Abstract
Objective
Although pathophysiologic alterations during acute HIV infection (AHI) may have long-term neuropsychiatric consequences, scant research has examined biobehavioral predictors of distinct depressive symptom trajectories from AHI through suppressive anti-retroviral therapy (ART), despite the fact that depressive symptoms have been associated with poorer adherence to ART and overall quality of life.
Methods
This analysis utilized data from the RV254/SEARCH010 Cohort, a large, well-characterized AHI cohort in Bangkok, Thailand. Longitudinal hierarchal density-based spatial clustering with uniform manifold approximation and projection was used to examine depressive symptom trajectories from AHI through 96 weeks of suppressive ART. Logistic regressions examined the immunologic and behavioral correlates of persistently high (versus low) depressive symptom trajectories.
Results
Participants (N = 502) were between the ages of 18 and 70, with a mean age of 27.7 (SD 7.4). The sample was predominantly male (98 %, n = 494). Three depressive symptom trajectories emerged: Cluster 1 (n = 257, 51 %) persistently high, Cluster 2 (n = 61, 12 %) transient, and Cluster 3 (n = 184, 37 %) persistently low. There were greater odds of persistently high (versus low) depressive symptoms for every log10copies/mL increase in plasma viral load (Odds Ratio [OR] = 1.27; 95 % Confidence Interval [CI] = 0.99–3.35) and sTNF-αRII (OR = 1.23, 95 % CI = 1.04, 1.45) at baseline. Recent amyl nitrite use also increased risk (OR = 2.39; 95 % CI = 1.17, 4.88).
Conclusions
Viral load, inflammation and substance use may be viable targets for reducing risk for depressive disorders in people with HIV, even in its earliest stages.
Keywords: Acute HIV infection, depression, Inflammation, Chemsex
Highlights
-
•
Alterations during acute HIV infection may have long-term mental health consequences.
-
•
Cluster analysis revealed 3 distinct depressive symptom trajectories.
-
•
Higher inflammation among individuals that reported persistent depressive symptoms.
-
•
Higher substance use among individuals that reported persistent depressive symptoms.
-
•
Viral load, inflammation and substance use may affect persistent depressive symptoms.
1. Background
Despite remarkable biomedical progress that has enabled people with HIV (PWH) to live long, healthy lives, depression has emerged as a key determinant of worsening disease states and poorer health outcomes (Rubin and Maki, 2019; Tran et al., 2019). Depression is two-to three-times more prevalent among PWH compared to demographically similar people without HIV and has been associated with higher viral load (Edwards et al., 2024), cognitive impairment (Williams et al., 2020), non-infectious comorbidities (Adebajo et al., 2023), and a two-fold increased risk of mortality despite prolonged use of suppressive anti-retroviral therapy (ART) (Kemp et al., 2024; Rubin and Maki, 2019).
Sexualized drug use, also referred to as chemsex, defined as the use of substances before or during sexual encounters to enhance or extend sexual pleasure, has significant implications for HIV risk acquisition (Íncera-Fernández et al., 2023). Substances including methamphetamine, gamma-hydroxybutyrate (GHB), erectile dysfunction medications and alkyl and butyl nitrites (poppers) are widely used during chemsex and have been associated with mental health symptoms (Íncera-Fernández et al., 2021). Other psychosocial risk factors, including stigma (Brown et al., 2023), early life stress (Masiano et al., 2022) and non-sexualized substance use (Earnshaw et al., 2020), are established determinants of elevated depressive symptoms in PWH. However, psychosocial factors alone are insufficient to explain the disproportionate prevalence and severity of depression in PWH (Mudra Rakshasa-Loots et al., 2022; Remien et al., 2019; Rendina et al., 2019; Yousuf et al., 2019).
Immune dysregulation has also been associated with depression in PWH, including higher blood plasma C-reactive protein, tumor necrosis factor – alpha (TNF-α), and neopterin (Orsolini et al., 2022; Poudel-Tandukar et al., 2014; Saloner et al., 2022). These changes are also detected in acute and early HIV, however, few studies have examined whether events in acute infection alter the trajectory in depressive symptoms after treatment with ART (Hellmuth et al., 2017). Studies like that of Gold et al. found that despite the fact that depression was prevalent in primary HIV infection, it was not improved over time in the presence of ART (Gold et al., 2014). Proinflammatory cytokines and activated monocytes, which are elevated in peripheral blood of PWH, are also associated with alterations in functional connectivity of key brain circuits involved in reward pathways, emotional processing, and the hypothalamic-pituitary-adrenal axis stress response (Mehta et al., 2018; Philippi et al., 2020; Rengasamy et al., 2022).
Furthermore, gut-immune dysfunction during acute HIV infection (AHI) persists after initiation of suppressive ART and associates with persistent elevations in soluble markers of inflammation and immune activation that have well documented neuropsychiatric consequences (Bosi et al., 2020; Carrico et al., 2022; Crowell et al., 2016; Del Guerra et al., 2013). In a cross-sectional study with 123 participants, Hellmuth et al. (2017) observed that higher plasma neopterin, a measure of monocyte/macrophage activation, was associated with greater symptoms of depression and anxiety during AHI (Hellmuth et al., 2017). There is also evidence that neuropsychiatric comorbidities predict blunted immune reconstitution following AHI. Paul and colleagues (2022) found that poorer mental health and reduced neurocognitive functioning during AHI was associated with persistent CD4/CD8 T-cell inversion due to incomplete CD4 T-cell recovery after suppressive ART (Paul et al., 2022). These studies indicate that AHI is a critical period where the potentially bi-directional connections between immune dysregulation and neuropsychiatric comorbidities may have long-term consequences in PWH.
Despite several studies examining the association between inflammation and depressive symptoms in PWH, a majority are cross-sectional in nature, or assess depressive symptoms in chronic HIV infection. An important knowledge gap that expands on the existing knowledge of inflammation and depressive symptoms, which is addressed in the present longitudinal study, is whether immune dysregulation that occurs during AHI influences long-term trajectories of depressive symptoms on suppressive ART. Furthermore, prior research relating to immune dysregulation and depressive symptoms often excludes individuals who use substances, despite the well-established role of substance use in the persistence of depressive symptoms. Our study highlights the clinical significance of early identification of individuals at risk for persistent depressive symptoms during the earliest stages of HIV infection.
