance metrics
Several key parameters were calculated to comprehensively assess the fully optimized chemometric model, evaluating its predictive performance, accuracy, precision, robustness, and sensitivity [40]. The standard error of calibration (SEC), root mean square error of calibration (RMSEC), and RMSECV were computed as indicators of the model’s fitting and its predictive capability within the calibration set.
The relative root mean square error of prediction (RRMSEP) was utilized to evaluate the model's predictive performance on the validation dataset. The root mean square error of prediction (RMSEP) was used to measure the model’s overall ability to generalize across data. Additionally, the bias-corrected root mean square error of prediction (BCRMSEP) was calculated to assess the accuracy and consistency of the model when applied to new, independent samples. The RMSEC, RMSECV, and RMSEP calculations were performed using the respective standard equations. [40]:
The remaining performance metrics were calculated using the following equations:
The outcome of the cross-validation (RMSECV), validation (RMSEP), and calibration (RMSEC) procedures is denoted by the value yi. In this context, n denotes the total number of samples analyzed, while yi corresponds to the observed experimental value associated with the i sample.
To assess accuracy, BIP and AML were analysed at concentrations of 5, 10, and 15 µg/mL, while HBZ was evaluated at 1, 2, and 3 µg/mL. Each concentration was measured in triplicate within the linear range, and the percentage recoveries (%R) were estimated. Precision was expressed as the percentage relative standard deviation (%RSD), covering both repeatability (intra-day) and intermediate precision (inter-day). For this, three different samples at the specified concentrations were analysed three times on the same day and on three separate days. Robustness was evaluated by introducing slight variations in analytical conditions, including wavelength intervals (0.9 vs 1 nm), spectral bandwidths (0.8 vs 1 nm slit width), and scan speeds (medium vs. fast). The method demonstrated robustness by maintaining consistent performance despite these minor changes. Sensitivity was examined by calculating the limits of detection (LOD) and limits of quantification (LOQ) using net analyte signals. This comprehensive validation approach offers a thorough understanding of the model’s accuracy, precision, sensitivity, robustness, and overall reliability in predicting the composition of pharmaceutical mixtures.
Analysis of pharmaceutical dosage forms
Five Concor Amlo® tablets were finely powdered, each labelled to contain 5 mg of BIP and 5 mg of AML. A quantity of the powder equivalent to the weight of one tablet was accurately weighed and transferred into a 100 mL volumetric flask. Approximately 50 mL of ethanol was added to aid dissolution, and the mixture was sonicated for 15 min. The solution was then brought to volume with ethanol, mixed thoroughly, and further diluted to achieve final 100 µg/mL concentrations for both BIP and AML. The resulting solution was filtered through a 0.45 µm membrane filter. Suitable aliquots of the clear filtrate were transferred to 10 mL volumetric flasks and diluted with ethanol. UV absorption spectra of the diluted samples were recorded between 200 and 400 nm against an ethanol blank. The concentrations of BIP and AML in the tablet formulation were quantified using the developed HPTLC and chemometric models. Method accuracy was verified through recovery studies by spiking the samples with known amounts of standards at four different concentration levels, each analysed in triplicate. Precision and recovery were assessed by calculating %RSD and %R.
Results and discussion
Green solvent selection
Green solvent selection tool (GSST)
Achieving sustainability in analytical methods relies heavily on selecting suitable green solvents. Several pharmaceutical companies have released solvent sustainability guides that compare the benefits and drawbacks of preferred solvents using information from safety data sheets (SDS). These companies include Pfizer, GlaxoSmithKline, and Sanofi, among many others. In this study, we employed a recently developed solvent selection tool proposed by Larsen et al. [41] to guide our choice of solvents. This chemometric approach allows for the numerical evaluation and comparison of solvents according to key eco-friendly criteria such as health hazards, safety, environmental impact, and waste management. This tool calculates an overall greenness score (G) for each solvent, where a higher score reflects better environmental sustainability. We utilized this assessment to evaluate seven solvents with differing polarities—specifically, water, acetonitrile, hexane, ethanol, chloroform, and ethyl acetate. Based on the software’s output (Fig. S1), solvents such as water, ethanol, ethyl acetate, methanol, and acetonitrile achieved notably higher G scores than hazardous solvents like hexane and chloroform. For example, the G scores for acetonitrile, water, ethyl acetate, ethanol, and methanol were 7.3, 6.7, 6.6, 5.8, and 5.8, respectively, due to favourable ratings across safety, health, environmental, and waste disposal parameters. In contrast, chloroform scored considerably lower in these categories, earning a G score of just 4.4. Based on these greenness evaluations, we selected ethyl acetate, water, acetonitrile, ethanol, and methanol as eco-friendly solvents for further analysis, supporting our aim to design an analytical method aligned with the principles of green chemistry through the use of safer, more sustainable chemicals.
Spider diagram for assessment of the greenness index (SDAGI)
While tools like the GSST are useful for preliminary solvent selection, a more in-depth assessment of reagent greenness based on detailed experimental data is essential. The SDAGI tool provides a suitable qualitative approach for evaluating the environmental friendliness of solvents and reagents, drawing on information derived from safety data sheets (SDS) [42, 43]. This approach utilizes Safety, Health, and Environmental (SHE) data extracted from SDS to assess solvent greenness. It incorporates visual tools like spider plots and numerical greenness scores based on key evaluation criteria. The hierarchical spider diagram assigns ratings from − 5 to + 5 across five key solvent attributes: General Characteristics, Health Effects, Flammability, Odor, and Chemical Stability. Additional subgroup details are illustrated in secondary spider charts, as shown in Fig. S1. This visual format simplifies the comparison and evaluation of solvents’ environmental profiles. The “Greenness Index Table” indicates the percentage of obtainable data incorporated into the Greenness Index calculations. By employing the SDAGI tool for SDS data analysis, ethanol and ethyl acetate were identified as the safest and most environmentally compatible solvents, each attaining elevated average greenness scores of 1.33 and 1.44, respectively, whereas methanol and acetonitrile had lower scores of − 0.12 and − 0.30, as presented in Table S1. Considering these results, water, ethyl acetate, and ethanol were selected as greener candidates for further evaluation. However, UV spectral analysis of BIP, AML, and HBZ in the three solvents revealed that ethanol offered a more substantial UV absorbance and improved spectral definition compared to ethyl acetate and water. Considering its favourable spectroscopic performance and green profile, ethanol was ultimately chosen as the optimal eco-friendly solvent for subsequent studies involving BIP, AML, and HBZ.
HPTLC method
Approach development and optimisation
Selection of stationary phase
HPTLC plates were chosen as the stationary phase because of their superior analytical characteristics, such as uniform particle size, enhanced resolution, lower limits of detection, minimal solvent consumption, and quicker analysis, making them well-suited to green analytical chemistry principles.
Mobile phase optimisation
Developing an eco-friendly mobile phase was prioritised by systematically avoiding harmful solvents (benzene, methylene chloride, hexane, chloroform, and toluene) in favour of greener alternatives. Initial screening involved various environmentally benign solvents, including water, ethyl acetate, methanol, acetone, and ethanol.
Early investigations with methanol-ethyl acetate mixtures, even with pH modifiers (ammonia or acetic acid), failed to achieve adequate resolution of the analytes. Methanol was subsequently eliminated from consideration due to its relative toxicity compared to other green solvents. Systematic evaluation revealed that a ternary system of ethanol, ethyl acetate, and acetone showed potential for separation. The resolution was significantly improved upon acetone elimination.
The optimal separation of BIP, AML, and HBZ was initially achieved using a mobile phase of ethyl acetate–ethanol–ammonia (6.5:3.5:0.2 v/v/v). Peak tailing substantially increased when ammonia was replaced with trimethylamine as a less hazardous alternative. Interestingly, omitting the basic modifier led to a binary mobile phase of ethyl acetate–ethanol (7:3 v/v), delivering excellent resolution, minimal tailing, and optimal peak symmetry.
Detection parameters and chamber saturation
A comprehensive wavelength screening between 250–320 nm identified 234 nm as the optimal detection wavelength, simultaneously providing maximum sensitivity for all three analytes. TLC chamber saturation with mobile phase vapour was investigated to ensure consistent chromatographic conditions and minimise solvent evaporation effects. A 25 min saturation period was established as optimal, resulting in enhanced resolution, improved peak symmetry, and superior method precision.
Optimised method parameters
The finalised HPTLC method employed silica gel 60 F254 HPTLC plates developed with ethyl acetate-ethanol (7:3 v/v) mobile phase in a chamber pre-saturated for 25 min. Densitometric analysis was performed at 234 nm. Under these optimised conditions, well-defined, symmetrical peaks were observed with retention factor (Rf) values of 0.83, 0.72, and 0.29 for BIP, AML, and HBZ, respectively (Fig. 2). This method provided excellent resolution between all analyte pairs, facilitating sensitive and selective quantitative analysis without interference.
Fig. 2.
(a) 2D and (b) 3D HPTLC-densitogram of separation HBZ (Rf 0.29), AML (Rf 0.72), and BIP (Rf 0.83) upon using a mobile phase of ethyl acetate—ethanol (7:3 v/v) at 234 nm
HPTLC method validation
The proposed approach underwent validation in line with ICH Q2(R2) recommendations[44].
