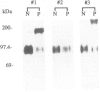
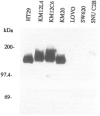
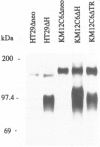
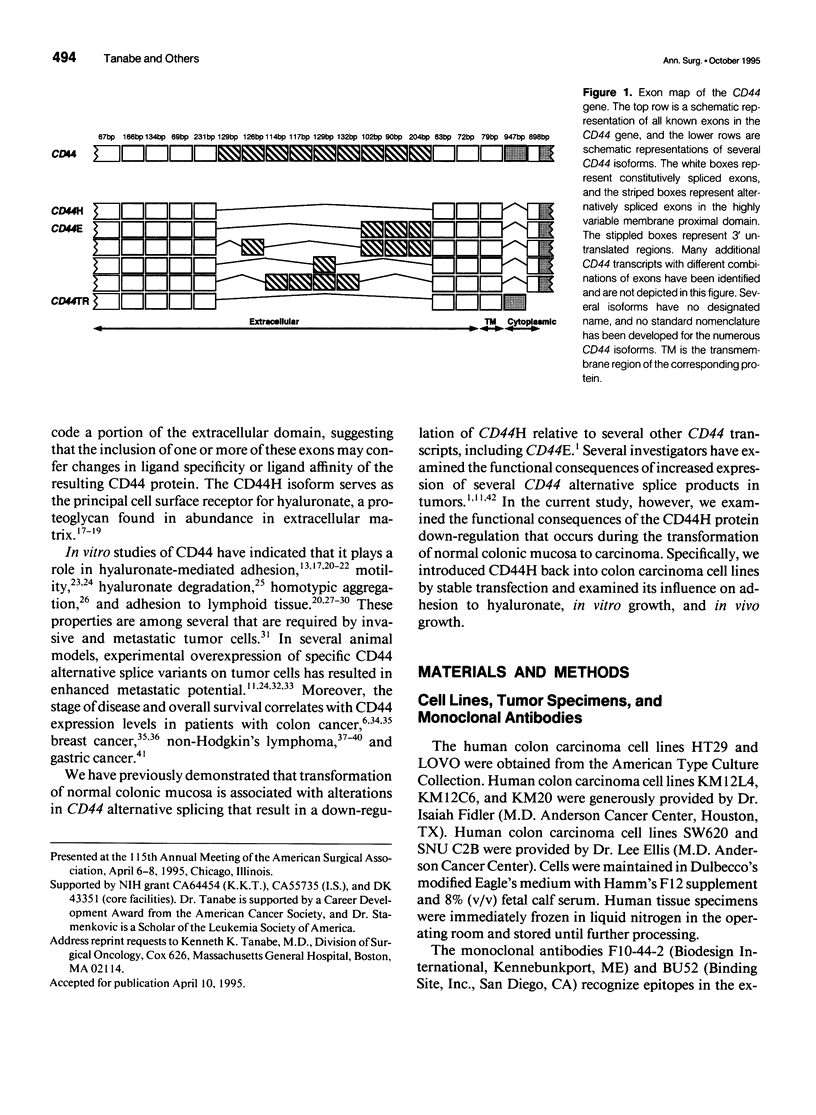
Abstract
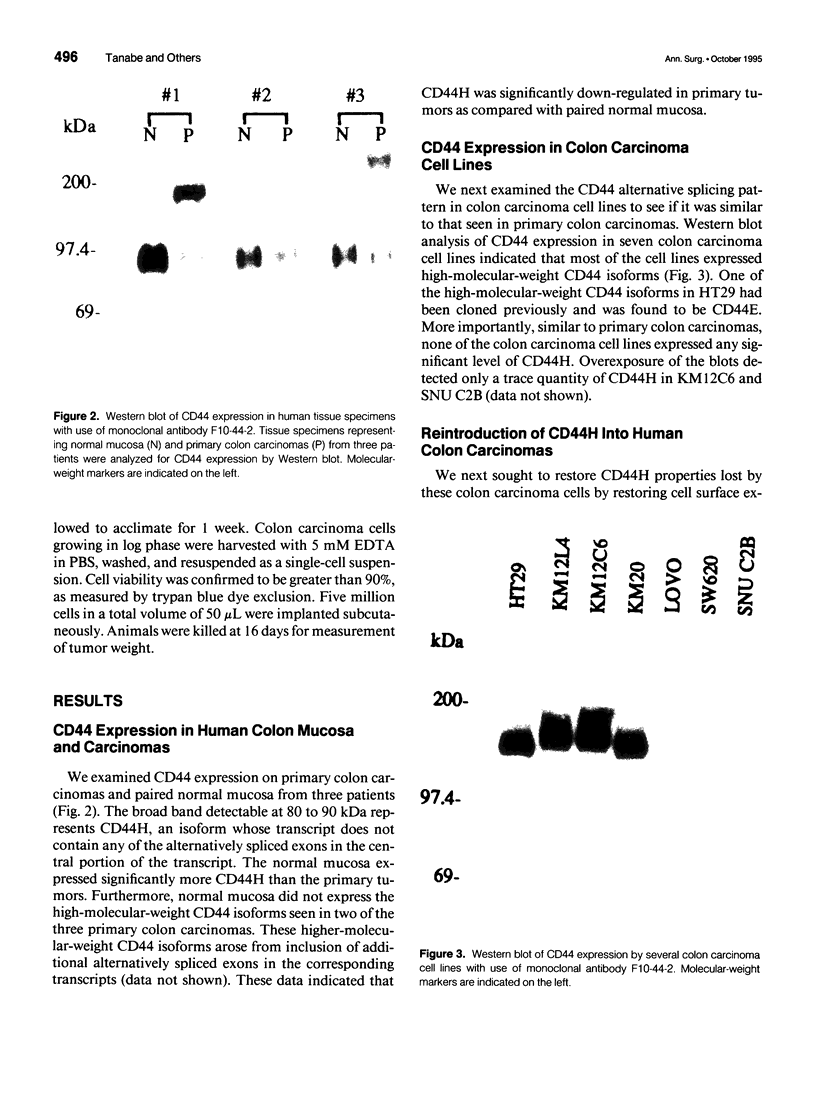
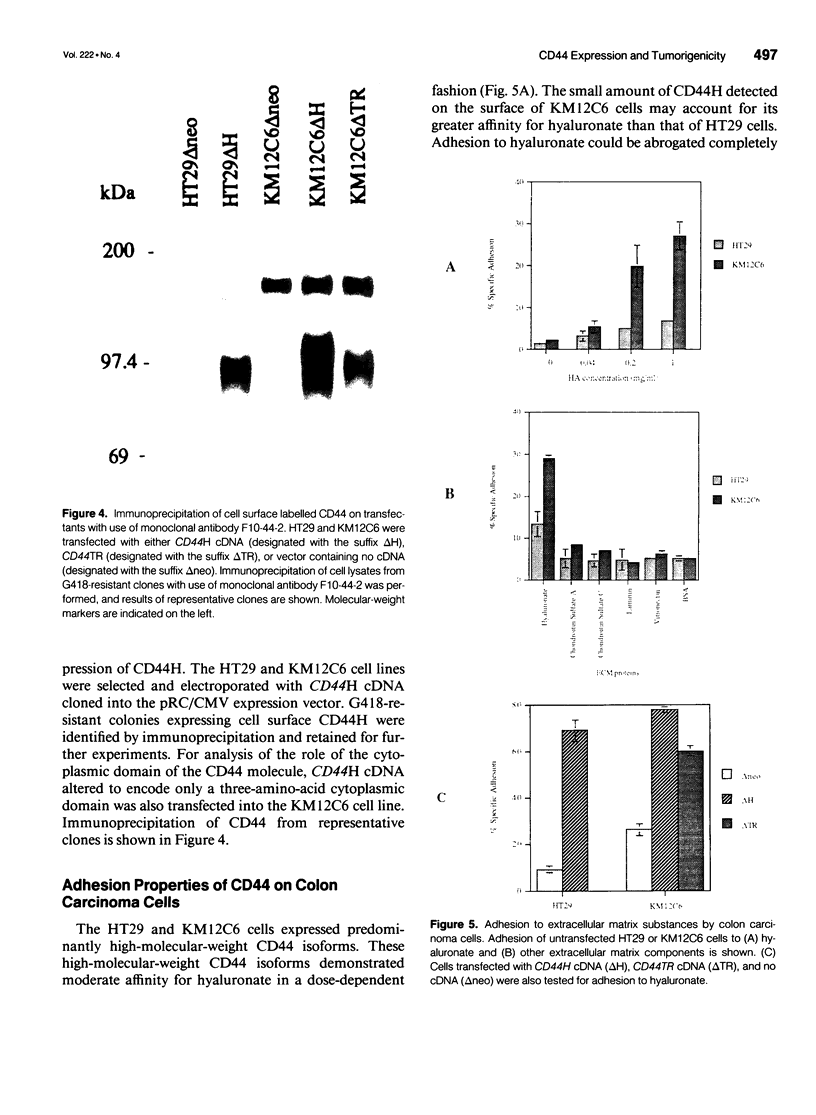
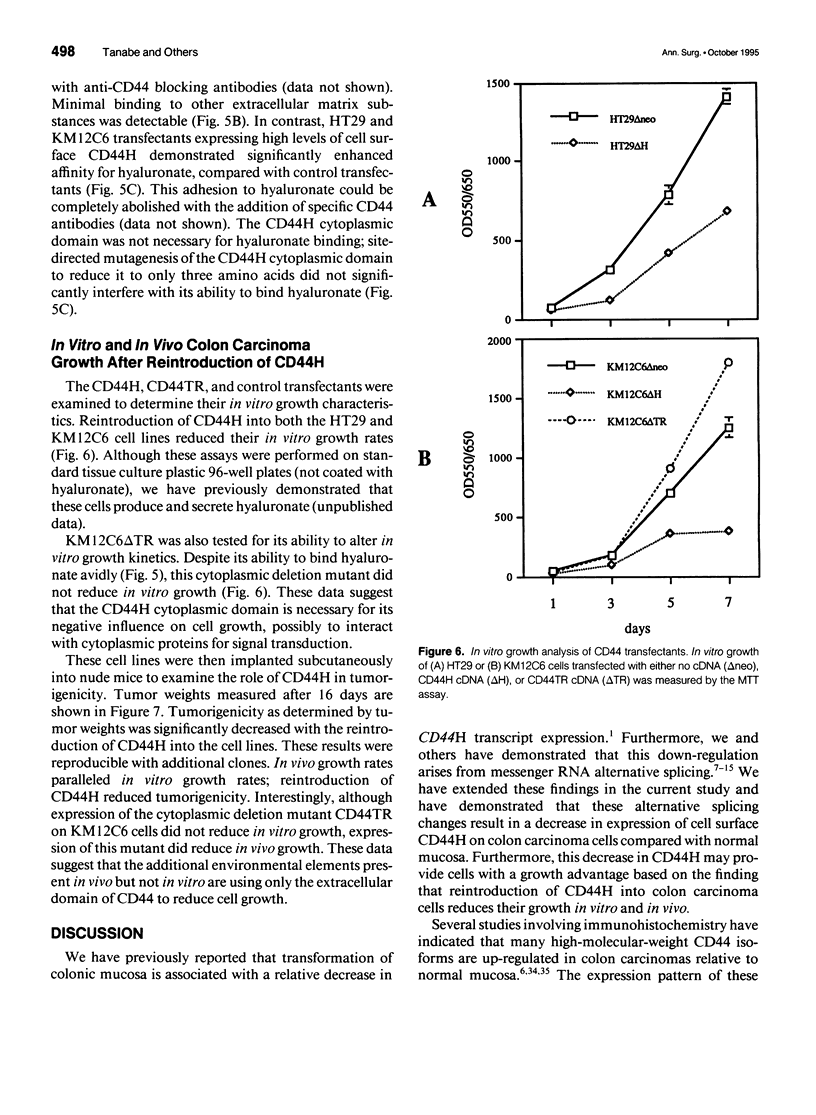
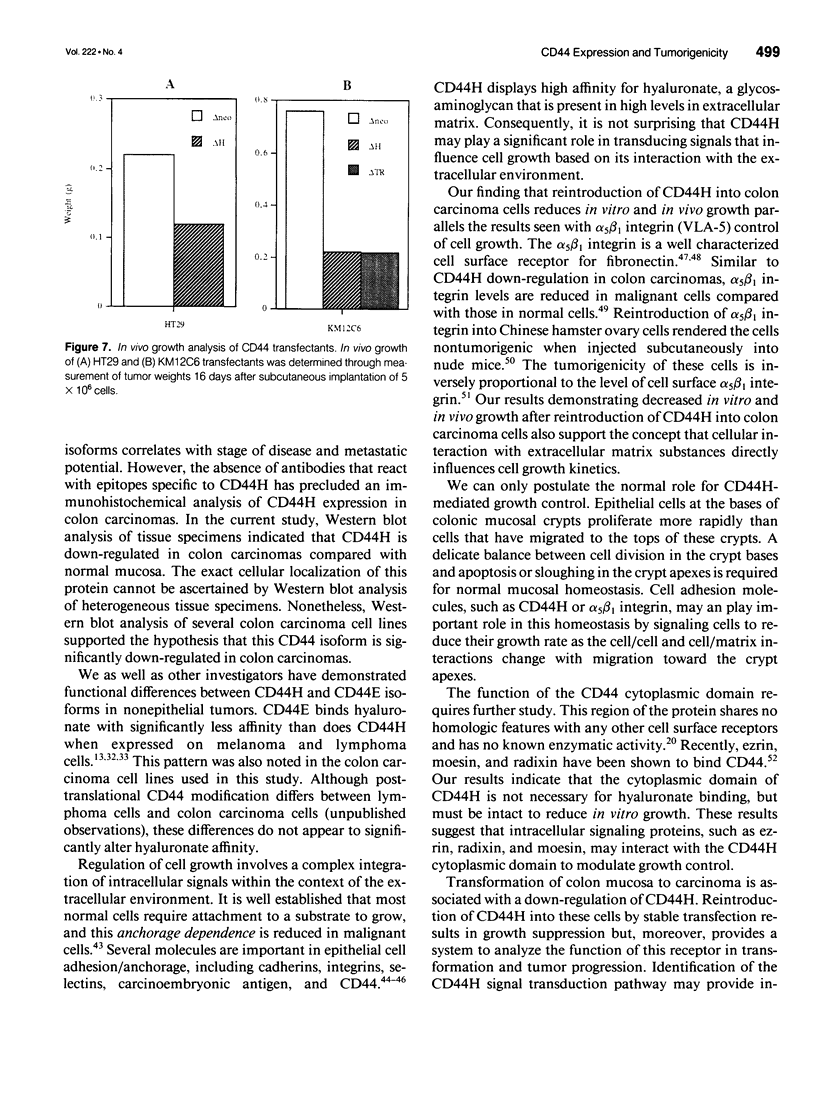
OBJECTIVE: The functional consequences of reintroduction of the CD44H cell adhesion molecule into colon carcinomas were investigated. BACKGROUND: CD44 is a cell surface adhesion molecule that is normally present in numerous isoforms as a result of messenger RNA alternative splicing. Individual CD44 isoforms differ in their ability to enhance tumorigenic or metastatic potential when overexpressed on tumor cells. Reverse transcriptase-polymerase chain reaction analysis demonstrates that CD44H is down-regulated during transformation of normal colon mucosa to carcinoma. The functional consequences of CD44H down-regulation in colon carcinomas has not been clarified. METHODS: Tumor cell lines and fresh tissue specimens were examined for CD44 expression by Western blot analysis. CD44H cDNA and site-directed mutants of CD44H cDNA were transfected into colon carcinoma cells. Stable transfectants were examined for adhesion to hyaluronate, in vitro growth, and in vivo growth. RESULTS: CD44H expression was nearly undetectable in primary colon carcinomas and colon carcinoma cell lines. In contrast, normal mucosa expressed high levels of CD44H. When CD44H was reintroduced into colon carcinoma cells, their in vitro and in vivo growth was significantly reduced. This CD44H-mediated growth rate reduction