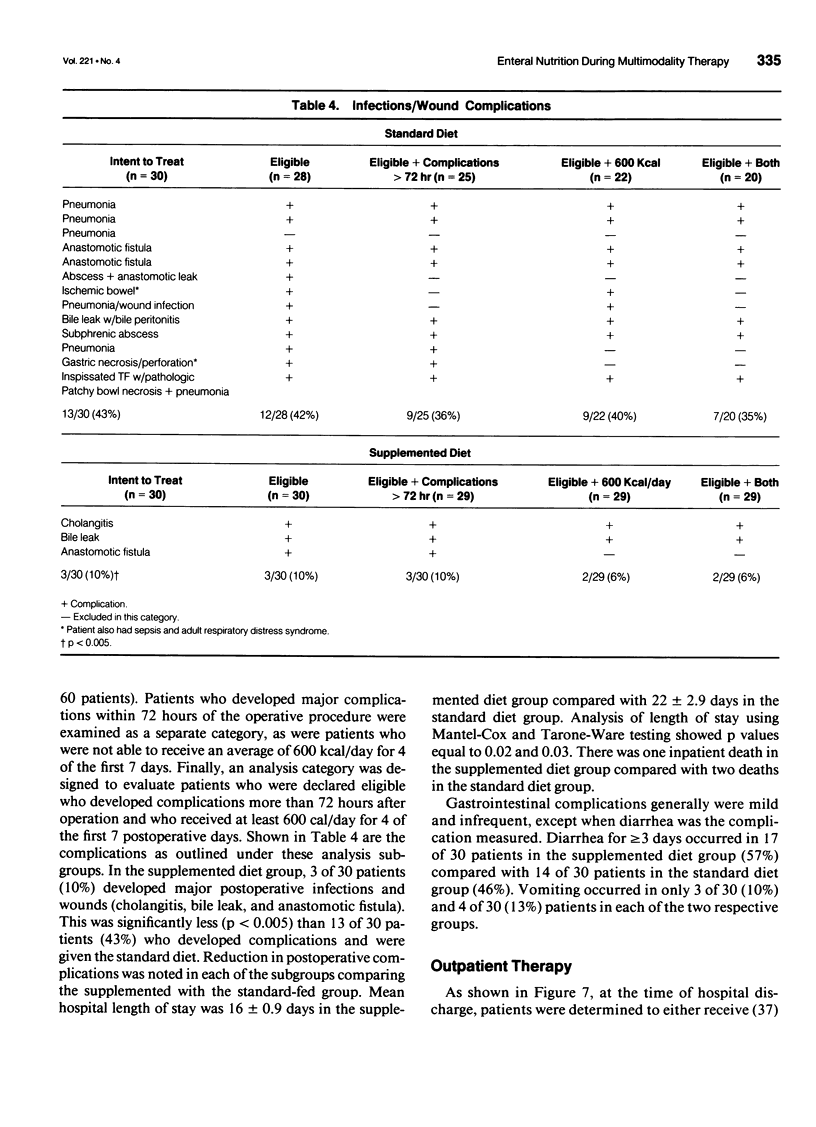
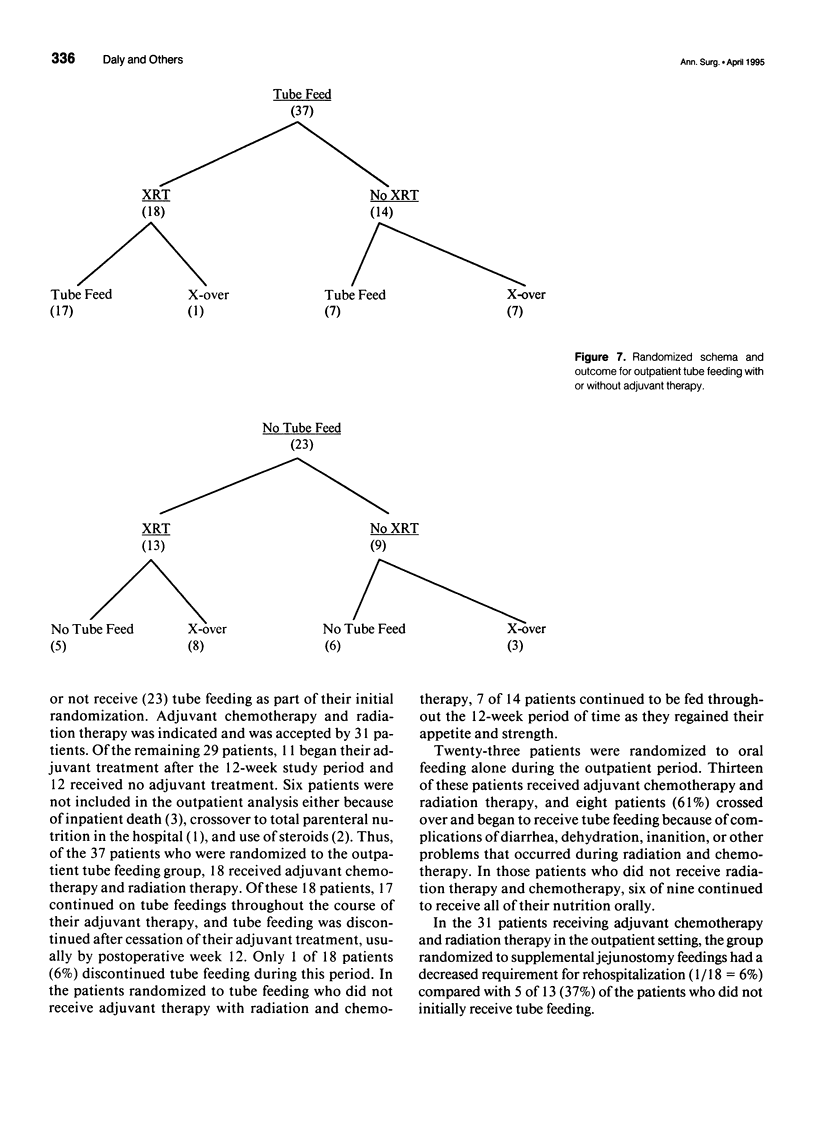
Abstract
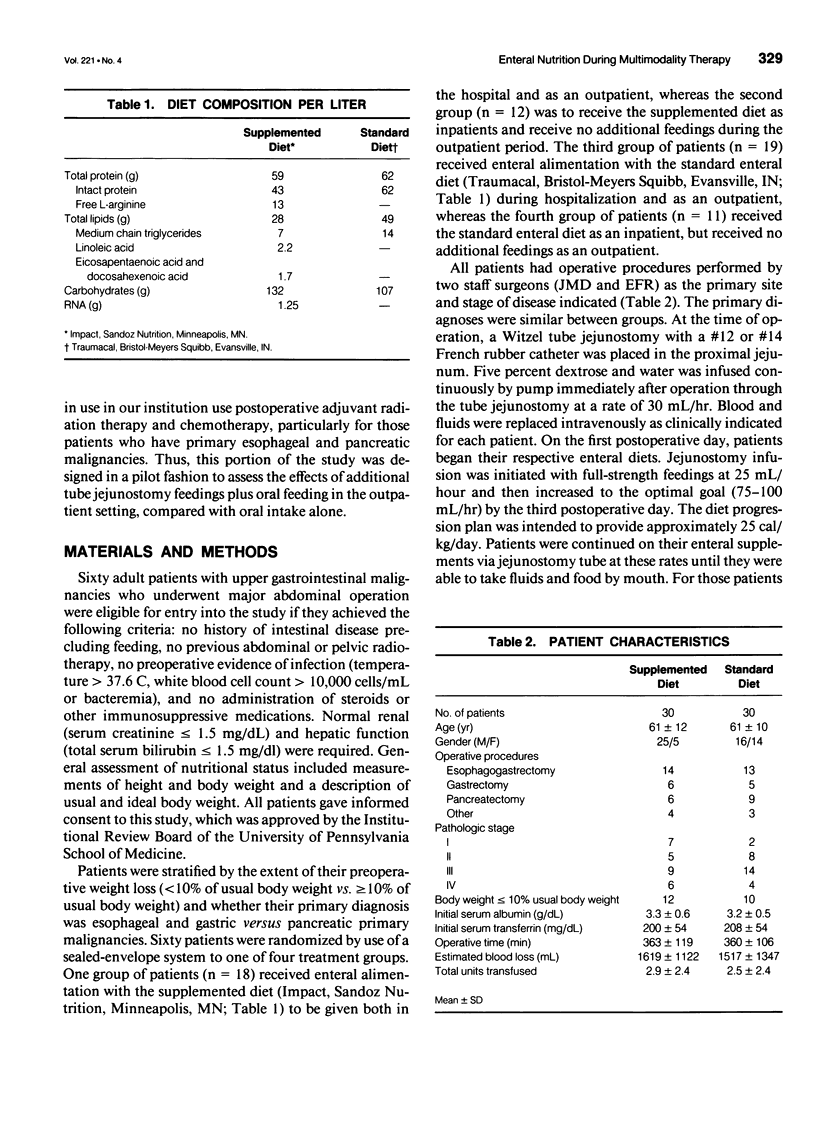
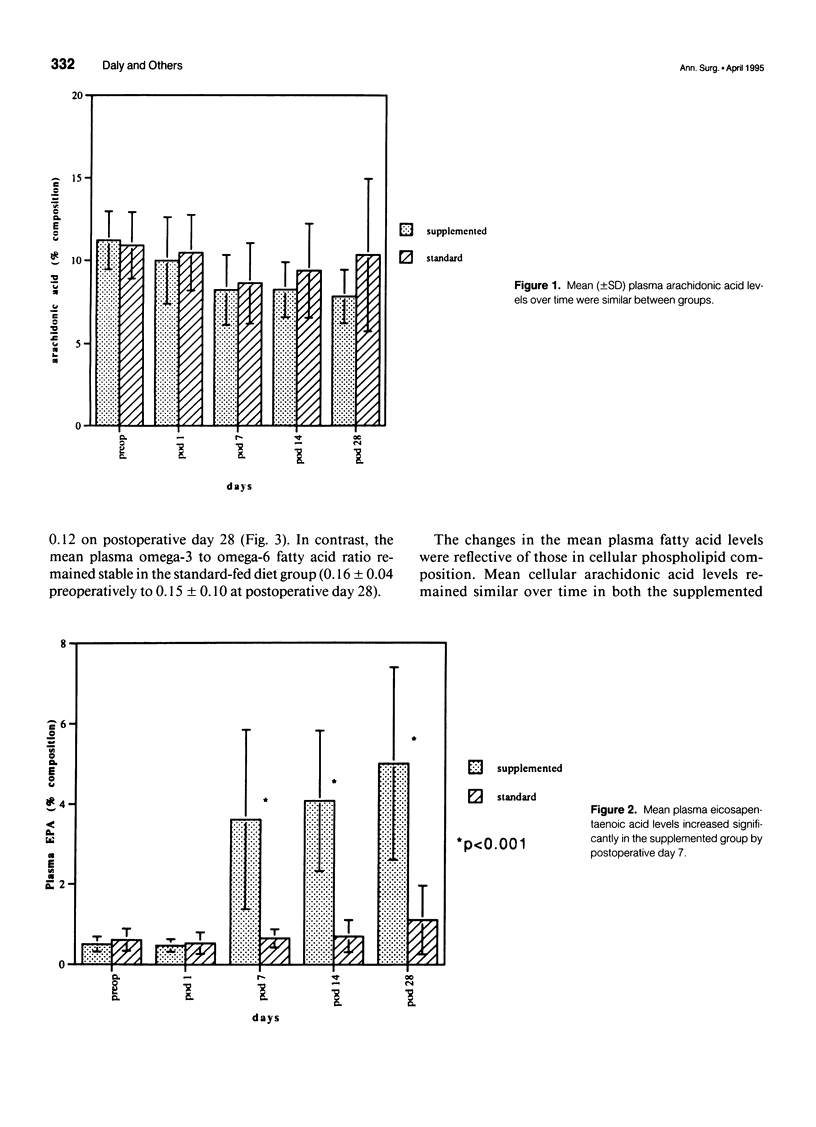
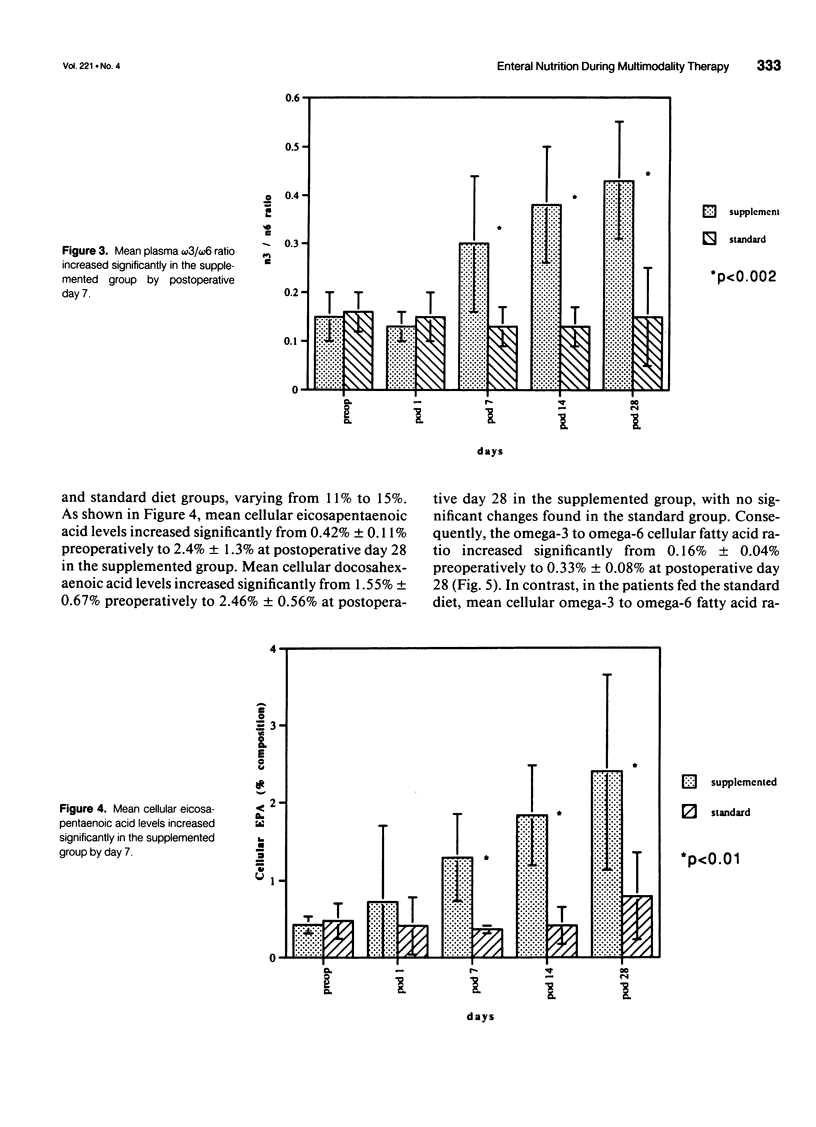
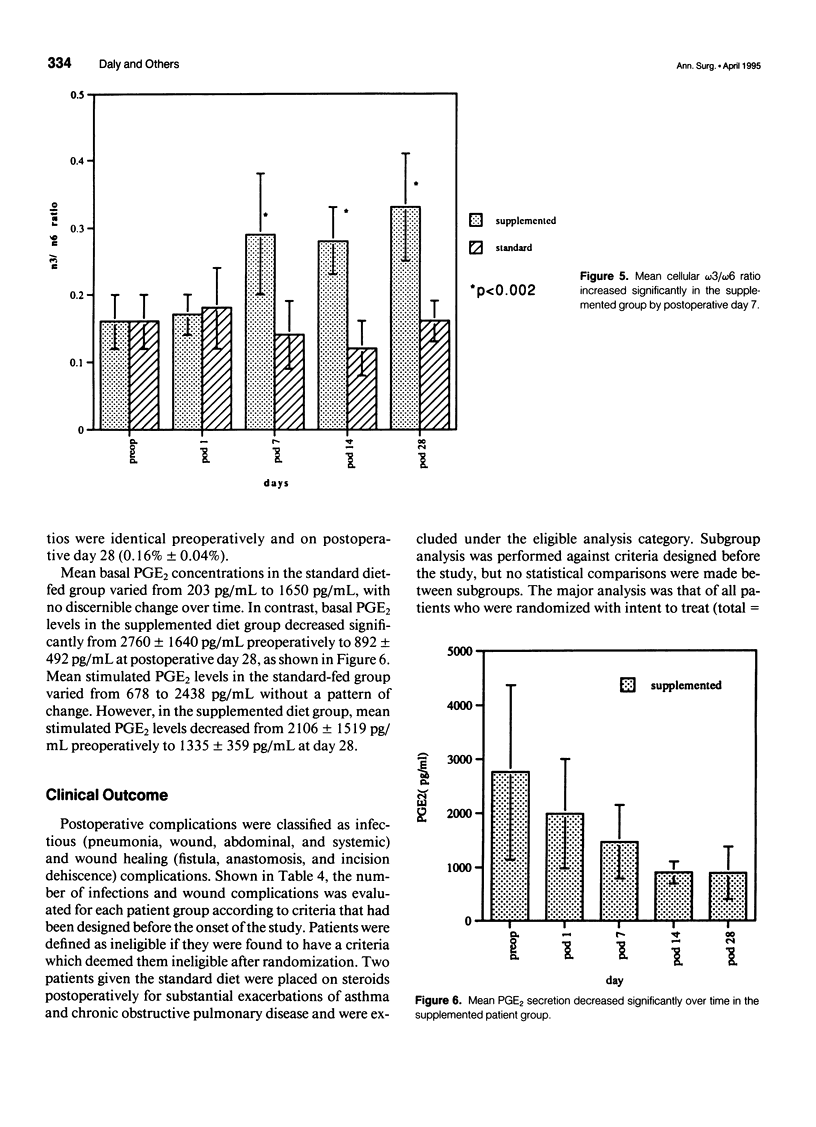
OBJECTIVE: The objective of this study was to evaluate long-term enteral nutrition support in postoperative cancer patients. BACKGROUND: Multimodality therapy for surgical patients with upper gastrointestinal malignancies may improve survival, but often results in substantial malnutrition, immunosuppression, and morbidity. The benefits of combined inpatient and outpatient enteral feeding with standard diets or diets supplemented with arginine, RNA + omega-3 fatty acids are unclear. METHODS: Sixty adult patients with esophageal (22), gastric (16), and pancreatic (22) lesions were stratified by disease site and percent usual weight and randomized to receive supplemental or standard diet via jejunostomy beginning on the first postoperative day (goal = 25 kcal/kg/day) until hospital discharge. Patients also were randomized to receive (n = 37) or not receive (n = 23) enteral jejunostomy feedings (1000 kcal/day overnight) for the 12- to 16-week recovery and radiation/chemotherapy periods. Plasma