Abstract
Heart failure (HF) remains a clinical challenge with cardiac dysfunction typically progressing even with treatment, and heart transplants only available to small numbers. We previously identified phosphoinositide 3-kinase (PI3K, p110α) as a master regulator of exercise-induced cardioprotection, and showed that gene therapy, incorporating a constitutively active form of PI3K (caPI3K) improved function of the failing mouse heart. However, this approach was not cardiac-specific and the gene therapy was challenging to manufacture. The aim of this study was to develop new PI3K-based gene therapies with more optimal properties for clinical translation. We generated and assessed adeno-associated viruses (AAV6) encoding various PI3K constructs, with different enhancers, promoters and transgene components in healthy adult male mice. The most promising AAV construct based on AAV expression, cardiac-specificity, and ease of manufacture contained a cardiac troponin T (cTnT) promoter together with a small region of the regulatory subunit of PI3K (iSH2), and an intron from the β-globin gene which enhances transcription (IVS2). This AAV (1 × 1012, 2 × 1012 vg) was administered to mice with myocardial ischemia/reperfusion injury (I/R: 1 h ischemia with reperfusion; AAV delivered 24 h post-I/R). Direct cardiac injections of PI3K-based AAVs were also performed in healthy adult female sheep. I/R mouse hearts treated with the AAV6-cTnT-IVS2-iSH2 displayed increased phosphorylation of Akt, but no improvement in cardiac function or structure was observed. AAV6-cTnT-IVS2-iSH2 successfully transduced healthy sheep hearts which increased endogenous PI3K catalytic activity. Further testing/optimization of the AAV (time of delivery and/or duration) will be required to assess the therapeutic potential of this approach.
Keywords: Heart failure, AAV, PI3K, Gene therapy, Mouse, Large animal
Graphical abstract
1. Introduction
Heart failure (HF) is a debilitating condition, which directly contributes to a lower quality of life and increased mortality and morbidity on a global scale. As life expectancy increases, so too does the burden on the heart and in turn the prevalence of HF and other life-threatening cardiac conditions. Five-year survival rates following a diagnosis of HF have been reported at less than 50 % [1], demonstrating the urgent need for the development of novel therapeutics.
The importance of exercise as both a preventative measure and a treatment for HF has been well established and physical inactivity has been shown to be a primary risk factor in the development of HF [[2], [3], [4]]. A large body of evidence also demonstrates that following a diagnosis of HF, the use of physical activity as a therapeutic intervention can be of benefit to patients by reversing cardiac remodeling, reducing mortality, decreasing hospitalization rates, and improving quality of life [[5], [6], [7], [8], [9]]. Unfortunately, due to the debilitating nature of HF, exercise therapy is not always an option. This is especially so in the later stages of the disease when the physiological impact of decreased cardiac function is greatest. Exercise compliance can also be low in patients with HF. While no therapeutic intervention is likely to mimic all the advantageous systemic effects of exercise, the development of therapies that can mimic the protective effects of exercise on the heart may provide significant benefit, particularly if this approach also allows patients with HF to increase their physical activity.
Phosphoinositide 3-kinase (PI3K, p110α) is a heterodimeric lipid kinase consisting of a regulatory subunit (p85) and a catalytic subunit (p110α). Activation of PI3K and subsequent downstream signaling requires interaction between the two subunits and the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). Akt is a key downstream effector of PI3K(p110α) which is phosphorylated when PI3K is activated and has been implicated in mediating cardiac protection [10]. In vitro studies have demonstrated that a small fragment of the regulatory subunit called inter-SH2 (iSH2) is sufficient and necessary for binding to the catalytic subunit p110α and the conversion of PIP2 to PIP3 [11]. Our laboratory has extensively demonstrated that PI3K is a critical mediator of exercise-induced physiological cardiac hypertrophy and cardioprotection [[12], [13], [14], [15], [16], [17]]. Exercise training induces an increase in PI3K activity in the mouse heart [18], and chronic exercise training is associated with physiological/adaptive cardiac hypertrophy in animal models [10]. Furthermore, transgenic mice with increased PI3K activity due to expression of a constitutively active form of PI3K (caPI3K) display physiological heart growth irrespective of exercise status, and display cardioprotection following a variety of cardiac insults including non-ischemic interventions (e.g. pressure overload, diabetes) and ischemic interventions (e.g. myocardial infarction) [[15], [16], [17],19]. The caPI3K transgene, also referred to as iSH2p110, consists of the iSH2 domain of p85 fused to p110α with a glycine linker and increases Akt phosphorylation in the mouse heart [20].
We previously reported improved cardiac function in a mouse model of pressure overload following administration of an AAV containing the same caPI3K construct used to generate the caPI3K transgenic mice, and attenuation of cardiac dysfunction in models of diabetes [17,21,22]. These findings demonstrated the potential of activating a key regulator of exercise-induced protection, PI3K, with a gene therapy to provide cardiac protection [17,21,22]. The development of a clinically viable AAV therapeutic product requires optimization to address factors such as cardiac specificity, vector transduction capacity and the cost of drug development. A disadvantage of the original caPI3K-AAV was that expression was driven by a promoter (cytomegalovirus, CMV) that was not cardiac-specific, and AAV yields were low due to the large construct size (~5.15 kb; maximum AAV capacity ~5.2 kb) [23,24]. Another broad challenge in the gene therapy field (independent of the gene being targeted), is the efficient transduction of the large animal heart and human heart in comparison to small animals [25]. The key aims of this study were to: 1) generate a commercially viable cardiac-specific gene therapy which could mimic the protective effects of PI3K, 2) assess whether the new gene therapy provides protection in a mouse model of I/R injury, and 3) in parallel evaluate cardiac AAV expression of the original caPI3K-AAV and new AAV gene constructs in a large animal model.
2. Materials and methods
2.1. Generation of AAVs
To improve cardiac-specificity and transduction efficiency, we incorporated a cardiomyocyte-specific cardiac troponin T promoter (cTnT) and an enhancer element into new AAV constructs. Expression cassettes (described in detail below) were cloned into a plasmid containing inverted terminal repeat (ITR) regions from AAV2 [26] (Fig. 1). Recombinant AAV6 vectors were produced by co-transfecting HEK293T cells with the corresponding plasmids and pDGM6 [26], a plasmid encoding AAV capsid proteins and adenoviral genes required for the packaging and production of functional AAV6 vectors. Purified AAV vectors were titered by qPCR using primers to detect either the cTnT477 promoter (Forward: CATGACTGTTCCCTGCATATC, reverse: TAAACCAGCTGTCCCTCTT) or the CMV promoter (Forward: GCGGTAGGCGTGTACGGTGG, reverse: CGTGGATGGCGTCTCCAGGC) by the Muscle Research and Therapeutics Laboratory, University of Melbourne.
Fig. 1.
PI3K-based constructs. A) AAV constructs encoding full length constitutively activated PI3K (caPI3K, also referred to as iSH2p110), driven by the CMV promoter (upper) or cTnT promoter (lower). B) Truncated PI3K constructs encoding the iSH2 fragment of p85, driven by either the CMV promoter (left) or cTnT promoter (right). C) Truncated PI3K constructs encoding the iSH2 fragment of p85 together with the intron IVS2, driven by either the CMV promoter (left) or cTnT promoter (right). D) Gel electrophoresis image of linearized plasmids containing the PI3K-based constructs or empty control constructs that were inserted into AAV6 vectors.
An AAV6 containing a caPI3K construct with a CMV promoter was produced (AAV6-CMV-caPI3K) as previously described [17] for comparison with five new PI3K-based constructs, which were designed to increase PI3K activity specifically in cardiac myocytes (through the inclusion of a cardiomyocyte-specific cardiac troponin T promoter, cTnT477) and/or enhance transcription of the AAV genome through the inclusion of an intronic element from the rabbit β-globin gene [27]. The truncated cTnT promoter consisted of 477 nucleotides from the promoter region of human TNNT2 (gcggccgcctcgagtctgctcccagctggccctcccaggcctgggttgctggcctctgctttatcaggattctcaagagggacagctggtttatgttgcatgactgttccctgcatatctgctctggttttaaatagcttatctgctagcctgctcccagctggccctcccaggcctgggttgctggcctctgctttatcaggattctcaagagggacagctggtttatgttgcatgactgttccctgcatatctgctctggttttaaatagcttatctgagcagctggaggaccacatgggcttatatggggcacctgccaaaatagcagccaacacccccccctgtcgcacattcctccctggctcaccaggccccagcccacatgcctgcttaaagccctctccatcctctgcctcacccagtccccgctgagactgagcagacgcctccaggatctgtcggcagctaattaat).
Construct 1) AAV6-cTnT-caPI3K: The ubiquitous CMV promoter within AAV6-CMV-caPI3K was replaced with the cTnT477 promoter to restrict expression to cardiomyocytes (Fig. 1A).
Constructs 2 & 3) AAV6-CMV-iSH2 & AAV6-cTnT-iSH2: iSH2, a region of the PI3K regulatory subunit p85 that interacts with the p110α catalytic subunit and is necessary and sufficient for its activation [11] was derived from the Mus musculus sequence for Pik3r1 (encoding p85α). Sequences encoding a PGG hinge and an HA tag were added to the C′ end as previously described [11]. In addition, a Kozak consensus sequence was inserted immediately upstream of the ATG start site [28]. iSH2 and IVS2-iSH2 sequences were synthesized by Genscript and subcloned into pAAV6-CMV-MCS-SpA [17] or pAAV6-cTnT477-MCS-SpA (see Supp Figs. 1–5). iSH2 is significantly smaller than the caPI3K transgene (~0.75 kb compared to ~3.9 kb; Fig. 1B).
Constructs 4 & 5) AAV6-CMV-IVS2-iSH2 & AAV-cTnT-IVS2-iSH2: Identical to constructs 2 & 3, but with an intron (IVS2) inserted immediately upstream of the Kozak sequence [28] and ATG start site. IVS2 is rabbit β-globin intron 2 and has been demonstrated to improve transgene expression [27]. The size of IVS2 + iSH2 combined is ~1.32 kb compared to the ~3.9 kb caPI3K transgene (Fig. 1C).
Further details and sequences of all constructs are provided within the data supplement (Supp Figs. 1–5).