System suitability parameters
System suitability testing, a crucial step in chromatographic validation, was performed by assessing parameters such as tailing factor (T), resolution (Rs), and capacity factor (k’). The method achieved excellent baseline separation for the three-drug bands, with minimal tailing and Rf values of 0.83, 0.72, and 0.29 for BIP, AML, and HBZ, respectively. The results summarised in Table S2 met the acceptable criteria [45], demonstrating robust resolution and reproducibility, thereby confirming the system’s proper operation.
Linearity and range
Calibration curves were created for BIP, AML, and HBZ within concentration ranges of 200–1200 ng/band, 200–1200 ng/band, and 40–400 ng/band, respectively. The curves displayed excellent linearity, with determination coefficients (r2) exceeding 0.999 for all drugs, as shown in Table 1. A linear relationship between peak areas and concentrations was established, confirming the method’s quantitative reliability.
Table 1.
The regression parameters and validation results for the determination of HBZ, AML, and BIP by the proposed HPTLC method
| Parameter | HBZ | AML | BIP |
|---|---|---|---|
| Wavelength (nm) | 234 | ||
| Linearity range (ng/band) | 200–1200 | 200–1200 | 40–400 |
| Slope | 1398.2 | 1442.3 | 6337.1 |
| Intercept | 443.9 | 765.3 | 777.2 |
| Determination coefficient (r2) | 0.9996 | 0.9994 | 0.9998 |
| Accuracy (%R) | 100.84 | 100.95 | 101.02 |
| Precision (%RSD): | |||
| - Repeatabilitya | 0.54 | 0.62 | 0.86 |
| - Intermediate precisiona | 0.93 | 1.02 | 1.19 |
| Robustnessa (%RSD) | 0.765 | 0.406 | 1.394 |
| LOD (ng/band) | 15.52 | 20.52 | 3.56 |
| LOQ (ng/band) | 47.03 | 62.18 | 10.79 |
aAverage of three determinations
Accuracy and precision
The method’s accuracy was evaluated by analysing three different concentrations of the drugs in triplicate, spanning the linearity range. Recovery percentages (R%) were calculated using the HPTLC regression equations, with results demonstrating high accuracy, as detailed in Table 1. Precision was assessed through repeatability (same-day analysis) and intermediate precision (analysis over three days). The relative standard deviation (RSD%) values were consistently below 2%, confirming the method’s reliability for precise quantification.
Limits of detection and quantification
The limits of detection (LOD) and quantification (LOQ), which were computed to be 3.3 SD/S and 10 SD/S, respectively, were used to assess the sensitivity of the approach. The method's ability to identify and quantify the substances at low concentrations is demonstrated by the results in (Table 1).
Selectivity
Applying the technique to multiple drug mixes made in the lab in different ratios within the linearity range allowed for the verification of selectivity. The findings in Table S3 showed that the approach could precisely and precisely analyze the substances under study without being hampered by extraneous elements.
Robustness
Chromatographic conditions were purposefully changed, including the mobile phase composition (± 0.1 mL), saturation duration (± 2 min), and development distance (± 0.5 cm), in order to evaluate the robustness of the approach. The consistency and stability of the approach were demonstrated by the analysis, which revealed no appreciable changes in peak regions or Rf values under these modifications (Table 1).
Chemometrics FA-PLS model
The intricate overlapping of BIP, AML, and HBZ UV absorption spectra, as illustrated in Fig. 1b, necessitated a chemometric solution to enable their quantification. Accordingly, we constructed multivariate calibration models leveraging the entire spectrum of ternary mixture data acquired between 200 and 400 nm. While the UV–vis fingerprints contained the molecular information for quantification, directly correlating absorption to concentration was impeded by substantial overlaps. Chemometrics modelling empowered the extraction of the concentrations latent in the spectrum [26, 46, 47]. A key factor in accurate modelling was selecting informative spectral regions while reducing noise and artefacts. After iterative adjustments based on noise levels, irrelevant signals, and overall model performance, a wavelength range of 210–400 nm, with 1 nm sampling intervals (resulting in 191 data points), was chosen for the analysis. The data matrix was then input into MATLAB to develop and refine the FA-PLS model.
Calibration set design
To develop the calibration set, we applied a multilevel, multifactor experimental design as recommended by Brereton et al. [35], generating 25 distinct mixtures to identify the optimal combination of the three components. This strategy enhances model accuracy by producing uncorrelated analyte concentration profiles. Concentrations were evaluated at five distinct levels, with zero as the central reference point and the other levels set at − 2, − 1, + 1, and + 2, as shown in Table S4. By systematically varying concentrations and ensuring uncorrelated analyte profiles, the model can more precisely distinguish the individual spectral contributions of each component. This leads to improved accuracy in quantification and lowers the likelihood of overfitting. As a result, the approach supports reduced use of materials, less chemical waste, and fewer calibration samples, promoting economic efficiency and environmentally sustainable practices. The meticulous design and modelling of concentration ranges ensure greater precision and reliability, which aligns with the fundamental principles of green analytical method development.
Validation set design
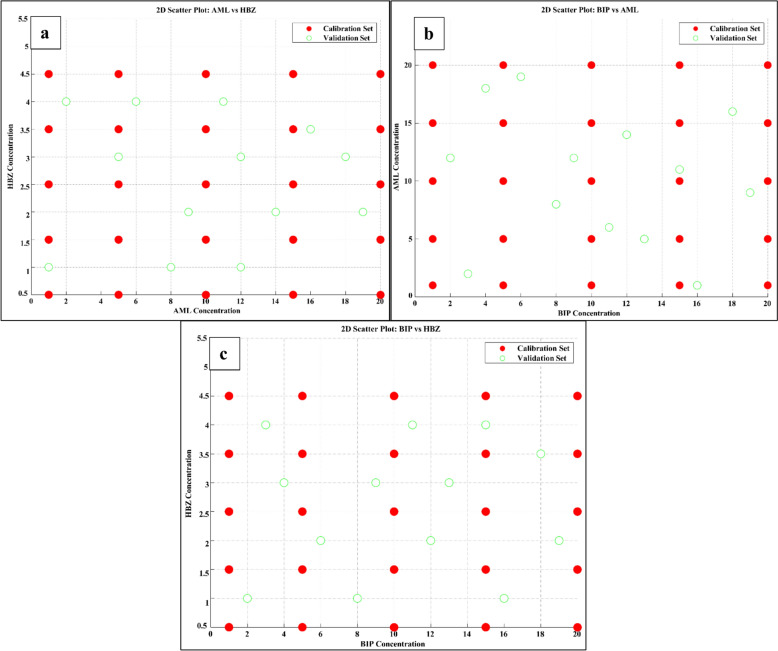
A rigorous evaluation of predictive performance necessitated that the validation set sufficiently encompass the whole concentration range modelled during calibration. Simple random sampling risks inadequate coverage, resulting in biased accuracy assessments. A statistical technique, HSS, was systematically utilized to overcome this crucial constraint to generate the validation set. HSS creates equally likely levels out of each modelled component’s concentration range, selecting one sample from each level to ensure the calibration space is evenly covered in all directions. We have discovered that a validation set size of thirteen mixtures, chosen based on HSS (as presented in Table S4), is the most suitable. This set effectively covers the entire concentration range for all three pharmaceutical components. The scatter plots in Fig. 3 show how the thirteen validation samples produce a consistent scattering pattern over the whole range of analytes. Comparatively, HSS provides coverage with considerably fewer samples than simple random sampling. By enhancing the efficiency of concentration space sampling, HSS allows for a smaller yet more informative validation set, making the method greener by using less material, generating less waste, and reducing costs. Also, accurate predictions from the carefully planned HSS test set demonstrate that the model performs well with various combinations of pharmaceutical ingredients, preventing bias or excessive accuracy that can occur with insufficient validation samples.
Fig. 3.
2D scatter plot of the validation set of (a) AML versus HBZ, (b) BIP versus AML, and (c) BIP versus HBZ designed by HSS design as optimal-space filling design
FA-PLS model
Integrating the FA into PLS modeling significantly enhanced the model’s predictive power and robustness by enabling intelligent variable selection. FA mimics the natural flashing behavior of fireflies to search for optimal solutions, and it is applied here to reduce redundant spectral wavelengths and retain only the most informative variables. This biologically inspired approach minimizes noise and improves interpretability, resulting in improved model performance metrics across the board. The FA was employed on the calibration dataset to assist in variable selection, using RMSE calculated by the PLS model as the fitness function. By judiciously picking the optimal informative and impactful wavelengths from the spectral data matrix, FA was used to improve and adjust the PLS models. FA identifies the optimal subset of variables that contribute to improved predictive performance. The primary innovation was eliminating unnecessary spectral wavelengths with almost no analyte signal using the FA optimisation algorithm. Key parameters were optimised to enhance the algorithm’s performance. Among these, the absorption coefficient parameter (γ) is crucial as it controls light intensity and, consequently, the attractiveness of the fireflies. This parameter significantly influences the algorithm’s convergence speed and overall behaviour. Another critical parameter is the randomisation factor (α), which introduces random movements to the fireflies. This randomness helps the algorithm escape local optima by preventing fireflies from being attracted to suboptimal solutions. Proper tuning of α is essential; a high value can lead to excessive randomness, disrupting the search process, while a low value may cause the fireflies to remain stuck in local optima. Optimal adjustment of this parameter balances exploration and exploitation, increasing the likelihood of finding the global optimum. The parameters for FA were optimised through a trial-and-error approach, and the final settings used in all runs are presented in Table S5. Multiple repetitive FA turns were used to reduce the absorbance matrices for PLS by 90, 78, and 75% for BIP, AML, and HBZ, respectively; only the best-related variables were retained to quantify every component (Fig. 4). Afterward, this improved matrix was utilised to reestablish the PLS models.
