Abstract
Event-related brain potentials (ERPs) are altered in patients with a variety of psychiatric disorders and may represent quantitative correlates of disease liability that are more amenable to genetic analysis than disease status itself. Results of a genomewide linkage screen are presented for amplitude of the N4 and P3 components of the ERP, measured at 19 scalp locations in response to a semantic priming task for 604 individuals in 100 pedigrees ascertained as part of the Collaborative Study on the Genetics of Alcoholism. N4 and P3 amplitudes in response to three stimuli (nonwords, primed words [i.e., antonyms], and unprimed words) all showed significant heritabilities, the highest being .54. Both N4 and P3 showed significant genetic correlations across stimulus type at a given lead and across leads within a stimulus, indicating shared genetic influences among the traits. There were also substantial genetic correlations between the N4 and P3 amplitudes for a given lead, even across stimulus type. N4 amplitudes showed suggestive evidence of linkage in several chromosomal regions, and P3 amplitudes showed significant evidence of linkage to chromosome 5 and suggestive evidence of linkage to chromosome 4.
Introduction
Event-related brain potentials (ERPs) are recordings of neuroelectric activity, usually in response to some task, made from electrodes on the scalp. ERPs have been shown to be altered in patients with a variety of psychiatric disorders and in members of their families, compared with the general population. In particular, alcoholic subjects have a reduction of amplitude of the P3 component, a positive peak ∼300–600 ms after a stimulus, that remains after long periods of abstinence from alcohol (Porjesz and Begleiter 1985). A similar reduction in P3 amplitude is also seen in the young alcohol-naive sons of alcoholic probands (Begleiter et al. 1984). P3 amplitude is thought to be related to stimulus significance and the reduced amplitude in alcoholics and their relatives implies difficulty in discriminating stimulus relevance (Porjesz and Begleiter 1996). Although some studies have not observed a reduction in P3 amplitude in family members of alcoholics, meta-analysis suggests that these inconsistencies may be due to differences in stimulus modality and task difficulty of the paradigms used to elicit the P3 (Polich et al. 1994). Similar neuroelectric alterations have been suggested in other psychiatric disorders. For example, characteristic ERP response patterns also have been demonstrated in schizophrenic patients and their first-degree relatives (Blackwood et al. 1991). Because these ERP phenomena occur not only in psychiatric patients themselves but also in their clinically unaffected relatives, the neuroelectric abnormalities may reflect processes that underlie liability to these complex, multifactorial disorders, rather than functional changes caused by disease progression. Thus, an assessment of the genetics of ERPs may provide insight into the underlying neuropathology involved in liability to various psychiatric conditions.
The current experiment consisted of a lexical decision task requiring subjects to indicate as rapidly as possible whether a letter string was or was not a word. Words preceded by semantically related words (antonyms) were more quickly recognized as words (primed) than those preceded by unrelated words (unprimed). N4 and P3 components were recorded at 19 scalp locations to three different stimuli: nonwords (jumbles), primed words (antonyms), and unprimed words. The N4 component is a negative component occurring ∼400–600 ms after an incongruent (unprimed) word among contextually related (primed) words. The more incongruent the unprimed word is, the greater the amplitude of the N4 peak (Kutas and Van Petten 1988). Although N4 is obtained to unprimed but not primed words in normal subjects, alcoholics manifest N4s to both primed and unprimed words (Porjesz and Begleiter 1996). The failure of priming to suppress N4 amplitude in the alcoholic subjects indicates semantic memory deficits and suggests that N4 amplitude, like P3 amplitude, may be an electrophysiological endophenotype indexing underlying genetic susceptibility to alcohol dependence. Abnormalities of N4 amplitude in semantic priming tasks have also been observed in individuals with schizophrenia (Grillon et al. 1991; Adams et al. 1993), disruptive behavior disorders (Knott et al. 1998), and autism (Strandberg et al. 1993). The purpose of the current study was to quantify the strength of genetic influences on these potential endophenotypes, assess the degree of pleiotropy between them, and localize quantitative trait loci (QTLs) influencing variation in N4 and P3 response. Such QTLs would become candidate loci for alcohol dependence. Toward this end, the heritabilities of N4 and P3 amplitudes were estimated for each lead and each stimulus type, bivariate quantitative genetic analyses were performed, and multipoint genomic linkage screens were conducted.