2.1.1. Amplification of plasmid DNA for AAV production
To amplify plasmids in bacterial cells, plasmid DNA was combined with DH5-α cells and incubated on ice for 30 min, followed by heat shock at 42 °C for 50 s and incubation on ice for an additional 2 min. Cell/plasmid suspensions were made up to 1 mL with pre-warmed Luria broth (LB) and incubated with orbital agitation for 1 h (37 °C, 225 RPM). Cells were pelleted (4500 RPM, 2 min), the supernatant removed, and cells resuspended in LB and incubated overnight on LB agar plates containing ampicillin. The next day, colonies were spread on new LB agar plates and incubated for an additional 6 h (37 °C). Aliquots of LB broth (4 mL) containing 1 % ampicillin were inoculated with a scrape of cells from each colony, and incubated overnight with orbital agitation (37 °C, 225 RPM). Plasmid DNA was purified using the QIAGEN miniprep kit according to the manufacturer's instructions. To verify the identity of selected colonies and mini-cultures, restriction digests were performed using HindIII (to linearize plasmids) or MscI (to generate two fragments of known size), according to the manufacturer's instructions (New England Biolabs). Digested DNA samples were separated on 0.7 % agarose gels at 100 V for 45 min alongside a 2-log ladder. Validated plasmids were amplified by inoculating LB with mini-cultures for overnight incubation at 37 °C, 225 RPM. Plasmid DNA was purified using QIAGEN maxiprep kits, as per the manufacturer's instructions.
2.1.2. Large-scale AAV6 vector production and purification
HEK 293 T cells were maintained at 37 °C, 5 % CO2 in Dulbecco's Modified Eagle's Medium (DMEM) containing penicillin/streptomycin, 2 mM l-glutamine and 10 % foetal bovine serum. For each vector preparation, twenty-five 15 cm cell culture dishes were seeded at a density of 7.875 × 106 cells/plate and incubated at 37 °C, 5 % CO2 overnight.
Transfection solutions- One aliquot each of Solutions A and B were prepared as outlined below and sterilised (0.22 μm filter). Solution A (total volume up to 900 μL with ddH2O: pAAV6 (22.5 μg), pDGM6 (45 μg), 2 M CaCl2 (112.5 μL)). Solution B (total volume: 900 μL): 2× HEPES-buffered saline, pH 7.05 (280 mM NaCl, 50 mM HEPES) (891 μL), phosphate mix, pH 7.05 (49.5 mM NaH2PO4, 100.5 mM Na2HPO4) (9 μL). To make the transfection solution, Solution B was continuously vortexed while Solution A was slowly added. The resulting solution was vortexed for an additional 15 s and incubated at RT for 15 min. Every 5 min, 4 μL was pipetted onto a cell culture dish and examined under a microscope at 100× magnification. If small black flecks of precipitate were observed, a greater volume of transfection solution was made (i.e. enough for the remaining 24 plates). If particles were too large, the solution was discarded and prepared again using a smaller volume of phosphate mix.
Cells were transfected at 50–60 % confluency. 1.8 mL of the transfection solution was pipetted onto each plate of cells and the plates swirled to mix. Cells were incubated at 37 °C, 5 % CO2 for 72 h, with a switch to serum-free media 24 h post-transfection. Cells producing rAAV vectors take on a spherical morphology and dislodge from the cell culture plate. To harvest vector, the media was collected and the plate vortexed to dislodge any remaining cells. Viral capsids were released from cells via a process of rapid freezing and thawing. Each tube of cells was frozen in a methanol bath containing dry ice, then warmed in a water bath at 37 °C. This process was repeated three times to disrupt cell membranes. The resulting supernatant was homogenised using a Model M-110L Microfluidizer Processor (Microfluidics, MA, USA) and passed through a 0.22 μm filter prior to purification on an HPLC column, as previously described [29]. Each preparation yielded approximately 1 mL of purified vector.
2.2. Animal care and experimentation
Mice care and experimental protocols were approved by the Alfred Research Alliance Animal Ethics Committee (application number E/1964/2019/B & E/1797/2018/B) and performed in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia, 8th edition, 2013). All AAV6 vectors in all protocols were systemically administered via tail vein injection (29G needle, 150 μL) to conscious male wild type C57BL/6 mice generated at the People And Cures (PAC) facility through the Alfred Research Alliance (Melbourne, Victoria, Australia).
All aspects of sheep animal care and experimentation were conducted in accordance with the guidelines of the Animal Ethics Committee of the Florey Institute of Neuroscience and Mental Health (application number 15-086-FINMH) and the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia, 8th edition, 2013).
2.3. Experimental protocols
For all mouse studies, mice were randomized using an automated method and exclusions were illustrated in flow charts [30].
2.4. Assessment of cardiac AAV expression and PI3K signaling with iSH2 ± IVS2
Following randomization and blinding, 8-week-old male C57Bl/6 mice were administered 2 × 1011, 1 × 1012 or 2 × 1012 vector genomes (vg) of AAV6-CMV-iSH2 or AAV6-CMV-IVS2-iSH2. Additionally, 2 × 1011 vg of an empty control vector (AAV6-CMV-Con) or saline were administered as controls. Mice were euthanized 8 weeks post-AAV administration.
2.5. Assessment of cardiac specificity and expression driven by the cTnT477 promoter versus CMV promoter
Following randomization and blinding, 8-week-old male C57Bl/6 mice were administered 2 × 1011, 1 × 1012 or 2 × 1012 vg AAV6-cTnT-caPI3K or 2 × 1011 vg of AAV6-CMV-caPI3K. 2 × 1011 vg of a control vector (AAV6-CMV-Con) or saline were administered as controls. Mice were euthanised 8 weeks post-AAV administration.
2.6. Assessment of AAV6-cTnT-IVS2-iSH2 in healthy adult mice
Following randomization and blinding, 8-week-old male C57Bl/6 mice were administered 2 × 1012 vg AAV6-cTnT-IVS2-iSH2 or saline. Mice were euthanised 8 weeks post-AAV administration. All animal exclusions are outlined in Supp Fig. 6.
2.7. Assessment of AAV6-cTnT-IVS2-iSH2 in a mouse model of ischemia/reperfusion injury
Ten-week old male C57Bl/6 mice underwent baseline echocardiography prior to cardiac-ischemic reperfusion (I/R) surgery (ligation of the left coronary artery for 60 min followed by reperfusion) as previously described [31]. Immediately following myocardial I/R surgery, a three-lead ECG recording of each mouse was used to confirm ST-segment elevation (and successful ligation). At 24 h post-surgery, mice were anaesthetized with isoflurane (induction: 3–4.5 % in room air, maintenance: 1.6–1.8 % in room air) and placed on a heated platform. Parasternal long-axis cine loops were obtained by an ultra-high frequency ultrasound probe (MS-550D) using the Vevo® 2100 or UHF57x probe with the F2 System (Visualsonics, Fujifilm, Canada). Analysis was performed using the manufacturer's VevoLAB software to discern inactive from active relative radial tissue displacement in the long axis. Inactive/zero relative tissue displacement provides a rigorous surrogate for ischemic area/infarct size and is used to exclude mice with small/irregular infarctions (resulting from e.g. missed ligation or collateral branching of the coronary arteries). Mice with ischemic area (tissue displacement) of 45 ± 10 % of the left ventricle were included in the study (N = 5 excluded) [32]. Following randomization and blinding, mice were subsequently administered 2 × 1012 vg or 1 × 1012 vg AAV6-cTnT-IVS2-iSH2, AAV6-cTnT-Con or saline. Mice underwent echocardiography followed by pressure-volume (PV) loop analysis and euthanasia 12-weeks post-surgery. All animal exclusions are outlined in Supp Figs. 7–8.
2.8. Echocardiographic assessment of left ventricular structure and function
Echocardiographic studies of the LV were performed using the same ultrasound system and probes described above to acquire parasternal long-axis cine loops of the LV. Analysis was performed using the manufacturer's VevoLAB software for assessment of systolic LV function. Image acquisition and data analyses were performed blinded, and all analyses were performed and validated by two independent observers.
2.9. Pressure-volume loop analysis
Mice were anaesthetized with isoflurane (3–4.5 % induction, 1.6–1.8 % maintenance) and placed in a supine position. An incision was made in the neck, and a Millar pressure-volume catheter (model SPR-839) was inserted into the right common carotid artery and advanced until the tip was just above the aortic valve. Steady-state blood pressure measurements were made, then the transducer was guided through to the LV to measure LV pressure and volume parameters. To determine end-systolic and end-diastolic PV relationships, the abdominal vena cava was occluded by gently pressing a cotton tip through the abdomen for 7–10 heart beats. Parallel conductance was corrected for by bolus I.V. infusion of 20 % w/v hypertonic saline (via the jugular vein). Alpha-calibration of the volume trace was performed by software adjustments for endpoint echocardiographic measures (cardiac output) for each animal.
2.10. Tissue dissection and morphological analysis
2.10.1. Healthy adult male mice
Animals were anaesthetized (pentobarbitone, 300–400 mg/kg i.p.) and euthanised by cervical dislocation. The atria were removed from the heart; the ventricles dissected (for protein extraction) and snap frozen in liquid nitrogen.
2.10.2. Adult male mice subjected to I/R injury
Prior to dissection, mice were anaesthetized with isoflurane as described above. PV loop measurements were performed followed by euthanasia through cardiac puncture and cervical dislocation. Hearts were removed, weighed and the apex and base were snap frozen in liquid nitrogen. The mid-ventricle was used for histology.
For all cohorts of mice, heart, atria, lungs, skeletal muscle, kidney, brain, liver and spleen were weighed and snap frozen for tissue analyses. Lower limbs were collected, digested (1 M NaOH for 6 h, 37 °C) and tibia measured with a Vernier calliper.
2.10.3. Histological analysis – cardiac fibrosis
From the I/R cohort, thinly sliced mid-ventricle transverse/short axis rings (1–2 mm) were dissected from the LV inferior to the ligation, and placed into cassettes for fixation in 10 % Neutral Buffered Formalin for 24 h. Subsequent tissue processing was performed by the Monash Histology Platform (Clayton, VIC). Tissue was processed, paraffin embedded and sectioned at a thickness of 4 μm and stained with Masson's Trichrome to measure interstitial fibrosis. Images were acquired by the Monash Histology Platform or in-house with bright field scanning using a 20× objective. The ImageJ package Fiji was used to perform colour deconvolution and objective measurement of area positivity for collagen staining (fibrosis, blue) as a percentage of total tissue (blue and red) area. All analyses were performed blinded.