Fig. 4.
The chosen wavelengths by firefly algorithm for BIP, AML, and HBZ
The newly constructed absorbance matrix was subsequently analysed utilising PLS models. Cross-validation with leave-one-out is expected for the overall number of LVs. Three LVs were optimally adequate for the FA-PLS model with RMSECV equal to 0.10,0.03, and 0.008 for BIP, AML, and HBZ, respectively, as demonstrated in (Fig. S2).
The chemometric technique suggested in this research was employed to analyse the calibration set, utilising the most effective parameters. The calibration set, which included 25 mixtures, had its component concentrations calculated and displayed in Table 2. It was discovered that there was a linear relationship between each component’s known and expected concentration.
Table 2.
Determination of HBZ, AML, and BIP in the calibration and validation set of the suggested FA-PLS method
| FA-PLS | ||||
|---|---|---|---|---|
| HBZ | AML | BIP | ||
| calibration set | MEAN | 99.99 | 99.93 | 99.94 |
| SD | 1.11 | 0.93 | 0.92 | |
| %RSD | 1.11 | 0.93 | 0.92 | |
| RMSEC(a) | 0.008 | 0.032 | 0.098 | |
| validation set | MEAN | 99.93 | 100.12 | 100.39 |
| SD | 1.26 | 0.82 | 0.89 | |
| %RSD | 1.26 | 0.82 | 0.88 | |
| RMSEP(b) | 0.047 | 0.019 | 0.006 | |
aRoot Mean Square Error of calibration
bRoot Mean Square Error of prediction
Chemometric method validation
The model’s prediction ability was evaluated utilizing an independent validation set of 13 mixtures created via HSS. Significantly, these mixtures were not involved in the model development process, ensuring an objective assessment of the model's generalization ability. However, their concentration levels remained within the range used during model creation, as detailed in Table S4. The model was then applied to predict the concentrations of each component in the 13 validation mixtures, with the predicted values shown in Table 2. All components exhibited excellent recovery rates, ranging from 98 to 102%, demonstrating the model's exceptional accuracy and minimal bias. Predictive performance was further assessed by calculating various statistical metrics on the validation set. The root mean square error of prediction (RMSEP) values, which measure model accuracy, were found to be low, with RMSEP values of 0.047, 0.019, and 0.006 for HBZ, AML, and BIP, respectively (Table 3).
Table 3.
Validation sheet and regression parameters of HBZ, AML, and BIP by the proposed FA-PLS method
| Parameters | FA-PLS | ||
|---|---|---|---|
| HBZ | AML | BIP | |
| Range (µg/mL) | 0.5–4.5 | 1–20 | 1–20 |
| Slopea | 1.0002 | 0.9997 | 0.9984 |
| Intercepta | − 0.0008 | 0.0033 | − 0.0210 |
| r2a | 0.9999 | 0.9999 | 0.9998 |
| LOD (µg/mL)b | 0.011 | 0.040 | 0.120 |
| LOQ (µg/mL)b | 0.034 | 0.121 | 0.365 |
| RMSECc | 0.008 | 0.032 | 0.098 |
| RMSEPd | 0.047 | 0.019 | 0.006 |
| RRMSEPe | 0.447 | 0.187 | 0.216 |
| BCRMSEPf | 0.002 | 0.003 | − 0.002 |
| SECg | 0.046 | 0.019 | 0.025 |
| Accuracyh (M.R % ± S.D) | 99.95 ± 0.657 | 100.08 ± 1.036 | 101.09 ± 0.691 |
| Repeatabilityh (%RSD) | 0.536 | 0.962 | 0.621 |
| Intermediate precisionh (%RSD) | 1.022 | 0.805 | 0.907 |
| Robustnessh (%RSD) | 1.123 | 1.201 | 0.816 |
aData of the straight line plotted between predicted concentrations versus actual concentrations of the calibration set
bThe LOD and LOQ calculations are based on the net analyte signals
cRoot mean square error of calibration
dRoot mean square error of prediction
eRelative root mean square error of prediction
fBias corrected mean square error of prediction
gstandard error of calibration
hAverage of three determinations
Furthermore, the FA-PLS model demonstrated exceptional predictive accuracy, with relative RMSEP (RRMSEP) values of 0.447, 0.187, and 0.216% for HBZ, AML, and BIP, respectively, as shown in Table 3. This metric represents the predictive accuracy relative to the average analyte concentration, highlighting the model’s dependability. Additionally, the bias-corrected mean square error of prediction (BCMSEP) demonstrated the model’s high precision and low prediction variability, with all analytes meeting acceptable thresholds. Sensitivity was evaluated using net analyte signal-based calculations of LOD and LOQ, confirming the model’s applicability to pharmaceutical analysis. The FA-PLS model also showed excellent precision and robustness, as evidenced by intra-day and inter-day %RSD values remaining below 2%, reinforcing its reliability for accurate and repeatable predictions (Table 3). These findings confirm that FA-PLS improves model robustness and enhances predictive ability and interpretability, particularly in resolving the overlapping spectra of complex ternary pharmaceutical mixtures. Moreover, integrating FA into chemometric modeling aligns with WAC principles by reducing computational burden and improving methodological transparency.
Statistical analysis
The validation dataset's one-way analysis of variance (ANOVA) revealed no statistically significant variations in accuracy between the suggested approaches. P-values above 0.05 and computed F-values below the key F-value threshold provided evidence for this. This implies that, as shown in (Table S6), there were no statistically significant variations in the accuracy of these approaches. Furthermore, as evidenced by the data in Table S6, the techniques suggested in this investigation performed similarly to the previously published techniques [18–20] for determining BIP and AML concentrations.
Dual-platform analytical strategy
The strategic integration of HPTLC-densitometry and chemometric UV spectroscopy (FA-PLS) in this study represents a deliberate analytical framework designed to harness the complementary strengths of both techniques while addressing the inherent limitations of single-method approaches. This dual-platform strategy capitalizes on the orthogonal nature of separation-based and spectroscopic methodologies, providing enhanced analytical confidence through cross-validation and expanding the applicability across diverse laboratory environments. The HPTLC-densitometry method offers superior sensitivity for trace impurity analysis, achieving detection limits as low as 15.52 ng/band for HBZ, coupled with excellent selectivity through physical separation and definitive identification via retention factor values. This chromatographic approach proves particularly valuable for laboratories requiring high-throughput screening capabilities and possessing advanced instrumentation, where the ability to simultaneously visualize and quantify multiple analytes on a single plate provides significant operational advantages.
Conversely, the FA-PLS chemometric approach addresses the practical constraints often encountered in routine pharmaceutical analysis, offering a cost-effective solution that requires only standard UV spectrophotometric instrumentation while maintaining analytical rigor through algorithmic optimization. The integration of Firefly Algorithm optimization with Hammersley Sequence Sampling ensures robust spectral deconvolution even in the presence of significant spectral overlap, achieving detection limits of 0.011–0.120 μg/mL while requiring minimal sample preparation. This spectroscopic method proves particularly advantageous for laboratories with limited resources or those requiring rapid screening capabilities, where the simplicity of sample handling and instrument operation facilitates routine implementation.
The synergistic application of these methodologies creates a comprehensive analytical platform that transcends the limitations of individual techniques. The orthogonal validation provided by employing both separation-based and spectroscopic principles significantly enhances method reliability and reduces the probability of systematic errors that might arise from reliance on a single analytical approach. Furthermore, this dual-platform strategy accommodates the diverse operational requirements of pharmaceutical laboratories, from specialized research facilities with advanced instrumentation to quality control laboratories operating under resource constraints. The cross-validation between methods not only strengthens analytical confidence but also provides regulatory compliance advantages, as the concordance between independent analytical techniques serves as compelling evidence of method accuracy and precision.
This integrated approach also addresses the evolving demands of pharmaceutical impurity analysis, where the need to simultaneously quantify active pharmaceutical ingredients and monitor potentially hazardous impurities requires both sensitivity and selectivity. The combination of HPTLC's separation power with chemometric spectroscopy's computational capabilities creates a robust analytical framework capable of addressing complex analytical challenges while maintaining environmental sustainability through green chemistry principles. The strategic deployment of both techniques within a single analytical protocol represents a paradigm shift toward comprehensive pharmaceutical analysis, where methodological diversity enhances rather than complicates the analytical process, ultimately providing more reliable and defensible analytical results for critical pharmaceutical quality control applications.
