Abstract
Background
C5 palsy is a debilitating complication that may occur after posterior cervical decompression or fusion surgery, characterized by acute deltoid and biceps weakness. While most cases resolve spontaneously, prolonged dysfunction imposes significant physical, psychological, and socioeconomic burdens. Virtual reality (VR) has emerged as a promising adjunct in neurorehabilitation, offering immersive environments that promote engagement and motor learning. However, its application in postoperative C5 palsy rehabilitation remains underexplored.
Methods
This single-center randomized controlled trial was conducted from January to December 2023 at a tertiary academic hospital. Adult patients (≥ 20 years) who developed new-onset C5 palsy after posterior cervical fusion were enrolled. C5 palsy was defined as a ≥ 2-grade drop in shoulder flexion or abduction strength postoperatively. Patients were randomly assigned to either a control group that received standard postoperative rehabilitation or a VR-assisted group that received the same standard rehabilitation plus an additional VR-based rehabilitation program. VR rehabilitation included interactive, game-based shoulder exercises delivered via head-mounted displays during initial hospitalization and follow-ups at 3, 6, 12, and 24 weeks. Primary outcomes were surface electromyography-derived maximal voluntary isometric contraction (MVIC), %MVIC, and fatigue index (FI) of the deltoid muscles. Secondary outcomes included the Medical Research Council (MRC) scale, Neck Disability Index (NDI), EuroQoL-5 Dimension (EQ-5D), Visual Analog Scale (VAS), and Hospital Anxiety and Depression Scale (HADS). Data were collected preoperatively and at each postoperative visit. Ten patients (VR = 4, Control = 6) completed the study.
Results
Final analysis included data from 4 patients in the VR group and 6 patients in the control group. The VR group demonstrated significantly greater efficiency in muscle activation, evidenced by lower %MVIC values at 24 weeks during both shoulder flexion (median 1.0 vs. 1.5; p = 0.025) and abduction (0.9 vs. 1.8; p = 0.014). Improvements in patient-reported quality of life (EQ-5D, p = 0.032) and arm pain reduction (VAS, p = 0.048) were observed. Depression scores (HADS-D) and anxiety scores (HADS-A) also trended lower in the VR group, particularly at 24 weeks (HADS-D: 4.0 vs. 9.5; p = 0.067). Functional metrics, including maximum arm elevation (from 90.0 cm to 145.0 cm) and apple placement count (from 25 to 55 per session), improved markedly in the VR group.
Conclusions
VR-assisted rehabilitation may contribute to improved neuromuscular efficiency, pain reduction, and psychological well-being in patients with postoperative C5 palsy. These preliminary findings suggest that immersive VR could be a promising adjunct in postoperative spinal rehabilitation, warranting further investigation in larger studies.
Trial registration
Clinical Research Information Service (CRIS), KCT0010436. Registered on April 21, 2025. Retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-025-01716-7.
Keywords: Virtual reality rehabilitation, C5 palsy, Postoperative recovery, Electromyography, Cervical surgery
Introduction
Background
C5 palsy is a common complication of cervical spine decompression surgery, characterized primarily by deltoid and biceps muscle weakness, often accompanied by sensory deficits or radicular pain [1]. The incidence of C5 palsy varies widely, with reports ranging from 1 to 30%, depending on the surgical approach and patient population [2, 3]. This condition typically manifests shortly after surgery and may take several months to resolve, causing significant anxiety and stress for patients, their families, and clinicians [4]. Clinically, C5 palsy is significant owing to its impact on patients’ ability to perform daily activities, particularly those involving shoulder abduction and flexion. Although the prognosis for recovery is generally favorable, with most patients showing substantial improvement within 6 months, its pathogenesis remains unclear [4–6]. Proposed mechanisms include direct nerve root injury during surgery, traction injury from spinal cord shift, ischemia due to reduced blood supply, and reperfusion injury [7, 8]. The socioeconomic impact of C5 palsy is considerable [9]. Prolonged periods of disability necessitate ongoing medical care and rehabilitation, which incur substantial costs. Additionally, the psychological burden on patients and their families, combined with the increased demand for healthcare resources, highlights the need for effective rehabilitation strategies to expedite recovery and alleviate anxiety [5, 10]. Given these challenges, there is a critical need for rehabilitation programs that support not only physical recovery but also psychological well-being.
Recent technological advancements have introduced innovative solutions, with virtual reality (VR) emerging as a promising tool in the medical field [11–14]. VR is increasingly being used across various healthcare domains, including pain management, psychiatric therapy, and physical rehabilitation. The immersive and interactive nature of VR enhances patient engagement, providing a more stimulating and motivating environment for rehabilitation [14, 15]. Several studies have explored the use of VR in medical rehabilitation, reporting positive outcomes [16–18]. For example, VR has been effectively used to manage chronic pain by diverting patients’ attention away from discomfort, thereby reducing perceived pain levels [14]. Additionally, VR-based therapy has shown benefits in treating mental health conditions such as anxiety and post-traumatic stress disorder by creating controlled, immersive environments where patients can safely confront and manage their symptoms [15].
Rationale
In physical rehabilitation, VR offers unique advantages. It can simulate various real-world scenarios and provide instant feedback, which is crucial for motor learning and recovery [19]. VR-based rehabilitation has the potential to shorten recovery times and improve functional outcomes by making exercises more engaging and less monotonous. This is particularly relevant for conditions such as C5 palsy, where sustained patient motivation is essential for effective rehabilitation. This study aimed to evaluate the efficacy of VR-assisted rehabilitation in patients with C5 palsy following cervical spine surgery. By comparing VR-assisted rehabilitation with conventional methods, we sought to determine whether VR provides superior outcomes in motor function recovery and psychological well-being. Specifically, this study was designed to contribute to the growing body of evidence supporting the integration of VR technology in medical rehabilitation, thereby offering a potential solution to enhance patient care and reduce the socioeconomic burden associated with postoperative complications such as C5 palsy.
In summary, this study investigated the application of VR technology in the rehabilitation of patients with C5 palsy, addressing the critical need for innovative, effective, and engaging rehabilitation programs. Through this research, we aimed to demonstrate the potential benefits of VR in accelerating recovery, reducing healthcare costs, and improving the overall quality of life for patients affected by this challenging condition.
Patients/Methods
Study design and setting
This study was designed as a prospective, randomized controlled trial conducted at a tertiary medical institution to assess the efficacy of VR-assisted rehabilitation in patients with C5 palsy following posterior cervical spine surgery. The study was approved by the institutional review board (approval number: 3-2022-0070), and all patients provided written informed consent.
Participants
Participants included adults aged 20 years and older scheduled for posterior cervical spinal fusion due to degenerative cervical conditions. The inclusion criteria were confirmed cervical spinal stenosis, cervical myelopathy, or recurrent cervical disc herniation unresponsive to conservative treatment for at least 3 months. Exclusion criteria encompassed patients with spinal or intervertebral disc infections, spinal tumors, active chronic infections, or human immunodeficiency virus (HIV), bleeding disorders, pre-existing shoulder conditions limiting abduction, or those unable to comply with follow-up visits. Surgeries were performed by an orthopedic spine surgeon with over 25 years of post-fellowship experience at a single tertiary hospital between January 2023 and December 2023. Patients who developed C5 palsy postoperatively were enrolled in the study.
Randomization
Randomization was conducted using a block randomization method to ensure equal allocation between the VR and control groups. A research nurse, who was not involved in patient enrollment, outcome assessment, or data analysis, generated the random allocation sequence in advance using the RAND() function in Microsoft Excel. The sequence was securely stored and accessed only after determining patient eligibility postoperatively, ensuring allocation concealment and minimizing selection bias.
