Abstract
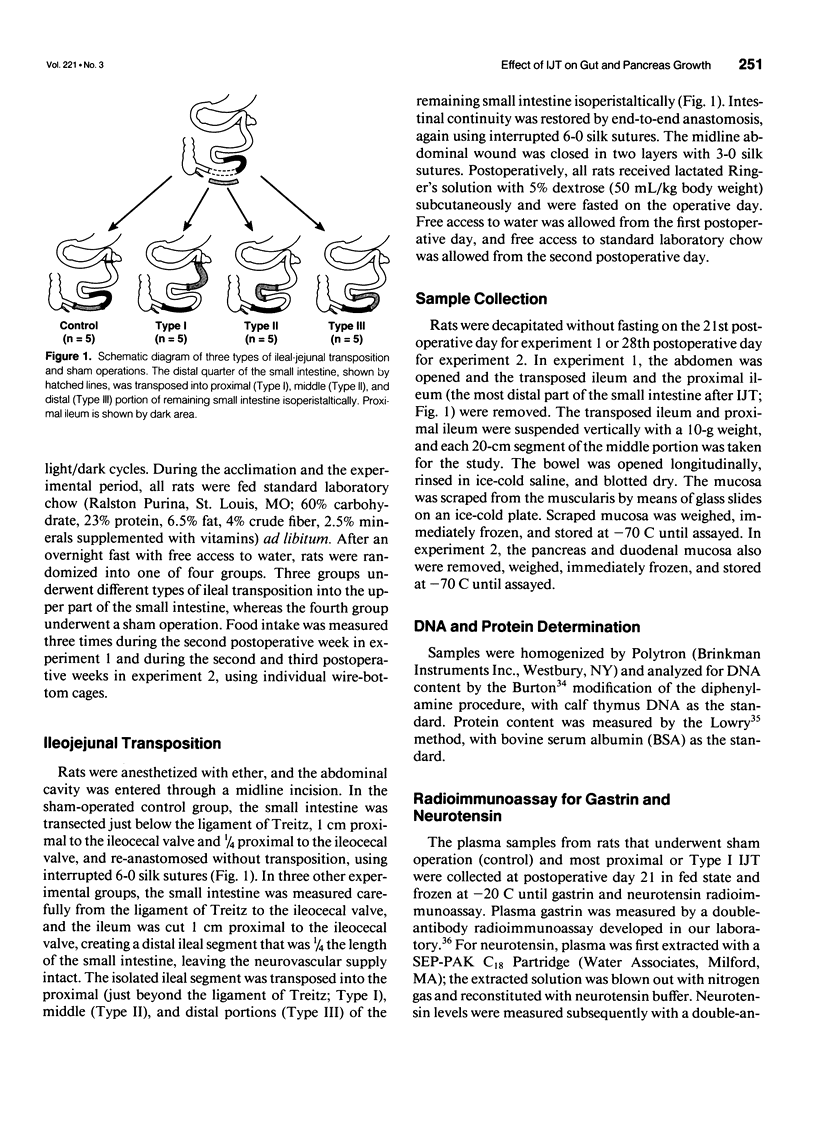
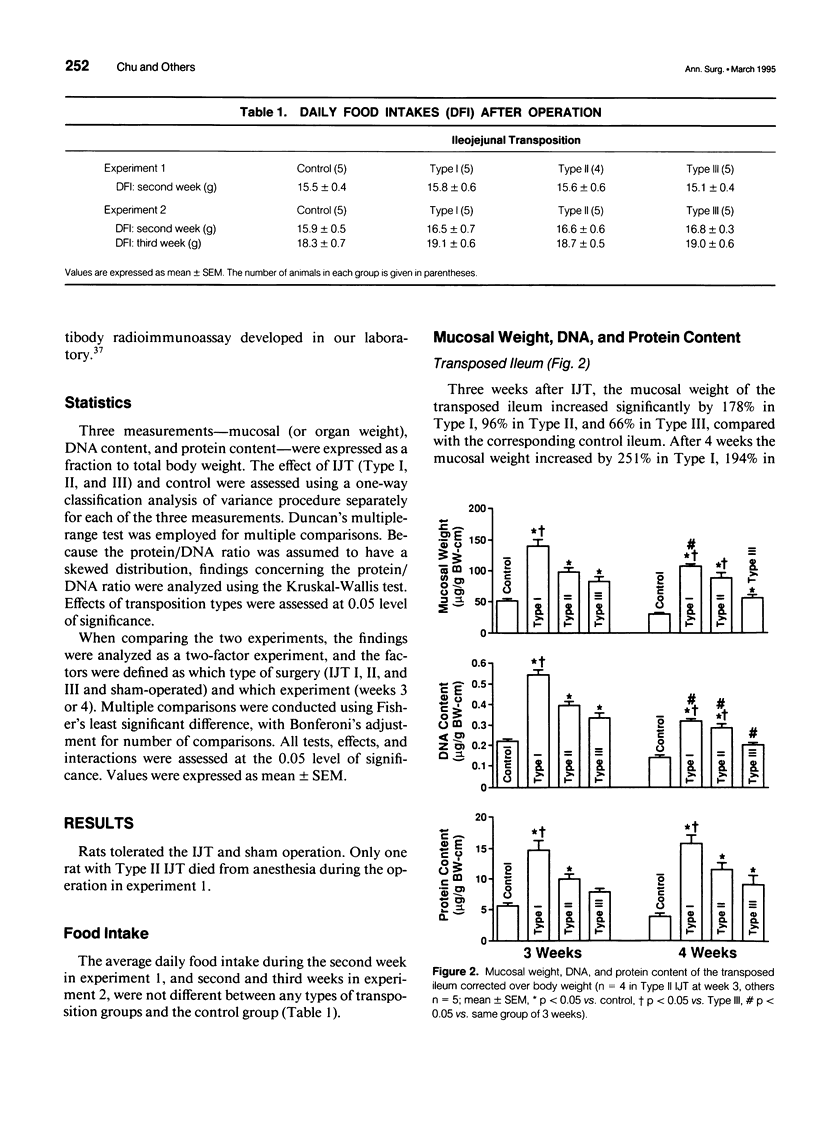
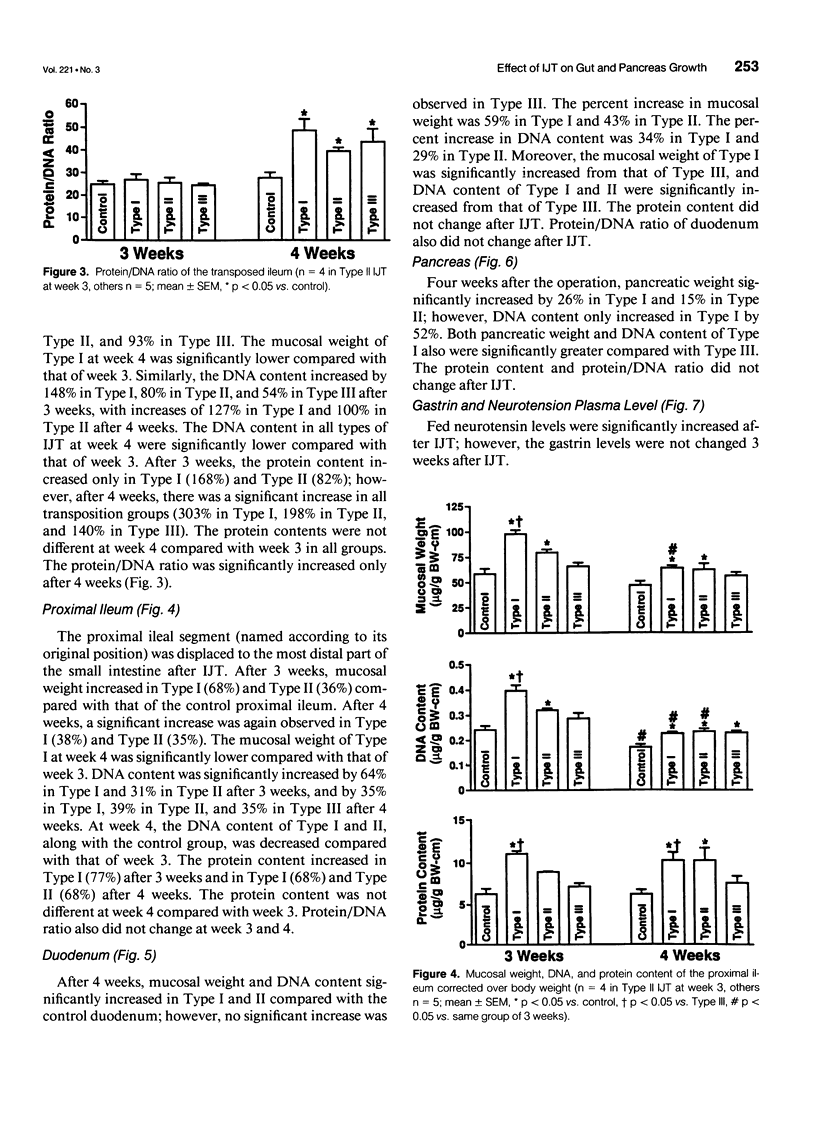
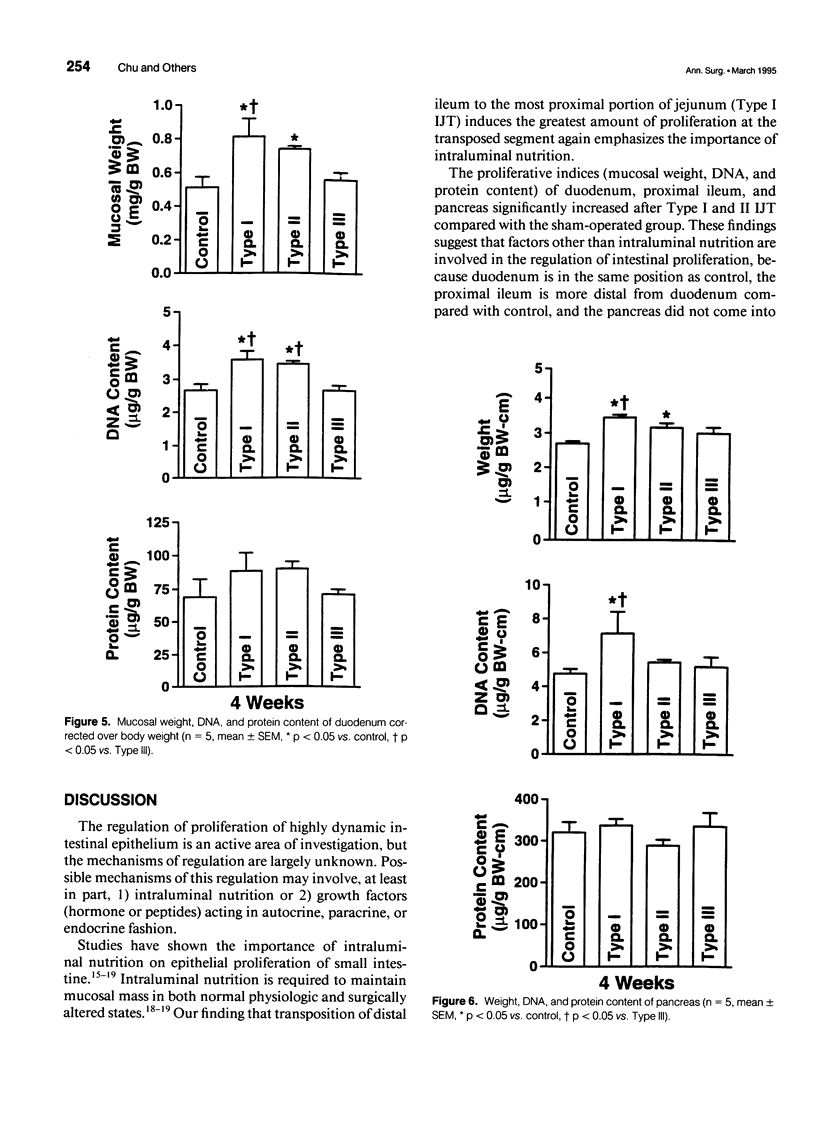
OBJECTIVE: The authors determined whether ileojejunal transposition (IJT) stimulates the growth of the pancreas or the nontransposed segment of small intestine, and ascertained whether this trophic effect is altered by the location of transposed gut segment. SUMMARY BACKGROUND DATA: Transposition of the ileum to the proximal small intestine stimulates a marked mucosal growth of the transposed ileal segment; the cellular mechanisms responsible for this adaptive hyperplasia are not known. METHODS: The distal quarter of the small intestine (distal ileum) was transposed into the proximal (Type I), middle (Type II), or distal (Type III) portions of the remaining small intestine. On postoperative day 28, the pancreas and scraped mucosa from the segments of transposed ileum, proximal ileum, and duodenum were obtained, weighed, and examined for DNA and protein content. RESULTS: All types of IJT increased mucosal weight and DNA content of the transposed ileum. Types I and II IJT produced a significant proliferation of the pancreas and mucosa of the duodenum and proximal ileum. The magnitude of proliferative increases was greatest in Type I IJT. CONCLUSIONS: Ileojejunal transposition appears to be an excellent model to examine the mechanisms by which intestinal epithelial cells proliferate in response to luminal nutrients or humoral factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol J. B., Williamson R. C. Postoperative adaptation of the small intestine. World J Surg. 1985 Dec;9(6):825–832. doi: 10.1007/BF01655386. [DOI] [PubMed] [Google Scholar]