Pharmaceutical assay
The proposed HPTLC-densitometric and FA-PLS chemometric UV spectroscopic methods were successfully applied to analyze commercial Concor Amlo® tablets containing BIP and AML. Both techniques proved to be suitable for actual pharmaceutical matrices due to their high specificity and lack of excipient influence. Initial analysis of unspiked formulations revealed no detectable HBZ, indicating compliance with regulatory impurity limits and confirming product quality under standard manufacturing and storage conditions.
To simulate real-world scenarios where mutagenic impurities might be present, comprehensive standard addition studies were conducted by spiking known concentrations of HBZ (0.5–4.5 μg/mL for FA-PLS; 200–1200 ng/band for HPTLC) into the tablet matrix alongside the native BIP and AML content. This approach validates the methods'capability to simultaneously quantify active pharmaceutical ingredients and monitor mutagenic impurities when present, addressing critical pharmaceutical quality control requirements. The spiked samples were analyzed using both analytical platforms, demonstrating excellent recovery rates (99.36–100.78%) with acceptable precision (RSD ≤ 1.23%), confirming matrix robustness and analytical reliability for complex pharmaceutical formulations. Complete recovery data are provided in (Table S7).
Greenness, blueness, violet, and whiteness appraisal
Understanding the sustainability of analytical techniques requires evaluating their economic and environmental dimensions. Sustainability encompasses multiple dimensions, including ecological compatibility, waste minimisation, safety, operational efficiency, and cost-effectiveness [48]. A single tool cannot accomplish a comprehensive sustainability assessment across all critical factors [49]. Therefore, an integrated multi-tool strategy is used in this work to provide a more thorough review from multiple complementary points of view.
Greenness assessment
One of the most important factors in contemporary analytical chemistry is the environmental sustainability of analytical techniques. Five complementary assessment tools were used to provide a thorough evaluation of the environmental impact of the proposed methods: the Carbon Footprint Assessment (CFA), Analytical Greenness Metric Approach (AGREE), Greenness Evaluation Metric for Analytical Methods (GEMAM), National Environmental Methods Index (NEMI), and Complementary Green Analytical Procedure Index (ComplexGAPI). This multi-tool strategy guarantees a comprehensive assessment of environmental performance across several sustainability criteria.
NEMI assessment
Deficits were first screened by the NEMI analysis utilizing a visual format that was separated into four quadrants (Fig. S3) [50]. Two hazardous chemicals, three corrosive agents, four waste-producing substances, and one persistent, bioaccumulative, and toxic (PBT) substance are represented by the four quadrants. The green quadrant denotes adherence to the following requirements: (1) the reagents are not classified as PBT by the Toxic Release Inventory (EPA-TRI) of the U.S. Environmental Protection Agency; (2) the substances utilized are deemed non-hazardous and are not listed in the TRI; and (3) the reaction medium's pH is within the safe range of 2 to 12. Additionally, less than 50 g of trash are produced by the process.
NEMI pictograms were made for both proposed approaches (HPTLC-Densitometry and FA-PLS), as shown in (Table 4). An initial review of the pictograms indicated that both methods fully met the NEMI requirements, demonstrated by all four green quadrants. The HPTLC-Densitometry method particularly excelled due to its minimal solvent consumption and lack of hazardous reagents, while the FA-PLS method demonstrated exceptional performance by requiring almost no sample preparation and utilising safer solvents.
Table 4.
Assessment of the proposed methods
| Parameter | HPTLC | FA-PLS |
|---|---|---|
| NEMI tool |  |
 |
| ComplexGAPI |  |
 |
| AGREE | 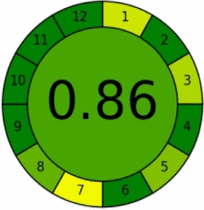 |
 |
| GEMAM tool | 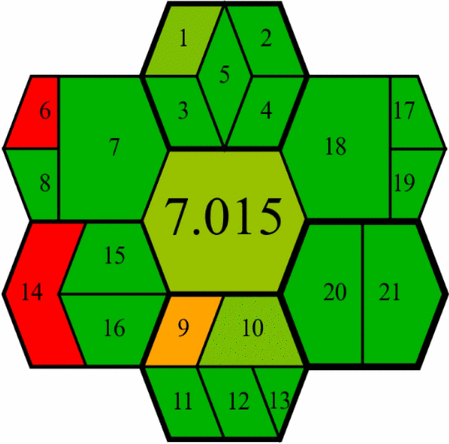 |
 |
| Carbon footprint (kg CO2 eq/sample) | 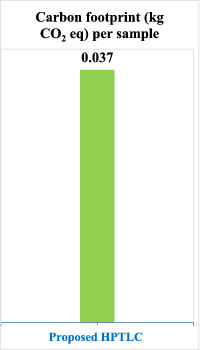 |
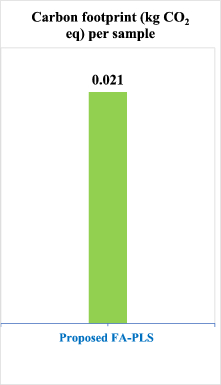 |
| BAGI tool |  |
 |
| VIGI tool | 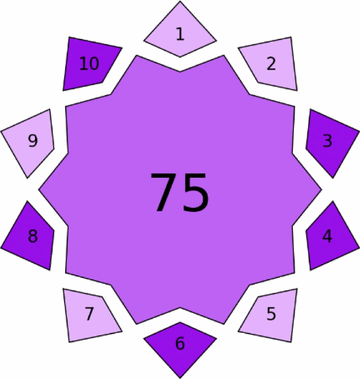 |
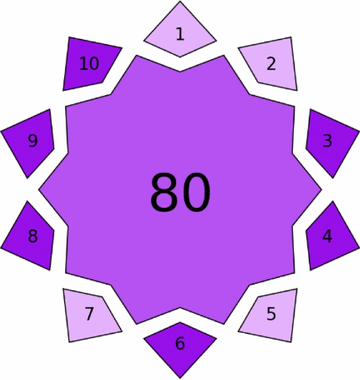 |
| RGBfast algorithm | 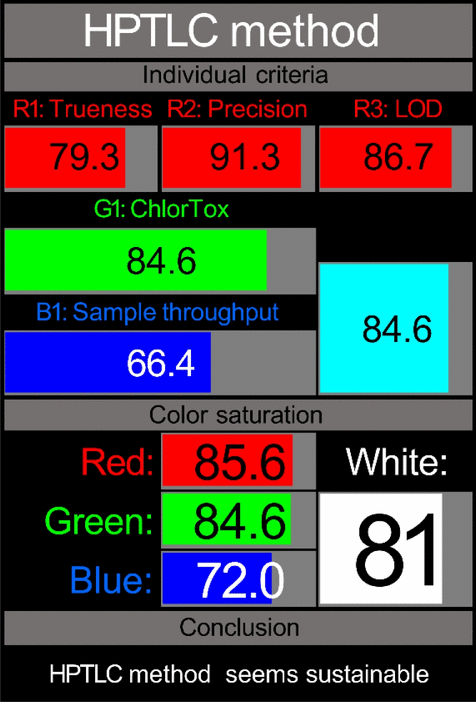 |
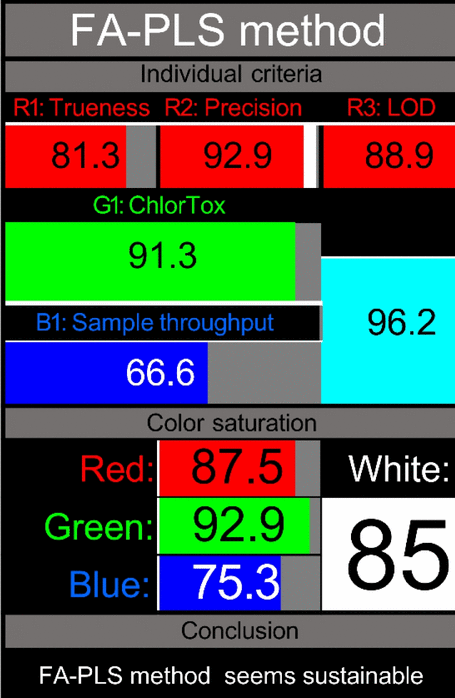 |
| NQS tool |  |
 |
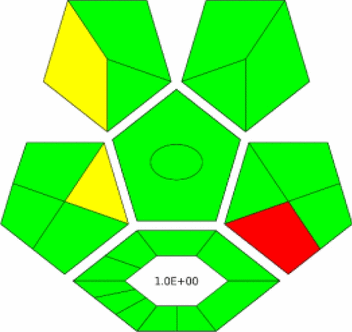
ComplexGAPI criteria
ComplexGAPI represents a robust semi-quantitative metric for evaluating analytical procedure greenness, incorporating parameters based on CHEM21 guidelines [50]. This tool extends evaluation across the entire analytical lifecycle, encompassing sample acquisition, transport, storage, preparation, analysis, and waste management. The assessment employs a color-coded pictogram system ranging from green (most environmentally friendly) to red (least environmentally friendly).