2.10.4. Immunofluorescence of HA-tagged iSH2 in cardiomyocytes
Paraffin-embedded mouse and sheep heart samples were sectioned (4 μm) and mounted onto Superfrost slides (Epredia #J1800AMNZ). Sections were dewaxed in xylene (3 × 10 min), xylene/ethanol (1:1, 5 min), rehydrated in 100 % ethanol, 95 % ethanol, and 70 % ethanol (2 × 5 min each), and sections rinsed in Milli-Q water (2 × 2 min). Sections were submerged in citrate buffer (10 mM, pH 6.0, Sigma-Aldrich #S1804) and microwaved (12 min) for heat-induced antigen retrieval, put on ice (10 min), microwaved (3 min), allowed to cool to RT (1 h) and air-dried (3 min). Sections were then incubated with 0.1 % pepsin (Sigma-Aldrich #P6887) in 0.01 N HCl (20 min) followed by washes in Milli-Q water (3 × 30 s). Slides were washed with PBS (2 × 2 min, 1 × 10 min), incubated in 1 % goat serum (PBS,0.4 % Triton X-100) for 10 min, and blocked with 5 % BSA in PBS containing0.2 % (w/v) gelatin and 0.25 % Triton X-100 at RT (1 h) followed by washes in PBS (3 × 10 min). The HA-tag primary antibody (C29F4, Cell Signaling #3724S, 1:800) was diluted in 1 % BSA in PBS containing 0.2 % (w/v) gelatin and 0.25 % Triton X-100 and incubated on the slides overnight at 4 °C. The samples were then washed with PBS (3 × 30 min) followed by the secondary antibody (Alexa Fluor 647 Goat Anti-Rabbit, Jackson ImmunoResearch #111-605-003, 1:142, diluted in 1 % BSA) for 4 h at RT, followed by PBS (3 × 30 min). Troponin T-C Antibody (cTNT) (10 μg/mL, Santa Cruz #sc-20025) was used to stain the myocytes and NucBlue™ Live ReadyProbes™ Reagent (Hoechst 33342, ThermoFisher #R37605) for nuclei labelling in 1 % BSA, overnight at 4 °C. The samples were then washed with PBS, mounted in Vectashield (Vector Laboratories #H-1000-10) and stored at 4 °C. Slides were imaged using the Leica Stellaris 8 confocal microscope (Leica Microsystems) with Z-stack imaging performed and the images was then collapsed using Leica Software (Leica Microsystems).
2.11. Western blotting analysis
Protein extraction and quantitation was performed as previously described [33]. Protein lysates from cardiac tissue containing 75–100 μg of protein were separated by SDS-PAGE. Protein was immobilized on a polyvinylidene difluoride (PVDF) membrane (MERCK, Frankfurt, Germany) using a wet tank transfer for 16 h, 9 V, 4 °C. Membranes were subsequently blocked in 5 % skim milk powder in Tris-buffered saline and 0.1 % Tween 20 (TBST). Membranes were probed with the following antibodies: HA-tag (C29F4) (Cell Signaling #3724, 1:2000), α-tubulin (Cell Signaling #2144, 1:5000) p-AKT (Ser473) (Cell Signaling #9271, 1:500) and AKT (Cell Signaling #9272, 1:1000). Membranes were incubated with anti-rabbit antibodies conjugated to HRP. Proteins were visualized using SuperSignal® West Pico PLUS Chemiluminescent Substrate (Thermo Scientific). Western blots were imaged using the G:BOX gel doc system (SYNGENE) and quantitation was performed using the GeneTools software (SYNGENE). All raw Western blot images are provided within the data supplement.
2.12. Immunoprecipitation & lipid kinase activity assay analysis
A lipid kinase activity assay was employed to assess PI3K activity in heart tissue as previously described [20]. 250 μg (I/R cohort) or 500 μg (healthy mice cohorts) of protein was immunoprecipitated using a HA-tag antibody (C29F4) (Cell Signaling #3724) and Protein A Sepharose CL-4B beads (GE Healthcare, 17-0780-01) according to the manufacturer's instructions. Beads were washed twice in HEPES buffer (1 M HEPES, 40 mM EDTA, 1 M DTT and 0.015 % IGEPAL) and split evenly into two separate tubes. A 1:1 ratio of phosphatidylinositol and phosphatidylserine stored in chloroform (10 μL) was evaporated into gel pellets using a SpeedVac vacuum concentrator (ThermoFisher), sonicated in 130 μL of HEPES buffer and mixed with one half of the immunoprecipitated protein attached to Sepharose beads. ATP and 10 μCi of [γ-32P]-ATP (PerkinElmer, BLU502A250UC) were subsequently added to the Sepharose beads. A reaction was generated by shaking (1400 RPM) for 10 min at 25 °C and stopped with 2 M HCl. 160 μL of 1:1 chloroform-methanol mixture was added to extract the lipids. The organic phase was collected (phospholipids) and resolved using a thin liquid chromatography (TLC) plate (Merck, 1.05553.0001) overnight in an acid solvent consisting of 65:35 isopropanol: 2 M acetic acid. The TLC plate was dried and added to a cassette underneath autoradiography film (GE Healthcare, 28906845) at −80 °C. The radiolabel signal was standardized against immunoglobulin G signals by running Western blots from the second half of the immunoprecipitate from each sample. All raw activity images are provided within the data supplement.
2.13. Direct cardiac AAV injection and dissection of healthy sheep
AAV was administered to three adult ewes (~40 kg) via direct cardiac injection. Briefly, sheep were anaesthetized with isoflurane, intubated and placed on artificial respiration [34]. The heart was exposed from the left lateral position, the pericardium was opened and 1–2 cm diameter circles were sutured into isolated positions on the surface of the LV myocardium, above and below the second branch of the left anterior descending artery, to mark sites for direct cardiac injection. 5 × 1012 to 1 × 1013 vg of AAV was administered via 10–15–20 μL injections evenly distributed within each sutured circle directly into the myocardium on a 45° angle. The pericardium, intercostal muscle, subcutaneous tissue and skin were closed, anaesthetic removed, and the animal recovered. Approximately 6 weeks following AAV administration sheep were euthanized, the heart was removed and myocardium tissue from the sutured sites and remote regions were snap frozen in liquid nitrogen for analysis.
-
•
Sheep #1 received 5 × 1012 vg of both AAV6-CMV-caPI3K and AAV6-cTnT-caPI3K at separate sites on the LV.
-
•
Sheep #2 received 1 × 1013 vg of both AAV6-CMV-iSH2 and AAV6-CMV-IVS2-iSH2 at separate sites on the LV.
-
•
Sheep #3 received 1 × 1013 vg of AAV6-CMV-iSH2, AAV6-cTnT-iSH2 & AAV6-cTnT-IVS2-iSH2 at separate sites on the LV.
2.13.1. Sheep LV DNA isolation and quantification of vector genomes
DNA isolation- Genomic DNA was isolated from frozen LV heart sheep samples using the methodologies previously outlined [35]. Briefly, small pieces of the samples were put in a lysis buffer (100 mM Tris–HCl, pH 8.5, 5 mM EDTA, 200 mM NaCl, 0.2 % SDS and 0.1 mg/mL of proteinase K) and homogenised using tissue processor (PRO200 Pro Scientific) for 10 s. Samples were incubated at 55 °C with shaking at 1400 rpm for 2 h, followed by overnight incubation at 55 °C to facilitate enzymatic digestion of proteins and other non-nucleic acid cellular components. DNA extraction was purified twice using phenol:chloroform:isoamyl alcohol (25:24:1, pH 8.0) followed by chloroform. After centrifugation, the aqueous phase containing DNA was subjected to ethanol precipitation by adding 2.5 volumes of cold absolute ethanol and 0.1 volume of 3 M sodium acetate. The DNA was left to precipitate overnight at −20 °C. The DNA pellet was collected by centrifugation and washed twice with 75 % ethanol to remove residual salts and impurities. It was then air-dried and resuspended in nuclease-free water, followed by incubation overnight at 4 °C. DNA was quantified on a Nanodrop™ Spectrometer (Thermo Fisher Scientific).
Vector genomes - To quantify AAV vector genomes in the sheep heart samples (#1–3), qPCR was conducted using 100 ng of genomic DNA on a real-time PCR instrument (Biorad CFX Opus 96) using SensiFAST™ SYBR® No-ROX Kit, following the manufacturer's protocol. Primers targeting iSH2 were used (forward, 5′-AGGAGCGGTACAGCAAAGAA-3′; reverse, 5′-TACTCAGCTGCCTGCTTCTT-3′). Known copy numbers (101–107) of the plasmid pAAV-CMV-iSH2-SpA were used to construct the standard curve for the absolute quantification of vector genome copies per microgram of genomic DNA, as described [36].
2.14. Statistical analysis & exclusions
Statistical analyses were performed using Graphpad Prism version 10 (La Jolla, CA, USA). Results are presented as mean ± SEM. Differences between groups were determined using unpaired t-tests or one-way analysis of variance (ANOVA) followed by the Tukey's posthoc test. A value of P < 0.05 was considered significant. Any animal or sample exclusions are described in the data supplement.
3. Results
3.1. Generation of new AAVs for cardiomyocyte-specific upregulation of PI3K activity and improved vector yields
We have previously shown that AAV6-mediated delivery of caPI3K improves heart function in mouse models of pressure overload and diabetic cardiomyopathy [17,21,22]. The AAV construct used in our previous studies is shown in Fig. 1A (upper). In the current study, we modified the caPI3K AAV construct by 1) replacing the ubiquitous CMV promoter with a cardiomyocyte-specific cTnT promoter (Fig. 1A, lower), 2) replacing the caPI3K transgene with a significantly smaller PI3K activator (the inter-SH2 domain of PI3K(p85α), iSH2; Fig. 1B), and 3) inserting an intron from the rabbit β-globin gene (IVS2) to enhance expression of iSH2 (Fig. 1C). iSH2 binds to the p110α catalytic subunit of PI3K and is both necessary and sufficient for activation of PI3K in cultured cells [11]. iSH2 AAV constructs were significantly smaller than the original caPI3K AAV construct (1.7–2.5 kb vs 5.15 kb; see Fig. 1D), which markedly improved vector yields (~3.6 × 1012 vg per 150 mm plate of HEK293T cells for iSH2 AAVs vs ~1.9 × 1011 vg per 150 mm plate for caPI3K AAVs).
3.2. Validation and characterization of iSH2 AAVs in the healthy mouse heart
Multiple constructs, including those with CMV, were used as controls and as a reference point. We confirmed expression of HA-tagged iSH2 in ventricular tissue of mice administered iSH2 AAVs, and investigated if inclusion of IVS2 enhanced iSH2 expression. AAV6-CMV-iSH2 (Fig. 1B) and AAV6-CMV-IVS2-iSH2 (Fig. 1C) were administered to 8-week old male mice at 2 × 1011 vg (low dose; n = 3/treatment), 1 × 1012 vg (mid dose; n = 3/treatment) or 2 × 1012 vg (high dose; n = 2–3/treatment) and tissue collected 8 weeks post-AAV administration. Both AAVs induced expression of iSH2 in a dose-dependent manner (Fig. 2A). Expression of iSH2 was >3-fold higher in mice that received AAV6-CMV-IVS2-iSH2 vs AAV6-CMV-iSH2 at both the low and mid doses (Fig. 2A), demonstrating that inclusion of IVS2 in AAV constructs can enhance expression of the delivered transgene. Animal numbers were insufficient to perform statistical analysis in mice that received a high dose (2 × 1012 vg) of AAV6-CMV-IVS2-iSH2 vs AAV6-CMV-iSH2, but the difference in expression was less apparent (Fig. 2A).
