Abstract
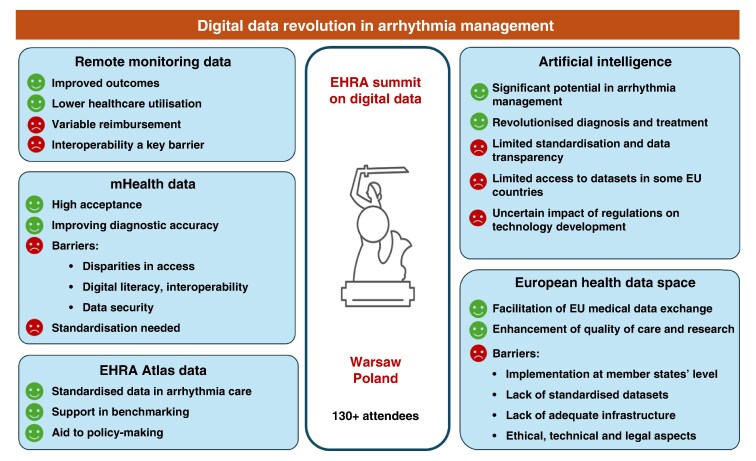
The 2024 European Heart Rhythm Association (EHRA) Summit in Warsaw focused on the digital transformation of arrhythmia management, convening over 130 stakeholders from academia, industry, and policy. This review summarises the current state (in 2025) and future directions of digital health in arrhythmia care, including remote monitoring (RM) of cardiac implantable electronic devices (CIEDs), mobile health (mHealth), artificial intelligence (AI), and integration into the European Health Data Space (EHDS). RM has become central to CIED follow-up, improving outcomes and reducing healthcare use. However, challenges in reimbursement, workforce adaptation, and data interoperability persist. The absence of standardised data exchange between device vendors and healthcare systems has led to initiatives like the World Forum on CIED follow-up to develop interoperability standards. mHealth tools, including apps and wearable devices, offer accurate arrhythmia detection but face regulatory, digital literacy, and privacy barriers. The EHDS aims to enable cross-border data sharing for personalised care and real-world research, though implementation must address ethical, legal, and infrastructural issues. AI shows promise in prediction, monitoring, and data integration, but lacks standardised, transparent validation. The ESC-EHRA Atlas in Heart Rhythm Disorders supports structured data collection to harmonize and benchmark care across Europe.
Overall, digital innovations, if coupled with regulatory alignment, interoperability standards, and equitable access, have the potential to shift arrhythmia management toward a more predictive, personalized, and efficient model of care.
Keywords: Arrhythmia, Digital health, Remote monitoring, CIEDs, Artificial intelligence, mHealth, European Health Data Space, EHRA, Interoperability, Health data integration
Graphical Abstract
Graphical Abstract.
Table of contents
Introduction
Remote monitoring of cardiac implantable electronic devices
Regional disparities in penetration, reimbursement and implementation of RM
CIED interoperability: Current status
Future directions
Mobile health in arrhythmia care
Available evidence, penetration, and performance
Barriers to wider implementation
Patient perspectives
Standardisation of available technology
European health data space: a tool to integrate healthcare data in arrhythmia patients across Europe
Basic concept
Integrating arrhythmia/device care by European health data space: opportunities, threats and challenges
AI and big data management in arrhythmia management
Education and training in the field of AI and digital literacy
Registries and large databases as a tool to integrate health data in arrhythmia patients
Regulatory standards and data security for big data and AI
The ESC-EHRA atlas in heart rhythm disorders
Design and data quality control
Current status and future perspectives
Conclusion
Supplementary material
Acknowledgements
Funding
References
What’s new.
This is the first comprehensive summary of an EHRA Summit presenting key discussions and expert insights focused on the digital transformation of arrhythmia care and enriched with newly published data following the meeting.
The paper highlights disparities in the implementation and uptake of remote monitoring for cardiac implantable electronic devices (CIEDs), reinforcing the need for universal standards to support secure, interoperable data exchange across vendors and platforms.
A concise overview is provided of the European Health Data Space (EHDS), including its core principles and implementation timelines, with relevance to arrhythmia and device care integration.
Organizational perspectives on mobile health (mHealth) solutions and artificial intelligence in arrhythmia care are presented, emphasizing both opportunities for innovation and barriers to widespread adoption.
Introduction
The European Heart Rhythm Association (EHRA), as a network of professionals dedicated to advancing arrhythmia care, plays a pivotal role in improving patient outcomes and shaping clinical practices across all member countries. With over 4500 members and close collaboration with National Cardiac Societies (NCS) and Arrhythmia Working Groups, EHRA facilitates high-quality care, professional development, and fosters innovation in the field of arrhythmia management. The annual EHRA summit is an event that brings together representatives from the NCSs, along with researchers, industry representatives, other healthcare professionals, and policymakers.
In recognition of the growing impact of digital transformation in cardiovascular healthcare and arrhythmias in particular, the annual EHRA Summit 2024, held in Warsaw on June 13–14 was dedicated to digital technologies in arrhythmia management, bringing together more than 130 attendees. Over 2 days, leading experts in the field delivered state-of-the-art presentations and participants engaged in interactive discussions aimed at assessing the current landscape, identifying barriers, and exploring strategies for implementing innovative digital solutions in arrhythmia care.
This paper provides a comprehensive review of the key insights from the summit, outlining the current status (2025) and future directions in telemonitoring and remote monitoring of implantable devices, artificial intelligence (AI) in arrhythmia care, mHealth applications, and the integration of healthcare data within the European Health Data Space (EHDS). It also highlights the role of structured data collection through initiatives like the ESC-EHRA Atlas in Heart Rhythm Disorders, which aims to standardise and enhance knowledge of arrhythmia care across EHRA member countries. By addressing these topics, this paper offers a current perspective on the digital transformation of arrhythmia management, emphasising both the opportunities and challenges that lie ahead in optimising patient care and advancing clinical research.