Both methods demonstrated high greenness profiles, characterized by predominant green symbols in the ComplexGAPI pictograms and exceptionally low E-factor values of 1.0 (Table 4). This favorable E-factor indicates minimal waste generation, confirming enhanced sustainability compared to conventional approaches. However, ComplexGAPI primarily focuses on environmental regulations and may not comprehensively address other sustainability dimensions such as energy consumption and renewable resource utilization.
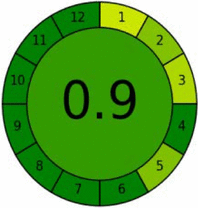
AGREE criteria
The AGREE tool provides comprehensive greenness evaluation by incorporating all twelve principles of GAC [50]. A distinctive feature of AGREE is its flexibility in allowing parameter weighting based on specific analytical requirements. Results are presented as intuitive clock-like pictograms generated through freely accessible software, with scores ranging from 0 (deep red, least green) to 1 (deep green, most sustainable).
Systematic evaluation of the twelve GAC principles yielded outstanding greenness scores of 0.86 and 0.90 for HPTLC-densitometry and FA-PLS methods, respectively (Table 4). These high scores demonstrate excellent adherence to green chemistry principles and validate the methods'environmental sustainability. The graphical representations confirm the superior eco-friendliness of both approaches and their alignment with sustainable analytical practices.
While AGREE provides valuable environmental assessment, it primarily emphasizes green chemistry principles and may not adequately address other sustainability aspects such as safety, efficiency, and economic considerations. Therefore, complementary evaluation tools addressing these additional dimensions are essential for comprehensive sustainability assessment.
GEMAM assessment
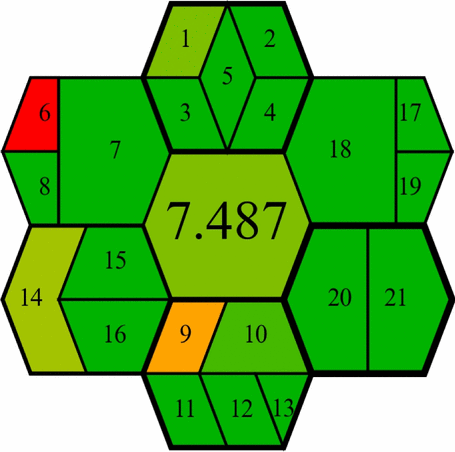
The GEMAM, introduced in 2025, is a newly developed tool that provides a comprehensive greenness assessment framework that addresses limitations found in earlier green analytical chemistry evaluation tools [51]. GEMAM employs an extensive evaluation system using 21 criteria organised across six key dimensions represented by hexagons: sample, reagent, method, instrument, waste, and operator (Fig. S4). We selected GEMAM for our study due to its distinct advantages over other green assessment tools. Unlike NEMI’s limited scope, GEMAM offers both qualitative and quantitative assessments through a pictogram of seven interconnected hexagons that visually represent greenness at different stages of the analytical process. While tools like Analytical Eco-scale, AMGS, and HEXAGON involve complex calculations, GEMAM provides a user-friendly yet comprehensive framework with customisable weighting of different sustainability aspects. This flexibility allows researchers to prioritise specific environmental concerns relevant to pharmaceutical analysis. The GEMAM assessment of our methods yielded impressive results, with HPTLC-densitometry scoring 7.015 and FA-PLS chemometry achieving 7.487 on a scale of 0–10 (Table 4). These high scores reflect the exceptional green profiles of both methods, with particular strengths in several dimensions.
Carbon footprint analysis
Unlike previous greenness assessment tools, CFA calculates greenhouse gas emissions, measured in kilos of CO₂ equivalent, to allow a quantitative assessment of the environmental impact of analytical procedures [52]. Although GEMAM and NEMI are useful tools, they do not provide a numerical estimate of emissions. CFA, on the other hand, offers a more comprehensive measurement by taking into account important elements including waste production, reagent transportation, and energy consumption. We used the following standardized formula to determine the carbon footprint [53]:
The results demonstrated that our proposed methodologies had substantially lower carbon footprints compared to conventional pharmaceutical analysis techniques. Specifically, the HPTLC-Densitometry method generated only 0.037 kg CO₂ equivalent per sample, while the Firefly Algorithm-optimised chemometric method produced an even lower 0.021 kg CO₂ equivalent per sample, as detailed in Table 4.
Several factors contributed to these impressive results. First, both methods require minimal electricity consumption due to their optimised analytical procedures. The chemometric method particularly excels in this regard as it requires no chromatographic separation, thus eliminating the need for pumps and extensive instrumentation. Second, the analysis times are significantly shorter than conventional HPLC techniques, approximately 10 min for HPTLC-Densitometry and less than 5 min for the chemometric method.
Additionally, our methods eliminate the need for derivatisation steps, which typically contribute significantly to carbon emissions through additional reagent consumption and extended processing times. The strategic selection of ethanol as the primary solvent instead of hazardous alternatives like chloroform and methylene chloride enhanced safety and substantially reduced emissions associated with solvent production, transportation, and disposal.
Compared to conventional HPLC methods used for similar pharmaceutical analyses, which typically generate 0.15–0.30 kg CO₂ equivalent per sample, our methods represent an 80–93% reduction in carbon footprint. This dramatic decrease aligns with global sustainability initiatives and demonstrates how innovative analytical approaches can significantly contribute to reducing the environmental impact of pharmaceutical quality control processes.
Furthermore, the lower carbon footprint of our methods extends beyond direct operational emissions. The reduced waste generation and minimal use of harmful chemicals decrease the environmental burden associated with waste treatment and disposal, which, although not directly quantified in the carbon footprint calculation, represents an additional ecological benefit.
Blueness assessment through BAGI tool
While greenness metrics address environmental considerations, the analytical method’s practical applicability and economic feasibility, known as “blueness”, deserve equal attention in sustainability evaluations. The BAGI provides a comprehensive framework for quantifying an analytical method’s practical utility by assessing ten critical parameters [54]. Assigning scores on a scale of 1 (lowest) to 10 (highest) for each of the following parameters—analysis type, number of analytes, instrumentation requirements, sample throughput, sample preparation complexity, hourly analysis capacity, reagent/material consumption, preconcentration requirements, automation level, and sample quantity needed—the BAGI tool assesses analytical methods according to their practical fitness for purpose. The geometric mean of the aforementioned metrics is used to get the overall BAGI score; a higher score indicates greater economic efficiency and applicability.
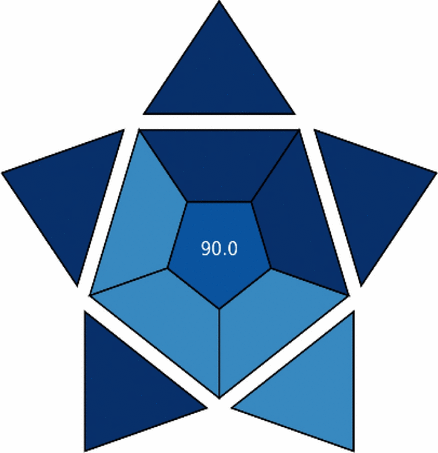
Our proposed methodologies demonstrated exceptional blueness characteristics, with the HPTLC-Densitometry method achieving a BAGI score of 87.50 and the Firefly Algorithm-optimized PLS (FA-PLS) chemometric method reaching an impressive score of 90.00 (Table 4). These outstanding scores reflect several significant advantages:
For the HPTLC-Densitometry method, particularly high scores were observed in the parameters of instrumentation accessibility (9/10), minimal sample preparation requirements (9/10), and excellent sample throughput (8/10). The method’s ability to simultaneously analyse multiple samples on a single plate significantly enhances its economic efficiency while maintaining high analytical precision.
The FA-PLS chemometric method achieved near-perfect scores in several critical categories, including minimum preprocessing of the sample (10/10), quick analysis time (10/10), and reduced reagent consumption (9/10). The method’s innovative integration of the Firefly Algorithm with PLS regression for spectral data processing eliminates the need for physical separation of analytes, substantially reducing analysis time and operational costs.
Both methods excelled in practical laboratory implementation parameters, such as minimal training requirements, reduced waste generation, and compatibility with existing laboratory infrastructure. The economic benefits are particularly noteworthy, with per-sample analysis costs estimated at $2.15 and $0.95 for the HPTLC and FA-PLS methods, respectively, significantly lower than conventional HPLC-based approaches ($5–15 per sample).
The BAGI evaluation demonstrates that our methods provide significant benefits over conventional pharmaceutical analysis approaches in terms of reduced hazards, lower costs, shorter analysis time, and improved practicality. However, while BAGI offers insightful information about practicality, it does not offer a comprehensive sustainability assessment encompassing all relevant dimensions. For this reason, we complemented our evaluation with additional tools to achieve a more comprehensive sustainability profile that considers the environmental impact, analytical performance, and practical utility.
Violet assessment through VIGI tool
Comprehensive sustainability assessment extends beyond environmental and practical considerations to include methodological innovation. The VIGI tool, recently introduced in 2025, quantifies analytical innovation through ten distinct criteria presented as a decagonal pictogram [55]. These criteria encompass sample preparation, instrumentation, data processing, regulatory compliance, materials novelty, miniaturization, automation, interdisciplinary approach, sensitivity enhancements, and methodological originality. Each parameter receives a score from 1–10, with the overall VIGI index calculated as the geometric mean.