and peripheral white blood cells were obtained for fatty acid levels and PGE2 production measurements. RESULTS: Mean plasma and cellular omega 3/omega 6 fatty acid levels (percent composition) increased significantly (p < 0.05) in the arginine + omega-3 fatty acid group by postoperative day 7 (0.30 vs. 0.13) and (0.29 vs. 0.14) and continued to increase over time. Mean PGE2 production decreased significantly (p < 0.05) from 2760 to 1600 ng/10(6) cells/mL at day 7 in the arginine + omega-3 fatty acid group, whereas no significant change over time was noted in the standard group. Infectious/wound complications occurred in 10% of the supplemented group compared with 43% of the standard group (p < 0.05); mean length of hospital stay was 16 vs. 22 (p < 0.05) days, respectively. Of the patients who received postoperative chemoradiation therapy, only 1 (6%) of the 18 patients randomized to receive tube feeding did not continue, whereas 8 (61%) of the 13 patients not randomized to tube feedings required crossover to jejunostomy nutritional support. CONCLUSIONS: Supplemental enteral feeding significantly increased plasma and peripheral white blood cell omega 3/omega 6 ratios and significantly decreased PGE2 production and postoperative infectious/wound complications compared with standard enteral feeding. For outpatients receiving adjuvant therapy, those initially randomized to oral feedings alone required rehospitalization more frequently, and 61% crossed over to supplemental enteral feedings.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Saito H., Trocki O., Ogle C. K. The importance of lipid type in the diet after burn injury. Ann Surg. 1986 Jul;204(1):1–8. doi: 10.1097/00000658-198607000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986 Mar-Apr;10(2):227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Barbul A., Sisto D. A., Wasserkrug H. L., Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981 Aug;90(2):244–251. [PubMed] [Google Scholar]
- Daly J. M., Dudrick S. J., Copeland E. M., 3rd Intravenous hyperalimentation. Effect on delayed cutaneous hypersensitivity in cancer patients. Ann Surg. 1980 Nov;192(5):587–592. doi: 10.1097/00000658-198011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Lieberman M. D., Goldfine J., Shou J., Weintraub F., Rosato E. F., Lavin P. Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery. 1992 Jul;112(1):56–67. [PubMed] [Google Scholar]
