Abstract
It is widely held that changes in the distribution of mutant mtDNAs underlie the progressive nature of mtDNA diseases, but there are few data documenting such changes. We compared the levels of 3243 A→G mutant mtDNA in blood at birth from Guthrie cards and at the time of diagnosis in a blood DNA sample from patients with mitochondrial encephalopathy, lactic acidosis, and strokelike episodes (MELAS) syndrome. Paired blood DNA samples separated by 9–19 years were obtained from six patients with MELAS. Quantification of mutant load, by means of a solid-phase minisequencing technique, demonstrated a decline (range 12%–29%) in the proportion of mutant mtDNA in all cases (P=.0015, paired t-test). These results suggest that mutant mtDNA is slowly selected from rapidly dividing blood cells in MELAS.
Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes (MELAS [MIM 540000]) is a neurological disease of either child or adult onset. Approximately 80% of patients with MELAS (Goto et al. 1990; Kobayashi et al. 1990), as well as ∼1% of patients with non–insulin-dependent diabetes mellitus (van den Ouweland 1994), carry the nucleotide (nt) 3243 A→G point mutation in the tRNA leucineUUR gene of mitochondrial DNA (mtDNA). The population frequency of the mutation is ∼1:15,000, making it one of the most common neurogenetic diseases (Majamaa et al. 1998).
Noninvasive diagnosis of MELAS by means of blood DNA has become a routine procedure (Hammans et al. 1991). However, 3243 A→G mutant mtDNA levels have been noted to be consistently lower in blood than in skeletal muscle (Ciafaloni et al. 1992), which may sometimes cause a false negative finding in diagnosis of MELAS. Furthermore, cross-sectional data demonstrated that the difference between levels in blood and skeletal muscle is proportional to age, which might be explained by declining mutant mtDNA levels in blood as a result of preferential selection of cells containing high levels of wild-type mtDNA (Poulton and Morten 1993). To test this hypothesis, we retrieved dried neonatal blood spots from six patients known to have 3243 A→G MELAS and compared longitudinally the level of the mutant mtDNA in blood at birth with that at the time of diagnosis, the two samples being separated by 9–19 years.
After informed patient consent, five Guthrie-card specimens with dried neonatal blood spots from unrelated patients diagnosed with MELAS syndrome were obtained from neonatal screening centers in the United Kingdom. A genomic DNA sample, extracted from leukocytes of each patient by routine methods at the time of diagnosis of MELAS, accompanied each Guthrie card. An additional patient, who has had two DNA samples taken 12 years apart, was included. For Guthrie-card specimens, a 3-mm diameter punch was cut, with a sterile scalpel and forceps from each card, directly into a 0.5-ml PCR tube. The samples were incubated twice in 0.5 ml ddH2O for 30 min at room temperature and were vacuum dried prior to PCR (Makowski et al. 1995). For controls, we included a Guthrie spot from a patient with no MELAS mutation and a piece of Guthrie card outside the blood spot. The PCR mix was added directly onto the card sample or, in the case of the DNA samples, to 50 ng of genomic DNA. The PCR and subsequent mutation analysis of the 62-bp PCR product by the solid-phase minisequencing technique (Syvänen et al. 1990) were carried out as described elsewhere (Suomalainen et al. 1993). To obtain a mixture of lymphocytes and monocytes, mutant load in individual blood cell series was determined for patient 6 by separating the buffy coat by use of a percoll gradient. Monocytes were separated by adherence to plastic tissue culture wells for 60 min at 37°C (Romani 1996).