Electrodiagnosis
Electrodiagnostic testing was selectively conducted approximately three weeks postoperatively in patients with bilateral weakness, atypical clinical progression, or when other potential causes, such as motor neuron disease or neuromuscular disorders unrelated to cervical spine pathology, needed to be ruled out following clinical confirmation of motor deterioration. This approach is not routinely applied to all cases of C5 palsy, but was used to exclude complex or unrelated neuromuscular conditions that could confound the results or be unevenly assigned to one group. Electrodiagnosis was conducted by a neurophysiologist with over 10 years of experience in nerve conduction studies and needle electromyography (EMG). Nerve conduction studies primarily assessed distal conduction of the median and ulnar nerves, with additional nerves examined on the basis of clinical suspicion. Needle EMG was performed on both paraspinal muscles at the C5-T1 spinal levels, as well as the rhomboid major, infraspinatus, supraspinatus, triceps, biceps, brachioradialis, flexor carpi radialis, abductor pollicis brevis, first dorsal interossei, and other relevant muscles on the affected side [20].
Surface electromyography
Surface EMG measurements, including MVIC, %MVIC, and FI, were collected only at preoperatively and at 24 weeks postoperatively to assess long-term muscle activation and fatigue recovery by an experienced physical therapist using a surface EMG device (BTS FREEEMG 1000; BTS Bioengineering, Lombardia, Italy). The device had a bandpass filter of 20–500 Hz, a notch filter of 60 Hz, and a sampling rate of 1,024 Hz.
Before the EMG assessment, the active range of motion for shoulder flexion and abduction was measured. The skin surface over the anterior and middle deltoid muscles was cleaned with alcohol, and hair was removed to minimize skin impedance. Bluetooth-enabled wireless surface EMG probes were attached parallel to the muscles, following established guidelines, including the Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) and the Anatomical Guide for the Electromyographer [21, 22]. The active electrode was positioned 5 cm distal to the acromion along the anterior margin of the anterior deltoid and at the greatest bulge of the middle deltoid, along the line between the acromion and the lateral epicondyle of the elbow. Surface EMG in this study was not intended as a diagnostic tool for C5 palsy but rather as a means to quantitatively evaluate neuromuscular recovery and task-oriented muscle activation during rehabilitation. Although surface EMG is not part of the standard diagnostic workflow for C5 palsy, it has been increasingly used in spinal cord injury and peripheral nerve disorders as a non-invasive biomarker of residual motor function and neural reorganization during recovery [23, 24].
Maximal voluntary isometric contraction (MVIC).
Patients were seated on a stationary stool and positioned with their shoulders externally rotated to 45°. They were instructed to abduct their shoulder to 50% of their active range of motion, exerting maximal effort against the therapist’s resistance for 5 s. Surface EMG signals were recorded bilaterally from the anterior and middle deltoid muscles using wireless electrodes placed according to SENIAM guidelines. MVIC was calculated as the root mean square of the middle 3 s of the EMG signal, excluding the first and last seconds. Data analysis was performed using BTS EMG-Analyzer software (BTS Bioengineering). Each measurement was repeated three times with a 30-second rest interval, and the average of these measurements was reported [25, 26].
-
2)
Percent maximal voluntary isometric contraction (%MVIC).
After a 1-minute rest following MVIC assessments, patients were instructed to abduct their shoulder in the same position as aforementioned while holding a 1-kg weight and maintaining 50% of their active range of motion for 5 s to measure the task-oriented EMG activity. The root mean square of the middle 3 s of the EMG signal was analyzed, and %MVIC was calculated as the ratio of task-oriented EMG activity to MVIC. Each task was performed three times with a 30-second rest interval, and the average %MVIC was calculated [27, 28].

A lower %MVIC indicated greater efficiency in completing tasks with reduced muscle activation.
-
3)
Fatigue index (FI).
Muscle endurance and contraction strength were evaluated using the FI. To evaluate muscle fatigue, FI was measured bilaterally from the anterior and middle deltoid muscles preoperatively and from the affected side at 24 weeks postoperatively. Patients performed the MVIC task for 1 min with resistive shoulder flexion (anterior deltoid) and abduction (middle deltoid). The median frequency (MDF) of the EMG signal for the initial 3 s (MDFinitial) and final 3 s (MDFfinal) was calculated, excluding the first and last 3 s to minimize noise and variability. A reduction in MDF over the duration of sustained muscle contraction is a well-recognized indicator of localized muscle fatigue due to decreased muscle fiber conduction velocity and altered motor unit recruitment patterns [29, 30]. Thus, FI was calculated based on changes in MDF from the beginning to the end of contraction. FI was determined as follows [29, 31]:

Surgical procedure and definition of C5 palsy
All participants exhibited multilevel spinal cord compression with foraminal stenosis and a marked reduction in interpedicular foraminal height due to disc height loss. Surgical interventions included staged anterior and posterior cervical spine fusion or laminoplasty. For cases of suspected bony compression during the anterior approach, uncinate process resection was performed at the affected segments.
C5 palsy was identified as a postoperative reduction of two or more grades on the Medical Research Council (MRC) scale for shoulder flexion or abduction compared to the preoperative baseline. Strength assessments were performed daily during the inpatient period by trained orthopedic clinicians. The diagnosis of C5 palsy was made when a noticeable motor deficit developed during hospitalization, typically within the first 3 to 5 days after surgery.
High-resolution VR equipment details
The immersive VR display system (VIVE Pro 1.0; HTC Corporation, Hong Kong, China) is a sophisticated VR headset featuring dual AMOLED 3.5-inch screens, each with a resolution of 1440 × 1600 pixels, delivering a combined total resolution of 2880 × 1600 pixels. The VR equipment included a headset, a controller (with an option to use either of the two provided), and two base stations. The controllers enhanced interactivity, enabling precise motion tracking and task execution in the virtual environment. The two base stations ensured accurate spatial tracking, covering a 5 × 5-m area. The headset operated at a refresh rate of 90 Hz with a 110-degree field of view, providing an immersive experience. It featured high-resolution certified audio capabilities, detachable headphones, and support for high-impedance headphones, ensuring high-quality sound. For sensory input tracking, the VR headset was equipped with a G-sensor (accelerometer), gyroscope, proximity sensor, and interpupillary distance sensor. It used SteamVR Tracking 1.0 (Valve Corporation, Bellevue, WAS, USA) for VR tracking software. The VR controllers were equipped with SteamVR Tracking 1.0 and included a multifunction trackpad, grip buttons, dual-stage triggers, system buttons, and menu buttons.
VR gameplay rules, procedure, and follow-up
The experimental group underwent VR-assisted rehabilitation using a head-mounted display with a handheld motion-tracking controller and a sensor for round-relative height detection. The VR system featured an interactive game designed to improve shoulder abduction and flexion. Each VR exercise cycle lasted 5 min and involved a mission where participants placed virtual apples into baskets. The process was as follows. At the start, baskets appeared and moved across the screen. Participants were instructed to pick apples from a box and place them into the moving baskets. The baskets alternated their movement every 10 s, randomly switching between left-to-right and right-to-left directions. The successful placement of an apple in a basket caused the basket’s height to increase by 2 cm; otherwise, the height remained unchanged. During each 5-minute session, 60 baskets appeared: 30 at the upper level and 30 at the lower level. The upper baskets started 80 cm above the ground and could rise to a maximum of 160 cm based on success. Each successful apple placement increased the basket’s height by 2 cm, while failures kept the height constant. The lower baskets started 60 cm above the ground. With each successful apple placement, the basket moved up by 2 cm; unsuccessful attempts caused the basket to rise by 2 cm.