Methods
The families in this study were ascertained through six separate sites as part of the Collaborative Study on the Genetics of Alcoholism (COGA). The COGA project (Begleiter et al. 1995) was designed to investigate genetic influences on susceptibility to alcohol dependence both through direct analyses of the disease phenotype and through related risk factors such as ERPs. The COGA sample includes a variety of phenotypic data from families ascertained through one or several alcoholic probands and from randomly ascertained control families. Genotypic and electrophysiological data are available for a subset of individuals in the densely affected families. Probands in these families met both DSM-III-R criteria for alcohol dependence (American Psychiatric Association 1987) and Feighner definite criteria for alcoholism (Feighner et al. 1972) and were also required to have two additional first-degree relatives who were alcohol dependent by the same criteria for a family to enter stage 2 of the study, in which a genome screen was conducted. In 100 families meeting these criteria, 604 individuals were examined in identical electrophysiological laboratories at the six COGA data collection sites. Although family members provided a detailed psychiatric history, no one was excluded on this basis. Family members also completed a neuropsychological battery and a family history questionnaire, with electroencephalogram (EEG)/ERP data and blood samples collected for subsequent analyses. These procedures were approved by the institutional review boards of all six COGA sites, and all participants gave informed consent. Subjects ranged in age from 16 to 70 years and included approximately equal numbers of males and females (51% versus 49%).
The experimental design used for ERP studies in the COGA project have been described in detail elsewhere, in studies documenting the consistency of measurements across the six COGA laboratories (Alexander et al. 1994; Cohen et al. 1994; Kuperman et al. 1995), and the protocols will only briefly be reviewed here. Subjects were seated in a dimly lit sound-attenuated chamber (Industrial Acoustics) and wore a fitted electrode cap (Electro-Cap International) containing the 21 leads of the 10–20 international system. The tip of the nose served as the reference and the forehead as ground. Electrical activity was amplified 10 K (Sensorium EPA-2 Electrophysiology Amplifier) over a bandwidth of 0.02–50 Hz and was continuously sampled (Concurrent 5550 computer) at a rate of 256 Hz. Vertical and horizontal eye movements were monitored, and artifact rejection was performed on-line. Digital filtering (8 Hz low-pass filter) of the accumulated data was performed off-line.
The current experiment consisted of a lexical decision task requiring subjects to indicate as rapidly as possible, with a button-press response, whether a letter string was or was not a word. Words preceded by semantically related words (antonyms) were more quickly recognized as words (primed) than those preceded by unrelated words (unprimed). Both N4 and P3 components were recorded at 19 scalp locations to three different stimuli: nonwords (jumbles), primed words (antonyms), and unprimed words. Target trials with a response time of >1,000 ms were rejected. Speed of response was emphasized but not at the expense of accuracy. ERPs were averaged across trials for each type of stimulus, and a semiautomatic peak picking procedure was used. The P3 component of the response was selected as the largest positive peak within a time window of 400–600 ms. The N4 component was selected as the largest negative peak immediately preceding the P3 peak, generally between 300 and 600 ms. Peak amplitude was measured relative to the prestimulus baseline (125 ms of EEG prior to stimulus onset), and peak latency was taken as the time point with the maximum positive or negative amplitude within the specified time window.
The 100 families examined ranged in size from 2 to 20 phenotyped individuals, with most pedigrees having two generations of family members examined and a few having three generations. The complexity of these pedigrees and their information content are illustrated by the number and variety of pairwise relationships within the families. These pedigrees encompass 1,759 phenotyped relative pairs, including 497 parent-child pairs, 758 sibling pairs, 335 avuncular pairs, and 104 first-cousin pairs, as well as a number of grandparent-grandchild, half-sibling, half-avuncular, and half-cousin pairs. It should be noted that these relative pair counts are provided to illustrate the complexity of the COGA pedigrees, since the maximum-likelihood analyses employed use entire pedigrees simultaneously.
