Abstract
OBJECTIVE: In this study, the impact of preoperative chemotherapy and radiation on the histopathology of a subgroup of patients with rectal adenocarcinoma was examined. As well, survival, disease-free survival and pelvic recurrence rates were examined, and compared with a concurrent control group. SUMMARY BACKGROUND DATA: The optimal treatment of large rectal carcinomas remains controversial; current therapy usually involves abdominoperineal resection plus postoperative chemoradiation; the combination can be associated with significant postoperative morbidity. In spite of these measures, local recurrences and distant metastases continue as serious problems. METHODS: Fluorouracil, cisplatin, and 4500 cGy were administered preoperatively over a 5-week period, before definitive surgical resection in 43 patients. In this group of patients, all 43 had biopsy-proven lesions > 3 cm (median diameter), involving the entire rectal wall (as determined by sigmoidoscopy and computed tomography scan), with no evidence of extrapelvic disease. The patients ranged from 31 to 81 years of age (median 61 years), with a male:female ratio of 3:1. A concurrent control group consisting of 56 patients (median: 62 years, male:female ration of 3:2) with T2 and T3 lesions was used to compare survival, disease-free survival, and pelvic recurrence rates. RESULTS: The preoperative chemoradiation therapy was well tolerated, with no major complications. All patients underwent repeat sigmoidoscopy before surgery; none of the lesions progressed while patients underwent therapy, and 22 (51%) were determined to have complete clinical response. At the time of resection, 21 patients (49%) had gross disease, 9 (22%) patients had only residual microscopic disease, and 11 (27%) had sterile specimens. Of the 30 patients with evidence of residual disease, 4 had positive lymph nodes. In follow-up, 39 of the 43 remain alive (median follow-up = 25 months), and only 1 of the 11 patients with complete histologic response developed recurrent disease. Six of the 32 patients with residual disease (2 with positive nodes) have developed metastatic disease in follow-up (median time to diagnosis 10 months, range 3-15 months). Three of these patients with metastases have died (median survival after diagnosis of metastases = 36 months). Local recurrence was seen in only 2 of 43 patients (< 5%). Cox-Mantel analysis of Kaplan-Meier distributions demonstrated increased survival (p = 0.017), increased disease-free survival (p = 0.046), and decreased pelvic recurrence (p = 0.031) for protocol versus control patients. CONCLUSIONS: This therapeutic regimen has provided enhanced local control and decreased metastases. Furthermore, the marked degree of tumor downstaging, as seen by a 27% incidence of sterile pathologic specimens and a low rate of positive lymph nodes in this group with initially advanced lesions, strongly suggest that less radical surgery and sphincter preservation may be used with increasing frequency.