The VR program consisted of two modes: active VR rehabilitation, where patients used only the affected arm, and assisted VR rehabilitation, which allowed the use of the unaffected arm to assist the affected arm. Patients performed VR exercises daily for 1 h during their first postoperative week of hospitalization. Approximately 1 week after surgery, patients were discharged and subsequently returned for outpatient VR rehabilitation sessions at 3, 6, 12, and 24 weeks postoperatively. During these follow-up visits, the patients participated in 1-hour VR exercise sessions in a controlled environment. Data collected included the maximum height reached by the arm and the number of apples placed in the basket per cycle (Supplementary Video 1). Both the VR and control groups received standardized postoperative exercise education starting from the immediate postoperative period, once C5 palsy was confirmed. Education included daily passive and active shoulder range of motion (ROM) exercises, all performed in a standing position with feet shoulder-width apart. For passive ROM exercise, patients were instructed to use a self-assisted method by leaning their body and affected arm against a wall to facilitate elevation of the shoulder through the available range. The movement was performed in both flexion and abduction directions. Patients were guided to raise the arm as high as possible, hold the position for 10 s, lower the arm, and rest for 10 s. Each session consisted of 10 repetitions per movement direction, and five sessions were performed daily. For active ROM, patients actively elevated the affected shoulder in flexion and abduction to their maximum available range and held the position for up to 10 s, or as long as tolerable. To avoid sudden loss of muscle tension and reduce the risk of ligament or tendon strain, patients were instructed to support the arm with the contralateral (sound-side) hand when lowering it. Each session consisted of five repetitions with 30-second intervals, and five sessions were prescribed daily. This standardized protocol reflects real-world clinical practice, where patients typically perform unsupervised home-based rehabilitation guided by initial education.
Primary outcomes and secondary outcomes
The primary outcome was the MVIC of the anterior and middle deltoid muscles, assessed using surface EMG data. Secondary outcomes included %MVIC, FI, MRC scales for affected shoulder flexion and abduction [32], and patient-reported outcomes, such as the Neck Disability Index (NDI) [33], EuroQol 5-Dimension Health Questionnaire (EQ-5D) [34], Visual Analog Scale (VAS) for neck and arm pain, and Hospital Anxiety and Depression Scale (HADS) [35].
The HADS consists of two subscales: HADS-A for anxiety and HADS-D for depression, each containing seven items scored from 0 to 3, with subscale scores summed to give a total ranging from 0 to 21. Higher scores indicate greater anxiety or depression, with scores above 10 suggesting clinically significant symptoms [36].
Data were collected at baseline and at 3, 6, 12, and 24 weeks postoperatively during outpatient follow-up visits. Secondary outcomes also incorporated functional assessments, such as the maximum height reached by the hand on the C5-palsy side and the number of apples placed in the basket during a single VR task cycle. These measures were compared between the “active” and “assisted” VR rehabilitation modes immediately postoperatively and at 3, 6, 12, and 24 weeks.
Statistical analysis/study size
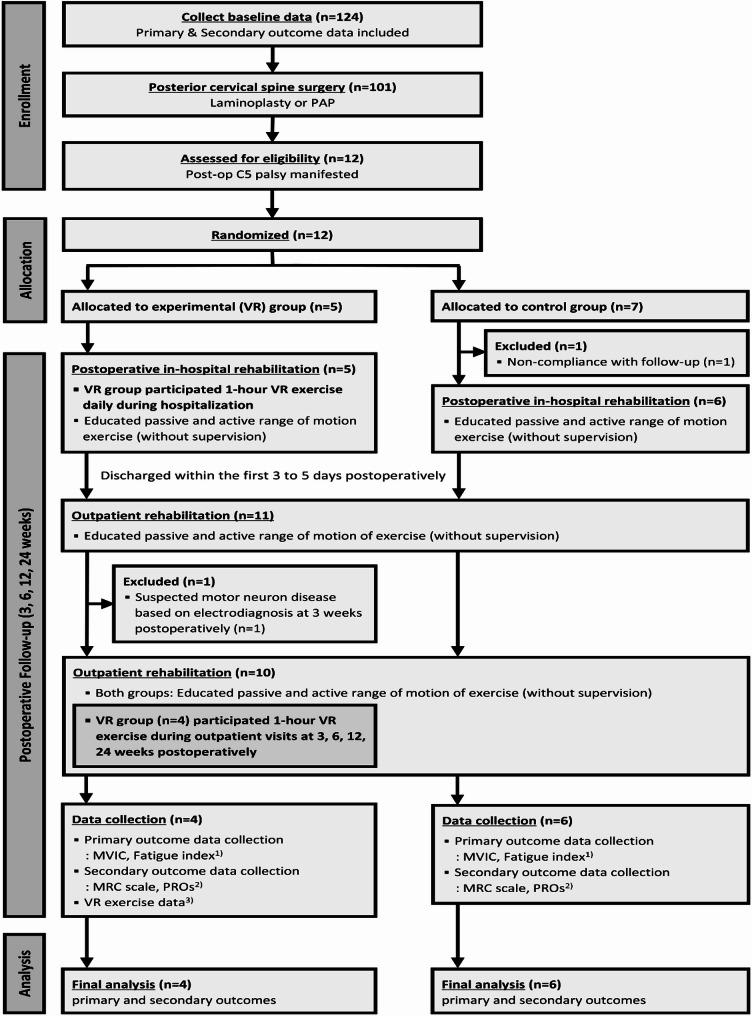
This study was designed as an exploratory, preliminary investigation into the feasibility and potential effects of VR-assisted rehabilitation in patients with postoperative C5 palsy. Due to the rarity of C5 palsy following posterior cervical surgery, a formal a priori power calculation was not performed. Instead, a pragmatic sample size of five participants per group was targeted to assess feasibility and explore trends. Intergroup comparisons for continuous variables were performed using the Mann–Whitney U test, and categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. The Wilcoxon signed-rank test was used to assess within-group differences over time. The primary outcome, maximal voluntary isometric contraction (MVIC), was assessed by calculating the 24-week/preoperative ratio. Given the small sample size, p-values were interpreted with caution. To further support the interpretation of between-group differences, Cohen’s d was calculated for key outcomes to estimate the magnitude of observed effects. Effect sizes were interpreted as small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8) according to standard conventions. This dual approach allows for a more nuanced interpretation of the data beyond statistical significance alone. Consequently, this study aimed to explore the potential effects of VR-assisted rehabilitation in a preliminary context, rather than to formally test a predefined hypothesis, due to the feasibility-oriented nature of the design and the limited patient population (Fig. 1).
Fig. 1.
CONSORT flow diagram for the VR-assisted rehabilitation study. This diagram outlines the randomized controlled trial process, including participant enrollment (N = 12), randomization into two groups (Control Group: N = 7; VR Group: N = 5), exclusions due to ineligibility or loss to follow-up (N = 2), completion of follow-up, and data analysis. All randomized participants completed the follow-up period and were included in the final analysis. Assessments included: (1) MVIC and FI using dynamic EMG analysis and power spectrum analysis; (2) patient-reported outcomes (PROs) such as NDI, EQ-5D, VAS, HADS-A, HADS-D; and, (3) performance metrics, including maximum height achieved and the number of apples placed in the basket during VR tasks. EQ-5D EuroQol 5-Dimension health questionnaire, FI fatigue index, HADS Hospital Anxiety and Depression Scale, PROs patient-reported outcomes, MVIC maximum voluntary isometric contraction, NDI Neck Disability Index, VAS Visual Analogue Scale, VR virtual reality
Results
Incidence and demographics of C5 palsy
During the study period, 124 patients underwent preoperative screening and 101 underwent posterior cervical spine surgery, among whom patients with postoperative C5 palsy were considered for enrollment. A total of 12 patients were enrolled, with two excluded from the final analysis: one from the VR group and one from the control group. Although exclusions are typically performed prior to randomization, in this study, the exclusion of the VR group participant occurred three weeks after allocation, as abnormal findings suggestive of motor neuron disease were only detectable at that time through needle electromyography, which generally requires at least three weeks following the onset of neurological deficits. In the control group, one patient was excluded after allocation due to non-compliance with follow-up, which occurred before any intervention was initiated. Data from 10 patients (Control Group: N = 6; VR Group: N = 4) were analyzed. Baseline characteristics were comparable between the Control and VR groups, including age (68.5 years [48.0–78.0] and 53.5 years [42.0–61.0], respectively, p = 0.054), height, weight, and comorbidities, such as diabetes mellitus (66.7% and 0%, respectively, p = 0.076). The median number of operated segments was 4.0 for both groups (Control: 3.0–6.0 and VR: 4.0, p = 0.694). Posterior-anteroposterior (PAP) fusion surgery was performed in all participants, while resection of the uncinate process was conducted in 60% of cases (66.7% in the Control group and 50% in the VR group). The duration of surgery (239.0 min [207.0–292.0] and 280.0 min [221.0–327.0], respectively, p = 0.336) and estimated blood loss (450.0 mL [150.0–700.0] and 425.0 mL [200.0–550.0], respectively, p = 0.747) were similar between the Control and VR groups (Table 1). To complement these group-level summaries, individual baseline data for each participant are listed in Table 2.