Additive genetic heritabilities and their standard errors were calculated by use of standard maximum-likelihood variance decomposition techniques, implemented in SOLAR (Almasy and Blangero 1998), with phenotypes regressed for age and sex prior to analyses. P values were obtained by comparing a model in which additive genetic heritability was estimated to one in which that parameter was fixed at 0. The difference in loge likelihood between these two models is distributed as a mixture of a χ2 distribution with 1 df and a point mass at 0 (Self and Liang 1987). Phenotypic, genetic, and environmental correlations were obtained through bivariate variance-component analyses, using a modified version of SOLAR (Almasy et al. 1997).
Individuals were genotyped for a complete genome screen, including 312 highly polymorphic microsatellite markers spaced at ∼15-cM intervals. Multipoint linkage analyses were conducted using a maximum-likelihood variance component method, implemented in SOLAR. The difference in log10 likelihood between a model in which a QTL effect is estimated and one in which it is fixed at 0 provides a LOD score.
Results
Table 1 summarizes the additive genetic heritabililies of N4 and P3 amplitudes in response to primed, unprimed, and nonsense words. Heritabilities for P3 amplitude were greater than those for N4 amplitude and tended to be greatest for occipital leads. Although a Bonferroni correction is unduly conservative, since these 114 traits are highly intercorrelated, the 35 traits in table 1 marked with four asterisks have heritabilities with P<.05 after a Bonferroni correction.
Table 1.
Heritabilities of N4 and P3 Amplitude in Response to Primed, Unprimed, and Nonsense Words ± SE[Note]
| N4 |
P3 |
|||||
| Lead | Primed | Unprimed | Nonsense | Primed | Unprimed | Nonsense |
| Fp1 | .22 ± .07** | .23 ± .08** | .25 ± .08** | .27 ± .08*** | .25 ± .08** | .21 ± .08** |
| Fp2 | .19 ± .07** | .16 ± .08* | .26 ± .08** | .24 ± .08** | .15 ± .07* | .15 ± .08* |
| F7 | .13 ± .07 | .18 ± .08* | .26 ± .08** | .09 ± .07 | .16 ± .07* | .09 ± .07 |
| F3 | .25 ± .07**** | .27 ± .08*** | .36 ± .08**** | .28 ± .07**** | .26 ± .08*** | .19 ± .09* |
| FZ | .23 ± .07** | .24 ± .08** | .38 ± .09**** | .26 ± .07**** | .24 ± .08** | .15 ± .08 |
| F4 | .22 ± .07** | .21 ± .08** | .38 ± .09**** | .24 ± .07*** | .22 ± .07** | .17 ± .07* |
| F8 | .21 ± .07** | .26 ± .08*** | .27 ± .07**** | .21 ± .07** | .18 ± .07** | .11 ± .07 |
| T7 | .09 ± .07 | .18 ± .08* | .26 ± .08*** | .15 ± .08 | .24 ± .08** | .15 ± .07* |
| C3 | .14 ± .07* | .16 ± .07* | .26 ± .08*** | .25 ± .08*** | .27 ± .08*** | .21 ± .08** |
| CZ | .21 ± .07** | .23 ± .08** | .32 ± .08**** | .28 ± .07**** | .27 ± .07**** | .23 ± .08** |
| C4 | .20 ± .07** | .21 ± .08** | .27 ± .08**** | .27 ± .08** | .27 ± .08**** | .22 ± .08** |
| T8 | .12 ± .07 | .19 ± .08** | .24 ± .07*** | .15 ± .07* | .21 ± .07** | .20 ± .07** |
| P7 | .20 ± .08** | .29 ± .08*** | .29 ± .08**** | .31 ± .09*** | .33 ± .08**** | .37 ± .08**** |
| P3 | .10 ± .07 | .18 ± .07* | .22 ± .07** | .26 ± .08*** | .26 ± .08*** | .33 ± .08**** |
| PZ | .15 ± .07* | .13 ± .07 | .20 ± .07** | .32 ± .08**** | .26 ± .07*** | .36 ± .09**** |
| P4 | .10 ± .07 | .18 ± .08* | .22 ± .07** | .26 ± .08** | .29 ± .08**** | .38 ± .08**** |
| P8 | .20 ± .08* | .30 ± .08**** | .33 ± .08**** | .30 ± .08*** | .35 ± .08**** | .45 ± .08**** |
| O1 | .25 ± .08** | .36 ± .09**** | .38 ± .08**** | .42 ± .09**** | .44 ± .08**** | .51 ± .08**** |
| O2 | .30 ± .08**** | .41 ± .09**** | .44 ± .09**** | .39 ± .09**** | .46 ± .09**** | .54 ± .08**** |
Note.— *P<.05, **P<.01, ***P<.001, and ****P<.00044.
