Abstract
Triple-negative breast cancer has previously been considered to a weak tumor immunogenicity. A strategy combining sono-immunotherapy and metal ion interference therapy holds promise for the tumor immune environment. 2,2-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH), functioning as a sonosensitizer, can produce alkyl radicals that can trigger sono-oxidative stress in neoplastic cells. Metal ion interference therapy has also demonstrated promise as an immunotherapy. However, the AIPH’s chemical instability and the metal ions are limited by the short half-life of blood components and the lack of specificity. Herein, we developed a multifunctional nanoparticle HA/AIPH@ZIF-8 (HAZ), which incorporates hyaluronic acid (HA) specifically targeting CD44 receptors. This design aims to facilitate the targeted accumulation of nanoparticles within tumor microenvironments. HAZ amplifies sono-oxidative stress and the release of mitochondrial DNA and nuclear DNA damage. Both dsDNA and zeolitic imidazolate framework (ZIF-8) derived zinc ions (Zn2+) to facilitate the STING pathway and immunogenic cell death (ICD) mediated maturation of dendritic cells, infiltration of cytotoxic T lymphocytes(CTLs), the nitrogen produced by the dehydration of AIPH can increase the acoustic cavitation effect, promoting HAZ penetration at the tumor. The real-time ultrasound imaging capabilities of HAZ are also attributed to the generation of nitrogen. In general, HAZ-mediated sonodynamic therapy (SDT) might offer contrast-enhanced imaging and an efficient anticancer sono-metalloimmunotherapy.
Keywords: Sono-metalloimmunotherapy, Acoustic cavitation, Ultrasonic imaging, STING pathway, Immunogenic cell death, mtDNA release
1. Introduction
Triple-negative breast cancer (TNBC) has previously been considered to have weak tumor immunogenicity compared with other tumor types [1]. Most patients suffer from the immune desert and are immune-excluded after prolonged immune anti-tumor therapy [2]. Recently, innovative antitumor therapeutic strategies: photothermal therapy [3], photodynamic therapy [4], and sonodynamic therapy [5] have been developed for anti-tumor. Among them, sonoimmunotherapy is considered an oxidative stress-mediated solid tumor therapy with the benefit of noninvasiveness, spatiotemporal control, deeper tissue penetration, and fewer adverse effects [6,7]. A water-soluble2,2-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH), which can easily generate nitrogen gas (N2) and alkyl radicals with stimulation of thermal, light, and ultrasound [8], alkyl radicals may directly promote apoptosis of tumor cells and consumption of oxygen to produce alkoxyl and peroxyl radicals [9], also N2 can be utilized for contrast-enhanced ultrasonic imaging and the acoustic cavitation effect. However, AIPH is chemically unstable and easily degrades. Consequently, there is a clear need to develop a nanocarrier to load AIPH.
Mitochondria are highly sensitive to free radicals; therefore, ultrasound-stimulated mitochondrial outer membrane permeabilization and release of mitochondrial DNA (mtDNA) may become a promising anti-tumor therapy [10,11]. Cytosolic mtDNA activates an immunostimulatory DNA sensor such as cyclic GMP-AMP synthase(cGAS) that can trigger stimulator of interferon genes(STING) signaling, inducing the generation of interferon-β (IFN-β) [12,13]. IFN-β is associated with innate immunity and adaptive immunity, which enhances the antigen cross-presentation capabilities of dendritic cells (DCs) and increases the cytotoxic activity of cytotoxic T lymphocytes(CTLs). Research indicates that the presence of zinc ions (Zn2+) activates the cGAS enzyme, suggesting that Zn2+ is particularly effective in triggering the murine cGAS-STING signaling pathway [14]. Moreover, part of metal ions are proven to trigger immune responses to combat tumors, a phenomenon known as metal ion interference therapy (MIIT), looking at the variety of ways that Zn2+ can cause disruptions and activate immune response [15], but the danger of significant toxicity and unfavorable treatment is increased by the brief half-life of blood components and the lack of specificity associated with metal ions. To enhance efficacy and decrease these adverse effects, a series of metal-based nanoparticles have been prepared, which could effectively activate immunotherapies and targeted degradation at the tumor microenvironment while minimizing the toxic side effects of MIIT [16].
According to the above studies, we developed a therapeutic method that combines zinc-based nanoparticles with ultrasound. Herein, we synthesized a hyaluronic acid (HA)-modified ZIF-8 that encapsulates AIPH, referred to as HAZ. The overexpression of HA-specific receptors, particularly CD44, in breast cancer cells promotes the targeted accumulation of HA-modified ZIF-8 nanoparticles within tumor cells [17]. Specialized ZIF-8 nanoparticles can transport drugs and control the release of encapsulated drugs through dissociation within the microenvironment [18]. By employing AIPH and ultrasound, we induce double-stranded DNA (dsDNA) damage, mitochondrial outer membrane permeabilization, and the release of mtDNA. This combination of dsDNA and cGAS, along with the stabilizing effect of Zn2+, leads to cGAS enzyme activity. Both of these mechanisms effectively activate the cGAS-STING axis. Moreover, the combined treatment is considered a potential inducer of immunogenic cell death that releases damage-associated molecular patterns (DAMPs); both mechanisms eventually trigger the maturation and migration of DC cells, which in turn activate CTLs to achieve sono-metalloimmunotherapy (Fig. 1).
Fig. 1.
Construction diagram of HA/AIPH@ZIF-8 and diagrammatic representation of US-mediated HAZ nanoparticles' therapeutic mechanisms for sono-metalloimmunotherapy.
2. Materials and methods
2.1. Reagents
Zinc nitrate hexahydrate (Zn (NO3)2⋅6H2O, 99 %) was bought from China National Medicines Corporation (Shanghai, China). 2-Methylimidazole (2-MIM), and HA were purchased from Aladdin (Shanghai, China). AIPH and IR783 were purchased from Macklin (Shanghai, China). Zn2+ ions probe N-(6-Methoxy-8-quinolyl)-p-toluenesulfonamide (TSQ) was purchased from AAT Bioquest (CA, USA). Enhanced ATP assay kit, JC-1, Calcein/PI Cell Viability/Cytotoxicity Assay Kit, and ROS assay kit were purchased from Beyotime (Shanghai, China). Anti-Phospho-TBK1 (Ser172), anti-TBK1, and anti-Phospho-Histone H2A.X(Ser139) antibodies were bought from Cell Signaling Technology (MA, USA). Anti-Phospho-IRF3 (Ser385) and anti-IRF3 were purchased from Immunoway (TX, USA). Anti-Calreticulin (CRT) antibodies and rabbit anti-GAPDH antibodies were purchased from Proteintech (Wuhan, China). Antibodies for flow cytometry were purchased from eBioscience (MA, USA). Annexin V-FITC/PI Cell Apoptosis Detection Kit and Fluorescein (FITC) Tunel Cell Apoptosis Detection Kit were purchased from Servicebio (Wuhan, China). Mouse IL-12 ELISA Kit, Mouse IFN-β ELISA Kit, and HMGB1 ELISA Kit were obtained from Bioswamp (Wuhan, China). Mouse IFN-β ELISA Kit was obtained from R&D Systems (MN, USA). 0.25 % trypsin-EDTA, RPMI-1640 medium, and fetal bovine serum were purchased from Gibco (USA).
2.2. Cells and animals
The 4T1 cell was supplied by Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China), which was cultured at 37 °C in a 5 % CO2 environment using RPMI-1640 medium (Gbico, USA) enriched with 10 % fetal bovine serum (FBS) (Gbico, USA).
Female Balb/c mice (6–8 weeks) were bought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The suspension of 4T1 cells (1 × 106 cells) was subcutaneously injected into each mouse's right flank. Each animal experiment was carried out according to the model animal research care guidelines established by the Institutional Animal Care and Use Committee of Qilu Hospital of Shandong University (DWLL-2023–105).
2.3. Synthesis of ZIF-8
10 mL of Zn (NO3)2·6H2O methanol solution (20 mg mL−1), followed by the gradual addition of 10 mL of 2-methylimidazole in a water mixture (180 mg mL−1). The combined solution was agitated at room temperature for 1 h. The product was acquired by centrifuging at a speed of 10,000 rpm for 30 min, followed by washing thrice
2.4. Synthesis of AIPH@ZIF-8
1 mg of AIPH was added to Zn (NO3)2·6H2O solution, then 10 mL of 2-MIM solution was added dropwise to the mixture and stirred (4 °C, dark, 4 h). The product was obtained through centrifugation at a speed of 10,000 revolutions per minute for 30 min, followed by washing thrice.