Table 1.
Demographic, surgical and postoperative data
| Variable | Total (N = 10) | Control (N = 6) | VR (N = 4) | P |
|---|---|---|---|---|
| N (%) or Median (range) | ||||
| Demographic data | ||||
| Sex (male, %) | 10 (100.0) | 6 (100.0) | 4 (100.0) | > 0.999 |
| Age (years) | 61.0 (42.0–78.0) | 68.5 (48.0–78.0) | 53.5 (42.0–61.0) | 0.054 |
| Height (cm) | 169.5 (160.0–190.0) | 169.0 (163.0–171.7) | 170.5 (160.0–190.0) | 0.668 |
| Weight (kg) | 70.0 (65.0–100.0) | 68.0 (65.0–91.9) | 80.0 (68.0–100.0) | 0.166 |
| Diabetes mellitus | 4 (40.0) | 4 (66.7) | 0 (0.0) | 0.076 |
| Hypertension | 3 (30.0) | 2 (33.3) | 1 (25.0) | > 0.999 |
| CVA | 1 (10.0) | 1 (16.7) | 0 (0.0) | > 0.999 |
| Surgical data | ||||
| Number of operated segments | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 4.0 (4.0–4.0) | 0.694 |
| Surgical procedure | ||||
| Resection of uncinate process | 6 (60.0) | 4 (66.7) | 2 (50.0) | 0.870 |
| Surgical approach | ||||
| PAP | 10 (100.0) | 6 (100.0) | 4 (100.0) | > 0.999 |
| Surgical level | 0.619 | |||
| C3-6 | 1 (10.0) | 1 (16.7) | 0 (0.0) | |
| C3-7 | 6 (60.0) | 2 (33.3) | 4 (100.0) | |
| C3-T1 | 1 (10.0) | 1 (16.7) | 0 (0.0) | |
| C3-T2 | 1 (10.0) | 1 (16.7) | 0 (0.0) | |
| C4-T1 | 1 (10.0) | 1 (16.7) | 0 (0.0) | |
| Duration of surgery (minutes) | 252.5 (207.0–327.0) | 239.0 (207.0–292.0) | 280.0 (221.0–327.0) | 0.336 |
| Estimated blood loss (mL) | 450.0 (150.0–700.0) | 450.0 (150.0–700.0) | 425.0 (200.0–550.0) | 0.747 |
| Postoperative data | ||||
| Palsy side (right side, %) | 4 (40.0) | 2 (33.3) | 2 (50.0) | > 0.999 |
| MRC grade at the time of paralysis | ||||
| Shoulder flexion | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 1.75 (1.0–2.0) | 0.640 |
| Shoulder abduction | 2.0 (1.0–2.0) | 1.67 (1.0–2.0) | 2.0 (2.0–2.0) | 0.175 |
Table 2.
Individual baseline data of the participants
| Patient No. | Group | Sex | Age (yr) | Height (cm) | Weight (kg) | Past history | Surgical data | Postoperative data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | HTN | CVA | No. of operated segment | Surgical level | Approach | Resection of uncinate process | Duration of surgery (min) | Estimated blood loss (mL) | Palsy side | MRC grade at the time of paralysis | |||||||
| Shoulder flexion | Shoulder abduction | ||||||||||||||||
| 1 | Control | M | 48 | 172 | 92 | + | – | – | 4 | C3-7 | PAP | + | 239 | 400 | Left | 1 | 2 |
| 2 | Control | M | 61 | 171 | 71 | + | – | – | 4 | C3-7 | PAP | + | 292 | 700 | Right | 1 | 2 |
| 3 | Control | M | 67 | 165 | 67 | + | – | – | 5 | C3-T1 | PAP | - | 237 | 700 | Left | 2 | 1 |
| 4 | Control | M | 70 | 169 | 65 | + | + | + | 4 | C4-T1 | PAP | + | 280 | 500 | Left | 4 | 1 |
| 5 | Control | M | 77 | 163 | 66 | – | + | – | 6 | C3-T2 | PAP | - | 207 | 400 | Left | 2 | 2 |
| 6 | Control | M | 78 | 169 | 69 | – | – | – | 3 | C3-6 | PAP | + | 239 | 150 | Right | 2 | 2 |
| 7 | VR | M | 42 | 190 | 100 | – | – | – | 4 | C3-7 | PAP | + | 266 | 300 | Left | 2 | 2 |
| 8 | VR | M | 47 | 160 | 68 | – | + | – | 4 | C3-7 | PAP | + | 294 | 550 | Right | 2 | 2 |
| 9 | VR | M | 60 | 170 | 78 | – | – | – | 4 | C3-7 | PAP | – | 327 | 550 | Left | 1 | 2 |
| 10 | VR | M | 61 | 171 | 82 | – | – | – | 4 | C3-7 | PAP | – | 221 | 200 | Right | 2 | 2 |
CVA cerebrovascular accident, PAP posterior-anteroposterior procedure, MRC Medical Research Council, min minimum, max maximum.
For the reader’s convenience, patient numbers were ordered by group and age, rather than by enrollment sequence. All participants had a preoperative MRC grade of 5 for both shoulder flexion and abduction, indicating no muscle weakness in these movements prior to surgery.
CVA cerebrovascular accident, DM diabetes mellitus, HTN hypertension, min minute, MRC Medical Research Council, VR virtual reality group, yr year.
Preoperative surface electromyography data
Prior to the initiation of any rehabilitation intervention, surface EMG data were collected from all participants to establish baseline muscle function. Preoperative MVIC values of the anterior deltoid during shoulder flexion were 415.2 µV (157.3–991.3) in the control group and 263.9 µV (92.1–545.8) in the VR group. The corresponding %MVIC values were 56.0% (14.2–81.4) and 74.4% (30.5–123.7), respectively. Similarly, for the middle deltoid, preoperative MVIC values were 422.5 µV (115.1–669.4) in the control group and 256.1 µV (154.8–628.1) in the VR group, with %MVIC values of 39.6% (11.6–61.1) and 47.3% (25.9–94.6). The FI of the anterior deltoid at baseline was 4.9% (1.9–5.5) in the control group and 3.9% (0.8–5.2) in the VR group. For the middle deltoid, the FI was 5.5% (–1.5–7.3) and 9.7% (1.1–10.2) for control and VR groups, respectively (Table 3).
Table 3.