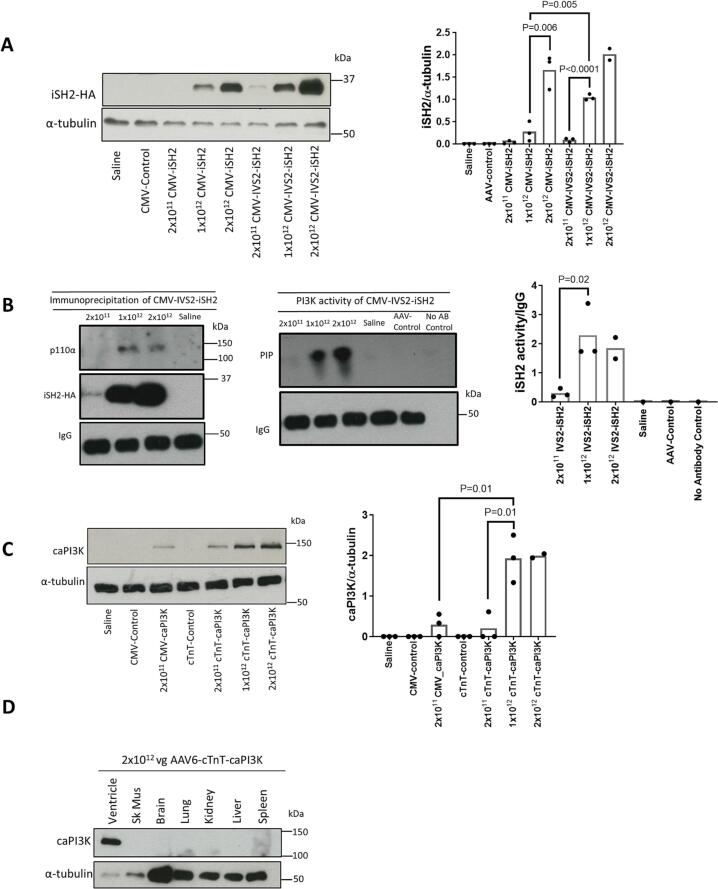
Fig. 2.
Validating the efficacy of iSH2, IVS2 and the cTnT promoter at multiple doses. A) Detection of iSH2 in cardiac tissue using an HA-tag antibody following intravenous administration of 2 × 1011 vg, 1 × 1012 vg or 2 × 1012 vg of AAV6-CMV-iSH2 or AAV6-CMV-IVS2-iSH2 in healthy mice. Saline and 2 × 1011 vg of empty AAV6-CMV-Control vector were administered as negative controls. N = 3 (Saline, AAV-control, 2 × 1011 vg, 1 × 1012 vg and 2 × 1012 vg of CMV-iSH2, 2 × 1011 vg and 1 × 1012 vg of CMV-IVS2-iSH2), N = 2 (2 × 1012 of CMV-IVS2-iSH2). B) Cardiac lysates from mice administered 2 × 1011 vg, 1 × 1012 vg or 2 × 1012 vg of AAV6-CMV-IVS2-iSH2 or saline were immunoprecipitated using an HA-tag antibody to purify iSH2-containing complexes and the resulting Western blots probed with an anti-p110α antibody (left panel). A lipid kinase activity assay using the iSH2-HA immunoprecipitates revealed iSH2-HA-associated PI3K activity, as reflected by the higher PIP signal (middle and right panel). N = 3 (2 × 1011 vg, 1 × 1012 vg or 2 × 1012 vg of IVS2-iSH2). C) Expression of caPI3K driven by a cTnT promoter in cardiac tissue following intravenous administration of 2 × 1011 vg, 1 × 1012 vg or 2 × 1012 vg. Saline, 2 × 1011 vg of AAV6-CMV-Control and AAV-cTnT-Control vectors were administered as negative controls and 2 × 1011 vg of AAV6-CMV-caPI3K was administered to compare expression of cTnT to the CMV promoter. N = 3 (Saline, AAV-control, 2 × 1011 vg CMV_caPI3K, cTnT-control, 2 × 1011 vg and 1 × 1012 vg of cTnT-caPI3K), N = 2 (2 × 1012 vg cTnT-caPI3K). D) Expression of caPI3K in cardiac tissue compared to non-cardiac tissues following intravenous administration of 2 × 1012 vg of AAV-cTnT-caPI3K in a healthy mouse. Statistical analysis performed using an unpaired t-test (only for data where n = 3). N.B. IgG Western image in panel B is duplicated for both panels (4 samples in left and 6 in the right) because it is the same sample with half the sample used for the activity assay and half used for the Western blot. N refers to number of animals.
To determine if iSH2 was capable of binding and activating PI3K in vivo, an HA-tag antibody was used to immunoprecipitate iSH2 from ventricular lysates of mice that received AAV6-CMV-IVS2-iSH2. The catalytic subunit p110α was detectable in immunoprecipitates from mice that received the medium (1 × 1012 vg) and high dose (2 × 1012 vg) of AAV6-CMV-IVS2-iSH2 (Fig. 2B). PI3K activity assays revealed lipid phosphorylation in mice that received AAV6-CMV-IVS2-iSH2, with higher activity in the mid and high dose groups compared with the low dose group, demonstrating that iSH2-bound p110α was catalytically active.
3.3. Cardiac-specific transgene expression with the cTnT477 promoter
AAV serotype 6 (AAV6) is a promising vector for cardiac gene therapy as it preferentially transduces striated muscle, including the heart and skeletal muscle [26]. We previously demonstrated that cardiac muscle can be efficiently transduced using an AAV6 vector carrying a CMV promoter with a caPI3K construct [17], with lower expression in skeletal muscle when 2 × 1011 vectors was administered. Since PI3K has tumorigenic properties in non-cardiac tissues, we investigated whether replacement of the CMV promoter in our original AAV6 vector encoding caPI3K with a cTnT promoter (Fig. 1A) would improve cardiac specificity. Here, we administered three doses of AAV6-cTnT-caPI3K to mice and observed comparable expression to the original AAV6-CMV-caPI3K vector in the heart at 2 × 1011 vg (Fig. 2C). caPI3K expression was enhanced further at 1 × 1012 vg of AAV6-cTnT-caPI3K (Fig. 2C). Intravenous tail vein administration of the highest dose (2 × 1012 vg) of the AAV6-cTnT-caPI3K vector led to strong expression in the heart with no evidence of expression in skeletal muscle, brain, lung, kidney, liver or spleen (Fig. 2D), confirming the cardiac specificity of the cTnT477 promoter. For the subsequent in vivo studies, we chose to proceed with the highest AAV dose (2 × 1012 vg) because this dose had safely been administered to mice by us and others [37], protein expression of iSH2 in the heart was greater than at 1 × 1012 vg (Fig. 2A), and given the heart disease model to be used in later studies (I/R injury) is associated with severe cardiac pathology, we considered this would maximize the chance of identifying a therapeutic effect.
3.3.1. Morphological characterization of healthy adult mice administered with AAV6-cTnT-iSH2
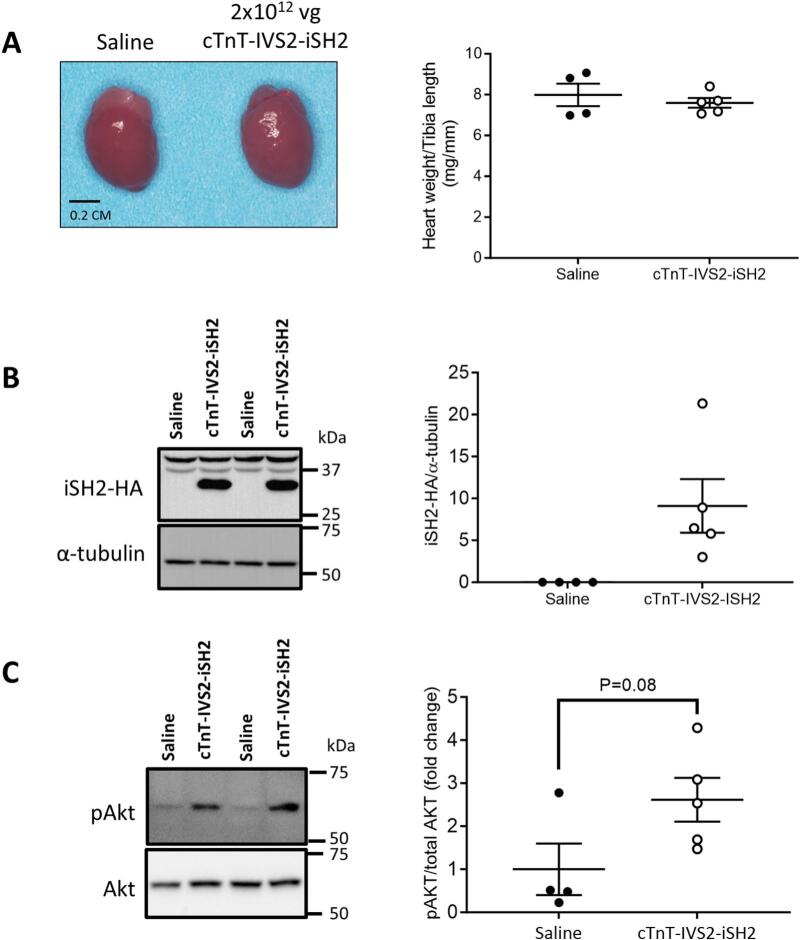
Having established efficacy of the iSH2 construct, the IVS2 enhancer and the cTnT promoter, we administered AAV6-cTnT-IVS2-iSH2 at a dose of 2 × 1012 vg or saline to a cohort of 10-week old healthy adult male mice. Heart size and weights were comparable between the AAV6-cTnT-IVS2-iSH2 and saline groups (Fig. 3A and Supp Table 1). There were no significant differences in atria, lung, kidney, liver or spleen weights between groups (Supp Table 1). Cardiac AAV expression of iSH2 was confirmed in hearts of all mice administered AAV6-cTnT-IVS2-iSH2 by Western blot detection of the HA tag (Fig. 3B). The phosphorylation of Akt relative to total Akt tended to be higher in hearts of mice that received AAV6-cTnT-IVS2-iSH2 (P = 0.08, Fig. 3C).