Full text
PDF



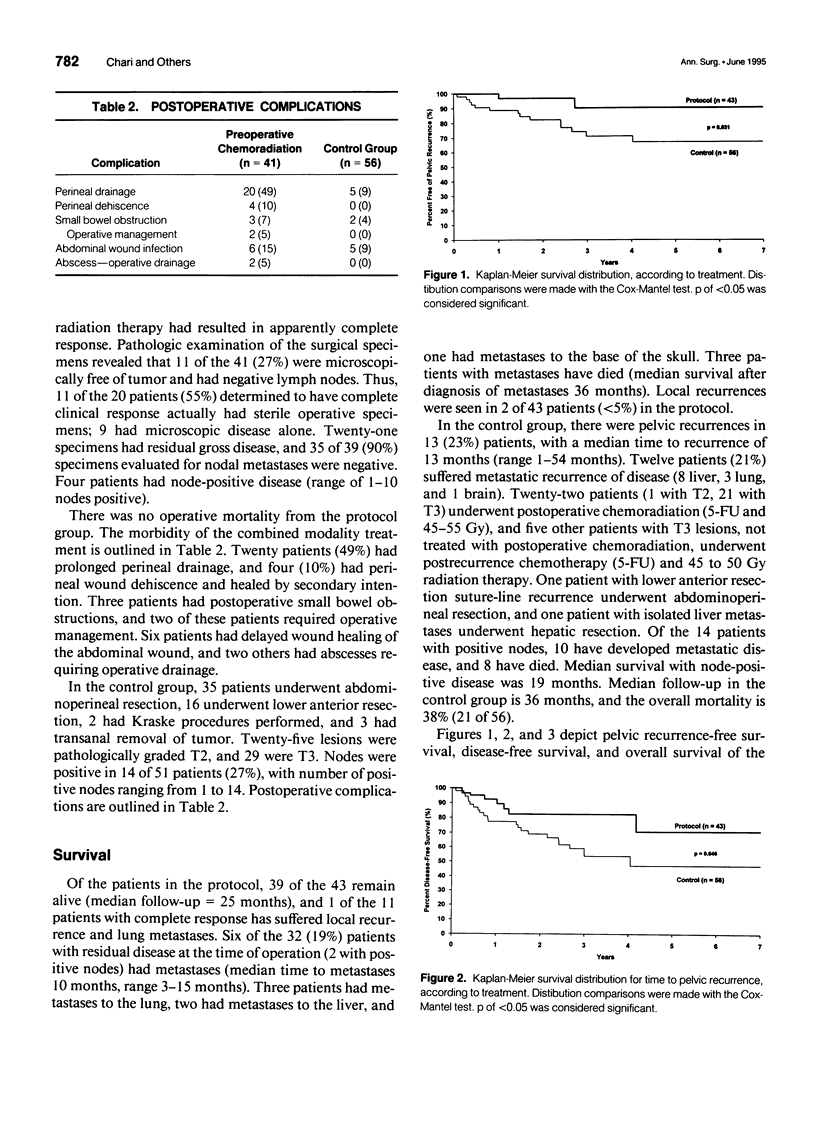
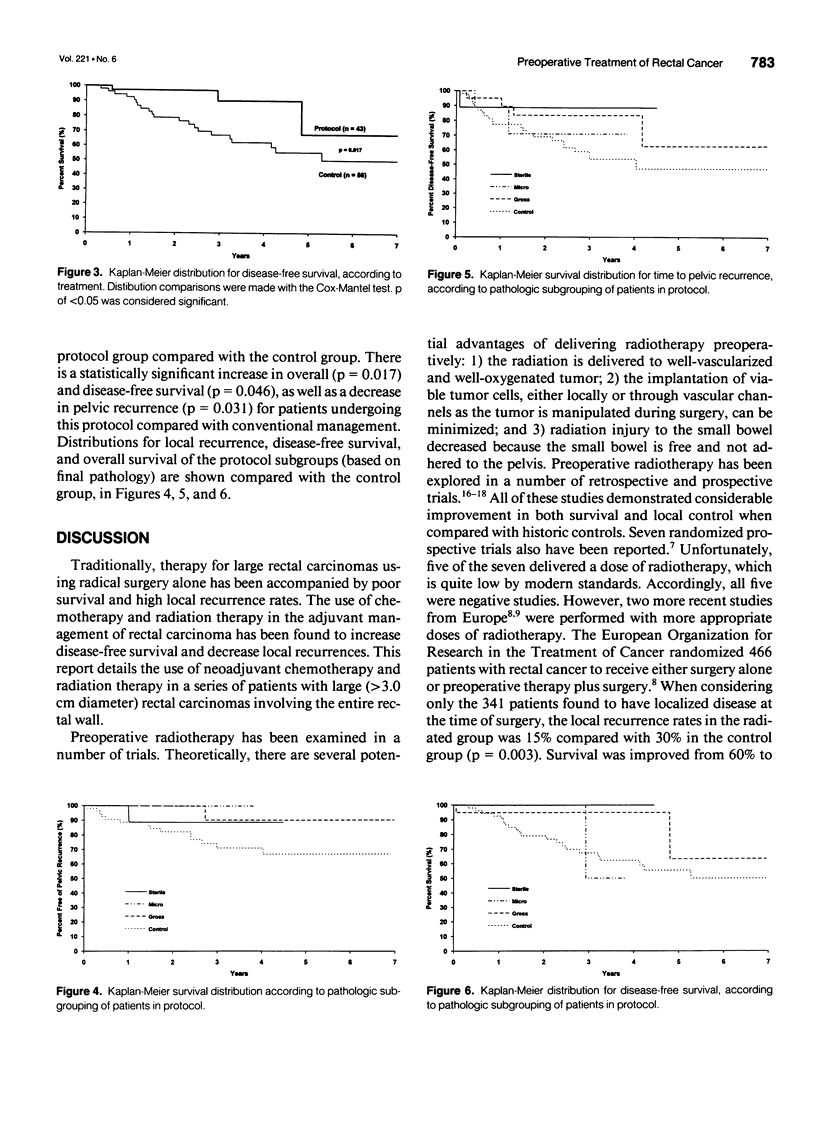



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg A. C., van der Kolk P. J., Dewit L., Bartelink H. Radiosensitization by cisplatin of RIF1 tumour cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Nov;50(5):871–884. doi: 10.1080/09553008614551291. [DOI] [PubMed] [Google Scholar]
- Beynon J., Mortensen N. J., Foy D. M., Channer J. L., Virjee J., Goddard P. Pre-operative assessment of local invasion in rectal cancer: digital examination, endoluminal sonography or computed tomography? Br J Surg. 1986 Dec;73(12):1015–1017. doi: 10.1002/bjs.1800731228. [DOI] [PubMed] [Google Scholar]
- Bleday R., Wong W. D. Recent advances in surgery for colon and rectal cancer. Curr Probl Cancer. 1993 Jan-Feb;17(1):1–65. doi: 10.1016/0147-0272(93)90003-k. [DOI] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T., Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994 Jan-Feb;44(1):7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- Byfield J. E., Frankel S. S., Sharp T. R., Hornbeck C. L., Callipari F. B. Phase I and pharmacologic study of 72-hour infused 5-fluorouracil and hyperfractionated cyclical radiation. Int J Radiat Oncol Biol Phys. 1985 Apr;11(4):791–800. doi: 10.1016/0360-3016(85)90313-x. [DOI] [PubMed] [Google Scholar]
- Dritschilo A., Piro A. J., Kelman A. D. The effect of cis-platinum on the repair of radiation damage in plateau phase Chinese hamster (V-79) cells. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1345–1349. doi: 10.1016/0360-3016(79)90667-9. [DOI] [PubMed] [Google Scholar]
- Galloway D. J., Cohen A. M., Shank B., Friedman M. A. Adjuvant multimodality treatment of rectal cancer. Br J Surg. 1989 May;76(5):440–447. doi: 10.1002/bjs.1800760507. [DOI] [PubMed] [Google Scholar]
- Gérard A., Buyse M., Nordlinger B., Loygue J., Pène F., Kempf P., Bosset J. F., Gignoux M., Arnaud J. P., Desaive C. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg. 1988 Nov;208(5):606–614. doi: 10.1097/00000658-198811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDELBERGER C., GRIESBACH L., MONTAG B. J., MOOREN D., CRUZ O., SCHNITZER R. J., GRUNBERG E. Studies on fluorinated pyrimidines. II. Effects on transplanted tumors. Cancer Res. 1958 Apr;18(3):305–317. [PubMed] [Google Scholar]
