Abstract
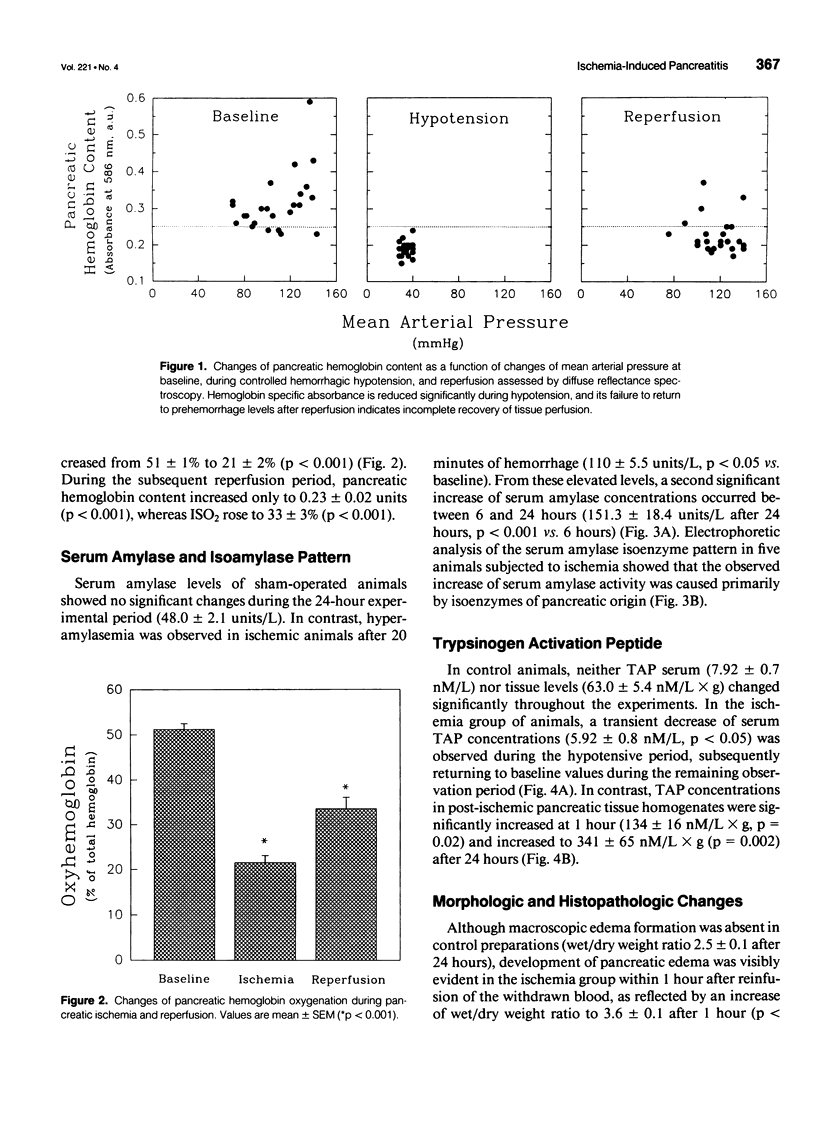
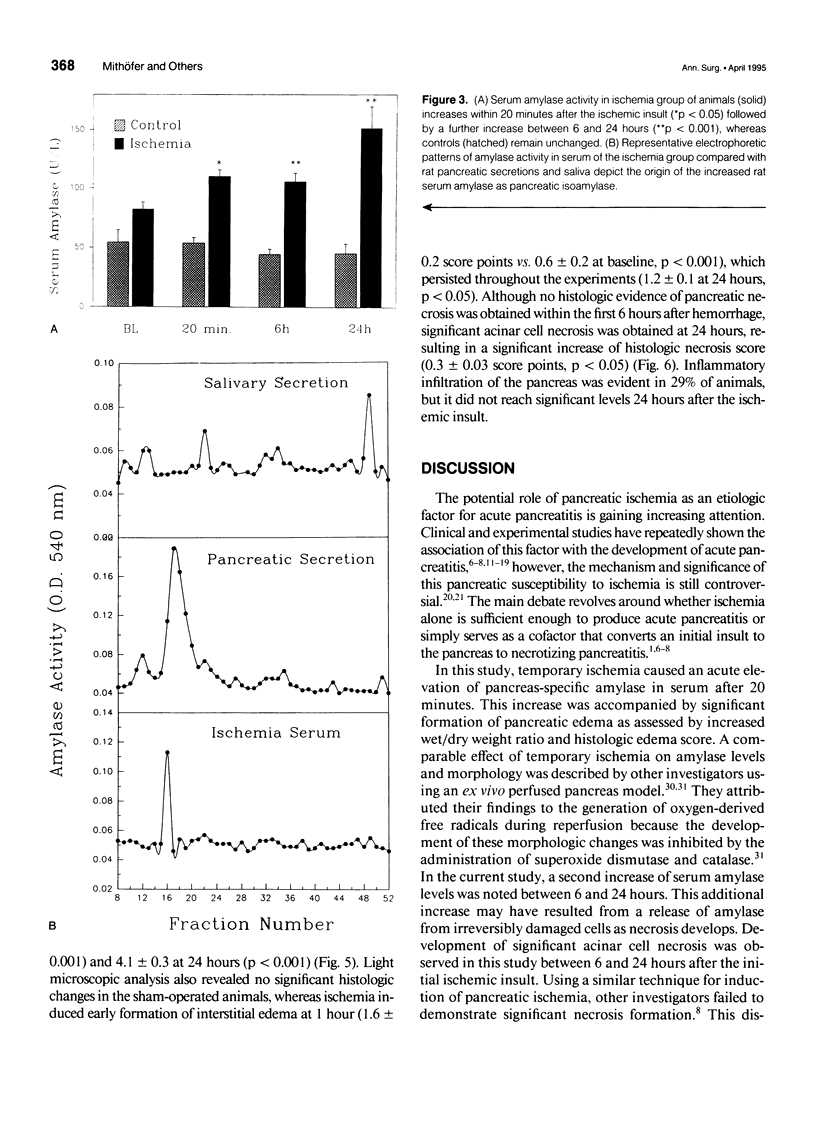
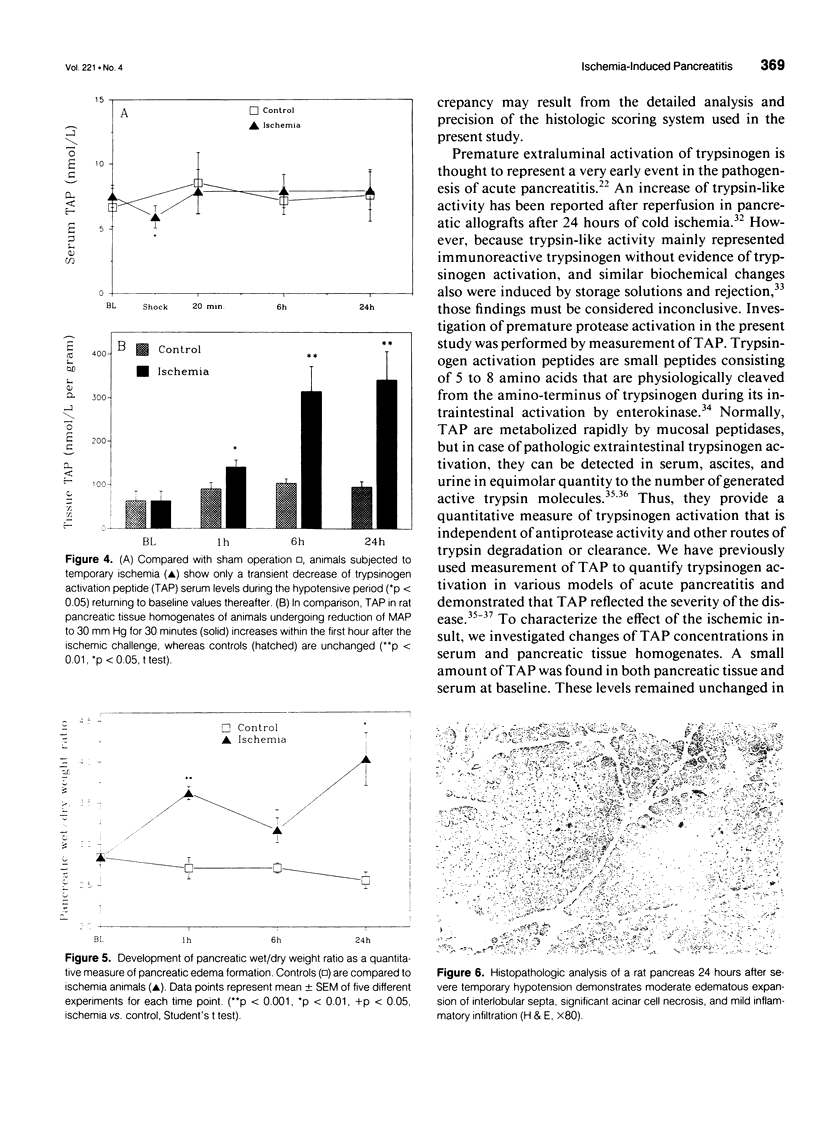
OBJECTIVE: The potential of pancreatic ischemia to cause acute pancreatitis as indicated by morphologic changes and ectopic trypsinogen activation was investigated. BACKGROUND: Experimental evidence has shown that pancreatic ischemia is important in the evolution of severe pancreatitis, but whether ischemia can initiate pancreatitis has been disputed. METHODS: Pancreatic ischemia was induced in rats by hemorrhagic hypotension (30 mm Hg for 30 min; n = 64). Changes of pancreatic microcirculatory perfusion were studied using diffuse reflectance spectroscopy. Serum amylase, trypsinogen activation peptide (TAP) in serum and pancreatic tissue, wet/dry weight ratio, and histology were determined over 24 hours and compared with sham-operated control subjects (n = 35). RESULTS: In control animals, serum amylase (47.9 +/- 2.1 units/L), serum (7.9 +/- 0.7 nmol/L) and tissue TAP (63.0 +/- 5.4 nmol/L x g), wet/dry weight ratio (2.8 +/- 0.1), and histology remained unchanged. Temporary hypotension markedly decreased pancreatic perfusion with incomplete recovery after reperfusion. Pancreatic isoamylase activity increased within 1 hour (110 +/- 5 units/L, p < 0.05) and further to 151 +/- 18 units/L at 24 hours. Tissue TAP was elevated at 1 hour (134 +/- 16 nmol/L x g, p < 0.05) and increased to 341 +/- 43 nmol/L x g (p < 0.001) after 24 hours, whereas serum TAP remained unchanged (8.3 +/- 0.5 nmol/L). Morphologic alterations included elevated wet/dry weight ratio (4.1 +/- 0.3, p < 0.01) and increased histologic scores for edema (p < 0.05) and acinar necrosis (p < 0.05) at 24 hours. Trypsinogen activation preceded the development of pancreatic necrosis. CONCLUSIONS: In addition to its potentiating role, severe pancreatic ischemia can play a pathogenetic role in the initiation of acute pancreatitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassi D., Kollias N., Fernandez-del Castillo C., Foitzik T., Warshaw A. L., Rattner D. W. