Abstract
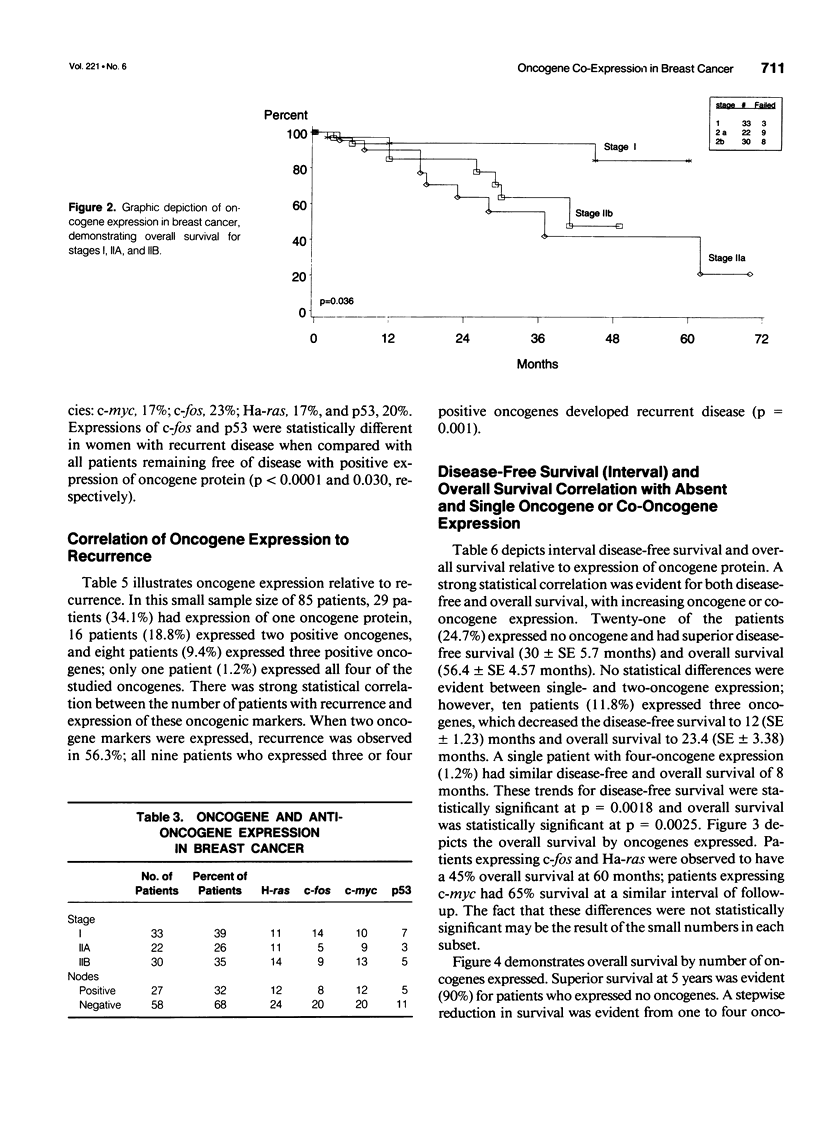
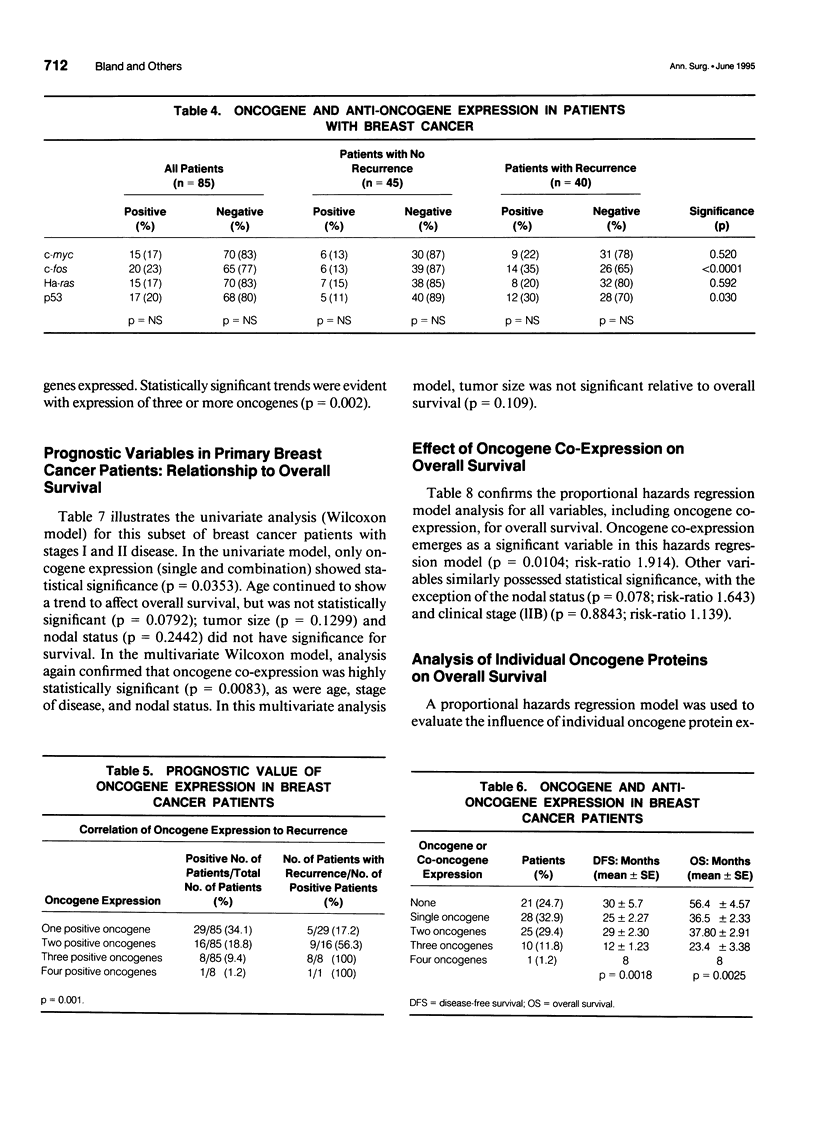
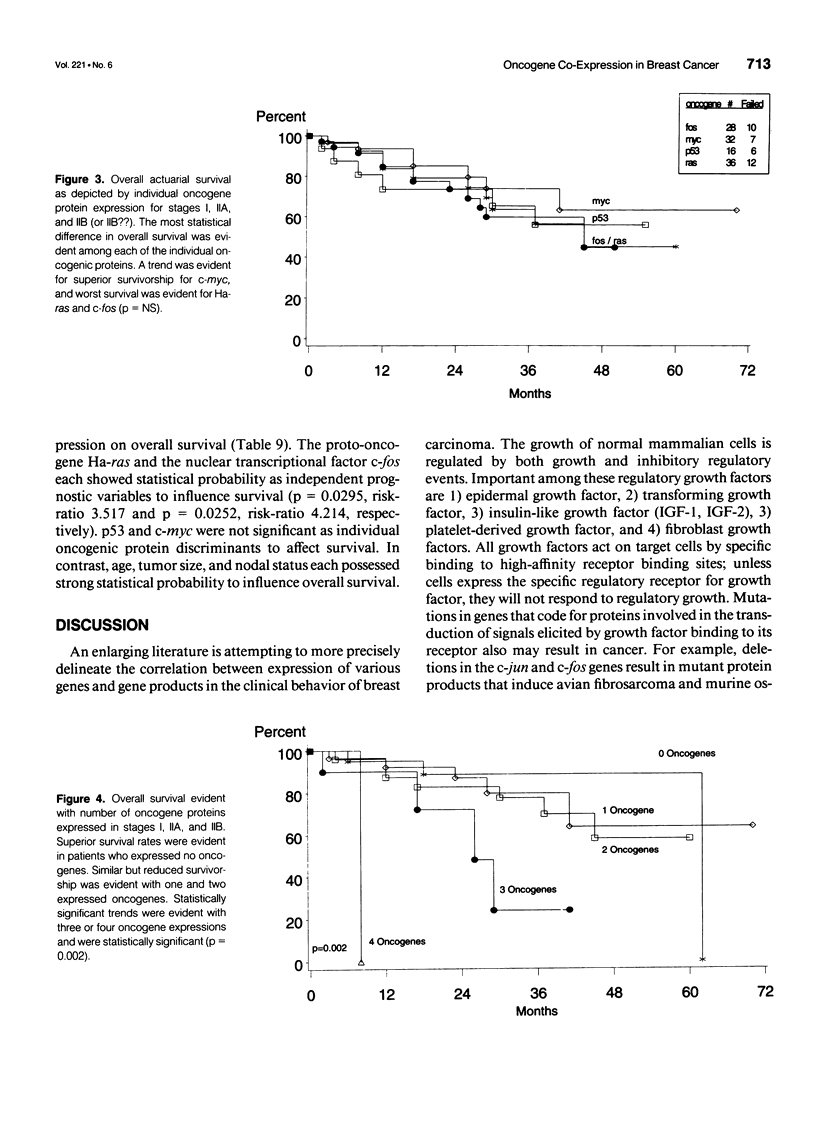
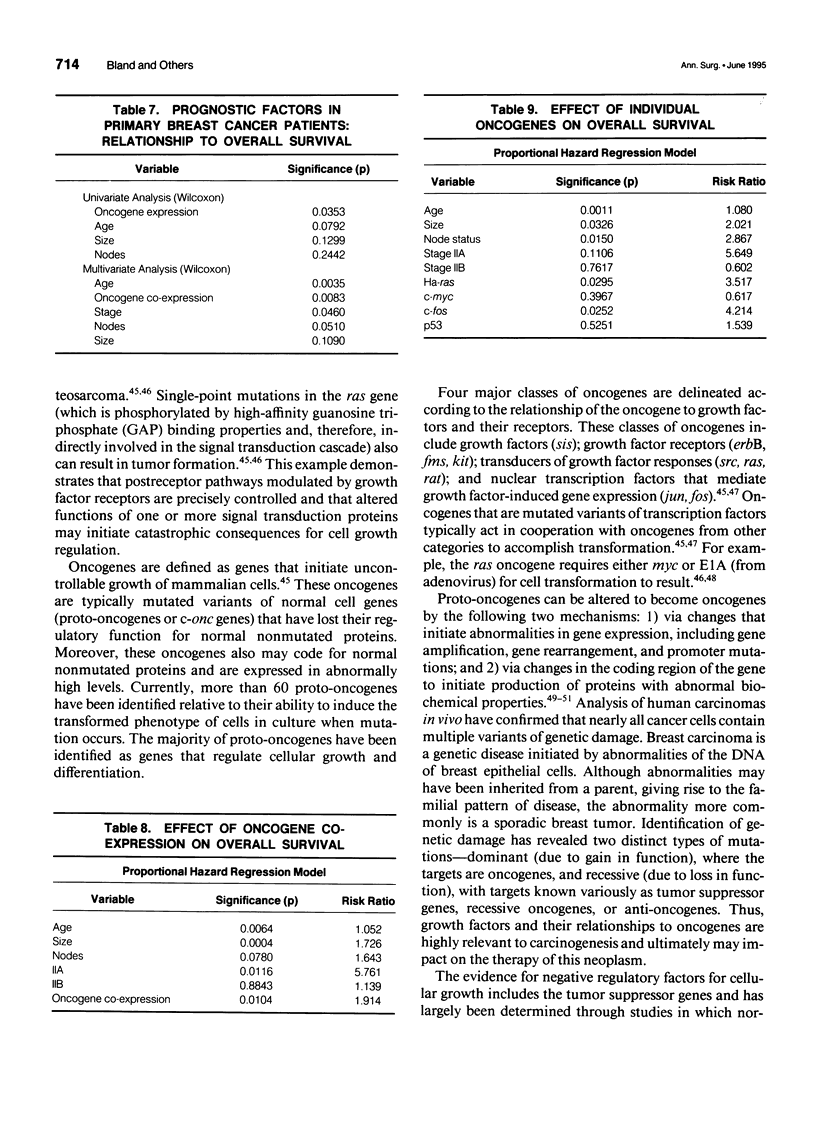
OBJECTIVE: A refinement of prognostic variables using traditional pathologic markers integrated with oncogene proteins, enzymes, and hormonal factors may enhance the ability to predict for recurrence or survival in patients with mammary carcinoma. Although various oncogenes and oncogene products have been identified in human breast carcinoma, their relationship to disease outcome remains controversial. METHODS: Using the monoclonal antibodies cS93.1, 9E1.0, F235-1.7.1, and PAb 1801 against each oncogene protein studied, the avidin-biotin complex immunoperoxidase method provided immunohistochemical staining of bound oncogene protein for c-fos, c-myc, Ha-ras, and p53, respectively. Analyses were made on archival pathology tissues of 85 breast cancer patients (stages I, IIA, and IIB). Forty patients (47%) had recurrence of disease; 45 remained free of