Abstract
Objectives
To pool and rank the efficacy of various stimulation therapies, including repetitive peripheral magnetic stimulation (rPMS), neuromuscular electrical stimulation (NMES), functional electrical stimulation (FES), transcranial magnetic stimulation (TMS), and combinations of these interventions on upper extremity function, activities of daily living (ADL), and spasticity after stroke relative to sham/conventional rehabilitation.
Literature Survey
MEDLINE, Scopus, Physiotherapy Evidence Database, Cochrane Central Register of Controlled Clinical Trials, and Google Scholar were searched from inception to July 2022.
Methodology
Randomized controlled trials comparing any of the interventions mentioned above (rPMS, NMES, FES, TMS, NMES+rPMS, NMES+TMS, FES+TMS, and conventional rehabilitation) on upper extremity function, ADL, or spasticity from five databases were systematically reviewed and collected. Two‐stage network meta‐analysis was applied.
Synthesis
Thirty‐four studies involving 1476 patients reporting upper extremity function with the Fugl‐Meyer Assessment were pooled. NMES combined with rPMS, NMES, NMES combined with TMS, TMS, and FES showed significantly higher improvement than conventional rehabilitation, with pooled mean differences (95% confidence intervals) of 14.69 (9.94–19.45), 9.09 (6.01–12.18), 6.10 (2.51–9.69), 4.07 (0.33–7.81), and 3.61 (0.14–7.07) respectively. NMES combined with rPMS had the highest probability for improving upper extremity function. NMES plus TMS had the highest probability for improving ADL, but none of the interventions showed significant differences in spasticity.
Conclusions
NMES plus rPMS might be the best intervention to improve upper extremity functions, with NMES plus TMS most likely to lead to improved ADL but the quality of the evidence is low.
INTRODUCTION
Rehabilitation is pivotal for the recuperation of patients after stroke, 1 of whom approximately 25% to 50% are partially or totally dependent to undertake daily life activities, 2 especially given deficits in upper extremity motor function. 3 Currently, various neurorehabilitation interventions have been incorporated into standard rehabilitation practices to enhance neuroplasticity and aid in the recovery of upper extremity function in post‐stroke patients. These interventions include neuromuscular electrical stimulation (NMES), functional electrical stimulation (FES), transcranial magnetic stimulation (TMS), and repetitive peripheral magnetic stimulation (rPMS). TMS is subject to several side effects, such as headache, discomfort, 4 , 5 and unintentional seizures, 4 and electrical stimulation (ES) may cause skin irritation and burns. 6 Recently, rPMS has grown in popularity given the limitations and side effects associated with ES and TMS. rPMS is a noninvasive method of delivering a rapidly pulsed, high‐intensity magnetic field to the extremities. In poststroke rehabilitation, rPMS aims to improve motor function and neuromodulation in movement.
Several previous systematic reviews and pairwise meta‐analyses have been conducted, 7 , 8 but there was a dearth of evidence for the use of rPMS in patients after stroke. More recent studies 9 , 10 , 11 , 12 have suggested that rPMS may improve upper extremity function, leading to a better recovery than TMS in patients after stroke. To our knowledge, there has not been a previous network meta‐analysis comparing the efficacy of single or multiple interventions, including rPMS, TMS, or ES, relative to each other or sham/conventional rehabilitation for improving upper extremity function in poststroke patients. Therefore, this network meta‐analysis was conducted with the following objectives: first, to pool and rank the efficacy of various stimulation therapies on upper extremity function relative to conventional rehabilitation; and second, to pool and rank the efficacy of the aforementioned interventions on activities of daily living (ADL) and spasticity in poststroke patients.
METHODS
This systematic review and network meta‐analysis was undertaken and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) extension for network meta‐analysis. 13 Its protocol was prospectively registered in International Prospective Register of Systematic Reviews (CRD42022351051).
Search strategy
We searched electronic databases, including MEDLINE via PubMed, Scopus, the Physiotherapy Evidence Database, and the Cochrane Central Register of Controlled Clinical Trials, from their inception to July 2022. The search terms were constructed based on relevant concepts as follows: (1) patients—stroke; (2) interventions/comparators—rPMS, TMS, NMES, FES; (3) outcomes— upper extremity function, ADL, spasticity; and (4) study type—randomized controlled trial (RCT), as shown in Appendix 1 in Data S1.
Selection of studies
RCTs were considered eligible based on the following inclusion criteria: (1) conducted in adult patients aged 18 years or older with a poststroke condition or any patients with neurological disorder >50% attributable to stroke; (2) compared any regimen of interventions (ie, rPMS, TMS, ES, or their combinations) with any control intervention (ie, sham procedure, placebo, conventional rehabilitation, or a combination thereof); and (3) assessed at least one of the following outcomes: upper extremity functions, ADL, and spasticity.
Studies were excluded if they had insufficient data for pooling or were published in languages that reviewers could not translate.
Interventions
The interventions included rPMS, TMS, and ES, or combinations thereof. rPMS was applied to the paretic upper limb using any regimen. The intensity was at least minimal muscle contraction with a frequency of 1–50 Hz and a duration of 1 minute. TMS was applied through the affected or unaffected cerebral hemisphere with an intensity of at least 50% of the motor threshold, focusing on upper extremity muscles and a frequency of 1–50 Hz. Two types of ES were considered: NMES used electrical current to produce minimal contraction of paretic muscles whereas FES used electrical stimulation during voluntary movement for functional purposes.
Outcomes of interest
The primary outcome of interest was the upper extremity function after receiving the intervention. The measurement time was categorized as short term (at the end of the course of intervention) and long term (1–3 months after the course of intervention). Most studies used the Fugl‐Meyer Assessment 14 (FMA), which measures the upper extremity's voluntary movement, reflex activity, grasp, and coordination. The total score ranges from 0 to 66 points; with higher score indicating better upper extremity performance.
The secondary outcomes of interest included ADL and spasticity. For ADL, most studies used the Barthel Index (BI) 15 which ranges from 0 to 100 points, with a higher score indicative of more independence in ADL. The BI measures independence for 10 items of daily activities (feeding, moving from a wheelchair to bed and returning, using the toilet, getting on and off the toilet, bathing oneself, walking on a level surface, ascending and descending stairs, dressing, and controlling bowels and bladder). Each item is categorized to three options: unable to perform the task (score = 0), needing assistance (score = 0, 5, or 10 according to the item), and being fully independent (score = 5, 10, or 15 according to the item). For spasticity, the Modified Ashworth Scale (MAS) 16 was used. MAS can range from 0 (no increase in muscle tone), 1 (slight increase in muscle tone, manifested by catch and release), 1+ (slight increase in muscle tone, manifested by catch, followed by minimal resistance), 2 (more marked increase in muscle tone through most of the range of motion), 3 (considerable increase in muscle tone, passive movement difficult), and 4 (affected part rigid in flexion or extension). A higher score reflects more spasticity.
Data extraction and risk of bias assessment
Data extraction was performed by two reviewers (A.K., M.S.) and covered six domains: general information, study characteristics, participant characteristics, interventions, outcomes, and data for pooling, see details in Appendices 2 and 3 in Data S1. The quality of the studies was also independently assessed by the same reviewers using the Revised Cochrane Risk‐of‐Bias Tool for Randomized Trials (RoB 2.0). 17 Five domains were assessed including randomization process, protocol deviations, missing outcome data, measurement of the outcome, and selection of the reported results. Each study was judged as low risk, high risk, or having some concerns. Any disagreements between reviewers were resolved by consensus (A.K., M.S., K.T., P.N., and A.T.).
Statistical analysis
Pairwise meta‐analysis was performed on each intervention pair that was present in at least three studies. The unstandardized mean difference (USMD) from each study was estimated and pooled across the studies using a random‐effects model if heterogeneity was present, otherwise a fixed‐effect model was used. Cochrane's Q test and the I 2 statistic were used for assessing heterogeneity. Heterogeneity was considered present if the p value of Cochrane's Q test <.1 or I 2 ≥ 25%. Covariables were explored as a source of heterogeneity by fitting each covariable in a meta‐regression model; if a covariate reduced τ 2 (tau squared) by at least 50%, subgroup analysis on that covariable was performed.
Network meta‐analysis with the consistency model was performed using a two‐stage approach as follows. First, USMDs and their variance–covariance were estimated for each study using the sham procedure/conventional rehabilitation as the reference group. Second, multivariate random‐effects meta‐analysis was applied to pool USMDs across the studies. The transitivity assumption was checked by reviewing characteristics of the studies. The consistency assumption was checked using the design‐by‐treatment interaction inconsistency model. 18 , 19 Inconsistency was considered present when the p value of the global test was <.05. 20 If this assumption was violated, a loop‐specific approach was applied to identify a specific loop of network meta‐analysis that caused inconsistency. 21 Characteristics of the studies within the loop were explored and sensitivity analysis performed by excluding studies with different characteristics to achieve global consistency; if not achieved, the inconsistency model with design‐by‐treatment interaction, which adjusted the effects of the treatments from different study designs, was used to estimate the relative treatment effects. The surface under the cumulative ranking curves (SUCRA) was used to rank the best treatment with highest upper extremity functions or ADL score, and lowest spasticity score. Subgroup analysis was performed according to duration of stroke (acute‐subacute vs. chronic stroke) and stroke severity (low vs. high baseline functional FMA) in the outcomes with sufficient data. Publication bias was assessed using a comparison‐adjusted funnel plot and Egger's test. All analyses were conducted using Stata version 17.0 (StataCorp. 2022. Stata Statistical Software: Release 17. StataCorp LLC, College Station, TX).