Fig. 3.
AAV6-cTnT-IVS2-iSH2 administration to a cohort of healthy adult mice. A) Representative hearts from control and treated groups (Left panel). Heart weight normalized to tibia length 8-weeks after administration of 2 × 1012 vg cTnT-IVS2-iSH2. B) Western blot representative images showing HA-tagged iSH2 normalized to α-tubulin. C) Phosphorylation of Akt as a ratio of total Akt from cardiac tissue. N = 4 (Saline), N = 5 (cTnT-IVS2-iSH2). Statistical analysis performed using an unpaired t-test. All data presented as mean ± SEM. N refers to number of animals.
3.4. AAV6-cTnT-IVS2-iSH2 increased the phosphorylation of Akt in non-infarcted ventricle but did not improve systolic function following ischemia/reperfusion injury at multiple doses (2 × 1012 vg, 1 × 1012 vg)
3.4.1. AAV administered at 2 × 1012 vg
To examine whether iSH2 could enhance PI3K signaling and improve heart function in a setting of cardiac injury, 10-week old male C57Bl/6 mice were subjected to one-hour of cardiac ischemia followed by reperfusion (I/R), and 24 h later administered AAV-cTnT-IVS2-iSH2 (2 × 1012 vg), an empty vector control (AAV6-cTnT-control; identical AAV lacking the IVS2-iSH2 insert, 2 × 1012 vg) or saline (Fig. 4A). One day post-I/R, and just prior to AAV administration, the area at risk of the heart assessed by echocardiography was comparable between groups (45 % ± 10 %, Fig. 4B). Robust expression of iSH2 was observed in the myocardium of 14 of 15 I/R injury mice that received the vector, and this corresponded with iSH2-specific PI3K activity (Fig. 4C–D), and iSH2 protein expression in cardiomyocytes (HA tag within iSH2 colocalized with troponin, Fig. 4C). One mouse with very low iSH2-HA expression was excluded from all subsequent analyses (See Supp Information for raw blots). Administration of AAV6-cTnT-IVS2-iSH2 was associated with a significant elevation in pAkt/tAkt in non-infarcted remote ventricle vs. saline and a trend vs. AAV-cTnT-control (Fig. 4E; unpaired t-test IVS2-iSH2 vs. AAV control, P = 0.1).
Fig. 4.
Administration of AAV6-cTNT-IVS2-iSH2 (2 × 1012 vg), saline or AAV6-cTnT-Control (AAV-Control) in a setting of cardiac dysfunction due to ischemia/reperfusion (I/R) injury. A) Schematic showing timing of the I/R surgery, echocardiography assessments and AAV intervention. B) The estimated area at risk (AAR) for each group after I/R surgery and AAV administration based on 24-hour post-surgery echocardiography analysis. N = 8 (Saline), N = 7 (AAV Control), N = 14 (IVS2-iSH2) C) Cardiac expression of iSH2-HA normalized to α-tubulin following administration of 2 × 1012 vg of AAV-cTnT-IVS2-iSH2, saline or AAV6-cTnT-Control (AAV-Control). N = 15 (Saline/AAV Control), N = 13 (IVS2-iSH2). Lower panel: Confocal stack images of mouse sections from a I/R mouse injected with saline or AAV6-cTNT-IVS2-iSH2. The samples were stained with Hoechst (blue, nuclei), HA TAG (red, HA TAG positive) and troponin (green, myocytes). Scale bars represent 50 μm, magnification ×1000. D) iSH2-HA-associated PI3K activity of all mice that received the AAV-cTnT-IVS2-iSH2 vector. N = 13 (IVS2-iSH2). E) Top: Representative Western blot probed with pAkt (Ser473) and total Akt antibodies in left ventricular lysates from I/R rAAV6:CON (CON) and rAAV6:IVS-iSHR2 (IVS-iSH2) treated mice. Bottom: Quantitation of pAkt/tAkt. N = 8 (Saline), N = 6 (AAV Control), N = 13 (IVS2-iSH2). F) Representative hearts from each group and the corresponding heart weight normalized to tibia length. N = 7 (Saline), N = 7 (AAV Control), N = 14 (IVS2-iSH2). G) Top: Masson's trichrome to assess the % of interstitial fibrosis in the heart. Bottom: Quantitation of % fibrosis. N = 7 (Saline), N = 7 (AAV Control), N = 14 (IVS2-iSH2). H) LV systolic function indicated by % ejection fraction (EF). N = 8 (Saline), N = 7 (AAV Control), N = 14 (IVS2-iSH2). I) Representative LV pressure-volume loops at 12-weeks following I/R surgery: Loops recorded during haemodynamic studies performed at endpoint for mice of each study group, alongside a sham-operated comparator for reference only (no infarction, negative control). J) LV Stroke Work assessed by PV loop. N = 6 (Saline), N = 6 (AAV Control), N = 12 (IVS2-iSH2). Statistical analysis performed using One-way ANOVA with Tukey's post-hoc test and #unpaired t-test. All data presented as mean ± SEM. N refers to number of animals. Animal exclusions are described in Supp Fig. 7. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Prior to assessing any effect of AAV6-cTnT-IVS2-iSH2 in the I/R model on morphology, organ weights from the saline and empty vector control group (AAV6-cTnT-control) were compared to check for any potential impact of the control AAV alone (Supp Table 2). Unexpectedly, morphological changes were identified in the AAV control group compared to the saline group, including a significant increase in spleen and kidney weights, as well as heart weight normalized to tibia length (Supp Table 2, Fig. 4F, including a trend for AAV6-cTnT-IVS2-iSH2 vs. saline, P = 0.12 by unpaired t-test). This finding was unexpected given there were no differences in organ weights from healthy adult mice administered saline or AAV6-cTnT-IVS2-iSH2 at the same dose (Supp Table 1). Observations in the I/R mice administered empty vector suggest the vector led to a non-specific effect in the cardiac stress setting. Given this finding, groups were compared by both ANOVA (comparison of all 3 groups) and unpaired t-test (comparison of AAV-cTnT-control and AAV6-cTnT-IVS2-iSH2 I/R).
The increase in HW/TL in I/R mice administered AAV6-cTnT-control vs saline, was not associated with any significant differences in cardiac function or performance based on echocardiography and PV-loop analyses performed 12 weeks after AAV administration (Supp Tables 3 & 4: AAV6-cTnT-control vs. saline). There were no significant differences in heart weight or other tissue weights between the I/R mice administered AAV6-cTnT-IVS2-iSH2 and AAV6-cTnT-control (Fig. 4F, Supp Table 5). There were also no differences in cardiac fibrosis (Masson's trichrome staining performed on mid-ventricle rings, Fig. 4G), or cardiac function based on echocardiography parameters such as ejection fraction between groups (Fig. 4H, Supp Table 6). Performance measures from PV-loop analyses, including stroke work, were largely unchanged (Fig. 4I–J, Supp Table 7) but there was a significant decrease in end-systolic pressure volume relationship (ESPVR) between the AAV-iSH2 and AAV-Control groups (Supp Table 7).
3.4.2. AAV administered at 1 × 1012 vg
Given the unexpected impact of the AAV-cTnT-control at 2 × 1012 vg on heart weight and other organ weights in comparison to saline administered I/R mice, we considered it important to repeat the study at a lower dose of AAV-cTnT-control and AAV-cTnT-IVS2-iSH2 (1 × 1012 vg). At this lower dose, we observed no significant increase in heart, kidney or spleen weights in the AAV-cTnT-control group vs. saline (Supp Fig. 9, Supp Table 8).
We subsequently focused on comparing the AAV6-cTnT-IVS2-iSH2 administered mice with AAV6-cTnT-control mice to investigate if iSH2 expression could improve morphological or functional parameters. Prior to AAV delivery (24 h post-I/R), the area at risk of the heart was similar between groups (Fig. 5A). At study endpoint, we confirmed strong AAV expression in the remote LV from I/R mice administered AAV-cTnT-IVS2-iSH2 (Fig. 5B), cardiac-specific AAV expression (Fig. 5C), and increased pAkt/total Akt (Fig. 5D). Despite this, there were no significant differences in measures of cardiac function by echocardiography or PV analysis (Figure 5E, Supp Tables 9–10), and no differences in heart weight, atria weight, lung weight or LV fibrosis (Fig. 5F–G). Expression of cardiac stress and contractile markers were also comparable between groups (Fig. 5H).
Fig. 5.
Administration of AAV6-cTNT-IVS2-iSH2 (1 × 1012 vg), saline or AAV6-cTnT-Control (AAV-Control) in a setting of ischemia/reperfusion (I/R) injury. A) The estimated percentage area at risk (AAR) in rAAV6:CON (CON) and rAAV6:IVS2-iSHR2 (IVS2-iSH2) treated mice after 24 h of I/R surgery. Each mouse has an AAR within the exclusion range indicated by the red dashed line (i.e. under 55 % and over 35 %). Lines represent the mean. Data analysed by unpaired t-test; no significant differences between groups. N = 8 (CON), N = 12 (IVS2-iSH2). B) Representative Western blot probed with an anti-HA antibody to confirm the presence of HA-iSH2 (HA-tag) in left ventricular lysates from I/R mice, and α-tubulin as a loading control. C) Representative Western blot probed with an anti-HA antibody to confirm the presence of HA-iSH2 (HA-tag) in left ventricular lysates from I/R mice treated with rAAV6:IVS2-iSH2, but not in non-cardiac tissues. D) Left: Representative Western blot probed with pAkt (Ser473) and total Akt antibodies in left ventricular lysates from I/R rAAV6:CON (CON) and rAAV6:IVS2-iSH2 (IVS2-iSH2) treated mice. Right: Quantitation of pAkt/tAkt expression left ventricular lysates from I/R rAAV6:CON (CON) and rAAV6:IVS2-iSHR2 (IVS2-iSH2) treated mice. Lines represent the mean. Data was analysed by an unpaired t-test. N = 8 (CON), N = 12 (IVS2-iSH2). E) Left: LV function assessed by echocardiography from ischemia-reperfusion mice at study endpoint; quantitation of ejection fraction at endpoint. Right: Stroke work assessed from pressure-volume loops. Lines indicate the mean. Data were analysed by unpaired t-test; no significant differences between groups. N = 7 (CON), N = 11 (IVS2-iSH2). F) Heart weight (HW), atria weight (AW) and lung weight (LW) normalized to tibia length (TL) at endpoint in I/R rAAV6:CON (CON) and rAAV6:IVS2-iSH2 (IVS2-iSH2) treated mice. Lines indicate the mean. Data were analysed by unpaired t-test; no significant differences between groups. N = 8 (CON), N = 12 (IVS-iSH2). G) Representative cross-sections of I/R rAAV6:CON and rAAV6:IVS2-iSH2 treated hearts stained with Masson's trichrome and quantification of left ventricular (LV) fibrosis. Scale bar = 0.5 mm. Lines indicate the mean. Data analysed by unpaired t-test; no significant differences between groups. N = 8 (CON), N = 10 (IVS-iSH2). H) Quantification of Nppa (atrial natriuretic peptide), Nppb (B-type natriuretic peptide), Atp2a2 (sarco(endo)plasmic reticulum calcium-ATPase 2; Serca2a), Myh6 (alpha myosin heavy chain) and Myh7 (beta myosin heavy chain) by qPCR in hearts of I/R rAAV6:CON (CON) and rAAV6:IVS2-iSHR2 (IVS2-iSH2) treated mice, normalized to Hprt1. N = 8 (CON), N = 12 (IVS2-iSH2). Statistical analysis performed using an unpaired t-test. All data presented as mean ± SEM. N refers to number of animals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. AAV6-iSH2 transduces the healthy sheep heart and activates PI3K by phosphorylating PIP
Assessment of the ability of AAV6 to transduce sheep heart was first performed using direct cardiac injections with AAVs containing the original caPI3K construct and the CMV or cTnT promoter, i.e., AAV6-CMV-caPI3K and AAV6-cTnT-caPI3K (Fig. 6A). Limitations due to large construct size and cost in producing these AAVs meant the highest dose we could assess was 5 × 1012 vg (this is relatively low for large animal studies [25]). No evidence of AAV expression was identified in the heart of sheep #1 with either AAV, whereas a clear signal was identified in the heart from a mouse which had received an i.v. injection of 2 × 1011 vg (Fig. 6A).