2.5. Synthesis of HA/AIPH@ZIF-8
5 mg AIPH@ZIF-8 was immersed in 5 mL HA solution (1 mg mL−1), followed by stirring (4℃, dark, 3 h), and the mixed system was dialyzed in the water phase for 24 h. The product was dehydrated using a vacuum at −80 °C to produce powder.
2.6. Synthesis of HA/ZIF-8
5 mg ZIF-8 was immersed in 5 mL HA solution (1 mg mL−1), followed by stirring (4℃, 3 h), and the mixed system was dialyzed in the water phase for 24 h. The product was dehydrated using a vacuum at −80 °C to produce powder.
2.7. Characterization of HA/AIPH@ZIF-8
The morphological characteristics of HAZ were analyzed using TEM (JEM-F200, JEOL, Tokyo, Japan). The nanoparticles' average hydrodynamic size, PDI, and zeta potential were assessed through DLS (Malvern Instruments Ltd., Worcestershire, UK). XRD patterns were acquired using a powder diffractometer for X-rays (DMAX-2500PC, Rigaku, Japan) in the range of 5–50 degrees (2θ). XPS results were gained from X-ray photoelectron spectroscopy (ESCALAB Xi plus, Thermo, USA). XRD patterns (DMAX-2500PC, Rigaku, Japan) were acquired using a powder diffractometer for X-rays in the range of 5–50 degrees(2θ). UV–Vis spectrum was obtained using a UV–Vis spectrometer (UV2600i, Shimadzu, Japan). The results of ICP-MS were gained from an inductively coupled plasma mass spectrometer (Agilent 7500a, Agilent, USA).
2.8. pH-responsive degradation assay
HAZ was dispersed into PBS (pH 6.5 or pH 7.4) and incubated at 37 °C under stirring. After 24 h, the precipitates were observed by TEM.
2.9. Detection of ABTS+•
ABTS+• exhibits a distinct absorption in the range of 400 to 1000 nm. HAZ were exposed to various intensities of US stimulation, and then those solutions were incubated with ABTS. Different concentrations of HAZ were also tested. The absorbance of ABTS+• was examined by UV–Vis spectrometer.
2.10. Intracellular uptake
4T1 cells were cultured in 24-well plates, then incubated with RPMI-1640, 40 μg mL−1 of ZIF-8, 40 μg mL−1 of ZIF-8/HA and Zn2+ ions (50 mM), respectively. Following an 8 h incubation period, the cells were treated with a 3 mM solution of the Zn2+ probe TSQ for 30 min at 37 °C. Subsequently, they were examined using a fluorescence microscope (ni-u, Nikon, Japan).
2.11. Hemolysis assay
Blood cells were mixed with varying amounts of HAZ and allowed to incubate for one hour at a temperature of 37 °C, after being resuspended in 1 ml of PBS. Following centrifugation, the optical density (OD) of each supernatant was measured using a microplate reader (infinite E plex, Tecan, Switzerland) at 545 nm.
2.12. Alkyl radical production in the cell
4T1 cells were cultured in 24-well plates and subsequently treated with (1) PBS, (2) HZ, (3) HAZ, (4) US, (5) HZ + US, (6) HAZ + US for 24 h, incubated with DCFH-DA at 37℃ for 30 min, lately imaged utilizing fluorescence microscopy and subsequently quantified through FCM.
2.13. Cytosolic mtDNA measurement
4T1 cells were cultured in 6-well plates and treated with (1) PBS, (2) HZ, (3) HAZ, (4) US, (5) HZ + US, (6) HAZ + US for 24 h. The cells were divided into two sections, with one portion utilized for the extraction of cytosolic mtRNA. This was achieved by lysing the cells with 0.1 % NP-40 (Beyotime, China) for 30 min at 4 °C. Following this, the mixture was centrifuged at 14,000 rpm for 20 min at 4 °C, allowing for the supernatant collection of the cytosolic mtDNA. Cytosolic mtDNA and total mtDNA were isolated utilizing a Genomic DNA Kit (TIANamp, China), and RT-PCR and agar gel electrophoresis were applied.
2.14. Assessment of live and dead cell staining
According to the Calcein/PI Assay Kit (Beyotime, China), 4T1 cells subjected to various treatments were incubated with Calcein-AM, which stains live cells green, and propidium iodide (PI), which stains dead cells red. Subsequently, the apoptosis of the cells was assessed using a fluorescence microscope.
2.15. Measurement of ATP content
According to the ATP Assay Kit (Beyotime, China), cells subjected to various treatments were lysed using a lysis solution. The sample solution was then collected, and subsequently, the working solution was combined with the sample. Relative light units were measured using a microplate reader (Infinite Eplex, Tecan, Switzerland).
2.16. HAZ-induced immunogenic cell death of 4T1 cells in vitro
4T1 cells were inoculated into a 24-well plate and incubated overnight. Then the medium was replaced with HZ and HAZ (40 μg mL−1) solution dispersed in PMI 1640 medium and co-cultured for 24 h. The cells were then irradiated with US (0.5 W cm−2, 1.0 MHz, 1 min). After 24 h, the supernatant from 4T1 cells was collected and analyzed for concentrations of IFN-β and HMGB1 by using ELISA kits.
2.17. In vitro culture and FCM of DCs
Bone marrow cells were extracted through the flushing of femoral and tibial bones and subsequently cultured in 6-well plates with PMI 1640 medium, enriched with 10 % FBS, 1 % penicillin–streptomycin, 20 ng mL−1 GM-CSF (Proteintech, China), and 10 ng mL−1 IL-4 (Proteintech, China). After 6 days, 4T1 cells from different treatments were added to BMDCs. After 24 h, BMDCs were stained with anti-CD11c-FITC, anti-CD80-PE, and anti-CD86-APC for flow cytometry analysis.
2.18. The effect of dsDNA and Zn2+ on DCs
100 ng DNA and 25 mM Zn2+ were added to BMDCs, respectively. After 24 h, BMDCs were stained with anti-CD11c-APC, anti-CD80-PE, and anti-CD86-FITC for flow cytometry analysis.
2.19. Immunofluorescence
4T1 cells and tumor tissues were cultured under various treatment conditions. The cells were subjected to incubation with primary antibodies overnight at 4 °C. Following a washing step, the cells were subsequently incubated with fluorescent secondary antibodies for one hour, finally stained with DAPI, and examined using a fluorescence microscope.
2.20. Western blot analysis
4T1 cells treated with (1) PBS, (2) HZ, (3) HAZ, (4) US, (5) HZ + US, (6) HAZ + US for 24 h, cells were lysed with PIRA supplemented with PMSF on ice, centrifuged, and collected for protein. Protein samples were subjected to separation through SDS-PAGE. Following this transfer, the membranes were incubated with primary antibodies specific to TBK1, P-TBK1(Ser172), IRF3, P-IRF3(Ser385), P-H2A.X(Ser139), and GAPDH. Finally, a Gel imaging system (Tanon-4800, Tanon, China) was used.
2.21. In vivo immune responses
Tumors, lymph nodes, and spleens from each cohort of mice were processed to acquire a suspension of single cells. The T cells in tumors were subjected to staining using viability Dye 780, anti-CD45-PerCP/Cyanine5.5, anti-CD3-FITC, anti-CD4-APC, and anti-CD8-PE for FCM. The T cells in the spleen were subjected to staining using anti-CD3-FITC, anti-CD4-APC, and anti-CD8-PE for flow cytometry analysis. The DCs in draining lymph nodes were stained with anti-CD11c-FITC, anti-CD80-PE, and anti-CD86-APC for FCM. Serum from mice blood was collected, and concentrations of IFN-β and HMGB1 were analyzed using ELISA kits.
2.22. In vivo biodistribution
When the tumor volume reached 200 mm3, HA/IR783@ZIF-8 was injected into mice. After 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h, the major organs were imaged by the IVIS spectrum (PerkinElmer, Waltham, USA).
2.23. Ultrasound imaging capability
The HAZ solution was irradiated with 1.5 W cm−2, 1 min, 1.0 MHz, then the solution was incubated at pH 6.5.