The confidence in the network meta‐analysis findings was assessed using the web application Confidence in Network Meta‐Analysis (CINeMA). Six domains were considered based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, that is, within‐study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence. 22 , 23
First, within‐study bias refers to bias in a study's design or conducting study that can systematically distort the estimated relative treatment effect, causing it to deviate from the true effect. This bias could be assessed using the RoB tool as mentioned. Second, reporting bias occurs when there is a systematic omission or distortion of study results, often due to publication bias (omitting non‐significant or “negative” studies), time‐lag bias (delaying publication of studies with unfavorable results), or outcome reporting bias (excluding unfavorable results from study reports). Third, the indirectness refers to the degree to which the included studies directly address the research question. This can arise when the study populations, interventions, outcomes, or study settings are not representative of the settings, populations, or outcomes for which inferences are being drawn. For example, we downgraded studies that included neurological patients rather than solely stroke patients. Fourth, imprecision of the effect size is assessed based on the equivalence zone. An estimate is considered imprecise if lower and upper limits of the 95% confidence interval (CI) fall outside the equivalence zone from no benefit to risk effect, and the other limit exceeds the equivalence zone; or both limits fall within the range of no benefit to risk. Fifth, heterogeneity, measured by tau, 2 reflects the variation in effect sizes across included studies. This variation is accounted for when calculating the prediction interval. Heterogeneity is significant if the prediction interval includes values that lead to different conclusions compared to those drawn from the 95% CI alone. Sixth, incoherence is the disagreement of effect sizes estimated from direct and indirect comparisons. Each domain was graded as no concern, some concern, and major concern. Finally, each comparison was summarized across domains and graded level of confidence of the GRADE approach as very low, low, moderate, and high. 22 , 23
RESULTS
Characteristics of included studies
Of 2096 identified studies, 81 full papers were screened, leaving 62 eligible and included in this review. Reasons for exclusions are shown in the PRISMA flow chart (Figure 1). Characteristics of the eligible studies are summarized in Table 1. There were five single interventions (ie, NMES, FES, TMS, rPMS, and conventional rehabilitation) and three combination interventions (ie, NMES+TMS, NMES+rPMS, and FES + TMS); see more details in Table 1 and Appendix 4 in Data S1. Percentage of males ranged from 3.6 to 93.8, and mean age from 45.5 to 74.5 years. Most studies (98%) included patients with ischemic stroke as the majority of their participants. The percentage of right hemiparesis with left dominant hemisphere lesion ranged from 26.7 to 77.8.
FIGURE 1.

Preferred reporting items for systematic reviews and meta‐analysis (PRISMA) flow diagram of study selection. ADL, activities of daily living; BI, Barthel Index; CENTRAL, Cochrane Central Register of Controlled Clinical Trials; FES, functional electrical stimulation; FMA, Fugl‐Meyer Assessment; MAS, Modified Ashworth Scale; NMES, neuromuscular electrical stimulation; PEDro, Physiotherapy Evidence Database; RCT, randomized controlled trial; Rehab, conventional rehabilitation; rPMS, repetitive electrical magnetic stimulation; SR, systematic review; SRMA, systematic review and meta‐analysis; TMS, transcranial magnetic stimulation.
TABLE 1.
Characteristics of included studies.
| References | Year | n | Mean age (year) | Male (%) | Mean duration of disease (months) | Ischemic stroke (%) | Left hemiparesis (%) | Intervention | Duration of intervention (days) | Upper extremity function‐measurement | ADL‐measurement | Spasticity‐measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ke et al. 11 | 2022 | 26 | 57 | 53.8 | 0.54 | 0 | 46.2 | rPMS vs. Rehab | 10 | FMA | ‐ | ‐ |
| Jiang et al. 10 | 2022 | 44 | 55.3 | 61.6 | 0.46 | 72.7 | 38.6 | NMES + rPMS vs. NMES | 14 | FMA | BI | ‐ |
| El Nahas. et al. 62 | 2022 | 64 | 46.0 | 75 | 46.35 | NA | NA | rPMS vs. Rehab | 8 | ‐ | ‐ | MAS |
| Krewer et al. 41 | 2014 | 40 | 54.5 | 60.3 | 7.15 | NA | 52.4 | rPMS vs. Rehab | 10 | FMA | BI | mTS |
| Luk et al. 44 | 2022 | 37 | 66.2 | 58.3 | 3.30 | 87.5 | 58.3 | TMS vs. Rehab | 10 | FMA, ARAT, BBT | ‐ | ‐ |
| Haghighi et al. 33 | 2021 | 24 | 52.2 | 50 | 3.10 | 45 | 55 | TMS vs. Rehab | 10 | FMA, BBT | ‐ | ‐ |
| Gottlieb et al. 31 | 2021 | 20 | 63.1 | 42.9 | 1.41 | 89.3 | 25 | FES + TMS vs. FES | 10 | FMA | ‐ | MAS |
| Sharma et al. 56 | 2020 | 96 | 53.8 | 72.9 | 0.16 | 100 | NA | TMS vs. Rehab | 14 | FMA | mBI | MAS |
| Kim et al. 40 | 2020 | 77 | 62.1 | 61.65 | 0.49 | 100 | 50.7 | TMS vs. Rehab | 14 | FMA, BBT | mBI | MAS |
| Chen et al. 27 | 2019 | 22 | 52.8 | 6.00 | 22.5 | 68.2 | TMS vs. Rehab | 10 | FMA, ARAT | ‐ | ‐ | |
| Tretriluxana et al. 87 | 2018 | 16 | 52.2 | 3.6 | 3.57 | NA | NA | TMS vs. Rehab | 1 | WMFT‐time | ‐ | ‐ |
| Harvey et al. 34 | 2018 | 199 | 58.7 | 65.3 | 7.71 | 78.9 | 53 | TMS vs. Rehab | 18 | FMA, ARAT, WMFT‐time | ‐ | ‐ |
| Wang et al. 54 | 2017 | 42 | 60.8 | 69.0 | 2.30 | 69.0 | 40.5 | TMS vs. Rehab | 24 | FMA, WMFT | ‐ | ‐ |
| Ozjeskin et al. 63 | 2017 | 21 | 60.3 | 61.9 | 17.81 | NA | 52.4 | TMS vs. Rehab | 10 | ‐ | ‐ | MAS |
| Meng et al. 58 | 2017 | 20 | 65 | 85 | NA | 100 | NA | TMS vs. Rehab | 14 | ‐ | BI | ‐ |
| Guan et al. 32 | 2017 | 42 | 58.5 | 71.43 | 0.23 | 100 | 54.8 | TMS vs. Rehab | 10 | FMA | BI | ‐ |
| Askin et al. 24 | 2017 | 40 | 57.8 | 72.5 | 26.4 | 100 | 57.5 | TMS vs. Rehab | 10 | FMA, BBT | FIM | MAS |
| Hosomi et al. 35 | 2016 | 39 | 62.8 | 59.2 | 1.84 | 61.62 | 61.5 | TMS vs. Rehab | 10 | FMA | FIM | ‐ |
| Ackerley et al. 88 | 2016 | 18 | 66 | 66.7 | 19.00 | 100 | 33.3 | TMS vs. Rehab | 10 | ARAT | ‐ | ‐ |
| Matsuura et al. 45 | 2015 | 20 | 73.4 | 55 | 0.32 | 100 | 50 | TMS vs. Rehab | 5 | FMA | ‐ | ‐ |
| Vaziri et al. 53 | 2014 | 12 | 56.7 | NA | 23.50 | NA | NA | TMS vs. Rehab | 10 | FMA | BI | ‐ |
| Rose et al. 47 | 2014 | 21 | 64.6 | 68.4 | 61.66 | NA | 47.4 | TMS vs. Rehab | 16 | FMA, WMFT, WMFT‐time |
