Simple Summary
Deoxynivalenol (DON) is a common mycotoxin that weakens immune function in pigs. However, its effects on cellular metabolism remain unclear. This study employs porcine alveolar macrophages (3D4/21 cells) to investigate DON-induced metabolic alterations using non-targeted metabolomics. MTT assays showed DON reduced cell viability in a concentration- and time-dependent manner. Metabolomic analysis identified 127 differential metabolites, revealing distinct metabolic profiles between control and DON-treated cells. These changes mainly affected purine metabolism, glutathione metabolism, and arginine–proline metabolism. Integration with transcriptomics data confirmed these pathways are important for DON-induced immunotoxicity. The study provides new insights into DON-induced metabolic reprogramming in immune cells and identifies candidate targets for alleviating mycotoxin-driven immunosuppression in swine.
Keywords: deoxynivalenol, pig, alveolar macrophages, metabolomics
Abstract
Deoxynivalenol (DON) is a common mycotoxin that causes immunosuppression in pigs. Its effects on cellular metabolism remain unclear. In this study, we investigate DON-induced metabolic alterations in porcine alveolar macrophage cell line 3D4/21 using non-targeted metabolomics. MTT assays showed DON reduced cell viability in a concentration- and time-dependent manner. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) revealed distinct metabolic profiles between control and DON-treated groups. Metabolomic analysis identified 127 differential metabolites (VIP > 1, p < 0.05), primarily in purine metabolism, glutathione metabolism, and arginine–proline metabolism. Integration with transcriptomic data confirmed that these pathways play key roles in DON-induced immunotoxicity. Specifically, changes in purine metabolism suggested disrupted nucleotide synthesis and energy balance, while glutathione depletion indicated weakened antioxidant defense. These findings provided a systems biology perspective on DON’s metabolic reprogramming of immune cells and identified potential therapeutic targets to reduce mycotoxin-related immunosuppression in swine.
1. Introduction
Mycotoxin contamination in feed is a major threat to global livestock health and causes severe economic losses in agriculture [1]. Among these toxins, deoxynivalenol (DON), a type B trichothecene produced by Fusarium species, is one of the most widespread contaminants in cereal crops worldwide [2]. Due to its high stability during storage and food processing, DON exposure through contaminated feed is almost unavoidable in the diet of domestic animals [3]. Even at subclinical levels, DON exposure reduces feed intake, slows growth, and decreases feed conversion efficiency, further harming productivity [4].
Pigs are especially susceptible to DON toxicity owing to their substantial dietary consumption of DON-contaminated cereals (wheat, corn, and barley) [5]. Compared with other domestic animals, pigs have a higher absorption rate of DON (exceeding 70%), mainly due to the short transit time of feed through the gastrointestinal tract and the efficient absorption of DON in the upper small intestine [4,6]. Moreover, only a small amount of DON can be converted to the less toxic metabolite DOM-1, resulting in high bioavailability of the toxin in the pig’s bloodstream [7]. Consequently, pigs are particularly susceptible to DON toxicity, even at low exposure levels. Previous research shows that even low DON levels (3.02 mg/kg) can reduce feed intake and growth in pigs [5], while in vitro studies report that DON as low as 0.8 μM impairs porcine immune cell function [8].
DON has strong effects on the porcine immune system in multiple ways. It directly damages immune organs such as the thymus and spleen, leading to atrophy of the thymic cortex, reduction in lymphocyte populations, and impaired development of germinal centers in the spleen [9,10,11]. In pig models, DON exposure leads to thymic atrophy and increased apoptosis of thymocytes, with significant reductions in cortical lymphocyte density [10,12]. In addition, DON has been reported to impair both T-cell and B-cell function in porcine peripheral blood mononuclear cells (PBMCs) [8]. However, the global metabolic alterations underlying DON-induced immunotoxicity remain poorly characterized.
Metabolomics, a powerful systems biology tool, provides a comprehensive view of how xenobiotic exposure affects cellular metabolism [13]. Non-targeted metabolomic approaches are particularly advantageous for elucidating unexpected pathways altered by toxic insults [14,15]. By mapping these metabolic alterations, researchers can pinpoint specific biomarkers for early detection of xenobiotic exposure, evaluate the severity of its toxic effects, and understand interindividual variability in susceptibility [13,16]. Nevertheless, limited data exist on DON-driven metabolic dysregulation in porcine immune cells, especially in the context of alveolar macrophage functionality.
Since alveolar macrophages play a key role in the first line of defense against pulmonary pathogens, the porcine alveolar macrophage cell line 3D4/21 has emerged as a valuable in vitro model to investigate DON-induced cellular and molecular alterations in pigs [17]. In this study, we employ an in vitro model using 3D4/21 porcine alveolar macrophages exposed to DON in combination with LC-MS/MS-based non-targeted metabolomics. Our work expands our understanding of DON’s immunometabolism toxicity and offers potential therapeutic targets for mitigating mycotoxin-related risks in swine.
2. Materials and Methods
2.1. Cell Culture
The 3D4/21 cells were obtained from the American Type Culture Collection and grown in RPMI 1640 medium (HyClone, Marlborough, MA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution (Gibco, New York, NY, USA). Cells were maintained at 37 °C with 5% CO2.
2.2. Cell Viability Assay
The 3D4/21 cells were seeded into 96-well plates at a density of 8 × 103 cells/mL and treated with 0, 1, or 2 μM DON (Sigma-Aldrich, St. Louis, MO, USA) for 12, 24, or 48 h. Cell viability was assessed using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Beyotime, Shanghai, China) as previously described [17]. Briefly, 100 μL of fresh medium and 10 μL of freshly prepared MTT solution (5 mg/mL) were added to each well. After incubation, 100 μL of formazan solubilization solution was added to dissolve the crystals. The absorbance was measured at 570 nm using a microplate reader, and cell viability was calculated accordingly.