- Daly J. M., Redmond H. P., Lieberman M. D., Jardines L. Nutritional support of patients with cancer of the gastrointestinal tract. Surg Clin North Am. 1991 Jun;71(3):523–536. doi: 10.1016/s0039-6109(16)45431-9. [DOI] [PubMed] [Google Scholar]
- Daly J. M., Reynolds J., Thom A., Kinsley L., Dietrick-Gallagher M., Shou J., Ruggieri B. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988 Oct;208(4):512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel W., Morrison M. H., Ayala A., Chaudry I. H. Modulation of macrophage membrane phospholipids by n-3 polyunsaturated fatty acids increases interleukin 1 release and prevents suppression of cellular immunity following hemorrhagic shock. Arch Surg. 1993 Jan;128(1):15–21. doi: 10.1001/archsurg.1993.01420130019004. [DOI] [PubMed] [Google Scholar]
- Fanslow W. C., Kulkarni A. D., Van Buren C. T., Rudolph F. B. Effect of nucleotide restriction and supplementation on resistance to experimental murine candidiasis. JPEN J Parenter Enteral Nutr. 1988 Jan-Feb;12(1):49–52. doi: 10.1177/014860718801200149. [DOI] [PubMed] [Google Scholar]
- Gottschlich M. M., Jenkins M., Warden G. D., Baumer T., Havens P., Snook J. T., Alexander J. W. Differential effects of three enteral dietary regimens on selected outcome variables in burn patients. JPEN J Parenter Enteral Nutr. 1990 May-Jun;14(3):225–236. doi: 10.1177/0148607190014003225. [DOI] [PubMed] [Google Scholar]