‐ |
MAS |
| Galvao et al. 30 | 2014 | 20 | 61 | 65 | 53.35 | 85 | 50 | TMS vs. Rehab | 10 | FMA | FIM | MAS |
| Hsu et al. 36 | 2013 | 12 | 59.6 | 66.7 | 0.70 | 100 | NA | TMS vs. Rehab | 10 | FMA, ARAT | ‐ | ‐ |
| Higgins et al. 89 | 2013 | 11 | 66.2 | 66.7 | 108.33 | NA | 22.2 | TMS vs. Rehab | 8 | BBT, WMFT, WMFT‐time | ‐ | ‐ |
| DiLazzaro et al. 90 | 2013 | 12 | 58.5 | 58.3 | 32.40 | NA | NA | TMS vs. Rehab | 10 | ARAT, JTT | ‐ | ‐ |
| Seniow et al. 49 | 2012 | 40 | 63.4 | 65 | 1.31 | 87.5 | 42.5 | TMS vs. Rehab | 15 | FMA, WMFT, WMFT‐time | ‐ | ‐ |
| Conforto et al. 64 | 2012 | 30 | 55.8 | 61.7 | 0.91 | 100 | 50 | TMS vs. Rehab | 10 | ‐ | ‐ | MAS |
| Sohn et al. 65 | 2010 | 13 | 54.2 | 61.5 | 53.88 | NA | 46.2 | TMS vs. Rehab | 10 | Motor function test | ‐ | MAS |
| Malcolm et al. 91 | 2007 | 19 | 67 | 42.1 | 45.60 | NA | 47.4 | TMS vs. Rehab | 10 | BBT, WMFT‐time | ‐ | ‐ |
| Kim et al. 92 | 2021 | 33 | NA | NA | 6.00 | NA | NA | TMS vs. Rehab | 20 | Motor function test | mBI | ‐ |
| Fletcher‐Smith et al. 57 | 2019 | 40 | 71 | 50 | 0.06 | NA | 55 | NMES vs. Rehab | 60 | ARAT | BI | ‐ |
| Dorsch et al. 93 | 2014 | 33 | 67.6 | 54.6 | 0.52 | NA | 42.6 | NMES vs. Rehab | 20 | Motor assessment scale | ‐ | ‐ |
| Sahin et al. 94 | 2012 | 42 | 59.8 | 57.1 | 30.05 | NA | NA | NMES vs. Rehab | 20 | ‐ | FIM | ‐ |
| Rosewillium et al. 61 | 2012 | 90 | 72.6 | 50.2 | 1.38 | NA | 49 | NMES vs. Rehab | 30 | ARAT | BI | ‐ |
| Lin et al. 42 | 2011 | 37 | 64.5 | 59.5 | 1.39 | 67.6 | 59.5 | NMES vs. Rehab | 15 | FMA | mBI | MAS |
| Church et al. 95 | 2006 | 176 | 74.5 | 50.6 | 0.33 | 93.6 | 63.6 | NMES vs. Rehab | 12 | ARAT | ‐ | ‐ |
| Mann et al. 96 | 2005 | 22 | 69.4 | 42.8 | 7.03 | 95.2 | 54.6 | NMES vs. Rehab | 84 | ARAT | ‐ | ‐ |
| Kimberley et al. 97 | 2004 | 16 | 60.1 | 68.8 | 33.44 | NA | 50 | NMES vs. Rehab | 10 | BBT, JTT | ‐ | ‐ |
| Powell et al. 60 | 1999 | 60 | 67.7 | 46.7 | 0.77 | NA | 63.3 | NMES vs. Rehab | 56 | ARAT | BI | MAS |
| Francisco et al. 29 | 1998 | 16 | 65.5 | 44.4 | 0.59 | 100 | 62.5 | NMES vs. Rehab | 29.4 | FMA | FIM | ‐ |
| Chae et al. 25 | 1998 | 28 | 59.7 | 46.4 | 0.52 | 89.3 | 53.6 | NMES vs. Rehab | 15 | FMA | FIM | ‐ |
| Niu et al. 46 | 2022 | 20 | 60.1 | 93.8 | 4.07 | 100 | NA | NMES vs. Rehab | 5 | FMA | ‐ | ‐ |
| Kirac‐Unal et al. 98 | 2018 | 27 | 65.6 | 51.9 | 0.46 | 100 | 52.6 | FES vs. Rehab | 20 | ARAT | FIM | ‐ |
| Karaahmet et al. 38 | 2019 | 21 | 56.9 | 62 | 1.37 | 85.6 | 52.1 | FES vs. Rehab | 20 | FMA | FIM | ‐ |
| Nakipoglu‐Yuzer et al. 59 | 2017 | 30 | 58.9 | 56.7 | 0.16 | 80 | 60 | FES vs. Rehab | 20 | UEFT | BI | ‐ |
| Jonsdottir et al. 37 | 2017 | 68 | 68.0 | 42.6 | 3.71 | 80.9 | NA | FES vs. Rehab | 25 | FMA, ARAT | ‐ | ‐ |
| Thorsen et al. 99 | 2013 | 11 | 48.8 | 8.55 | FES vs. Rehab | 25 | ARAT | ‐ | ‐ | |||
| Karakus et al. 100 | 2013 | 28 | 59.0 | 53.6 | 3.29 | 85.7 | 53.6 | FES vs. Rehab | 10 | Total motricity index | ‐ | ‐ |
| Mohamed‐Faisal et al. 101 | 2012 | 30 | NA | NA | 0.92 | NA | NA | FES vs. Rehab | 24 | ARAT, BBT | ‐ | ‐ |
| Mangold et al. 66 | 2009 | 23 | 59.6 | 73.9 | 1.61 | 82.5 | 69.6 | FES vs. Rehab | 12 | ‐ | eBI | MAS |
| Chan et al. 26 | 2009 | 20 | 45.5 | 55 | 15.10 | NA | NA | FES vs. Rehab | 15 | FMA | FIM | MAS |
| Shin et al. 102 | 2008 | 14 | 57.6 | 85.7 | 19.15 | NA | 50 | FES vs. Rehab | 50 | BBT | ‐ | ‐ |
| Jian et al. 43 | 2005 | 48 | 59.4 | 70.8 | NA | 68.8 | NA | NMES vs. Rehab | 36 | FMA | ‐ | ‐ |
| Schick et al. 48 | 2022 | 12 | 65 | 66.7 | 3.45 | 100 | 41.7 | FES vs. Rehab | 15 | FMA, BBT | ‐ | ‐ |
| Chen et al. 9 | 2020 | 40 | 50.8 | 77.1 | 1.50 | NA | 51.4 | FES vs. NMES | 10 | FMA | BI | ‐ |
| Khan et al. 39 | 2019 | 60 | 63.5 | 65 | 0.55 | 100 | 30 | rPMS vs. TMS | 12 | FMA | BI | |
| Tosun et al. 52 | 2022 | 25 | 58.5 | 56 | 1.69 | 100 | 48 | TMS vs. FES vs. Rehab | 10 | Total motricity index | BI | MAS |
| Du et al. 28 | 2022 | 240 | 58.3 | 51.7 | 47.73 | 74.6 | 56.7 | NMES + TMS vs. TMS vs. Rehab | 20 | FMA | mBI | MAS |
| Tarri et al. 50 | 2022 | 24 | 50.1 | 66.7 | 2.32 | NA | NA | NMES + TMS vs. NMES vs. TMS vs. Rehab | 5 | FMA | ‐ | ‐ |
| Tilkici et al. 51 | 2017 | 40 | 62.9 | 45 | 10.50 | 77.5 | 50 | NMES + TMS vs. NMES | 15 | FMA | FIM | MAS |
| Blesneag et al. 55 | 2015 | 16 | 69 | 62.5 | 0.33 | NA | NA | NMES vs. Rehab | 10 | FMA | ‐ | ‐ |
Abbreviations: ARAT, Action Research Arm Test; BBT, Box and Block Test of manual dexterity; BI, Barthel Index; eBI, Extended Barthel Index; FES, functional electrical stimulation; FIM, Functional Independence Measure; FMA, Fugl‐Meyer Assessment; JTT, Jebsen Taylor Hand Function Test; MAS, Modified Ashworth Scale; mBI, Modified Barthel index, mTS, Modified Tardieu scale; NA, not applicable; NMES, neuromuscular electrical stimulation; Rehab, conventional rehabilitation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation; UEFT, upper extremity function test; WMFT, Wolf Motor Function Test.
Risk of bias
For the overall risk of bias, 41 studies (66.1%) were judged to have some concerns, primarily related to bias in the randomization process, whereas 10 studies (16.1%) were judged to have a low risk of bias and 11 (17.7%) studies were judged to have high risk of bias in the randomization process; see Appendix 5 in Data S1.
Upper extremity function
Thirty‐four studies 9 , 10 , 11 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 reported FMA at the end of the intervention course, and another 10 studies 25 , 30 , 35 , 36 , 40 , 44 , 49 , 54 , 55 , 56 reported FMA at 1–3 months post intervention. Pairwise meta‐analysis was performed on three comparisons with FMA measured at the end of intervention course from at least three studies, that is, NMES (N = 5), FES (N = 6), and TMS (N = 19) versus conventional rehabilitation. NMES, FES, and TMS significantly improved FMA compared with conventional rehabilitation with USMDs of 7.28 (95% CI, 2.68–11.88), 5.37 (95% CI, 1.25–9.49), and 2.97 (95% CI, 0.30–5.64), respectively, with moderate to high heterogeneity detected (Appendix 6 in Data S1). The source of heterogeneity was explored identifying baseline FMA, duration of intervention, TMS type, and side of lesion as potential causes; see details in Appendix 6 in Data S1. TMS significantly improved FMA during the 1–3‐month follow‐up period when compared to conventional rehabilitation, with an USMD of 3.55 (95% CI, 1.04–6.95; I 2 = 16.23%).