As such, the present analyses leveraged the RV254/SEARCH010 cohort of people diagnosed with acute HIV to determine if plasma viral load, soluble markers of immune dysregulation, and self-reported substance use during AHI predicted persistent depressive symptoms over 96 weeks of suppressive ART.
2. Methods
Data for 502 individuals included as part of this analysis were collected between 2009 and 2020, participants included in this analysis had depression data available for all three timepoints selected. Study design and methods for the RV254/SEARCH010 cohort have been described in prior publications (Ananworanich et al., 2012; Hellmuth et al., 2016; Kore et al., 2015; Teigler et al., 2018). Briefly, RV254/SEARCH010 is one of the largest prospective cohorts of individuals who have undergone extensive clinical phenotyping from pre-ART AHI through chronic HIV, subsequent follow-up visits while starting ART during during the AHI period, at a median 20 days on ART. Eligible participants include individuals that are 1) 18 years of age or older, 2) have a confirmed diagnosis of AHI (Fiebig stage I-V), 3) have no prior history of ART, and 4) have an ambulatory outpatient status. Individuals with active and severe psychiatric illness were excluded (e.g., schizophrenia, bipolar disorder). Additionally, individuals that had or developed conditions that could preclude them from participation in future research in HIV cure studies, including immune dysregulatory conditions like Hepatitis B or C were also excluded or withdrawn by investigators. Enrollment was voluntary and participants provided written informed consent following a thorough explanation of study activities. This study was approved by institutional review boards at all participating institutions.
2.1. Measures
2.1.1. Sociodemographics
Participants were asked to complete questionnaires that included age, sexual orientation, and educational attainment. For analysis, men that identified as gay or bisexual were categorized as sexual minority men (SMM) and compared to other enrolled participants
2.1.2. ART Regimen (Days from Exposure to ART, Standard ART vs ART + Maraviroc Regimen)
Before 2017, study participants received efavirenz, tenofovir disoproxil fumarate, and either lamivudine or emtricitabine, with a subset randomized to an intensified protocol that also included raltegravir plus maraviroc for 24 weeks (Ananworanich et al., 2012). From 2017 forward, newly enrolled participants were prescribed abacavir, lamivudine, and dolutegravir (DTG), with a subset receiving maraviroc intensification for 96 weeks. Individuals enrolled before 2017 were switched to the DTG-based regimen. The change in ART regimen was implemented for the entire cohort in 2017 (unless medically contraindicated). Participants were virally controlled at the time of the switch. Of note, prior analyses revealed no change in cognitive or affective symptoms following the switch to DTG (Chan et al., 2020). Days from HIV exposure to ART was included as a continuous variable. ART vs ART + maraviroc was dichotomized. Of note, ART regimen distribution across the PHQ-9 clusters was not significantly different.
2.1.3. Plasma viral load during AHI
HIV-RNA quantification was performed using the COBAS AMPLICOR HIV-1 monitor test v1.5 or COBAS Taqman HIV-1 test v2.0 (Roche molecular systems). Viral loads were then transformed by applying a log base 10 transformation
2.1.4. Fiebig Stage. Fiebig stages I–V were determined using a hierarchical algorithm applied to nucleic acid testing, sequential immunoassay, p24 antigen, and Western blot testing. The staging included the following: Fiebig I: RNA+, p24 antigen−, negative by immunoglobin M (IgM)–sensitive enzyme immunoassay (EIA); Fiebig II: RNA+, p24 antigen+, negative by IgM-sensitive EIA; Fiebig III: RNA+, positive by IgM-sensitive EIA, Western blot; and Fiebig V: RNA+, Western blot + without p31 protein band.
2.1.4. Sexualized drug use at AHI
Participants were asked if they had used certain substances (e.g., methamphetamine, amyl nitrites, or erectile dysfunction (ED) medications) in the past four months. Responses were coded in a binary (yes/no) fashion. Participants also indicated if they had used erectile dysfunction (ED) medications in the past 4 months as these are commonly used to enhance sexual pleasure while under the influence of other substances
2.1.6. Soluble Markers of Immune Activation and Inflammation. Immune marker data were available for a subset of participants (n = 166–199), as cellular immunophenotyping was an optional procedure within the parent study. Venipuncture was performed using standard sterile techniques; blood was collected into EDTA tubes and processed on the day of collection. Plasma was separated by centrifugation within 24 h and peripheral blood mononuclear cells were cryopreserved within 8 h at −135 °C or lower. Cryopreserved ACD (acid-citrate-phosphate) plasma was thawed on ice, clarified by centrifugation at 10,844 g for 10 min, and sterilized with 0.05 % Tween 20 for 15 min at room temperature. Plasma was then measured for 84 biomarker levels using the Milliplex MAP Human Cytokine/Chemokine Magnetic Bead Panels I, II, III, and Milliplex MAP Human Soluble Cytokine Receptor Panel according to the manufacturer's instructions in duplicates (EMD Millipore). Samples were analyzed on a 5-parameter logistic curve with a standard acceptance range of 80 %–120 % on a BioPlex 200 running BioPlex Manager v6 (Bio-Rad). Inter- and intra-assay variation was monitored and all testing adhered to rigorous quality assurance protocols.
Baseline data were log10 transformed for the purposes of analyses. Of the 84 immune markers measured, the soluble markers of immune activation and inflammation used in these analyses were selected due to their relevance to depressive symptoms a priori and statistical significance. The full range of immune markers explored is detailed in previous manuscripts (Paul et al., 2022; Teigler et al., 2018). Total ns per immune marker are noted in Table 1.
Table 1.