- Gottschlich M. M. Selection of optimal lipid sources in enteral and parenteral nutrition. Nutr Clin Pract. 1992 Aug;7(4):152–165. doi: 10.1177/0115426592007004152. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Kinsella J. E., Lokesh B., Broughton S., Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990 Jan-Feb;6(1):24–62. [PubMed] [Google Scholar]
- Kudsk K. A., Croce M. A., Fabian T. C., Minard G., Tolley E. A., Poret H. A., Kuhl M. R., Brown R. O. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992 May;215(5):503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. D., Fanslow W. C., Drath D. B., Rudolph F. B., Van Buren C. T. Influence of dietary nucleotide restriction on bacterial sepsis and phagocytic cell function in mice. Arch Surg. 1986 Feb;121(2):169–172. doi: 10.1001/archsurg.1986.01400020055006. [DOI] [PubMed] [Google Scholar]
- Law D. K., Dudrick S. J., Abdou N. I. Immunocompetence of patients with protein-calorie malnutrition. The effects of nutritional repletion. Ann Intern Med. 1973 Oct;79(4):545–550. doi: 10.7326/0003-4819-79-4-545. [DOI] [PubMed] [Google Scholar]
- Mills C. D., Shearer J., Evans R., Caldwell M. D. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992 Oct 15;149(8):2709–2714. [PubMed] [Google Scholar]
- Moore F. A., Feliciano D. V., Andrassy R. J., McArdle A. H., Booth F. V., Morgenstein-Wagner T. B., Kellum J. M., Jr, Welling R. E., Moore E. E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992 Aug;216(2):172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen J. L., Buzby G. P., Matthews D. C., Smale B. F., Rosato E. F. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann Surg. 1980 Nov;192(5):604–613. doi: 10.1097/00000658-198019250-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzini R. P., Kumar S., Kulkarni A. D., Rudolph F. B., Van Buren C. T. Dietary nucleotides reverse malnutrition and starvation-induced immunosuppression. Arch Surg. 1990 Jan;125(1):86–90. doi: 10.1001/archsurg.1990.01410130092012. [DOI] [PubMed] [Google Scholar]
- Reynolds J. V., Daly J. M., Shou J., Sigal R., Ziegler M. M., Naji A. Immunologic effects of arginine supplementation in tumor-bearing and non-tumor-bearing hosts. Ann Surg. 1990 Feb;211(2):202–210. doi: 10.1097/00000658-199002000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. V., Daly J. M., Zhang S., Evantash E., Shou J., Sigal R., Ziegler M. M. Immunomodulatory mechanisms of arginine. Surgery. 1988 Aug;104(2):142–151. [PubMed] [Google Scholar]
