Abstract
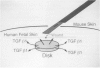
OBJECTIVE: Fetal skin wounds heal without scarring. To determine the role of TGF-beta 1 in fetal wound healing, mRNA expression of TGF-beta 1 was analyzed in human fetal and adult skin wounds. METHODS: Human fetal skin transplanted to a subcutaneous location on an adult athymic mouse that was subsequently wounded heals without scar, whereas human adult skin heals with scar formation in that location. In situ hybridization for TGF-beta 1 mRNA expression and species-specific immunohistochemistry for fibroblasts, macrophages, and neutrophils were performed in human adult wounds, fetal wounds, and fetal wounds treated with a TGF-beta 1 slow release disk. RESULTS: Transforming growth factor-beta 1 mRNA expression was induced by wounding adult skin. No TGF-beta 1 mRNA upregulation was detected in human fetal skin after wounding. However, when exogenous TGF-beta 1 was added to human fetal skin, induction of TGF-beta 1 mRNA expression in human fetal fibroblasts occurred, an adult-like inflammatory response was detected, and the skin healed with scar formation. CONCLUSIONS: Transforming growth factor-beta 1 is an important modulator in scar formation. Anti-TGF-beta 1 strategies may promote scarless healing in adult wounds.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzick N. S., Harrison M. R., Glick P. L., Beckstead J. H., Villa R. L., Scheuenstuhl H., Goodson W. H., 3rd Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985 Aug;20(4):315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- Adzick N. S., Longaker M. T. Scarless wound healing in the fetus: the role of the extracellular matrix. Prog Clin Biol Res. 1991;365:177–192. [PubMed] [Google Scholar]
- Adzick N. S., Lorenz H. P. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg. 1994 Jul;220(1):10–18. doi: 10.1097/00000658-199407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Krummel T. M., Michna B. A., Thomas B. L., Sporn M. B., Nelson J. M., Salzberg A. M., Cohen I. K., Diegelmann R. F. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988 Jul;23(7):647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Lane A. T., Scott G. A., Day K. H. Development of human fetal skin transplanted to the nude mouse. J Invest Dermatol. 1989 Dec;93(6):787–791. doi: 10.1111/1523-1747.ep12284423. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Adzick N. S., Crombleholme T. M., Langer J. C., Duncan B. W., Bradley S. M., Stern R., Ferguson M. W., Harrison M. R. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990 Jan;25(1):63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Longaker M. T., Perkocha L. A., Jennings R. W., Harrison M. R., Adzick N. S. Scarless wound repair: a human fetal skin model. Development. 1992 Jan;114(1):253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Hopkinson-Woolley J., McCluskey J. Growth factors and cutaneous wound repair. Prog Growth Factor Res. 1992;4(1):25–44. doi: 10.1016/0955-2235(92)90003-z. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Richart R. M. A comparison of biotin- and 35S-based in situ hybridization methodologies for detection of human papillomavirus DNA. Lab Invest. 1989 Oct;61(4):471–476. [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992 Jan 25;339(8787):213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994 May;107(Pt 5):1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. M., Lorenz H. P., Meuli M., Lin R. Y., Adzick N. S. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg. 1995 Feb;30(2):198–203. doi: 10.1016/0022-3468(95)90560-x. [DOI] [PubMed] [Google Scholar]
- Syrjänen S., Partanen P., Mäntyjärvi R., Syrjänen K. Sensitivity of in situ hybridization techniques using biotin- and 35S-labeled human papillomavirus (HPV) DNA probes. J Virol Methods. 1988 Mar-Apr;19(3-4):225–238. doi: 10.1016/0166-0934(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991 Sep;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Wikner N. E., Persichitte K. A., Baskin J. B., Nielsen L. D., Clark R. A. Transforming growth factor-beta stimulates the expression of fibronectin by human keratinocytes. J Invest Dermatol. 1988 Sep;91(3):207–212. doi: 10.1111/1523-1747.ep12464997. [DOI] [PubMed] [Google Scholar]