2.3. Metabolite Extraction
After treating 3D4/21 cells with or without DON (2 μM) for 24 h (six biological replicates per group), the cells were collected by centrifugation and stored in liquid nitrogen until further processing. The samples were first removed from liquid nitrogen, and 1 mL of −80 °C pre-cooled methanol was added to the sample containing 1 × 107 cells. The mixture was vortexed for 30 s, then centrifuged at 4 °C and 13,000 rpm for 20 min. 1 mL of −80 °C pre-cooled HPLC-grade water was added to the remaining pellet, followed by one freeze–thaw cycle in liquid nitrogen, vortexed for 30 s, and centrifuged again (4 °C, 13,000 rpm, 20 min). Supernatants were pooled and centrifuged at 4 °C and 13,000 rpm for 20 min to ensure clarity. The clear supernatant was transferred to a fume hood and evaporated to dryness. The dried residue was reconstituted in 100 μL of initial mobile phase, sonicated for 5 min to ensure complete dissolution, and centrifuged at 4 °C and 13,000 rpm for 20 min prior to subsequent analysis.
2.4. Metabolite Identification and Data Analysis
Metabolite identification was performed using the LC-MS/MS by BIOMS Biotechnology (Beijing, China). Chromatographic separation was conducted on an ExionLC system (AB Sciex, Framingham, MA, USA) equipped with a Waters HSS T3 column (100 × 2.1 mm, 1.8 μM, Waters, Milford, MA, USA). Mass spectrometric analysis was carried out using a TripleTOF 5600 + system (AB Sciex) under both positive and negative ion modes with Information Dependent Acquisition (IDA) in high-sensitivity mode and dynamic background subtraction. In negative ion mode, mass spectrometry was performed with the following ion source parameters: sheath gas flow rate of 30 psi, Gas1 and Gas2 flow rates of 55 psi each, ion source temperature of 550 °C, and ion spray voltage of −4500 V. The data acquisition time was 14 min. The TOF MS scan range was set from m/z 100 to 1200, and each MS1 scan was followed by 12 product ion (MS/MS) scans. The MS/MS scan range was m/z 50~1200, with an accumulation time of 0.05 s for each MS/MS scan. The collision energy was set at −40 eV (spread ±20 eV). In positive ion mode, the same parameters were used except that the ion spray voltage was set at +5500 V and the collision energy was adjusted to +40 eV.
The raw data obtained from mass spectrometry detection were imported into Progenesis QI (v3.0) software for data preprocessing and metabolite identification. Metabolites were identified by referencing the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/, accessed on 21 May 2024) and the Human Metabolome Database (HMDB, http://www.hmdb.ca, accessed on 21 May 2024). Quality control (QC) analysis was carried out to ensure the accuracy and reliability of the data. Metabolites with a coefficient of variation (CV) less than 30% in QC samples, a variable importance in the projection (VIP) value greater than 1 and a p-value less than 0.05 were selected as differential metabolites (DMs). To reveal the differences in metabolites among different components, principal component analysis (PCA) was carried out. To remove noise and identify metabolites that contribute most to group separation, orthogonal partial least squares discriminant analysis (OPLS-DA) was performed. The quality of the model was assessed by cross-validation, with R2Y and Q2 representing the explained variables and predictability of the model, respectively. Metabolic pathway enrichment was conducted using MetaboAnalyst (v5.0) (https://www.metaboanalyst.ca/, accessed on 27 May 2024) and KEGG (https://www.genome.jp/kegg/, accessed on 27 May 2024) to identify potentially dysregulated metabolic pathways.
2.5. Integrative Analysis of Metabolomics and Transcriptomics
Transcriptomic data were obtained from prior analysis of 3D4/21 cells treated with 2 μM DON for 24 h [17]. Differentially expressed genes (DEGs) filtered by log2 (fold change) ≥ 1 and FDR < 0.05. For metabolomics, 50 DMs with top VIP scores (VIP > 1) were selected, while 50 DEGs with the lowest FDR values were chosen for integration. Data were standardized (Z-score), and Pearson’s correlation coefficients (PCCs) between each DM-DEG pair were calculated in R (v4.2.1). Pairs with |PCC| ≥ 0.5 and p < 0.05 (adjusted by Benjamini–Hochberg) were considered significant. Pathway enrichment of significant pairs was performed via MetaboAnalyst. Correlation networks were visualized using Cytoscape (v3.9.1), with node sizes reflecting pathway enrichment and edge colors indicating positive/negative correlations.
2.6. Statistical Analysis
All experimental data are expressed as mean ± standard deviation (SD). Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparisons test, with p < 0.05 considered significant. Different letters (e.g., a, b, and c) indicate statistically significant differences between groups.
3. Results
3.1. Viability of 3D4/21 Cells Following DON Exposure
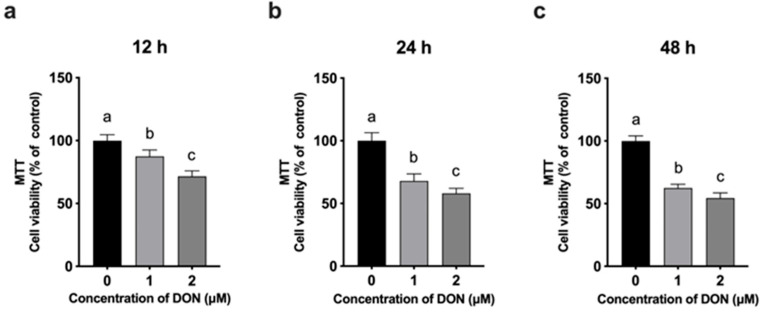
The cytotoxic effects of DON on 3D4/21 cells were evaluated using an MTT assay after treatment with 0, 1, and 2 μM DON for 12, 24, and 48 h. As shown in Figure 1a, DON exposure for 12 h led to a significant, dose-dependent decrease in cell viability. A similar trend was observed at 24 and 48 h (Figure 1b,c), with higher DON concentrations causing progressively reduced viability compared to the untreated control.