Data from 34 studies involving 1476 patients were included in a network meta‐analysis using the inconsistency model. The consistency assumption checking is detailed in Appendix 8 in Data S1. For the FMA outcome measured at the end of intervention course (N = 34), the network meta‐analysis map consisted of eight interventions (ie, NMES, FES, TMS, rPMS, NMES+TMS, NMES+rPMS, FES+TMS, and conventional rehabilitation) with 13 pairwise comparisons, see Figure 2A. Five interventions, that is, NMES+rPMS, NMES, NMES+TMS, TMS, and FES, showed significantly improved FMA when compared to conventional rehabilitation, with USMDs of 14.69 (95% CI, 9.94–19.45), 9.09 (95% CI, 6.01–12.18), 6.10 (95% CI, 2.51–9.69), 4.07 (95% CI, 0.33–7.81), and 3.61 (95% CI, 0.14–7.07), respectively; see Table 2. The interventions with the top four ranked by SUCRA for increasing FMA were NMES+rPMS (SUCRA = 99.8), NMES (81.9), NMES+TMS (63.1), and TMS (43.5), respectively; see Table 2 and Figure 3A.
FIGURE 2.
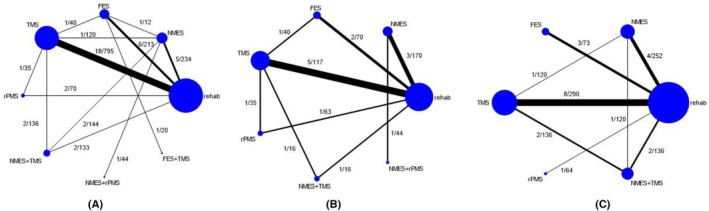
Network maps of the (A) Fugl‐Meyer Assessment, (B) Barthel Index, and (C) Modified Ashworth Scale outcomes assessed at the end of intervention course, with nodes and edges weighted by the number of studies and included patients, respectively. FES, functional electrical stimulation; NMES, neuromuscular electrical stimulation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation.
TABLE 2.
Multiple treatment comparisons of interventions on upper extremity function measured by FMA at the end of intervention courses.
| Reference treatment | Unstandardized mean difference | |||||||
|---|---|---|---|---|---|---|---|---|
| Rehab | FES+TMS | NMES+rPMS | NMES+TMS | rPMS | TMS | FES | NMES | |
| Rehab | 10, 0.0 | 0.41 (−9.48 to 10.29) | 14.69 (9.94 to 19.45) | 6.10 (2.51 to 9.69) | 3.14 (−4.54 to 10.81) | 4.07 (0.33 to 7.81) | 3.61 (0.14 to 7.07) | 9.09 (6.01 to 12.18) |
| FES+TMS | −0.41 (−10.29 to 9.48) | 22.4, 0.3 | 14.29 (3.32 to 25.25) | 5.69 (−4.82 to 16.21) | 2.73 (−9.78 to 15.24) | 3.66 (−6.91 to 14.23) | 3.20 (−6.06 to 12.46) | 8.69 (−1.66 to 19.04) |
| NMES+rPMS | −14.69 (−19.45 to −9.94) | −14.29 (−25.25 to −3.32) | 99.8, 98.7 | −8.59 (−14.55 to −2.63) | −11.56 (−20.58 to −2.53) | −10.62 (−16.67 to −4.58) | −11.09 (−16.96 to −5.22) | −5.60 (−9.22 to −1.98) |
| NMES+TMS | −6.10 (−9.69 to −2.51) | −5.69 (−16.21 to 4.82) | 8.59 (2.63 to 14.55) | 63.1, 0.1 | −2.96 (−11.44 to 5.51) | −2.03 (−5.75 to 1.69) | −2.49 (−7.49 to 2.50) | 2.99 (−1.74 to 7.73) |
| rPMS | −3.14 (−10.81 to 4.54) | −2.73 (−15.24 to 9.78) | 11.56 (2.53 to 20.58) | 2.96 (−5.51 to 11.44) | 37.9, 0.7 | 0.93 (−7.06 to 9.47) | 0.47 (−7.95 to 8.89) | 5.96 (−2.31 to 14.22) |
| TMS | −4.07 (−7.81 to −0.33) | −3.66 (−14.23 to 6.91) | 10.62 (4.58 to 16.67) | 2.03 (−1.69 to 5.75) | −0.93 (−9.47 to 7.06) | 43.5, 0.0 | −0.46 (−5.56 to 4.63) | 5.02 (0.18 to 9.87) |
| FES | −3.61 (−7.07 to −0.14) | −3.20 (−12.46 to 6.06) | 11.09 (5.22 to 16.96) | 2.49 (−2.50 to 7.49) | −0.47 (−8.89 to 7.95) | 0.46 (−4.63 to 5.56) | 41.5 to 0.0 | 5.49 (0.87 to 10.11) |
| NMES | −9.09 (−12.18 to −6.01) | −8.69 (−19.04 to 1.66) | 5.60 (1.98 to 9.22) | −2.99 (−7.73 to 1.74) | −5.96 (−14.22 to 2.31) | −5.02 (−9.87 to −0.18) | −5.49 (−10.11 to −0.87) | 81.9, 0.2 |
Note: Results in the off‐diagonal cells are the mean difference and 95% confidence intervals of the FMA from the network meta‐analysis. Each diagonal cell contains the surface under the cumulative ranking and probability of being the best treatment of each intervention.
Abbreviations: FES, functional electrical stimulation; FMA, Fugl‐Meyer Assessment; NMES, neuromuscular electrical stimulation; Rehab, conventional rehabilitation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation.
FIGURE 3.
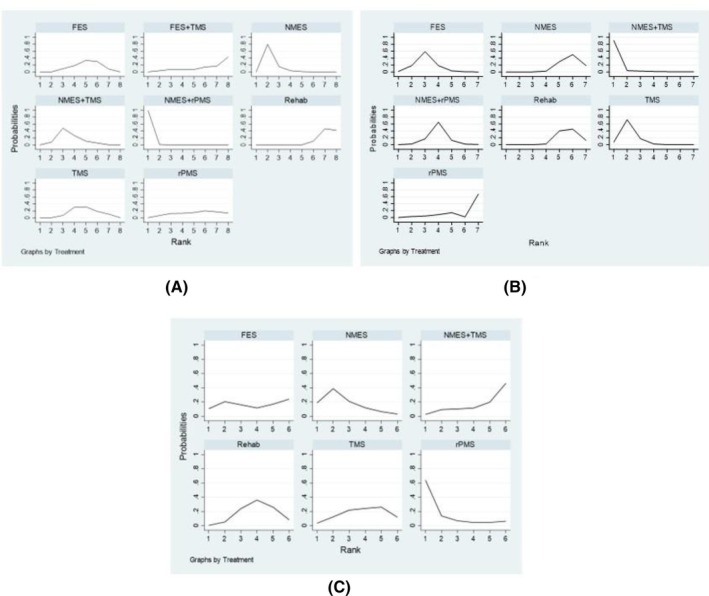
Ranking curves showing the probability of being the best intervention in terms of the (A) Fugl‐Mayer Assessment, (B) Barthel index, and (C) Modified Ashworth Scale outcomes at the end of intervention. FES, functional electrical stimulation; NMES, neuromuscular electrical stimulation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation.
Subgroup analyses were performed by stroke subtypes (see Appendix 7A–E in Data S1) and TMS frequency low (≤1 Hz) and high (≥5 Hz) (see Appendix 7A,F–G in Data S1). For stroke subtypes, NMES+rPMS was top rank in improving FMA scores in acute/subacute and severe stroke (baseline FMA < 25) with USMDs of 11.93 (95% CI, 8.27–15.59) and 14.63 (95% CI, 8.87–20.40), respectively (Appendix 7B,D in Data S1). In chronic stroke, NMES was ranked first with a significant USMD of 12.00 (95% CI, 8.22–15.77) relative to conventional rehabilitation, whereas none of the interventions significantly improved FMA in the subgroup with less severe stroke (baseline FMA ≥25); see Appendix 7C,E in Data S1. High‐frequency TMS (≥5 Hz) showed a trend toward greater FMA improvement than low‐frequency TMS (≤1 Hz), with USMDs of 10.6 (95% CI, 4.7–16.5) and 4.07 (95% CI, −1.12 to 9.26), respectively (Appendix 7F,G in Data S1).
The CINeMA framework assigned very low to low confidence ratings to all pairwise comparisons. This was primarily due to incoherence major concerns and some additional concerns regarding within‐study bias, imprecision, and heterogeneity (Appendix 9 in Data S1).
Activities of daily living
Twelve studies 9 , 10 , 32 , 39 , 41 , 52 , 53 , 57 , 58 , 59 , 60 , 61 reported BI at the end of intervention course. Two comparisons were available for pairwise meta‐analysis: NMES and TMS versus conventional rehabilitation. Only TMS showed significantly improved BI compared to conventional rehabilitation, with an USMD of 7.94 (95% CI, 0.44–15.44; I 2 = 79.11%), see Appendix 6 in Data S1.
Data from 12 RCTs involving 503 patients were pooled applying network meta‐analysis with an inconsistency model, which included nine pairwise comparisons among seven interventions, as shown in Figure 2B. The consistency assumption checking is detailed in Appendix 8 in Data S1. BI showed significant improvement in three interventions relative to conventional rehabilitation, that is, NMES+TMS, TMS, and FES, with USMDs of 30.89 (95% CI, 5.18–56.62), 12.25 (95% CI, 6.01–18.49), and 8.34 (95% CI, 2.37–14.31), respectively, see Table 3. In the ranking by SUCRA, the top four interventions were NMES+TMS (SUCRA = 96.2), TMS (80.8), FES (66.0), and NMES+rPMS (50.6), respectively, as shown in Table 3 and Figure 3B.
