Abstract
This study investigated dietary regimens on Hu sheep meat quality. Compared to control, supplementary feeding (SF) increased body weight (63.83 %), eye muscle area (89.72 %), and dry matter content (5.99 %) (P < 0.01), while restricted feeding (RF) impaired meat color and reduced intramuscular fat (38.89 %) (P < 0.01). SF elevated concentrations of 67 volatile flavor compounds versus control and 48 compounds versus RF, enhancing sweet- and fatty-flavor associated volatiles. Control and RF exhibited similar flavor profiles. Correlation analysis identified 90 significant associations between fatty acids/amino acids and key volatile compounds. RF upregulated lipolytic enzymes ATGL and CPT1A (P < 0.05), decreased muscle fatty acid content, reduced muscle fiber size (8.85 %), and increased fiber density (113.50 %) (P < 0.01). Conversely, SF activated the AMPK-mTOR-S6K1 pathway, enhancing protein synthesis and amino acid levels. These findings demonstrate that dietary interventions modulate flavor precursors through metabolic pathways, providing scientific support for developing high-quality mutton products.
Keywords: Sheep, Supplementary feeding, Restricted feeding, Meat quality, Flavor
Highlights
-
•
Supplementary feeding enhances meat quality, whereas restricted feeding degrades it.
-
•
SF group had higher volatile compound levels, enhancing sweet/fatty flavors, while CON/RF groups had similar profiles.
-
•
Supplementary feeding boosts lipid/protein anabolism, restricted feeding suppresses both, shaping flavor precursors.
1. Introduction
The quality of lamb meat is mostly determined by its taste and flavor, with flavor being formed by various volatile compounds. Numerous precursors and intermediate reaction products produce a multitude of volatile compounds through processes such as the lipid oxidation, Maillard reaction, and protein degradation. Varying levels of precursor compounds result in distinct meat flavor (Frank et al., 2017). Flavor perception is described as a “complex combination of the olfactory, gustatory, and trigeminal sensations experienced during tasting”. Through contact with olfactory receptors, the human olfactory system is able to distinguish between a large array of odors. Experiments commonly use high-resolution techniques to minimize the impact of subjectivity. Solid-phase microextraction (SPME), as a solvent-free methodology based on equilibrium partitioning between the sample matrix and coated fiber, has gained widespread application in meat science for sensitive determination of flavor-active compounds (C₄-C₂₀) in muscle tissue, processed meats, and derived commodities. Gas chromatography-mass spectrometry (GC–MS) is a frequently employed technique for the analysis of volatile constituents found in food.
Lipid oxidation serves as a pivotal biochemical pathway driving the generation of volatile flavor compounds, including aliphatic alcohols, ester derivatives, and reactive aldehydes with distinct carbonyl functionalities (Mariutti & Bragagnolo, 2017). Therefore, the flavor of sheep with different amounts of intramuscular fat content differs significantly (Li et al., 2022). Both breed and feed are the key factors affecting flavor. Different breeds of pigs and poultry have distinct volatile metabolites, and extensive research has been conducted on the flavor differences that arise from these species (Jin et al., 2021). Introducing pomegranate by-products into the diet of sheep leads to the deposition of different flavor precursors, resulting in a range of varied meat flavors (Natalello et al., 2023). Grazing and captive feeding yield different meat flavors (Zhang et al., 2022). The organoleptic properties of ovine meat remain insufficiently characterized in the context of nutritional intervention strategies, particularly regarding comparative analyses of supplementary feeding versus restricted feeding regimens and their modulation of flavor-related biochemical pathways. Furthermore, bioactive compounds such as polyphenols and minerals present in feedstuffs may influence key meat matrix components through protein conformational changes and lipid stabilization, potentially modulating flavor precursor dynamics (Cheng et al., 2025).
Supplementary feeding with high-concentrate diets is widely adopted to enhance growth performance in sheep production systems, its long-term implementation may lead to excessive fat deposition and metabolic disorders such as rumen acidosis (Fernandes et al., 2012). Conversely, restricted feeding regimes, though less common in intensive systems, hold significance under specific scenarios: seasonal forage shortages during autumn-winter transitions in the northern regions of China (Fu et al., 2022) and nutritional stress during post-weaning periods when feed resources are limited (Wu et al., 2016). Moderate feed restriction has been demonstrated to improve feed conversion efficiency and meat quality (Lopes et al., 2014), while severe restriction (55–60 % of ad libitum intake) serves as an experimental model to investigate metabolic plasticity and stress-induced flavor compound formation (Lopes et al., 2014; Zhao et al., 2016). Notably, the volatile flavor profiles and metabolism of flavor precursors dynamics under contrasting feeding strategies remain unexplored in sheep.
The Hu sheep, a indigenous kind of sheep in China, is primarily found in the Jiaxing and Taihu regions of Zhejiang province. This breed is known for its exceptional fertility and rapid growth rate, making it highly sought after by consumers due to its delectable and nutritious meat. Notably, its meat exhibits distinct flavor characteristics, including elevated levels of key sweet and fatty-flavor compounds such as hexanal and nonanal compared to other Chinese indigenous breeds (Li et al., 2022), making it an ideal model for investigating diet-flavor relationships. The meat quality of Hu sheep is influenced by the feed ratio and feeding quantity during the feeding process (Zhao et al., 2024). However, it is still unclear whether supplementary feeding and restricted feedings have any effect on the flavor of mutton.
This study systematically examines the biochemical and flavor alterations in Ovis aries (Hu sheep) meat quality parameters induced by supplementary feeding and restricted feeding. For the experimental design, we established three groups: control group, supplementary feeding group, and restricted feeding group. This study investigates the effects of adjusting concentrate-to-forage ratios and feeding levels on chemical parameters, volatile flavor compounds, and precursor substances (fatty acids and amino acids) in Hu sheep meat. We further analyze the expression of regulatory enzymes related to lipid metabolism and protein synthesis to elucidate underlying mechanisms. This study aims to systematically compare the effects of supplementary and restricted feeding on meat quality, flavor compounds, and lipid metabolism in Hu sheep, thereby providing actionable insights for precision feeding strategies.
2. Materials and methods
2.1. Animal ethics statement
All experiments received approval from the Animal Ethics Committee of Nanjing Agricultural University (AEC approval No. PZ2022045). The sampling procedures were as per the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
2.2. Animals, experimental design, and sample collection
A total of 15 female Hu sheep lambs with uniform paternal genetics, all at 90 days of age and with similar body weights, were selected from an established closed breeding nucleus herd and randomly allocated to three groups: control (CON), supplementary feeding (SF), and restricted feeding (RF). Each sheep was housed in an individual pen. The CON group was fed a basal diet according to the National Academies Press, using a compound feed with a ratio of concentrate (commercial mixed diet) to forage of 4:6. The SF group received a compound feed with a ratio of concentrate to forage of 7:3. The daily feeding amount was the same as the CON group. The RF group was fed at 55 % of the CON group's feeding amount (Fan et al., 2018). Its ratio of concentrate to forage was also 4:6. Table S1 displays the dietary composition and nutritional value. After one week of acclimatization, the study commenced with a 60-day trial period (Zhao et al., 2016). Sheep were fed twice daily at 09:00 and 18:00 following standard farm protocols, with unrestricted access to drinking water throughout the study. The pens were maintained in a clean, dry, and periodically sanitized condition.
Following the feeding trial, the humane slaughter of sheep on the farm is carried out by qualified professionals under the supervision of veterinarians in compliance with animal welfare standards. Sheep were housed in holding pens overnight and subjected to a 16 h fasting period, with free access to drinking water throughout the process. The jugular blood samples were obtained post-16 h fasting. Serum was separated via centrifugation (3000 ×g, 10 min, 4 °C), aliquoted, snap-frozen in liquid nitrogen, and preserved at −80 °C for downstream analysis. According to animal welfare protocols, the humane slaughter of sheep on the farm is carried out by qualified professionals under the supervision of veterinarians in compliance with animal welfare standards. The sheep were slaughtered using electrical stunning followed by exsanguination. The left longissimus thoracis muscle was collected within 30 min after slaughter and cutting off any visible surface fat and connective tissue. Muscle samples were divided into two portions: one subjected to meat quality analysis (chilling at 4 °C), and the remainder snap-frozen in liquid nitrogen and archived at −80 °C for long-term preservation.
2.3. Biochemical analysis of serum
Serum levels of glucose (GLU), triglycerides (TG), total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL—C), and low-density lipoprotein cholesterol (LDL-C) were quantified using an automatic biochemical analyzer (7020, Hitachi, Tokyo, Japan) with commercial kits (Medicalsystem Biotechnology Co., Ltd., China). Assays employed the following enzymatic methods: GLU (H108, Hexokinase method); TG (H201, GPO-PAP method); CHOL (H202, CHOD-PAP method); HDL-C (H206, Direct method – catalase clearance); LDL-C (H207, Direct method - surfactant clearance).
2.4. Meat quality evaluation
Meat quality evaluation focused on longissimus thoracis sections spanning the 6th–12th rib interface. A 1:1 scale tracing of the 12th–13th rib cross-section was made using transparent paper to determine eye muscle area (EMA) via the formula: EMA = muscle width × height × 0.7. 45 min after slaughter, the pH analysis was performed using an automatic temperature-compensated portable pH meter (Seven2Go™, METTLER TOLEDO), calibrated with pH 4.00/7.00 buffers. Triplicate measurements per sample were averaged for final pH values. A chroma meter (CR-400, KONICA MINOLTA Japan, aperture size of 8.0 mm, illuminant D65, observer angle of 2°) was utilized to measure the redness (a*), yellowness (b*), and brightness (L*) of the meat, After the newly cut surface of the sample bloomed about 40 min at approximate 20 °C, the chroma meter was calibrated with the white board for measurement, The color of each sample was tested three times in three different directions. The contents of moisture, dry matter, and crude fat in the meat were determined using recognized AOAC methods. Moisture and dry matter profiles were quantified through oven desiccation at 103 ± 2 °C until mass stabilization, while lipid content was determined via standardized Soxhlet solvent extraction.