local-regional or distant disease at mean follow-up of 48 months (range 6-180 months). Molecular biological data were merged with clinicopathologic demographics 1) to determine the frequency of single or co-expression of oncogenes in this patient population; 2) to evaluate the value of these molecular protein markers to predict probability of recurrence; and 3) to determine worth of the studied oncogenes to correlate with traditional clinical pathologic parameters and overall survival. RESULTS: In this study, oncogene expression had statistical correlation for recurrence with increasing co-expression: one oncogene 17.2%, two oncogenes 56.3%, three or four oncogenes, 100% (p = 0.001). Increasing oncogene or co-oncogene expression correlated with statistically significant reduction in disease-free and overall survival; with no expression of oncogenes, disease-free survival was 30 (SE +/- 5.7) months and overall survival was 56.4 (SE +/- 4.57) months. With expression of three oncogenes, disease-free survival was 12 (SE +/- 1.23) months (p = 0.0018) and overall survival was 23.4 (SE +/- 3.38) months (p = 0.0025). In univariate Wilcoxon analysis, oncogene expression was the most significant variable to determine survival (p = 0.035); in multivariate analysis, age and oncogene co-expression each emerged as the most significant variables for overall survival. For the proportional hazards regression model, oncogene co-expression was significant (p = 0.0104, risk-ratio 1.914) and correlated with age and tumor size as significant variables. Ha-ras and c-fos both emerged as important individual oncogene proteins to affect survival (p = 0.0925, risk-ratio 3.517 and p = 0.025, risk-ratio 4.214, respectively). The proto-oncogene c-myc and the antitumor suppressor gene p53 did not have significant effects as individual oncogenes to influence survival. CONCLUSIONS: Approximately one fifth of the breast cancer patients in this analysis (disease-free and recurrent) expressed only a single oncogene marker (c-fos, c-myc, Ha-ras, or p53); one quarter of patients with recurrent disease expressed only one oncogene protein. Single oncogene expression did not possess independent prognostic significance for prediction of recurrence. Further, p53 mutations did not function as independent correlates for prognosis. The co-expression of the studied proto-oncogenes (c-myc, Ha-ras) and the nuclear transcriptional protein (c-fos) functioned as a strong prognostic correlate for recurrence and survival; the effect of individual oncogenes to predict survival was greatest for Ha-ras and c-fos. Immediate or early co-expression of three oncogene proteins in neoplastic transformation endowed cells of invasive carcinoma with an aggressive phenotype. This aggressive phenotype was evident in a small percentage of the studied population (11%) and predicted adverse disease-free and overall survival. These findings suggest that oncogene co-expression possesses significant prognostic and potential therapeutic value; incorporation of this molecular technology into future prospective randomized trials is advisable.