TABLE 3.
Multiple treatment comparisons of interventions on ADL measured by BI at the end of intervention courses.
| Reference treatment | Unstandardized mean difference | ||||||
|---|---|---|---|---|---|---|---|
| Rehab | NMES+rPMS | NMES+TMS | rPMS | TMS | FES | NMES | |
| Rehab | 22.0, 0.0 | 4.79 (−0.30 to 9.87) | 30.89 (5.18 to 56.62) | −4.67 (−22.01 to 12.67) | 12.25 (6.01 to 18.49) | 8.34 (2.37 to 14.31) | −0.21 (−1.85 to 14.42) |
| NMES+rPMS | −4.76 (−9.87 to 0.30) | 50.6, 0.1 | 26.11 (−0.10 to 52.33) | −9.46 (−27.52 to 8.61) | 7.46 (−0.58 to 15.51) | 3.55 (−4.29 to 11.40) | −5.00 (−9.82 to −0.18) |
| NMES+TMS | −30.89 (−56.62 to −5.18) | −26.11 (−52.33 to 0.10) | 96.2, 90.5 | −35.57 (−66.59 to −4.55) | −18.65 (−45.12 to 7.81) | −22.56 (−48.96 to 3.84) | −31.11 (−56.89 to −5.34) |
| rPMS | 4.67 (−12.67 to 22.01) | 9.46 (−8.61 to 27.52) | 35.57 (4.55 to 66.59) | 15.0, 0.2 | 16.92 (−1.51 to 35.34) | 13.01 (−5.33 to 31.35) | 4.46 (−12.96 to 21.87) |
| TMS | −12.25 (−18.49 to −6.01) | −7.46 (−15.51 to 0.58) | 18.65 (−7.81 to 45.12) | −16.92 (−35.34 to 1.51) | 80.8, 7.7 | −3.91 (−12.54 to 4.73) | −12.46 (−18.91 to −6.02) |
| FES | −8.34 (−14.31 to −2.37) | −3.55 (−11.40 to 4.29) | 22.56 (−3.84 to 48.96) | −13.01 (−31.35 to 5.33) | 3.91 (−4.73 to 12.54) | 66.0, 1.5 | −8.55 (−14.75 to −2.36) |
| NMES | 0.21 (−14.42 to 1.85) | 5.00 (0.18 to 9.82) | 31.11 (5.34 to 56.89) | −4.46 (−21.87 to 12.96) | 12.46 (6.02 to 18.91) | 8.55 (2.36 to 14.75) | 19.3, 0.0 |
Note: Results in the off‐diagonal cells are the mean difference and 95% confidence intervals of the BI from the network meta‐analysis. Each diagonal cell contains the surface under the cumulative ranking and probability of being the best treatment of each intervention.
Abbreviations: ADL, activities of daily living; BI, Barthel Index; FES, functional electrical stimulation; NMES, neuromuscular electrical stimulation; Rehab, conventional rehabilitation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation.
Sensitivity analysis in the subgroup of studies with acute/subacute stroke showed that NMES+TMS was also in the top rank, with an USMD of 30.9 (95% CI, 5.17–56.62) relative to conventional rehabilitation. rPMS and TMS came in the second and third ranks in improving BI, with significant USMDs of 21.65 (95% CI, 7.17–36.11) and 12.24 (95% CI, 6.01–18.47), respectively, see Appendix 10A,B in Data S1.
The major confidence rating of each pairwise comparison assessed with the CINeMA framework was very low due to major concerns predominantly in the incoherence domain and some concerns in the within‐study bias, imprecision, and heterogeneity domains, see Appendix 9 in Data S1.
Spasticity
Fifteen studies 24 , 26 , 28 , 30 , 31 , 42 , 51 , 52 , 59 , 60 , 62 , 63 , 64 , 65 , 66 reported MAS at the end of the intervention course, and three studies 30 , 63 , 64 reported at 1–3 months thereafter.
Pairwise meta‐analysis was performed on the end‐of‐intervention MAS in three comparisons, that is, NMES, FES, and TMS versus conventional rehabilitation; only the TMS versus conventional rehabilitation comparison was able to be performed on the 1–3‐month postintervention MAS. The pooled USMDs were nonsignificant in all comparisons, see Appendix 6 in Data S1.
Fifteen studies involving 683 patients assessed with MAS at the end of intervention course were pooled by applying a consistency network meta‐analysis model, including eight pairwise comparisons among six interventions; see Figure 2. All interventions showed no significant differences in spasticity compared to conventional rehabilitation, as shown in Table 4. All subgroup analyses also showed no significant differences; see Appendix 11A–D in Data S1.
TABLE 4.
Multiple treatment comparisons of interventions on spasticity measured by MAS at the end of intervention courses.
| Reference treatment | Unstandardized mean difference | |||||
|---|---|---|---|---|---|---|
| Rehab | NMES+TMS | rPMS | TMS | FES | NMES | |
| Rehab | 38.5, 0.5 | 0.14 (−0.45,0.73) | −0.51 (−1.40,0.38) | −0.02 (−0.36,0.33) | −0.03 (−0.67,0.61) | −0.20 (−0.64,0.24) |
| NMES+TMS | −0.14 (−0.73,0.45) | 25.9, 3.0 | −0.65 (−1.72,0.42) | −0.16 (−0.76,0.44) | −0.17 (−1.04,0.70) | −0.34 (−1.00,0.32) |
| rPMS | 0.51 (−0.38,1.40) | 0.65 (−0.42,1.72) | 80.2, 62.0 | 0.49 (−0.46,1.45) | 0.48 (−0.62,1.58) | 0.31 (−0.68,1.30) |
| TMS | 0.02 (−0.33,0.36) | 0.16 (−0.44,0.76) | −0.49 (−1.45,0.46) | 42.4 to 4.0 | −0.01 (−0.74,0.72) | −0.19 (−0.70,0.33) |
| FES | 0.03 (−0.61,0.67) | 0.17 (−0.70,1.04) | −0.48 (−1.58,0.62) | 0.01 (−0.72,0.74) | 45.0,11.3 | −0.17 (−0.95,0.61) |
| NMES | 0.20 (−0.24,0.64) | 0.34 (−0.32,1.00) | −0.31 (−1.30,0.68) | 0.19 (−0.33,0.70) | 0.17 (−0.61,0.95) | 67.9, 19.2 |
Note: Results in the off‐diagonal cells are the mean difference and 95% confidence intervals of the MAS from the network meta‐analysis. Each diagonal cell contains the surface under the cumulative ranking and probability of being the best treatment of each intervention.
Abbreviations: FES, functional electrical stimulation; MAS, Modified Ashworth Scale; NMES, neuromuscular electrical stimulation; Rehab, conventional rehabilitation; rPMS, repetitive electrical magnetic stimulation; TMS, transcranial magnetic stimulation.
The overall confidence rating was low to very low due to major concerns predominantly in the imprecision domain and some concerns mainly in the within‐study bias, imprecision, and heterogeneity domains, see Appendix 9 in Data S1.
Publication bias
The comparison‐adjusted funnel plots for all outcomes are shown in Appendix 12 in Data S1. The comparison‐adjusted funnel plot was considered asymmetric for all outcomes. This might be caused by heterogeneity in the TMS versus conventional rehabilitation comparison in all three outcomes and NMES versus conventional rehabilitation comparisons in the FMA and MAS outcomes.
DISCUSSION
Multiple interventions are currently applied for poststroke rehabilitation to improve upper extremity functions, ADL, and spasticity. For the upper extremity outcome, our pairwise meta‐analysis showed benefit of NMES, FES, and TMS when compared to conventional rehabilitation. Our results support the previous systematic reviews and meta‐analyses, which found these interventions (NMES, 67 , 68 FES, 69 and TMS 70 , 71 , 72 , 73 , 74 , 75 ) could improve upper extremity functions in patients after stroke. Although there were insufficient data to draw conclusions on the use of single intervention rPMS, rPMS combined with NMES may be the best intervention for improving FMA, especially in acute/subacute stroke and in patients with severe stroke with low baseline FMA. This suggests timely rehabilitation influences the benefit of intervention in acute/subacute stroke patients, compared to chronic stroke patients. 76 In addition, high‐frequency TMS (≥5 Hz) trended toward greater FMA improvement than low‐frequency (≤1 Hz).
Nevertheless, rPMS machines are quite expensive and may not be available in small hospitals. NMES is a less expensive intervention than rPMS and was ranked second, which could improve upper extremity function. A rPMS or TMS machine can cost US $15,000–75,000, whereas the cost of a NMES or FES machine is approximately $100–3500. Furthermore, an interesting finding from our results is that NMES alone was more efficacious than NMES+TMS in improving FMA; this may be due to the varied types of TMS used. Hiragami et al. 77 reported that a score change in FMA of 12.4 points was clinically meaningful in stroke patients. Our results found that only the patients who received NMES+rPMS reached this minimal clinically important difference.