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg. 1994 Sep;179(3):257–263. [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Feiner H. Pancreatitis after cardiac surgery; a morphologic study. Am J Surg. 1976 Jun;131(6):684–688. doi: 10.1016/0002-9610(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Fernández-del Castillo C., Harringer W., Warshaw A. L., Vlahakes G. J., Koski G., Zaslavsky A. M., Rattner D. W. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991 Aug 8;325(6):382–387. doi: 10.1056/NEJM199108083250602. [DOI] [PubMed] [Google Scholar]
- Fernández-del Castillo C., Schmidt J., Rattner D. W., Lewandrowski K., Compton C. C., Jehanli A., Patel G., Hermon-Taylor J., Warshaw A. L. Generation and possible significance of trypsinogen activation peptides in experimental acute pancreatitis in the rat. Pancreas. 1992;7(3):263–270. doi: 10.1097/00006676-199205000-00001. [DOI] [PubMed] [Google Scholar]
- Fernández-del Castillo C., Schmidt J., Warshaw A. L., Rattner D. W. Interstitial protease activation is the central event in progression to necrotizing pancreatitis. Surgery. 1994 Sep;116(3):497–504. [PubMed] [Google Scholar]
- Foitzik T., Lewandrowski K. B., Fernández-del Castillo C., Rattner D. W., Warshaw A. L. Evidence for extraluminal trypsinogen activation in three different models of acute pancreatitis. Surgery. 1994 Jun;115(6):698–702. [PubMed] [Google Scholar]
- Gmaz-Nikulin E., Nikulin A., Plamenac P., Hegewald G., Gaon D. Pancreatic lesions in shock and their significance. J Pathol. 1981 Nov;135(3):223–236. doi: 10.1002/path.1711350307. [DOI] [PubMed] [Google Scholar]