The effect of VR on muscle activities
| Preop | POD 24 wks | POD 24 wks/Preop | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action | Variable | Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | ||
| Shoulder flexion | MVICant.del | 415.2 (157.3–991.3) | 263.9 (92.1–545.8) | 0.456 | 0.673 | 419.3 (151.5–663.9) | 232.6 (48.1–425.3) | 0.337 | 0.877 | 1.2 (0.2–2.9) | 0.8 (0.5–1.0) | 0.594 | 0.71 | ||
| %MVICant.del | 56.0 (14.2–81.4) | 74.4 (30.5–123.7) | 0.241 | 0.701 | 86.8 (14.2–130.1) | 75.4 (63.3–98.7) | 0.594 | 0.152 | 1.5 (1.0–2.0) | 1.0 (0.8–2.2) | 0.337 | 0.456 | |||
| MVICmid.del | 422.5 (115.1–669.4) | 256.1 (154.8–628.1) | 0.915 | 0.233 | 408.3 (83.2–602.2) | 409.9 (46.4–642.6) | 0.915 | 0.004 | 1.0 (0.2–2.8) | 0.8 (0.3–3.7) | 0.915 | 0.001 | |||
| %MVICmid.del | 39.6 (11.6–61.1) | 47.3 (25.9–94.6) | 0.594 | 0.596 | 60.9 (11.7–79.1) | 47.7 (17.5–67.3) | 0.594 | 0.399 | 1.2 (1.0–2.0) | 0.8 (0.7–1.1) | 0.025* | 1.64 | |||
| FIant.del | 4.9 (1.9–5.5) | 3.9 (0.8–5.2) | 0.551 | 0.526 | -0.3 (-8.9–10.7) | 3.4 (0.6–13.8) | 0.594 | 0.651 | -1.2 (-2.5–2.2) | 3.6 (0.1–3.6) | 0.136 | 1.269 | |||
| Shoulder abduction | MVICant.del | 417.8 (163.5–988.2) | 266.4 (92.4–543.0) | 0.456 | 0.679 | 420.3 (154.2–654.9) | 234.2 (46.6–427.3) | 0.337 | 0.874 | 1.2 (0.2–2.9) | 0.8 (0.5–1.0) | 0.594 | 0.7 | ||
| %MVICant.del | 51.9 (43.9–129.8) | 68.7 (36.2–105.9) | > 0.999 | 0.177 | 73.9 (59.8–136.6) | 81.4 (52.5–95.1) | 0.915 | 0.222 | 1.4 (0.7–2.6) | 1.1 (0.9–1.9) | 0.749 | 0.408 | |||
| MVICmid.del | 422.8 (115.0–737.1) | 256.8 (155.0–633.9) | 0.915 | 0.265 | 405.9 (86.8–603.4) | 409.0 (46.4–655.1) | 0.915 | 0.006 | 1.0 (0.2–2.7) | 0.8 (0.3–3.6) | 0.915 | 0.003 | |||
| %MVICmid.del | 45.0 (37.3–63.2) | 68.3 (39.5–84.0) | 0.11 | 1.145 | 91.9 (44.8–125.0) | 60.0 (25.2–75.6) | 0.11 | 1.268 | 1.8 (1.0–3.2) | 0.9 (0.6–0.9) | 0.014* | 1.966 | |||
| FImid.del | 5.5 (-1.5–7.3) | 9.7 (1.1–10.2) | 0.371 | 0.499 | 6.0 (-14.8–18.0) | 7.8 (2.2–10.5) | > 0.999 | 0.358 | 0.0 (-8.3–3.3) | 1.0 (1.0–2.0) | 0.766 | 0.625 | |||
Values are mean rank (min-max)
MVIC Maximum Voluntary Isometric Contraction (kg), %MVIC task-related EMG activity normalized to MVIC (%), ant.del anterior deltoid, mid. del middle deltoid, FI fatigue index, POD postoperative day, Preop. preoperative day 1, wks weeks. VR virtual reality
*, P < 0.05.
Muscle strength and activities
Recovery of muscle strength, assessed via the MRC scale, showed substantial improvements in deltoid function in both groups by 24 weeks postoperatively. Median MRC grades for shoulder flexion and abduction reached 4.5–5.0, indicating near-complete recovery. No significant differences were observed in MRC grade recovery trajectories between the VR and Control groups. Surface EMG–based assessments revealed no significant group differences in absolute MVIC values during either shoulder flexion or abduction at baseline or 24 weeks. However, at 24 weeks, the %MVIC of the middle deltoid during flexion was significantly lower in the VR group than in the Control group (1.2 [1.0–2.0] vs. 0.8 [0.7–1.1], p = 0.025), indicating greater muscle efficiency. Similarly, the %MVIC ratio for shoulder abduction (POD 24 weeks/Preop) was significantly lower in the VR group than in the Control group (1.8 [1.0–3.2] vs. 0.9 [0.6–0.9], p = 0.014). FI, reflecting muscular endurance, remained comparable between groups at all time points (Table 3).
Patient-reported outcomes
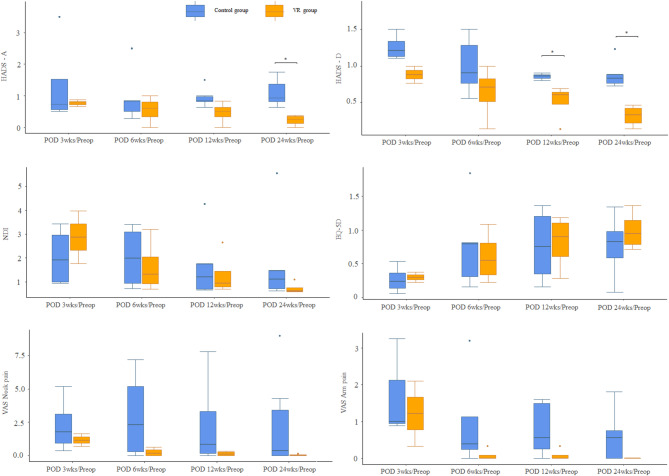
The VR group demonstrated notable improvements in patient-reported outcomes compared with the Control group (Table 4; Fig. 2). (a) NDI: Functional disability scores improved significantly in the VR group compared with the Control group from preoperative values to 24 weeks postoperatively (40.0 [18.0–78.0] vs. 20.0 [12.0–22.0], p = 0.069). (b) EQ-5D index: Quality of life scores favored the VR group over the Control group at 24 weeks (0.5 [0.0–0.7] vs. 0.7 [0.6–0.8], p = 0.032). (c) VAS (pain): Significant reductions in arm pain were observed in the VR group compared with the Control group at 12 weeks (35.0 [0.0–80.0] vs. 0.0 [0.0–10.0], p = 0.048), with sustained results at 24 weeks. Neck pain reduction, though more pronounced in the VR group than in the Control group, was not statistically significant. (d) HADS: Anxiety (HADS-A) and depression (HADS-D) scores were lower in the VR group than in the Control group at 24 weeks, although the differences were not statistically significant. These findings suggest that VR-assisted rehabilitation provides both physical and psychological benefits for patients with C5 palsy (Table 4). Ratio analyses revealed significant improvements in HADS-D (depression subscale) at 12 and 24 weeks (12 weeks: VR: 0.6 [0.5–0.9] vs. Control: 0.9 [0.8–1.1], p < 0.05; 24 weeks: VR: 0.3 [0.2–0.6] vs. Control: 0.8 [0.6–1.0], p < 0.01). Similarly, the HADS-A (anxiety subscale) ratio at 24 weeks (POD 24 weeks/Preop) was significantly lower in the VR group than in the Control group (0.5 [0.3–0.7] vs. 0.9 [0.7–1.1], p < 0.05), indicating reduced anxiety.
Table 4.