Figure 1.
Effects of different concentrations of DON (0, 1, and 2 μM) on the viability of 3D4/21 cells assessed by MTT assay at 12 h (a), 24 h (b), and 48 h (c) after treatment. Data are presented as mean ± SD (n = 8). Different letters indicate significant differences among groups (p < 0.05, one-way ANOVA followed by Tukey’s multiple comparisons).
A concentration of 2 μM DON for 24 h was chosen for metabolomic analysis, as it caused significant cytotoxicity without excessive cell death, enabling the detection of biologically relevant metabolic changes. Furthermore, this concentration and time point were consistent with those used in our previous studies [17], ensuring comparability across experiments.
3.2. PCA Principal Component Analysis
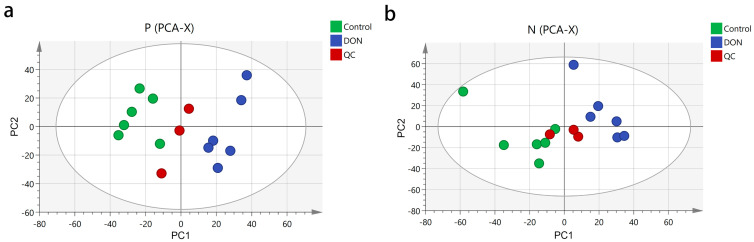
To investigate the metabolic characteristics of 3D4/21 cells after DON treatment, we conducted metabolomic profiling. Principal component analysis (PCA) showed distinct clustering patterns between the control and DON-treated groups, indicating significant metabolic perturbations induced by DON exposure. The peak areas of each metabolite in positive and negative ion modes were compared separately using SIMCA-P 14.1 software and subjected to principal component analysis. The three quality control (QC) samples were closely clustered near the center point in both the positive ion mode (Figure 2a) and the negative ion mode (Figure 2b). The samples of the same group were gathered in a relatively concentrated area and could be well distinguished from the other groups, confirming the reliability of the analytical method.
Figure 2.
Principal component analysis score plot of the control and DON groups. Cells were treated with 2 μM DON for 24 h. (a) PCA score plot for the two groups analyzed in the positive ion mode. (b) PCA score pt for the two groups analyzed in the negative ion mode. PC1 was the first principal component; PC2 was the second principal component.
3.3. OPLS-DA Analysis and Iterative Validation
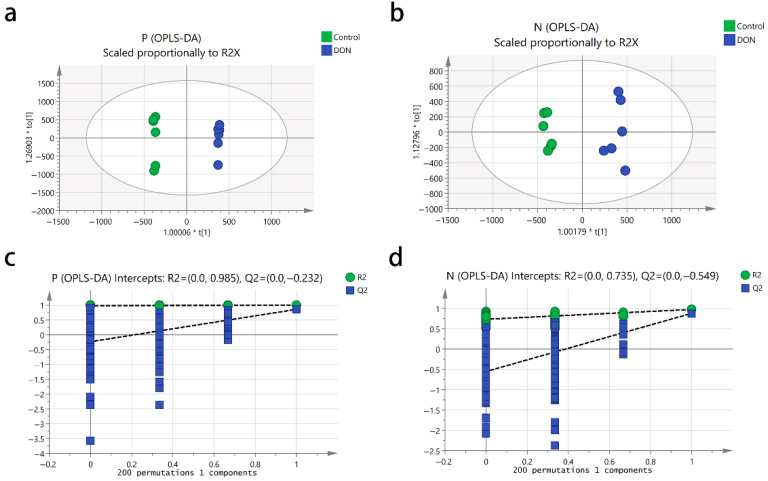
To filter out orthogonal signals and establish the OPLS-DA model, we analyzed the metabolic differences between the control and DON-treated groups. The OPLS-DA score plots demonstrated clear separation between the two groups and tight clustering within each group (Figure 3a,b). In positive ion mode, the model achieved an R2Y of 0.99 and a Q2 of 0.86 (Figure 3a), while in negative ion mode, the R2Y and Q2 values were 0.97 and 0.88, respectively (Figure 3b). The OPLS-DA model quality was then validated through 200 iterations of cross-validation (Figure 3c,d). Permutation test results showed regression line intercepts for predictive ability at −0.232 (positive ion mode) and −0.549 (negative ion mode), confirming a well-fitting model without overfitting.
Figure 3.
Orthogonal partial least squares discriminant analysis (OPLS-DA) score and permutation test plot in the positive ion mode (a,c) and the negative ion mode (b,d) for the control and DON groups. Cells were treated with 2 μM DON for 24 h. The intercept limit of Q2 was calculated by the regression line, which was the plot of Q2 from the permutation test in the OPLS-DA model.
3.4. Metabolic Pathway Analysis
We identified 127 DMs between the control and DON treatment groups, using thresholds of CV < 30%, VIP > 1, and p < 0.05 (Table 1). Among these, 36 metabolites were upregulated and 41 metabolites were downregulated in the positive ion mode (Table S1), while 19 metabolites were upregulated and 26 metabolites were downregulated in the negative ion mode (Table S2).
Table 1.