required an intact cytoplasmic domain. CONCLUSIONS: Transformation of normal mucosa to colon carcinoma is associated with a down-regulation of CD44H, which consequently may enhance the growth rate and tumorigenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Peach R., Aruffo A., Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994 Jul 1;180(1):53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch M., Mitchell S., Hart I. R. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991 Dec 15;51(24):6660–6667. [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Kansas G. S., Niloff J., DeFranzo B., Kim Y., Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993 Aug 15;53(16):3830–3838. [PubMed] [Google Scholar]
- Cooper D. L., Dougherty G., Harn H. J., Jackson S., Baptist E. W., Byers J., Datta A., Phillips G., Isola N. R. The complex CD44 transcriptional unit; alternative splicing of three internal exons generates the epithelial form of CD44. Biochem Biophys Res Commun. 1992 Jan 31;182(2):569–578. doi: 10.1016/0006-291x(92)91770-q. [DOI] [PubMed] [Google Scholar]
- Culty M., Miyake K., Kincade P. W., Sikorski E., Butcher E. C., Underhill C., Silorski E. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990 Dec;111(6 Pt 1):2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culty M., Nguyen H. A., Underhill C. B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992 Feb;116(4):1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Cooper D. L., Memory J. F., Chiu R. K. Ligand binding specificity of alternatively spliced CD44 isoforms. Recognition and binding of hyaluronan by CD44R1. J Biol Chem. 1994 Mar 25;269(12):9074–9078. [PubMed] [Google Scholar]