Mice were anesthetized and injected subcutaneously with either 5 mg kg−1 or PBS. After 2 h, tumor areas were exposed to the US (1.5 W cm−2, 1 min, 1.0 MHz) and imaged by a US system (LOGIQ E9, GE, USA) with a 9L linear transducer for contrast-enhanced ultrasound (CEUS) imaging in vivo.
2.24. In vivo anti-tumor effect
After the tumor attained 60 mm3, mice bearing tumors were systematically assigned to six distinct groups (n = 5) through a randomization process, the dosage of HAZ and HZ injected was 5 mg kg−1, accompanied by ultrasound irradiation (1.5 W cm−2, 1 min, 1.0 MHz) was applied two hours after injection. The body weight and tumor volume of the mice were assessed bi-daily. At the end of the observation period, tumors were preserved for H&E staining, TUNEL assays, and immunofluorescence. Additionally, blood samples were obtained and subjected to analysis for biosecurity purposes.
2.25. Statistics
All data were expressed as mean ± standard deviation (SD) and analyzed utilizing GraphPad Prism version 8.0. The significance of the data was evaluated according to one-way ANOVA or student’s t-test (n ≥ 3, p* < 0.05, p** < 0.01, p*** < 0.001, p**** < 0.0001, ns, no significant).
3. Results and discussion
3.1. Synthesis and characterization
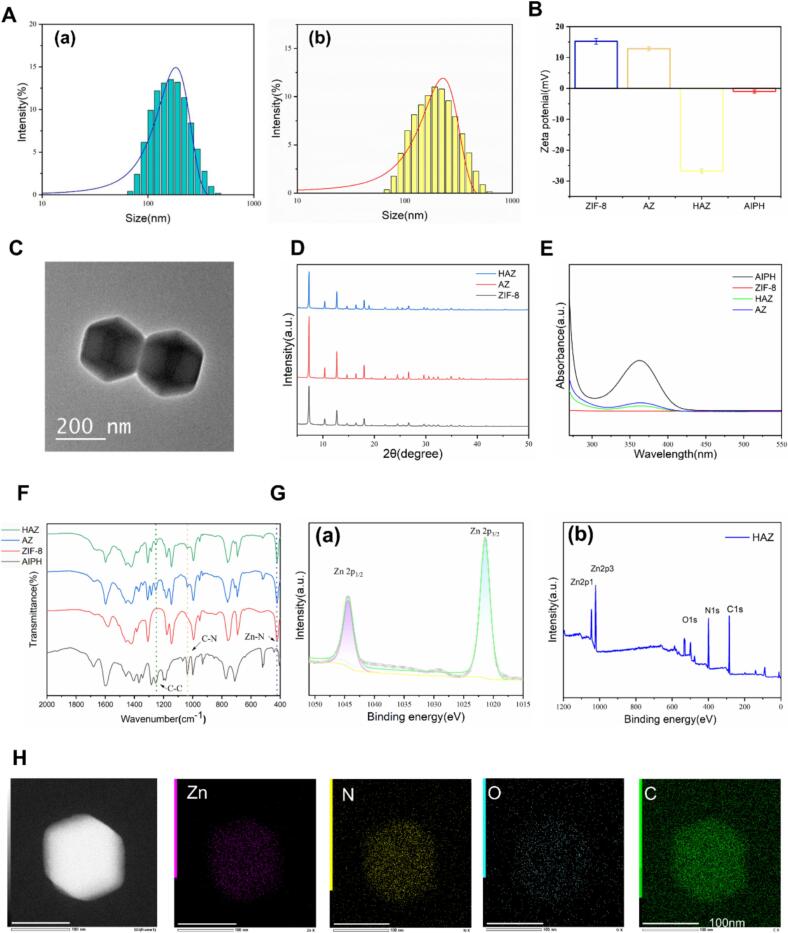
HA/AIPH@ZIF-8 were assembled via metal Zn2+ ions with organic 2-methylimidazole(2-MIM) and AIPH was wrapped, then modified with HA. The average hydrodynamic sizes of AIPH@ZIF-8 (AZ) and HA/AIPH@ZIF-8 (HAZ) were confirmed by to be 162.0 ± 8.0 nm dynamic light scattering (DLS)and 191.8 ± 8.8 nm, respectively, with polydispersity index (PDI) at 0.107 ± 0.021 and 0.182 ± 0.099 (Fig. 2A). The zeta potentials of ZIF-8 and AZ were 15.23 ± 0.96 mV and 12.87 ± 0.65 mV, and the negative zeta potential change, −26.7 ± 0.64 mV of HAZ, confirmed the successful modification of HA on AZ (Fig. 2B). As observed by transmission electron microscopy (TEM), HAZ was stable, with regular dodecahedron structures (Fig. 2C). Over a day, HAZ's size and zeta potential were not affected significantly, indicating exceptional physical stability of the nanoparticle (Fig. S1 and S2). The diffraction peaks of AZ and HAZ were found to be in high agreement with ZIF-8 in the powder X-ray diffraction (XRD) pattern, demonstrating a negligible effect of AIPH on the coordination of Zn2+ ions and 2-methylimidazole (Fig. 2D). As shown in Fig. 2E, AIPH was responsible for the absorption peak seen in the HAZ UV–vis spectra at 366 nm. Based on the absorption peak in UV spectra, the encapsulation efficiency of AIPH in AZ was calculated to be 81.1 %. Moreover, the loading of AIPH and the coating of HA were further verified by Fourier transform infrared (FTIR) spectra; the absorption bands at 422 cm−1 of ZIF-8 were attributed to Zn–N stretching mode. Following AIPH loading, the peaks of AIPH, the C-N stretching vibration peak at 1038 cm−1, and the C-C stretching vibration peak at 1250 cm−1 were visible in the AZ and HAZ (Fig. 2F). X-ray photoelectron spectroscopy (XPS) shows the Zn 2p, O 1 s, and N 1 s peaks, and the XPS spectrum relating to Zn in HAZ at 1044.3 and 1021.3 eV could be attributed to Zn 2p 1/2 and 2p 3/2 (Fig. 2G). The allocation of zinc (Zn), carbon (C), oxygen (O), and nitrogen (N) elements is demonstrated by an energy dispersive spectrometer (EDS) (Fig. 2H and S3). As shown in Fig. S4, testifying to the pH-responsive biodegradable property and Zn2+ release, immersed HAZ in PBS solutions exhibiting various pH levels, HAZ still maintained regular dodecahedron structures without obvious degradation (PH = 7.4), in contrast, HAZ in acidic PBS(PH = 6.5) degraded and aggregated obviously. Zn2+ release under acidic conditions was measured using inductively coupled plasma mass spectrometry (ICP-MS), with a maximum release of 71 % within 24 h. Regarding the time-varying release of AIPH at different pH values, after 24 h, the total release rates of AIPH at pH = 6.5 reached about 60 % (Fig. S5).
Fig. 2.
HA/AIPH@ZIF-8 synthesis and characterization. (A) Hydrodynamic size of (a) AZ and(b) HAZ. (B) Zeta potentials of ZIF-8, AZ, HAZ, and AIPH. (C) TEM image of HAZ. (D) XRD patterns of ZIF-8, AZ and HAZ. (E) UV − vis spectra of ZIF-8, AZ, HAZ, and AIPH. (F) FTIR spectra of ZIF-8, AZ, HAZ, and AIPH. (G) XPS scans of (a) Zn 2p peaks in HAZ, (b) XPS spectra of HAZ. (H) Mapping analysis of HAZ (Scale bar: 100 nm).