Full text
PDF



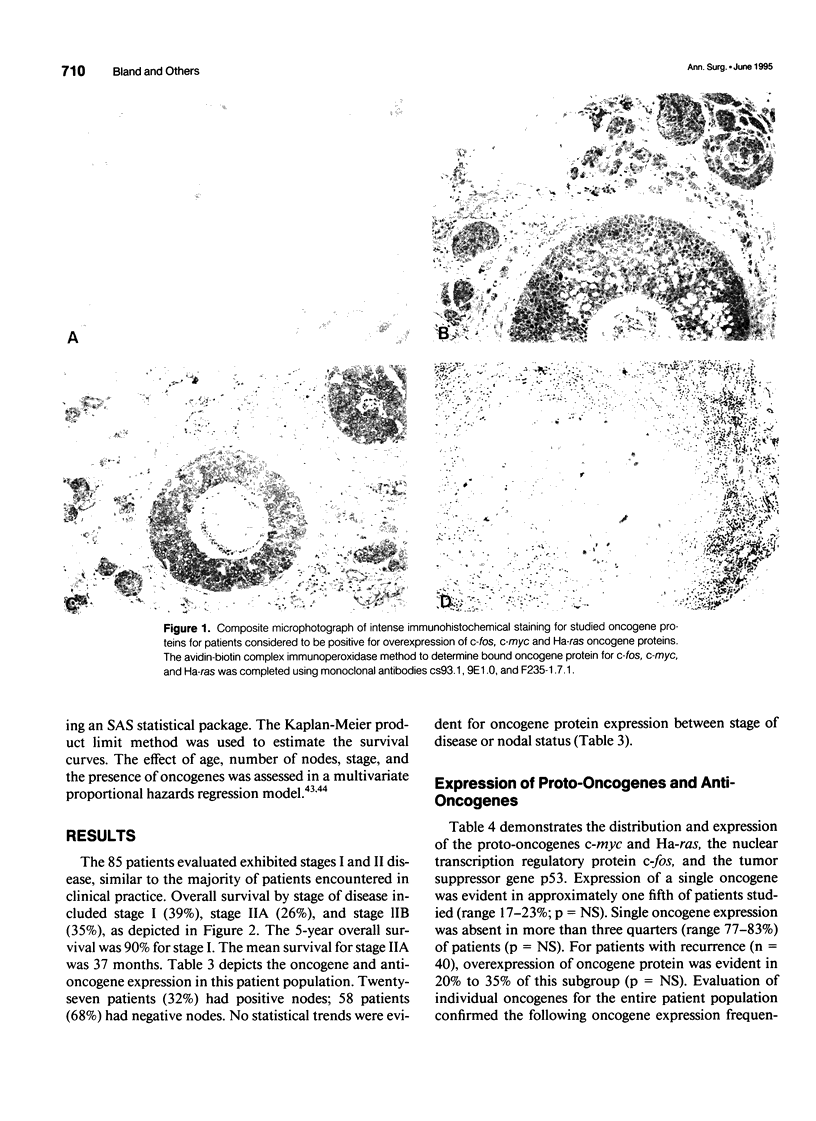




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnane J., Gaudray P., Simon M. P., Simony-Lafontaine J., Jeanteur P., Theillet C. Proto-oncogene amplification and human breast tumor phenotype. Oncogene. 1989 Nov;4(11):1389–1395. [PubMed] [Google Scholar]
- Allred D. C., Clark G. M., Elledge R., Fuqua S. A., Brown R. W., Chamness G. C., Osborne C. K., McGuire W. L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993 Feb 3;85(3):200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Andres A. C., Bchini O., Schubaur B., Dolder B., LeMeur M., Gerlinger P. H-ras induced transformation of mammary epithelium is favoured by increased oncogene expression or by inhibition of mammary regression. Oncogene. 1991 May;6(5):771–779. [PubMed] [Google Scholar]
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Bartkova J., Camplejohn R. S., Gullick W. J., Smith P. J., Millis R. R. Overexpression of the c-erbB-2 oncoprotein: why does this occur more frequently in ductal carcinoma in situ than in invasive mammary carcinoma and is this of prognostic significance? Eur J Cancer. 1992;28(2-3):644–648. doi: 10.1016/s0959-8049(05)80117-0. [DOI] [PubMed] [Google Scholar]
- Barton C. M., Staddon S. L., Hughes C. M., Hall P. A., O'Sullivan C., Klöppel G., Theis B., Russell R. C., Neoptolemos J., Williamson R. C. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991 Dec;64(6):1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bchini O., Andres A. C., Schubaur B., Mehtali M., LeMeur M., Lathe R., Gerlinger P. Precocious mammary gland development and milk protein synthesis in transgenic mice ubiquitously expressing human growth hormone. Endocrinology. 1991 Jan;128(1):539–546. doi: 10.1210/endo-128-1-539. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Borg A., Tandon A. K., Sigurdsson H., Clark G. M., Fernö M., Fuqua S. A., Killander D., McGuire W. L. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990 Jul 15;50(14):4332–4337. [PubMed] [Google Scholar]
- Caleffi M., Teague M. W., Jensen R. A., Vnencak-Jones C. L., Dupont W. D., Parl F. F. p53 gene mutations and steroid receptor status in breast cancer. Clinicopathologic correlations and prognostic assessment. Cancer. 1994 Apr 15;73(8):2147–2156. doi: 10.1002/1097-0142(19940415)73:8<2147::aid-cncr2820730820>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Callahan R., Campbell G. Mutations in human breast cancer: an overview. J Natl Cancer Inst. 1989 Dec 6;81(23):1780–1786. doi: 10.1093/jnci/81.23.1780. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Clair T., Miller W. R., Cho-Chung Y. S. Prognostic significance of the expression of a ras protein with a molecular weight of 21,000 by human breast cancer. Cancer Res. 1987 Oct 15;47(20):5290–5293. [PubMed] [Google Scholar]
- Clark G. M., Dressler L. G., Owens M. A., Pounds G., Oldaker T., McGuire W. L. Prediction of relapse or survival in patients with node-negative breast cancer by DNA flow cytometry. N Engl J Med. 1989 Mar 9;320(10):627–633. doi: 10.1056/NEJM198903093201003. [DOI] [PubMed] [Google Scholar]
- Clark G. M., McGuire W. L., Hubay C. A., Pearson O. H., Marshall J. S. Progesterone receptors as a prognostic factor in Stage II breast cancer. N Engl J Med. 1983 Dec 1;309(22):1343–1347. doi: 10.1056/nejm198312013092240. [DOI] [PubMed] [Google Scholar]