- Bury K. D. Carbohydrate digestion and absorption after massive resection of the small intestine. Surg Gynecol Obstet. 1972 Aug;135(2):177–187. [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dworkin L. D., Levine G. M., Farber N. J., Spector M. H. Small intestinal mass of the rat is partially determined by indirect effects of intraluminal nutrition. Gastroenterology. 1976 Oct;71(4):626–630. [PubMed] [Google Scholar]
- Fuller P. J., Beveridge D. J., Taylor R. G. Ileal proglucagon gene expression in the rat: characterization in intestinal adaptation using in situ hybridization. Gastroenterology. 1993 Feb;104(2):459–466. doi: 10.1016/0016-5085(93)90414-8. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Grey V. L., Morin C. L. Role of food in appearance of growth-stimulating activity in rat proximal intestine after small-bowel resection. Dig Dis Sci. 1988 Jan;33(1):78–82. doi: 10.1007/BF01536635. [DOI] [PubMed] [Google Scholar]
- Grönqvist B., Engström B., Grimelius L. Morphological studies of the rat small intestine after jejuno-ileal transposition. Acta Chir Scand. 1975;141(3):208–217. [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W., Sharp J. G. Compensation by the residual intestine after intestinal resection in the rat. I. Influence of amount of tissue removed. Gastroenterology. 1977 Apr;72(4 Pt 1):692–700. [PubMed] [Google Scholar]