For the ADL outcome, our pairwise meta‐analysis showed benefits for TMS relative to conventional rehabilitation, which was similar to the findings from a previous systematic review and meta‐analysis. 72 However, our results showed no benefit from NMES in improving ADL, contrary to a recent systematic review and meta‐analysis. 78 The difference may be due to discrepancies in the number of studies and method of calculation. In our review, we used the USMD of BI, but the previous review 78 used the SMDs of many scores. Findings from network meta‐analysis indicated that NMES+TMS might be the best combined intervention, whereas TMS was the best single intervention. The subgroup analysis in acute/subacute stroke patients also showed that NMES+TMS was the best combined intervention, but the best single intervention was rPMS. Thus, rPMS may be beneficial in the acute/subacute stroke group. rPMS can recruit peripheral afferents, potentially influencing cerebral activation and neuroplasticity that may help improve motor control in stroke patients. 79 , 80
For the spasticity outcome, neither pairwise meta‐analysis nor network meta‐analysis showed benefit from the use of NMES, FES, and TMS when compared to conventional rehabilitation, a finding similar to those from previous systematic reviews and meta‐analyses. 81 , 82 Although rPMS was the first‐ranked intervention in the network meta‐analysis, it was not significantly different compared to other interventions. Therefore, the treatment of choice for spasticity may be oral or injectable antispasticity drugs. 83
The treatment rankings from network meta‐analysis of the upper extremity functions and ADL outcomes were different in that the top‐ranked intervention was NMES+rPMS for FMA but NMES+TMS for BI. FMA is a clinician‐reported measurement, but BI is patient reported. Therefore, the results from FMA may be more objective. To explore the discrepancy between these measures in the same patients, a sensitivity analysis was attempted by performing network meta‐analysis of only the studies that reported both outcomes, but NMES+rPMS and NMES+TMS fell into two disconnected loops in the network meta‐analysis. Therefore, we could not assess the comparability between the results of both outcomes. However, the results of a pairwise comparison between NMES+rPMS and NMES based on a single study 10 showed the same direction of greater improvement in both FMA and BI in the NMES+rPMS group than the NMES group. Thus, NMES+rPMS may have benefits in improving not only upper extremity functions but also ADL.
Strengths and limitations
This study is the first systematic review and network meta‐analysis to evaluate the effects of rPMS along with other interventions used in poststroke rehabilitation. However, it has some limitations. First, the number of studies for some comparisons, particularly those involving rPMS, was limited, potentially impacting the precision of the estimated treatment effects. Therefore, studies were included only if >50% of participants were stroke patients. To assess the robustness of our findings, a sensitivity analysis was conducted by excluding two studies with assorted patient types: El Nahas et al. 62 (64% stroke patients, 36% limb spasticity) and Krewer et al. 41 (95% stroke patients, 5% traumatic brain injury). This exclusion did not alter the intervention ranking, see Appendix 13 in Data S1.
Because the typical indications of rPMS are musculoskeletal and neurogenic pain, 84 treatment with rPMS is currently new in patients after stroke and not widely used. More high‐quality RCTs are needed to confirm the effects of rPMS in poststroke patients. We also found non‐RCTs 12 , 85 that were ineligible in our systematic review and network meta‐analysis but which suggested that adding rPMS to conventional rehabilitation had a beneficial effect on upper extremity function. Moreover, we found an ongoing RCT 86 of rPMS on upper extremity function that could be added to the meta‐analysis in the future. Second, consistency in some network meta‐analysis models could not be achieved by removing studies with characteristics dissimilar to the others included; inconsistency models were applied instead. Lastly, there was evidence of publication bias in the comparison of NMES versus conventional rehabilitation on the spasticity outcome; readers should interpret or apply these results with caution.
CONCLUSION
Network meta‐analysis suggests that NMES combined with rPMS may be the most effective intervention for improving upper extremity function after stroke, although few studies have investigated this combination. NMES alone may be a cost‐effective alternative, and NMES plus TMS may offer the greatest improvements in ADL. However, evidence supporting the efficacy of these interventions in reducing spasticity is insufficient. The low certainty of these conclusions, due to inconsistencies in the network meta‐analysis, necessitates further well‐designed RCTs.
DISCLOSURE
All authors declare that they have no conflicts of interest.
This journal‐based CME activity is designated for 1.0 AMA PRA Category 1 Credit TM. Effective January 2024, learners are no longer required to correctly answer a multiple‐choice question to receive CME credit. Completion of an evaluation is required, which can be accessed using this link, https://onlinelearning.aapmr.org/. This activity is FREE to AAPM&R members and available to nonmembers for a nominal fee. CME is available for 3 years after publication date. For assistance with claiming CME for this activity, please contact (847) 737–6000. All financial disclosures and CME information related to this article can be found on the Online Learning Portal (https://onlinelearning.aapmr.org/) prior to accessing the activity.
Supporting information
Data S1. Supporting Information.
Keesukphan A, Suntipap M, Thadanipon K, et al. Effects of electrical and magnetic stimulation on upper extremity function after stroke: A systematic review and network meta‐analysis. PM&R. 2025;17(8):978‐993. doi: 10.1002/pmrj.13356
Answer questions and earn CME credit.
REFERENCES
- 1. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98‐e169. [DOI] [PubMed] [Google Scholar]
- 2. Ugur HG, Erci B. The effect of home care for stroke patients and education of caregivers on the caregiver burden and quality of life. Acta Clin Croat. 2019;58(2):321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nichols‐Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. 2005;36(7):1480‐1484. [DOI] [PubMed] [Google Scholar]
- 4. Dobek CE, Blumberger DM, Downar J, Daskalakis ZJ, Vila‐Rodriguez F. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975‐2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graef P, Dadalt MLR, Rodrigués D, Stein C, Pagnussat AS. Transcranial magnetic stimulation combined with upper‐limb training for improving function after stroke: a systematic review and meta‐analysis. J Neurol Sci. 2016;369:149‐158. [DOI] [PubMed] [Google Scholar]
- 6. Nussbaum EL, Houghton P, Anthony J, Rennie S, Shay BL, Hoens AM. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can. 2017;69(5):1‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakai K, Yasufuku Y, Kamo T, Ota E, Momosaki R. Repetitive peripheral magnetic stimulation for impairment and disability in people after stroke. Cochrane Database Syst Rev. 2019;11(11):CD011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Momosaki R, Yamada N, Ota E, Abo M. Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database Syst Rev. 2017;6(6):CD011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X, Liu X, Cui Y, et al. Efficacy of functional magnetic stimulation in improving upper extremity function after stroke: a randomized, single‐blind, controlled study. J Int Med Res. 2020;48(6):300060520927881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang YF, Zhang D, Zhang J, Hai H, Zhao YY, Ma YW. A randomized controlled trial of repetitive peripheral magnetic stimulation applied in early subacute stroke: effects on severe upper‐limb impairment. Clin Rehabil. 2022;36(5):693‐702. [DOI] [PubMed] [Google Scholar]
- 11. Ke J, Wei J, Zheng B, et al. Effect of high‐frequency repetitive peripheral magnetic stimulation on motor performance in intracerebral haemorrhage: a clinical trial. J Stroke Cerebrovasc Dis. 2022;31(7):106446. [DOI] [PubMed] [Google Scholar]
- 12. Obayashi S, Takahashi R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation. 2020;46(4):569‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777‐784. [DOI] [PubMed] [Google Scholar]
- 14. Fugl‐Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post‐stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13‐31. [PubMed] [Google Scholar]
- 15. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61‐65. [PubMed] [Google Scholar]
- 16. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206‐207. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods. 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson D, Boddington P, White IR. The design‐by‐treatment interaction model: a unifying framework for modelling loop inconsistency in network meta‐analysis. Res Synth Methods. 2016;7(3):329‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thakkinstian A. Advanced Meta‐Analysis. Clinical Epidermiology and Biostatistics. Mahidol University; 2019. [Google Scholar]
- 21. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683‐691. [DOI] [PubMed] [Google Scholar]