The genetic influences on these phenotypes are strongly correlated. Table 2 shows the total phenotypic, genetic, and environmental correlations across stimulus type and wavelength. Each correlation was averaged across the 19 leads. Within wavelength, the average genetic correlation across stimulus type was .88. This is not significantly different from 1 and suggests that the same genes influence N4 in response to nonsense, antonym, and unprimed stimuli and that the same genes influence P3 in response to nonsense, antonym, and unprimed stimuli. Within stimulus type, across wavelength, the average of the genetic correlations was .77, suggesting substantial, but not complete, overlap in the genes influencing N4 and P3 amplitudes in response to the lexical decision task. The SDs on the genetic correlations averaged 0.13 and ranged from 0.03 to 0.35, whereas SDs on the environmental correlations averaged 0.05 and ranged from 0.03 to 0.10.
Table 2.
Phenotypic, Genetic, and Environmental Correlations (Averaged Across 19 Leads) for Between-Stimulus Type and Between-Wavelength Comparisons
| Wavelengths and Stimuli | Phenotypic | Genetic | Environmental |
| P3 nonsense P3 antonym | .67 | .80 | .62 |
| P3 nonsense P3 unprimed | .68 | .81 | .62 |
| P3 antonym P3 unprimed | .68 | .91 | .60 |
| N4 nonsense N4 antonym | .65 | .86 | .59 |
| N4 nonsense N4 unprimed | .73 | .94 | .66 |
| N4 antonym N4 unprimed | .62 | .97 | .53 |
| P3 nonsense N4 nonsense | .55 | .74 | .49 |
| P3 antonym N4 antonym | .63 | .72 | .61 |
| P3 unprimed N4 unprimed | .60 | .85 | .52 |
| P3 nonsense N4 antonym | .43 | .72 | .36 |
| P3 nonsense N4 unprimed | .41 | .81 | .28 |
| P3 antonym N4 nonsense | .38 | .44 | .35 |
| P3 antonym N4 unprimed | .38 | .68 | .28 |
| P3 unprimed N4 nonsense | .42 | .61 | .34 |
| P3 unprimed N4 antonym | .43 | .76 | .33 |
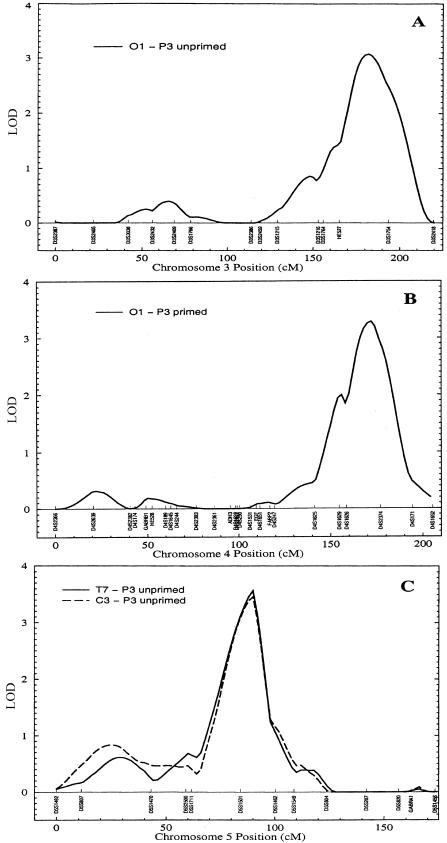
Table 3 lists all LOD scores ⩾2 in the genome screens. Four LOD scores over the conventional significance cutoff of 3 were observed in three chromosomal regions. The LOD profiles of these peaks are illustrated in figure 1. Multiple LODs >2 in a single chromosomal region were only observed in the chromosome 4 and 5 areas showing the highest overall LODs. Three traits produced peak LODs >2 around 170 cM on chromosome 4. These included both P3 and N4 amplitudes but were restricted to occipital leads and primed stimuli. Eleven traits had LODs >2 within 10 cM of the chromosome 5 linkage peak. These included P3 amplitudes in response to primed and unprimed words at primarily central and frontal leads.