Statistical results of differential metabolites.
| Detection Mode | Differential Metabolomics | ||
|---|---|---|---|
| All | Up | Down | |
| DON vs. Control (Negative mode) | 45 | 19 | 26 |
| DON vs. Control (Positive mode) | 77 | 36 | 41 |
The heatmap of differential metabolites revealed clear clustering between control and DON-treated groups in both positive (Figure S1) and negative ion modes (Figure S2), indicating that DON treatment significantly altered the metabolic profile of 3D4/21 cells. Furthermore, we ranked 127 differential metabolites by log2FC. In positive ion mode, ophthalmic acid, lysyl-Hydroxyproline and γ-Glutamylcysteinylserine were upregulated, while (±)-2-Methylthiazolidine, tyrosyl-Asparagine, valyl-Threonine, L-proline, and racemethionine were downregulated (Table 2). In negative ion mode, nitazoxanide, 5-Hexenyl glucosinolate, 2-Deoxy-6-O-sulfo-2-(sulfoamino)-D-glucopyranose, hypotaurocyamine and nicotinate D-ribonucleoside were upregulated, whereas nicotinic acid mononucleotide, asparagusic acid syn-S-oxide and gemcitabine were downregulated (Table 3).
Table 2.
Top 10 upregulated and top 10 downregulated metabolites after DON treatment in positive ion modes.
| Compound Name | Formula | log2(FC) | Regulation |
|---|---|---|---|
| Harzianopyridone | C14H19NO5 | 6.89 | Up |
| Dribendazole | C15H19N3O2S | 5.07 | Up |
| Ophthalmic acid | C11H19N3O6 | 4.7 | Up |
| Lysyl-Hydroxyproline | C11H21N3O4 | 3.91 | Up |
| γ-Glutamylcysteinylserine | C11H19N3O7S | 2.65 | Up |
| LENAMPICILLIN | C21H23N3O7S | 2.55 | Up |
| 2-Acetylpyrrolidine | C6H11NO | 2.4 | Up |
| Gravacridonediol methyl ether | C20H21NO5 | 2.25 | Up |
| 3,11,12-Trihydroxy-1(10)-spirovetiven-2-one | C15H24O4 | 2.04 | Up |
| Miserotoxin | C9H17NO8 | 2.00 | Up |
| Oxybutynin Chloride | C22H31ClNO3 | −4.83 | Down |
| Coutaric acid | C18H27N3O4 | −2.82 | Down |
| (±)-2-Methylthiazolidine | C4H9NS | −2.24 | Down |
| Cefminox | C16H21N7O7S3 | −2.18 | Down |
| THTC | C5H8O2S | −2.17 | Down |
| Tyrosyl-Asparagine | C13H17N3O5 | −2.15 | Down |
| Valyl-Threonine | C9H18N2O4 | −2.14 | Down |
| L-Proline | C5H9NO2 | −2.06 | Down |
| Racemethionine | C5H11NO2S | −2.03 | Down |
| 2-O-alpha-D-Glucopyranosyl-D-glucopyranose | C12H22O11 | −1.87 | Down |
Table 3.
Top 10 upregulated and top 10 downregulated metabolites after DON treatment in negative ion modes.
| Compound Name | Formula | log2(FC) | Regulation |
|---|---|---|---|
| Flurocitabine | C9H10FN3O4 | 4.76 | Up |
| [(1-oxo-1H-isochromen-3-yl)methoxy]sulfonic acid | C10H8O6S | 3.22 | Up |
| Nitazoxanide | C12H9N3O5S | 2.37 | Up |
| 5-Hexenyl glucosinolate | C13H23NO9S2 | 2.32 | Up |
| Carbofenotion | C11H16ClO2PS3 | 2.23 | Up |
| 2-Deoxy-6-O-sulfo-2-(sulfoamino)-D-glucopyranose | C6H13NO11S2 | 2.05 | Up |
| Hypotaurocyamine | C3H9N3O2S | 1.95 | Up |
| Nicotinate D-ribonucleoside | C11H14NO6+ | 1.87 | Up |
| 1-(6-Oxo-6H-benzo[c]chromen-3-yl)-1H-pyrrole-2,5-dione | C17H9NO4 | 1.73 | Up |
| Methasulfocarb | C9H11NO4S2 | 1.66 | Up |
| (2R,5Z)-4-Methyl-5-[2-(phosphonooxy)ethylidene]-2,5-dihydro-1,3-thiazole-2-carboxylic acid | C7H10NO6PS | −3.42 | Down |
| 2-(2,6-dihydroxy-3,4-dimethoxycyclohexylidene)acetonitrile | C10H15NO4 | −2.79 | Down |
| Nicotinic acid mononucleotide | C11H15NO9P | −2.78 | Down |
| Flunidazole | C11H10FN3O3 | −2.42 | Down |
| Asparagusic acid syn-S-oxide | C4H6O3S2 | −2.39 | Down |
| Ethyl glucuronide | C8H14O7 | −2.21 | Down |
| MFCD00215956 | C20H11NO2 | −2.17 | Down |
| DIAMIDAFOS | C8H13N2O2P | −2.06 | Down |
| AY6315000 | C9H10FNO2 | −2.03 | Down |
| Gemcitabine | C9H11F2N3O4 | −1.96 | Down |
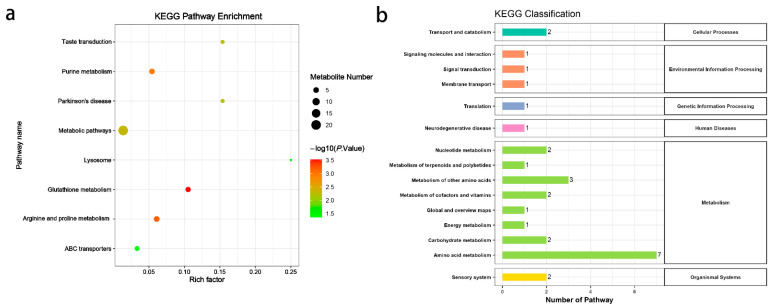
Enrichment analysis revealed that the differential metabolites were involved in eight metabolic pathways, including lysosomes, parkinsonism, taste transduction, purine metabolism, glutathione metabolism, arginine and proline metabolism, and ABC transporter proteins (Figure 4a). These pathways were classified under KEGG categories as follows: cellular processes (transport and catabolism); environmental information processing (signaling molecules and interaction; signal transduction; membrane transport); genetic information processing (translation); human diseases (neurodegenerative disease); metabolism (nucleotide metabolism; metabolism of terpenoids and polyketides; metabolism of other amino acids; metabolism of cofactors and vitamins; global and overview maps; energy metabolism; carbohydrate metabolism; amino acid metabolism); and organismal systems (sensory system) (Figure 4b).