Demographic, HIV clinical information and substance use information at enrollment.
| Cluster 1 Persistently High Depressive Symptoms (n = 257, 51.19 %) |
Cluster 2 Transient Depressive Symptoms (n = 61, 12.15 %) |
Cluster 3 Persistently Low Depressive Symptoms (n = 184, 36.65 %) |
Total Sample (N = 502, 100 %) |
Padj-value |
|
|---|---|---|---|---|---|
| Demographic Information | |||||
| Age (M, SD) | 27.7 (7.4) | 27.9 (8.0) | 27.8 (8.1) | 27.8 (7.8) | 0.964 |
| Sexual Minority Status | 0.230 | ||||
| Sexual Minority Men | 243 (94.6 %) | 54 (88.5 %) | 170 (92.4 %) | 467 (93.0 %) | |
| Other | 14 (5.4 %) | 7 (11.5 %) | 14 (7.6 %) | 35 (7.0 %) | |
| Educational Attainment | 0.442 | ||||
| Lower than Primary School | 2 (0.8 %) | 2 (3.3 %) | 1 (0.5 %) | 5 (1.0 %) | |
| Primary School | 6 (2.3 %) | 0 (0.0 %) | 3 (1.6 %) | 9 (1.8 %) | |
| Secondary School | 13 (5.1 %) | 4 (6.6 %) | 13 (7.1 %) | 30 (6.0 %) | |
| High School/Basic Technical School | 66 (25.7 %) | 17 (27.9 %) | 44 (23.9 %) | 127 (25.3 %) | |
| Advanced Technical School | 18 (7.0 %) | 4 (6.6 %) | 12 (6.5 %) | 34 (6.8 %) | |
| Bachelor's Degree | 120 (46.7 %) | 30 (49.2 %) | 99 (53.8 %) | 249 (49.6 %) | |
| Master's Degree or Higher | 32 (12.5 %) | 4 (6.6 %) | 12 (6.5 %) | 48 (9.6 %) | |
| HIV Clinical Information | |||||
| Days from Exposure to ART (M, SD) | 21.3 (8.9) | 20.4 (8.1) | 21.9 (11.4) | 21.4 (9.8) | 0.554 |
| Baseline Plasma Viral Load (log10; M, SD) | 5.9 (1.1) | 5.68 (1.1) | 5.68 (1.2) | 5.84 (1.1) | 0.009 |
| Plasma Viral Load <200 copies/mL at Week 96, n (%) | 254 (98.8 %) | 61 (100 %) | 184 (100 %) | 499 (99.4 %) | 0.237 |
| CD4/CD8 Ratio (M, SD) | 0.81 (0.5) | 0.77 (0.5) | 0.80 (0.46) | 0.80 (0.49) | 0.834 |
| Fiebig Stage | 0.413 | ||||
| I | 34 (13.2 %) | 11 (18 %) | 29 (39.2 %) | 74 (14.7 %) | |
| II | 64 (24.9 %) | 10 (16.4 %) | 38 (33.9 %) | 112 (22.3 %) | |
| III | 123 (47.9 %) | 30 (49.2 %) | 77 (41.8 %) | 230 (45.8 %) | |
| IV | 26 (10.1 %) | 7 (11.5 %) | 26 (14.1 %) | 59 (11.8 %) | |
| V | 10 (3.9 %) | 3 (4.9 %) | 14 (7.6 %) | 27 (5.4 %) | |
| Sexualized Drug Use | |||||
| Any Amyl Nitrite Use | 0.048 | ||||
| No | 161 (81.3 %) | 40 (83.3 %) | 115 (91.3 %) | 316 (84.9 %) | |
| Yes | 37 (18.7 %) | 8 (16.7 %) | 11 (8.7 %) | 56 (15.1 %) | |
| Any ED Medication Use | 0.086 | ||||
| No | 177 (89.4 %) | 45 (93.8 %) | 121 (96.0 %) | 343 (92.2 %) | |
| Yes | 21 (10.6 %) | 3 (6.3 %) | 5 (4.0 %) | 29 (7.8 %) | |
| Any Methamphetamine Use |
0.134 | ||||
| No | 154 (77.8 %) | 40 (83.3 %) | 109 (86.5 %) | 303 (81.5 %) | |
| Yes | 44 (22.2 %) | 8 (16.7 %) | 17 (13.5 %) | 69 (18.5 %) | |
| Immune Markers (pg/mL) | |||||
| Neopterin (M, SD) [n = 194] | 3.9 (2.6) | 4.2 (2.7) | 3.5 (2.2) | 3.8 (2.5) | 0.418 |
| IFN-γ (M, SD) [n = 166] | 2.1 (2.8) | 1.4 (2.4) | 2.8 (3.1) | 2.2 (2.9) | 0.126 |
| hsIL-6 (M, SD) [n = 199] | 3.5 (2.8) | 2.7 (2.3) | 3.0 (2.4) | 3.3 (2.7) | 0.223 |
| sTNF-αRII (M, SD) [n = 166] | 3.8 (2.5) | 4.0 (3.0) | 2.8 (1.8) | 3.5 (2.4) | 0.034 |
| PHQ-9 Scores | |||||
| Week 0 | 13.22 (4.77) | 9.02 (1.46) | 4.49 (2.56) | 9.51 (5.54) | <0.001 |
| Week 24 | 7.18 (4.61) | 4.31 (1.32) | 3.18 (3.37) | 5.37 (4.33) | <0.001 |
| Week 96 | 7.89 (4.22) | 3.57 (2.03) | 2.56 (2.33) | 5.40 (4.25) | <0.001 |
2.1.7Depressive Symptom Severity. To assess depressive symptom severity, participants completed the Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001), which has been validated for use in Thailand (Lotrakul et al., 2008). Items are rated from “0” (not at all) to “3” (nearly every day). Item level and total scores were included in the analysis. Total depression scores acquired at baseline (week 0), week 24, and week 96 were utilized construct trajectories.
2.2. Statistical analysis
The analytic approach involved several steps. First, to examine depressive symptom trajectories on the PHQ-9, we utilized a robust approach for cluster discovery using dimensionality reduction and hierarchical density-based clustering. Uniform Manifold Approximation and Projection (UMAP) is a data reduction process adept at maintaining the integrity of the global data structure (McInnes et al., 2020). The application of UMAP yielded a two-dimensional representation that revealed distinct groupings, indicative of underlying data stratification. Subsequently, we leveraged hierarchical density based spatial clustering with noise (HDBSCAN) to identify clusters in this reduced dimension space (dos Santos et al., 2021). The clustering solution is iteratively optimized to minimize noise and increase interpretability of resulting cluster formations. The combination of UMAP and HDBSCAN provide a powerful approach for clustering while preserving the natural structure of the data. One of the significant advantages of HDBSCAN is its ability to infer the optimal number of clusters from the data structure itself, eliminating the need to predetermine the number and shape of clusters. Furthermore, outliers are not forced into a predetermined cluster structure. The quality of the cluster solution was examined using the silhouette score with values exceeding 0.70 indicating distinct and homogenous subgroup formation within our data. The silhouette score serves as an indicator of cluster quality, gauging the degree of similarity of individual data points within their own cluster compared to other clusters (Shutaywi and Kachouie, 2021). Each data point is analyzed for its average intra-cluster distance (denoted as a), which quantifies the mean distance to all other points within the same cluster. Concurrently, the average nearest inter-cluster distance (denoted as b), or the mean distance to points in the nearest cluster that the data point is not a member of, is calculated to measure cluster separation.