- Clark G. M., McGuire W. L. Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol. 1988 Apr;15(2 Suppl 1):20–25. [PubMed] [Google Scholar]
- Coon J. S., Deitch A. D., de Vere White R. W., Koss L. G., Melamed M. R., Reeder J. E., Weinstein R. S., Wersto R. P., Wheeless L. L. Interinstitutional variability in DNA flow cytometric analysis of tumors. The National Cancer Institute's Flow Cytometry Network Experience. Cancer. 1988 Jan 1;61(1):126–130. doi: 10.1002/1097-0142(19880101)61:1<126::aid-cncr2820610122>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Corliss D. A., White W. E., Jr Fluorescence of yeast vitally stained with ethidium bromide and propidium iodide. J Histochem Cytochem. 1981 Jan;29(1):45–48. doi: 10.1177/29.1.6162881. [DOI] [PubMed] [Google Scholar]
- Dati C., Muraca R., Tazartes O., Antoniotti S., Perroteau I., Giai M., Cortese P., Sismondi P., Saglio G., De Bortoli M. c-erbB-2 and ras expression levels in breast cancer are correlated and show a co-operative association with unfavorable clinical outcome. Int J Cancer. 1991 Apr 1;47(6):833–838. doi: 10.1002/ijc.2910470607. [DOI] [PubMed] [Google Scholar]
- Dressler L. G., Seamer L. C., Owens M. A., Clark G. M., McGuire W. L. DNA flow cytometry and prognostic factors in 1331 frozen breast cancer specimens. Cancer. 1988 Feb 1;61(3):420–427. doi: 10.1002/1097-0142(19880201)61:3<420::aid-cncr2820610303>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., O'Grady P., Devaney D., O'Siorain L., Fennelly J. J., Lijnen H. R. Tissue-type plasminogen activator, a new prognostic marker in breast cancer. Cancer Res. 1988 Mar 1;48(5):1348–1349. [PubMed] [Google Scholar]
- Elledge R. M., McGuire W. L., Osborne C. K. Prognostic factors in breast cancer. Semin Oncol. 1992 Jun;19(3):244–253. [PubMed] [Google Scholar]
- Ernberg I. T. Oncogenes and tumor growth factors in breast cancer. A minireview. Acta Oncol. 1990;29(3):331–334. doi: 10.3109/02841869009090009. [DOI] [PubMed] [Google Scholar]
- Feuer E. J., Wun L. M., Boring C. C., Flanders W. D., Timmel M. J., Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993 Jun 2;85(11):892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Fisher E. R., Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol. 1988 Jul;6(7):1076–1087. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Love S. B., Wright C., Barnes D. M., Gusterson B., Harris A. L., Altman D. G. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer. 1991 Mar;63(3):434–438. doi: 10.1038/bjc.1991.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. R., Lippman M. E., Veronesi U., Willett W. Breast cancer (3). N Engl J Med. 1992 Aug 13;327(7):473–480. doi: 10.1056/NEJM199208133270706. [DOI] [PubMed] [Google Scholar]
- Hayes D. F. Tumor markers for breast cancer. Ann Oncol. 1993 Dec;4(10):807–819. doi: 10.1093/oxfordjournals.annonc.a058385. [DOI] [PubMed] [Google Scholar]