Remote monitoring of cardiac implantable electronic devices
Several observational studies have reported an improvement in clinical outcomes through the application of remote monitoring.1–3 Additionally, the randomised IN-TIME trial demonstrated mortality reduction in heart failure patients undergoing remote monitoring (RM) of their ICD.4 This finding was later confirmed in a meta-analysis of three trials.5 Contrary to that, in the randomised REM-HF trial RM was not associated with significant reduction in mortality and unplanned cardiovascular hospitalisation.6 These divergent results can be partially explained by the higher proportion of patients with mild symptoms and the use of technology from multiple manufacturers in REM-HF vs. IN-TIME similar to the real-world practice. RM has also been reported to decrease other adverse events such as inappropriate shocks.7 Importantly, randomised trials and observational studies demonstrated that it can substantially diminish utilisation of healthcare resources,8 in-office follow-ups and the workload of healthcare professionals.9,10 For these reasons, RM is recommended as a standard of care for patients with CIEDs by recent consensus statements.11 The increasing number of patients routinely using RM and the vast volume of transmissions create challenges for the device clinic staff, with an urgent need for adapting the workforce, but also for improving appropriate in-hospital workflows, alert management, and patient education, as shown by a recent systematic review.12
Regional disparities in penetration, reimbursement and implementation of RM
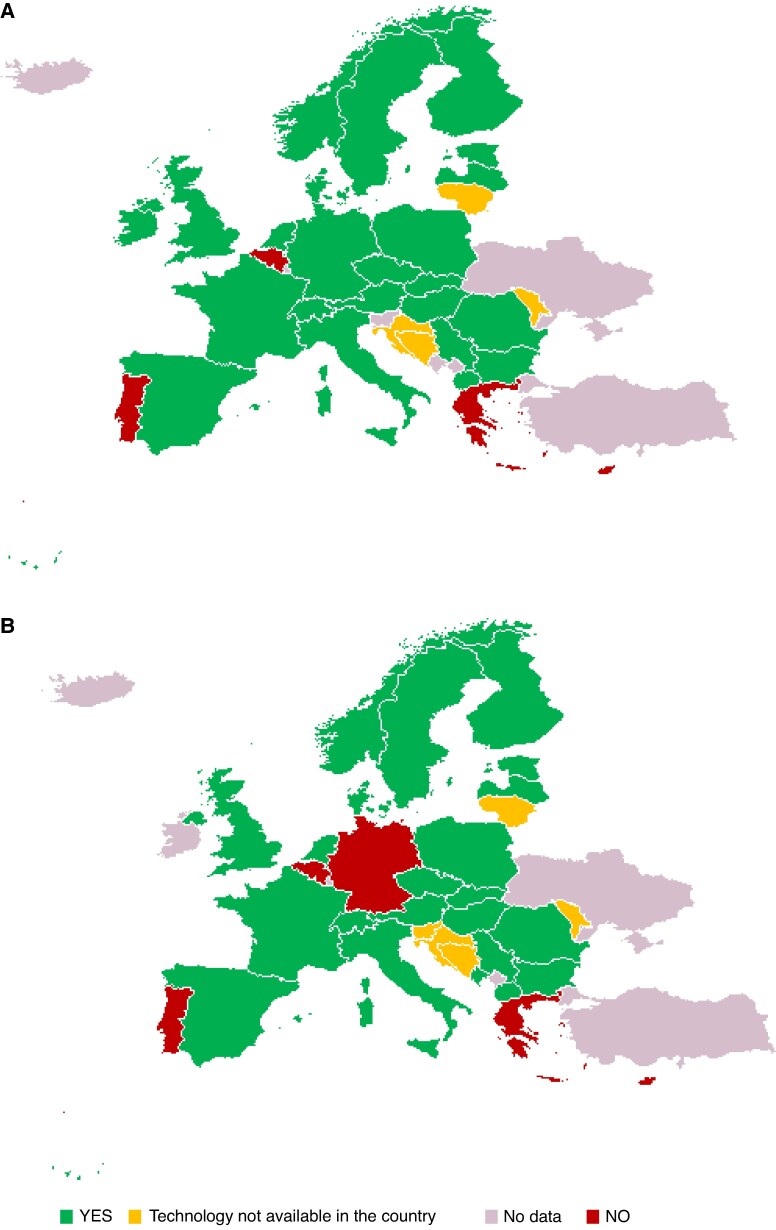
RM penetration is very difficult to evaluate, however, it is not uniformly used in Europe and worldwide as shown by an international survey.13 Reimbursement issues seem to be the major hurdle for implementing RM in CIEDs patients, and appropriate funding can lead to the desired change of care.14,15 For example, the proportion of ICD patients followed remotely in France has increased to about 90% since the Health ministry has provided nationwide coverage for this technology. Reimbursement provisions are, however, very disparate across Europe and European Society of Cardiology (ESC) member countries (Figure 1, Supplementary material online, Table S1).
Figure 1.
Current status of reimbursement for remote monitoring of ICD/CRT-D (A) and other CIEDs (panel B) in the European countries. Category definitions: YES: Fully reimbursed by mandatory or voluntary, public or private, insurance, or reimbursement level decided on a case-by-case basis or at the region level. NO: Technology is fully covered with patients’ private expenditure (out-of-pocket) or other sources, or there is no agreed reimbursement scheme. Technology not available in the country: the technology is not available in the country. No data: no information is available. Source: The 2025 ESC-EHRA Atlas on Heart Rhythm Disorders (ref.16).
CIED interoperability: Current status
Despite the existence of a common international standard for reporting CIED data [ISO/IEEE 11073-10103:2014, based on the Implantable Device Cardiac Observation (IDCO) nomenclature of 2011], the integration of interrogation and remotely transmitted data from CIEDs in healthcare workflows faces significant interoperability challenges (Box 1). In contrast to the Digital Imaging and Communications in Medicine (DICOM) standard used for the interoperable exchange of medical images, CIED manufacturers use proprietary file formats for the readouts of their devices. This situation significantly hinders seamless communication across electronic health records, vendor platforms, and third-party data management systems. Other technical hurdles that negate efficient clinical workflow (and even create medicolegal risks for healthcare providers) are the slow pace of updates to the nomenclature in the light of technological device progress (although IDCO allows for vendor-specific plug-ins), uncertainty about the reliable connectivity of devices (again with different technical deficiencies among different manufacturers), the shared responsibilities to restore lost connectivity (patient, manufacturer and clinic), the priority reporting of alerts, the handling of alerts of different urgency,17 and synchronisation of clinical or demographical data with the data on the telemonitoring platform and/or the CIED itself. Many aspects could be helped by bidirectional communication among CIED vendor servers, third-party data management systems, and electronic health records (EHRs), which currently do not exist. All these shortcomings lead to increased workloads for clinicians and staff due to the need for manual data transfer and verification among different systems.18 The potential benefit of third-party platforms over traditional manufacturer systems has been demonstrated in terms of clinical and organisational impacts, which is in line with the 2023 international expert consensus suggesting the use of such services.11 The recent nationwide EVIDENCE-RM study reported a notable 26% reduction in 1-year all-cause mortality and reduced number and duration of hospitalisations compared to the so-called ‘conventional’ remote monitoring.19
Box 1 The problem of non-interoperability in cardiac implantable electronic devices (CIED).
CIED information can be retrieved by in-office interrogation or telemonitoring; however, data currently CANNOT be viewed in a single platform and discrete data (e.g. battery voltage, pacing and sensing values, etc.) CANNOT be retrieved uniformly because:
Despite an existing standard for CIED information since 2011 (ISO/IEEE 11073-10103:2014 (reviewed in 2019) based on the IDCO profile, there are different file formats and inhomogeneous adherence to the data format standard
There are differences between vendors, type of CIED, type of programmer, in-office or telemonitoring data interrogations, etc.
The lack of standard data flow leads to suboptimal care for patients, frustration for physicians and impossibility of generating quality CIED cross-vendor registries. This is in contrast to medical images, which can all be viewed by a DICOM viewer [National Electrical Manufacturers Association (NEMA) standard PS3 & ISO standard 12052:2017].
Future directions
For these reasons, in 2022, the four major worldwide arrhythmia sister societies have taken the initiative to create a World Forum on CIED Follow-up. Under the lead of Prof. Hein Heidbuchel (EHRA Past-President) and Prof. David Slotwiner [Heart Rhythm Society (HRS)], the forum has brought together all global stakeholders in the field, including the five major CIED manufacturers and more than fifteen middleware companies (which now offer different solutions to help streamline the fragmented data flow). The primary policy goal of the World Forum was to overcome the described interoperability barriers, ultimately providing benefits to both patients and healthcare providers.