2.5. Hematoxylin and eosin (H&E) staining and oil red O staining
Longissimus thoracis specimens underwent dual processing protocols: (a) 4 % paraformaldehyde fixation (16 ± 2 h/4 °C) followed by paraffin embedding, with 5 μm sections subjected to H&E staining; (b) Fresh-frozen tissues were cryosectioned at 8 μm, fixed in 10 % formalin (10 min/RT), and rinsed with 60 % isopropanol. Sections were stained with 0.3 % Oil Red O solution (pre-filtered, 15 min). Excess dye was removed by 60 % isopropanol (1 min) and distilled water washes, followed by counterstained with Mayer's hematoxylin (30 s), rinsed in running tap water (5 min), and briefly dipped in 0.1 % acid alcohol (1–2 s) for differentiation and aqueous mounting. All microscopic observations were conducted using an Olympus BX53 system (Olympus Corporation, Tokyo, Japan), with morphometric parameters (cross-sectional area, fiber diameter) quantified through Image-Pro Plus 6.0.
2.6. Analysis of amino acids profile
Approximately 100 mg of sample was weighed into borosilicate glass vials. Each vial received 10 mL of 6 mol/L hydrochloric acid, flash-frozen in liquid nitrogen, and subjected to nitrogen purging for 3 ± 0.5 min to remove oxygen. Hydrolysates generated under controlled thermal hydrolysis (110 °C, 22–24 h) were volumetrically standardized to 50 mL using ultrapure water. Aliquots (1 mL) underwent nitrogen-assisted solvent evaporation followed by reconstitution in 2 mL 0.02 M HCl. Post-filtration through 0.22 μm PVDF membranes (Millipore SLGV033RS), amino acid profiles were resolved via Hitachi L-8900 analyzer employing cation-exchange chromatography with ninhydrin detection.
2.7. Analysis of fatty acids profile
Muscle lipids were extracted using a modified Folch method. Briefly, 2 g of longissimus thoracis tissue was weighed into 15 mL borosilicate glass tubes and spiked with 200 μL heptadecanoic acid (C17:0, 10 mg/mL in methanol) as internal standard prior to homogenization in 2 mL chloroform:methanol (2:1, v/v). After adding 1 mL hydrochloric acid (8.3 mol/L), the mixture was incubated at 80 °C for 2 h for hydrolysis. Following cooling to room temperature, 1 mL sodium hydroxide solution (2 % w/v) was added and incubated at 65 °C for 30 min. The solution was then rapidly cooled, and 2 mL boron trifluoride-methanol solution (14–15 % w/v) was added for methylation at 65 °C for 30 min. After final cooling, 2 mL saturated sodium chloride solution and 2 mL n-hexane were added. The mixture was vortexed vigorously for 2 min and centrifuged at 1000 ×g for 5 min to achieve phase separation. The upper n-hexane layer containing fatty acid methyl esters (FAMEs) was collected, filtered through a 0.22-μm PTFE membrane, and analyzed via gas chromatography (Agilent 6890 N) equipped with a CP-Sil 88 capillary column (100 m × 0.25 mm × 0.25 μm) coupled to an Agilent 5977 mass spectrometer. Chromatographic conditions: Helium carrier gas (1.0 mL/min), split ratio 10:1, injection volume 1 μL. Oven temperature program: 100 °C (5 min hold) → 4 °C/min to 240 °C (15 min hold). FAMEs were identified and quantified against a certified 37-component reference standard (Supelco 18,919-1AMP, Sigma, MO, USA) using the internal standard method.
2.8. Electronic nose analysis
E-nose profiling was conducted on a PEN3 multisensory array (Winmuster Airsense Analytic Inc., Germany) equipped with 10 discrete metal oxide semiconductor (MOS) detectors (sensor specifications in Table S2). Muscle specimens (1.0 g longissimus thoracis) underwent thermal equilibration (95 °C, 30 min) in 10 mL hermetic vials prior to dynamic headspace sampling. Volatile organic compounds were delivered to the sensor chamber at 400 mL/min for 150 s (1 Hz data acquisition). Electronic nose data were acquired over 150 s. Analysis was performed using the 121–125 s interval, representing the stable plateau phase where sensor responses reached equilibrium. Post-acquisition system sanitization involved 100 s active air scrubbing until sensor resistances stabilized at baseline levels. Triplicate measurements were performed per biological replicate.
2.9. Analysis of volatile flavor compounds
Samples were immediately frozen in liquid nitrogen post-harvest then stored at −80 °C until analysis. For processing, samples were ground into powder under liquid nitrogen. A 0.2 g aliquot of the powder was transferred to a 20 mL headspace vial (Agilent, Palo Alto, CA, USA) containing 0.2 g NaCl to inhibit enzymatic activity. Vials were sealed with crimp caps fitted with TFE‑silicone septa (Agilent). During SPME analysis, vials were equilibrated at 60 °C for 5 min, followed by exposure of a 120 μm DVB/CWR/PDMS fiber (Agilent) to the headspace at 60 °C for 15 min. After sampling, the fiber was desorbed in the injection port of an Agilent 8890 gas chromatograph (GC) at 250 °C (splitless mode, 5 min). Volatile organic compounds (VOCs) were identified and quantified using an Agilent 8890 GC coupled to a 7000D mass spectrometer (MS) equipped with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm). Helium carrier gas was maintained at a constant linear velocity of 1.2 mL/min. The oven temperature program: 40 °C (3.5 min), 10 °C / min to 100 °C, 7 °C/min to 180 °C, 25 °C/min to 280 °C (5 min hold). Mass spectra were recorded in electron ionization (EI) mode at 70 eV. MS parameters: quadrupole 150 °C, ion source 230 °C, transfer line 280 °C. Analytes were quantified via selected ion monitoring (SIM). The relative odor activity value (rOAVi) was calculated according to the equation: rOAVi = Ci/OTi. Ci is the relative concentration of the volatile compound, and OTi is the odor threshold in water (Li et al., 2022).
2.10. Isolation of total RNA and quantitative polymerase chain reaction (qPCR)
Total RNA isolation was executed with TRIzol reagent (Tsingke Biotech, China), followed by cDNA synthesis from 1 μg RNA aliquots using Vazyme's reverse transcriptase master mix under standardized protocols. qPCR amplification was conducted on a Stratagene Mx3000P thermocycler with 2 μL 1:20 cDNA dilutions. The housekeeping gene HPRT1 served as endogenous control due to its stable expression across experimental conditions, with all target-specific primers (sequences detailed in Table S3) commercially synthesized by Tsingke Biotech (Beijing).
2.11. Extraction of total proteins and western blot analysis
Samples were lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 % (w/v) sodium deoxycholate, 0.1 % SDS, 1 % NP-40) and protein concentrations were quantified using a BCA Protein Assay Kit (TransGen Biotech, China) according to the manufacturer's instructions. For SDS-PAGE, 30–50 μg of protein per lane was electrophoresed on 10 % gels. Proteins were then transferred to nitrocellulose membranes (0.45 μm pore size) using a semi-dry transfer system (Bio-Rad). Western blot analysis was performed with antibodies listed in Table S4. Membranes were imaged on a Tannon-5200 imaging system (Shanghai, China) and band densities were analyzed using ImageJ with α-tubulin as the loading control.
2.12. Statistical analysis
Quantitative data are presented as the mean ± SEM. Statistical processing was conducted with complementary platforms (SPSS 22.0 and GraphPad Prism v8.0), employing one-way ANOVA for intergroup comparisons. Pearson correlation analysis was performed using SPSS software (version 22.0). Analytical thresholds followed biological research standards, with statistical significance defined as P < 0.05.
3. Results
3.1. Meat quality
Table 1 presents the measurements of the meat quality for the three groups of longissimus thoracis muscle in Hu sheep. There were significant differences in body weight, eye muscle area, moisture content, and dry matter content among the three groups (P < 0.05). The intramuscular lipid content in the CON and SF groups showed significantly higher values compared to the RF group (P < 0.05). Additionally, the L* value in the CON and SF groups showed significantly lower values than that in the RF group (P < 0.05). The SF group had a considerably greater a* value than the RF group (P < 0.05). The photographs of the recently harvested longissimus thoracis muscle were in agreement with the measurements of the colorimeter (Fig. S1 A). The Oil Red O staining results in the longissimus thoracis muscle agreed with the intramuscular lipid content identified using the Soxhlet extraction method (Fig. S1B).
Table 1.
Effects of supplementary feeding and restricted feeding on the meat quality of the longissimus thoracis muscles of Hu sheep.
| Trait | CON | SF | RF | SEM | P-value |
|---|---|---|---|---|---|
| Weight (kg) | 18.80 ± 3.66b | 30.8 ± 1.48a | 12.50 ± 2.65c | 2.14 | <0.001 |
| L*45 min | 36.8 ± 1.03b | 36.1 ± 1.30b | 43.4 ± 4.17a | 1.08 | 0.001 |
| a*45 min | 17.4 ± 1.46ab | 18.1 ± 0.70a | 15.6 ± 1.80b | 0.43 | 0.043 |
| b*45 min | 4.2 ± 0.84 | 4.3 ± 0.55 | 5.0 ± 0.81 | 0.20 | 0.261 |
| pH45min | 6.53 ± 0.21 | 6.54 ± 0.14 | 6.67 ± 0.68 | 0.04 | 0.304 |
| Eye muscle area (cm2) | 9.44 ± 2.25b | 17.91 ± 3.09a | 6.08 ± 0.65c | 1.49 | <0.001 |
| Moisture (%) | 76.33 ± 0.69b | 74.40 ± 0.65c | 78.42 ± 1.40a | 0.47 | <0.001 |
| Dry matter (%) | 23.87 ± 0.88b | 25.30 ± 0.65a | 21.58 ± 1.40c | 0.48 | <0.001 |
| Fat (%) | 3.42 ± 0.52a | 4.27 ± 1.27a | 2.09 ± 0.54b | 0.32 | 0.011 |
Values are mean ± SD. a, b, c Mean values with unlike letters statistically significant (P < 0.05) for parameters where the overall one-way ANOVA was significant (P < 0.05). Parameters without letters show no significant difference among groups (ANOVA P ≥ 0.05). CON = control group; SF = supplementary feeding group; RF = restricted feeding group. SEM = standard error of the mean.