Figure 4.
KEGG pathway enrichment analysis (a) and KEGG classification (b) of differential metabolites in 3D4/21 cells exposed to 2 µM DON for 24 h.
3.5. Integration of Metabolomics Results with Transcriptomics
To provide mechanistic insight into links between gene expression and functional metabolic outcomes, we integrated our previous transcriptomic analysis of DON-induced 3D4/21 cells with current metabolomics data [17]. In the positive ion mode, specific correlation analyses (Figure S3) showed that the metabolite HMDB0039733 exhibited the strongest positive correlation with the gene PDIA4, with a correlation coefficient of 0.9994, while HMDB0039733 displayed the strongest negative correlation with the gene TXNIP, with a coefficient of −0.9989. Additionally, in the positive ion mode (Figure S4), the metabolite HMDB0125517 showed the strongest positive correlation with the gene OTUD1 (0.9977), and the metabolite CSID54753 had the strongest negative correlation with the gene BRCA1 (−0.9986). In addition, pathway enrichment analysis indicated that both differential genes and metabolites co-regulated purine metabolism, metabolic pathways, lysosome, glutathione metabolism, and arginine–proline metabolism (Figure 5).
Figure 5.
Integrated KEGG pathway enrichment analysis of metabolomic and transcriptomic data in 3D4/21 cells exposed to 2 µM DON for 24 h. Meta represents metabolomic data, and Tra represents transcriptomic data.
4. Discussion
Deoxynivalenol (DON) is a well-known threat to porcine health and swine industry productivity because it damages macrophages. However, the global metabolomic perturbations underlying DON-induced immunotoxicity remain poorly understood. In this study, we employ non-targeted metabolomics, cell viability assays, and multi-omics integration to characterize the metabolic disturbances caused by DON in 3D4/21 cells. Our findings extend current knowledge of DON as a potent immunosuppressive mycotoxin in pigs.
The concentration- and time-dependent reduction in 3D4/21 cell viability corroborates our previous evidence of DON’s direct cytotoxic effects in 3D4/21 cells [17]. Consistent with our previous results, DON treatment led to a decrease in cell viability at 2 μM after 24 h exposure. Unexpectedly, a significant decrease also occurred at 1 µM after only 12 h, showing that DON exerts harmful effects even at low concentrations and that 3D4/21 cells are highly sensitive to it. In porcine alveolar macrophages (PAMs), 1 µM DON did not affect viability but did alter immune signaling [18]. Previous studies have shown that DON exerts variable effects depending on both the cell type and concentration. For example, in granulosa cells, low concentrations of DON have been reported to promote cell proliferation, potentially through enhanced IGF-I signaling, while higher concentrations inhibit proliferation, likely due to cytotoxic effects [19,20,21]. These findings underscore that the biological impact of DON is not only dose-dependent but also highly influenced by the cellular context, indicating that different cell types may respond to DON exposure via distinct mechanisms.
In our study, all samples are processed using a standardized methanol–water extraction protocol alongside QC samples. These QC samples clustered tightly in PCA plots, and all metabolites showed a CV below 30%, minimizing the likelihood of systematic errors such as ion suppression. Moreover, PCA and OPLS-DA analyses showed clear separation between control and DON-treated groups, indicating profound metabolic dysregulation [22]. The robust model fit (Q2 > 0.85) and absence of overfitting (permutation test results) validate the reliability of our metabolomic data, supporting the biological relevance of identified DMs.
DON drives oxidative stress management and rewires sulfur amino acid and NAD+ metabolism in porcine macrophages, metabolic axes closely tied to their inflammatory phenotype [23,24]. In positive ion mode, we observed elevated levels of ophthalmic acid, a sensitive proxy for glutathione depletion and oxidative stress [25], together with γ-glutamylcysteinylserine, a dipeptide that reinforces glutathione biosynthesis under inflammatory challenge [26]. Concomitant declines in racemethionine [27], L-Proline [28], and related dipeptides imply a constrained sulfur and aminoacid supply that supports macrophage methylation and stress-responsive metabolism. In negative ion mode, accumulation of nitazoxanide [29] points to an immunomodulatory signal, whereas the opposite shifts in nicotinate D-ribonucleoside (up) and nicotinic acid mononucleotide (down) reflect remodeling of the NAD salvage network that shapes macrophage effector programs [23]. Increased hypotaurocyamine [30] is consistent with taurine/hypotaurine antioxidative pathways, and the reduction in gemcitabine [31] aligns with lowered exposure to a nucleoside analog known for myeloid immunosuppression. Two high-abundance features were annotated as harzianopyridone, a pyridone alkaloid produced [32], and oxybutynin chloride, a compound with antimuscarinic activity [33]. As there is no evidence of endogenous synthesis of these compounds in macrophages or any mechanistic association with DON or PAMs, their observed increase is likely attributable to background contamination. This finding does not impact our conclusions regarding DON-induced immunotoxicity. These results emphasize the need for further validation to confirm compound identities and determine any residual biological relevance.