The World Forum moved to concrete action under the umbrella of the standards-setting organization Health Level Seven (HL7). Inside HL7, the CardX community is focused on cardiovascular health and has also prioritized CIED interoperability use case. The aim is to create new Fast Healthcare Interoperability Resource (FHIR) standards to tackle the problems described above. FHIR enables secure and efficient data exchange. With the World Forum being a member of CardX together with the major CIED vendors, there is a unique collaboration where clinician’s needs can be met with the technical developments along newly set standards. The first use case being tackled is the ‘connectivity use case’ so that a loss of remote connection is reported in a uniform way and can be dealt with efficiently. CardX aims to have a first Implementation Guide ready for testing in 2025, which could pave the way for industry-wide adoption in the future.
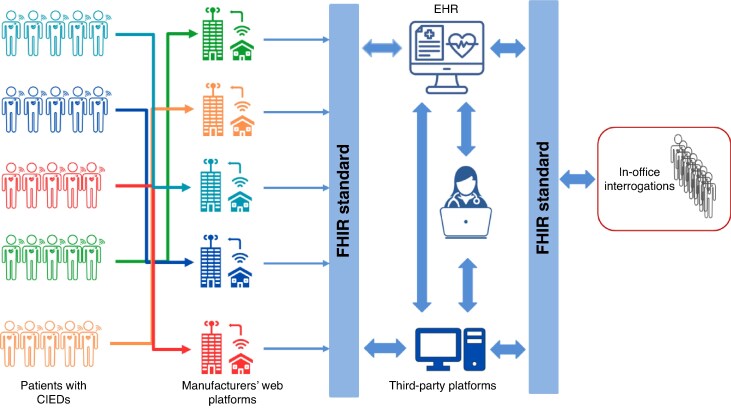
Standardization of data into the IDCO format now needs to be complemented by standardisation of data exchange protocols. This will facilitate automated data integration and hence alleviate the burden of inefficient follow-up, improve data accuracy, and allow more rapid clinical decision-making. Integrating CIED data flow with other data sources and therapeutic interventions (e.g. medication, lab data, monitored symptoms, etc.) will lead to more personalised remote monitoring based on patient characteristics, i.e. moving from a device-centric perspective to a patient-centric approach (Figure 2).
Figure 2.
Interoperable data flow framework for patients with CIED remote monitoring. This diagram illustrates the integration of remote monitoring data from diverse CIED manufacturers through their respective web platforms. Using the FHIR (Fast Healthcare Interoperability Resources) standard, data are standardised and shared with electronic health records (EHRs), third-party platforms, and clinicians. The model ensures a unified, interoperable system for clinical decision-making and enables both remote and in-office interrogations. It highlights the importance of standardised data exchange for effective multi-vendor device monitoring and patient management. CIED, cardiac implantable electronic device; EHR, electronic health record; FHIR, Fast Healthcare Interoperability Resource.
Mobile health in arrhythmia care
Available evidence, penetration, and performance
Mobile health (mHealth) solutions have revolutionised arrhythmia care, offering novel opportunities for early detection, continuous monitoring, and personalised management.20 Devices such as smartphone applications and wearable ECG or photoplethysmography (PPG) monitors are widely used, with penetration expanding globally. For example, wearable ECG devices like the Apple Watch and AliveCor’s KardiaMobile have demonstrated high sensitivity and specificity in detecting atrial fibrillation (AF). A meta-analysis published in 2020 showed that smartphone-based applications had a pooled sensitivity of 94% and specificity of 96% for detecting AF, underlining their clinical utility.21 Similarly, the 2019 Apple Heart Study reported a positive predictive value of 84% for detecting AF among participants receiving irregular pulse notifications.22
Advancements in wearable technology, including ECG and PPG-based devices, have further enhanced diagnostic yields and patient adherence. Previously published studies emphasise the superior diagnostic accuracy of ECG patches over traditional Holter monitoring, particularly for short-term AF screening. However, the need for clinician oversight to interpret results and prevent over-reliance on automated algorithms remains critical.23 Additionally, a recent ESC consensus stresses the importance of standardised assessments, highlighting frameworks like CEN-ISO/TS 82304-2 to evaluate clinical effectiveness and safety.24
Barriers to wider implementation
Several barriers hinder the broader adoption of mHealth solutions in arrhythmia care. First, there are challenges related to interoperability and integration with existing EHR systems. Without standardised protocols, seamless data exchange remains problematic. Second, as in other fields of digital transformation, data security and privacy concerns pose significant obstacles. A recent analysis found that over 80% of health apps share data with third parties, raising concerns about compliance with regulations like the General Data Protection Regulation (GDPR).24
Cost considerations also play a critical role. While the upfront cost of wearable devices may be manageable for some patients, the ongoing subscription fees or physician reviews are prohibitive for others, particularly in low- and middle-income countries. Additionally, regulatory hurdles vary significantly across regions, complicating the approval process for novel devices. For instance, under the European Union Medical Device Regulations (EU MDR), many previously Class I mHealth solutions are now classified as Class IIa, increasing the burden on innovators.
Patient perspectives
Patients’ perspectives on mHealth solutions are multifaceted, encompassing satisfaction with ease of use, perceived utility, and concerns over data security. Surveys reveal high acceptance rates among tech-savvy individuals, with reported high levels of comfort and acceptance of wearable monitors, as supported by findings in a recent EHRA practical guide.23 Moreover, mHealth fosters a sense of empowerment by enabling patients to actively participate in their care, as evidenced by improved medication adherence and lifestyle modifications.
However, some patients report anxiety related to false-positive results or over-reliance on technology, which may lead to unnecessary healthcare utilisation. Frequent alerts for benign arrhythmias can cause psychological distress and erode trust in mHealth tools.25 Additionally, disparities in access to devices—due to cost, availability, or poor technological literacy—can exacerbate existing inequities in arrhythmia care. For instance, older patients, who are often at higher risk for arrhythmias, may lack the technological literacy required to use mHealth tools effectively.26 Language barriers and cultural differences further complicate adoption in diverse patient populations. The COVID-19 pandemic highlighted the potential of remote monitoring but also revealed disparities in access and infrastructure.23
Engaging patients in designing and implementing mHealth solutions is critical to address these concerns. Approaches involving patient feedback at each stage of design and development have shown promise in improving usability and satisfaction. Educational initiatives to enhance digital health literacy can further facilitate adoption and optimise outcomes.24
Standardisation of available technology
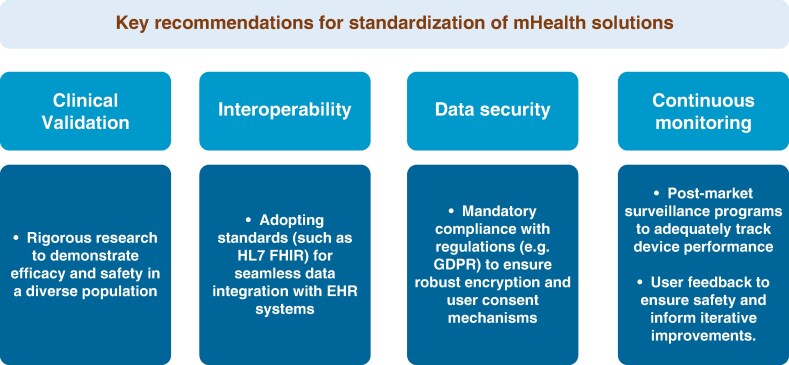
Standardising the classification and assessment of mHealth technologies is essential for ensuring their reliability, safety, and clinical utility. Current efforts, such as the EHDS, aim to establish unified frameworks for data sharing and governance across the EU. A recent ESC consensus document, developed in collaboration with EHRA has proposed guidelines for evaluating mHealth devices based on parameters like data accuracy, clinical validation, and user experience.24 It also emphasises the need for harmonised certification processes, leveraging tools like CEN-ISO/TS 82304-2 to ensure that health apps meet rigorous safety and quality standards (Figure 3). By addressing variability in classification systems and regulatory requirements, these efforts can accelerate innovation while maintaining patient safety.