3.2. Serum biochemical parameters
Serum biochemical profiles are presented in Fig. S1. The SF group demonstrated significantly elevated glucose (GLU) concentrations compared to RF group (P < 0.05), along with a marked increase in triglycerides (TG) relative to CON group (P < 0.05). However, cholesterol (CHOL), high-density lipoprotein cholesterol (HDL—C) and low-density lipoprotein cholesterol (LDL-C) levels remained statistically comparable across all experimental groups.
3.3. Characteristics of volatile compounds
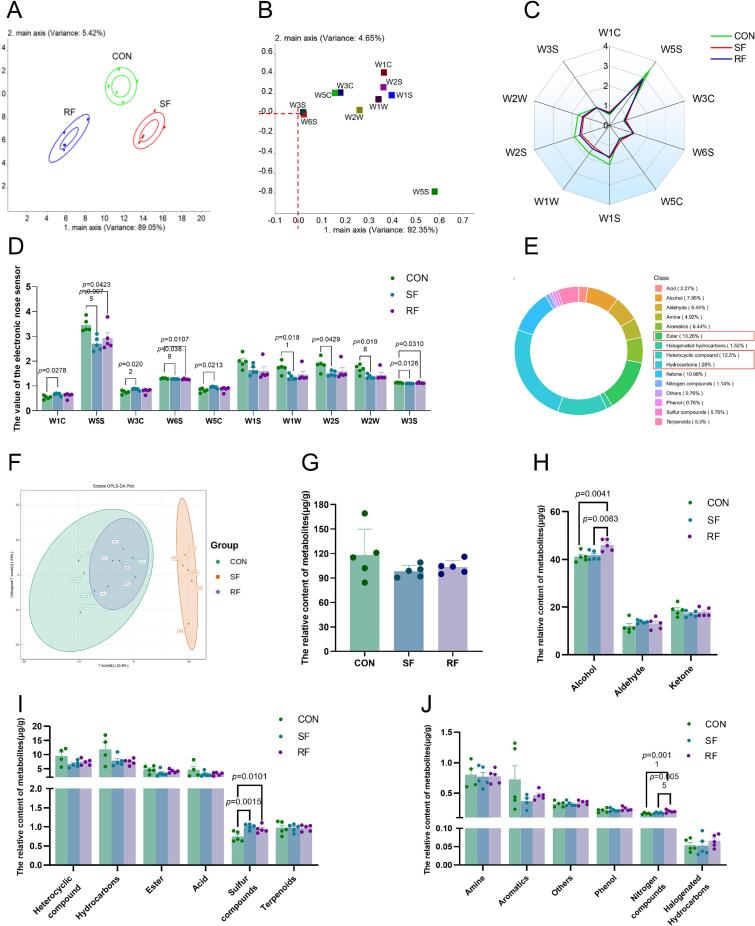
Analysis of three groups of mutton using an electronic nose showed notable distinctions among them (Fig. 1A). Nitrogen oxides (W5S), methyl compounds (W2S), and aromatic components (W1C) were key discriminators of mutton volatile profiles, as their corresponding electronic nose sensors showed the highest loadings on PC1 and PC2 (Fig. 1B). Notably, the volatile compound profiles (Fig. 1C) and the values of 10 sensors (Fig. 1D) were comparable between the SF and RF groups. However, the CON group exhibited higher response value. The subsequent gas chromatography–mass spectrometry (GC–MS) investigation indicated that hydrocarbons, esters, and heterocyclic compounds were the main constituents of mutton, as shown in Fig. 1E. The OPLS-DA analysis revealed that the CON and RF groups exhibited similarities. However, they were distinct from the SF group (Fig. 1F). There was no significant difference in the total relative amount of identified chemicals across the three groups, as shown in Fig. 1G. When examining the classification in greater detail, it was found that the RF group had larger levels of alcohol and nitrogen-containing compounds compared to the CON and SF groups. On the other hand, the SF and RF groups had higher levels of sulfur compounds compared to the CON group (Fig. 1H-J).
Fig. 1.
Effects of supplementary and restricted feeding on flavor characteristics. PCA (rincipal component analysis) for E-nose analysis of three groups (A); Loading plot of electronic nose sensors. Each point represents an individual metal oxide semiconductor (MOS) sensor in the array. The position of a sensor relative to the origin indicates its contribution to the principal components: sensors farther from the origin contribute more significantly to sample discrimination (B); Radar plot of lamb flavor (C); The detection value of 10 sensors of electronic nose (D); The proportion of vari ous compounds was detected by GC–MS (E); PLS-DA analysis of the three groups of samples (F); The relative content of classified compounds (G-J). The values are presented as mean ± SEM (n = 5).
3.4. Differential flavor compounds and key flavor compounds
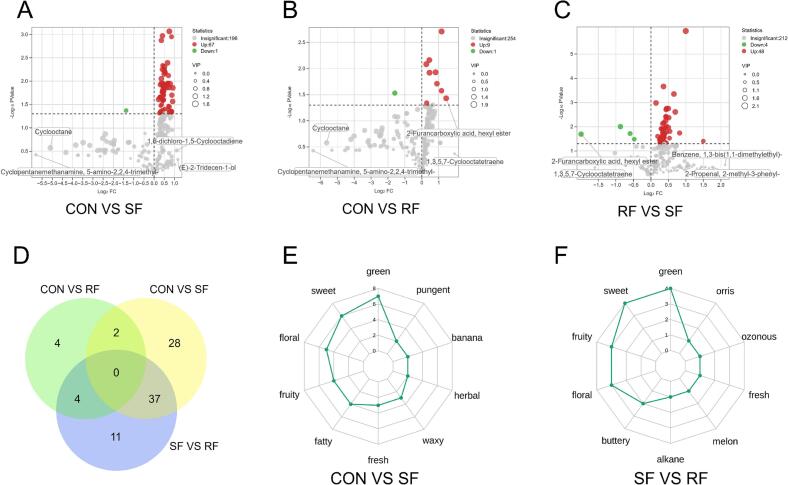
The analysis of the volatile compounds discovered in all three groups showed that the SF group had 67 compounds with higher concentrations and one with lower concentrations compared to the CON group (P < 0.05) (Fig. 2A). Similarly, the RF group had 9 compounds with higher concentrations and one with lower concentrations (P < 0.05) (Fig. 2B). In contrast to the RF group, the SF group exhibited 48 compounds with elevated content and 4 compounds with lower concentrations (P < 0.05) (Fig. 2C). Two compounds were found to be present in both differential analyses (Fig. 2D). By identifying the 67 compounds in the SF group that had a higher concentrations than the CON group with sensory taste characteristics, the top 10 most frequently occurring flavor characteristics are displayed in Fig. 2E. These features include green, sweet aroma, floral, fruity, fatty, fresh, waxy, herbal, banana, and pungent. Furthermore, when annotating the 48 compounds in the SF group that exhibited increased content compared to the RF group, the top 10 flavor descriptors that were observed most frequently are green, sweet aroma, fruity, flowery, buttery, alkane, melon, fresh, ozonous, and orris (Fig. 2F). Based on odor activity values, a total of 29 key compounds that greatly impact the flavor of mutton were picked (rOAV ≥1) (Table 2). Out of them, six compounds exhibited notable variations in their content among the three groups.
Fig. 2.
The difference compounds between the three groups and the flavor presented by the difference compounds. Statistics on the change of different volatile flavor compounds in the three groups (P < 0.05) (A-C); Venn diagram showing the same different compounds between different groups (D); Annotable volatile flavor compounds-enriched flavor intensity radar map for compounds with significantly increased content in SF group (E-F). The values are presented as mean ± SEM (n = 5).
Table 2.
OAV value of key volatile components identified by GC–MS in three group Hu sheep.