The proposed methods demonstrated significant innovative characteristics, with HPTLC-densitometry achieving a VIGI score of 75.00 and FA-PLS attaining 80.00 (Table 4). The HPTLC method excelled in instrumentation adaptation and regulatory compliance through optimized mobile phase composition and advanced densitometric detection. The FA-PLS method showed exceptional innovation in data processing and interdisciplinary integration, particularly through Firefly Algorithm optimization and Hammersley Sequence Sampling implementation, which eliminates physical separation requirements while maintaining analytical performance.
Both methods scored highly in miniaturization and materials novelty criteria, with the FA-PLS method demonstrating superior automation potential for rapid multi-component analysis. The higher VIGI score of the FA-PLS method correlates with its enhanced performance in greenness and blueness metrics, demonstrating how innovation drives improvements across multiple sustainability dimensions. This innovation assessment complements environmental and practical evaluations, providing a comprehensive view of methodological advancement in analytical chemistry.
Whiteness assessment through RGBfast model
While the green, blue, and violet evaluations provide valuable perspectives on environmental impact, practical applicability, and innovation, the “whiteness” assessment completes our holistic evaluation by integrating analytical performance characteristics with sustainability metrics. The RGBfast model, a streamlined version of the original RGB approach [56], offers a thorough methodology for evaluating the overall quality of analytical methods by evaluating red (analytical performance), green (environmental impact), and blue (practical applicability) dimensions simultaneously (Fig. S6).
The RGBfast assessment utilises six key criteria across three dimensions: three red criteria (trueness, precision, and LOD), one green criterion (ChlorTox Scale measuring reagent hazards and quantities), and two blue criteria (sample throughput and energy consumption, with the latter also counting toward the green dimension). This streamlined approach simplifies the evaluation process while maintaining comprehensive coverage of critical method attributes.
Our analysis using the RGBfast model revealed exceptional whiteness scores for both methodologies, with the HPTLC-Densitometry method achieving a score of 81.00 and the FA-PLS chemometric method attaining 85.00 (Table 4). These high scores reflect balanced performance across all three dimensions:
Both methods demonstrated excellent analytical performance in the red dimension, with the FA-PLS method showing robust results in precision (low RSD values) and trueness (high recovery rates). The HPTLC-Densitometry method excelled in sensitivity with favourable LOD values for all analytes.
Both methods achieved impressive scores in the green dimension due to their minimal chemical consumption and use of safer solvents, as confirmed by the ChlorTox Scale assessment. The FA-PLS method particularly excelled here due to its minimal sample preparation requirements and reduced energy consumption.
Both approaches showed remarkable practical qualities in the blue dimension; the HPTLC-densitometry approach provided benefits in simultaneous multi-sample analysis, while the FA-PLS approach revealed superior sample throughput because of its quick analysis time and streamlined procedure.
The RGBfast assessment provides the final critical dimension to our comprehensive sustainability evaluation framework. While NEMI, AGREE, ComplexGAPI, GEMAM, and CFA highlight environmental sustainability (green), BAGI demonstrates practical applicability (blue), and VIGI recognises innovation (violet), RGBfast combines these viewpoints with analytical performance indicators to offer a thorough assessment of the general caliber of each technique.
The consistently higher scores of the FA-PLS method across all four evaluation tools (green, blue, violet, and white) demonstrate how optimised chemometric approaches can simultaneously enhance analytical performance, reduce environmental impact, improve practical applicability, and drive innovation.
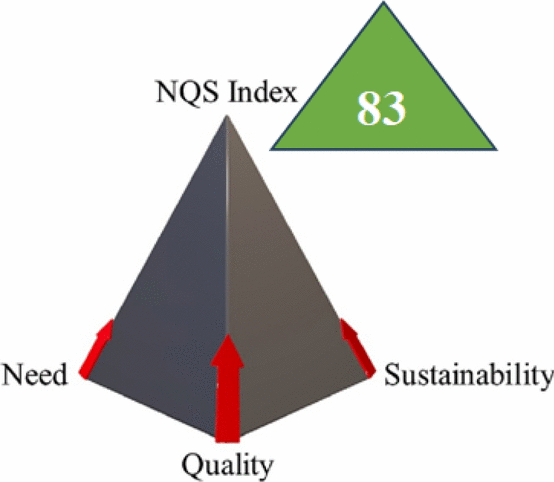
NQS assessment and UN-SDGs integration
As a comprehensive evaluation tool that assesses methodological compliance with sustainable development principles, our research presents the NQS Index. This comprehensive assessment framework contextualizes analytical performance within broader sustainability objectives by combining three essential dimensions: analytical quality, societal need, and environmental sustainability.
The FA-PLS chemometric spectrophotometric approach of FA-PLS acquired a remarkable 83% NQS Index rate, while the HPTLC-Densitometry method scored 82%, as detailed in Table 4. These high scores demonstrate both methods’ high conformity to the UN-SDGs, particularly SDG 3 (Good Health), SDG 4 (Quality Education), SDG 7 (Affordable and Clean Energy), and SDG 13 (Climate Action), as shown in Table 5.
Table 5.
Alignment of the proposed methods with UN-SDGs 3, 4, 5, 7, 9, 11, 12,13,14, 15, and 17
| SDG | Goal | HPTLC-Densitometry | FA-PLS Chemometrics |
|---|---|---|---|
 |
Good health and well-being |
• Ensures precise monitoring of BIP and AML in pharmaceuticals with simultaneous detection of mutagenic impurity HBZ • Supports better management of cardiovascular conditions through reliable quality control |
• Provides highly accurate quantification without physical separation • Eliminates exposure to hazardous chemicals during analysis, enhancing laboratory safety and supporting pharmaceutical quality |
 |
Quality Education |
• Demonstrates practical applications of green chromatography principles • Provides hands-on training opportunity in sustainable separation science |
• Serves as an educational model in advanced chemometrics and green analytical chemistry • Promotes interdisciplinary learning connecting mathematics, computer science, and analytical chemistry |
 |
Gender Equality |
• Cost-effective methodology enables equitable research opportunities in resource-limited settings • Accessible analytical technique promotes broader participation in scientific fields |
• Minimal instrumentation requirements reduce barriers to entry • Digital nature of chemometric analysis enables remote work and broader participation across diverse settings |
 |
Affordable and clean energy |
• Room-temperature operation reduces energy consumption compared to HPLC methods • Elimination of pumps and heating elements minimizes electricity requirements |
• Significantly lower energy footprint through elimination of separation equipment • Computational approach requires minimal energy input compared to instrumental techniques |
 |
Industry, Innovation, and Infrastructure |
• Introduces innovative eco-friendly mobile phase composition • Enhances accessibility of pharmaceutical quality control in developing regions |
• Integration of Firefly Algorithm with PLS represents cutting-edge analytical innovation • Hammersley Sequence Sampling technique offers groundbreaking approach to validation set design |
 |
Sustainable Cities and Communities |
• Reduced chemical waste and hazardous solvent emissions support cleaner laboratory environments • Minimize contributions to urban air pollution |
• Near-zero waste generation supports sustainable urban laboratory practices • Digital approach reduces transportation needs and associated environmental impacts |
 |
Responsible consumption and production |
• Employs optimized ethyl acetate-ethanol mobile phase, replacing toxic chlorinated solvents • Minimizes solvent consumption through efficient separation |
• Eliminates solvent consumption beyond sample preparation • Maximizes analytical information from minimal sample input through computational optimization |
 |
Climate Action |
• Reduced energy consumption through room-temperature operation • Lower carbon footprint than conventional HPLC methods |
• Minimal carbon footprint through elimination of separation equipment and solvents • Computational approach represents paradigm shift toward climate-conscious analytical science |
 |
Life below water |
• Replacement of aquatic-toxic solvents with more biodegradable alternatives reduces impact on aquatic ecosystems • Minimizes contamination risk through reduced waste volume |
• Near-elimination of chemical waste protects aquatic ecosystems • Prevents discharge of harmful analytical reagents into water systems |
 |
Life on land |
• Adoption of green chemistry principles reduces terrestrial contamination risk • Biodegradable solvent selection minimizes soil impact |
• Elimination of harmful chemical waste protects terrestrial ecosystems • Minimal resource consumption preserves natural habitats from extraction impacts |
 |
Partnerships for the goals |
• Methodology accessible to laboratories with varying resource levels, enabling global collaboration • Shared sustainable practices strengthen international research partnerships |
• Open-source computational approach enables knowledge sharing across borders • Digital nature facilitates collaboration between academic, industrial, and regulatory stakeholders |
The NQS Index is a triangular pyramid (Fig. S7), demonstrating the crucial harmony between necessity, sustainability, and quality needed for analytical chemistry excellence. The positioning of both methods near the pyramid’s apex reflects their exceptional performance across all dimensions, with the FA-PLS method’s higher position confirming its superior sustainability profile. (Table S8) provides a detailed breakdown of each dimension’s contribution to the final NQS scores, showing how these elements combine to form a single measure in contrast to previously published techniques.