- Haas G. S., Warshaw A. L., Daggett W. M., Aretz H. T. Acute pancreatitis after cardiopulmonary bypass. Am J Surg. 1985 Apr;149(4):508–515. doi: 10.1016/s0002-9610(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Hurley P. R., Cook A., Jehanli A., Austen B. M., Hermon-Taylor J. Development of radioimmunoassays for free tetra-L-aspartyl-L-lysine trypsinogen activation peptides (TAP). J Immunol Methods. 1988 Jul 22;111(2):195–203. doi: 10.1016/0022-1759(88)90127-5. [DOI] [PubMed] [Google Scholar]
- Klar E., Endrich B., Messmer K. Microcirculation of the pancreas. A quantitative study of physiology and changes in pancreatitis. Int J Microcirc Clin Exp. 1990 Feb;9(1):85–101. [PubMed] [Google Scholar]
- Klar E., Mall G., Messmer K., Herfarth C., Rattner D. W., Warshaw A. L. Improvement of impaired pancreatic microcirculation by isovolemic hemodilution protects pancreatic morphology in acute biliary pancreatitis. Surg Gynecol Obstet. 1993 Feb;176(2):144–150. [PubMed] [Google Scholar]
- Klar E., Messmer K., Warshaw A. L., Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990 Nov;77(11):1205–1210. doi: 10.1002/bjs.1800771104. [DOI] [PubMed] [Google Scholar]
- Knoefel W. T., Kollias N., Warshaw A. L., Waldner H., Nishioka N. S., Rattner D. W. Pancreatic microcirculatory changes in experimental pancreatitis of graded severity in the rat. Surgery. 1994 Nov;116(5):904–913. [PubMed] [Google Scholar]
- Kyogoku T., Manabe T., Tobe T. Role of ischemia in acute pancreatitis. Hemorrhagic shock converts edematous pancreatitis to hemorrhagic pancreatitis in rats. Dig Dis Sci. 1992 Sep;37(9):1409–1417. doi: 10.1007/BF01296012. [DOI] [PubMed] [Google Scholar]
- Källén R., Borgström A. Biochemical characterization of reperfusion pancreatitis in porcine pancreatic allografts after six hours of cold storage. Transplantation. 1991 Apr;51(4):754–759. doi: 10.1097/00007890-199104000-00003. [DOI] [PubMed] [Google Scholar]