Following hierarchical clustering, independent samples t-tests were used to compare the highest and lowest depressive symptom cluster trajectories on select demographic, clinical, behavioral, and immune indices. Odds ratios (ORs) and 95 % confidence intervals (CIs) were computed to estimate the strength of differences observed among the highest and lowest symptom cluster trajectories (Table 2). The threshold for all significance tests was set at p < 0.05 (two tailed). All statistical analyses were performed using SPSS Version 29 (IBM Corp., 2023). False discovery rate (Benjamini-Hochberg procedure) was utilized to adjust for multiple comparisons (Benjamini and Hochberg, 1995).
Table 2.
Logistic regression analyses for predictors of persistent depressive symptom trajectories (N = 441).
| OR (95 % CI) | p-value | |
|---|---|---|
| Any Erectile Dysfunction Medication Usea | 2.855 (1.045–7.78) | 0.040∗ |
| Any Amyl Nitrite Usea | 2.39 (1.17–4.88) | 0.017∗ |
| Any Methamphetamine Usea | 1.820 (0.99–3.35) | 0.055 |
| Plasma Viral Load (Log10)b | 1.272 (1.08–1.50) | 0.005∗ |
| sTNF-αRII (Log10) | 1.229 (1.04–1.45) | 0.016∗ |
For multivariate analyses, all variables were included in the model; ∗significant at p < 0.05; apast 4 months use; blog10 plasma viral load at baseline.
3. Results
Participants were between the ages of 18 and 70, with a mean age of 27.7 (Standard deviation [SD] = 7.4). The sample was 98 % male (n = 494), with 467 (93 %) identifying as SMM. Approximately half of the sample had attained a bachelor's degree (n = 219, 49.7 %). At the AHI enrollment visit, the median CD4+ T-cell count was 361 cells/mm3 (Interquartile range [IQR] = 266–495), median CD8+ T-cell count was 532 cells/mm3 (IQR = 340–887), median CD4/CD8 ratio was 0.70 (IQR 0.42–1.03), and mean log10 plasma viral load was 5.84 copies/mL (IQR 5.24–6.75). Participants had an estimated mean of 20.76 days (SD = 9.71, range 14–25) since acquisition of HIV, and had gone an estimated mean 21.4 days (SD = 9.8, range 15–26) between HIV acquisition and ART start. Most participants were in Fiebig stages I-III (I–14.7 %, II–22.3 % and III–45.8 %). Amyl nitrite use was endorsed by 15 % (n = 56) of participants, whereas 8 % (n = 29) reported erectile dysfunction medication use, and 19 % endorsed methamphetamine use (n = 69) in the past four months.
3.1. Hierarchical trajectory clustering of depressive symptoms
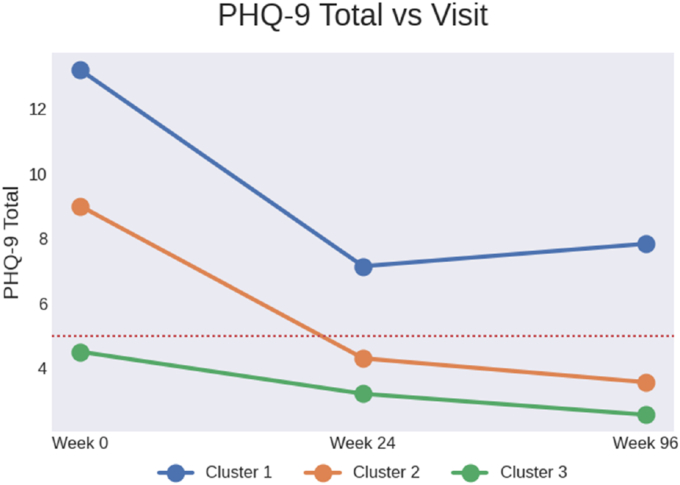
Hierarchical clustering revealed three distinct depressive symptom trajectories. The largest subgroup (Cluster 1, n = 257; “Persistently High Depressive Symptoms”) accounted for 51.2 % of the sample. Participants in this cluster reported high levels of depressive symptoms at enrollment that persisted throughout the 96 weeks of observation post ART. Cluster 2 (n = 61; “Transient Depressive Symptoms”) accounted for 12.1 % of the sample and was characterized by mild to moderate depressive symptoms at enrollment that decreased over follow up assessments. Cluster 3 (n = 184; “Persistently Low Depressive Symptoms”) accounted for 36.7 % of the sample. Participants in this cluster reported minimal depressive symptoms at enrollment that declined over follow-up. The mean PHQ-9 score for the persistently high depressive symptoms cluster at enrollment was 13.22 (SD = 4.77) compared to an mean score of 4.49 (SD = 2.56) in the persistently low depressive symptoms cluster. The mean PHQ-9 score at 96 weeks was 7.89 (SD = 4.22) in the persistently high depressive symptoms cluster, and 2.56 (SD = 2.33) in the persistently low depressive symptoms cluster. Subsequent analyses focused on comparisons between the persistently high versus low depressive symptom clusters.
3.2. Inferential comparisons between the persistent and low depressive symptom clusters
Table 1 provides demographic, HIV clinical, substance use, and immune marker data stratified by cluster. Table 2 presents logistic regression analyses for predictors of depressive symptom trajectories. Cluster 3 was comprised of significantly more individuals (p = 0.003) initiating a DTG-based ART regimen (33.0 %) than clusters 1 (19.5 %) and 2 (18.3 %). These regimens did not change notably over the course of the analysis period. In bivariate analyses, there were 27 % greater odds of persistent (versus low) depressive symptoms for every log10copies/mL increase in plasma viral load (OR = 1.27; 95 % CI = 0.99, 3.35) at AHI. Similarly, there were 23 % higher odds of high persistent (versus low) depressive symptoms for every log10 increase in sTNF-αRII (OR = 1.23, 95 % CI = 1.04, 1.45) at AHI. The odds of persistent depressive symptoms were also more than two-fold greater among participants who reported any ED medication use (OR = 2.86, 95 % CI = 1.05, 7.78) and any amyl nitrite (OR = 2.39; 95 % CI = 1.17, 4.88) use in the past 4 months at the time of enrollment. However, the significant difference for ED medication use did not survive FDR correction.