Comparison of patient–reported outcomes and muscle strength between control and VR groups
| PROs & MRC scale | Preop | POD 3 wks | POD 6 wks | POD 12 wks | POD 24 wks | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | Control (N = 6) | VR (N = 4) | P | Cohen’s d | ||
| NDI | 31.0 (12.0–76.0) | 25.0 (18.0–36.0) | > 0.999 | 0.59 | 52.0 (34.0–66.0) | 68.0 (64.0–72.0) | 0.247 | 1.461 | 48.0 (24.0–56.0) | 44.0 (14.0–60.0) | 0.902 | 0.256 | 41.0 (20.0–60.0) | 31.0 (14.0–48.0) | 0.334 | 0.656 | 40.0 (18.0–78.0) | 20.0 (12.0–22.0) | 0.069 | 1.481 | |
| Equation 5D | 0.6 (0.1–0.8) | 0.8 (0.6–1.0) | 0.285 | 0.967 | 0.1 (0.0–0.4) | 0.2 (0.2–0.2) | 0.481 | 0.143 | 0.2 (0.1–0.6) | 0.5 (0.2–0.8) | 0.389 | 0.446 | 0.4 (0.1–0.6) | 0.7 (0.2–0.8) | 0.069 | 0.957 | 0.5 (0.0–0.7) | 0.7 (0.6–0.8) | 0.032* | 1.737 | |
| VAS_Neck | 15.0 (10.0–70.0) | 70.0 (40.0–90.0) | 0.067 | 1.635 | 50.0 (17.0–80.0) | 53.0 (41.0–65.0) | > 0.999 | 0.172 | 46.0 (0.0–72.0) | 10.0 (0.0–25.0) | 0.262 | 1.141 | 29.5 (0.0–78.0) | 4.0 (0.0–19.0) | 0.322 | 1.074 | 21.5 (0.0–90.0) | 0.0 (0.0–9.0) | 0.337 | 1.07 | |
| VAS_Arm | 56.0 (0.0–90.0) | 55.0 (30.0–70.0) | > 0.999 | 0.079 | 63.5 (51.0–80.0) | 41.5 (20.0–63.0) | 0.487 | 0.996 | 22.0 (0.0–64.0) | 0.0 (0.0–10.0) | 0.073 | 1.43 | 35.0 (0.0–80.0) | 0.0 (0.0–10.0) | 0.048* | 1.739 | 29.5 (0.0–90.0) | 0.0 (0.0–0.0) | 0.072 | 1.336 | |
| HADS-A/21 | 10.0 (2.0–21.0) | 11.0 (4.0–18.0) | > 0.999 | < 0.001 | 5.5 (4.0–14.0) | 13.0 (12.0–14.0) | 0.34 | 1.651 | 5.0 (2.0–18.0) | 7.0 (0.0–12.0) | > 0.999 | 0.226 | 8.5 (3.0–18.0) | 6.5 (0.0–9.0) | 0.392 | 0.791 | 10.0 (3.0–21.0) | 3.5 (0.0–6.0) | 0.109 | 1.399 | |
| HADS-D/21 | 12.0 (7.0–21.0) | 10.5 (7.0–17.0) | 0.667 | 0.305 | 11.0 (9.0–17.0) | 13.0 (13.0–13.0) | 0.481 | 0.397 | 12.0 (5.0–19.0) | 9.0 (1.0–11.0) | 0.27 | 0.845 | 10.0 (6.0–19.0) | 7.0 (1.0–10.0) | 0.241 | 1.04 | 9.5 (6.0–21.0) | 4.0 (1.0–7.0) | 0.067 | 1.541 | |
| MRC grade for flexion | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | > 0.999 | 3.0 (2.0–4.0) | 2.5 (2.0–3.0) | 0.556 | 0.497 | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.909 | 0.072 | 3.5 (3.0–5.0) | 4.0 (3.0–5.0) | 0.568 | 0.408 | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 0.878 | 0.183 | ||
| MRC grade for abduction | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | > 0.999 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.817 | 0.212 | 3.0 (3.0–4.0) | 3.0 (3.0–4.0) | 0.878 | 0.183 | 4.0 (3.0–5.0) | 4.5 (4.0–5.0) | 0.556 | 0.497 | 5.0 (4.0–5.0) | 4.5 (4.0–5.0) | 0.350 | 0.667 | ||
Values are mean rank (min-max)
NDI neck disability index, Eq. 5D EuroQol-5 dimension scale, VAS visual analog scale, HADS-A hospital anxiety and depression scale - anxiety subscale, HADS-D hospital anxiety and depression scale - depression subscale, MRC Medical Research Council, POD postoperative day, Preop preoperative day 1, wks weeks, FI fatigue index, VR virtual reality, min minimum, max maximum, PROs patient-reported outcomes. *, P < 0.05
Fig. 2.
Proportions of patients with clinically meaningful changes in patient-reported outcomes at postoperative time points compared to the preoperative baseline. HADS-A Hospital Anxiety and Depression Scale - Anxiety, HADS-D Hospital Anxiety and Depression Scale - Depression, NDI Neck Disability Index, EQ-5D EuroQol 5-Dimension health questionnaire, VAS Visual Analog Scale, Preop Preoperative, POD postoperative day
For other patient-reported outcomes, including the NDI, EQ-5D, and VAS scores for neck and arm pain, ratio analyses did not reveal statistically significant differences between the groups at any postoperative time point. However, trends generally favored the VR group over the Control group. For instance, the NDI ratio at 24 weeks (POD 24 weeks/Preop) was lower in the VR group than in the Control group (0.5 [0.4–0.6] vs. 0.7 [0.6–0.8]), and the EQ-5D ratio was higher in the VR group than in the Control group (1.4 [1.3–1.5] vs. 1.2 [1.1–1.3]) (Fig. 2).
VR-specific rehabilitation metrics
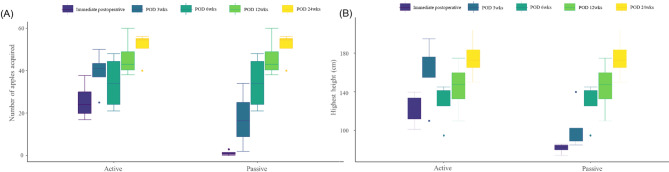
In the VR group, rehabilitation performance metrics showed significant improvements over time, reflecting enhanced motor coordination and engagement throughout the program. Patients participated in gamified VR exercises, where they placed virtual apples into baskets at varying heights. Performance metrics were recorded at each follow-up interval.
At the start of the program, the maximum height reached by the affected hand was 90.0 cm (median, range 80.0–95.0 cm). By 24 weeks postoperatively, this height increased to 145.0 cm (median, range 130.0–160.0 cm). Similarly, the number of successfully placed apples increased substantially over time. Initially, the median number of apples placed in the basket during a single cycle was 25 (range: 20–30). By 24 weeks, this number increased to 55 (range, 50–60), with most patients achieving near-maximal success rates (Fig. 3).
Fig. 3.
Changes in performance metrics over time in the VR rehabilitation group, comparing active and assisted modes. (A) Number of apples collected and (B) maximum height of the paralyzed arm elevation. POD Postoperative day, wks weeks
Discussion
C5 palsy following cervical spine decompression or fusion surgery presents significant clinical and psychological challenges. Despite preoperatively counseling about the potential risks of such complications, the development of C5 palsy often induces substantial anxiety and distress [4, 5, 9]. This psychological burden arises from the sudden and often unexpected weakness in the deltoid and biceps muscles, which severely limits shoulder abduction and flexion, impairing daily activities and overall quality of life [3]. However, the pathophysiology of C5 palsy is not fully understood, with proposed mechanisms including direct nerve root traction, ischemic injury, and reperfusion injury [1, 9]. These factors complicate the development of targeted rehabilitation strategies.