Table 3.
LOD Scores ⩾2 in Genome Screen[Note]
| Chromosome and Distance(in cM) from p-ter | Peak StimulusLead | LOD |
| 2: | ||
| 158 | P3 Primed P7 | 2.16 |
| 3: | ||
| 182 | P3 Unprimed O1 | 3.08 |
| 4: | ||
| 172 | P3 Primed O1 | 3.29 |
| 168 | N4 Primed O2 | 2.20 |
| 168 | P3 Primed O2 | 2.76 |
| 146 | P3 Unprimed O1 | 2.10 |
| 5: | ||
| 90 | P3 Unprimed T7 | 3.57 |
| 90 | P3 Unprimed C3 | 3.46 |
| 68 | P3 Primed PZ | 2.05 |
| 84 | P3 Primed C3 | 2.34 |
| 82 | P3 Primed C4 | 2.42 |
| 82 | P3 Primed CZ | 2.69 |
| 90 | P3 Unprimed F3 | 2.92 |
| 90 | P3 Unprimed F4 | 2.34 |
| 90 | P3 Unprimed FZ | 2.21 |
| 90 | P3 Unprimed F7 | 2.35 |
| 86 | P3 Unprimed C4 | 2.69 |
| 86 | P3 Unprimed CZ | 2.92 |
| 6: | ||
| 144 | N4 Unprimed O2 | 2.60 |
| 11: | ||
| 240 | N4 Nonsense F8 | 2.39 |
| 13: | ||
| 26 | P3 Nonsense C3 | 2.19 |
| 60 | P3 Primed C4 | 2.18 |
| 17: | ||
| 86 | P3 Primed C4 | 2.58 |
| 20: | ||
| 0 | P3 Nonsense C4 | 2.46 |
Note.— LOD scores ⩾3 are italicized.
Figure 1 .
Multipoint LOD plots for phenotypes with LOD >3 on chromosomes 3 (A), 4 (B), and 5 (C)
Because these traits are so highly intercorrelated, rendering standard corrections for multiple testing inappropriate, we conducted simulation studies to estimate the significance of the observed LOD scores, given the large number of traits analyzed in the linkage screen. A marker unlinked to the trait loci was simulated using marker allele frequencies drawn from the genotyped polymorphisms, and linkage analyses were performed with all 114 phenotypes. A total of 36,000 markers were simulated, producing >4 million LOD scores. Table 4 details the results of these simulations. The highest LOD observed in our linkage screen was 3.57. LODs ⩾3.57 occurred 58 times in simulations of unlinked markers for a pointwise P of .0016. However, the observed chromosome 5 linkage derives strong support not only from the magnitude of the highest LOD scores in the genome screen (LODs 3.57 and 3.46) but also from the large number of high LOD scores (11) within an 8-cM region. In our simulations, two or more LODs ⩾3.46 with the same marker occurred 12 times (pointwise P=.0003), and 11 or more LODs ⩾2 occurred 11 times (pointwise P=.0003). Three simulated markers produced 11 LODs ⩾2, of which two LODs were ⩾3.46 (pointwise P=.000083). To correct the single-marker pointwise P values for the genomewide screen that was actually performed, genomewide P values were calculated using the method of Feingold et al. (1993), with the chromosome lengths and marker density from the COGA genome screen and crossover rate estimated from the types of relative pairs contained in the COGA data set. With this correction, the conjunction of linkage findings on chromosome 5 is expected to occur by chance less than once in every 20 genome screens of these 114 traits (i.e., experimentwide, genomewide P<.05).