| Volatile compound | Threshold (ug/kg) |
Odor | OAV value |
SEM | P valve | ||
|---|---|---|---|---|---|---|---|
| CON | SF | RF | |||||
| 3(2H)-Furanone, dihydro-2-methyl- | 0.01 | sweet, solvent, bread, buttery, nutty | 2,852,000.00 ± 168,136.85 | 2,738,000.00 ± 235,202.04 | 2,888,000.00 ± 343,467.61 | 64,274.71 | 0.644 |
| 2,4-Undecadienal | 0.01 | green, buttery, spicy, baked, fruity, fatty, aldehydic, chicken | 38,640.00 ± 14,655.65a | 28,780.00 ± 3306.36a | 10,673.80 ± 14,572.25b | 4233.78 | 0.010 |
| 1-Octen-3-ol | 2 | mushroom | 6288.00 ± 363.21b | 6340.00 ± 302.65b | 7064.00 ± 380.76a | 126.41 | 0.007 |
| Cyclohexanone, 2,2,6-trimethyl- | 0.10 | pungent, thujone, labdanum, honey, cistus | 4832.00 ± 541.64 | 5504.00 ± 765.36 | 4818.00 ± 1115.69 | 218.58 | 0.369 |
| β-Ionone | 0.01 | floral, woody, sweet, fruity, berry, tropical, beeswax | 1215.20 ± 1258.94 | 1392.80 ± 2631.74 | 512.80 ± 333.66 | 417.79 | 0.694 |
| 5-Heptenal, 2,6-dimethyl- | 16.00 | fresh, ozonous, melon, fresh air, sweet, green | 325.20 ± 107.14 | 471.00 ± 25.80 | 398.00 ± 134.16 | 28.76 | 0.112 |
| Dodecanenitrile | 0.09 | citrus, orange, peel, metallic, spicy | 129.86 ± 53.76 | 194.00 ± 90.27 | 285.00 ± 115.97 | 27.49 | 0.055 |
| (E)-2-Octenal | 3.00 | fresh, cucumber, fatty, green, herbal, banana, waxy, leafy | 181.40 ± 16.29 | 186.00 ± 10.17 | 204.00 ± 18.48 | 4.51 | 0.087 |
| Nonanal | 1.00 | aldehyde, citrus, orange peel | 195.40 ± 62.64 | 179.40 ± 77.53 | 202.60 ± 12.14 | 14.10 | 0.814 |
| 2-Hexenal | 17.00 | sweet, almond, fruity, green, leafy, apple, plum, vegetable | 130.60 ± 20.06 | 139.20 ± 14.41 | 146.60 ± 17.04 | 4.49 | 0.374 |
| .alpha.-Irone | 2.00 | orris, floral, berry, violet, woody, powdery | 129.68 ± 28.10 | 139.20 ± 40.20 | 163.60 ± 23.58 | 8.43 | 0.252 |
| 2-methoxy-Phenol | 1.60 | nutty | 132.40 ± 20.45 | 134.80 ± 17.37 | 149.60 ± 20.07 | 5.05 | 0.347 |
| Linalool | 6.00 | floral, green | 101.74 ± 15.20 | 106.02 ± 10.55 | 104.08 ± 5.61 | 2.71 | 0.834 |
| 10-Undecenal | 3.50 | waxy, aldehydic, rose, mandarin, citrus, soapy, fatty | 223.82 ± 235.13 | 44.86 ± 22.96 | 40.48 ± 12.64 | 39.84 | 0.092 |
| 2(3H)-Furanone, 5-hexyldihydro- | 1.10 | fresh, oily, waxy, peach, coconut, buttery, sweet | 38.06 ± 10.47 | 43.04 ± 19.95 | 63.36 ± 20.18 | 5.10 | 0.091 |
| 2-Undecanone | 6.20 | waxy, fruity, creamy, fatty, orris, floral | 166.80 ± 225.75 | 11.57 ± 6.32 | 9.85 ± 3.52 | 36.86 | 0.134 |
| Heptanoic acid, methyl ester | 4.00 | sweet, fruity, green, orris, waxy, floral, berry | 7.07 ± 0.83 | 8.40 ± 1.31 | 7.76 ± 1.21 | 0.31 | 0.220 |
| Diallyl Sulfur compounds | 100.00 | sulfury, onion, garlic, horseradish, metallic | 5.51 ± 1.35b | 7.95 ± 0.56a | 7.37 ± 0.84a | 0.36 | 0.005 |
| 4-Nonanone | 8.20 | – | 6.77 ± 0.55 | 7.53 ± 1.11 | 7.49 ± 1.13 | 0.25 | 0.400 |
| .beta.-Myrcene | 15.00 | musty, balsamic, spice | 9.86 ± 5.32 | 7.31 ± 0.36 | 8.86 ± 1.35 | 0.81 | 0.465 |
| (E)-2-Decenal | 5.00 | waxy, fatty, earthy, green, cilantro, mushroom, aldehydic, fried, chicken, fatty, tallow | 4.36 ± 1.66 | 6.07 ± 1.36 | 5.39 ± 1.83 | 0.43 | 0.285 |
| 6-Methyl-3,5-heptadiene-2-one | 100.00 | – | 4.82 ± 0.49 | 5.69 ± 0.78 | 5.29 ± 0.68 | 0.18 | 0.156 |
| 2(3H)-Furanone, dihydro-5-propyl- | 400.00 | sweet, coconut, nutty, caramel, tonka, hay | 3.67 ± 0.43b | 3.78 ± 0.47b | 5.02 ± 0.76a | 0.21 | 0.005 |
| 1-Decanol | 23.00 | fatty, waxy, floral, orange, sweet, watery | 2.86 ± 2.31 | 3.53 ± 4.47 | 2.47 ± 1.27 | 0.73 | 0.854 |
| .alpha.-Ionone | 3.78 | sweet, woody, floral, violet, orris, tropical, fruity | 2.54 ± 1.13 | 3.04 ± 0.84 | 3.14 ± 0.27 | 0.21 | 0.495 |
| Geraniol | 6.60 | sweet, floral, fruity, rose, waxy, citrus | 2.54 ± 0.78 | 2.51 ± 0.79 | 2.40 ± 0.78 | 0.19 | 0.955 |
| Acetic acid, 2-ethylhexyl ester | 47.00 | earthy, herbal, humus, undergrowth | 8.21 ± 3.10a | 2.45 ± 2.87b | 6.25 ± 0.73a | 0.87 | 0.010 |
| 2-methyl-3-Pentanol | 420.00 | – | 1.34 ± 0.15b | 1.54 ± 0.06a | 1.38 ± 0.12b | 0.04 | 0.040 |
| Benzene, n-butyl- | 14.00 | – | 1.15 ± 0.11 | 1.38 ± 0.19 | 1.24 ± 0.14 | 0.04 | 0.095 |
Values are mean ± SD. a, b, c Mean values with unlike letters statistically significant (P < 0.05) for parameters where the overall one-way ANOVA was significant (P < 0.05). Parameters without letters show no significant difference among groups (ANOVA P ≥ 0.05). CON = control group; SF = supplementary feeding group; RF = restricted feeding group. SEM = standard error of the mean. Flavor character: Reference from Flavordb database (https://cosylab.iiitd.edu.in/flavordb/) and Foodflavorlab database (http://foodflavorlab.cn/#/home); RI: retention index; Threshold: odor threshold values were taken from the VCF online database (https://www.vcf-online.nl) and literature (Sohail et al., 2022); OAV: odor activity value.
3.5. Fatty acid and amino acid composition of the longissimus thoracis muscle
Table 3 displays the fatty acid composition of the longissimus thoracis muscle. The C16:0, C17:0, C18:1n9t, C18:2n6c, C18:3n6, and C20:2n6 fatty acid contents were higher in the SF group. The RF group had higher C20:0, C23:0, and C22:6n3 fatty acid contents. The above changes in fatty acid content resulted in higher levels of saturated fatty acids and unsaturated fatty acids, but lower levels of n-3 PUFA in the SF group, so the SF group had a higher ratio of n-6/n-3 PUFA ratio.
Table 3.
Effects of supplementary feeding and restricted feeding on the fatty acids profile of the longissimus thoracis of Hu sheep (mg/100 g muscle tissue).
| Trait | CON | SF | RF | SEM | P-value |
|---|---|---|---|---|---|
| Saturated fatty acid | |||||
| C10:0 | 0.39 ± 0.21 | 0.04 ± 0.01 | 0.06 ± 0.07 | 0.12 | 0.548 |
| C14:0 | 3.93 ± 1.25 | 3.49 ± 1.47 | 2.99 ± 1.05 | 0.32 | 0.523 |
| C15:0 | 1.89 ± 0.28 | 1.83 ± 0.40 | 1.98 ± 0.33 | 0.08 | 0.787 |
| C16:0 | 215.04 ± 6.41b | 239.72 ± 15.07a | 181.66 ± 12.35c | 6.96 | <0.001 |
| C17:0 | 8.83 ± 0.60b | 10.28 ± 1.13a | 8.51 ± 0.96b | 0.30 | 0.023 |
| C18:0 | 204.97 ± 20.72 | 217.66 ± 13.11 | 213.48 ± 32.01 | 5.74 | 0.688 |
| C20:0 | 2.14 ± 0.26b | 1.86 ± 0.46b | 2.80 ± 0.48a | 0.14 | 0.010 |
| C22:0 | 3.69 ± 1.90 | 5.42 ± 1.64 | 3.45 ± 3.08 | 0.60 | 0.364 |
| C23:0 | 2.61 ± 0.93ab | 1.79 ± 0.43b | 3.53 ± 0.56a | 0.25 | 0.006 |
| C24:0 | 8.66 ± 1.54 | 7.33 ± 1.23 | 8.39 ± 2.18 | 0.43 | 0.447 |
| SFA | 451.71 ± 25.71ab | 489.95 ± 25.30a | 427.51 ± 38.16b | 9.93 | 0.020 |
| Monounsaturated fatty acid | |||||
| C14:1 | 2.68 ± 1.83 | 2.40 ± 1.45 | 1.92 ± 1.19 | 0.37 | 0.734 |
| C16:1 | 12.40 ± 1.88 | 13.45 ± 3.17 | 10.03 ± 2.62 | 0.73 | 0.147 |
| C17:1 | 18.78 ± 3.29 | 21.30 ± 4.55 | 17.84 ± 2.04 | 0.91 | 0.297 |
| C18:1n9t | 14.04 ± 1.09b | 29.70 ± 5.75a | 10.23 ± 2.52b | 2.42 | <0.001 |
| C18:1n9c | 347.11 ± 21.33 | 325.93 ± 43.04 | 305.05 ± 54.32 | 11.01 | 0.318 |
| C22:1n9 | 1.04 ± 0.44 | 0.94 ± 0.50 | 0.96 ± 0.29 | 0.10 | 0.918 |
| C24:1 | 3.68 ± 1.11 | 3.68 ± 0.75 | 4.88 ± 1.50 | 0.32 | 0.208 |
| MUFA | 399.72 ± 20.15 | 397.39 ± 52.61 | 350.91 ± 58.35 | 12.70 | 0.219 |
| Polyunsaturated fatty acid | |||||
| C18:2n6c | 215.42 ± 30.07b | 308.87 ± 36.96a | 174.52 ± 37.79b | 17.21 | <0.001 |
| C18:3n6 | 2.50 ± 0.75b | 3.71 ± 0.51a | 1.85 ± 0.32b | 0.24 | <0.001 |
| C18:3n3 | 9.29 ± 0.77 | 7.82 ± 1.53 | 9.50 ± 2.09 | 0.42 | 0.220 |
| C20:2n6 | 1.54 ± 0.55b | 2.75 ± 0.89a | 1.48 ± 0.34b | 0.22 | 0.013 |
| C20:3n6 | 10.58 ± 0.85 | 11.56 ± 1.41 | 10.53 ± 1.58 | 0.34 | 0.404 |
| C20:3n3 | 0.98 ± 0.27 | 0.83 ± 0.17 | 1.19 ± 0.62 | 0.10 | 0.381 |
| C20:4n6 | 161.27 ± 20.94 | 181.67 ± 18.48 | 188.24 ± 37.40 | 7.14 | 0.293 |
| C20:5n3 | 3.69 ± 1.14 | 3.49 ± 0.46 | 4.56 ± 1.10 | 0.26 | 0.209 |
| C22:6n3 | 3.78 ± 0.90b | 2.99 ± 0.53b | 5.75 ± 1.82a | 0.42 | 0.010 |
| PUFA | 409.06 ± 52.63b | 523.69 ± 49.67a | 397.64 ± 67.10b | 20.42 | 0.008 |
| n-3 PUFA | 17.75 ± 2.62ab | 15.12 ± 2.41b | 21.01 ± 4.05a | 0.98 | 0.035 |
| n-6 PUFA | 391.32 ± 51.59b | 508.57 ± 49.77a | 376.63 ± 64.43b | 20.64 | 0.005 |
| n-6/n-3 | 22.26 ± 3.03b | 34.51 ± 7.88a | 18.19 ± 2.69b | 2.22 | <0.001 |
| UFA | 808.79 ± 50.79b | 921.08 ± 71.83a | 748.54 ± 108.98b | 27.18 | 0.017 |
Values are mean ± SD. a, b, c Mean values with unlike letters statistically significant (P < 0.05) for parameters where the overall one-way ANOVA was significant (P < 0.05). CON = control group; SF = supplementary feeding group; RF = restricted feeding group. SEM = standard error of the mean. SFA: Saturated fatty acids = C10:0 + C14:0 + C15:0 + C16:0 + C17:0 + C18:0 + C20:0 + C22:0 + C23:0 + C24:0. MUFA: Monounsaturated fatty acids = C14:1 + C16:1 + C17:1 + C18:1n9t + C18:1n9c + C20:1n9 + C24:1. PUFA: Polyunsaturated fatty acids = C18:2n6c + C18:3n3 + C18:3n6 + C20:2n6 + C20:3n6 + C20:3n3 + C20:4n6 + C20:5n3 + C22:6n3. n-3 PUFA = C18:3n3 + C20:3n3 + C20:5n3 + C22:6n3. n-6 PUFA = C18:2n6c + C18:3n6 + C20:2n6 + C20:3n6 + C20:4n6. TFA: Total fatty acids = SFA + MUFA + PUFA.