Fig. 6.
Administration of AAVs containing caPI3K and iSH2 to three healthy sheep. A) AAV cardiac injection sites collected for tissue analysis from Sheep 1 that received 5 × 1012 vg AAV6-CMV-caPI3K and AAV6-cTnT-caPI3K. Expression of caPI3K, endogenous p110α and α-tubulin from the corresponding injection sites and from the heart of a mouse that was administered AAV6-CMV-caPI3K (lower panel). B) AAV cardiac injection sites and remote region collected for tissue analysis from Sheep 2 that received 1 × 1013 vg AAV6-CMV-iSH2 and AAV6-CMV-IVS2-iSH2 (left). iSH2-HA expression of administration sites, remote regions and positive control mice using an HA-tag antibody (right and lower left). Vector genomes and normalized iSH2-HA expression relative to tissue vector genomes (lower middle and right). C) AAV cardiac injection sites and remote region collected for tissue analysis from Sheep 3 that received 1 × 1013 vg AAV6-CMV-iSH2, AAV6-cTnT-iSH2 and AAV6-cTnT-IVS2-iSH2 (left). iSH2 expression of administration sites, remote regions and positive control mice using an HA tag antibody (right and middle left). Vector genomes and normalized iSH2-HA expression relative to tissue vector genomes (middle center and right). Lower panel: Confocal stack images of sheep sections from a remote region of the heart or site injected with AAV6-cTNT-iSH2. The samples were stained with Hoechst (blue, nuclei), HA TAG (red, HA TAG positive) and troponin (green, myocytes). Scale bars represent 50 μm, magnification ×1000. D) iSH2-HA-associated PI3K activity (PIP) and IgG in sheep that received iSH2 vectors (Sheep 2 and 3) and positive control mice (upper) and quantitation (lower panel). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we examined the ability of the smaller AAV constructs to transduce the sheep heart, i.e., iSH2 constructs with the CMV or cTnT promoter ± IVS2 (Fig. 6B & C). Successful AAV expression of the sheep heart was observed at all sites of administration with the smaller AAV vectors. In sheep #2, 1 × 1013 vg of AAV6-CMV-iSH2 or AAV6-CMV-IVS2-iSH2 was directly injected into separate regions of the LV (Fig. 6B, left panel). The concentration of viral genomes in each region was comparable (2.2 × 106 vg/μg DNA for AAV6-CMV-iSH2, 2.9 × 106 vg/μg DNA for AAV6-CMV-iSH2) and yielded a similar level of iSH2-HA protein expression (Fig. 6B). No viral genomes or iSH2-HA protein were detected in remote regions. The degree of expression from direct injection was comparable to the expression from the heart of a mouse that had received 1 × 1012 vg of the same constructs intravenously (Fig. 6B, right panel). In sheep #3, 1 × 1013 vg of AAV6-CMV-iSH2, AAV6-cTnT-iSH2 and AAV6-cTnT-IVS2-iSH2 was administered directly into three separate regions of the heart (Fig. 6C, left panel). Heart tissue sampled from these regions showed a variable level of AAV gene delivery (5.6 × 106 vg/μg DNA for AAV6-CMV-iSH2, 2.7 × 106 vg/μg DNA for AAV6-cTnT-iSH2 and 7.1 × 105 vg/μg DNA for AAV6-cTnT-IVS2-iSH2, Fig. 6C). Consistent with our experiments in mice (Fig. 1), we observed expression of iSH2-HA protein from all three constructs, with more iSH2-HA protein in tissue transduced with AAV6-cTnT-IVS2-iSH2 vs cTnT-iSH2 (i.e. in the presence of the intron), despite fewer viral genomes being present (Fig. 6C). By immunofluorescence, iSH2 protein expression was detected in cardiomyocytes in an injected region of the sheep heart (HA tag within iSH2 colocalized with troponin, Fig. 6C, lower panel). iSH2 was immunoprecipitated from heart lysates from sheep 2 & 3 using the HA tag antibody. iSH2-associated PI3K activity was observed in tissue from all injection sites whilst no iSH2-related PI3K activity was observed in a sample from a remote cardiac region (Fig. 6D). In summary, efficient transduction of the sheep heart was observed at all sites of administration with the iSH2 AAV vectors.
4. Discussion
There is growing interest in developing and optimising therapeutics that can recapitulate the benefits of exercise on the heart by enhancing critical molecular signaling pathways such as the IGF1-PI3K-Akt cascade. The role of PI3K in regulating heart growth is well established, and numerous studies have demonstrated its capacity to provide cardioprotection following a variety of cardiac insults using transgenic mouse models with increased or decreased PI3K [[13], [14], [15], [16], [17]]. Transgenic mice with a constitutively activated PI3K allele (caPI3K) have increased cardiac PI3K activity and pAkt/total Akt, and were protected to a similar degree to exercise trained mice in response to pressure overload or dilated cardiomyopathy [13,17]. This suggests that cardiac PI3K can mimic, at least in part, the beneficial effects of exercise. In contrast, transgenic mice with a dominant negative PI3K allele have reduced PI3K activity, and display worse outcomes following a cardiac insult [19,20]. The key aims of this study were to 1) generate a commercially viable gene therapy which could mimic the protective effects of PI3K, 2) assess whether the new gene therapy provides protection in a mouse model of I/R injury, and 3) in parallel evaluate AAV expression in a large animal heart. In this study, we report that AAV6-mediated expression of a PI3K(p85) fragment, iSH2, is sufficient to activate PI3K signaling in hearts of healthy mice and sheep, and in mice following a cardiac injury. This demonstrated that fragments of large kinase proteins can mediate downstream signaling in vivo and may provide a viable approach to circumventing the limited packaging capacity and high costs of AAV therapies. We also report that IVS2, a rabbit β-globin intron, can enhance expression of AAV6-delivered constructs in the heart. The new iSH2 gene therapy (1 × 1012 vg) did not improve cardiac function in a setting of I/R injury, despite an increase in the phosphorylation of Akt. Further studies will be required to determine if there is therapeutic potential of the AAV6-cTnT-IVS2-iSH2 construct in other cardiac disease settings.
AAV is one of the most successful vectors for gene delivery due to its positive safety profile, capacity to mediate long-term transgene expression (>1 year) following a once-off delivery and very mild host immune response [25,38]. However, the limited packaging capacity of AAV and high costs associated with production of AAVs still pose barriers to the delivery of large transgenes and economic viability, respectively. It has previously been demonstrated that AAVs have a maximum package size of ~5 kb and attempts to insert transgenes exceeding the packaging capacity become truncated after ~5.2 kb at the 5` end of the vector genome [23]. We previously reported that an AAV-caPI3K vector that is 5.15 kb in size transduced the mouse heart and improved heart function in a setting of pressure overload [17], and prevented a further decline in cardiac dysfunction in settings of diabetic cardiomyopathy [21,22]. Although the 5.15 kb transgene still provided efficacy at the upper limit of the AAV packaging capacity, production of an AAV containing such a large transgene was inefficient, and labour intensive to produce. To overcome this limitation and assess if a smaller fragment of PI3K could still be functional in the hearts of mice and sheep, we generated an AAV containing an inter-SH2 domain (iSH2): a small fragment that sits between the two SH2 domains of the p85 regulatory subunit of PI3K. The role and sequence of this subunit was first described by Klippel et al.[11] who demonstrated that in cultured cells, the iSH2 domain alone was capable of binding to the p110α catalytic subunit of PI3K and generate PI3K activity. Moreover, p85 lacking iSH2 was unable to bind to p110α and form a functional heterodimer. Our studies replicated these findings in animal models by demonstrating that iSH2 could bind p110α and generate PI3K activity. The phosphorylation of Akt, a key downstream event of PI3K signaling, tended to be higher (P = 0.08) in hearts of healthy adult mice administered AAV-cTnT-IVS2-iSH2. Using the much smaller iSH2 construct (~2.3 kb size) instead of the caPI3K construct (~5.15 kb) drastically improved the efficiency of AAV vector production (~18-fold increase in production yield) and subsequently reduced the production cost.
A number of different AAV serotypes have been used to transduce the animal and human heart via different routes of administration (e.g. AAV1, AAV2, AAV6, AAV8 and AAV9) [25]. Here, we chose to use AAV6 because: 1) it selectively transduced the mouse heart after intravenous administration [17], 2) AAV6-cTnT incorporating another gene was shown to enhance heart function [39] and 3) we wanted to use an AAV with good potential for transducing the human heart. In a study designed to guide the development of AAV vectors for human heart disease, AAV6 provided better results (cardiac-specificity and efficient gene transfer) than AAV8 or AAV9 in primates (rhesus macaques) [40]. Further, results from more recent work using a capsid-shuffled rAAV library, suggested that AAV6-based vectors have the most potential to be cardiotropic in human cells [41].