4. Discussion
Findings from this prospective cohort study demonstrate that approximately half of participants enrolled into RV254/SEARCH 010 exhibited persistent elevations in depressive symptoms from AHI through 96 weeks of suppressive ART. Plasma viral load, inflammation, and amyl nitrite use during AHI were associated with persistently high (versus low) depressive symptoms despite suppressive ART. These findings provide empirical evidence for the presence of an early risk phenotype for persistently high depressive symptoms after initiating ART. Additionally, findings underscore the potential benefits of biobehavioral interventions at the time of ART initiation focused on reducing viral load resulting in peripheral inflammation and sexualized drug use.
Findings from the current study corroborate previous results from our group and others implicate inflammation and immune activation in the pathogenesis of depression among PWH (Carrico et al., 2022; Chan et al., 2020; Gold et al., 2014; Poudel-Tandukar et al., 2014; Rubin and Maki, 2019). Our findings are consistent with Musinguzi et al. (2018), who reported an association between TNF-α and a higher risk of depression in ART-naïve PWH (Musinguzi et al., 2018). Similarly, we observed that the odds of persistent depressive symptoms increased with every log10 increase in sTNF-αRII. Studies in other large cohorts, including the Multicenter AIDS Cohort Study (MACS) have also demonstrated that markers of immune activation were associated with depressive symptoms in a sample of SMM (Lu et al., 2019). Specifically, sTNF-R2, sIL-2Ra, sCD27, B-cell activating factor, IP-10, sIL-6R, sCD14 and sGP130 were significantly associated with 9 % higher odds of depressive symptoms in PWH. Findings from this cohort study demonstrate that HIV-associated pathophysiologic alterations during AHI may have enduring consequences for depressive disorders, which underscores a clear need for early, integrated HIV and mental health treatment.
A novel finding from this study is the association between amyl nitrite use and persistently high depressive symptoms. Amyl nitrites or poppers, are often used during chemsex, along with erectile dysfunction medications, to enhance sexual pleasure. The use of poppers and erectile dysfunction medications for sexual purposes has been on the rise among SMM around the globe, including in Southeast Asia, where it is referred to as “hi-fun” and has been associated with higher psychological distress among people without HIV (Giorgetti et al., 2017; Maxwell et al., 2019; Witzel et al., 2023). Furthermore, vasoactive peptides, like amyl nitrites, are currently being investigated and leveraged to transiently disrupt the blood brain barrier, induce vascular permeability and improve drug delivery to the brain (Smith-Cohn et al., 2022). Given that amyl nitrites are known vasodilators, their use during the time of acute HIV may increase vascular remodeling, facilitating both entry of the virus; intensifying the risk of neurotoxicity and localized inflammation in the brain (Cha et al., 2016; Smith-Cohn et al., 2022; Soontornniyomkij et al., 2016). Interventions to address sexualized drug use could have important implications for reducing depressive symptoms among SMM with HIV.
Our findings have significant clinical implications and may contribute to our understanding of the biological underpinnings of depressive symptoms in PWH. Individuals in Cluster 1 (“Persistently high depressive symptoms”) exhibited moderately high depressive symptoms that declined to mild but sustained levels over time as evidenced by mean PHQ-9 scores. This persistence of symptoms suggests an enduring psychological burden that extends beyond the initial stressor of an HIV diagnosis and highlights a subgroup at risk for chronic, depressive symptoms that may be associated with other poor health outcomes. Furthermore, the association between baseline amyl nitrite use and Cluster 1 membership highlights a need for early intervention and integrated mental health screening at the time of HIV diagnosis, given the potential impact of substance use on long-term mental health trajectories among PWH.
Beyond our psychosocial findings, our study provides evidence linking baseline immune dysregulation to long-term depressive symptoms. Elevated levels of sTNF-RII at baseline were associated with Cluster 1 membership. Higher levels of sTNF-RII have been observed in HIV infection, and in vitro studies demonstrate that HIV gene products can induce the release of these inflammatory products in circulation (Deeks et al., 2004, 2013; Memiah et al., 2021). Once released into circulation, these pro-inflammatory cytokines are able to cross the blood brain barrier and act as neuromodulators that affect neurotransmitter metabolism and can lead to HPA axis dysregulation (Memiah et al., 2021). sTNF-RII in particular, has been implicated in pathways involving serotonin biosynthesis (Memiah et al., 2021; Mudra Rakshasa-Loots et al., 2023). For example, elevated sTNF-RII has been associated with increased activity of indoleamine 2,3-dioxygenase, leading to increased tryptophan degradation (Davidson et al., 2022; Huang et al., 2002; Memiah et al., 2021; Xue et al., 2023). The depletion of tryptophan may result in reduced serotonin availability, contributing to the development of depression (Davidson et al., 2022; Xue et al., 2023). Our findings suggest that sTNF-RII may be a biologically plausible link between inflammation and depressive symptoms. sTNF-RII may serve as a useful biomarker of susceptibility to depressive symptoms or as a prognostic indicator of persistent depressive symptoms in individuals initiating ART during AHI. However, further research clarifying its mechanistic role and the directionality of the association is warranted.
Our research enhances the existing literature by identifying biobehavioral determinants of persistent depressive symptoms from AHI through 96 weeks of ART. Participants experiencing persistently high depressive symptoms were comparable to those with low depressive symptoms for several key confounders such as sociodemographic characteristics, Fiebig stage, and timing of ART initiation. Despite these strengths, it is important to consider several limitations. First, our sample was predominantly comprised of Thai SMM individuals, which limits generalizability. Second, we focused on information acquired during AHI in the RV254/SEARCH 010 cohort to predict depressive symptoms reported over 96 weeks of suppressive ART. Although our baseline data provides sufficient temporality to examine biobehavioral determinants of depressive symptoms over 96 weeks, more comprehensive studies with time-varying exposures are needed. Depression is a complex condition with multifaceted origins, suggesting the need for further investigations to explore the impact of risk and protective factors that may be exacerbated post-ART initiation. Third, few participants reported taking psychotropic medications typically prescribed for depressive disorders and/or engagement in mental health care, therefore, we were unable to adjust for this in the analyses. Fourth, the impact of persistent substance use is not included in the analysis and represents a limitation of the data, but should be considered in future research.