- Dougherty G. J., Landorp P. M., Cooper D. L., Humphries R. K. Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med. 1991 Jul 1;174(1):1–5. doi: 10.1084/jem.174.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fox S. B., Fawcett J., Jackson D. G., Collins I., Gatter K. C., Harris A. L., Gearing A., Simmons D. L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994 Aug 15;54(16):4539–4546. [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Guo Y. J., Liu G., Wang X., Jin D., Wu M., Ma J., Sy M. S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994 Jan 15;54(2):422–426. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hofmann M., Rudy W., Zöller M., Tölg C., Ponta H., Herrlich P., Günthert U. CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res. 1991 Oct 1;51(19):5292–5297. [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Radaszkiewicz T., Ossekoppele G. J., Van Krieken J. H., Pals S. T. Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA-1 (CD11a/18), and ICAM-1 (CD54). Leukemia. 1990 Aug;4(8):595–599. [PubMed] [Google Scholar]
- Jackson D. G., Buckley J., Bell J. I. Multiple variants of the human lymphocyte homing receptor CD44 generated by insertions at a single site in the extracellular domain. J Biol Chem. 1992 Mar 5;267(7):4732–4739. [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Jalkanen S., Joensuu H., Söderström K. O., Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J Clin Invest. 1991 May;87(5):1835–1840. doi: 10.1172/JCI115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Klemi P. J., Toikkanen S., Jalkanen S. Glycoprotein CD44 expression and its association with survival in breast cancer. Am J Pathol. 1993 Sep;143(3):867–874. [PMC free article] [PubMed] [Google Scholar]