- Kodner I. J., Shemesh E. I., Fry R. D., Walz B. J., Myerson R., Fleshman J. W., Schechtman K. B. Preoperative irradiation for rectal cancer. Improved local control and long-term survival. Ann Surg. 1989 Feb;209(2):194–199. doi: 10.1097/00000658-198902000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook J. E., Moertel C. G., Gunderson L. L., Wieand H. S., Collins R. T., Beart R. W., Kubista T. P., Poon M. A., Meyers W. C., Mailliard J. A. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991 Mar 14;324(11):709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- Mendenhall W. M., Bland K. I., Rout W. R., Pfaff W. W., Million R. R., Copeland E. M., 3rd Clinically resectable adenocarcinoma of the rectum treated with preoperative irradiation and surgery. Dis Colon Rectum. 1988 Apr;31(4):287–290. doi: 10.1007/BF02554362. [DOI] [PubMed] [Google Scholar]
- Meterissian S., Skibber J., Rich T., Roubein L., Ajani J., Cleary K., Ota D. M. Patterns of residual disease after preoperative chemoradiation in ultrasound T3 rectal carcinoma. Ann Surg Oncol. 1994 Mar;1(2):111–116. doi: 10.1007/BF02303553. [DOI] [PubMed] [Google Scholar]
- Minsky B. D., Cohen A. M., Kemeny N., Enker W. E., Kelsen D. P., Reichman B., Saltz L., Sigurdson E. R., Frankel J. Enhancement of radiation-induced downstaging of rectal cancer by fluorouracil and high-dose leucovorin chemotherapy. J Clin Oncol. 1992 Jan;10(1):79–84. doi: 10.1200/JCO.1992.10.1.79. [DOI] [PubMed] [Google Scholar]
- Minsky B. D., Kemeny N., Cohen A. M., Enker W. E., Kelsen D. P., Reichman B., Saltz L., Sigurdson E. R., Frankel J. Preoperative high-dose leucovorin/5-fluorouracil and radiation therapy for unresectable rectal cancer. Cancer. 1991 Jun 1;67(11):2859–2866. doi: 10.1002/1097-0142(19910601)67:11<2859::aid-cncr2820671126>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mohiuddin M., Marks G. High dose preoperative irradiation for cancer of the rectum, 1976-1988. Int J Radiat Oncol Biol Phys. 1991 Jan;20(1):37–43. doi: 10.1016/0360-3016(91)90135-q. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Martenson J. A., Wieand H. S., Krook J. E., Macdonald J. S., Haller D. G., Mayer R. J., Gunderson L. L., Rich T. A. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994 Aug 25;331(8):502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- Paty P. B., Enker W. E., Cohen A. M., Lauwers G. Y. Treatment of rectal cancer by low anterior resection with coloanal anastomosis. Ann Surg. 1994 Apr;219(4):365–373. doi: 10.1097/00000658-199404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Rectal Cancer Study Group. Cancer. 1990 Jul 1;66(1):49–55. doi: 10.1002/1097-0142(19900701)66:1<49::aid-cncr2820660111>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Påhlman L., Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990 Feb;211(2):187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P., Garb J. L., Park W. C., Stark A. J., Chabot J. R., Friedmann P. Long-term results and complications of preoperative radiation in the treatment of rectal cancer. Surgery. 1988 Feb;103(2):161–167. [PubMed] [Google Scholar]