Traditional rehabilitation approaches have often been passive, focusing on basic strengthening and range of motion exercises without sufficient stimulation to promote neural plasticity and muscle re-education [37]. In contrast, this study introduces VR-assisted rehabilitation as a novel approach to enhance motor recovery and alleviate the psychological burden associated with C5 palsy. One important consideration is that the VR group received approximately one additional hour of rehabilitation per session compared to the control group. Prior research has demonstrated that greater physical therapy duration is associated with improved functional outcomes, as noted in a systematic review by Peiris et al. [38]. Therefore, it is possible that the enhanced outcomes observed in the VR group may be partly due to the increased duration of task-specific motor activity, rather than the VR modality per se. However, unlike the therapist-supervised sessions analyzed in that review, both groups in our study received only education-based home rehabilitation, and only the VR group was provided with interactive, gamified feedback and progressive task difficulty. This distinction suggests that both duration and qualitative differences in rehabilitation approach may have contributed to the improved outcomes. Research has indicated that VR technology has the potential to revolutionize rehabilitation by providing an immersive and interactive environment that engages multiple sensory modalities, including visual, auditory, and proprioceptive feedback. Multisensory engagement enhances neuroplasticity and facilitates effective motor learning [13, 14, 19].
In this study, patients in the VR group exercised with real-time feedback, enabling them to track their progress and adjust their efforts accordingly. These patients demonstrated significant improvements in muscle activity compared with the Control group, likely due to enhanced neuromuscular performance. Although the VR program did not directly improve the MVIC of the deltoid muscle in patients with C5 palsy, the observed reduction in %MVIC in the VR group, when accompanied by improved MRC grades and shoulder function, may suggest more coordinated or efficient neuromuscular activation [39]; however, this interpretation should be viewed cautiously and warrants further study. These findings underscore the ability of VR to deliver targeted, high-intensity neurostimulation, which is crucial for recovering functional muscle activity and may contribute to improved performance in daily activities. These results align with previous studies demonstrating the efficacy of VR in enhancing motor function in various neurological conditions [40].
However, the lack of improvement in MVIC itself may be attributed to considerable interindividual variability, sparse assessment intervals, and the small sample size of this pilot study, which limits the generalizability of the findings. Future research with larger sample sizes is needed to explore whether VR programs significantly impact MVIC and FI and to identify the optimal timing for VR implementation following injury.
Although this study did not quantify patient engagement or motivation, the VR group’s favorable trend in HADS scores may reflect a greater emotional receptivity to the rehabilitation process, as previously reported in studies exploring gamified therapies [19, 39, 41]. Interactive tasks, such as “putting apples in the basket,” not only provided physical challenges but also made the exercises more enjoyable and engaging, leading to better adherence and improved outcomes. Beyond the physical benefits, the psychological advantages of VR-assisted rehabilitation are noteworthy. Patients with C5 palsy often face uncertainty regarding the extent of their paralysis and prospects for recovery. This uncertainty, coupled with the variable timeline for neurological recovery–which can range from weeks to months–contributes to heightened anxiety and a risk of depression [42]. Thus, addressing these psychological aspects is crucial. The HADS is particularly relevant in this context, with HADS-A assessing anxiety and HADS-D evaluating depression [43]. In this study, the VR-assisted rehabilitation group showed a notable reduction in both HADS-A and HADS-D scores compared with the control group, indicating its effectiveness in alleviating psychological distress during recovery. We posit that the immersive nature of VR likely distracted patients from their discomfort and disability, fostering a more positive rehabilitation experience. Furthermore, the ability of VR to provide a safe and controlled environment for practicing motor skills can boost patient confidence and reduce psychological barriers to recovery [44, 45].
Limitations
This study has several limitations. First, the small sample size limits both the generalizability and statistical power of the results. Due to the low incidence of postoperative C5 palsy and the challenges of enrolling affected patients, who are often physically and emotionally vulnerable, this study was conducted as a preliminary pilot trial without a predefined power calculation. While appropriate statistical methods, including non-parametric tests, were applied, the findings should be interpreted with caution and viewed as an initial step toward future, larger-scale research. Second, group-level baseline imbalances may have influenced the outcomes. The VR group included relatively younger patients and had fewer comorbidities, notably a complete absence of diabetes, which could independently contribute to improved recovery. Although no statistically significant differences were observed in baseline characteristics (Table 1), such clinical imbalances are relevant and may confound outcome interpretation. Given the small sample size and exploratory nature of this pilot study, random variability in group composition was unavoidable. These limitations highlight the need for cautious interpretation and underscore the importance of future trials with larger, randomized cohorts and stratified or covariate-adjusted analyses. Third, although both groups received standardized rehabilitation education comprising range-of-motion exercises, the VR group received an additional hour of guided intervention. This discrepancy in total therapy dosage may have contributed to outcome differences beyond the VR modality itself. Fourth, while preoperative electrodiagnostic testing helped exclude patients with underlying neuromuscular diseases, the interpretation of surface EMG findings, such as %MVIC and MDF, as indicators of neuromuscular efficiency and fatigue remains exploratory. These parameters should be validated in larger, more homogeneous cohorts with appropriate control comparisons. Fifth, the use of a single VR system limited the frequency and customization of interventions. Future studies may benefit from more flexible VR platforms that allow frequent, home-based rehabilitation supported by web or Bluetooth interfaces, enabling remote monitoring and tailored feedback. Lastly, the rapid advancement of VR and digital rehabilitation technologies presents both opportunities and challenges. Integration with artificial intelligence and machine learning could support adaptive, personalized rehabilitation strategies that enhance patient engagement and functional recovery.
Conclusions
This pilot study suggests that VR-assisted rehabilitation may offer potential benefits for improving motor function and emotional well-being in patients with postoperative C5 palsy, though larger, adequately powered studies are necessary to confirm these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
J.K. conceived and designed the study. K.S.S., J.P., and T.C. contributed to data acquisition and patient management. S.H.M., H.S.K., and S.Y.P. supervised surgical procedures. B.H.L. and Y.A. performed the clinical evaluations and coordinated follow-up visits. S.Y. conducted all physical therapy sessions and performed surface EMG assessments. J.K. and J.P. analyzed and interpreted the data. J.K. drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version for submission.Kyung Soo Suk and Jinyoung Park contributed equally as co-first authors.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Yonsei University College of Medicine institutional review board (approval number: 3-2022-0070).
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests in relation to the present study, including financial, consultant, institutional, and other relationships.