- Hayes D. F. What would you do if this were your ... wife, sister, mother, self? J Clin Oncol. 1991 Jan;9(1):1–3. doi: 10.1200/JCO.1991.9.1.1. [DOI] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Isola J., Visakorpi T., Holli K., Kallioniemi O. P. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992 Jul 15;84(14):1109–1114. doi: 10.1093/jnci/84.14.1109. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kute T. E., Muss H. B., Cooper M. R., Case L. D., Buss D., Stanley V., Gregory B., Galleshaw J., Booher K. The use of flow cytometry for the prognosis of stage II adjuvant treated breast cancer patients. Cancer. 1990 Oct 15;66(8):1810–1816. doi: 10.1002/1097-0142(19901015)66:8<1810::aid-cncr2820660828>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lipponen P., Ji H., Aaltomaa S., Syrjänen S., Syrjänen K. p53 protein expression in breast cancer as related to histopathological characteristics and prognosis. Int J Cancer. 1993 Aug 19;55(1):51–56. doi: 10.1002/ijc.2910550110. [DOI] [PubMed] [Google Scholar]
- Liu E., Thor A., He M., Barcos M., Ljung B. M., Benz C. The HER2 (c-erbB-2) oncogene is frequently amplified in in situ carcinomas of the breast. Oncogene. 1992 May;7(5):1027–1032. [PubMed] [Google Scholar]
- Marks J. R., Humphrey P. A., Wu K., Berry D., Bandarenko N., Kerns B. J., Iglehart J. D. Overexpression of p53 and HER-2/neu proteins as prognostic markers in early stage breast cancer. Ann Surg. 1994 Apr;219(4):332–341. doi: 10.1097/00000658-199404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. R., Humphrey P. A., Wu K., Berry D., Bandarenko N., Kerns B. J., Iglehart J. D. Overexpression of p53 and HER-2/neu proteins as prognostic markers in early stage breast cancer. Ann Surg. 1994 Apr;219(4):332–341. doi: 10.1097/00000658-199404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen O., Bonderup O., Carl J., Panduro J., Pedersen K. O. The prognostic value of estrogen and progesterone receptors in female breast cancer. A single center study. Acta Oncol. 1991;30(6):691–695. doi: 10.3109/02841869109092441. [DOI] [PubMed] [Google Scholar]
- Mooi W. J., Peterse J. L. Progress in molecular biology of breast cancer. Eur J Cancer. 1992;28(2-3):623–625. doi: 10.1016/s0959-8049(05)80112-1. [DOI] [PubMed] [Google Scholar]
- Morrison B. W. The genetics of breast cancer. Hematol Oncol Clin North Am. 1994 Feb;8(1):15–27. [PubMed] [Google Scholar]
- Neville A. M., Bettelheim R., Gelber R. D., Säve-Söderbergh J., Davis B. W., Reed R., Torhorst J., Golouh R., Peterson H. F., Price K. N. Factors predicting treatment responsiveness and prognosis in node-negative breast cancer. The International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992 May;10(5):696–705. doi: 10.1200/JCO.1992.10.5.696. [DOI] [PubMed] [Google Scholar]
- Ostrowski J. L., Sawan A., Henry L., Wright C., Henry J. A., Hennessy C., Lennard T. J., Angus B., Horne C. H. p53 expression in human breast cancer related to survival and prognostic factors: an immunohistochemical study. J Pathol. 1991 May;164(1):75–81. doi: 10.1002/path.1711640113. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P. P., Groshen S., Saigo P. E., Kinne D. W., Hellman S. Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: a study of 644 patients with median follow-up of 18 years. J Clin Oncol. 1989 Sep;7(9):1239–1251. doi: 10.1200/JCO.1989.7.9.1239. [DOI] [PubMed] [Google Scholar]