Through metabolic pathway analysis, we identified metabolic pathways associated with immune regulation. Glutathione (GSH) metabolism emerged as a critical pathway, with reduced GSH levels potentially compromising antioxidant defenses and exacerbating oxidative stress [34,35]. This aligns with DON’s known ability to induce reactive oxygen species (ROS) production and mitochondrial damage in porcine lymphocytes [10]. Depletion of GSH might disrupt redox balance, amplifying cellular injury and immune dysfunction [36]. Notably, our study reveals that disturbances in arginine and proline metabolism constitute another key pathway affected by DON exposure. Arginine is a critical substrate for both nitric oxide synthase (NOS) and polyamine synthesis—processes essential for macrophage effector functions [37]. Disruption of arginine metabolism by DON may therefore impair the production of immune-related proteins in 3D4/21 cells, potentially reducing their functional capacity. As alveolar macrophages, 3D4/21 cells serve as a primary defense against pulmonary pathogens [38,39]. Consequently, dysfunction in these cells due to altered arginine metabolism could weaken this critical immune barrier in pigs. In addition, perturbations in purine metabolism suggest impaired nucleotide synthesis and energy homeostasis, which may contribute to reduced cell viability and dysfunctional immune responses [40]. Elevated purine metabolites could reflect increased nucleic acid turnover due to cellular stress or apoptosis, which is consistent with extensive apoptosis observed in lymphocytes after DON exposure in pigs [41,42].
Integrated transcriptomic–metabolomic analysis highlighted joint enrichment of purine, glutathione, and arginine/proline pathways, implying that their interaction drives DON immunotoxicity. In piglet models, DON exposure causes thymic atrophy and lymphocyte apoptosis [43], which may be related to energy metabolism disorders (purine metabolism) and oxidative stress (glutathione metabolism) identified in this study. For example, GSH depletion can overproduce ROS and damage mitochondrial function [44], while purine metabolism disorders reduce ATP supply, leading to energy crisis in immune cells and accelerating apoptosis [45,46]. The metabolite HMDB0039733 (γ-glutamylcysteinylserine) correlated strongly with PDIA4 and inversely with TXNIP, supported by stringent criteria (|PCC| ≥ 0.5, adjusted p < 0.05) and consistent patterns across replicates. PDIA4, a protein disulfide isomerase involved in endoplasmic reticulum (ER) stress responses [47], and TXNIP, a thioredoxin-interacting protein that modulates redox balance [48]. Their strong correlations indicate that PDIA4 upregulation may counteract ER stress induced by DON [17], while TXNIP downregulation could attempt to relieve inhibition of thioredoxin, a key antioxidant enzyme, to compensate for glutathione depletion. OTUD1 and BRCA1 showed strong correlations with specific metabolites under stringent statistical thresholds. Functionally, OTUD1 is involved in deubiquitination [49], while BRCA1 plays a key role in DNA repair [50], supporting their potential involvement in DON-induced toxicity. However, the specific roles of these genes in response to DON require further validation.
5. Conclusions
DON exposure significantly impairs 3D4/21 cell line viability and induces metabolic reprogramming. Key affected pathways include purine metabolism, glutathione metabolism, and arginine–proline metabolism. These disruptions suggest oxidative stress, energy imbalance, and immune dysfunction. Integrated metabolomic and transcriptomic analyses confirm their central role in DON-induced immunotoxicity. The study offers novel insights into DON’s effects on immune cells and identifies potential metabolic targets for mitigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15152324/s1. Table S1. The differential metabolites between the DON and control groups in the positive ion mode; Table S2. The differential metabolites between the DON and control groups in the negative ion mode; Figure S1. Heatmap of differential metabolites between the control and DON-treated groups in positive ion mode; Figure S2. Heatmap of differential metabolites between the control and DON-treated groups in negative ion mode; Figure S3. Correlation patterns between differentially expressed genes and differential metabolites (positive ion mode) in 3D4/21 cells exposed to 2 μM DON for 24 h; Figure S4. Correlation patterns between differentially expressed genes and differential metabolites (negative ion mode) in 3D4/21 cells exposed to 2 μM DON for 24 h.
Author Contributions
Conceptualization, L.S. and J.Z.; methodology, J.Z. and B.Y.; investigation, Y.H.; writing—original draft preparation, Y.H., B.Y. and W.W.; writing—review and editing, J.Z. and L.S.; supervision, J.Z.; project administration, J.Z. and L.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable. This study was conducted using established cell lines and did not involve human participants or animal subjects.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (No. 32372972) and the National Natural Science Foundation of China (No. 32202651).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Magnoli A.P., Poloni V.L., Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019;29:99–108. doi: 10.1016/j.cofs.2019.08.009. [DOI] [Google Scholar]
- 2.Gab-Allah M.A., Choi K., Kim B. Type B Trichothecenes in Cereal Grains and Their Products: Recent Advances on Occurrence, Toxicology, Analysis and Post-Harvest Decontamination Strategies. Toxins. 2023;15:85. doi: 10.3390/toxins15020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf-Hall C.E., Hanna M.A., Bullerman L.B. Stability of Deoxynivalenol in Heat-Treated Foods. J. Food Prot. 1999;62:962–964. doi: 10.4315/0362-028X-62.8.962. [DOI] [PubMed] [Google Scholar]