Figure 3.
Key recommendations for standardization of mHealth solutions. EHR, electronic health record; FHIR, Fast Healthcare Interoperability Resource; GDPR, General Data Protection Regulation.
European health data space: a tool to integrate healthcare data in arrhythmia patients across Europe
Basic concept
The EHDS is a regulatory framework established by the EU to facilitate the secure, standardised exchange and use of electronic health data across Member States, ensuring compliance with other regulations, including the GDPR and other regulations (https://www.european-health-data-space.com/). EHDS defines two primary data use categories: primary use which pertains to the direct processing of electronic health data for healthcare service delivery, and secondary use which governs access to health data for research, innovation, public health, and policy-making purposes.
Under primary use, all individuals shall have the right to digital access, free of charge, to their EHRs, including patient summaries, e-prescriptions, laboratory results, imaging, and discharge reports, to ensure continuity of care. This process will be regulated by Digital Health Authorities at each member state, which will ensure compliance with the standards and will also participate in the establishment of cross-border digital infrastructure—MyHealth@EU.
Under secondary use, health data may be processed for research, policy-making, innovation, and regulatory decisions. This activity will be governed by national Health Data Access Bodies (HDABs), which will be responsible for providing access to research institutions, public health agencies, regulatory bodies, and other eligible entities, and restrict data use for commercial advertising and other non-medical purposes.
Integrating arrhythmia/device care by European health data space: opportunities, threats and challenges
The EHDS represents a transformative opportunity to integrate arrhythmia and device care across Europe (https://www.european-health-data-space.com/). By enabling seamless access to EHRs, device data, and population-level analytics, the EHDS can revolutionize arrhythmia management and enhance patient outcomes (Figure 4, Supplementary material online, Figure S1).
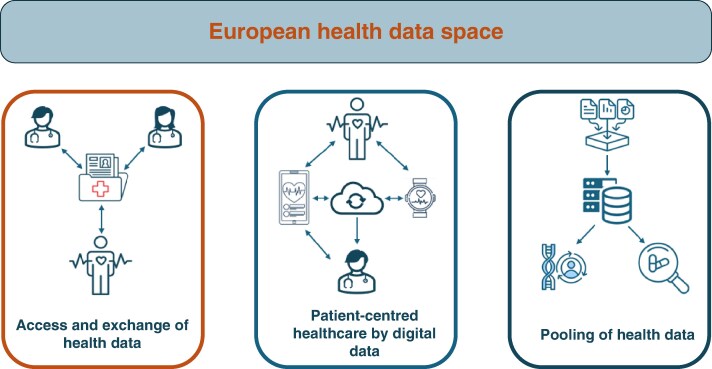
Figure 4.
The main pillars of the European health data space concept (https://digital-strategy.ec.Europa.eu/en/news/transformation-health-and-care-digital-single-market-gaining-more-support). The figure illustrates three core components: (i) Access and exchange of health data—enabling individuals and healthcare providers to securely share medical records across borders; (ii) Patient-centred healthcare by digital data—promoting integrated care through digital tools such as electronic health records, wearables, and cloud-based platforms; and (iii) Pooling of health data—facilitating secondary use of anonymised health data for research, innovation, policy-making, and public health, while ensuring privacy and security.
The creation and implementation of EHDS offers arrhythmia/CIED patients and healthcare providers numerous important opportunities (Box 2). Firstly, EHDS will facilitate cross-border exchange of patients’ health data, enabling clinicians to access medical histories and device data regardless of where care is provided thus enhancing continuity of care. This option is particularly important for arrhythmia patients who rely on precise monitoring and timely interventions, such as pacemaker adjustments or AF management.11,27 Secondly, the EHDS supports the harmonisation of data across national registries, simplifying compliance with device safety regulations and improving post-market surveillance of implanted devices, to maximise patients’ safety, thus streamlining registries and reporting.28 Such harmonisation also enables Europe-wide quality benchmarking, fostering clinical excellence. In addition, aggregating data from millions of patients offers an unprecedented resource for research into arrhythmia epidemiology, treatment efficacy, and device performance. AI-powered analytics could identify early predictors of arrhythmia occurrence or adverse events, or optimise device algorithms, promoting personalised approach (e.g. by creation of digital twins) with the potential to improve patient care as suggested by observational data.29–37 Accessing EHDS resources to identify eligible patient populations, monitor outcomes, and assess the effectiveness of new therapeutic modalities in larger populations will also allow conducting data-driven clinical trials, utilising real-world data.38 In this perspective, collaborative networks will allow researchers and clinicians to share data and insights for improved outcome research. This could be of great value for all the arrhythmic disorders, but of extraordinary value for rare diseases and conditions.
Box 2 Opportunities and challenges posed by the European Health Data Space.
Opportunities:
Enhancing continuity of care
Optimizing healthcare resources
Patient empowerment through data and digital tools
Streamlining registries and reporting
Conduct of large-scale data-driven trials
Fostering research and innovation
Challenges:
Data privacy concerns
Ethical considerations
Secure data access and usage
Complex regulatory landscape
Interoperability challenges
Data quality and standardisation
Resource allocation
Healthcare inequities
Patients will be able to access and manage their EHRs, fostering greater engagement and facilitating informed decision-making.20,25,39,40 Importantly, EHDS is expected to facilitate optimisation of healthcare resources. It has been estimated that the benefits of primary use of health data can be quantified at 5.5 billion Euros over 10 years, including savings for healthcare providers and patients in health costs thanks to an increased uptake of telemedicine, faster deployment of cross-border sharing of health data and faster growth of the digital health and wellness applications markets. The secondary use of health data will potentially lead to savings of at least 5.4 billion Euros over 10 years from better use of data for research, innovation and policy-making.41
However, such a vast system involving many stakeholders and complex regulation is associated with significant threats and challenges that need to be addressed in the process of implementation (Box 2). The cross-border exchange of sensitive health data introduces significant risks of breaches or misuse, raising data privacy concerns. Ensuring compliance with GDPR and maintaining public trust in the EHDS framework will require robust security measures and transparent governance. Balancing the benefits of data sharing with ethical concerns, such as patient consent and data ownership, is another delicate task. Developing frameworks that respect individual rights while enabling the meaningful use of data also remain a critical hurdle.42 On the other hand, uneven adoption of the EHDS across member states may exacerbate disparities in arrhythmia care. Less-resourced regions may struggle to implement necessary infrastructure, potentially disadvantaging patients in these areas. Providing secure data access and usage and ensuring that only authorised individuals can access sensitive data also represents a critical challenge.42 Despite the promise of standardised data exchange, variability in national EHR systems and device platforms poses another important challenge. In addition, the implementation of the EHDS is subject to diverse regulatory frameworks across EU member states. Regulatory misalignments could delay the integration of critical health data.41 Less-resourced healthcare systems may face difficulties in allocating funds and expertise, potentially leaving them behind in the adoption curve.