Table 4 displays the amino acid composition of the longissimus thoracis muscle. The content of lysine, phenylalanine, threonine, leucine, isoleucine, essential amino acids (EAA), arginine, aspartic acid, alanine, tyrosine, non-essential amino acids (NEAA) and total amino acids were notably higher in the SF group compared to the RF group. The content of Valine, Histidine, Cystine, and EAA/NEAA (%) was considerably elevated in both the CON and SF groups. Additionally, the content of proline was significantly higher in the SF group compared to the CON and RF groups. The levels of glutamic acid, glycine, serine, and flavor amino acids were similar among all three groups.
Table 4.
Effects of supplementary feeding and restricted feeding on the amino acid profile of the longissimus thoracis of Hu sheep (mg/g).
| Trait | CON | SF | RF | SEM | P-value |
|---|---|---|---|---|---|
| Essential amino acids (EAA) | |||||
| Lysine | 14.66 ± 1.16ab | 15.83 ± 0.72a | 13.26 ± 1.51b | 0.40 | 0.016 |
| Phenylalanine | 6.53 ± 0.55ab | 7.10 ± 0.31a | 5.85 ± 0.68b | 0.19 | 0.010 |
| Threonine | 7.62 ± 0.56ab | 8.07 ± 0.35a | 6.98 ± 0.76b | 0.18 | 0.036 |
| Methionine | 3.53 ± 0.53 | 3.71 ± 0.80 | 3.24 ± 0.61 | 0.17 | 0.537 |
| Valine | 8.00 ± 0.70a | 8.79 ± 0.40a | 7.11 ± 0.82b | 0.24 | 0.006 |
| Leucine | 13.36 ± 1.14ab | 14.43 ± 0.65a | 12.09 ± 1.41b | 0.37 | 0.020 |
| Isoleucine | 7.32 ± 0.76ab | 8.09 ± 0.41a | 6.54 ± 0.81b | 0.24 | 0.012 |
| Histidine | 6.00 ± 0.65a | 6.23 ± 0.38a | 4.65 ± 0.93b | 0.25 | 0.007 |
| EAA | 67.03 ± 5.87ab | 72.27 ± 3.93a | 59.71 ± 7.50b | 1.98 | 0.019 |
| Non-essential amino acids (NEAA) | |||||
| Arginine | 10.34 ± 0.83ab | 11.08 ± 0.55a | 9.57 ± 0.92b | 0.25 | 0.031 |
| Asparticacid | 15.07 ± 1.16ab | 16.16 ± 0.69a | 13.69 ± 1.53b | 0.39 | 0.020 |
| Glutamicacid | 24.79 ± 1.89 | 26.10 ± 1.22 | 23.23 ± 1.95 | 0.52 | 0.064 |
| Glycine | 6.95 ± 0.40 | 7.34 ± 0.37 | 6.77 ± 0.41 | 0.11 | 0.102 |
| Alanine | 9.26 ± 0.75ab | 9.87 ± 0.45a | 8.57 ± 0.79b | 0.22 | 0.033 |
| Proline | 5.53 ± 0.26b | 5.98 ± 0.30a | 5.25 ± 0.29b | 0.11 | 0.005 |
| Serine | 6.29 ± 0.41 | 6.85 ± 0.30 | 6.10 ± 0.58 | 0.14 | 0.051 |
| Tyrosine | 5.63 ± 0.45ab | 6.00 ± 0.34a | 5.11 ± 0.56b | 0.15 | 0.028 |
| Cystine | 1.63 ± 0.08a | 1.71 ± 0.12a | 1.44 ± 0.17b | 0.04 | 0.016 |
| NEAA | 85.74 ± 6.14ab | 91.09 ± 4.25a | 79.70 ± 6.90b | 1.88 | 0.031 |
| EAA/NEAA (%) | 78.11 ± 1.39a | 79.31 ± 0.87a | 74.71 ± 3.1b | 0.71 | 0.010 |
| Flavor amino acids | 59.19 ± 4.59 | 62.69 ± 3.39 | 54.82 ± 5.44 | 1.39 | 0.054 |
| Total amino acids | 152.78 ± 12.00ab | 163.36 ± 8.15a | 139.41 ± 14.40b | 3.85 | 0.024 |
Values are mean ± SD. a, b, c Mean values with unlike letters statistically significant (P < 0.05) for parameters where the overall one-way ANOVA was significant (P < 0.05). CON = control group; SF = supplementary feeding group; RF = restricted feeding group. SEM = standard error of the mean. EAA = Lys + Phe + Thr + Met + Val + Leu + Ile + His. NEAA = Arg + Asp + Glu + Gly + Ala + Pro + Ser + Tyr + Cys. Flavor amino acids = Met + Asp + Glu + Ala + Ser.
3.6. Correlation analysis between flavor precursors and key volatile flavor compounds
Pearson correlation analysis was conducted to examine the relationships between 29 key volatile flavor compounds and flavor precursors (fatty acids and amino acids). The analysis revealed 50 statistically significant negative correlations between amino acids and volatile compounds. Notably, three compounds – dihydro-5-propyl-2(3H)-furanone, 2-octen-1-ol, and dodecanenitrile - showed consistent negative correlations with nearly all amino acids analyzed (Fig. 3A). Interestingly, the restricted feeding group exhibited significantly higher concentrations of both dihydro-5-propyl-2(3H)-furanone and 1-octen-3-ol compared to the control and supplementary feeding groups, potentially attributable to their lower amino acid content.
Fig. 3.
Correlation Analysis between Flavor Precursors and Key Volatile Flavor Compounds. Correlations between fatty acids and volatile flavor compounds (A); Correlations between amino acids and volatile flavor compounds. (B). *P < 0.05, **P < 0.01.
Regarding fatty acids, we identified 25 significant positive correlations and 15 negative correlations with volatile compounds. Particularly strong positive correlations were observed between: (1) C18:3n6/C17:0 and 2-methyl-3-pentanol; (2) C22:0 and (E)-2-decenal; and (3) C20:5n3 and (E)-2-octenal (Fig. 3B). The elevated C18:3n6 levels in the supplementary feeding group likely contributed to the higher 2-methyl-3-pentanol content observed in this group.