- 4.Jia B., Lin H., Yu S., Liu N., Yu D., Wu A. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J. Hazard. Mater. 2023;451:131172. doi: 10.1016/j.jhazmat.2023.131172. [DOI] [PubMed] [Google Scholar]
- 5.Serviento A.M., Brossard L., Renaudeau D. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behavior in finishing pigs. J. Anim. Sci. 2018;96:5209–5221. doi: 10.1093/jas/sky378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy K.E., Kim M., Kim K.H., Ji S.Y., Baek Y., Chun J.L., Jung H.J., Choe C., Lee H.J., Kim M., et al. Effect of commercially purified deoxynivalenol and zearalenone mycotoxins on microbial diversity of pig cecum contents. Anim. Biosci. 2021;34:243–255. doi: 10.5713/ajas.20.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panisson J.C., Wellington M.O., Bosompem M.A., Nagl V., Schwartz-Zimmermann H.E., Columbus D.A. Urinary and Serum Concentration of Deoxynivalenol (DON) and DON Metabolites as an Indicator of DON Contamination in Swine Diets. Toxins. 2023;15:120. doi: 10.3390/toxins15020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierron A., Kleber A., Mayer E., Gerner W. Effect of DON and ZEN and their metabolites DOM-1 and HZEN on B cell proliferation and antibody production. Front. Immunol. 2024;15:1338937. doi: 10.3389/fimmu.2024.1338937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Meis J., Aurélio Farias-de-Oliveira D., Nunes Panzenhagen P.H., Maran N., Villa-Verde D.M., Morrot A., Savino W. Thymus atrophy and double-positive escape are common features in infectious diseases. J. Parasitol. Res. 2012;2012:574020. doi: 10.1155/2012/574020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Z., Guo C., He H., Zuo Z., Hu Y., Yu S., Zhong Z., Liu H., Zhu L., Xu S., et al. Effects of deoxynivalenol on mitochondrial dynamics and autophagy in pig spleen lymphocytes. Food Chem. Toxicol. 2020;140:111357. doi: 10.1016/j.fct.2020.111357. [DOI] [PubMed] [Google Scholar]
- 11.Kuang Y., Wu Z., Liu Y. Deoxynivalenol induces spleen damage, apoptosis, and inflammation in mice by increasing mitochondrial reactive oxygen species: Protective effects of curcumin. Food Chem. Toxicol. 2025;196:115200. doi: 10.1016/j.fct.2024.115200. [DOI] [PubMed] [Google Scholar]
- 12.He Y., Wang G., Liu Y., Shi W., Han Z., Wu J., Jiang C., Wang S., Hu S., Wen H., et al. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2012;160:455–462. doi: 10.1016/j.vetmic.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C.H., Patterson A.D., Idle J.R., Gonzalez F.J. Xenobiotic metabolomics: Major impact on the metabolome. Annu. Rev. Pharmacol. Toxicol. 2012;52:37–56. doi: 10.1146/annurev-pharmtox-010611-134748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z.Z., Pacheco J.A., Gao Y., Deng S., Peterson B., Shi X., Zheng S., Tahir U.A., Katz D.H., Cruz D.E., et al. Nontargeted and Targeted Metabolomic Profiling Reveals Novel Metabolite Biomarkers of Incident Diabetes in African Americans. Diabetes. 2022;71:2426–2437. doi: 10.2337/db22-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez T., Daneshian M., Kamp H., Bois F.Y., Clench M.R., Coen M., Donley B., Fischer S.M., Ekman D.R., Fabian E., et al. Metabolomics in toxicology and preclinical research. Altex. 2013;30:209–225. doi: 10.14573/altex.2013.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estévez J., Vilanova E., Sogorb M.A. Chapter 66—Biomarkers for Testing Toxicity and Monitoring Exposure to Xenobiotics. In: Gupta R.C., editor. Biomarkers in Toxicology. 2nd ed. Academic Press; San Diego, CA, USA: 2019. pp. 1165–1174. [Google Scholar]
- 17.Zhang J., Zhao Q., Xue Z., Zhang S., Ren Z., Chen S., Zhou A., Chen H., Liu Y. Deoxynivalenol induces endoplasmic reticulum stress-associated apoptosis via the IRE1/JNK/CHOP pathway in porcine alveolar macrophage 3D4/21 cells. Food Chem. Toxicol. 2023;180:114033. doi: 10.1016/j.fct.2023.114033. [DOI] [PubMed] [Google Scholar]
- 18.Liu D., Wang Q., He W., Chen X., Wei Z., Huang K. Two-way immune effects of deoxynivalenol in weaned piglets and porcine alveolar macrophages: Due mainly to its exposure dosage. Chemosphere. 2020;249:126464. doi: 10.1016/j.chemosphere.2020.126464. [DOI] [PubMed] [Google Scholar]
- 19.Ranzenigo G., Caloni F., Cremonesi F., Aad P.Y., Spicer L.J. Effects of Fusarium mycotoxins on steroid production by porcine granulosa cells. Anim. Reprod. Sci. 2008;107:115–130. doi: 10.1016/j.anireprosci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Pizzo F., Caloni F., Schreiber N.B., Cortinovis C., Spicer L.J. In vitro effects of deoxynivalenol and zearalenone major metabolites alone and combined, on cell proliferation, steroid production and gene expression in bovine small-follicle granulosa cells. Toxicon. 2016;109:70–83. doi: 10.1016/j.toxicon.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Pizzo F., Caloni F., Schutz L.F., Totty M.L., Spicer L.J. Individual and combined effects of deoxynivalenol and alpha-zearalenol on cell proliferation and steroidogenesis of granulosa cells in cattle. Environ. Toxicol. Pharmacol. 2015;40:722–728. doi: 10.1016/j.etap.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Yu L., Li Z., Xiao Y., Jiang H., Tang Y.L., Chen Y., Xue H. Dysregulated arginine metabolism in precursor B-cell acute lymphoblastic leukemia in children: A metabolomic study. BMC Pediatr. 2024;24:540. doi: 10.1186/s12887-024-05015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie N., Zhang L., Gao W., Huang C., Huber P.E., Zhou X., Li C., Shen G., Zou B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wculek S.K., Dunphy G., Heras-Murillo I., Mastrangelo A., Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell. Mol. Immunol. 2022;19:384–408. doi: 10.1038/s41423-021-00791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soga T., Baran R., Suematsu M., Ueno Y., Ikeda S., Sakurakawa T., Kakazu Y., Ishikawa T., Robert M., Nishioka T., et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y., Fujii J. The Emerging Roles of gamma-Glutamyl Peptides Produced by gamma-Glutamyltransferase and the Glutathione Synthesis System. Cells. 