To improve cardiac transgene expression, we trialled inserting β-globin intron 2 (IVS2) between the promoter and transgene to determine if it was capable of improving AAV-mediated cardiac transgene expression. IVS1 & 2 were initially reported to improve transcriptional efficiency in plasmid vector systems more than thirty years ago [42]. In the current study, the impact of IVS2 was prominent at the 1 × 1012 vg dose with which we observed a 3-fold increase in cardiac transgene expression. The efficacy of IVS2 in improving transgene expression has also been observed through microinjection of plasmid DNA [43], and more recently it was shown to provide greater than a 5-fold increase in AAV-mediated transduction of the liver in mice [27]. In line with these findings, we show that incorporation of IVS2 into a cardiac promoter can improve transgene expression from AAVs in the heart. This is significant, because the human and large animal heart has been challenging to transduce [25,38,44,45]. In the Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease phase 2b (CUPID2) clinical trial which administered AAV1 encoding a SERCA2a transgene or placebo to 243 HF patients, no improvement in cardiac function or LV remodeling was identified. One explanation provided for the lack of efficacy was inadequate transduction efficiency with AAV-mediated expression of SERCA2a occurring in only a small proportion of cardiomyocytes [46]. Thus, any tool which can enhance AAV-mediated transduction such as IVS2 may prove valuable in improving transduction of the human heart. The value of our truncated PI3K AAV constructs was highlighted when we tested our AAV tools in the sheep heart. The sheep studies were performed in parallel to the mouse studies because we had previously shown that AAV6-CMV-caPI3K provided protection in the mouse heart. Thus, if we had achieved efficient transduction of the sheep heart with AAV6-CMV-caPI3K that may still have been a viable option for assessing the therapeutic potential of the AAV in a sheep heart disease model (even in the absence of a positive result with our new AAV constructs). On performing these studies, we were unable to detect any transduction of our AAVs containing caPI3K, but identified a strong signal with constructs containing iSH2. Furthermore, the addition of IVS2 appeared to improve the transduction of AAV6-cTnT-iSH2 in the sheep heart, but this needs to be confirmed in further studies.
Having developed a new AAV gene therapy tool with the capacity to generate PI3K activity, and efficiently transduce the mouse heart, we examined the therapeutic potential of this AAV in a mouse model of I/R injury. Phosphorylation of Akt was significantly higher in hearts of I/R mice treated with AAV-cTnT-IVS2-iSH2 vs. control. Despite the increased phosphorylation of Akt in the I/R model, this was not associated with positive outcomes based on morphology, cardiac function, or cardiac fibrosis. A number of factors may have contributed to the lack of functional improvement. At the highest dose of AAV (2 × 1012 vg), mild systemic toxicity may have limited the ability to identify any cardiac protection. We had commenced with this dose (2 × 1012 vg) because it did not appear to be associated with any phenotype in healthy adult mice in which organ weights and appearance were indistinguishable from control saline mice. However, in the I/R model, spleen, kidney and normalized heart weight ratio were all modestly higher in mice receiving AAV in comparison to saline. This does not represent a significant immune response since spleen weights were only marginally elevated (~90–95 mg in the AAV groups vs 82 mg in the saline group). Further, there was no evidence of exacerbated depressed cardiac function or performance in I/R mice administered AAV6-cTnT-control versus saline. However, given normalized heart and kidney weights were elevated in the I/R cohorts receiving AAV, we cannot exclude this may have impacted the results. For this reason, we proceeded to assess a lower AAV dose (1 × 1012 vg). At this dose, we observed no significant differences in spleen, kidney or normalized heart weight ratio. Yet, despite elevated pAkt/tAkt in the heart, we still observed no obvious improvement in any cardiac markers (morphology, function or histology). This raises the question as to whether the activation of the PI3K-Akt pathway was potentially too high or too low. Based on our prior work in the caPI3K transgenic mouse models and exercise models, both of which were associated with physiological hypertrophy and protection, there is no evidence to suggest that activation of PI3K or Akt was too high (pAkt/total Akt elevated in caPI3K and exercise ~2–7 fold) [19,[47], [48], [49]]. We cannot exclude the possibility that the phosphorylation of Akt was too low in the I/R model (elevation on average ~2-fold). However, of note, we previously identified an improvement in heart function in a pressure overload model with AAV6-CMV-caPI3K, and the phosphorylation of Akt was ~1.6–1.7- fold versus control [17]. Akt has previously been observed to protect against an I/R injury in the mouse [50], though the degree of Akt activation was not quantitatively assessed. Another possibility is that our prior AAV study with the CMV promoter enhanced Akt phosphorylation in non-cardiomyocytes in addition to cardiomyocytes. However, immunofluorescence studies in mice administered AAV6-CMV-caPI3K were consistent with transduction in cardiomyocytes alone [17]. Thus, we consider this unlikely.
Strategies to consider in future work include the timing of AAV delivery and transduction. Given we administered AAV one day after I/R surgery, irreversible cardiac damage may already have occurred by the time the heart was sufficiently expressing iSH2 (3 weeks following the cardiac insult). In comparing our study to prior AAV studies in which protection was observed, there are some notable differences. Cardiac protection was observed following administration of AAV6/9 vectors with complement component 1q (C1q) and tumour necrosis factor related protein 5 (CTRP5) or a novel muscle-specific micropeptide (DWORF) which enhances SERCA2a activity [51,52]. The time point of AAV administration was a key difference from our study, as the AAV vectors were administered three and eight weeks prior to the cardiac insult, respectively. Further, in the study assessing AAV-DWORF, the AAV was administered prior to adulthood at postnatal day 5.
Since commencing our own work, AAV technology has advanced and could be considered in future work. One study engineered an AAV capsid (AAVMYO) which was shown to enhance expression in cardiomyocytes [53]. Moreover, given that cardiac function is governed by a multitude of signaling pathways, approaches such as dual-AAV mediated therapies that can target multiple protective pathways simultaneously are likely to confer greater functional improvements. This has already shown promise in improving outcomes as well as facilitating the administration of transgenes too large for a single AAV vector in numerous non-cardiac AAV-based therapeutic trials [54].
In summary, in this report we have described the development and optimization of a truncated PI3K AAV gene therapy (AAV6-cTnT-IVS2-iSH2) which can efficiently transduce the mouse and sheep heart. Despite not identifying cardiac protection in the I/R model, we consider the findings important for informing and progressing the research field, Future studies in cardiac stress settings should administer AAV6-cTnT-IVS2-iSH2 at an earlier time point and/or longer duration to assess the therapeutic potential of this new gene therapy tool.
4.1. Limitations
1) Only male mice were used in this study for the following reasons: i) AAV costs are substantial, particularly for constructs with caPI3K. Thus, for practical reasons it was decided to focus on one sex. ii) Given our prior work demonstrating AAV6-CMV-caPI3K provided cardiac protection was only performed in male mice, we commenced with male mice. iii) If we had identified cardiac protection in our first cohort of I/R male mice at 2 × 1012 vg, we had planned to assess efficacy in female mice. Given the unexpected non-specific effect, it was decided it was critical to repeat the study in male mice at a lower dose. We acknowledge that future work should be performed in both sexes. 2) For practicalities of housing sheep, we only tested AAV constructs in female sheep which are significantly smaller than male sheep and more docile. 3) For the exploratory studies including initial testing of constructs in mice and sheep, some n numbers are low, limiting statistical power or preventing statistics from being performed. Despite this, collectively we consider the majority of the exploratory results convincing, and importantly the mouse I/R studies were well powered. However, in studies moving forward we would incorporate additional experiments including assessment of the percentage of the mouse heart transduced at different AAV doses, and additional testing in sheep heart.
CRediT authorship contribution statement
Sebastian Bass-Stringer: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Daniel G. Donner: Methodology, Investigation, Formal analysis, Data curation. Clive N. May: Resources, Methodology. Aya Matsumoto: Supervision, Investigation, Formal analysis, Data curation. Emma I. Masterman: Investigation, Formal analysis, Data curation. Aascha A. D'Elia: Methodology, Investigation, Formal analysis, Data curation. Yi Ching Chen: Investigation. Helen Kiriazis: Methodology, Investigation, Formal analysis, Data curation. Jieting Luo: Investigation. Roger Chooi: Formal analysis. Clara Liu Chung Ming: Investigation, Formal analysis, Data curation. Paul Gregorevic: Resources, Methodology. Colleen J. Thomas: Writing – review & editing, Supervision, Resources, Methodology. Bianca C. Bernardo: Writing – review & editing, Supervision, Investigation, Formal analysis, Data curation. Kate L. Weeks: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Julie R. McMullen: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Funding
This work was supported by a grant from the National Health and Medical Research Council [grant number 1163732 to JRM and CJT], grant from the Victorian Medical Research Acceleration Fund, Institute Seed Funding from private philanthropists, Dr David Thurin AM and Mrs Lisa Thurin, and in part by the Victorian Government's Operational Infrastructure Support Program. SB was supported by a joint Baker Heart and Diabetes Institute-La Trobe University doctoral scholarship. KLW is supported by a Future Leader Fellowship from the National Heart Foundation of Australia [grant number 102539]. BCB was supported by an Alice Baker and Eleanor Shaw Fellowship (The Baker Foundation, Australia). JRM was supported by a National Health and Medical Research Council Senior Research Fellowship [grant number 1078985], Baker Fellowship (The Baker Foundation, Australia), and Cardiovascular Research Capacity Program - Research Leadership Grants (NSW Health).
Declaration of competing interest
None.