In summary, this longitudinal study is among the first to demonstrate that higher plasma viral load, greater systemic inflammation, and sexualized drug use during AHI predict persistently high (versus low) depressive symptom trajectories over 96 weeks of suppressive ART. Findings underscore that viral load, inflammation and sexualized drug use are viable targets for Fig. 1 reducing risk for depressive disorders in PWH, even in the earliest stages of HIV.
Fig. 1.
Depressive symptom trajectories identified in the group based multi-trajectory analysis aRed dotted line represents cut off for mild depressive symptoms. Total scores of 5, 10, 15 and 20 on the Patient Health Questionnaire-9 (PHQ-9) correspond to cutoff points for mild, moderate, moderately severe and severe depression, respectively.
CRediT authorship contribution statement
Jennifer V. Chavez: Writing – original draft, Conceptualization, Writing – review & editing. Jacob Bolzenius: Formal analysis, Data curation, Writing – original draft, Visualization. Phillip Chan: Writing – review & editing. Kyu Cho: Writing – original draft, Software, Formal analysis. Julie Mannarino: Writing – original draft, Supervision, Project administration, Writing – review & editing. Carlo Sacdalan: Writing – review & editing, Investigation, Project administration, Data curation. Shelli Farhadian: Writing – review & editing. Lydie Trautmann: Supervision, Investigation, Writing – review & editing. Lishomwa C. Ndhlovu: Writing – review & editing. Somporn Tipsuk: Writing – review & editing, Investigation. Trevor A. Crowell: Writing – review & editing. Shelly J. Krebs: Writing – review & editing, Data curation. Bonnie Slike: Data curation, Writing – review & editing. Duanghathai Suttichom: Writing – review & editing, Data curation. Donn J. Colby: Data curation, Writing – review & editing. Nittaya Phanuphak: Writing – review & editing, Investigation, Funding acquisition, Data curation. Eugène Kroon: Writing – review & editing. Sandhya Vasan: Writing – review & editing. Somchai Sriplienchan: Data curation, Writing – review & editing, Funding acquisition. Serena Spudich: Writing – review & editing, Supervision, Investigation, Funding acquisition. Robert Paul: Funding acquisition, Conceptualization, Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation. Adam W. Carrico: Writing – review & editing, Investigation, Supervision, Conceptualization, Writing – original draft, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements & Grant Funding
We would like to thank the study participants who committed so much of their time for this study. The participants were from the RV254/SEARCH 010, which is supported by cooperative agreements (W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD) and by an intramural grant from the Thai Red Cross AIDS Research Centre and, in part, by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institute of Health (DAIDS, NIAID, NIH) (grant AAI21058-001-01000). Research reported in this publication was also supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health under award number 1R36MH139364-01. Antiretroviral therapy for RV254/SEARCH 010 participants was supported by the Thai Government Pharmaceutical Organization, Gilead Sciences, Merck and ViiV Healthcare.
Data availability
Data will be made available on request.
References
- Adebajo S.B., Adebiyi R., Chama J., Bello S., ONONAKU U., Aka A., Lai S., Baral S.D., Dyer T.V., Crowell T.A., Nowak R.G., Charurat M. Depression and sexual stigma are associated with cardiometabolic risk among sexual and gender minorities living with HIV in Nigeria. J. Acquir. Immune Defic. Syndr. 2023;92:50–58. doi: 10.1097/QAI.0000000000003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananworanich J., Schuetz A., Vandergeeten C., Sereti I., de Souza M., Rerknimitr R., Dewar R., Marovich M., van Griensven F., Sekaly R., Pinyakorn S., Phanuphak N., Trichavaroj R., Rutvisuttinunt W., Chomchey N., Paris R., Peel S., Valcour V., Maldarelli F., Chomont N., Michael N., Phanuphak P., Kim J.H. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bosi A., Banfi D., Bistoletti M., Giaroni C., Baj A. Int JTryptophanRes. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. 2020;13 doi: 10.1177/1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.J., Gao C., Kaur A., Qiao S., Li X. Social support, internalized HIV stigma, resilience and depression among people living with HIV: a moderated mediation analysis. AIDS Behav. 2023;27:1106–1115. doi: 10.1007/s10461-022-03847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico A.W., Cherenack E.M., Rubin L.H., McIntosh R., Ghanooni D., Chavez J.V., Klatt N.R., Paul R.H. Through the looking-glass: psychoneuroimmunology and the microbiome-gut-brain axis in the modern antiretroviral therapy era. Psychosom. Med. 2022;84:984–994. doi: 10.1097/PSY.0000000000001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H.J., Kim Y.J., Jeon S.Y., Kim Y.-H., Shin J., Yun J., Han K., Park H.-K., Kim H.S. Neurotoxicity induced by alkyl nitrites: impairment in learning/memory and motor coordination. Neurosci. Lett. 2016;619:79–85. doi: 10.1016/j.neulet.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Chan P., Goh O., Kroon E., Colby D., Sacdalan C., Pinyakorn S., Prueksakaew P., Reiss P., Ananworanich J., Valcour V., Spudich S., Paul R., the RV254/SEARCH 010 Research Team Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res. Ther. 2020;17:1. doi: 10.1186/s12981-019-0257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell T.A., Fletcher J.L., Sereti I., Pinyakorn S., Dewar R., Krebs S.J., Chomchey N., Rerknimitr R., Schuetz A., Michael N.L., Phanuphak N., Chomont N., Ananworanich J., Group Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J. Int. AIDS Soc. 2016;19 doi: 10.7448/IAS.19.1.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M., Rashidi N., Nurgali K., Apostolopoulos V. The role of tryptophan metabolites in neuropsychiatric disorders. Int. J. Mol. Sci. 2022;23:9968. doi: 10.3390/ijms23179968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S.G., Kitchen C.M.R., Liu L., Guo H., Gascon R., Narváez A.B., Hunt P., Martin J.N., Kahn J.O., Levy J., McGrath M.S., Hecht F.M. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Deeks S.G., Tracy R., Douek D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guerra F.B., Fonseca J.L.I., Figueiredo V.M., Ziff E.B., Konkiewitz E.C. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J. Neurovirol. 2013;19:314–327. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- dos Santos J.A., Syed T.I., Naldi M., Campello R.J.G.B., Sander J. Hierarchal Density-Based Clustering Using MapReduce. IEEE Transactions on Big Data. 2021 [Google Scholar]