AI and big data management in arrhythmia management
AI has emerged as a transformative force in arrhythmia management, revolutionising how healthcare professionals diagnose, monitor, and treat heart rhythm disorders.43 The integration of AI, particularly in handling large datasets, has the potential to enhance the efficiency, precision, and scalability of arrhythmia management as well as to facilitate the introduction of personalised arrhythmia care. These algorithms not only detect arrhythmias such as AF and ventricular tachycardia but also have the potential to predict future episodes based on historical trends in patient data. This predictive capability enables earlier intervention and more tailored treatment plans.
In the realm of big data, AI excels in managing and interpreting the overwhelming volume of information generated by wearable devices, remote monitoring systems, and EHRs. Wearable technologies continuously collect data from patients, creating a real-time repository of cardiac metrics. In this context, AI-driven platforms can analyse this data to identify clinically significant events with the ultimate goal of reducing the burden on healthcare providers and enabling timely responses to different clinical situations, as shown in observational studies.44,45 In this context, AI’s role in arrhythmia management and big data handling has the potential to redefine the field.45,46 By enabling faster diagnoses, predictive insights, and personalised care, AI not only improves clinical outcomes but also empowers patients to take a proactive role in managing their heart health.
However, standardised reporting of AI-related research in the field of arrhythmia is extremely important for the understanding and improvement of transparency of research. To ensure this, EHRA has recently created an AI checklist which was applied to a number of studies on arrhythmias and the different items in the checklist were found to be only moderately well reported.47 This highlights the need for a more systematic approach in the future and underscores the main limitations and challenges for the wider application of AI. Among these is the limited external validation in many published models, which is an obstacle to generalizability, potentially limiting their wider clinical implementation.47 Most of the available AI models are not entirely transparent because of their ‘black box’ nature, lacking clarity on how the various algorithms make decisions. Implementing explainable AI and federated learning in the field of electrophysiology is the key to mitigation of these challenges and to the better understanding of the underlying processes thus increasing trust in AI.47
Education and training in the field of AI and digital literacy
Education and familiarising healthcare professionals, researchers, and regulators with the different AI tools and algorithms is crucial to the wider implementation of AI into research and its responsible translation into clinical practice. In that regard, scientific organisations like the ESC and EHRA play a central role by providing structured educational initiatives, training, and certification programs ensuring safe and effective use of various AI models for arrhythmia care. Digital literacy of all the stakeholders can be improved by EHRA/ESC-led activities. These include digital skills workshops, continuing medical education modules as a part of a systematic approach to education.
Registries and large databases as a tool to integrate health data in arrhythmia patients
The integration of health data from arrhythmia patients into registries and large databases plays a pivotal role in advancing both clinical practice and research. With the increasing availability of patient health information from wearable devices, implantable cardiac monitors and EHRs, centralising these data in structured databases offers unprecedented opportunities to enhance arrhythmia management, decision-making, and facilitate research.46,48
In the clinical setting, registries dedicated to arrhythmia patients serve as a comprehensive repository of demographic, diagnostic, treatment, and outcomes data. Integrating data from patients with AF into large-scale registries can help clinicians identify factors associated with treatment success or complications, such as anticoagulation therapy efficacy and procedural outcomes of catheter ablation. Furthermore, these registries can help risk-stratify patients, allowing for more personalised interventions and improved long-term management of arrhythmias.
For research, large databases are the cornerstone for uncovering new insights into the pathophysiology, progression, and treatment of arrhythmias.48 When health data from diverse populations is aggregated, researchers gain access to a more extensive pool of information, enabling statistically robust analyses and the identification of previously unrecognized patterns.
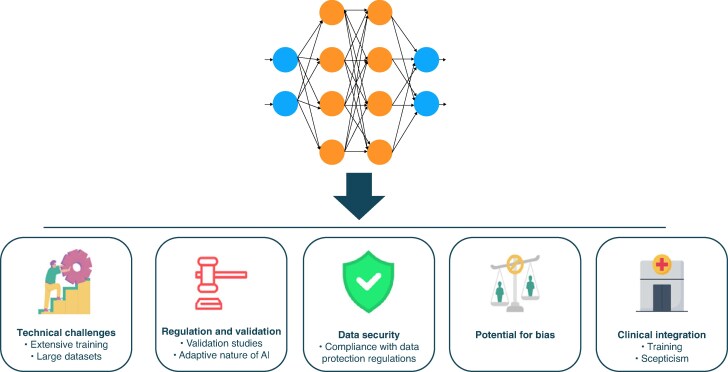
Multicentre collaborations facilitate the creation of large-scale databases and the conduct of observational studies, expediting the translation of research findings into clinical practice.49 Ensuring data standardisation, maintaining patient privacy and addressing interoperability between different systems is critical for the success of such initiatives. Advancements in data governance frameworks and secure data-sharing protocols are crucial for overcoming these barriers. By integrating health data from arrhythmia patients into registries and large databases, healthcare providers and researchers can open new opportunities to advance precision medicine, improve outcomes and drive innovations in the management of cardiac arrhythmias. However, this process faces several obstacles and challenges that must be addressed to ensure safe and effective utilization (Figure 5, Supplementary material online, Table S2).
Figure 5.
Key challenges in implementing artificial intelligence (AI) in healthcare. The figure summarizes critical barriers to the adoption of AI-based models in clinical practice. These include technical challenges, such as the need for extensive training and large annotated datasets; regulatory and validation requirements, especially due to the adaptive nature of AI; data security, requiring strict compliance with privacy laws; the potential for algorithmic bias, which may impact clinical fairness; and finally, clinical integration, which is often hindered by limited training and user scepticism. Addressing these factors is essential for safe, effective, and equitable deployment of AI in medicine.
Regulatory standards and data security for big data and AI
The implementation of AI and big data management into various sectors necessitates adherence to a complex regulatory framework designed to ensure safety, privacy, and ethical standards in the EU. Key regulations include the MDR, the AI Act, and the GDPR, each addressing different facets of AI and big data utilization (Box 3). The regulatory standards governing AI and big data in the EU are designed to balance innovation and the protection of individual rights and public safety. Compliance with the MDR, AI Act, GDPR, and security directives requires a comprehensive understanding of the requirements of each regulation and a proactive approach to integrating these standards into organisational practices.
Box 3 Regulatory standards for big data and AI.
Medical Device Regulation (MDR)
The MDR (Regulation (EU) 2017/745) governs the production and distribution of medical devices within the EU. For AI-based medical devices, compliance with MDR is mandatory, ensuring that these devices meet stringent safety and performance requirements. The MDR emphasises a risk-based classification system, rigorous clinical evaluation, and continuous post-market surveillance. Manufacturers must demonstrate that their AI-enabled devices consistently perform as intended without compromising patient safety.
Artificial Intelligence Act
The proposed AI Act aims to establish a comprehensive regulatory framework for AI applications, categorising them based on risk levels. High-risk AI systems, including certain medical devices, are subject to strict obligations such as conformity assessments, transparency requirements and human oversight mechanisms. The AI Act complements existing regulations like the MDR by addressing specific challenges posed by AI technologies, ensuring that AI systems are safe, transparent and respect fundamental rights.