Acknowledgements
The authors thank Dr Yow Keat Tham for assistance with the formal randomization and blinding of mice, Hongwei Qian for technical assistance producing the AAVs, and Celeste Tai for administrative assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmccpl.2025.100478.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Taylor C.J., Ordonez-Mena J.M., Roalfe A.K., Lay-Flurrie S., Jones N.R., Marshall T., et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. 2019;364 doi: 10.1136/bmj.l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J., Ogden L.G., Bazzano L.A., Vupputuri S., Loria C., Whelton P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 3.Djousse L., Driver J.A., Gaziano J.M. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302(4):394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayor M., Vasan R.S. Preventing heart failure: the role of physical activity. Curr Opin Cardiol. 2015;30(5):543–550. doi: 10.1097/HCO.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn K.E., Pina I.L., Whellan D.J., Lin L., Blumenthal J.A., Ellis S.J., et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hambrecht R., Gielen S., Linke A., Fiehn E., Yu J., Walther C., et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor C.M., Whellan D.J., Lee K.L., Keteyian S.J., Cooper L.S., Ellis S.J., et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A., Parashar A., Kumbhani D., Agarwal S., Garg J., Kitzman D., et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8(1):33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisloff U., Stoylen A., Loennechen J.P., Bruvold M., Rognmo O., Haram P.M., et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 10.Bernardo B.C., Ooi J.Y.Y., Weeks K.L., Patterson N.L., McMullen J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev. 2018;98(1):419–475. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 11.Klippel A., Escobedo J.A., Hu Q., Williams L.T. A region of the 85-kilodalton (kDa) subunit of phosphatidylinositol 3-kinase binds the 110-kDa catalytic subunit in vivo. Mol Cell Biol. 1993;13(9):5560–5566. doi: 10.1128/mcb.13.9.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bass-Stringer S., Tai C.M.K., McMullen J.R. IGF1-PI3K-induced physiological cardiac hypertrophy: implications for new heart failure therapies, biomarkers, and predicting cardiotoxicity. J Sport Health Sci. 2020;10(6):637–647. doi: 10.1016/j.jshs.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMullen J.R., Amirahmadi F., Woodcock E.A., Schinke-Braun M., Bouwman R.D., Hewitt K.A., et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104(2):612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius L., Du X.J., Woodcock E.A., Kiriazis H., Lin R.C., Marasco S., et al. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol. 2009;175(3):998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R.C., Weeks K.L., Gao X.M., Williams R.B., Bernardo B.C., Kiriazis H., et al. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol. 2010;30(4):724–732. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie R.H., Love J.E., Huynh K., Bernardo B.C., Henstridge D.C., Kiriazis H., et al. Enhanced phosphoinositide 3-kinase(p110alpha) activity prevents diabetes-induced cardiomyopathy and superoxide generation in a mouse model of diabetes. Diabetologia. 2012;55(12):3369–3381. doi: 10.1007/s00125-012-2720-0. [DOI] [PubMed] [Google Scholar]
- 17.Weeks K.L., Gao X., Du X.J., Boey E.J., Matsumoto A., Bernardo B.C., et al. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5(4):523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 18.Perrino C., Naga Prasad S.V., Mao L., Noma T., Yan Z., Kim H.S., et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116(6):1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMullen J.R., Shioi T., Zhang L., Tarnavski O., Sherwood M.C., Kang P.M., et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100(21):12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shioi T., Kang P.M., Douglas P.S., Hampe J., Yballe C.M., Lawitts J., et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19(11):2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakoso D., De Blasio M.J., Qin C., Rosli S., Kiriazis H., Qian H., et al. Phosphoinositide 3-kinase (p110alpha) gene delivery limits diabetes-induced cardiac NADPH oxidase and cardiomyopathy in a mouse model with established diastolic dysfunction. Clin Sci (Lond) 2017;131(12):1345–1360. doi: 10.1042/CS20170063. [DOI] [PubMed] [Google Scholar]
- 22.Prakoso D., De Blasio M.J., Tate M., Kiriazis H., Donner D.G., Qian H., et al. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110alpha) attenuates cardiac remodeling in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2020;318(4):H840–H852. doi: 10.1152/ajpheart.00632.2019. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18(1):80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grieger J.C., Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79(15):9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bass-Stringer S., Bernardo B.C., May C.N., Thomas C.J., Weeks K.L., McMullen J.R. Adeno-associated virus gene therapy: translational progress and future prospects in the treatment of heart failure. Heart Lung Circ. 2018;27(11):1285–1300. doi: 10.1016/j.hlc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10(8):828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Muthuramu I., Somanathan S., Zhang H., Bell P., He Z., et al. Developing a second-generation clinical candidate AAV vector for gene therapy of familial hypercholesterolemia. Mol Ther Methods Clin Dev. 2021;22:1–10. doi: 10.1016/j.omtm.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blankinship M.J., Gregorevic P., Allen J.M., Harper S.Q., Harper H., Halbert C.L., et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10(4):671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Weeks K.L., Henstridge D.C., Salim A., Shaw J.E., Marwick T.H., McMullen J.R. CORP: practical tools for improving experimental design and reporting of laboratory studies of cardiovascular physiology and metabolism. American Journal of Physiology-Heart and Circulatory Physiology. 2019;317(3):H627–H639. doi: 10.1152/ajpheart.00327.2019. [DOI] [PubMed] [Google Scholar]
- 31.Gao X.M., Wu Q.Z., Kiriazis H., Su Y., Han L.P., Pearson J.T., et al. Microvascular leakage in acute myocardial infarction: characterization by histology, biochemistry, and magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2017;312(5):H1068–h1075. doi: 10.1152/ajpheart.00073.2017. [DOI] [PubMed] [Google Scholar]
- 32.Donner D., Kiriazis H., Gao X., Du X. A novel non-invasive measurement of myocardial infarct size in the mouse using cardiac ultrasound tissue displacement mapping. J Mol Cell Cardiol. 2020;140:6–7. [Google Scholar]
- 33.Sapra G., Tham Y.K., Cemerlang N., Matsumoto A., Kiriazis H., Bernardo B.C., et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat Commun. 2014;5:5705. doi: 10.1038/ncomms6705. [DOI] [PubMed] [Google Scholar]
- 34.Thomas C.J., Lim N.R., Kedikaetswe A., Yeap Y.Y., Woodman O.L., Ng D.C., et al. Evidence that the MEK/ERK but not the PI3K/Akt pathway is required for protection from myocardial ischemia-reperfusion injury by 3′,4′-dihydroxyflavonol. Eur J Pharmacol. 2015;758:53–59. doi: 10.1016/j.ejphar.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Weeks K.L., Kiriazis H., Wadley G.D., Masterman E.I., Sergienko N.M., Raaijmakers A.J.A., et al. A gene therapy targeting medium-chain acyl-CoA dehydrogenase (MCAD) did not protect against diabetes-induced cardiac pathology. J Mol Med. 2024;102(1):95–111. doi: 10.1007/s00109-023-02397-2. [DOI] [PubMed] [Google Scholar]
- 36.Prasad K.M., Xu Y., Yang Z., Acton S.T., French B.A. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2011;18(1):43–52. doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva-Pinheiro P., Cerutti R., Luna-Sanchez M., Zeviani M., Viscomi C. A single intravenous injection of AAV-PHPB-hNDUFS4 ameliorates the phenotype of Ndufs4 (−/−) mice. Mol Ther Methods Clin Dev. 2020;17:1071–1078. doi: 10.1016/j.omtm.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki N., Kok C.Y., Westhaus A., Alexander I.E., Lisowski L., Kizana E. In search of adeno-associated virus vectors with enhanced cardiac tropism for gene therapy. Heart Lung Circ. 2023;32(7):816–824. doi: 10.1016/j.hlc.2023.06.704. [DOI] [PubMed] [Google Scholar]
- 39.Kolwicz S.C., Jr., Odom G.L., Nowakowski S.G., Moussavi-Harami F., Chen X., Reinecke H., et al. AAV6-mediated cardiac-specific overexpression of ribonucleotide reductase enhances myocardial contractility. Mol Ther. 2016;24(2):240–250. doi: 10.1038/mt.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao G., Bish L.T., Sleeper M.M., Mu X., Sun L., Lou Y., et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther. 2011;22(8):979–984. doi: 10.1089/hum.2011.042. [DOI] [PubMed] [Google Scholar]
- 41.Kok C.Y., Tsurusaki S., Cabanes-Creus M., Igoor S., Rao R., Skelton R., et al. Development of new adeno-associated virus capsid variants for targeted gene delivery to human cardiomyocytes. Mol Ther Methods Clin Dev. 2023;30:459–473. doi: 10.1016/j.omtm.2023.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchman A.R., Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988;8(10):4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee S., Min L., Karuturi R.K., Lufkin T. The role of post-transcriptional RNA processing and plasmid vector sequences on transient transgene expression in zebrafish. Transgenic Res. 2010;19(2):299–304. doi: 10.1007/s11248-009-9312-x. [DOI] [PubMed] [Google Scholar]
- 44.Lyon A.R., Babalis D., Morley-Smith A.C., Hedger M., Suarez Barrientos A., Foldes G., et al. Investigation of the safety and feasibility of AAV1/SERCA2a gene transfer in patients with chronic heart failure supported with a left ventricular assist device - the SERCA-LVAD TRIAL. Gene Ther. 2020;27(12):579–590. doi: 10.1038/s41434-020-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajjar R.J., Ishikawa K. Introducing genes to the heart: all about delivery. Circ Res. 2017;120(1):33–35. doi: 10.1161/CIRCRESAHA.116.310039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Desai A.S., et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387(10024):1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 47.Tham Y.K., Bernardo B.C., Ooi J.Y., Weeks K.L., McMullen J.R. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89(9):1401–1438. doi: 10.1007/s00204-015-1477-x. [DOI] [PubMed] [Google Scholar]
- 48.Bass-Stringer S., Bernardo B.C., Yildiz G.S., Matsumoto A., Kiriazis H., Harmawan C.A., et al. Reduced PI3K(p110α) induces atrial myopathy, and PI3K-related lipids are dysregulated in athletes with atrial fibrillation. J Sport Health Sci. 2025;14 doi: 10.1016/j.jshs.2025.101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weeks K.L., Tham Y.K., Yildiz S.G., Alexander Y., Donner D.G., Kiriazis H., et al. FoxO1 is required for physiological cardiac hypertrophy induced by exercise but not by constitutively active PI3K. Am J Physiol Heart Circ Physiol. 2021;320(4):H1470–H1485. doi: 10.1152/ajpheart.00838.2020. [DOI] [PubMed] [Google Scholar]
- 50.Fujio Y., Nguyen T., Wencker D., Kitsis R.N., Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng M., Liu Y., Zhang X.Q., Xu Y.W., Zhao Y.T., Yang H.B. CTRP5-overexpression attenuated ischemia-reperfusion associated heart injuries and improved infarction induced heart failure. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.603322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makarewich C.A., Bezprozvannaya S., Gibson A.M., Bassel-Duby R., Olson E.N. Gene therapy with the DWORF micropeptide attenuates cardiomyopathy in mice. Circ Res. 2020;127(10):1340–1342. doi: 10.1161/CIRCRESAHA.120.317156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinmann J., Weis S., Sippel J., Tulalamba W., Remes A., El Andari J., et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat Commun. 2020;11(1):5432. doi: 10.1038/s41467-020-19230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tornabene P., Trapani I., Minopoli R., Centrulo M., Lupo M., de Simone S., et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci Transl Med. 2019;11(492) doi: 10.1126/scitranslmed.aav4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2