Table 4.
Results of Linkage Analyses of 36,000 Simulated Markers with the 114 ERP Phenotypes
|
P |
|||
| Result | No.Observed | Pointwise | Genomewide |
| LOD ⩾2.00 | 4,984 | .1384 | NS |
| LOD ⩾3.08 | 260 | .0072 | NS |
| LOD ⩾3.29 | 147 | .0041 | NS |
| LOD ⩾3.46 | 79 | .0022 | NS |
| LOD ⩾3.57 | 58 | .0016 | NS |
| 11+ LODs ⩾2.00 | 11 | .0003 | .0423 |
| 2+ LODs ⩾3.46 | 12 | .0003 | .0423 |
| 11 LODs ⩾2, with 2 ⩾3.46 | 3 | .000083 | .0232 |
Discussion
The interpretation of these results is complicated by the unresolved statistical issue of correcting for multiple testing in linkage screens of highly intercorrelated traits. Given a limited number of traits, say five or fewer, the best solution is obviously to conduct a single multivariate linkage screen, taking into account the additional degrees of freedom introduced by the multiple traits. However, in the present situation, the number of phenotypes involved does not lend itself to this approach. Another seemingly attractive strategy would be to conduct principal-components analyses to create genetically orthogonal traits that extract the maximum genetic information in the minimum number of factors, reducing the number of linkage screens to be conducted. Because the principal components are uncorrelated with each other, this strategy not only reduces the number of traits but also renders appropriate a Bonferroni correction based on the number of components analyzed. Olson et al. (1999) used a principal-components approach in analyses of 16 P3 and N1 amplitudes in response to a visual target detection task for Genetic Analysis Workshop 11. They found that, although the first four principal components accounted for the majority of the trait variation (74%), these components showed little evidence for linkage, and it was the remaining minor components (accounting for <6% of the variance each), which showed strong evidence of linkage. The fact that minor components cannot be safely ignored suggests that the principal components approach to reducing multiple testing may discard valuable linkage information.
In the absence of a clearly accepted method for dealing with the problem of genome screens of multiple correlated traits, we have chosen to present our linkage results in as complete a form as possible, to provide the reader with enough detail to accurately evaluate the regions of signal. LOD scores >3 were observed in three regions on chromosomes 3, 4, and 5. Chromosome 5 had both the highest LOD scores and the highest concentration of LOD scores >2 across multiple traits, making it the most promising of the three candidate regions. Our simulation analyses of the distribution of LOD scores for this suite of traits under the null hypothesis of no linkage indicate that clustering of false positive results of this magnitude in a single chromosomal region is highly unlikely. Taking into account the number of traits analyzed and the number of tests performed in the genomewide linkage screen, the P value estimated for the conjunction of linkage findings on chromosome 5, 11 or more LODs ⩾2 of which at least two are ⩾3.46 (experimentwide and genomewide P=.0232), suggests that we have met the standard criterion for statistical significance.
In a related study, this area of chromosome 5 also showed suggestive LOD scores (2–2.9) in a linkage screen of P3 amplitude in response to visual target stimuli (Begleiter et al. 1998). As with the present study, the previously reported visual P3 linkage signals on chromosome 5 were highest for central and temporal leads. Bivariate analyses with both the visual and lexical P3 amplitudes at the T7 lead produce a QTL-specific genetic correlation of .68 (P=.009), supporting a hypothesis of pleiotropy and suggesting that a single QTL in this region of chromosome 5 influences responses to both tasks. An examination of the online genetic databases reveals no compelling candidate genes in this region of chromosome 5. However, it is worth noting that this is the same area that has previously been linked to a gene influencing schizophrenia (Sherrington et al. 1988; Owen et al. 1990). Although this finding has been controversial and difficult to replicate (Aschauer et al. 1990), it is supported by the observation of trisomy of this region of chromosome 5 in an uncle-nephew pair with schizophrenia (Bassett et al. 1988; Gilliam et al. 1989). Also in this area is the gene ENC1, which codes for a nuclear matrix protein expressed in primary neurons and thought to be involved in neuronal differentiation (Kim et al. 1998).