- Källén R., Montgomery A., Borgström A. Protease activation following reperfusion of porcine pancreatic allografts. J Surg Res. 1992 Nov;53(5):495–501. doi: 10.1016/0022-4804(92)90096-i. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Barenholz Y. Pancreatic hydrolases and the formation of a myocardial depressant factor in shock. Am J Physiol. 1972 Nov;223(5):1103–1109. doi: 10.1152/ajplegacy.1972.223.5.1103. [DOI] [PubMed] [Google Scholar]
- PFEFFER R. B., LAZZARINI-ROBERTSON A., Jr, SAFADI D., MIXTER G., Jr, SECOY C. F., HINTON J. W. Gradations of pancreatitis, edematous, through hemorrhagic, experimentally produced by controlled injection of microspheres into blood vessels in dogs. Surgery. 1962 Jun;51:764–769. [PubMed] [Google Scholar]
- Printz H., Saluja A., Leli U., Sengupta A., Steer M. Effects of hemorrhagic shock, aspirin, and ethanol on secretagogue-induced experimental pancreatitis. Int J Pancreatol. 1990 Apr;6(3):207–217. doi: 10.1007/BF02924289. [DOI] [PubMed] [Google Scholar]
- Prinz R. A. Mechanisms of acute pancreatitis. Vascular etiology. Int J Pancreatol. 1991 Summer;9:31–38. doi: 10.1007/BF02925576. [DOI] [PubMed] [Google Scholar]
- Redha F., Uhlschmid G., Ammann R. W., Freiburghaus A. U. Injection of microspheres into pancreatic arteries causes acute hemorrhagic pancreatitis in the rat: a new animal model. Pancreas. 1990 Mar;5(2):188–193. doi: 10.1097/00006676-199003000-00011. [DOI] [PubMed] [Google Scholar]
- Rose D. M., Ranson J. H., Cunningham J. N., Jr, Spencer F. C. Patterns of severe pancreatic injury following cardiopulmonary bypass. Ann Surg. 1984 Feb;199(2):168–172. doi: 10.1097/00000658-198402000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Fernández-del Castillo C., Rattner D. W., Lewandrowski K., Compton C. C., Warshaw A. L. Trypsinogen-activation peptides in experimental rat pancreatitis: prognostic implications and histopathologic correlates. Gastroenterology. 