- Hanson W. R. Proliferative and morphological adaptation of the intestine to experimental resection. Scand J Gastroenterol Suppl. 1982;74:11–20. [PubMed] [Google Scholar]
- Jackson B. M., Reeder D. D., Thompson J. C. Dynamic characteristics of gastrin release. Am J Surg. 1972 Feb;123(2):137–142. doi: 10.1016/0002-9610(72)90323-6. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Copeland E. M., Dudrick S. J., Lichtenberger L. M., Castro G. A. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. Gastroenterology. 1975 May;68(5 Pt 1):1177–1183. [PubMed] [Google Scholar]
- Kotler D. P., Levine G. M., Shiau Y. F. Effects of luminal nutrition and metabolic status on in vivo glucose absorption. Am J Physiol. 1981 Jun;240(6):G432–G436. doi: 10.1152/ajpgi.1981.240.6.G432. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Steiger E., Zinno R. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology. 1974 Nov;67(5):975–982. [PubMed] [Google Scholar]
- Lilja P., Wiener I., Inoue K., Thompson J. C. Changes in circulating levels of cholecystokinin, gastrin, and pancreatic polypeptide after small bowel resection in dogs. Am J Surg. 1983 Jan;145(1):157–163. doi: 10.1016/0002-9610(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Lund P. K., Ulshen M. H., Rountree D. B., Selub S. E., Buchan A. M. Molecular biology of gastrointestinal peptides and growth factors: relevance to intestinal adaptation. Digestion. 1990;46 (Suppl 2):66–73. doi: 10.1159/000200369. [DOI] [PubMed] [Google Scholar]