General Data Protection Regulation (GDPR)
The GDPR (Regulation (EU) 2016/679) is a cornerstone of data protection law in the EU, regulating the processing of personal data. Organizations utilizing AI and big data must ensure compliance with GDPR principles, including lawfulness, fairness, transparency, data minimization and purpose limitation. Given that AI systems often rely on large datasets, adherence to GDPR is crucial to protect individual privacy rights and maintain public trust.
Security Considerations (NIS Directive)
The Directive on Security of Network and Information Systems (NIS Directive) complements GDPR by establishing cybersecurity requirements for operators of essential services and digital service providers. Ensuring robust cybersecurity measures is vital to protect sensitive data from breaches and to maintain the integrity and availability of AI systems.
Navigating this regulatory environment presents challenges, including potential overlaps between the MDR and the AI Act and the need for alignment with GDPR provisions.50 Organisations must conduct thorough conformity assessments, implement comprehensive data protection strategies, and establish robust cybersecurity protocols. In this context, engaging with regulatory bodies and staying informed about evolving guidelines are essential steps to ensure compliance and foster innovation responsibly.
The ESC-EHRA atlas in heart rhythm disorders
The concept for the ESC-EHRA Atlas in Heart Rhythm Disorders was conceived in 2022 following a series of inquiries made to the EHRA concerning various aspects of arrhythmia care delivery and the need of registries and large databases of health data from arrhythmia patients in Europe. EHRA used to collect and present such data annually in the form of EHRA White Book until 2017. Meanwhile, the ESC Atlas Project emerged as the primary source of data for general cardiovascular disease statistics, followed by more focused subspecialty Atlases developed in collaboration with ESC Associations.51–53 While the ESC Atlas of Cardiology covers all general aspects of cardiovascular care delivery in Europe, including basic arrhythmia care, it can only cover the key aspects and data points of all cardiology subspecialties. Therefore, a subspecialty Atlas project, covering aspects of care for a specific field like electrophysiology and electrotherapy in more detail, would be a valuable addition to the ESC Atlas of Cardiology. Recognizing the opportunity, EHRA leadership authorised the National Cardiac Societies Committee to approach the ESC Atlas leadership and team to revive the EHRA White Book initiative54 under the new format of the 2025 ESC-EHRA Atlas on Heart Rhythm Disorders.
Design and data quality control
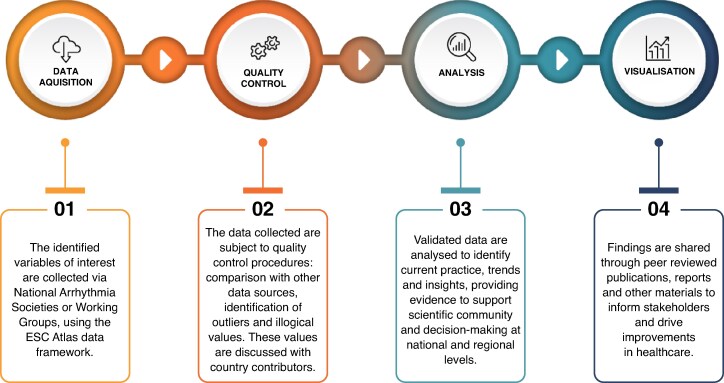
The ESC-EHRA Atlas adopts the ESC Atlas framework and resources, focusing on various facets of the organisation and delivery of arrhythmia care (Figure 6). A dedicated task force within the NCSs was established to prepare a comprehensive list of variables broadly encompassing the following Sections: Infrastructure and Human Resources (e.g. Hospitals, Education, Certification), Procedures and resources—Electrophysiology (e.g. Ablations) or CIED (e.g. Implantations, Lead extraction), Organisation of care and reimbursement (e.g. Formal networks, Reimbursement). The goal was to collect high-quality data derived from national registries, administrative databases, and similarly reliable sources. Also, the task force opted to limit the total number of variables and their level of detail to mitigate potential problems with missing data or unreliable estimations. Finally, almost 100 variables, both quantitative and qualitative, were accepted and incorporated into a standardized spreadsheet. These variables underwent both an external review with the EHRA NCS representatives, as well as an internal quality control, including an alignment with the ESC General Atlas of Cardiology in terms of variable definition and areas covered.
Figure 6.
The ESC atlas of cardiology four-step data framework.
Representatives from the National Arrhythmia Societies or Working Groups of ESC-affiliated countries were invited to actively participate in the ESC-EHRA Atlas and form country-specific task forces designed to gather data and complete the spreadsheets. To acknowledge their input and engagement, members of country-specific task forces will be listed as contributors in the official publication.
The ESC-EHRA Atlas incorporates several layers of data quality control, including clear definitions of variables, internal quality checks within the spreadsheet, cross-validation with other data sources, systematic benchmarking across data points reported by countries, and predefined queries sent to participating countries.16
Current status and future perspectives
The entire process has already been communicated to participating countries by a variety of channels, including face-to-face meetings held at EHRA-organised conferences, dedicated email communication and voluntary individual meetings at the 2024 EHRA Summit in Warsaw. A separate mailing was used to disseminate the spreadsheets and conduct data queries.
The initial results of the project were presented for the first time during the 2025 EHRA Congress in Vienna. The 1st edition of the ESC-EHRA Atlas on Heart Rhythm Disorders is available as publication in the Europace and online via a dedicated webpage as an open access paper to ensure broad dissemination and easy access to the ESC-EHRA Atlas.16
ESC Atlas of Cardiology project demonstrates the value of systematic healthcare data collection over a consistent period for the scientific community, as well as for the policymakers and regulators. By providing evidence of the cardiovascular care capacity at the country level, and reporting the procedures performed, it delivers essential information alongside clinical practice guidelines. This curated and extended body of evidence supports the development of healthcare policies tailored to each country`s context and aligned with scientific advancements. Building on this foundation, the ESC-EHRA Atlas expands the ESC Atlas repository by adding a comprehensive arrhythmia care database. This addition will enable mapping the evolution of the field over time and collecting high-quality evidence at the country level to support adapted arrhythmia care policies.
ESC and EHRA already plan to cooperate on subsequent editions of the Atlas, which will follow a similar methodology and be published bi-annually. This approach will streamline the whole process by allowing contributors in participating countries to prepare beforehand and reduce the amount of missing or below-par data. Furthermore, it will allow longitudinal comparisons and identification of trends in successive editions. Constant re-evaluation of variables included, variable definition, and ways of data presentation are underway and will further enhance this ESC-EHRA landmark project.
Conclusion
The EHRA Summit 2024 and this updated review highlighted the transformative potential of digital innovation in arrhythmia care. Among the immediate priorities for EHRA, the ESC, and national stakeholders are adopting interoperable standards (e.g. FHIR), adapting remote monitoring workflows, and actively engaging in the implementation of the EHDS. Implementing structured programs for education and certification of researchers, healthcare professionals and other stakeholders in the field of AI and managing big data also represents an important priority.
Key research gaps include the validation of AI tools, comparative assessment of certified vs. non-certified mHealth solutions, and strategies to address digital literacy and care disparities. Integrating diverse data sources into patient-centred, clinically actionable models remains a priority for research.