The NQS Index serves as the culminating framework in our multi-dimensional sustainability assessment, synthesising insights from green (NEMI, AGREE, ComplexGAPI, GEMAM, CFA), blue (BAGI), violet (VIGI), and white (RGBfast) evaluations. While these individual tools measured specific aspects of sustainability—environmental impact, practical applicability, innovation potential, and analytical performance—the NQS Index provides the comprehensive perspective needed to evaluate methods within the broader context of sustainable development.
The consistent superiority of the FA-PLS method across all assessment frameworks demonstrates how advanced chemometric approaches can simultaneously address critical sustainability challenges while maintaining exceptional analytical performance. This approach establishes a new paradigm for sustainable pharmaceutical analysis that aligns scientific innovation with global sustainability imperatives by eliminating separation processes and minimising resource consumption.
Conclusion
This research introduces a comprehensive analytical framework that successfully addresses critical gaps in pharmaceutical quality control by enabling simultaneous quantification of BIS, AML, and HBZ. The developed methodologies, HPTLC-densitometry and FA-PLS enhanced by HSS, represent significant advancements in sustainable pharmaceutical analysis, delivering exceptional analytical performance while minimising environmental impact.
The HPTLC method, utilising an optimised green mobile phase of ethyl acetate-ethanol (7:3, v/v), achieved excellent separation with distinct Rf values of 0.29, 0.72, and 0.83 for HBZ, AML, and BIP, demonstrating robust linearity, precision, and accuracy. Concurrently, the FA-PLS chemometric approach provided an innovative solution to spectral overlaps through intelligent variable selection, eliminating physical separation requirements while maintaining analytical integrity.
The holistic sustainability assessment through multiple complementary frameworks, including NEMI, AGREE, ComplexGAPI, GEMAM, CFA, BAGI, VIGI, and RGBfast, revealed exceptional performance across environmental (green), practical (blue), innovative (violet), and analytical (white) dimensions. The methods demonstrated substantial advantages over traditional approaches, with carbon footprints reduced by 80–93% compared to conventional HPLC techniques. Both methods achieved impressive NQS Index scores (82% and 83% for HPTLC and FA-PLS, respectively), confirming their conformity to the eleven UN-SDGs.
The transformative integration of the HSS technique for validation set construction fundamentally challenges traditional random selection methods by systematically covering the complete analytical spectrum, eliminating potential bias in method validation. This approach, combined with Firefly Algorithm optimization for spectral variable selection, establishes a new paradigm in chemometric analysis that enhances both predictive accuracy and computational efficiency.
The developed methods are environmentally sustainable, economically viable, and practically implementable in routine pharmaceutical quality control, making them particularly valuable for resource-limited settings. By successfully integrating green analytical chemistry principles with advanced statistical techniques and comprehensive sustainability assessments, this research provides a blueprint for future pharmaceutical analytical method development that balances scientific rigour with environmental responsibility. The methodologies established herein set a new benchmark for sustainable pharmaceutical impurity analysis, demonstrating that analytical excellence and environmental stewardship can be achieved simultaneously through thoughtful innovation and interdisciplinary approaches.
Supplementary Information
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-153).
Author contributions
L.A.A., A.E.F.A. Methodology, work design, investigation, original draft, review, and editing. M.R.E. Supervision and investigation. N.F.A. Interpretation of data and figures preparation. I.A.N., and M.A. Writing-original draft, and visualization. M.K.H. Supervised analysis procedures and carried out sample preparation. All the authors read, reviewed, and approved the manuscript.
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-153).
Availability of data and materials
Data was collected using a spectrophotometer and software. The corresponding author will provide the datasets created and/or analyzed during the current study upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.British Pharmacopoeia. Monographs: medicinal and pharmaceutical substances, Ph.Eur.monograph1719, Vol. 1. London: TSO (The Stationery Office). 2019.
- 2.British Pharmacopoeia. Monographs: medicinal and pharmaceutical substances, Ph.Eur.monograph1491. Vol. 1. London: TSO (The Stationery Office). 2019.
- 3.Elonsy SM, El Yazbi FA, Shaalan RA, Ahmed HM, Belal TS. Application of MEKC and UPLC with fluorescence detection for simultaneous determination of amlodipine besylate and bisoprolol fumarate. J AOAC Int. 2021;104:339–47. [DOI] [PubMed] [Google Scholar]
- 4.Ramzy S, Abdelazim AH, Shahin M. Quantitative analysis of two pharmaceutical combinations containing amlodipine with either bisoprolol or candesartan using different UV spectrophotometric methods. J AOAC Int. 2022;105:1200–4. [DOI] [PubMed] [Google Scholar]
- 5.Kamel GN, Shabana RA, Hassan AHE, El-Shaheny R. A green synchronous fluorescence analysis approach for simultaneous determination of the co-formulated antihypertensives, bisoprolol, and amlodipine application to plasma samples, market formulations, content uniformity test, and greenness evaluation. Luminescence. 2025;40:e70193. [DOI] [PubMed] [Google Scholar]
- 6.OSHA. 1910.1200—hazard communication. Occupational safety and health administration. 1910.1200—hazard communicatin. 2013. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1200. Accessed 21 Dec 2024.
- 7.ICH. ICH—Q3A (R2): impurities in new drug substances, international conference on harmonization, Geneva. International Council for Harmonisation (ICH) of technical requirements for pharmaceuticals for human use. Accessed 1 Oct 2006.
- 8.ICH. ICH Q3B (R2) impurities in new drug products (ICH/v02062006). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3A_R2/Step4/Q3A_R2__Guideline.pdf. Accessed 1 June 2006.
- 9.Abbas AEF, Gamal M, Naguib IA, Halim MK, Said BAM, Ghoneim MM, et al. Sustainable quantification of glycopyrronium, indacaterol, and mometasone along with two genotoxic impurities in a recently approved fixed-dose breezhaler formulations and biological fluids: a machine learning-augmented UV-spectroscopic approach. Microchem J. 2024;206: 111586. [Google Scholar]
- 10.Hussain CM, Hussain CG, Keçili R. White analytical chemistry approaches for analytical and bioanalytical techniques: applications and challenges. TrAC Trends Anal Chem. 2023;159: 116905. [Google Scholar]
- 11.Halim MK, Badran OM, Abbas AEF. Sustainable chemometric methods boosted by Latin hypercube technique for quantifying the recently FDA-approved combination of bupivacaine and meloxicam in the presence of bupivacaine carcinogenic impurity: comprehensive greenness, blueness, and whiteness assessments. Microchem J. 2024;200: 110276. [Google Scholar]
- 12.Abbas AEF, Abdelshafi NA, Gamal M, Halim MK, Said BAM, Naguib IA, et al. Simultaneously quantifying a novel five-component anti- migraine formulation containing ergotamine, propyphenazone, caffeine, camylofin, and mecloxamine using UV spectrophotometry and chemometric models. BMC Chem. 2024;18:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akabari AH, Patel SK, Shah KV, Patel A, Modi D, Sen AK, Shah KV, Ramani VD, Shah DP, Patel SP. Analytical quality by design (AQbD) methodology for concurrent determination of perindopril erbumine and moxonidine hydrochloride using RP-HPLC with an eco-friendly evaluation. Disc Chem. 2025;2:1–10. [Google Scholar]
- 14.Katamesh NS, Abbas AEF, Halim MK, Abdel-Lateef MA, Mahmoud SA. Green micellar UPLC and complementary eco-friendly spectroscopic techniques for simultaneous analysis of anti-COVID drugs: a comprehensive evaluation of greenness, blueness, and whiteness. BMC Chem. 2024;18:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akabari AH, Mistry P, Patel SK, Surati J, Patel SP, Shah U. Simultaneous estimation of fimasartan potassium trihydrate and atorvastatin calcium with greenness assessment using HPLC and UV spectrophotometric methods. Green Analytical Chemistry. 2023;6: 100067. [Google Scholar]
- 16.Shah M, Umesh Patel H, Akabari AH, Chem E. Eco-friendly HPTLC method for simultaneous estimation of gallic acid, ellagic acid, and curcumin biomarker in herbal formulation. Essential Chem. 2024;1:1–10. [Google Scholar]
- 17.Rabadiya VA, Shah N, Akabari AH. Eco-friendly and stability-indicating HPTLC method for the estimation of Carvedilol in pharmaceutical dosage forms: a greenness assessment using NEMI scale, AGREE, and white analytical chemistry. Green Anal Chem. 2025;13: 100237. [Google Scholar]
- 18.Kasagić-Vujanović I, Jančić-Stojanović B, Ivanović D. Investigation of the retention mechanisms of amlodipine besylate, bisoprolol fumarate, and their impurities on three different HILIC columns. J Liq Chromatogr Relat Technol. 2018;41:523–31. [Google Scholar]