3.2. US irradiation induced free radical generation
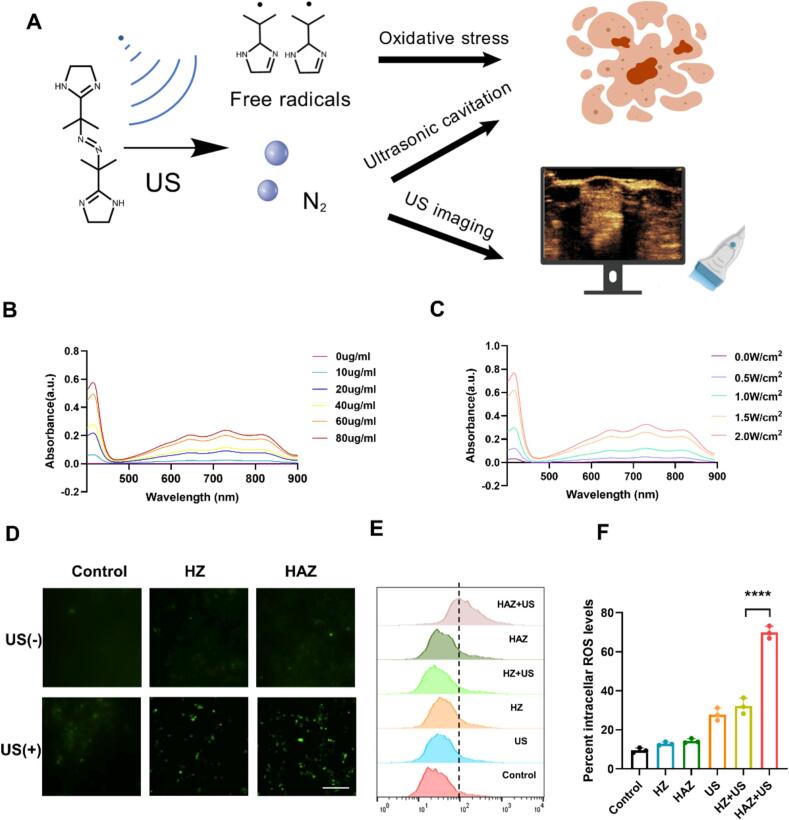
The exposure of AIPH to irradiation from the Ultrasound (US) can facilitate the formation of alkyl radicals (•R) and N2(Fig. 3A). These radicals subsequently react with 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), resulting in the production of ABTS+•, which exhibits absorption peaks within the range of 500 nm to 900 nm. As the concentration of AIPH increases, the hue of the solution undergoes a gradual transition from transparency to a blue coloration. (Fig. S6). As shown in Fig. 3B and C, when ABTS was combined with HAZ and exposed to US irradiation (1 MHz, 0.5 W cm−2, 1 min), then the mixture incubated at pH 6.5, the supernatant was collected and measured by UV–vis spectroscopy, the absorption peak of ABTS+• gradually increased in a concentration-dependent manner, uniformly, the absorption peak increased within US intensity. These data suggested that HAZ responds efficiently to low pH and US, resulting in the release of •R.
Fig. 3.
US irradiation induced free radical generation. (A) AIPH decomposition under US irradiation. (B, C) The UV–visible spectra of the ABTS+• were obtained following the reaction involving the combination of ABTS with (B) different HAZ concentrations and (C) Different US intensities. (D)Intercellular alkyl radical levels by measured with the DCFH-DA (Scale bar: 100 μm). (E, F) FCM results (E) of intercellular •R in 4T1 cells and the percentage of positive cell rate (F) were obtained using Flowjo. Data are shown as means ± SD(n = 3), ****p < 0.0001.
To further confirm that the •R can induce free radical generation, the dichloro-dihydro-fluorescein diacetate (DCFH-DA) was employed to evaluate the intracellular levels of free radicals. Following ultrasound irradiation, the cells demonstrated significantly enhanced green fluorescence in fluorescence microscopy relative to the Control group (Fig. 3D and Fig. S7). In agreement with the results from fluorescence microscopy, FCM results revealed that HAZ intracellularly generates free radicals after US irradiation (Fig. 3E and F), suggesting that HAZ after US irradiation could induce •R in cells. Due to the ultrasonic cavitation effect generated by microjets after bubble destruction [17]. N2 may enhance the delivery of therapeutic drugs to tumors due to an increased capacity for permeation.
3.3. Cellular uptake and distribution of HAZ
To measure the intracellular distribution of HAZ, a Zn2+ ions probe, N-(6-Methoxy-8-quinolyl)-p-toluenesulfonamide (TSQ) was used [18]. Following the 2-hour incubation period with 4T1 cells, the blue fluorescence is observably higher than the Control group and ZIF-8 group, but a little lower than the positive control (50 mM of free Zn2+). However, after blocking with HA solution, the blue fluorescence observably decreased (Fig. 4A and Fig. S8). HA/IR783@ZIF-8 was further investigated for its distribution in vivo. Then, the content of HA/IR783@ZIF-8 was imaged and quantified at 1, 2, 4, 8, 10, 12, and 24 h after injecting the NPs. As shown in Fig. 4B, the fluorescence intensity of the ex vivo organ photograph indicated that the tumor area peaked at 2 h post-injection. Notably, the retention time of HA/IR783@ZIF-8 within the tumor exceeded 24 h, suggesting that NPs can accumulate steadily in the tumor over extended periods, which is more conducive to the effect of therapy (Fig. 4C and Fig. S9). ICP-MS was also used to determine Zn2+ in tumor tissue. After therapy, the HAZ injection group was 113.4 times greater than the Control group (Fig. 4D). The rapid cellular uptake of HAZ can be ascribed to the high affinity of HA for the CD44 receptors located on the surface of breast cancer cells, demonstrating its potent targeting ability [19]. This selective recognition facilitates an increased accumulation of the HAZ within tumor cells. Therefore, due to the potential enhanced nanoparticle-induced endothelial leakiness (NanoEL) effects [20] or effects of permeability and retention(EPR) [21], the injected HAZ could also accumulate in the tumors.
Fig. 4.
Biodistribution, intracellular uptake, and CEUS images of HAZ. (A) Integrated image of intracellular Zn2+ ion was detected by a Zn2+ probe (scale bar: 100 μm). (B) Fluorescence imaging was conducted on isolated tumors and main organs at various time points following the injection of HA/IR783@ZIF-8. (C) The measurement of the fluorescence intensity within the tumor region derived from B. (D) ICP-MS analysis of Zn2+ content in tumor tissue post-treatment of HA/AIPH@ZIF-8. (E) US imaging in vitro. (F) and (G) (a) US imaging of different treatments in vivo, the red line delineates the location of the tumor, and (b) relative contrast intensity (Scale bars: 10 mm). The means ± SD (n = 3), **p < 0.01, ****p < 0.0001, and ns, no significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Both livers and kidneys exhibited the highest levels of fluorescence intensity, proving that NPs were primarily metabolized by the hepatic system and subsequently excreted via the renal system.
3.4. Bubble generation and imaging Performance of HAZ
Recently, multifarious gas delivery systems have been manufactured. The gas delivery system described herein facilitates the controlled generation of gases through in situ exogenous activation using ultraviolet (UV), near-infrared (NIR), and US [[22], [23], [24]]. The ultrasound imaging capabilities of HAZ solutions were investigated in vitro to evaluate the responsive nitrogen generation characteristics of HAZ. The contrast-enhanced ultrasonic (CEUS) mode increased following the US irritation of HAZ (Fig. 4E). The phenomenon observed was ascribed to the acoustic impedance mismatch induced by N2 to the gas and its ambient media [25]. According to the CEUS images shown in Fig. 4F, the injection with PBS resulted in no ultrasonic signals in the tumor area. As presented in Fig. 4G, 2 h after the injection of HAZ and PBS, a US (1 MHz, 1.5 W cm−2, 1 min) stimulus was applied, the ultrasonic imaging under the US contrast signal was observed in the tumor region, after ultrasound exposure, there was an observed increase in both tumor perfusion area and contrast intensity. Notably, the CEUS time of HAZ in the tumor site prolonged beyond 24 h. The collective results testified that HAZ is suitable for enhancing ultrasonic signals in tumor tissue.
3.5. US irradiation induced DNA oxidative damage and mtDNA leakage
HAZ demonstrated a lethal effect on 4T1 cells that relied on the dosage provided. According to the results of the CCK-8 study, the half-maximal inhibitory concentration (IC50) value of HAZ was 50.7 μg mL−1 (Fig. S10). HAZ + US-induced oxidative stress responses and cytotoxicity were evaluated. As shown in S11, the ability to trigger apoptosis was confirmed through flow cytometry; the HAZ + US group exhibited the highest cytotoxicity, approximately 52.2 %, based on the findings from calcein-AM/PI dual-staining (Fig. 5A), PI-labeled apoptotic cells (red) significantly increased after being treated with HAZ + US. The suppressive effects of various therapeutic interventions on the growth of 4T1 cells were assessed utilizing the EdU assay, and EDU-labeled proliferative cells (red) significantly dropped (Fig. 5B). This data confirmed the specific sono-toxicity of synergistic therapy. The cell viability of HAZ (40 μg mL−1) and US (1 MHz, 0.5 W cm−2, 1 min) was 79 % and nearly 100 %, respectively; neither US irradiation nor HAZ exhibited significant toxic effects when considered independently, thereby affirming the security of US irradiation and the biological compatibility of HAZ in isolation.