- Maxton D. G., Cynk E. U., Thompson R. P. Small intestinal response to 'elemental' and 'complete' liquid feeds in the rat: effect of dietary bulk. Gut. 1987 Jun;28(6):688–693. doi: 10.1136/gut.28.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus J. P., Isselbacher K. J. Effect of fasting versus feeding on the rat small intestine. Morphological, biochemical, and functional differences. Gastroenterology. 1970 Aug;59(2):214–221. [PubMed] [Google Scholar]
- Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133(3):233–248. [PubMed] [Google Scholar]
- Obertop H., Nundy S., Malamud D., Malt R. A. Onset of cell proliferation in the shortened gut. Rapid hyperplasia after jejunal resection. Gastroenterology. 1977 Feb;72(2):267–270. [PubMed] [Google Scholar]
- Sagor G. R., Al-Mukhtar M. Y., Ghatei M. A., Wright N. A., Bloom S. R. The effect of altered luminal nutrition on cellular proliferation and plasma concentrations of enteroglucagon and gastrin after small bowel resection in the rat. Br J Surg. 1982 Jan;69(1):14–18. doi: 10.1002/bjs.1800690106. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Newman J., Fujimura M., Greeley G. H., Jr, Townsend C. M., Jr, Thompson J. C. Role of neurotensin in pancreatic secretion. Surgery. 1984 Aug;96(2):146–153. [PubMed] [Google Scholar]