- Rudolph F. B., Kulkarni A. D., Schandle V. B., Van Buren C. T. Involvement of dietary nucleotides in T lymphocyte function. Adv Exp Med Biol. 1984;165(Pt B):175–178. doi: 10.1007/978-1-4757-0390-0_35. [DOI] [PubMed] [Google Scholar]
- Saito H., Trocki O., Wang S. L., Gonce S. J., Joffe S. N., Alexander J. W. Metabolic and immune effects of dietary arginine supplementation after burn. Arch Surg. 1987 Jul;122(7):784–789. doi: 10.1001/archsurg.1987.01400190050010. [DOI] [PubMed] [Google Scholar]
- Tartter P. I., Martinelli G., Steinberg B., Barron D. Changes in peripheral T-cell subsets and natural-killer cytotoxicity in relation to colorectal cancer surgery. Cancer Detect Prev. 1986;9(3-4):359–364. [PubMed] [Google Scholar]
- Waymack J. P., Rapien J., Garnett D., Tweddell J. S., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. Arch Surg. 1986 Jan;121(1):50–55. doi: 10.1001/archsurg.1986.01400010056007. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Robb E., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. II. Effect on mortality rate following septic challenge. Arch Surg. 1987 Aug;122(8):935–939. doi: 10.1001/archsurg.1987.01400200085016. [DOI] [PubMed] [Google Scholar]