Fig. 5.
US irradiation-induced DNA oxidative damage and mtDNA leakage. (A) Fluorescence microscope images of tumor cells under different treatment conditions were obtained by Calcein-AM (green) and PI (red) staining (scale bar: 100 μm). (B) Fluorescence images show the ability of cells to proliferate under different treatments (Scale bar:100 μm). (C) Immunofluorescence imaging of H2AX in 4T1 cells subjected to various treatments (Scale bar: 100 μm). (D) Expression level of H2AX on 4T1 cells subjected to various treatments. (E) Intracellular ATP content after a variety of treatments. (F) RT-qPCR findings for the cytosolic mtDNA of 4T1 cells. (G) Images of DNA agar gel electrophoresis show mtDNA in the cytosolic or total lysates of 4T1 cells subjected to various treatments. The means ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Oxidative stress is the main reason for DNA double-strand breaks (DSBs), with phosphorylated histone H2AX serving as an indicator for the presence of DSBs [26]. The levels of H2AX in 4T1 cells were assessed through immunofluorescence and western blot (WB) to evaluate DNA damage. The results indicated a significantly higher green fluorescence signal in cells incubated with HAZ + US compared to those treated with other treatments (Fig. 5C). Subsequently, WB results demonstrated that HAZ + US enhanced upregulation of H2AX (Fig. 5D and Fig. S12), suggesting that HAZ combined with sonodynamic therapy (SDT)-induced oxidative stress was sufficient to trigger severe DNA damage.
The decrease of mitochondrial membrane potential is a significant signal of cell apoptosis [27]. As shown in Fig. S13, an assessment of HAZ + US-induced mitochondrial damage was conducted using the JC-1(5,5́,6,6́tetrachloro-1,1́,3,3́-tetraethyl-imidacarbocyanine), which is utilized for monitoring mitochondrial membrane potential (MMP). The findings validated that HAZ + US effectively decreased polymer (red) in the mitochondrial matrix and promoted monomer (green) formation. Moreover, the ATP levels in group HAZ + US following treatment were lower than other groups (Fig. 5E). Unlike nuclear DNA, mtDNA does not possess protective histone proteins, rendering it more vulnerable to damage [28]. The RT-qPCR analysis indicated that more mtDNA (represented by Dloop, CytB, ND1, and ND2) was increased in the cytoplasm of HAZ + US-treated breast cancer cells, confirming the mtDNA leakage (Fig. 5F). To further investigate, the cytosolic mtDNA and total mtDNA were analyzed using agarose gel electrophoresis. The HAZ + US groups showed a considerable increase in mtDNA in the cytoplasm, but there was no discernible change in total DNA (Fig. 5G). To sum up, HAZ + US showed high-efficiency lethality to tumor cells by causing DNA damage and mitochondrial damage.
3.6. Zn-based nanoparticle-mediated SDT triggered ICD and GAS-STING signaling pathway
Dying tumor cells release DAMPs, including high mobility group protein 1 (HMGB1) and calreticulin (CRT). These DAMPs facilitate the ability of antigen presentation by DCs, which in turn activate CTLs to target neoplastic cells effectively [34]. CRT moves from the endoplasmic reticulum (ER) to the cell membrane, which is regarded as an“eat me” signal for DCs [35]. As illustrated in Fig. 6A, our investigation focused on how HAZ mediated US activates ICD and the STING pathway. Immunofluorescence staining showed that HAZ + US would lead to the expression of CRT on 4T1 cells, as indicated by the red fluorescence observed on the cell membrane when viewed through the fluorescence microscope. (Fig. 6B). Extracellular HMGB1 interacted with pattern recognition receptors (PRRs) on the bone marrow cells’ membrane, activating immune responses to kill tumor cells [36]. There is a nearly complete co-localization of HMGB1(red fluorescence) and nuclei (blue fluorescence) in the cells derived from the control group; however, the overlap was considerably reduced in the HAZ + US group (Fig. 6C). ELISA results showed that HMGB1 in 4T1 cell culture medium and mouse serum significantly increased (Fig. 6D and E). All results indicated that Zn-based nanoparticles mediated SDT to activate ICD and the migration of DAMPs to activate anti-tumor immunity.
Fig. 6.
HAZ-mediated SDT triggered ICD and GAS-STING signaling pathway. (A) Schematic representation of the mechanism underlying STING pathway activation and ICD in tumor cells. (B, C) Immunofluorescence photographs for CRT (B) and HMGB1 (C) of 4T1 cells exposed to various managements (Scale bar: 100 μm). (D, E) ELISA assay for (D) extracellular secretion of HMGB1 by 4T1 and (E) serum HMGB1 in mice exposed to various managements. (F) WB detection of TBK1, p-TBK1 and IFN-β in 4T1 cells with different treatments. Quantitative analyses of Western blot results for (G) p-TBK1 and (H) IFN-β protein expression in differently treated 4T1 cells (n = 3). (I) Immunofluorescence image for IFN-β on 4T1 cells exposed to various managements (Scale bar: 15 μm). (J, K) ELISA for (J) extracellular secretion of IFN-β by 4T1 and (K) serum IFN-β in mice after different treatments. The means ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. (L) A depiction of the experimental method created to evaluate BMDCs. (M) FCM analysis of mature BMDCs in vitro (gated on CD11c+).
During a typical cGAS-STING signaling process, tumor cell-derived dsDNA is taken up by dendritic cells and attaches itself to the cytosolic cGAS dimers, triggering their enzymatic activity to catalyze the reaction between guanosine triphosphate (GTP) and adenylate triphosphate (ATP) to produce cGAMP [29,30]. To trigger various immunostimulatory signals, particularly IFN-β in DCs, cGAMP binds to the STING dimers to induce STING oligomerization, then activates the TBK1-IRF3 axis, which would significantly aid in dendritic cells-mediated cross-priming of T cells to develop strong antitumor immunity [31,32]. Furthermore, by encouraging cGAS-DNA phase separation, free zinc ions increased the activity of the cGAS enzyme in cells and in vitro [14,33]. Fig. S14 illustrates that while both tumor DNA and Zn2+ can raise the proportions of mature DCs when compared to Control, Group Tumor DNA + Zn2+ demonstrated a more notable increase. WB results suggested that Zn2+ and alkyl radicals synergically upregulate the phosphorylated expression of TBK1 and IRF-3(Fig. 6F-G and S15). The aforementioned findings suggest that mtDNA and DNA may trigger the cGAS-STING-IRF3 pathway. Furthermore, the cellular expression of IFN-β also increases (Fig. 6H), and the red fluorescence (IFN-β) in the cytoplasm of HAZ + US is observably higher than the others (Fig. 6I). ELISA results show that IFN-β of tumor cell culture medium and mouse serum significantly increased (Fig. 6J and K). Refer to Fig. 6L, to assess the therapeutic effectiveness of US in conjunction with HAZ in eliciting an immune response at the cellular level, murine bone marrow-derived dendritic cells (BMDCs) were isolated. WB results suggested that coculture BMDCs upregulate the phosphorylated TBK1, phosphorylated IRF-3 and IFN-β (Fig. S16). This data proved that the 4T1 cell debris generated after HAZ + US activated the STING pathway to improve the maturation of BMDCs. CD80 and CD86 are representative mature markers of DCs. As illustrated in Fig. 6M and S17, the proportion of mature DCs characterized by CD11c+, CD80+, and CD86+ following the ratio of BMDCs co-cultured with HAZ + US 4T1 cells reached 25.2 ± 2.57 %. This figure was markedly higher than those recorded in the HAZ group (18.6 ± 1.47 %), the US group (14.6 ± 1.25 %), and the Control group (10.3 ± 3.26 %). Immune activation is further dependent on cytokine secretion. ELISA was utilized to assess the relevant cytokines in the BMDCs' supernatants. After HAZ combined with US, the 4T1 cells triggered BMDCs to release the highest levels of cytokines, involving IFN-β, TNF-α and IL-12 (Fig. S18). The data showed that the cGAS-STING-IRF3 axis eventually increases IFN-β release.