1992 Sep;103(3):1009–1016. doi: 10.1016/0016-5085(92)90036-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Ferńandez-del Castillo C., Rattner D. W., Lewandrowski K. B., Messmer K., Warshaw A. L. Hyperoncotic ultrahigh molecular weight dextran solutions reduce trypsinogen activation, prevent acinar necrosis, and lower mortality in rodent pancreatitis. Am J Surg. 1993 Jan;165(1):40–45. doi: 10.1016/s0002-9610(05)80402-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Rattner D. W., Lewandrowski K., Compton C. C., Mandavilli U., Knoefel W. T., Warshaw A. L. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992 Jan;215(1):44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath J. A., Jr, Gorczynski R. J., Lefer A. M. Pancreatic perfusion in the pathophysiology of hemorrhagic shock. Am J Physiol. 1974 Feb;226(2):443–451. doi: 10.1152/ajplegacy.1974.226.2.443. [DOI] [PubMed] [Google Scholar]
- Spormann H., Sokolowski A., Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989 May;184(5):507–513. doi: 10.1016/S0344-0338(89)80143-8. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987 Jan 15;316(3):144–150. doi: 10.1056/NEJM198701153160306. [DOI] [PubMed] [Google Scholar]
- Svensson L. G., Decker G., Kinsley R. B. A prospective study of hyperamylasemia and pancreatitis after cardiopulmonary bypass. Ann Thorac Surg. 1985 May;39(5):409–411. doi: 10.1016/s0003-4975(10)61945-5. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Bellini C. A., Lee K. H. Electrophoretic identification of an isoenzyme of amylase which increases in serum in liver diseases. Gastroenterology. 1976 Apr;70(4):572–576. [PubMed] [Google Scholar]
- Warshaw A. L., O'Hara P. J. Susceptibility of the pancreas to ischemic injury in shock. Ann Surg. 1978 Aug;188(2):197–201. doi: 10.1097/00000658-197808000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]