- Earnshaw V.A., Eaton L.A., Collier Z.K., Watson R.J., Maksut J.L., Rucinski K.B., Kelly J.F., Kalichman S.C. HIV stigma, depressive symptoms, and substance use. AIDS Patient Care STDS. 2020;34:275–280. doi: 10.1089/apc.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.A., Brijkumar J., Dudgeon M., Robichaux C., Johnson B., Rautman L., Powers R.A., Sun Y.V., Pillay S., Ordonez C., Castillo-Mancilla J., Tanser F.C., Asghar Z., Mee P., Moodley P., Sunpath H., Kuritzkes D.R., Marconi V.C., Moosa M.-Y.S. Depression: an individual-level early warning indicator of virologic failure in HIV patients in South Africa. Public Health Action. 2024;14:76–81. doi: 10.5588/pha.24.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti R., Tagliabracci A., Schifano F., Zaami S., Marinelli E., Busardò F.P. When “Chems” Meet Sex: a Rising Phenomenon Called “ChemSex.”. Curr. Neuropharmacol. 2017;15:762–770. doi: 10.2174/1570159X15666161117151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.A., Grill M., Peterson J., Pilcher C., Lee E., Hecht F.M., Fuchs D., Yiannoutsos C.T., Price R.W., Robertson K., Spudich S. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18:1124–1132. doi: 10.1007/s10461-013-0688-5. [DOI] [PubMed] [Google Scholar]
- Hellmuth J., Colby D., Valcour V., Suttichom D., Spudich S., Ananworanich J., Prueksakaew P., Sailasuta N., Allen I., Jagodzinski L.L., Slike B., Ochi D., Paul R., RV254/SEARCH 010 Study Group Depression and anxiety are common in acute HIV infection and associate with plasma immune activation. AIDS Behav. 2017;21:3238–3246. doi: 10.1007/s10461-017-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J., Fletcher J.L.K., Valcour V., Kroon E., Ananworanich J., Intasan J., Lerdlum S., Narvid J., Pothisri M., Allen I., Krebs S.J., Slike B., Prueksakaew P., Jagodzinski L.L., Puttamaswin S., Phanuphak N., Spudich S. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87:148–154. doi: 10.1212/WNL.0000000000002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., Fuchs D., Widner B., Glover C., Henderson D.C., Allen-Mersh T.G. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br. J. Cancer. 2002;86:1691–1696. doi: 10.1038/sj.bjc.6600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Version 29.0. 2023. IBM SPSS statistics for windows. [Google Scholar]
- Íncera-Fernández D., Gámez-Guadix M., Moreno-Guillén S. Mental health symptoms associated with sexualized drug use (Chemsex) among men who have sex with men: a systematic review. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph182413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Íncera-Fernández D., Román F.J., Moreno-Guillén S., Gámez-Guadix M. Understanding sexualized drug use: substances, reasons, consequences, and self-perceptions among men who have sex with other men in Spain. Int. J. Environ. Res. Publ. Health. 2023;20:2751. doi: 10.3390/ijerph20032751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C.G., Pence B.W., Velloza J., Concepcion T., Moitra M., Iroezindu M., Bahemana E., Kibuuka H., Semwogerere M., Owuoth J., Maswai J., Langat R., Esber A.L., Dear N.F., Parikh A., Crowell T.A., Ake J.A., Polyak C.S., Collins P.Y., AFRICOS Study Group Cumulative exposure to depressive symptoms and all-cause mortality among adults with HIV in Kenya, Nigeria, Tanzania, and Uganda. AIDS. 2024;38:1228–1236. doi: 10.1097/QAD.0000000000003891. [DOI] [PubMed] [Google Scholar]
- Kore I., Ananworanich J., Valcour V., Fletcher J.L., Chalermchai T., Paul R., Reynolds J., Tipsuk S., Ubolyam S., Rattanamanee S., Jagodzinski L., Kim J., Spudich S. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2015;70:393–399. doi: 10.1097/QAI.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrakul M., Sumrithe S., Saipanish R. Reliability and validity of the Thai version of the PHQ-9. BMC Psychiatry. 2008;8:46. doi: 10.1186/1471-244X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Surkan P.J., Irwin M.R., Treisman G.J., Breen E.C., Sacktor N., Stall R., Wolinsky S.M., Jacobson L.P., Abraham A.G. Inflammation and risk of depression in HIV: prospective findings from the multicenter AIDS cohort Study. Am. J. Epidemiol. 2019;188:1994–2003. doi: 10.1093/aje/kwz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiano S.P., Yu X., Tembo T., Wetzel E., Mphande M., Khama I., Mkandawire A., Chitani M., Liwimbi O., Udedi M., Mazenga A., Nyasulu P., Abrams E., Ahmed S., Kim M.H. The relationship between adverse childhood experiences and common mental disorders among pregnant women living with HIV in Malawi. J. Affect. Disord. 2022;312:159–168. doi: 10.1016/j.jad.2022.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S., Shahmanesh M., Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int. J. Drug Pol. 2019;63:74–89. doi: 10.1016/j.drugpo.2018.11.014. [DOI] [PubMed] [Google Scholar]
- McInnes L., Healy J., Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiV preprint. 2020 [Google Scholar]