3.7. HAZ-Mediated SDT enhanced Anti-Tumor effect
The effectiveness of HAZ in combating tumors, when used in conjunction with ultrasound, was also assessed in mice with 4T1 tumors. On day 12, mice were euthanized and tumors were subsequently excised for further analysis (Fig. 7A). The HAZ combined with the US group, demonstrated a prominent tumor inhibition effect in comparison to the others. The tumor growth inhibition (TGI) observed in HAZ + US group, HAZ group, and HZ + US group were 86.3 %, 59.5 %, and 28.0 % respectively (Fig. 7B and C), and the tumor growth rate of HAZ + US was considerably lower in comparison to the other experimental groups (Fig. 7D and Fig. S19), the US group showed nearly no inhibitory impact. However, there was no significant variation in body weight observed among the different groups (Fig. 7E). As presented in Fig. 7F and Fig. S20, histological analysis using H&E staining evaluated necrosis and apoptosis in the tumor specimens. A marked increase in necrotic cells, characterized by structural disintegration and nuclear shrinkage, was observed in the HAZ + US group. TUNEL staining indicated a markedly elevated occurrence of apoptotic cells (green fluorescence) in the HAZ + US group. Ki67, a symbol of cell proliferation, revealed that the proliferative cells (red fluorescence) of the HAZ + US group were dramatically lower than others. The above results further demonstrated that the HAZ-mediated US could effectively maximize the antitumor efficacy. Furthermore, the expression levels of p-IRF3 (indicated by red fluorescence) in the HAZ + US group were markedly higher in comparison to those observed in both the HAZ and HZ + US groups.
Fig. 7.
HAZ-mediated SDT enhanced anti-tumor effect. (A) Illustration for tumor experimentation and therapeutic schedule. (B) Photographs of tumors removed from each group. (C) Tumor weight of each group at the end of the treatments. (D) Tumor volume measurements for various treatment modalities over 12 days. (E) Weight variations among different groups of mice for 12 days. (F) H&E and TUNEL staining of tumors was conducted on tumors subjected to various treatment modalities. IHC staining of tumors was treated with various strategies (Scale bars: 100 μm). The means ± SD (n = 5), ***p < 0.001 and ****p < 0.0001.
A biosafety assessment was performed to analyze the potential for clinical application of HAZ. In vitro analysis indicated no significant differences in hemolysis rates with increasing concentrations of HAZ (Fig. S21). Moreover, we verified biosecurity using normal hepatic stellate cells (LX-2 cells) and normal kidney cells (HK-2 cells). Over a 24 h incubation period, there is no obvious cytotoxicity in LX-2 cells and HK-2 cells with different concentrations of HAZ (Fig. S22). In vivo studies demonstrated a 100 % survival rate across the HAZ + US group of mice. Blood samples and major organs were collected, and indicators of hepatic and renal function confirmed that HAZ does not induce significant toxicity to the livers or kidneys (Fig. S23). Histological examination of major organs utilizing H&E revealed no evident inflammation or lesions, further supporting the favorable biocompatibility of all treatment modalities (Fig. S24).
3.8. HAZ-mediated SDT enhanced immune reaction
The cGAS-STING pathway can augment the cross-presentation capabilities of DCs through the production of IFN-β. This process subsequently provokes CD8+ T cells, which are crucial in inhibiting tumor growth [[37], [38], [39]].
FCM gating strategy is shown in Fig. S25. To further confirm the maturation of BMDCs in vivo, a BALB/c mouse with a tumor was established (Fig. 8A), DCs were extracted from the draining lymph nodes. In alignment with the in vitro findings, the percentage of mature DCs in lymph nodes following HAZ combined with US treatment was recorded at 24.5 ± 4.87 %. The percentages of mature DCs in the various experimental groups were as follows: the HZ group exhibited a maturation percentage of 11.6 ± 0.66 %, the HZ + US group showed 16.0 ± 0.78 %, the HAZ group recorded 15.2 ± 2.33 %, the US group had a maturation percentage of 11.0 ± 0.44 %, and the Control group demonstrated a maturation percentage of 6.62 ± 1.28 % (Fig. 8B and C). Furthermore, the stimulation of the cGAS-STING pathway and ICD induction facilitated using HAZ combined with US significantly enhanced the immune response both in vivo and in vitro, thereby achieving a synergistic effect in sono-metalloimmunotherapy.
Fig. 8.
HAZ-mediated SDT enhanced immune response. (A) A depiction of the experimental method created to evaluate DCs and T cells in vivo. (B, C) FCM analysis (B) and measurement (C) of mature DCs in the lymph node (gated on CD11c+). (D, E) FCM analysis (D) and measurement (E) of CD8+ T cells (gated on CD45+) in tumors subjected to different treatments. (F, G) FCM measurement of splenic T cells (gated on CD3+) subjected to different treatments. The means ± SD (n = 3), *p < 0.05 and **p < 0.01. (H)Immunofluorescence images of CD8 proteins in tumors after different treatments (Scale bars: 100 μm).
Mature DCs are integral to antigen presentation and immunomodulation mechanisms, which can effectively capture and present antigens to T cells, thereby facilitating the activation of T cell-mediated immune responses [40]. CD8+ T cells, commonly referred to as CTLs, are pivotal effector cells in the context of antitumor immunotherapy. The group receiving HAZ combined with US exhibited a significantly higher proportion of CD8+ T cells in both tumor and spleen tissues compared to other experimental groups. The tumor infiltration of CD8+ T cells in the HAZ + US group (16.9 ± 2.51 %) was 1.83-fold greater than that in the HZ + US group (9.3 ± 0.79 %), 2.22 times greater than in the HAZ group (7.6 ± 3.37 %), and 3.75 times greater than in the Control group (4.5 ± 1.83 %) (Fig. 8D and E), moreover, CD4/CD8 ratio elevated in tumor (Fig. S26). Therefore, the percentage of CD8+ T cells in the HAZ + US group (25.5 ± 1.71 %) is significantly higher compared to the control (8.58 ± 3.18 %), with a 2.97-fold increase in the spleen (Fig. 8F and G). In Fig. 8H and Fig. S27, consistent with FCM data, there was a remarkable increase in the presence of tumor-infiltrating CD8+ T cells (indicated by green fluorescence) in the HAZ + US group in comparison to the other experimental groups. These results strongly prove that the combination therapy of US and HAZ facilitates the efficient penetration of CTLs into breast cancer tissues.
3.9. Abscopal effect on distant tumor
We also evaluated the abscopal effect of combination therapy on the bilateral 4T1 tumor model and the experimental timetable was displayed in Fig. 9A. There was no significant variation in body weight observed among the different groups. (Fig. 9B). Because of low immune activation, the Control group barely slowed the growth of distant tumors. However, the HAZ combined with the US group demonstrated a prominent tumor inhibition effect in comparison to the others. According to statistics, the tumors in the Control group were 2.4 times heavier than those in the HAZ + US group. (Fig. 9C-H). Moreover, the percentage of tumor-infiltrating T cells in the distant tumor of the HAZ + US group is significantly higher compared to the Control, which could explain the improved systemic immunity (Fig. 9I). As expected, HAZ-mediated SDT, which may be facilitated by the STING pathway and ICD, can increase CTLs infiltration throughout the body, killing distant tumor cells.
Fig. 9.
HAZ-mediated SDT enhanced the abscopal effect. (A) Illustration for tumor experimentation and therapeutic schedule. (B) Weight variations among different groups of mice. (C) Photographs of primary tumors removed from each group. (D) Primary tumor volume measurements for various treatments. (E) Primary tumor weight among different groups of mice. (F) Photographs of distant tumors removed from each group. (G) Distant tumor volume measurements for various treatment modalities. (H) Distant tumor weight of each group at the end of the treatments. (I) FCM analysis of T cells (gated on CD3+) in distant tumors subjected to different treatments. The means ± SD (n = 5), *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
3.10. RNA sequencing analysis of HAZ-Mediated SDT
To clarify how HAZ + US in situ tumor vaccines strengthen anti-tumor immune responses, transcriptome analysis of treated tumors was performed using RNA sequencing (RNAseq). As shown in Fig. 10A, in contrast to the Control group, 1680 down-regulated genes (blue) and 2087 up-regulated genes (red) were identified in 4T1 cells. The Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses were used to analyze the potential mechanism therapeutic pathways HAZ-mediated SDT. In the GO charts (Fig. 10B), these pathways include ATP binding, mitochondrion, apoptotic process, DNA damage response, innate immune response, and T cell mediated cytotoxicity significantly changed after HAZ + US treatment. These results confirm that HAZ-induced SDT influences several cellular functions and immune response to produce its therapeutic properties. KEGG analysis (Fig. 10C) indicates that the HAZ + US enhances the expression of genes linked to multiple pathways, such as apoptosis, DNA replication, the p53 signaling pathway, and the MAPK signaling pathway. Given the vital function of these pathways for cell proliferation and response to stress and damage, HAZ + US may change them to facilitate the death of cancer cells. As shown in Fig. 10D and E, the SDT treatment significantly upregulates the apoptosis-related genes in tumor cells, including hypermethylated in cancer 1 (HIC1), C-X-C motif chemokine ligand 10 (CXCL10). HIC1 regulates the apoptotic DNA-damage response dependent on the p53 pathway to promote tumor suppression. CXCL10 triggers tumor cell apoptosis via the non-canonical STING-NFκB pathway [41,42]. The downregulation of mt-Nd3 leads to mitochondrial complex I deficiency and reduction of ATP synthesis [43]. Hence, under US irradiation, the HAZ + US may cause apoptosis via DNA damage and mitochondrial dysfunction.