Regulatory and infrastructural development should focus on data protection (GDPR), certification pathways (MDR, AI Act), and national investment in secure, interoperable data systems aligned with EHDS goals. Along with that, initiatives such as the ESC-EHRA Atlas on Heart Rhythm Disorders will serve as a critical instrument for mapping care delivery, supporting benchmarking, and informing evidence-based policy. With aligned efforts across stakeholders and empowered patients, a more predictive, personalised, and equitable model of arrhythmia care is easily achievable.
Supplementary Material
Acknowledgements
We wish to thank all the participants at the EHRA summit in Warsaw. Special thanks to the EHRA staff for the excellent organization of the meeting.
Contributor Information
Vassil Traykov, Department of Invasive Electrophysiology, Acibadem City Clinic Tokuda University Hospital, Sofia, Bulgaria.
Helmut Puererfellner, Department of Cardiology, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Haran Burri, Cardiology Department, Cardiac Pacing Unit, University Hospital of Geneva, Geneva, Switzerland.
Csaba Laszlo Foldesi, Gottsegen National Cardiovascular Centre, Budapest, Hungary.
Daniel Scherr, Division of Cardiology, Department of Internal Medicine, Medical University of Graz, Graz, Austria.
David Duncker, Department of Cardiology and Angiology, Hannover Heart Rhythm Centre, Hannover Medical School, Hannover, Germany.
Elena Arbelo, Cardiology Department, Arrhythmia Section, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain; IDIBAPS, Institut d’Investigació August Pi i Sunyer (IDIBAPS), Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart (ERN GUARD-Heart).
Giovanni Luca Botto, Department of Medicine, ASST Rhodense Rho-Garbagnate Hospitals, Milan, Italy.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Italy.
Hein Heidbuchel, Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium; Research Group Cardiovascular Diseases, GENCOR, University of Antwerp, Antwerp, Belgium.
Katarzyna Malaczynska-Rajpold, Royal Brompton Hospital, Guy's and St Thomas’ NHS Foundation Trust, London, UK; Lister Hospital, East and North Hertfordshire NHS Trust, Stevenage, UK.
Michal M Farkowski, Department of Cardiology, Ministry of Interior and Administration National Medical Institute, Warsaw, Poland; II Department of Heart Arrhythmia, National Institute of Cardiology, Warsaw, Poland.
Nikolaos Dagres, Department of Cardiology, Angiology and Intensive Care Medicine, German Heart Center Charite, Berlin, Germany.
Piotr Szymanski, Clinical Cardiology Center, National Institute of Medicine MSWiA, Warsaw, Poland.
Radu Huculeci, European Society of Cardiology, Brussels, Belgium.
Ruben Casado-Arroyo, Cardiac Electrophysiology Laboratory, Université Libre de Bruxelles-Erasme Hospital, Brussels, Belgium.
Serge Boveda, Heart Rhythm Management Department, Clinique Pasteur, Toulouse, France; Brussels University VUB, Brussels, Belgium.
José L Merino, Hospital Universitario La Paz, IdiPaz, Madrid, Spain.
Supplementary material
Funding
The conference was financed entirely by the European Heart Rhythm Association (EHRA). No funding was received for the preparation of this manuscript.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Akar JG, Bao H, Jones PW, Wang Y, Varosy PD, Masoudi FA et al. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circ Arrhythm Electrophysiol 2015;8:1173–80. [DOI] [PubMed] [Google Scholar]
- 2. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–67. [DOI] [PubMed] [Google Scholar]
- 3. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–10. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–90. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon-Moreau L et al. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J et al. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017;38:2352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ricci RP, Pignalberi C, Landolina M, Santini M, Lunati M, Boriani G et al. Ventricular rate monitoring as a tool to predict and prevent atrial fibrillation-related inappropriate shocks in heart failure patients treated with cardiac resynchronisation therapy defibrillators. Heart 2014;100:848–54. [DOI] [PubMed] [Google Scholar]
- 8. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19:416–25. [DOI] [PubMed] [Google Scholar]
- 9. García-Fernández FJ, Osca Asensi J, Romero R, Fernández Lozano I, Larrazabal JM, Martínez Ferrer J et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J 2019;40:1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hindricks G, Dagres N. Remote monitoring, healthcare costs, and workload for healthcare professionals. Eur Heart J 2019;40:1847–9. [DOI] [PubMed] [Google Scholar]
- 11. Ferrick AM, Raj SR, Deneke T, Kojodjojo P, Lopez-Cabanillas N, Abe H et al. 2023 HRS/EHRA/APHRS/LAHRS expert consensus statement on practical management of the remote device clinic. Europace 2023;25:euad123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Botto GL, Sinagra G, Bulava A, Gargaro A, Timmel T, Giacopelli D et al. Predicting worsening heart failure hospitalizations in patients with implantable cardioverter defibrillators: is it all about alerts? A pooled analysis of nine trials. Europace 2024;26:euae032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allred J, Seiler A, Lyons M, Roberts P, Tsiperfal A, van Heel L et al. Current practices in managing patients with cardiac implantable electronic devices: results of an international survey. Heart Rhythm O2 2025;6:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helms TM, Boriani G, Brunner-La Rocca HP, Klein C, Koehler F, Krzesiński P et al. The present and future of cardiological telemonitoring in Europe: a statement from seven European countries. Herzschrittmacherther Elektrophysiol 2025;36:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boriani G, Burri H, Svennberg E, Imberti JF, Merino JL, Leclercq C. Current status of reimbursement practices for remote monitoring of cardiac implantable electrical devices across Europe. Europace 2022;24:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farkowski MM, Scherr D, Boriani G, Kazakevich D, Haim M, Huculeci R, et al. Arrhythmia care in ESC member countries—the 2025 ESC-EHRA atlas on Heart Rhythm disorders. Europace 2025;27:euaf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boriani G, Imberti JF, Bonini N, Carriere C, Mei DA, Zecchin M et al. Remote multiparametric monitoring and management of heart failure patients through cardiac implantable electronic devices. Eur J Intern Med 2023;115:1–9. [DOI] [PubMed] [Google Scholar]
- 18. Slotwiner DJ, Serwer GA, Allred JD, Bhakta D, Clark R, Durand J et al. 2024 HRS perspective on advancing workflows for CIED remote monitoring. Heart Rhythm O2 2024;5:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varma N, Marijon E, Vicaut É, Boveda S, Abraham A, Ibnouhsein I, et al. Impact of a universal monitoring system (“third party”) on outcomes of ICD patients: a nationwide study. Heart Rhythm 2024:S1547-5271(24)03612-9. [DOI] [PubMed] [Google Scholar]