3.7. Lipid metabolism of the longissimus thoracis muscle
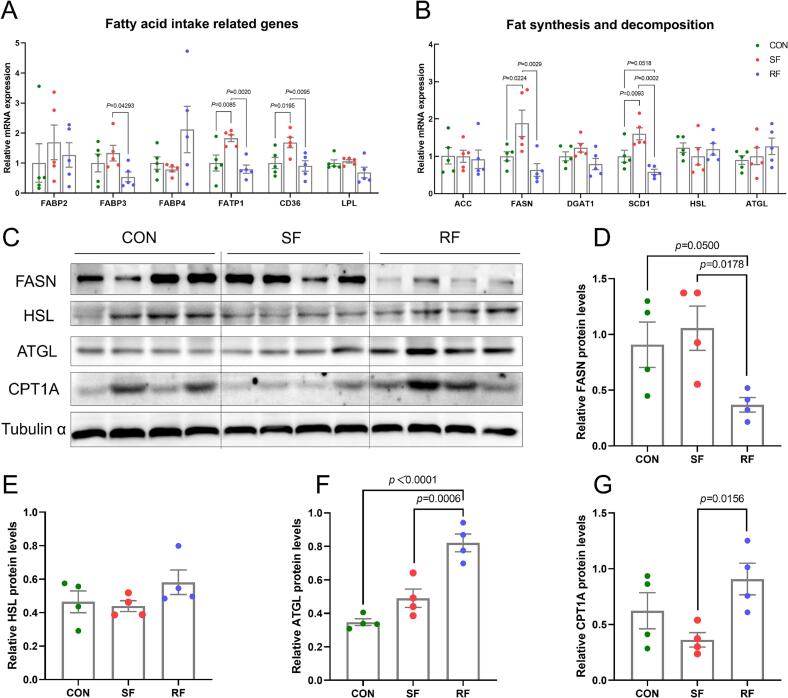
As shown in Fig. 4AB, the mRNA levels of fatty acid transport-related proteins FATP1 and CD36, as well as lipid synthesis-related proteins FASN and SCD1, were significantly higher in the SF group compared to the CON and RF groups (P < 0.05). Additionally, the mRNA levels of FABP3 were significantly higher in the SF group in comparison to the RF group (P < 0.05). The protein expression level of FASN was significantly higher in the CON and SF groups than in the RF group (P < 0.05), while the protein expression level of ATGL was significantly lower than that in the RF group (P < 0.01). The expression level of the fatty acid oxidation-associated protein CPT1A in the SF group was significantly lower than that in the RF group (P < 0.05) (Fig. 4C-G).
Fig. 4.
Effects of supplementary and restricted feeding on lipid metabolism related genes and proteins expression in longissimus thoracis muscle of Hu sheep. The mRNA expression of fatty acid intake related genes (A); The mRNA expression of lipid synthesis and decomposition related genes (B); Western blot (WB) bands of lipid metabolism related proteins (C); Quantitative results of corresponding WB bands (D-G). The values are presented as mean ± SEM (n = 4/5).
3.8. Protein synthesis and development of the longissimus thoracis muscle
The implementation of feed restriction resulted in a notable reduction in both the cross-sectional area and diameter of muscle fibers while also leading to an increase in muscle fiber density (P < 0.01) (Fig. 5A–F). The protein expression of MYOD1 in the SF group was significantly higher than that in the RF group (P < 0.05) (Fig. 5I). Similarly, the expression of PAX7 protein in the SF group was significantly higher than that in the CON and RF groups (P < 0.05) (Fig. 5J). The AMP-activated protein kinase (AMPK)-mechanistic target of rapamycin (mTOR)-ribosomal protein S6 kinase 1 (S6K1) pathway plays a crucial role in governing protein synthesis in response to the body's energy detection. The alterations in this pathway were observed, and the P-AMPK/AMPK ratio in the SF group was considerably lower compared to the RF group (P < 0.01) (Fig. 5N). The P-mTOR/mTOR value in the CON and SF groups exhibited a statistically significant increase compared to the RF group (P < 0.05) (Fig. 5Q). Additionally, the P-S6K1/S6K1 value in the SF group showed a statistically significant increase related to the RF group (P < 0.05) (Fig. 5T). Importantly, the SF group's higher amino acid pool (Table 4) driven by mTOR-S6K1 activation provides direct substrate reservoirs for flavor compound formation, mechanistically linking protein anabolism to flavor precursor enrichment.
Fig. 5.
Effects of supplementary and restricted feeding on muscle development and protein anabolism in longissimus thoracis muscle of Hu sheep. HE staining of longissimus thoracis muscle sections (A); Myofiber diameter, cross-sectional area and density (B—F); Western blot (WB) bands of muscle development related proteins (G); Quantitative results of corresponding WB bands (H-J); Western blot (WB) bands of AMPK-mTOR-S6K1 signaling pathway related proteins (K); Quantitative results of corresponding WB bands (L-T). The values are presented as mean ± SEM (n = 5).
4. Discussion
During this study, we discovered that supplementary feeding improved the quality of meat in Hu sheep. The mutton in SF group exhibited more pronounced sweetness, fruitiness, and fat flavor. Restricted feeding activated AMPK, resulting in a reduction in lipid and protein synthesis in muscle. Nevertheless, the muscles of the SF exhibited higher levels of fat production and protein anabolism. The results of our study will enhance comprehension regarding the impact of various feed compositions on the growth, development, meat quality, and muscle metabolism of Hu sheep. These findings demonstrate that dietary regimens influence meat flavor characteristics by modulating metabolic pathways that control flavor precursor production. This will aid in establishing a scientific and practical foundation for producing premium mutton that meets the demand for high-quality functional livestock products.
Body weight, eye muscle area, and nutritional content are indicators of animals' growth performance and nutrient consumption. The concentrate is a feed that has a higher amount of protein and energy. When grazing animals are given concentrate as a dietary supplementation, it enhances their performance by boosting their intake of essential nutrients (Fernandes et al., 2012). Dietary restriction leads to a constant daily gain of sheep and even negative daily gain under high-intensity nutritional restriction conditions, which may cause stunting, stagnation or even death of animals, which is caused by insufficient supply of nutrients (Zhao et al., 2016). The results showed that supplementary feeding led to an increase in dry matter content, eye muscle area, and body weight while reducing moisture content.
As rising affluence drives demand for premium livestock products, meat color has emerged as a key determinant of perceived quality in consumer markets. The alteration in meat color is indicative of the physiological, biochemical, and microbiological transformations occurring in the muscle. The intensity of the color primarily relies on the quantity and oxidation level of myoglobin present in the muscle. Myoglobin oxygenates to form bright-red oxymyoglobin, while oxidation of Fe2+ to Fe3+ yields brown metmyoglobin. This oxidation process has an impact on the a* value (Luciano et al., 2011). On the other hand, the b* value is impacted by the consumption of dietary carotenoids and the content of intramuscular fat (Nieto et al., 2010). Generally, meat with a higher a* value and a lower b* value tends to be more appealing to customers. The meat color can be altered by both the nature of the diet and the way of feeding (Zhang et al., 2022). Restricted feeding resulted in a decrease in L* value compared to both control and supplementary feeding. Additionally, restricted feeding led to a decrease in a* value compared to supplementary feeding. These changes can be attributed to the reduced synthesis of myoglobin produced by malnutrition. However, alternative research has demonstrated that restricted feeding does not impact the L*, a*, and b* value of meat (Abdullah & Musallam, 2007). This disparity in findings may be attributed to variations in the extent of restricted consumption.
The serum biochemical indices of animals indicate the extent to which nutrients are metabolically processed. Glucose is considered a crucial indicator of energy equilibrium within an animal's body, and its level decreases in the presence of an energy deficit (Teixeira et al., 2021). The serum glucose level was dramatically lower in the RF group than in the SF group. This is presumably because the RF group had lower calorie intake and utilized their glucose reserves. The higher concentrate intake promotes the fermentation of carbohydrates in the rumen, thus increasing the production of propionic acid, which is used to synthesize glucose in the liver resulting in higher blood glucose level (Piantoni & VandeHaar, 2023). Triglycerides (TG) represent the body's usage of lipids. The experiment found that the TG content in the SF group was significantly higher than that in the CON group. This could be attributed to the enhanced digestion and absorption of lipids caused by supplementary feeding.
An electronic nose, a detection system that mimics biological olfaction, is sensitive to volatile compounds in a sample, and subtle changes in volatile compounds can cause the sensor to respond differently. Electronic nose and GC–MS have been commonly employed to analyze volatile flavor compounds in meat products in order to reduce the impact of subjective sensory assessment. Both the electronic nose and GC–MS showed a comparable pattern in terms of the overall volatility of the three groups, while the CON group having the most intense odor. However, the sensing value of the electronic nose for the more finely classified compounds does not seem to be consistent with the detection content of GC–MS, which may be that the detection and classification of the same compound by the electronic nose is different from the results of GC–MS. The apparent discrepancy between the electronic nose indicating the higher response value in the CON group and GC–MS showing no difference in total volatile abundance likely arises from the electronic nose's sensitivity to the overall odor profile and key odor-active compounds (potentially present in lower concentrations but with high odor activity values, whereas GC–MS quantifies total chemical abundance regardless of sensory impact. Although supplemented-fed Hu sheep did not stand out in terms of total volatile compound content, analysis of differences with control and restricted feeding showed that the number of compounds with significantly higher levels was much more than that of compounds with reduced levels. This is consistent with Lu et al.'s study that greater protein intake enriched the volatiles of the lamb (Dou et al., 2023).
The relative odor activity value (rOAV) is an approach employed to identify the critical flavor compounds in food. It involves considering the relative content and Odor Threshold Value of these compounds to estimate their contribution to the overall flavor characteristics of the sample. Generally, a value of rOAV >1 means that the component directly contributes to the flavor of the sample (Huang et al., 2022). The 29 compounds with rOAV ≥1 have been identified in the longissimus thoracis of sheep. Particularly, Nonanal, (E)-2-Octenal, 1-Octen-3-ol, and Hexenal were considered significant components contributing to the characteristic flavor of sheep (Qi et al., 2022). Out of the 29 compounds, six exhibited group-wise rOAV value differences. Interestingly, three of these six compounds were shown to be more abundant in the RF group in comparison with the SF group, despite the mutton of SF group having a higher amount of dry matter and lower water content. This phenomenon could be due to the sorption of volatile flavor compounds by muscle lipids and proteins. Perez-Juan discovered that the volatilization of flavor compounds can be decreased by physically or chemically adsorbing various types of flavor compounds with a certain spatial conformation and structure (Pérez-Juan et al., 2007). Additions of arginine to the diet reduce the concentration of aldehydes in the volatile flavor compounds of mutton (Dou et al., 2023). Fat in the meat also entraps lipophilic flavor compounds and retards flavor volatilization (Frank et al., 2017).