- Mehta N.D., Haroon E., Xu X., Woolwine B.J., Li Z., Felger J.C. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav. Immun. 2018;73:725–730. doi: 10.1016/j.bbi.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memiah P., Nkinda L., Majigo M., Humwa F., Haile Z.T., Muthoka K., Zuheri A., Kamau A., Ochola L., Buluku G. Mental health symptoms and inflammatory markers among HIV infected patients in Tanzania. BMC Public Health. 2021;21:1113. doi: 10.1186/s12889-021-11064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudra Rakshasa-Loots A., Bakewell N., Sharp D.J., Gisslén M., Zetterberg H., Alagaratnam J., Wit F.W.N.M., Kootstra N.A., Winston A., Reiss P., Sabin C.A., Vera J.H. Biomarkers of central and peripheral inflammation mediate the association between HIV and depressive symptoms. Transl. Psychiatry. 2023;13:1–9. doi: 10.1038/s41398-023-02489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudra Rakshasa-Loots A., Whalley H.C., Vera J.H., Cox S.R. Neuroinflammation in HIV-associated depression: evidence and future perspectives. Mol Psychiatry. 2022:1–14. doi: 10.1038/s41380-022-01619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musinguzi K., Obuku A., Nakasujja N., Birabwa H., Nakku J., Levin J., Kinyanda E. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 2018;19:1. doi: 10.1186/s12865-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsolini L., Pompili S., Tempia Valenta S., Salvi V., Volpe U. C-Reactive protein as a biomarker for major depressive disorder? Int. J. Mol. Sci. 2022;23:1616. doi: 10.3390/ijms23031616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Cho K., Bolzenius J., Sacdalan C., Ndhlovu L.C., Trautmann L., Krebs S., Tipsuk S., Crowell T.A., Suttichom D., Colby D.J., Premeaux T.A., Phanuphak N., Chan P., Kroon E., Vasan S., Hsu D., Carrico A., Valcour V., Ananworanich J., Robb M.L., Ake J.A., Sriplienchan S., Spudich S. Individual differences in CD4/CD8 T-Cell ratio trajectories and associated risk profiles modeled from acute HIV infection. Psychosom. Med. 2022;84:976–983. doi: 10.1097/PSY.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Reyna L., Nedderman L., Chan P., Samboju V., Chang K., Phanuphak N., Ratnaratorn N., Hellmuth J., Benjapornpong K., Dumrongpisutikul N., Pothisri M., Robb M.L., Ananworanich J., Spudich S., Valcour V., Paul R., SEARCH 010/RV254 and RV304/SEARCH 013 study teams Resting-state neural signatures of depressive symptoms in acute HIV. J. Neurovirol. 2020;26:226–240. doi: 10.1007/s13365-020-00826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel-Tandukar K., Bertone-Johnson E.R., Palmer P.H., Poudel K.C. C-reactive protein and depression in persons with human immunodeficiency virus infection: the positive living with HIV (POLH) Study. Brain Behav. Immun. 2014;42:89–95. doi: 10.1016/j.bbi.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Remien R.H., Stirratt M.J., Nguyen N., Robbins R.N., Pala A.N., Mellins C.A. Mental health and HIV/AIDS: the need for an integrated response. AIDS. 2019;33:1411–1420. doi: 10.1097/QAD.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina H.J., Weaver L., Millar B.M., López-Matos J., Parsons J.T. Psychosocial well-being and HIV-Related immune health outcomes among HIV-positive older adults: support for a biopsychosocial model of HIV stigma and health. J. Int. Assoc. Phys. AIDS Care. 2019;18 doi: 10.1177/2325958219888462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy M., Brundin L., Griffo A., Panny B., Capan C., Forton C., Price R.B. Cytokine and reward circuitry relationships in treatment-resistant depression. Biol Psychiatry Glob Open Sci. 2022;2:45–53. doi: 10.1016/j.bpsgos.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L.H., Maki P.M. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr. HIV AIDS Rep. 2019;16:82–95. doi: 10.1007/s11904-019-00421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R., Savini N., Letendre S.L., Moore D.J., Montoya J.L. Neopterin relates to lifetime depression in older adults with HIV on suppressive antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2022;89:454. doi: 10.1097/QAI.0000000000002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutaywi M., Kachouie N.N. Silhouette analysis for performance evaluation in machine learning with applications to clustering. Entropy. 2021;23:759. doi: 10.3390/e23060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Cohn M.A., Burley N.B., Grossman S.A. Transient opening of the blood-brain barrier by vasoactive peptides to increase CNS drug delivery: reality versus wishful thinking? Curr. Neuropharmacol. 2022;20:1383–1399. doi: 10.2174/1570159X20999220131163504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V., Kesby J.P., Morgan E.E., Bischoff-Grethe A., Minassian A., Brown G.G., Grant I., Translational Methamphetamine AIDS Research Center (TMARC) Group Effects of HIV and methamphetamine on brain and behavior: evidence from human studies and animal models. J. Neuroimmune Pharmacol. 2016;11:495–510. doi: 10.1007/s11481-016-9699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teigler J.E., Leyre L., Chomont N., Slike B., Jian N., Eller M.A., Phanuphak N., Kroon E., Pinyakorn S., Eller L.A., Robb M.L., Ananworanich J., Michael N.L., Streeck H., Krebs S.J. Distinct biomarker signatures in HIV acute infection associate with viral dynamics and reservoir size. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran B.X., Ho R.C.M., Ho C.S.H., Latkin C.A., Phan H.T., Ha G.H., Vu G.T., Ying J., Zhang M.W.B. Depression among patients with HIV/AIDS: research development and effective interventions (GAPRESEARCH) Int. J. Environ. Res. Publ. Health. 2019;16:1772. doi: 10.3390/ijerph16101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.E., Ipser J.C., Stein D.J., Joska J.A., Naudé P.J.W. Peripheral immune dysregulation in the ART era of HIV-associated neurocognitive impairments: a systematic review. Psychoneuroendocrinology. 2020;118 doi: 10.1016/j.psyneuen.2020.104689. [DOI] [PubMed] [Google Scholar]
- Witzel T.C., Charoenyang M., Bourne A., Guadamuz T.E. Hi-fun among men who have sex with men in Bangkok: a scoping study exploring key informants' perspectives on hi-fun contexts, harms and support strategies. PLOS Glob Public Health. 2023;3 doi: 10.1371/journal.pgph.0002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., Li G., Zheng Q., Gu X., Shi Q., Su Y., Chu Q., Yuan X., Bao Z., Lu J., Li L. Tryptophan metabolism in health and disease. Cell Metab. 2023;35:1304–1326. doi: 10.1016/j.cmet.2023.06.004. [DOI] [PubMed] [Google Scholar]
- Yousuf A., Mohd Arifin S.R., Musa R., Md Isa M.L. Depression and HIV disease progression: a mini-review. Clin. Pract. Epidemiol. Ment. Health. 2019;15:153–159. doi: 10.2174/1745017901915010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.