- Joensuu H., Ristamäki R., Klemi P. J., Jalkanen S. Lymphocyte homing receptor (CD44) expression is associated with poor prognosis in gastrointestinal lymphoma. Br J Cancer. 1993 Aug;68(2):428–432. doi: 10.1038/bjc.1993.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., He Q., Miyake K., Hamann A., Hyman R., Kincade P. W. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992 Jan 1;175(1):257–266. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Hamou M. F., de Tribolet N., Jaufeerally R., Hofmann M., Diserens A. C., Van Meir E. G. Variant CD44 adhesion molecules are expressed in human brain metastases but not in glioblastomas. Cancer Res. 1993 Nov 15;53(22):5345–5349. [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. W., Kruyt P. M., Sewnath M., Oosting J., Seldenrijk C. A., Weidema W. F., Offerhaus G. J., Pals S. T. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994 Nov 26;344(8935):1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Nagasaka S., Tanabe K. K., Bruner J. M., Saya H., Sawaya R. E., Morrison R. S. Alternative RNA splicing of the hyaluronic acid receptor CD44 in the normal human brain and in brain tumors. J Neurosurg. 1995 May;82(5):858–863. doi: 10.3171/jns.1995.82.5.0858. [DOI] [PubMed] [Google Scholar]
- Pals S. T., Horst E., Ossekoppele G. J., Figdor C. G., Scheper R. J., Meijer C. J. Expression of lymphocyte homing receptor as a mechanism of dissemination in non-Hodgkin's lymphoma. Blood. 1989 Mar;73(4):885–888. [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schreiner C., Fisher M., Hussein S., Juliano R. L. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991 Mar 15;51(6):1738–1740. [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- St John T., Meyer J., Idzerda R., Gallatin W. M. Expression of CD44 confers a new adhesive phenotype on transfected cells. Cell. 1990 Jan 12;60(1):45–52. doi: 10.1016/0092-8674(90)90714-p. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tanabe K. K., Ellis L. M., Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993 Mar 20;341(8847):725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- Tanabe K. K., Nishi T., Saya H. Novel variants of CD44 arising from alternative splicing: changes in the CD44 alternative splicing pattern of MCF-7 breast carcinoma cells treated with hyaluronidase. Mol Carcinog. 1993;7(4):212–220. doi: 10.1002/mc.2940070403. [DOI] [PubMed] [Google Scholar]
- Thomas L., Byers H. R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992 Aug;118(4):971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Sato N., Sagara J., Kawai A., Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994 Jul;126(2):391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölg C., Hofmann M., Herrlich P., Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993 Mar 11;21(5):1225–1229. doi: 10.1093/nar/21.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielenga V. J., Heider K. H., Offerhaus G. J., Adolf G. R., van den Berg F. M., Ponta H., Herrlich P., Pals S. T. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993 Oct 15;53(20):4754–4756. [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]