- 20. Svennberg E, Caiani EG, Bruining N, Desteghe L, Han JK, Narayan SM et al. The digital journey: 25 years of digital development in electrophysiology from an europace perspective. Europace 2023;25:euad176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 2018;3:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar P et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caiani EG, Kemps H, Hoogendoorn P, Asteggiano R, Böhm A, Borregaard B et al. Standardized assessment of evidence supporting the adoption of mobile health solutions: a clinical consensus statement of the ESC Regulatory Affairs Committee: developed in collaboration with the European Heart Rhythm Association (EHRA), the Association of Cardiovascular Nursing & Allied Professions (ACNAP) of the ESC, the Heart Failure Association (HFA) of the ESC, the ESC Young Community, the ESC Working Group on e-Cardiology, the ESC Council for Cardiology Practice, the ESC Council of Cardio-Oncology, the ESC Council on Hypertension, the ESC Patient Forum, the ESC Digital Health Committee, and the European Association of Preventive Cardiology (EAPC). Eur Heart J Digit Health 2024;5:509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linz D, Andrade JG, Arbelo E, Boriani G, Breithardt G, Camm AJ et al. Longer and better lives for patients with atrial fibrillation: the 9th AFNET/EHRA consensus conference. Europace 2024;26:euae070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boriani G, Maisano A, Bonini N, Albini A, Imberti JF, Venturelli A et al. Digital literacy as a potential barrier to implementation of cardiology tele-visits after COVID-19 pandemic: the INFO-COVID survey. J Geriatr Cardiol 2021;18:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varma N, Braunschweig F, Burri H, Hindricks G, Linz D, Michowitz Y et al. Remote monitoring of cardiac implantable electronic devices and disease management. Europace 2023;25:euad233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Defaye P, Biffi M, El-Chami M, Boveda S, Glikson M, Piccini J et al. Cardiac pacing and lead devices management: 25 years of research at EP europace journal. Europace 2023;25:euad202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuijt E, Scherr D, Plank G, Schotten U, Heijman J. Evolution in electrophysiology 100 years after einthoven: translational and computational innovations in rhythm control of atrial fibrillation. Europace 2025;27:euae304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thangaraj PM, Benson SH, Oikonomou EK, Asselbergs FW, Khera R. Cardiovascular care with digital twin technology in the era of generative artificial intelligence. Eur Heart J 2024;45:4808–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhagirath P, Strocchi M, Bishop MJ, Boyle PM, Plank G. From bits to bedside: entering the age of digital twins in cardiac electrophysiology. Europace 2024;26:euae295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petzl AM, Jabbour G, Cadrin-Tourigny J, Pürerfellner H, Macle L, Khairy P et al. Innovative approaches to atrial fibrillation prediction: should polygenic scores and machine learning be implemented in clinical practice? Europace 2024;26:euae201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crespin E, Rosier A, Ibnouhsein I, Gozlan A, Lazarus A, Laurent G et al. Improved diagnostic performance of insertable cardiac monitors by an artificial intelligence-based algorithm. Europace 2023;26:euad375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hygrell T, Viberg F, Dahlberg E, Charlton PH, Kemp Gudmundsdottir K, Mant J et al. An artificial intelligence-based model for prediction of atrial fibrillation from single-lead sinus rhythm electrocardiograms facilitating screening. Europace 2023;25:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiraishi Y, Goto S, Niimi N, Katsumata Y, Goda A, Takei M et al. Improved prediction of sudden cardiac death in patients with heart failure through digital processing of electrocardiography. Europace 2023;25:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Pooter J, Timmers L, Boveda S, Combes S, Knecht S, Almorad A et al. Validation of a machine learning algorithm to identify pulmonary vein isolation during ablation procedures for the treatment of atrial fibrillation: results of the PVISION study. Europace 2024;26:euae116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu JR, Power JR, Zannad F, Lam CSP. Artificial intelligence and digital tools for design and execution of cardiovascular clinical trials. Eur Heart J 2025;46:814–26. [DOI] [PubMed] [Google Scholar]
- 39. Boriani G, Mei DA, Lip GYH; ARISTOTELES Consortium . Artificial intelligence in patients with atrial fibrillation to manage clinical complexity and comorbidities: the ARISTOTELES project. Eur Heart J 2025;46:775–7. [DOI] [PubMed] [Google Scholar]
- 40. Hillmann HAK, Angelini E, Karfoul N, Feickert S, Mueller-Leisse J, Duncker D. Accuracy and comprehensibility of chat-based artificial intelligence for patient information on atrial fibrillation and cardiac implantable electronic devices. Europace 2024;26:euad369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. A European Health Data Space . Harnessing the power of health data for people, patients and innovation. Available from: https://health.ec.europa.eu/publications/communication-commission-european-health-data-space-harnessing-power-health-data-people-patients-and_en (23 December 2024, date last accessed).
- 42. Song M, Elson J, Bastola D. Digital age transformation in patient-physician communication: 25-year narrative review (1999-2023). J Med Internet Res 2025;27:e60512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crea F. The growing role of artificial intelligence and of wearable devices in the management of arrhythmias. Eur Heart J 2021;42:3889–93. [DOI] [PubMed] [Google Scholar]
- 44. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C et al. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circ Heart Fail 2018;11:e004669. [DOI] [PubMed] [Google Scholar]
- 45. Dhingra L. Intelligent Decision Support System for Cardiac Arrhythmia Management using AI and ECG Data. 2023 IEEE International Conference on ICT in Business Industry & Government (ICTBIG); 8-9 Dec. 2023.
- 46. Nagarajan VD, Lee SL, Robertus JL, Nienaber CA, Trayanova NA, Ernst S. Artificial intelligence in the diagnosis and management of arrhythmias. Eur Heart J 2021;42:3904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Svennberg E, Han JK, Caiani EG, Engelhardt S, Ernst S, Friedman P et al. State of the art of artificial intelligence in clinical electrophysiology in 2025. A scientific statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC working group in e-cardiology. Europace 2025;27:euaf071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torp-Pedersen C, Goette A, Nielsen PB, Potpara T, Fauchier L, John Camm A et al. ‘Real-world’ observational studies in arrhythmia research: data sources, methodology, and interpretation. A position document from European Heart Rhythm Association (EHRA), endorsed by Heart Rhythm Society (HRS), Asia-Pacific HRS (APHRS), and Latin America HRS (LAHRS). Europace 2020;22:831–2. [DOI] [PubMed] [Google Scholar]
- 49. Bulgarelli L, Núñez-Reiz A, Deliberato RO. Building electronic health record databases for research. In: Celi L, Majumder M, Ordóñez P, Osorio J, Paik K, Somai M (eds.), Leveraging Data Science for Global Health. Cham, Switzerland: Springer Nature Switzerland; 2020. p. 55–64. [Google Scholar]
- 50. Pecchia L, Maccaro A, Matarrese MAG, Folkvord F, Fico G. Artificial intelligence, data protection and medical device regulations: squaring the circle with a historical perspective in Europe. Health Technol 2024;14:663–70. [Google Scholar]
- 51. Timmis A, Aboyans V, Vardas P, Townsend N, Torbica A, Kavousi M et al. European society of cardiology: the 2023 atlas of cardiovascular disease statistics. Eur Heart J 2024;45:4019–62.. [DOI] [PubMed] [Google Scholar]
- 52. Barbato E, Noc M, Baumbach A, Dudek D, Bunc M, Skalidis E et al. Mapping interventional cardiology in Europe: the European Association of percutaneous cardiovascular interventions (EAPCI) atlas project. Eur Heart J 2020;41:2579–88. [DOI] [PubMed] [Google Scholar]
- 53. Seferović PM, Jankowska E, Coats AJS, Maggioni AP, Lopatin Y, Milinković I et al. The heart failure association atlas: rationale, objectives, and methods. Eur J Heart Fail 2020;22:638–45. [DOI] [PubMed] [Google Scholar]
- 54. Arribas F, Auricchio A, Wolpert C, Merkely B, Merino JL, Boriani G et al. The EHRA white book. Europace 2012;14:iii1–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.