Amino acids and fatty acids serve as crucial precursors for the generation of volatile flavor compounds. As an important flavor compound in pork, nonanal is directly produced by oleic acid in the cooking process of β-oxidation (Tanimoto et al., 2015). 1-Octen-3-ol and 1-octene-3-one are the factors that cause the meat flavor of mushrooms in stewed mutton and roast mutton and can be directly produced by linoleic acid heating (Frankel, 1983; Qi et al., 2022). Similarly, the decomposition of amino acids caused by the Maillard reaction mediates the generation of aldehydes and heterocyclic compounds (Van Boekel, 2006). The primary cause of the fatty flavor in pork is the production of saturated and unsaturated aldehydes through intricate processes involving polyunsaturated fatty acids (Song et al., 2017). The strong positive correlations observed between C20:5n3 and (E)-2-octenal, and between C22:0 and (E)-2-decenal, should be interpreted cautiously due to the high number of correlations tested without multiplicity correction, which increases the risk of false positives. Nevertheless, these associations remain mechanistically plausible as they likely result from oxidative cleavage of these fatty acids' carbon chains during heating, yielding 2,4-decadienal. This mechanism resembles observations in heat-processed duck meat where C22:6, C20:5n3, and C20:4n6 generate decanal (Liu et al., 2023). Notably, negative correlations were observed between nearly all amino acids (except glycine, methionine, and proline) and 1-octen-3-ol/dodecanenitrile. The observed negative correlations between specific amino acids and volatile compounds likely arise from dual biochemical mechanisms. Competitive participation in degradation pathways may redirect amino acids away from volatile aldehyde formation through reactions with α-dicarbonyl intermediates (Van Boekel, 2006). Concurrently, protein matrices rich in specific amino acid residues can physically adsorb volatile compounds, as evidenced by actomyosin-mediated retention of up to 37 % of aldehydes like hexanal via non-covalent interactions (Pérez-Juan et al., 2007; Zhang et al., 2021).
On the one hand, lipid metabolism is intricately linked to animal growth, development, and overall body health, on the other hand, it affects meat quality, such as intramuscular lipid content affecting meat tenderness and flavor. Varying energy levels in meals can impact the metabolism of body fat, consequently influencing the expression of crucial enzymes and genes involved in metabolism (Jing et al., 2020). Fatty acids are conveyed into the cytoplasm by fatty acid transporters, including fatty acid translocase (CD36), fatty acid binding protein 4 (FABP4), and fatty acid transporter protein (FATP). Within the cytoplasm, surplus fatty acids are channeled into triglycerides and phospholipid. In cells, lipids can be generated autonomously. Acetyl-CoA is converted into fatty acids through the catalytic activity of stearoyl-CoA desaturase (SCD), fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC). Subsequently, triglycerides are synthesized. Hormone-sensitive lipase (HSL) and fatty triglyceride lipase (ATGL) have the ability to hydrolyze down triglycerides (TAG) into glycerol and fatty acids. Fatty acids are transported into mitochondria via carnitine palmitoyl transferase 1 (CPT1) in order to undergo beta-oxidation to generate energy. The crucial factor for the accumulation of lipids in animal bodies is the dynamic equilibrium between the production and breakdown of fats (Sleeth et al., 2010). Supplementary feeding in this experiment induced transcriptional activation of enzymes FATP1 and CD36, which are involved in the intake of fatty acids. Additionally, it upregulated the mRNA expression levels of enzymes FASN and SCD1, which are mediating the synthesis of lipids. In Hu sheep with restricted diet, the protein expression of lipid synthetase FASN decreased, but the protein expressions of lipolytic enzymes ATGL and fatty acid oxidase CPT1A increased. During periods of low-calorie intake, the body suppresses the creation of lipids and increases its ability to burn lipids in order to meet the energy challenge for maintenance. This finding aligns with prior findings in Tibetan sheep and small-tailed Han sheep (Jing et al., 2020). Understanding these adaptive metabolic responses also provides a foundation for optimizing resource efficiency in livestock systems, such as the enzymatic valorization of processing by-products like hemoglobin (Cheng et al., 2024).
Dietary restriction has an impact on various aspects of skeletal muscle in both humans and mice, including myogenesis, muscle fiber quantity and type, mitochondrial biogenesis and function, and satellite cell function (Fulco et al., 2008). In the livestock industry, poultry and sheep that are given low-energy diets observe a reduction in skeletal muscle development (Zhao et al., 2016). In agreement with prior research, feed restriction led to a reduction in the cross-sectional diameter, area, and density of muscular fibers in the longissimus thoracis muscle. Satellite cells are myogenic stem cells in skeletal muscle. Under typical circumstances, satellite cells in the resting state have the potential for self-renewal and regeneration. Postnatal muscle fiber growth is dependent on the multiplication of satellite cells (Halevy et al., 2004). The myogenic protein MyoD1 orchestrates transcriptional programming in both body growth and muscle development. It possesses the capacity to transform many types of cells, such as fibroblasts and adipocytes, into myoblasts, which subsequently have the capability to mature into adult muscle fiber satellite cells (Jeong et al., 2021). The results of the research demonstrated that the levels of protein expression for the proliferative state marker proteins Pax7 and MYOD1 were higher in the SF group. This implies that concentrate has a greater level of nutrients and energy, which facilitates the development and growth of muscle fibers in Hu sheep.
Muscle development and hypertrophy of muscle fibers require increased protein synthesis. AMP-activated protein kinase is a crucial nutrition and energy sensor that orchestrates metabolic homeostasis, cell development, and autophagy. Elevated AMP: ATP ratio during dietary deprivation can stimulate AMPK activation by promoting phosphorylation at Thr172 (Mihaylova & Shaw, 2011). Research has demonstrated that AMPK is involved in the development of skeletal muscle and the lipid mobilization under energy deficiency. The findings indicate that restricted feeding activates AMPK, in line with the data presented in Fulco et al. (Fulco et al., 2008). mTOR kinase—a highly conserved member of the serine/threonine kinase family—orchestrates key cellular processes including protein synthesis, lipid metabolism, autophagy, and proliferation (Dou et al., 2023). AMPK suppresses the initiation of the mTOR signaling cascade, thereby blocking downstream targets from activation (Sun et al., 2020). Ribosomal protein S6 kinase, polypeptide 1 (S6K1), is a protein that is activated through phosphorylation and acts as a downstream target of mTOR. It plays a role in controlling cell growth, differentiation, and proliferation, as well as stimulating protein synthesis (Holz et al., 2021). The experiment found that phosphorylated mTOR and S6K1 levels were significantly higher in the SF group than in the RF group. This suggests that the additional nutrients in the SF group stimulated protein formation by activating the AMPK-mTOR-S6K1 signaling pathway, thereby promoting the development of the longissimus thoracis muscle in Hu sheep. These synthesized proteins subsequently serve as the primary source of amino acids, which are critical precursors for flavor compound formation during cooking. Thus, the dietary modulation of the AMPK-mTOR-S6K1 axis directly influences the pool of amino acid-derived flavor precursors in muscle tissue.
Our study reveals a novel role for the AMPK-mTOR-S6K1 axis in orchestrating flavor precursor dynamics in Hu sheep. While this pathway is established in skeletal muscle protein synthesis, we demonstrate that supplementary feeding-induced mTOR/S6K1 activation elevates key flavor precursors—including essential amino acids and flavor-associated amino acids—through enhanced protein anabolism. These precursors serve as direct substrates for volatile compound generation via Maillard reactions and Strecker degradation during cooking. Critically, the enriched muscle protein matrix (particularly hydrophobic residues) exhibits selective adsorption of lipophilic flavor compounds through non-covalent interactions, thereby Critically, the enriched muscle protein matrix (particularly hydrophobic residues) exhibits selective adsorption of lipophilic flavor compounds through non-covalent interactions, thereby modulating their volatilization their volatilization. Conversely, restricted feeding activates AMPK, suppressing mTOR-S6K1 signaling and reducing precursor availability, consistent with observed decreases in overall amino acid content under dietary restriction. This establishes the AMPK-mTOR-S6K1 axis as a molecular bridge directly linking dietary energy status to flavor biochemistry, extending its role beyond growth regulation to actively coordinate flavor precursor production.
5. Conclusion
The findings demonstrate that supplementary feeding serves as a viable approach to enhancing the meat quality of Hu sheep. Supplementary feeding improved lamb flavor, and the mutton in SF group exhibited more pronounced sweetness, fruitiness, and fat flavor due to its increased number of amino acids and fatty acids possibly, which act as flavor precursors. Restricted feeding induced the activation of AMPK, resulting in a decrease in lipid and protein synthesis in muscle. Nevertheless, the muscles of the SF group exhibited higher levels of fat production and protein anabolism. An interesting observation was that the decrease in certain flavor precursor levels in the restricted feeding group compared to the control group did not result in significant alterations in volatile flavor compound concentrations. The results of our study will enhance comprehension regarding the impact of various feed compositions on the growth, development, meat quality, and muscle metabolism of Hu sheep. This will aid in establishing a scientific and practical foundation for producing premium mutton that meets the demand for high-quality functional livestock products.
CRediT authorship contribution statement
Huangbing Sun: Methodology, Investigation, Data curation. Jianghao Wang: Software, Investigation. Haoran Kuang: Software, Resources. Guoqiang Fan: Visualization, Validation, Methodology. Xiaojing Yang: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study received support from the Project of Northern Agriculture and Livestock Husbandry Technology Innovation Center, Chinese Academy of Agricultural Sciences (BFGJ2022002) and Inner Mongolia Zhongnong North Agriculture and Animal Husbandry Technology Co., Ltd.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2025.102865.
Appendix A. Supplementary data
-
•Supplementary materialmmc1.docx (1.9MB, docx)
Data availability
Data will be made available on request.