Although multiple testing issues necessitate caution and restraint in the interpretation of these results, the present study is a valuable first step toward the goal of localizing genes that influence variations in brain function between individuals, as indexed by neuroelectric measures, and that potentially influence liability to correlated psychiatric disorders. Further evidence supporting these results, particularly the promising candidate region on chromosome 5, may be obtained from several sources. Independent replication of the present results, although ideal, may be the most difficult avenue of confirmation. As Suarez et al. (1994) have discussed, replication of a specific result generally requires a substantially larger sample size than was used in the original study. On the other hand, thorough investigation of coincident results from different phenotypes through bivariate genetic analyses may both increase the evidence for linkage and narrow the prospective candidate region (Almasy et al. 1997; Williams et al. 1999a, 1999b). Such bivariate analyses have suggested that the QTL detected on chromosome 5 in these analyses also influences P3 amplitude measured in a visual target detection task. Finally, these results may be extended and confirmed through fine-mapping of the candidate region, detection of disequilibrium, and, ultimately, identification of one or more functional polymorphisms.
References
- Adams J, Faux SF, Nestor PG, Shenton M, Marcy B, Smith S, McCarley RW (1993) ERP abnormalities during semantic processing in schizophrenia. Schizophr Res 10:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JE, Polich J, Bloom FE, Bauer LO, Kuperman S, Rohrbaugh J, Morzorati S, O’Connor SJ, Porjesz B, Begleiter H (1994) P300 from an auditory oddball task: inter-laboratory consistency. Int J Psychophysiol 17:35–46 [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J (1998) Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Dyer TD, Blangero J (1997) Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol 14:953–958 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders (DSM-III-R). American Psychiatric Press, Washington, DC [Google Scholar]
- Aschauer HN, Aschauer-Treiber G, Isenberg KE, Todd RD, Knesevich MA, Garver DL, Reich T, Cloninger CR (1990) No evidence for linkage between chromosome 5 markers and schizophrenia. Hum Hered 40:109–115 [DOI] [PubMed] [Google Scholar]
- Bassett AS, McGillivray BC, Jones BD, Pantzar JT (1988) Partial trisomy chromosome 5 cosegregating with schizophrenia. Lancet 1:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B (1984) Event-related brain potentials in boys at risk for alcoholism. Science 225:1493–1496 [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, Van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li TK, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J Jr, Crowe R, Bloom FE (1998) Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol 108:244–250 [DOI] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li TK, Schuckit MA, Edenberg HJ, Rice JP (1995) The Collaborative Study on the Genetics of Alcoholism. Alcohol Health Res World 19:228–236 [PMC free article] [PubMed] [Google Scholar]
- Blackwood DHR, St. Clair DM, Muir WJ, Duffy JC (1991) Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry 48:899–909 [DOI] [PubMed] [Google Scholar]
- Blangero J, Almasy L, Williams JT, Porjesz B, Reich T, Begleiter H, and COGA collaborators (1997) Incorporating quantitative traits in genomic scans of psychiatric diseases: alcoholism and event-related potentials. Psychiatr Genet 74:602 [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Bauer L, Kuperman S, O’Connor SJ, Rohrbaugh J, Begleiter H (1994) Visual P300: an interlaboratory consistency study. Alcohol 11:583–587 [DOI] [PubMed] [Google Scholar]
- Feighner JF, Robins E, Guze S, Woodruff R, Winokur G, Munoz R (1972) Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatr 26:57–63 [DOI] [PubMed] [Google Scholar]