2023;12:2831. doi: 10.3390/cells12242831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji J., Xu Y., Zheng M., Luo C., Lei H., Qu H., Shu D. Methionine Attenuates Lipopolysaccharide-Induced Inflammatory Responses via DNA Methylation in Macrophages. ACS Omega. 2019;4:2331–2336. doi: 10.1021/acsomega.8b03571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phang J.M., Pandhare J., Liu Y. The metabolism of proline as microenvironmental stress substrate. J. Nutr. 2008;138:2008S–2015S. doi: 10.1093/jn/138.10.2008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo-Salazar M., Sanchez-Munoz F., Springall Del Villar R., Navarrete-Vazquez G., Hernandez-DiazCouder A., Mojica-Cardoso C., Garcia-Jimenez S., Toledano-Jaimes C., Bernal-Fernandez G. Nitazoxanide Exerts Immunomodulatory Effects on Peripheral Blood Mononuclear Cells from Type 2 Diabetes Patients. Biomolecules. 2021;11:1817. doi: 10.3390/biom11121817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizota T., Hishiki T., Shinoda M., Naito Y., Hirukawa K., Masugi Y., Itano O., Obara H., Kitago M., Yagi H., et al. The hypotaurine-taurine pathway as an antioxidative mechanism in patients with acute liver failure. J. Clin. Biochem. Nutr. 2022;70:54–63. doi: 10.3164/jcbn.21-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C., Tan X., Hu X., Zhou M., Yan J., Ding C. Tumor Microenvironment following Gemcitabine Treatment Favors Differentiation of Immunosuppressive Ly6C(high) Myeloid Cells. J. Immunol. 2020;204:212–223. doi: 10.4049/jimmunol.1900930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan R.A.A., Najeeb S., Hussain S., Xie B., Li Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms. 2020;8:817. doi: 10.3390/microorganisms8060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sathyan G., Chancellor M.B., Gupta S.K. Effect of OROS controlled-release delivery on the pharmacokinetics and pharmacodynamics of oxybutynin chloride. Br. J. Clin. Pharmacol. 2001;52:409–417. doi: 10.1046/j.0306-5251.2001.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muro P., Zhang L., Li S., Zhao Z., Jin T., Mao F., Mao Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024;15:1390351. doi: 10.3389/fendo.2024.1390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lushchak V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labarrere C.A., Kassab G.S. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol. 2022;13:979719. doi: 10.3389/fmicb.2022.979719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durante W., Johnson F.K., Johnson R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weingartl H.M., Sabara M., Pasick J., van Moorlehem E., Babiuk L. Continuous porcine cell lines developed from alveolar macrophages: Partial characterization and virus susceptibility. J. Virol. Methods. 2002;104:203–216. doi: 10.1016/S0166-0934(02)00085-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Zhang X., Luo Y., Liu R., Sun Y., Zhao S., Yu M., Cao J. Large Fragment InDels Reshape Genome Structure of Porcine Alveolar Macrophage 3D4/21 Cells. Genes. 2022;13:1515. doi: 10.3390/genes13091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan Y., Ma Z., Qian W. Utilizing integrated bioinformatics and machine learning approaches to elucidate biomarkers linking sepsis to fatty acid metabolism-associated genes. Sci. Rep. 2024;14:28972. doi: 10.1038/s41598-024-80550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikami O., Yamaguchi H., Murata H., Nakajima Y., Miyazaki S. Induction of apoptotic lesions in liver and lymphoid tissues and modulation of cytokine mRNA expression by acute exposure to deoxynivalenol in piglets. J. Vet. Sci. 2010;11:107–113. doi: 10.4142/jvs.2010.11.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camici M., Garcia-Gil M., Pesi R., Allegrini S., Tozzi M.G. Purine-Metabolising Enzymes and Apoptosis in Cancer. Cancers. 2019;11:1354. doi: 10.3390/cancers11091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruedas-Torres I., Rodríguez-Gómez I.M., Sánchez-Carvajal J.M., Pallares F.J., Barranco I., Carrasco L., Gómez-Laguna J. Activation of the extrinsic apoptotic pathway in the thymus of piglets infected with PRRSV-1 strains of different virulence. Vet. Microbiol. 2020;243:108639. doi: 10.1016/j.vetmic.2020.108639. [DOI] [PubMed] [Google Scholar]
- 44.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Z., Xie N., Illes P., Di Virgilio F., Ulrich H., Semyanov A., Verkhratsky A., Sperlagh B., Yu S.G., Huang C., et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021;6:162. doi: 10.1038/s41392-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torchia N., Brescia C., Chiarella E., Audia S., Trapasso F., Amato R. Neglected Issues in T Lymphocyte Metabolism: Purine Metabolism and Control of Nuclear Envelope Regulatory Processes. New Insights into Triggering Potential Metabolic Fragilities. Immuno. 2024;4:521–548. [Google Scholar]
- 47.Wang Z., Zhang H., Cheng Q. PDIA4: The basic characteristics, functions and its potential connection with cancer. Biomed. Pharmacother. 2020;122:109688. doi: 10.1016/j.biopha.2019.109688. [DOI] [PubMed] [Google Scholar]
- 48.Qayyum N., Haseeb M., Kim M.S., Choi S. Role of Thioredoxin-Interacting Protein in Diseases and Its Therapeutic Outlook. Int. J. Mol. Sci. 2021;22:2754. doi: 10.3390/ijms22052754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu L., Lin Y., Feng J., Qi Y., Wang X., Lin Q., Shi W., Zheng E., Wang W., Hou Z., et al. The deubiquitinating enzyme OTUD1 antagonizes BH3-mimetic inhibitor induced cell death through regulating the stability of the MCL1 protein. Cancer Cell Int. 2019;19:222. doi: 10.1186/s12935-019-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng C.X., Wang R.H. Roles of BRCA1 in DNA damage repair: A link between development and cancer. Hum. Mol. Genet. 2003;12:R113–R123. doi: 10.1093/hmg/ddg082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).