- 22. Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta‐analysis. Campbell Syst Rev. 2020;16(1):e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta‐analysis. PLoS Med. 2020;17(4):e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aşkın A, Tosun A, Demirdal ÜS. Effects of low‐frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: a randomized controlled trial. Somatosens Mot Res. 2017;34(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 25. Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29(5):975‐979. [DOI] [PubMed] [Google Scholar]
- 26. Chan MK, Tong RK, Chung KY. Bilateral upper limb training with functional electric stimulation in patients with chronic stroke. Neurorehabil Neural Repair. 2009;23(4):357‐365. [DOI] [PubMed] [Google Scholar]
- 27. Chen YJ, Huang YZ, Chen CY, et al. Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: a pilot randomized controlled trial. BMC Neurol. 2019;19(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du J, Wang S, Cheng Y, et al. Effects of neuromuscular electrical stimulation combined with repetitive transcranial magnetic stimulation on upper limb motor function rehabilitation in stroke patients with hemiplegia. Comput Math Methods Med. 2022;2022:9455428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Francisco G, Chae J, Chawla H, et al. Electromyogram‐triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79(5):570‐575. [DOI] [PubMed] [Google Scholar]
- 30. Galvão SCB, Dos Santos RBC, Dos Santos PB, Cabral ME, Monte‐Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper‐limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95(2):222‐229. [DOI] [PubMed] [Google Scholar]
- 31. Gottlieb A, Boltzmann M, Schmidt SB, et al. Treatment of upper limb spasticity with inhibitory repetitive transcranial magnetic stimulation: a randomized placebo‐controlled trial. NeuroRehabilitation. 2021;49(3):425‐434. [DOI] [PubMed] [Google Scholar]
- 32. Guan YZ, Li J, Zhang XW, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one‐year longitudinal randomized trial. CNS Neurosci Ther. 2017;23(12):940‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haghighi FM, Yoosefinejad AK, Razeghi M, Shariat A, Bagheri Z, Rezaei K. The effect of high‐frequency repetitive transcranial magnetic stimulation on functional indices of affected upper limb in patients with subacute stroke. J Biomed Phys Eng. 2021;11(2):175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey RL, Edwards D, Dunning K, et al. Randomized sham‐controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke. 2018;49(9):2138‐2146. [DOI] [PubMed] [Google Scholar]
- 35. Hosomi K, Morris S, Sakamoto T, et al. Daily repetitive transcranial magnetic stimulation for poststroke upper limb paresis in the subacute period. J Stroke Cerebrovasc Dis. 2016;25(7):1655‐1664. [DOI] [PubMed] [Google Scholar]
- 36. Hsu YF, Huang YZ, Lin YY, et al. Intermittent theta burst stimulation over ipsilesional primary motor cortex of subacute ischemic stroke patients: a pilot study. Brain Stimul. 2013;6(2):166‐174. [DOI] [PubMed] [Google Scholar]
- 37. Jonsdottir J, Thorsen R, Aprile I, et al. Arm rehabilitation in post stroke subjects: a randomized controlled trial on the efficacy of myoelectrically driven FES applied in a task‐oriented approach. PLoS One. 2017;12(12):e0188642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karaahmet OZ, Gurcay E, Unal ZK, Cankurtaran D, Cakci A. Effects of functional electrical stimulation‐cycling on shoulder pain and subluxation in patients with acute‐subacute stroke: a pilot study. Int J Rehabil Res. 2019;42(1):36‐40. [DOI] [PubMed] [Google Scholar]
- 39. Khan F, Rathore C, Kate M, et al. The comparative efficacy of theta burst stimulation or functional electrical stimulation when combined with physical therapy after stroke: a randomized controlled trial. Clin Rehabil. 2019;33(4):693‐703. [DOI] [PubMed] [Google Scholar]
- 40. Kim WS, Kwon BS, Seo HG, Park J, Paik NJ. Low‐frequency repetitive transcranial magnetic stimulation over Contralesional motor cortex for motor recovery in subacute ischemic stroke: a randomized sham‐controlled trial. Neurorehabil Neural Repair. 2020;34(9):856‐867. [DOI] [PubMed] [Google Scholar]
- 41. Krewer C, Hartl S, Müller F, Koenig E. Effects of repetitive peripheral magnetic stimulation on upper‐limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double‐blind, sham‐controlled study. Arch Phys Med Rehabil. 2014;95(6):1039‐1047. [DOI] [PubMed] [Google Scholar]
- 42. Lin Z, Yan T. Long‐term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J Rehabil Med. 2011;43(6):506‐510. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, You WX, Sun D. Effects of functional electric stimulation on shoulder subluxation and upper limb motor function recovery of patients with hemiplegia resulting from stroke. J First Mil Med Univ. 2005;25(8):1054‐1055. [PubMed] [Google Scholar]
- 44. Luk KY, Ouyang HX, Pang MYC. Low‐frequency rTMS over Contralesional M1 increases Ipsilesional cortical excitability and motor function with decreased interhemispheric asymmetry in subacute stroke: a randomized controlled study. Neural Plast. 2022;2022:3815357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuura A, Onoda K, Oguro H, Yamaguchi S. Magnetic stimulation and movement‐related cortical activity for acute stroke with hemiparesis. Eur J Neurol. 2015;22(12):1526‐1532. [DOI] [PubMed] [Google Scholar]
- 46. Niu CM, Chou CH, Bao Y, et al. A pilot study of synergy‐based FES for upper‐extremity poststroke rehabilitation. Neurosci Lett. 2022;780:136621. [DOI] [PubMed] [Google Scholar]
- 47. Rose DK, Patten C, McGuirk TE, Lu X, Triggs WJ. Does inhibitory repetitive transcranial magnetic stimulation augment functional task practice to improve arm recovery in chronic stroke? Stroke Res Treat. 2014;2014:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schick T, Kolm D, Leitner A, Schober S, Steinmetz M, Fheodoroff K. Efficacy of Four‐Channel functional electrical stimulation on moderate arm paresis in subacute stroke patients—results from a randomized controlled trial. Healthcare (Switzerland). 2022;10(4):704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seniów J, Bilik M, Leśniak M, Waldowski K, Iwański S, Członkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double‐blind, placebo‐controlled study. Neurorehabil Neural Repair. 2012;26(9):1072‐1079. [DOI] [PubMed] [Google Scholar]
- 50. Tarri M, Brihmat N, Gasq D, et al. Five‐day course of paired associative stimulation fails to improve motor function in stroke patients. Ann Phys Rehabil Med. 2018;61(2):78‐84. [DOI] [PubMed] [Google Scholar]
- 51. Tilkici M, Alemdaroglu E, Mandiroglu S, Ordu Gokkaya NK, Ucan H, Aykan SA. The effect of upper extremity electrical stimulation in addition to conventional rehabilitation in individuals with chronic stroke: randomized controlled study. J PMR Sci. 2017;20(3):126‐133. [Google Scholar]
- 52. Tosun A, Türe S, Askin A, et al. Effects of low‐frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil. 2017;24(5):361‐367. [DOI] [PubMed] [Google Scholar]
- 53. Vaziri PM, Bahrpeyma F, Firoozabadi M, et al. Low frequency repetitive transcranial magnetic stimulation to improve motor function and grip force of upper limbs of patients with hemiplegia. Iran Red Crescent Med J. 2014;16(8):e13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang HB, Long H, Yuan H, et al. Effect of low ‐frequency repetitive transcranial magnetic stimulation combining task‐oriented training on upper limb motor function recovery after stroke. Chin J Contemp Neurol Neurosurg. 2017;17(4):254‐260. [Google Scholar]
- 55. Blesneag AV, Slăvoacă DF, Popa L, et al. Low‐frequency rTMS in patients with subacute ischemic stroke: clinical evaluation of short and long‐term outcomes and neurophysiological assessment of cortical excitability. J Med Life. 2015;8(3):378‐387. [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma H, Vishnu VY, Kumar N, et al. Efficacy of low‐frequency repetitive transcranial magnetic stimulation in ischemic stroke: a double‐blind randomized controlled trial. Arch Rehabil Res Clin Transl. 2020;2(1):100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fletcher‐Smith JC, Walker DM, Allatt K, et al. The ESCAPS study: a feasibility randomized controlled trial of early electrical stimulation to the wrist extensors and flexors to prevent post‐stroke complications of pain and contractures in the paretic arm. Clin Rehabil. 2019;33(12):1919‐1930. [DOI] [PubMed] [Google Scholar]
- 58. Meng ZY, Song WQ. Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction. Neural Regen Res. 2017;12(4):610‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakipoğlu Yuzer GF, Köse Dönmez B, Özgirgin N. A randomized controlled study: effectiveness of functional electrical stimulation on wrist and finger flexor spasticity in hemiplegia. J Stroke Cerebrovasc Dis. 2017;26(7):1467‐1471. [DOI] [PubMed] [Google Scholar]
- 60. Powell J, Pandyan AD, Granat M, Cameron M, Stott DJ. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30(7):1384‐1389. [DOI] [PubMed] [Google Scholar]
- 61. Rosewilliam S, Malhotra S, Roffe C, Jones P, Pandyan AD. Can surface neuromuscular electrical stimulation of the wrist and hand combined with routine therapy facilitate recovery of arm function in patients with stroke? Arch Phys Med Rehabil. 2012;93(10):1715‐1721.e1. [DOI] [PubMed] [Google Scholar]