- Feingold E, Brown PO, Siegmund D (1993) Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet 53:234–251 [PMC free article] [PubMed] [Google Scholar]
- Gilliam TC, Freimer NB, Kaufmann CA, Powchik PP, Bassett AS, Bengtsson U, Wasmuth JJ (1989) Deletion mapping of DNA markers to a region of chromosome 5 that cosegregates with schizophrenia. Genomics 5:940–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Glazer WM (1991) N400 and semantic categorization in schizophrenia. Biol Psychiatry 29:467–480 [DOI] [PubMed] [Google Scholar]
- Kim TA, Lim J, Ota S, Raja S, Rogers R, Rivnay B, Avraham H, Avraham S (1998) NRP/B, a novel nuclear matrix protein, associates with p110(RB) and is involved in neuronal differentiation. J Cell Biol 141:553–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Kotsopoulos S, Lusk S, Walker S, Beggs K, Hiebert A (1998) Event-related potential correlates of primed and unprimed words in children co-morbid for disruptive behavior disorders and academic delay. Eur Child Adolesc Psychiatry 7:209–218 [DOI] [PubMed] [Google Scholar]
- Kuperman S, Porjesz B, Arndt S, Bauer L, Begleiter H, Cizadlo T, O’Connor S, Rohrbaugh J (1995) Multi-center N400 ERP consistency using a primed and unprimed word paradigm. Electroencephalogr Clin Neurophysiol 94:462–470 [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C (1988) Event-related brain potential studies of language. Introduction. Adv Psychophysiol 3:139–187 [Google Scholar]
- Olson JM, Rao S, Jacobs K, Elston RC (1999) Linkage of chromosome 1 markers to alcoholism-related phenotypes by sib pair linkage analysis of principal components. Genet Epidemiol 17:S271–276 [DOI] [PubMed] [Google Scholar]
- Owen M, Craufurd D, St Clair D (1990) Localisation of a susceptibility locus for schizophrenia on chromosome 5. Br J Psychiatry 157:123–127 [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE (1994) Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull 115:55–73 [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H (1985) Human brain electrophysiology and alcoholism. In: Tarter DV, Thiel DV (eds) Alcohol and the brain. Plenum, New York, pp. 139–182 [Google Scholar]
- ——— (1996) Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B (eds) Alcohol and alcoholism. Vol 2. The pharmacology of alcohol and alcohol dependence. Oxford University Press, New York, pp 207–247 [Google Scholar]
- Self SF, Liang K-Y (1987) Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J Am Stat Assoc 82:605–610 [Google Scholar]
- Sherrington R, Brynjolfsson J, Petursson H, Potter M, Dudleston K, Barraclough B, Wasmuth J, Dobbs M, Gurling H (1988) Localization of a susceptibility locus for schizophrenia on chromosome 5. Nature 336:164–167 [DOI] [PubMed] [Google Scholar]
- Strandburg RJ, Marsh JT, Brown WS, Asamow RF, Guthrie D, Higa J (1993) Event-related potentials in high-functioning adult autistics: linguistic and nonlinguistic visual information processing tasks. Neuropsychologia 31:413–434 [DOI] [PubMed] [Google Scholar]
- Suarez BK, Hampe CL, Van Eerdewegh P (1994) Problems of replicating linkage claims in psychiatry. In: ES Gershon, CR Cloninger (eds) Genetic approaches to mental disorders. American Psychiatric Press, Washington, DC [Google Scholar]
- Wijsman EM, Amos CI (1997) Genetic analysis of simulated oligogenic traits in nuclear and extended pedigrees: summary of GAW10 contributions. Genet Epidemiol 14:719–735 [DOI] [PubMed] [Google Scholar]
- Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, Goate A, VanEerdewegh P, Almasy L, Blangero J (1999a) Joint multipoint linkage analysis of multivariate quantitative and qualitative traits. II. Alcoholism and event-related potentials. Am J Hum Genet 65:1148–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, VanEerdewegh P, Almasy L, Blangero J (1999b) Joint multipoint linkage analysis of multivariate quantitative and qualitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet 65:1134–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]