- Sigalet D. L., Lees G. M., Aherne F., Van Aerde J. E., Fedorak R. N., Keelan M., Thomson A. B. The physiology of adaptation to small bowel resection in the pig: an integrated study of morphological and functional changes. J Pediatr Surg. 1990 Jun;25(6):650–657. doi: 10.1016/0022-3468(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Spector M. H., Levine G. M., Deren J. J. Direct and indirect effects of dextrose and amino acids on gut mass. Gastroenterology. 1977 Apr;72(4 Pt 1):706–710. [PubMed] [Google Scholar]
- Taylor R. G., Verity K., Fuller P. J. Ileal glucagon gene expression: ontogeny and response to massive small bowel resection. Gastroenterology. 1990 Sep;99(3):724–729. doi: 10.1016/0016-5085(90)90961-y. [DOI] [PubMed] [Google Scholar]
- Tilson M. D., Livstone E. M. Early proliferative activity: its occurrence in the crypts of small bowel and colon after partial small-bowel resection. Arch Surg. 1980 Dec;115(12):1481–1485. doi: 10.1001/archsurg.1980.01380120049012. [DOI] [PubMed] [Google Scholar]
- Ulshen M. H., Herbst C. A. Effect of proximal transposition of the ileum on mucosal growth and enzyme activity in orally nourished rats. Am J Clin Nutr. 1985 Nov;42(5):805–814. doi: 10.1093/ajcn/42.5.805. [DOI] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Weser E., Tawil T. Epithelial cell loss in remaining intestine after small bowel resection in the rat. Gastroenterology. 1976 Sep;71(3):412–415. [PubMed] [Google Scholar]
- Wittmann T., Crenner F., Koenig M., Grenier J. F. Adaptive changes in postprandial motility after intestinal resection and bypass. Electromyographic study in rats. Dig Dis Sci. 1988 Nov;33(11):1370–1376. doi: 10.1007/BF01536990. [DOI] [PubMed] [Google Scholar]