References
- Abdullah A.Y., Musallam H.S. Effect of different levels of energy on carcass composition and meat quality of male black goats kids. Livestock Science. 2007;107(1):70–80. doi: 10.1016/j.livsci.2006.09.028. [DOI] [Google Scholar]
- Cheng C.P., Chen L., Zhang D.Q., Yu J.Y., Zhu M., Li C.…Li S.B. Value-added utilization of hemoglobin and its hydrolysis products from livestock and poultry blood processing by-products: A review. Trends in Food Science & Technology. 2024;151 doi: 10.1016/j.tifs.2024.104645. [DOI] [Google Scholar]
- Cheng C.P., Xie X.R., Li S.B., Chen P.Y., Huang C.Y., Zheng X.C.…Zhang D.Q. Analysis of bioactive substances in mutton and their effects on the quality of minced mutton. Food Research International. 2025;200 doi: 10.1016/j.foodres.2024.115474. [DOI] [PubMed] [Google Scholar]
- Dou L., Sun L.A., Liu C., Su L., Chen X.Y., Yang Z.H.…Jin Y. Effect of dietary arginine supplementation on protein synthesis, meat quality and flavor in growing lambs. Meat Science. 2023;204 doi: 10.1016/j.meatsci.2023.109291. [DOI] [PubMed] [Google Scholar]
- Fan Y.X., Wang Z., Ren C.F., Ma T.W., Deng K.P., Feng X.…Zhang Y.L. Effect of dietary energy restriction and subsequent compensatory feeding on testicular transcriptome in developing rams. Theriogenology. 2018;119:198–207. doi: 10.1016/j.theriogenology.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Fernandes S.R., Monteiro A.L.G., Dittrich R.L., Salgado J.A., Silva C.J.A.D., Silva M.G.B.D.…Pinto P.H.N.J.R.B.D.Z. Vol. 41. 2012. Early weaning and concentrate supplementation on the performance and metabolic profile of grazing lambs; pp. 1292–1300. 5. [DOI] [Google Scholar]
- Frank D., Kaczmarska K., Paterson J., Piyasiri U., Warner R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Science. 2017;133:61–68. doi: 10.1016/j.meatsci.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Frankel E.N. Volatile lipid oxidation products. Progress in Lipid Research. 1983;22(1):22. doi: 10.1016/0163-7827(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Fu Z., Sun L., Wang Z., Liu Y., Hao J., Gao C., Ge G. Effect of different regions on fermentation profiles, microbial communities, and their metabolomic pathways and properties in Italian ryegrass silage. Frontiers in Microbiology. 2022;13:1076499. doi: 10.3389/fmicb.2022.1076499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M., Cen Y., Zhao P., Hoffman E.P., McBurney M.W., Sauve A.A., Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Developmental Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Allouh M.Z., Rosser B.W.C., Rinkevich Y., Reshef R.…Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Developmental Dynamics: An Official Publication of the American Association of the Anatomists. 2004;231(3):489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Holz M.K., Ballif B.A., Gygi S.P., Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2021;184(8):2255. doi: 10.1016/j.cell.2021.03.060. [DOI] [PubMed] [Google Scholar]
- Huang W., Fang S., Wang J., Zhuo C., Luo Y., Yu Y.…Ning J. Sensomics analysis of the effect of the withering method on the aroma components of Keemun black tea. Food Chemistry. 2022;395 doi: 10.1016/j.foodchem.2022.133549. [DOI] [PubMed] [Google Scholar]
- Jeong J., Choi K.-H., Kim S.-H., Lee D.-K., Oh J.-N., Lee M.…Lee C.-K. Combination of cell signaling molecules can facilitate MYOD1-mediated myogenic transdifferentiation of pig fibroblasts. Journal of Animal Science and Biotechnology. 2021;12(1):64. doi: 10.1186/s40104-021-00583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Cui H., Yuan X., Liu L., Liu X., Wang Y.…Wen J. Identification of the main aroma compounds in Chinese local chicken high-quality meat. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129930. [DOI] [PubMed] [Google Scholar]
- Jing X., Zhou J., Degen A., Wang W., Guo Y., Kang J.…Long R. Comparison between Tibetan and small-tailed Han sheep in adipocyte phenotype, lipid metabolism and energy homoeostasis regulation of adipose tissues when consuming diets of different energy levels. The British Journal of Nutrition. 2020;124(7):668–680. doi: 10.1017/S0007114520001701. [DOI] [PubMed] [Google Scholar]
- Li J., Yang Y., Tang C., Yue S., Zhao Q., Li F., Zhang J. Changes in lipids and aroma compounds in intramuscular fat from Hu sheep. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132611. [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang H., Yang Y., Liu T., Guo Z., Fan W.…Zhou Z. Vol. 10. Advanced Science; Weinheim, Baden-Wurttemberg, Germany: 2023. Metabolome-based genome-wide association study of duck meat leads to novel genetic and biochemical insights. (18), e2300148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes L.S., Martins S.R., Chizzotti M.L., Busato K.C., Oliveira I.M., Machado Neto O.R.…Ladeira M.M. Meat quality and fatty acid profile of Brazilian goats subjected to different nutritional treatments. Meat Science. 2014;97(4):602–608. doi: 10.1016/j.meatsci.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Luciano G., Vasta V., Monahan F.J., Lopez-Andres P., Biondi L., Lanza M., Priolo A. Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chemistry. 2011;124(3):1036–1042. doi: 10.1016/j.foodchem.2010.07.070. [DOI] [Google Scholar]
- Mariutti L.R.B., Bragagnolo N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Research International (Ottawa, Ont.) 2017;94:90–100. doi: 10.1016/j.foodres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalello A., Menci R., Luciano G., Monahan F., Gravador R.S., Valenti B.…Priolo A. Effect of dietary pomegranate by-product on lamb flavour. Meat Science. 2023;198 doi: 10.1016/j.meatsci.2023.109118. [DOI] [PubMed] [Google Scholar]
- Nieto G., Díaz P., Bañón S., Garrido M.D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Science. 2010;85(1):82–88. doi: 10.1016/j.meatsci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Pérez-Juan M., Flores M., Chemistry F.T.J.F. Vol. 105. 2007. Binding of aroma compounds by isolated myofibrillar proteins: Effect of protein concentration and conformation; pp. 932–939. 3. [DOI] [Google Scholar]
- Piantoni P., VandeHaar M.J. Symposium review: The impact of absorbed nutrients on energy partitioning throughout lactation. Journal of Dairy Science. 2023;106(3):2167–2180. doi: 10.3168/jds.2022-22500. [DOI] [PubMed] [Google Scholar]
- Qi S., Wang P., Zhan P., Tian H. Characterization of key aroma compounds in stewed mutton (goat meat) added with thyme (Thymus vulgaris L.) based on the combination of instrumental analysis and sensory verification. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131111. [DOI] [PubMed] [Google Scholar]
- Sleeth M.L., Thompson E.L., Ford H.E., Zac-Varghese S.E.K., Frost G. Free fatty acid receptor 2 and nutrient sensing: A proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutrition Research Reviews. 2010;23(1):135–145. doi: 10.1017/S0954422410000089. [DOI] [PubMed] [Google Scholar]
- Sohail A., Al-Dalali S., Wang J., Xie J., Shakoor A., Asimi S.…Patil P. Aroma compounds identified in cooked meat: A review. Food Research International (Ottawa, Ont.) 2022;157 doi: 10.1016/j.foodres.2022.111385. [DOI] [PubMed] [Google Scholar]
- Song S., Tang Q., Fan L., Xu X., Song Z., Hayat K.…Wang Y. Identification of pork flavour precursors from enzyme-treated lard using Maillard model system assessed by GC-MS and partial least squares regression. Meat Science. 2017;124:15–24. doi: 10.1016/j.meatsci.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Sun X., Han F., Lu Q., Li X., Ren D., Zhang J.…Li J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating Sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69(6):1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- Tanimoto S., Kitabayashi K., Fukusima C., Sugiyama S., Hashimoto T. Effect of storage period before reheating on the volatile compound composition and lipid oxidation of steamed meat of yellow tailSeriola quinqueradiata. Fisheries Science. 2015;81(6):1145–1155. doi: 10.1007/s12562-015-0921-4. [DOI] [Google Scholar]
- Teixeira A.B.M., Schuh B.R.F., Daley V.L., Pinto P.H.N., Fernandes S.R., de Freitas J.A. Performance, biochemical and physiological parameters of Dorper × Santa Ines lambs fed with three levels of metabolizable energy. Tropical Animal Health and Production. 2021;53(3):353. doi: 10.1007/s11250-021-02797-x. [DOI] [PubMed] [Google Scholar]
- Van Boekel M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnology Advances. 2006;24(2):230–233. doi: 10.1016/j.biotechadv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wu L., Li J., Li Y., Li T., He Q., Tang Y.…Liao P. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from different province in China. Journal of Animal Science and Biotechnology. 2016;7:63. doi: 10.1186/s40104-016-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kang D.C., Zhang W.G., Lorenzo J.M. Recent advantage of interactions of protein-flavor in foods: Perspective of theoretical models, protein properties and extrinsic factors. Trends in Food Science & Technology. 2021;111:405–425. doi: 10.1016/j.tifs.2021.02.060. [DOI] [Google Scholar]
- Zhang X., Han L., Hou S., Raza S.H.A., Wang Z., Yang B.…Al Hazani T.M.I. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chemistry. 2022;374 doi: 10.1016/j.foodchem.2021.131611. [DOI] [PubMed] [Google Scholar]
- Zhao J.X., Liu X.D., Li K., Liu W.Z., Ren Y.S., Zhang J.X. Different dietary energy intake affects skeletal muscle development through an Akt-dependent pathway in Dorper × small thin-tailed crossbred ewe lambs. Domestic Animal Endocrinology. 2016;57:63–70. doi: 10.1016/j.domaniend.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Zhao L., Yuan L., Li F., Zhang X., Tian H., Ma Z.…Wang W. Whole-genome resequencing of Hu sheep identifies candidate genes associated with agronomic traits. Journal of Genetics and Genomics. 2024;51(8):866–876. doi: 10.1016/j.jgg.2024.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.