Fig. 10.
RNA sequencing analysis of HAZ-mediated SDT. (A) Genes in HAZ + US-treated 4T1 cells were found to be differentially regulated compared to the Control, as illustrated in the volcano plot (P < 0.05, |fold change| ≥ 1.2). (B) GO term analysis of RNaseq-based biological process profiles following HAZ + US treatment. (C) KEGG term of variations in pathway profiles depending on RNaseq following SDT treatment (D) Heatmap of genes that had distinct expressions in HAZ + US-treated 4T1 cancer cells compared to the Control. (E) GSEA enrichment plots of genes with differential expression in the HAZ + US group versus the Control group.
To more effectively inhibit tumors, HAZ-mediated SDT therapy helps the body develop a strong and long-lasting immune response against tumors. Many immune response-related genes that are associated with anti-tumor and antigen processing are significantly heightened, consisting of H2-M3, H2-Q6, H2-Q7, and H2-DMa. To ensure efficient immune surveillance, this procedure depends on the cooperative activity of several genes and signaling pathways, major histocompatibility complex class I (MHC-I) molecules(H2-Q6 and H2-M3) that were up-regulated [44]. Conventional type 1 dendritic cells can present exogenous antigens to CD8+ T cells via MHC-I molecules, a process known as cross-presentation. CD8+ T cells activated through cross-presentation can recognize and kill tumor cells expressing the corresponding antigen [45]. These results imply that the HAZ + US influences many different kinds of biological processes and signaling pathways related to DNA repair, apoptosis, mitochondrial function, and immune response, ultimately resulting in the death of cancer cells and offering a possible therapeutic immune response.
4. Conclusion
In summary, we have successfully engineered ZIF-8 and alkyl radical generator-loaded nanoparticles for tumor-targeting sono-metalloimmunotherapy. Compared with conventional ultrasonic sensitizers, the HAZ-mediated SDT simultaneously achieves targeted delivery and release of Zn2+ and alkyl radicals, and the exceptional imaging capability of HAZ may make it possible to monitor the formation of HAZ at the location of the tumor. The alkyl radicals induce mitochondrial membrane destruction and damage to nuclear DNA, resulting in the leakage of dsDNA, including mtDNA and nuclear DNA. These dsDNA and ZIF-8-derived Zn2+ synergistically amplified the dsDNA/cGAS-STING pathway. Moreover, alkyl radicals from HAZ also effectively promote ICD. Both strategies enhance the population of mature DCs in draining lymph nodes as well as tumor-infiltrating CTL cells, thereby facilitating a robust antitumor immune response. We demonstrate that the HAZ-mediated SDT should effectually limit tumor growth in vivo while exhibiting negligible systemic toxicity. The HAZ-mediated SDT may provide effective immune treatment and US imaging guidance, which is expected to advance the domain of metal-based ultrasonic contrast agents.
CRediT authorship contribution statement
Xinyu Zeng: Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Xiaoxuan Wang: Methodology, Formal analysis. Yading Zhao: Methodology. Lu Guo: Writing – review & editing, Investigation. Xiao Sun: Supervision. Mengmeng Shang: Supervision. Shuting Huang: Formal analysis. Rui Liu: Software. Jialu Zhang: Resources. Shan Xiao: Investigation. Dandan Shi: Project administration. Ning Cong: Visualization. Jie Li: Supervision, Project administration, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
Qilu Hospital of Shandong University’s Laboratory Animal Ethics Committee authorized the protocols for the animal experiments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (82471992) and the Taishan Scholar Foundation of Shandong Province. The artwork of this study was drawn by the Generic Diagramming Platform (https://www.biogdp.com).
Footnotes
This article is part of a special issue entitled: ‘Biomaterial Assembly and Theranostics’ published in Ultrasonics Sonochemistry.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2025.107494.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Adams S., Gatti-Mays M.E., Kalinsky K., Korde L.A., Sharon E., Amiri-Kordestani L., Bear H., McArthur H.L., Frank E., Perlmutter J., Page D.B., Vincent B., Hayes J.F., Gulley J.L., Litton J.K., Hortobagyi G.N., Chia S., Krop I., White J., Sparano J., Disis M.L., Mittendorf E.A. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019;5:1205. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou D., Zhang G., Li J., Zhuang Z., Shen P., Fu X., Wang L., Qian J., Qin A., Tang B.Z. Near-infrared II agent with excellent overall performance for imaging-guided photothermal thrombolysis. ACS Nano. 2024 doi: 10.1021/acsnano.4c06965. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Xu X., Wei H., Wu D., Zeng L. Pt/Pd dual-modified porphyrin metal-organic frameworks for NIR-II photothermal-enhanced photodynamic/catalytic therapy. J. Colloid Interface Sci. 2025;678:42–52. doi: 10.1016/j.jcis.2024.08.154. [DOI] [PubMed] [Google Scholar]
- 5.Son S., Kim J.H., Wang X., Zhang C., Yoon S.A., Shin J., Sharma A., Lee M.H., Cheng L., Wu J., Kim J.S. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020;49:3244–3261. doi: 10.1039/C9CS00648F. [DOI] [PubMed] [Google Scholar]
- 6.Lin D., Wang Z., Long W., Xu M., Liu A., Gao Y., Wen Z., Liu C., He J., Cheng Y., Jiang S., Chen J., Liu Q., Zhang L., You R., Yin L., Guan Y. Nanosonosensitizer‐augmented sono‐immunotherapy for glioblastoma by non‐invasive opening of the blood–brain barrier. Adv. Funct. Mater. 2023 [Google Scholar]
- 7.Zhang Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021 doi: 10.1039/d1cs00403d. [DOI] [PubMed] [Google Scholar]
- 8.Shen S., Zhu C., Huo D., Yang M., Xue J., Xia Y. A hybrid nanomaterial for the controlled generation of free radicals and oxidative destruction of hypoxic cancer cells. Angew. Chem. Int. Ed. Engl. 2017;56:8801–8804. doi: 10.1002/anie.201702898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z.-H., Saito Y., Yoshida Y., Niki E. Effect of oxygen concentration on free radical-induced cytotoxicity. Biosci. Biotechnol. Biochem. 2008;72:1491–1497. doi: 10.1271/bbb.80002. [DOI] [PubMed] [Google Scholar]
- 10.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Li H., Niu G., Li Y., Huang Z., Cheng S., Zhang K., Li H., Fu Q., Jiang Y. Boosting sono-immunotherapy of prostate carcinoma through amplifying domino-effect of mitochondrial oxidative stress using biodegradable cascade-targeting nanocomposites. ACS Nano. 2024 doi: 10.1021/acsnano.3c12511. [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Gupta R., Blanco L.P., Yang S., Shteinfer-Kuzmine A., Wang K., Zhu J., Yoon H.E., Wang X., Kerkhofs M., Kang H., Brown A.L., Park S.-J., Xu X., Zandee Van Rilland E., Kim M.K., Cohen J.I., Kaplan M.J., Shoshan-Barmatz V., Chung J.H. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–1536. doi: 10.1126/science.aav4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C., Shang G., Gui X., Zhang X., Bai X., Chen Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–398. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du M., Chen Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Wang Y., Zhao L., Stenzel M.H., Jiang Y. Metal ion interference therapy: metal-based nanomaterial-mediated mechanisms and strategies to boost intracellular “ion overload” for cancer treatment. Mater. Horiz. 2024;11:4275–4310. doi: 10.1039/D4MH00470A. [DOI] [PubMed] [Google Scholar]