Footnotes
Kyung Soo Suk and Jinyoung Park have contributed equally as co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barak U, Sheinis D, Sidon E, Shemesh S, Amitai A, Ohana N. C5 palsy following cervical spine decompression. Isr Med Assoc J. 2021;23(8):521–5. [PubMed] [Google Scholar]
- 2.Imagama S, Matsuyama Y, Yukawa Y, Kawakami N, Kamiya M, Kanemura T, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br. 2010;92(3):393–400. [DOI] [PubMed] [Google Scholar]
- 3.Nassr A, Eck JC, Ponnappan RK, Zanoun RR, Donaldson WF 3rd, Kang JD. The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976). 2012;37(3):174–8. [DOI] [PubMed] [Google Scholar]
- 4.Jack A, Ramey WL, Dettori JR, Tymchak ZA, Oskouian RJ, Hart RA, et al. Factors associated with C5 palsy following cervical spine surgery: A systematic review. Global Spine J. 2019;9(8):881–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traynelis VC, Fontes RBV, Kasliwal MK, Ryu WHA, Tan LA, Witiw CD, et al. Risk factors for C5 palsy: a systematic review and multivariate analysis. J Neurosurg Spine. 2024;40(2):216–28. [DOI] [PubMed] [Google Scholar]
- 6.Choi JH, Birring PS, Lee J, Hashmi SZ, Bhatia NN, Lee YP, Correction. A comparison of short-term outcomes after surgical treatment of multilevel degenerative cervical myelopathy in the geriatric patient population: an analysis of the National surgical quality improvement program database 2010–2020. Asian Spine J. 2024;18(3):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsumi K, Yamazaki A, Watanabe K, Ohashi M, Shoji H. Analysis of C5 palsy after cervical open-door laminoplasty: relationship between C5 palsy and foraminal stenosis. J Spinal Disord Tech. 2013;26(4):177–82. [DOI] [PubMed] [Google Scholar]
- 8.Thompson SE, Smith ZA, Hsu WK, Nassr A, Mroz TE, Fish DE, et al. C5 palsy after cervical spine surgery: A multicenter retrospective review of 59 cases. Global Spine J. 2017;7(1 Suppl):s64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto M, Mochizuki M, Aiba A, Okawa A, Hayashi K, Sakuma T, et al. C5 palsy following anterior decompression and spinal fusion for cervical degenerative diseases. Eur Spine J. 2010;19(10):1702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande N, Stino AM, Smith BW, Little AA, Yang LJS, Park P, et al. Defining postoperative C5 palsy and recovery: a systematic review. J Neurosurg Spine. 2023;38(4):457–64. [DOI] [PubMed] [Google Scholar]
- 11.Cano Porras D, Sharon H, Inzelberg R, Ziv-Ner Y, Zeilig G, Plotnik M. Advanced virtual reality-based rehabilitation of balance and gait in clinical practice. Ther Adv Chronic Dis. 2019;10:2040622319868379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallari B, Spaeth EK, Goh H, Boyd BS. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res. 2019;12:2053–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menin A, Torchelsen R, Nedel L. An analysis of VR technology used in immersive simulations with a serious game perspective. IEEE Comput Graph Appl. 2018;38(2):57–73. [DOI] [PubMed] [Google Scholar]
- 14.Pandrangi VC, Gaston B, Appelbaum NP, Albuquerque FC Jr., Levy MM, Larson RA. The application of virtual reality in patient education. Ann Vasc Surg. 2019;59:184–9. [DOI] [PubMed] [Google Scholar]
- 15.Park MJ, Kim DJ, Lee U, Na EJ, Jeon HJ. A literature overview of virtual reality (VR) in treatment of psychiatric disorders: recent advances and limitations. Front Psychiatry. 2019;10:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan A, Imam YZ, Muneer M, Al Jerdi S, Gill SK. Virtual reality in stroke recovery: a meta-review of systematic reviews. Bioelectronic Med. 2024;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017;11(11):Cd008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin MF, Weiss PL, Keshner EA. Emergence of virtual reality as a tool for upper limb rehabilitation: incorporation of motor control and motor learning principles. Phys Ther. 2015;95(3):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riva G, Serino S. Virtual reality in the assessment, Understanding and treatment of mental health disorders. J Clin Med. 2020;9(11). [DOI] [PMC free article] [PubMed]
- 20.Rubin DI. Brachial and lumbosacral plexopathies: A review. Clin Neurophysiol Pract. 2020;5:173–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank B Underwood. Anatomical guide for the electromyographer: the limbs and trunk. 4th ed: Charles C Thomas Pub Ltd; 2006;86(7):1043. .
- 22.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–74. [DOI] [PubMed] [Google Scholar]
- 23.Kubota S, Kadone H, Shimizu Y, Takahashi H, Koda M, Miura K, et al. Robotic shoulder rehabilitation with the hybrid assistive limb in a patient with delayed recovery after postoperative C5 palsy: A case report. Front Neurol. 2021;12:676352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturma A, Hruby LA, Prahm C, Mayer JA, Aszmann OC. Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: protocols for clinical application. Front Neurosci. 2018;12:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, Jo ME. Comparison of maximum voluntary isometric contraction of the biceps on various posture and respiration conditions for normalization of electromyography data. J Phys Ther Sci. 2016;28(11):3007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meldrum D, Cahalane E, Conroy R, Fitzgerald D, Hardiman O. Maximum voluntary isometric contraction: reference values and clinical application. Amyotroph Lateral Scler. 2007;8(1):47–55. [DOI] [PubMed] [Google Scholar]
- 27.Bouillon LE, Wilhelm J, Eisel P, Wiesner J, Rachow M, Hatteberg L. Electromyographic assessment of muscle activity between genders during unilateral weight-bearing tasks using adjusted distances. Int J Sports Phys Ther. 2012;7(6):595–605. [PMC free article] [PubMed] [Google Scholar]
- 28.Brorsson S, Nilsdotter A, Thorstensson C, Bremander A. Differences in muscle activity during hand-dexterity tasks between women with arthritis and a healthy reference group. BMC Musculoskelet Disord. 2014;15(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrov GV, Arabadzhiev TI, Mileva KN, Bowtell JL, Crichton N, Dimitrova NA. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med Sci Sports Exerc. 2006;38(11):1971–9. [DOI] [PubMed] [Google Scholar]
- 30.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol (1985). 2004;96(4):1486–95. [DOI] [PubMed] [Google Scholar]
- 31.González-Izal M, Malanda A, Navarro-Amézqueta I, Gorostiaga EM, Mallor F, Ibañez J, et al. EMG spectral indices and muscle power fatigue during dynamic contractions. J Electromyogr Kinesiol. 2010;20(2):233–40. [DOI] [PubMed] [Google Scholar]
- 32.Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the medical research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40(8):665–71. [DOI] [PubMed] [Google Scholar]
- 33.Gabel CP, Cuesta-Vargas AI, Osborne JW, Burkett B, Melloh M. Confirmatory factory analysis of the neck disability index in a general problematic neck population indicates a one-factor model. Spine J. 2014;14(8):1410–6. [DOI] [PubMed] [Google Scholar]
- 34.Soer R, Reneman MF, Speijer BL, Coppes MH, Vroomen PC. Clinimetric properties of the EuroQol-5D in patients with chronic low back pain. Spine J. 2012;12(11):1035–9. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 36.Stern AF. The hospital anxiety and depression scale. Occup Med. 2014;64(5):393–4. [DOI] [PubMed] [Google Scholar]
- 37.Pennington Z, Lubelski D, Westbroek EM, Ahmed AK, Ehresman J, Goodwin ML, et al. Time to recovery predicted by the severity of postoperative C5 palsy. J Neurosurg Spine. 2020;32(2):191–9. [DOI] [PubMed] [Google Scholar]
- 38.Peiris CL, Taylor NF, Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabil. 2011;92(9):1490–500. [DOI] [PubMed] [Google Scholar]
- 39.Hunter SK, Duchateau J, Enoka RM. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev. 2004;32(2):44–9. [DOI] [PubMed] [Google Scholar]
- 40.Massetti T, da Silva TD, Crocetta TB, Guarnieri R, de Freitas BL, Bianchi Lopes P, et al. The clinical utility of virtual reality in neurorehabilitation: A systematic review. J Cent Nerv Syst Dis. 2018;10:1179573518813541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Yu S. The technology to enhance patient motivation in virtual reality rehabilitation: A review. Games Health J. 2024;13(4):215–33. [DOI] [PubMed] [Google Scholar]
- 42.De Miguel-Rubio A, Alba-Rueda A, Millán-Salguero EM, De Miguel-Rubio MD, Moral-Munoz JA, Lucena-Anton D. Virtual reality for upper limb rehabilitation in patients with obstetric brachial palsy: systematic review and Meta-Analysis of randomized controlled trials. J Med Internet Res. 2023;25:e47391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The hospital anxiety and depression scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateni H, Carruthers J, Mohan R, Pishva S. Use of virtual reality in physical therapy as an intervention and diagnostic tool. Rehabil Res Pract. 2024;2024:1122286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long Y, Ouyang R-g, Zhang J-q. Effects of virtual reality training on occupational performance and self-efficacy of patients with stroke: a randomized controlled trial. J Neuroeng Rehabil. 2020;17(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.