- 62. El Nahas N, Kenawy FF, Abd Eldayem EH, et al. Peripheral magnetic theta burst stimulation to muscles can effectively reduce spasticity: a randomized controlled trial. J Neuroeng Rehabil. 2022;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Özkeskin M, Öztürk V, Cakmur R, Bilge K, Küçük F. The effects of navigated repetitive transcranial magnetic simulation and Brunnstrom movement therapy on upper extremity proprioceptive sense and spasticity in stroke patients: a double‐blind randomized trial. J Basic Clin Health Sci. 2017;1(2):29‐35. [Google Scholar]
- 64. Conforto AB, Anjos SM, Saposnik G, et al. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012;259(7):1399‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sohn MK, Kim BO, Kim SG, Choi PS, Hwang SH. The effect of high frequency repetitive transcranial magnetic stimulation on the motor function in post‐stroke patients. J Korean Acad Rehabil Med. 2010;34(2):168‐173. [Google Scholar]
- 66. Mangold S, Schuster C, Keller T, Zimmermann‐Schlatter A, Ettlin T. Motor training of upper extremity with functional electrical stimulation in early stroke rehabilitation. Neurorehabil Neural Repair. 2009;23(2):184‐190. [DOI] [PubMed] [Google Scholar]
- 67. Yang JD, Liao CD, Huang SW, et al. Effectiveness of electrical stimulation therapy in improving arm function after stroke: a systematic review and a meta‐analysis of randomised controlled trials. Clin Rehabil. 2019;33(8):1286‐1297. [DOI] [PubMed] [Google Scholar]
- 68. Monte‐Silva K, Piscitelli D, Norouzi‐Gheidari N, Batalla MAP, Archambault P, Levin MF. Electromyogram‐related neuromuscular electrical stimulation for restoring wrist and hand movement in poststroke hemiplegia: a systematic review and meta‐analysis. Neurorehabil Neural Repair. 2019;33(2):96‐111. [DOI] [PubMed] [Google Scholar]
- 69. Eraifej J, Clark W, France B, Desando S, Moore D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta‐analysis. Syst Rev. 2017;6(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tang Z, Han K, Wang R, Zhang Y, Zhang H. Excitatory repetitive transcranial magnetic stimulation over the Ipsilesional hemisphere for upper limb motor function after stroke: a systematic review and meta‐analysis. Front Neurol. 2022;13:918597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He Y, Li K, Chen Q, Yin J, Bai D. Repetitive transcranial magnetic stimulation on motor recovery for patients with stroke: a PRISMA compliant systematic review and meta‐analysis. Am J Phys Med Rehabil. 2020;99(2):99‐108. [DOI] [PubMed] [Google Scholar]
- 72. Xiang H, Sun J, Tang X, Zeng K, Wu X. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta‐analysis of randomized controlled trials. Clin Rehabil. 2019;33(5):847‐864. [DOI] [PubMed] [Google Scholar]
- 73. Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta‐analysis. Am J Phys Med Rehabil. 2014;93(5):422‐430. [DOI] [PubMed] [Google Scholar]
- 74. Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta‐analysis. Stroke. 2012;43(7):1849‐1857. [DOI] [PubMed] [Google Scholar]
- 75. Zhang L, Xing G, Fan Y, Guo Z, Chen H, Mu Q. Short‐ and long‐term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta‐analysis. Clin Rehabil. 2017;31(9):1137‐1153. [DOI] [PubMed] [Google Scholar]
- 76. Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83(11):1629‐1637. [DOI] [PubMed] [Google Scholar]
- 77. Hiragami S, Inoue Y, Harada K. Minimal clinically important difference for the Fugl‐Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J Phys Ther Sci. 2019;31(11):917‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kristensen MGH, Busk H, Wienecke T. Neuromuscular electrical stimulation improves activities of daily living post stroke: a systematic review and meta‐analysis. Arch Rehabil Res Clin Transl. 2022;4(1):100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sato A, Liu X, Torii T, Iwahashi M, Iramina K. Modulation of motor cortex excitability by peripheral magnetic stimulation of different stimulus sites and frequencies. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:6413‐6416. [DOI] [PubMed] [Google Scholar]
- 80. Beaulieu LD, Massé‐Alarie H, Camiré‐Bernier S, Ribot‐Ciscar É, Schneider C. After‐effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol Clin. 2017;47(4):275‐291. [DOI] [PubMed] [Google Scholar]
- 81. Stein C, Fritsch CG, Robinson C, Sbruzzi G, Plentz RD. Effects of electrical stimulation in spastic muscles after stroke: systematic review and meta‐analysis of randomized controlled trials. Stroke. 2015;46(8):2197‐2205. [DOI] [PubMed] [Google Scholar]
- 82. Xu P, Huang Y, Wang J, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for stroke with spasticity: a systematic review and meta‐analysis. J Neurol. 2021;268(11):4013‐4022. [DOI] [PubMed] [Google Scholar]
- 83. Thibaut A, Chatelle C, Ziegler E, Bruno MA, Laureys S, Gosseries O. Spasticity after stroke: physiology, assessment and treatment. Brain Inj. 2013;27(10):1093‐1105. [DOI] [PubMed] [Google Scholar]
- 84. Han‐soo L. FDA OKs Remed's neuromuscular stimulation device. May 10, 2021. Accessed June 02, 2023. https://www.koreabiomed.com/news/articleView.html?idxno=11115
- 85. Chen S, Li Y, Shu X, et al. Electroencephalography Mu rhythm changes and decreased spasticity after repetitive peripheral magnetic stimulation in patients following stroke. Front Neurol. 2020;11:546599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kinoshita S, Ikeda K, Yasuno S, et al. Dose–response of rPMS for upper limb hemiparesis after stroke. Medicine (Baltimore). 2020;99(24):e20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tretriluxana J, Thanakamchokchai J, Jalayondeja C, Pakaprot N, Tretriluxana S. The persisted effects of low‐frequency repetitive transcranial magnetic stimulation to augment task‐specific induced hand recovery following subacute stroke: extended study. Ann Rehabil Med. 2018;6:777‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ackerley SJ, Byblow WD, Barber PA, MacDonald H, McIntyre‐Robinson A, Stinear CM. Primed physical therapy enhances recovery of upper limb function in chronic stroke patients. Neurorehabil Neural Repair. 2016;30(4):339‐348. [DOI] [PubMed] [Google Scholar]
- 89. Higgins J, Koski L, Xie H. Combining rTMS and task‐oriented training in the rehabilitation of the arm after stroke: a pilot randomized controlled trial. Stroke Res Treat. 2013;2013:539146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Di Lazzaro V, Rothwell JC, Talelli P, et al. Inhibitory theta burst stimulation of affected hemisphere in chronic stroke: a proof of principle, sham‐controlled study. Neurosci Lett. 2013;553:148‐152. [DOI] [PubMed] [Google Scholar]
- 91. Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint‐induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86(9):707‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim HN, Han SY, Jeong JW, Ma SR, Yang BL. Effects of treatment with bilateral upper limb approach combined with electromyography‐induced neuromuscular electrical stimulation on upper limb function and daily living in hemiplegic patients after stroke. Annals of the Romanian Society for Cell Biology; 2021:1027‐1032. Bucharest: Association of Cell Biology Romania. [Google Scholar]
- 93. Dorsch S, Ada L, Canning CG. EMG‐triggered electrical stimulation is a feasible intervention to apply to multiple arm muscles in people early after stroke, but does not improve strength and activity more than usual therapy: a randomized feasibility trial. Clin Rehabil. 2014;28(5):482‐490. [DOI] [PubMed] [Google Scholar]
- 94. Sahin N, Ugurlu H, Albayrak I. The efficacy of electrical stimulation in reducing the post‐stroke spasticity: a randomized controlled study. Disabil Rehabil. 2012;34(2):151‐156. [DOI] [PubMed] [Google Scholar]
- 95. Church C, Price C, Pandyan AD, Huntley S, Curless R, Rodgers H. Randomized controlled trial to evaluate the effect of surface neuromuscular electrical stimulation to the shoulder after acute stroke. Stroke. 2006;37(12):2995‐3001. [DOI] [PubMed] [Google Scholar]
- 96. Mann GE, Burridge JH, Malone LJ, Strike PW. A pilot study to investigate the effects of electrical stimulation on recovery of hand function and sensation in subacute stroke patients. Neuromodulation. 2005;8(3):193‐202. [DOI] [PubMed] [Google Scholar]
- 97. Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154(4):450‐460. [DOI] [PubMed] [Google Scholar]
- 98. Kirac‐Unal Z, Gencay‐Can A, Karaca‐Umay E, Cakci FA. The effect of task‐oriented electromyography‐triggered electrical stimulation of the paretic wrist extensors on upper limb motor function early after stroke: a pilot randomized controlled trial. Int J Rehabil Res. 2019;42(1):74‐81. [DOI] [PubMed] [Google Scholar]
- 99. Thorsen R, Cortesi M, Jonsdottir J, et al. Myoelectrically driven functional electrical stimulation may increase motor recovery of upper limb in poststroke subjects: a randomized controlled pilot study. J Rehabil Res Dev. 2013;50(6):785‐794. [DOI] [PubMed] [Google Scholar]
- 100. Karakuş D, ErsöZ M, Koyuncu G, Türk D, Şaşmaz FM, AkyüZ M. Effects of functional electrical stimulation on wrist function and spasticity in stroke: a randomized controlled study. Turk Fiziksel Tip Rehab Derg. 2013;59(2):97‐102. [Google Scholar]
- 101. Mohamed Faisal CK, Priyabandani NOP, Ajith S. Efficacy of functional neuromuscular electrical stimulation (FNMES) in the improvement of hand functions in acute stroke survivals. Nitte university. J Health Sci. 2012;2(4):16‐21. [Google Scholar]
- 102. Shin HK, Cho SH, Jeon HS, et al. Cortical effect and functional recovery by the electromyography‐triggered neuromuscular stimulation in chronic stroke patients. Neurosci Lett. 2008;442(3):174‐179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