- 16.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azagury A., Khoury L., Enden G., Kost J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014;72:127–143. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Okabe M., Yoshida T., Yoshii R., Sawataisi M., Takaya K. Zinc detection in the islet of Langerhans by SIMS. Appl. Surf. Sci. 2003;203–204:714–717. doi: 10.1016/S0169-4332(02)00797-3. [DOI] [Google Scholar]
- 19.Kim T., Han H.S., Yang K., Kim Y.M., Nam K., Park K.H., Choi S.Y., Park H.W., Choi K.Y., Roh Y.H. Nanoengineered polymeric RNA nanoparticles for controlled biodistribution and efficient targeted cancer therapy. ACS Nano. 2024;18:7972–7988. doi: 10.1021/acsnano.3c10732. [DOI] [PubMed] [Google Scholar]
- 20.Peng F., Setyawati M.I., Tee J.K., Ding X., Wang J., Nga M.E., Ho H.K., Leong D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019;14:279–286. doi: 10.1038/s41565-018-0356-z. [DOI] [PubMed] [Google Scholar]
- 21.Meng H., Xue M., Xia T., Ji Z., Tarn D.Y., Zink J.I., Nel A.E. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5:4131–4144. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu B., Chen Z., Wu X., Shi H., Jin X., Song B., Cui M., Zhao Y., Zhao Y., He Y., Wang H., Dong F. Photoactivated gas-generating nanocontrast agents for long-term ultrasonic imaging-guided combined therapy of tumors. ACS Nano. 2024;18:15590–15606. doi: 10.1021/acsnano.4c01041. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C., Xin L., Li J., Cao J., Sun Y., Wang X., Luo J., Zeng Y., Li Q., Zhang Y., Zhang T., Huang P. Metal–organic framework (MOF)‐based ultrasound‐responsive dual‐sonosensitizer nanoplatform for hypoxic cancer therapy. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202101946. [DOI] [PubMed] [Google Scholar]
- 24.Yan J., Wang Y., Song X., Yan X., Zhao Y., Yu L., He Z. The advancement of gas‐generating nanoplatforms in biomedical fields: current frontiers and future perspectives. Small Methods. 2022;6 doi: 10.1002/smtd.202200139. [DOI] [PubMed] [Google Scholar]
- 25.Jung E., Kang C., Lee J., Yoo D., Hwang D.W., Kim D., Park S.-C., Lim S.K., Song C., Lee D. Molecularly engineered theranostic nanoparticles for thrombosed vessels: H2O2 -activatable contrast-enhanced photoacoustic imaging and antithrombotic therapy. ACS Nano. 2018;12:392–401. doi: 10.1021/acsnano.7b06560. [DOI] [PubMed] [Google Scholar]
- 26.Lukas J., Lukas C., Bartek J. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 27.Lv C., Kang W., Liu S., Yang P., Nishina Y., Ge S., Bianco A., Ma B. Growth of ZIF-8 nanoparticles In Situ on Graphene oxide nanosheets: a multifunctional nanoplatform for combined ion-interference and photothermal therapy. ACS Nano. 2022;16:11428–11443. doi: 10.1021/acsnano.2c05532. [DOI] [PubMed] [Google Scholar]
- 28.West A.P., Shadel G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017;17:363–375. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C., Guan J., Lu S., Jin Q., Rousseau B., Lu T., Stephens D., Zhang H., Zhu J., Yang M., Ren Z., Liang Y., Liu Z., Han C., Liu L., Cao X., Zhang A., Qiao J., Batten K., Chen M., Castrillon D.H., Wang T., Li B., Diaz L.A., Li G.-M., Fu Y.-X. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell. 2021;39:96–108.e6. doi: 10.1016/j.ccell.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing Y., Peng A., Yang J., Cheng Z., Yue Y., Liu F., Li F., Liu Y., Liu Q. Precisely activating cGAS-STING pathway with a novel peptide-based nanoagonist to potentiate immune checkpoint blockade cancer immunotherapy. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024;11 doi: 10.1002/advs.202309583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Liu Y., Xue C., Hu Y., Zhao Y., Cai K., Li M., Luo Z. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat. Commun. 2022;13:5685. doi: 10.1038/s41467-022-33301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J., Wang G., Xie L., Tian H., Li J., Li B., Sang W., Li W., Zhang Z., Dai Y. Engineering radiosensitizer-based metal-phenolic networks potentiate STING pathway activation for advanced radiotherapy. Adv. Mater. Deerfield Beach Fla. 2022;34 doi: 10.1002/adma.202105783. [DOI] [PubMed] [Google Scholar]
- 33.Sun W., Wang H., Qi Y., Li M., Zhang R., Gao Z., Cui J., Yu D. Metal-phenolic vehicles potentiate cycle-cascade activation of pyroptosis and cGAS-STING pathway for tumor immunotherapy. ACS Nano. 2024;18:23727–23740. doi: 10.1021/acsnano.4c08613. [DOI] [PubMed] [Google Scholar]
- 34.Li Q., Lan P. Activation of immune signals during organ transplantation. Signal Transduct. Target. Ther. 2023;8:110. doi: 10.1038/s41392-023-01377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 36.Wang F., Lai W., Xie D., Zhou M., Wang J., Xu R., Zhang R., Li G. Nanoparticle-mediated celastrol ER targeting delivery amplify immunogenic cell death in melanoma. J. Adv. Res. 2024 doi: 10.1016/j.jare.2024.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynn G.M., Sedlik C., Baharom F., Zhu Y., Ramirez-Valdez R.A., Coble V.L., Tobin K., Nichols S.R., Itzkowitz Y., Zaidi N., Gammon J.M., Blobel N.J., Denizeau J., De La Rochere P., Francica B.J., Decker B., Maciejewski M., Cheung J., Yamane H., Smelkinson M.G., Francica J.R., Laga R., Bernstock J.D., Seymour L.W., Drake C.G., Jewell C.M., Lantz O., Piaggio E., Ishizuka A.S., Seder R.A. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao L., Tian H., Fang M., Xu Z., Tang D., Chen J., Yin J., Xiao H., Shang K., Han H., Li X. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121856. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z., Yue Y., Li Q., Wang Y., Wu X., Li X., Li H., Shi L., Guo D., Liu Y. Design of calixarene‐based ICD inducer for efficient cancer immunotherapy. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202213967. [DOI] [Google Scholar]
- 41.Wu J., Liu N., Chen J., Tao Q., Lu C., Li Q., Chen X., Peng C. Clofarabine induces tumor cell apoptosis, GSDME-related pyroptosis, and CD8+ T-cell antitumor activity via the non-canonical P53/STING pathway. J. Immunother. Cancer. 2025;13 doi: 10.1136/jitc-2024-010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W.Y., Wang D.H., Yen R.C., Luo J., Gu W., Baylin S.B. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Borna N.N., Kishita Y., Shimura M., Murayama K., Ohtake A., Okazaki Y. Identification of a novel MT-ND3 variant and restoring mitochondrial function by allotopic expression of MT-ND3 gene. Mitochondrion. 2024;76 doi: 10.1016/j.mito.2024.101858. [DOI] [PubMed] [Google Scholar]
- 44.Harding J., Vintersten-Nagy K., Yang H., Tang J.K., Shutova M., Jong E.D., Lee J.H., Massumi M., Oussenko T., Izadifar Z., Zhang P., Rogers I.M., Wheeler M.B., Lye S.J., Sung H.-K., Li C., Izadifar M., Nagy A. Immune-privileged tissues formed from immunologically cloaked mouse embryonic stem cells survive long term in allogeneic hosts. Nat. Biomed. Eng. 2024;8:427–442. doi: 10.1038/s41551-023-01133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luri-Rey C., Teijeira Á., Wculek S.K., de Andrea C., Herrero C., Lopez-Janeiro A., Rodríguez-Ruiz M.E., Heras I., Aggelakopoulou M., Berraondo P., Sancho D., Melero I. Cross-priming in cancer immunology and immunotherapy. Nat. Rev. Cancer. 2025;25:249–273. doi: 10.1038/s41568-024-00785-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.