- Sahin A. A., Ro J., Ro J. Y., Blick M. B., el-Naggar A. K., Ordonez N. G., Fritsche H. A., Smith T. L., Hortobagyi G. N., Ayala A. G. Ki-67 immunostaining in node-negative stage I/II breast carcinoma. Significant correlation with prognosis. Cancer. 1991 Aug 1;68(3):549–557. doi: 10.1002/1097-0142(19910801)68:3<549::aid-cncr2820680318>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sawan A., Randall B., Angus B., Wright C., Henry J. A., Ostrowski J., Hennessy C., Lennard T. W., Corbett I., Horne C. H. Retinoblastoma and p53 gene expression related to relapse and survival in human breast cancer: an immunohistochemical study. J Pathol. 1992 Sep;168(1):23–28. doi: 10.1002/path.1711680105. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Watson P. H., Dubik D. c-myc oncogene expression in estrogen-dependent and -independent breast cancer. Clin Chem. 1993 Feb;39(2):353–355. [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Benini E., Daidone M. G., Veneroni S., Boracchi P., Cappelletti V., Di Fronzo G., Veronesi U. p53 as an independent prognostic marker in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1993 Jun 16;85(12):965–970. doi: 10.1093/jnci/85.12.965. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Battifora H., Cline M. J. Expression of p21 ras oncoproteins in human cancers. Cancer Res. 1986 Mar;46(3):1465–1470. [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Battifora H., Cline M. J. Expression of p21 ras oncoproteins in human cancers. Cancer Res. 1986 Mar;46(3):1465–1470. [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Chirgwin J. M., McGuire W. L. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990 Feb 1;322(5):297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Ullrich A., McGuire W. L. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989 Aug;7(8):1120–1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- Thompson A. M., Anderson T. J., Condie A., Prosser J., Chetty U., Carter D. C., Evans H. J., Steel C. M. p53 allele losses, mutations and expression in breast cancer and their relationship to clinico-pathological parameters. Int J Cancer. 1992 Feb 20;50(4):528–532. doi: 10.1002/ijc.2910500405. [DOI] [PubMed] [Google Scholar]
- Thor A. D., Moore DH I. I., Edgerton S. M., Kawasaki E. S., Reihsaus E., Lynch H. T., Marcus J. N., Schwartz L., Chen L. C., Mayall B. H. Accumulation of p53 tumor suppressor gene protein: an independent marker of prognosis in breast cancers. J Natl Cancer Inst. 1992 Jun 3;84(11):845–855. doi: 10.1093/jnci/84.11.845. [DOI] [PubMed] [Google Scholar]
- Toikkanen S., Helin H., Isola J., Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. J Clin Oncol. 1992 Jul;10(7):1044–1048. doi: 10.1200/JCO.1992.10.7.1044. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Swallow J. E., Brammar W. J., Whittaker J. L., Walker R. A. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1(4):423–430. [PubMed] [Google Scholar]
- Winstanley J., Cooke T., Murray G. D., Platt-Higgins A., George W. D., Holt S., Myskov M., Spedding A., Barraclough B. R., Rudland P. S. The long term prognostic significance of c-erbB-2 in primary breast cancer. Br J Cancer. 1991 Mar;63(3):447–450. doi: 10.1038/bjc.1991.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zeillinger R., Kury F., Czerwenka K., Kubista E., Sliutz G., Knogler W., Huber J., Zielinski C., Reiner G., Jakesz R. HER-2 amplification, steroid receptors and epidermal growth factor receptor in primary breast cancer. Oncogene. 1989 Jan;4(1):109–114. [PubMed] [Google Scholar]