- Richmond R. C., Powers E. L. Radiation sensitization of bacterial spores by cis-dichlorodiammineplatinum(II). Radiat Res. 1976 Nov;68(2):251–257. [PubMed] [Google Scholar]
- Rotman M., Aziz H. Concomitant continuous infusion chemotherapy and radiation. Cancer. 1990 Feb 1;65(3 Suppl):823–835. doi: 10.1002/1097-0142(19900201)65:3+<823::aid-cncr2820651330>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Schaldenbrand J. D., Siders D. B., Zainea G. G., Thieme E. T. Preoperative radiation therapy for locally advanced carcinoma of the rectum. Clinicopathologic correlative review. Dis Colon Rectum. 1992 Jan;35(1):16–23. doi: 10.1007/BF02053333. [DOI] [PubMed] [Google Scholar]
- Shingleton W. W., Prosnitz L. R. Adjuvant therapy of colorectal cancer. Curr Probl Cancer. 1985 Oct;9(10):1–34. doi: 10.1016/s0147-0272(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Stevens K. R., Jr, Allen C. V., Fletcher W. S. Preoperative radiotherapy for adenocarcinoma of the rectosigmoid. Cancer. 1976 Jun;37(6):2866–2874. doi: 10.1002/1097-0142(197606)37:6<2866::aid-cncr2820370644>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Stevens K. R., Jr, Fletcher W. S., Allen C. V. Anterior resection and primary anastomosis following high dose preoperative irradiation for adenocarcinoma of the recto-sigmoid. Cancer. 1978 May;41(5):2065–2071. doi: 10.1002/1097-0142(197805)41:5<2065::aid-cncr2820410555>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- VERMUND H., HODGETT J., ANSFIELD F. J. Effects of combined roentgen irradiation and chemotherapy on transplanted tumors in mice. Am J Roentgenol Radium Ther Nucl Med. 1961 Mar;85:559–567. [PubMed] [Google Scholar]
- Wallner K. E., Li G. C. Effect of cisplatin resistance on cellular radiation response. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):587–591. doi: 10.1016/0360-3016(87)90076-9. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Koness R. J., Vezeridis M. P., Cohen S. I., Wrobleski D. E. Pelvic resection of recurrent rectal cancer. Ann Surg. 1994 Oct;220(4):586–597. doi: 10.1097/00000658-199410000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zák M., Drobník J. Effect of cis-dichlorodiamine platinum (II) on the post-irradiation lethality in mice after irradiation with X-rays. Strahlentherapie. 1971 Jul;142(1):112–115. [PubMed] [Google Scholar]