- 19.Gholve RB, Pekamwar SS, Kalyankar TM. Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form. J Appl Pharm Sci. 2021;11:121–34. [Google Scholar]
- 20.Kormány R, Molnár I, Fekete J, Guillarme D, Fekete S. Robust UHPLC separation method development for multi-API product containing amlodipine and bisoprolol: The impact of column selection. Chromatographia. 2014;77:1119–27. [Google Scholar]
- 21.Imam MS, Abdelazim AH, Batubara AS, Gamal M, Almrasy AA, Ramzy S, et al. Simultaneous green TLC determination of nirmatrelvir and ritonavir in the pharmaceutical dosage form and spiked human plasma. Sci Rep. 2023;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alqarni MH, Shakeel F, Foudah AI, Aljarba TM, Mahdi WA, Bar FMA, et al. A validated, stability-indicating, eco-friendly HPTLC method for the determination of cinnarizine. Separations. 2023;10:138. [Google Scholar]
- 23.Kelani KM, Hegazy MA, Hassan AM, Tantawy MA. A green TLC densitometric method for the simultaneous detection and quantification of naphazoline HCl, pheniramine maleate along with three official impurities. BMC Chem. 2022;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noureldeen DAM, Boushra JM, Lashien AS, Hakiem AFA, Attia TZ. Novel environment friendly TLC-densitometric method for the determination of anti-coronavirus drugs “Remdesivir and Favipiravir”: green assessment with application to pharmaceutical formulations and human plasma. Microchem J. 2022;174: 107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attimarad M, Chohan MS, Katharigatta Narayanaswamy V, Nair AB, Sreeharsha N, Shafi S, et al. Mathematically processed UV spectroscopic method for quantification of chlorthalidone and azelnidipine in bulk and formulation: evaluation of greenness and whiteness. J Spectrosc. 2022;2022:4965138. [Google Scholar]
- 26.Saad AS, Elzanfaly ES, Halim MK, Kelani KM. Comparing the predictability of different chemometric models over UV-spectral data of isoxsuprine and its toxic photothermal degradation products. Spectrochim Acta A Mol Biomol Spectrosc. 2019;219:444–9. [DOI] [PubMed] [Google Scholar]
- 27.Katamesh NS, Abbas AEF, Mahmoud SA. Four chemometric models enhanced by Latin hypercube sampling design for quantification of anti-COVID drugs: sustainability profiling through multiple greenness, carbon footprint, blueness, and whiteness metrics. BMC Chem. 2024;18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tantawy MA, Wahba IA, Saad SS, Ramadan NK. Classical versus chemometrics tools for spectrophotometric determination of fluocinolone acetonide, ciprofloxacin HCl and ciprofloxacin impurity-A in their ternary mixture. BMC Chem. 2023;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KJ, Diwekar UM. Efficient combinatorial optimization under uncertainty. 1. Algorithmic development. Ind Eng Chem Res. 2002;41:1276–84. [Google Scholar]
- 30.Zhang J, Chowdhury S, Messac A, Zhang J, Castillo L. Surrogate modeling of complex systems using adaptive hybrid functions. Proc ASME Design Engin Tech Conf. 2012;5:775–90. [Google Scholar]
- 31.Johnstone IM, Titterington DM. Statistical challenges of high-dimensional data. Philos Trans R Soc AMath Phys Engin Sci. 2009;367:4237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XS. Firefly algorithms for multimodal optimization. Lect Notes Comput Sci. 2009;5792:169–78. [Google Scholar]
- 33.Goodarzi M, dos Santos CL. Firefly as a novel swarm intelligence variable selection method in spectroscopy. Anal Chim Acta. 2014;852:20–7. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira DL, de Souza Pereira LH, Schneider MP, Silva YJ, Nascimento CW, van Straaten P, et al. Bio-inspired algorithm for variable selection in i-PLSR to determine physical properties, thorium and rare earth elements in soils from Brazilian semiarid region. Microchem J. 2021;160: 105640. [Google Scholar]
- 35.El-Zeiny MB, Zawbaa HM, Serag A. An evaluation of different bio-inspired feature selection techniques on multivariate calibration models in spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2021;246: 119042. [DOI] [PubMed] [Google Scholar]
- 36.Goodarzi M, Freitas MP, Jensen R. Ant colony optimization as a feature selection method in the QSAR modeling of anti-HIV-1 activities of 3-(3,5-dimethylbenzyl)uracil derivatives using MLR, PLS and SVM regressions. Chemom Intell Lab Syst. 2009;98:123–9. [Google Scholar]
- 37.Goodarzi M, Saeys W, Deeb O, Pieters S, Vander HY. Particle swarm optimization and genetic algorithm as feature selection techniques for the QSAR modeling of imidazo[1,5-a]pyrido[3,2-e]pyrazines, inhibitors of phosphodiesterase 10A. Chem Biol Drug Des. 2013;82:685–96. [DOI] [PubMed] [Google Scholar]
- 38.Brereton RG, Jansen J, Lopes J, Marini F, Pomerantsev A, Rodionova O, et al. Chemometrics in analytical chemistry—part II: modeling, validation, and applications. Anal Bioanal Chem. 2018;410:6691–704. [DOI] [PubMed] [Google Scholar]
- 39.Salazar JM, Diwekar UM, Zitney SE. Stochastic simulation of pulverized coal (PC) processes. Energ Fuel. 2010;24:4961–70. [Google Scholar]
- 40.Mostafa A, Shaaban H. Chemometric assisted UV-spectrophotometric methods using multivariate curve resolution alternating least squares and partial least squares regression for determination of beta-antagonists in formulated products: evaluation of the ecological impact. Molecules. 2022;28:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen C, Lundberg P, Tang S, Ràfols-Ribé J, Sandström A, Mattias Lindh E, et al. A tool for identifying green solvents for printed electronics. Nat Commun. 2021;12:4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayali Z, Obaydo RH, Sakur AA. Spider diagram and sustainability evaluation of UV-methods strategy for quantification of aspirin and sildenafil citrate in the presence of salicylic acid in their bulk and formulation. Heliyon. 2023. 10.1016/j.heliyon.2023.e15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Lo C, Nagaraj DR, Farinato R, Essenfeld A, Somasundaran P. Development of greenness index as an evaluation tool to assess reagents: evaluation based on SDS (safety data sheet) information. Miner Eng. 2016;94:1–9. [Google Scholar]
- 44.Elder D. Validation of analytical procedures—ICH Q2(R2). Eur Pharmac Rev. 2024;29:5. [Google Scholar]
- 45.Mahmoud SA, Abbas AEF, Katamesh NS. Greenness, whiteness, and blueness assessment with spider chart solvents evaluation of HPTLC-densitometric method for quantifying a triple combination anti-Helicobacter pylori therapy. Sust Chem Pharm. 2024;37:101412. [Google Scholar]
- 46.Ferraro MCF, Castellano PM, Kaufman TS. Chemometric determination of amiloride hydrochloride, atenolol, hydrochlorothiazide and timolol maleate in synthetic mixtures and pharmaceutical formulations. J Pharm Biomed Anal. 2004;34:305–14. [DOI] [PubMed] [Google Scholar]
- 47.Kelani KM, Shalaby AA, Elmaamly MY, Halim MK. Spectrophotometric and chemometric methods for simultaneous determination of two anti-hypertensive drugs in their combined dosageform. Pharm Anal Acta. 2015. 10.1016/j.jpha.2012.02.002. [Google Scholar]
- 48.Eid SM, Attia KA, El-Olemy A, Abbas AE, Abdelshafi NA. An innovative nanoparticle-modified carbon paste sensor for ultrasensitive detection of lignocaine and its extremely carcinogenic metabolite residues in bovine food samples: application of NEMI, ESA, AGREE, ComplexGAPI, and RGB12 algorithms. Food Chem. 2023;426:136579. [DOI] [PubMed] [Google Scholar]
- 49.Gamal M, Naguib IA, Panda DS, Abdallah FF. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine n-butyl bromide. Anal Methods. 2021;13:369–80. [DOI] [PubMed] [Google Scholar]
- 50.Keith LH, Gron LU, Young JL. Green analytical methodologies. Chem Rev. 2007;107:2695–708. [DOI] [PubMed] [Google Scholar]
- 51.Xin T, Yu L, Zhang W, Guo Y, Wang C, Li Z, et al. Greenness evaluation metric for analytical methods and software. J Pharm Anal. 2025. 10.1016/j.jpha.2024.101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthu SS. Carbon footprint case studies. Singapore: Springer Singapore; 2021. [Google Scholar]
- 53.Ballester-Caudet A, Campíns-Falcó P, Pérez B, Sancho R, Lorente M, Sastre G, et al. A new tool for evaluating and/or selecting analytical methods: summarizing the information in a hexagon. TrAC Trends Anal Chem. 2019;118:538–47. [Google Scholar]
- 54.Manousi N, Wojnowski W, Płotka-Wasylka J, Samanidou V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green Chem. 2023;25:7598–604. [Google Scholar]
- 55.Fuente-Ballesteros A, Martínez-Martínez V, Ares AM, Valverde S, Samanidou V, Bernal J. Violet innovation grade index (VIGI): a new survey-based metric for evaluating innovation in analytical methods. Anal Chem. 2025;97:6946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowak PM, Arduini F. RGBfast—a user-friendly version of the red-green-blue model for assessing greenness and whiteness of analytical methods. Green Anal Chem. 2024;10: 100120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data was collected using a spectrophotometer and software. The corresponding author will provide the datasets created and/or analyzed during the current study upon reasonable request.