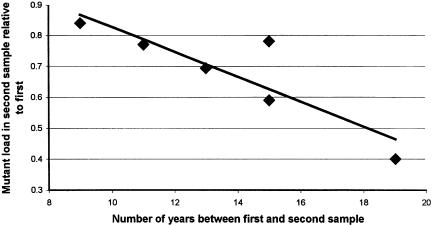
We observed progressive decline of mutant mtDNA in all patients with MELAS (table 1, fig. 1). The observed decrease ranged from 12% to 29% and was statistically significant (P=.0015, paired t-test). The mean rate of decline per year was 1.4% (range 0.6–1.9). In patient 6, the mutant load was not significantly different between blood cell types, being 24%, 20%, and 20% in monocytes, lymphocytes, and total blood leukocytes, respectively. Thus, even extreme alterations in the blood components could not underlie a fall in level of 3243 A→G mutant mtDNA of the magnitude that we documented.
Table 1.
Quantification of 3243 A→G Mutant mtDNA in Guthrie Card Samples at Birth and Blood Samples at the Time of Diagnosis from Patients with MELAS: Results of a Solid-Phase Minisequencing Assay
| Subject | Age(years) | Cpm C(mutant)a | Cpm T(wild type) | MutantmtDNA(%)b |
| 1 | Birth | 23603 | 13696 | 58 |
| 1 | 15 | 34275 | 33355 | 45 |
| 2 | Birth | 26183 | 8973 | 70 |
| 2 | 15 | 29267 | 33502 | 41 |
| 3 | Birth | 42530 | 9270 | 79 |
| 3 | 11 | 36124 | 18389 | 61 |
| 4 | Birth | 44029 | 10978 | 76 |
| 4 | 9 | 34320 | 15303 | 64 |
| 5 | Birth | 12894 | 42136 | 20 |
| 5 | 19 | 6135 | 59298 | 8 |
| 6 | 23 | 28526 | 41239 | 36 |
| 6 | 35 | 16518 | 53456 | 20 |
| Controlc | 347 | 408 | ||
| Controld | 48 | 52 |
When the MELAS A→G mutation at nt 3243 is present, two Cs are incorporated in the assay.
The specific activities of the 3H-labeled nucleotides were for dCTP 64 Ci/mmol and for dTTP 102 Ci/mmol. The percentage of mutant mtDNA was calculated according to the following formula: Cpm C/[cpm c+(cpm t×1.25)]×100%=% of mutant mtDNA, in which the coefficient 1.25 corrects for incorporation of two Cs in the case of the mutation, as well as the difference between the specific activities of the two dNTPs.
Card with no blood.
No DNA.
Figure 1.
Mutant load in blood, declining over the time period between the two samples.
The 3243 A→G mutation causes a defect in mitochondrial translation leading to impaired oxidative phosphorylation (OXPHOS), with deficient activity of respiratory chain complexes I and/or IV (Koga et al. 1988; Ciafaloni et al. 1992). Therefore, a possible mechanism of clearance of mutant mtDNA from rapidly dividing blood cells is by natural selection along Darwinian principles: cells with a high mutant mtDNA load have impaired OXPHOS capacity and may divide less frequently than cells with low mutant mtDNA content. Our long-term results show that the selection seems to be a general feature of mtDNA carrying the 3243 A→G mutation in blood, having a constant rate of decline. Our results agree well with another study (t' Hart et al. 1996), which observed a decline in the mutant 3243 A→G mtDNA in paired blood samples taken 1.5–6 years apart from diabetes pedigrees. A recent study reported no evidence of loss of A3243G mutant mtDNA from hair follicles, which are rapidly dividing but do not have a high energy requirement (Sue et al. 1998). These data contrast with those on other tissues and cultured cells, in which mutant mtDNA may accumulate (Larsson et al. 1990, 1992; Weber et al. 1997). In a study of Leber’s hereditary optic neuropathy, the heteroplasmic mutant mtDNA amount remained constant for >3.5 years (Ghosh et al. 1996). The authors concluded that no measurable selection pressures operated for or against the 3460 mutant mtDNA in this short time period and that random genetic drift was occurring. These data suggest that, in rapidly dividing tissues, mtDNA drifts randomly if the mutant mtDNA does not cause a severe respiratory defect, whereas in the case of 3243 A→G mutant mtDNA with a generalized severe effect on respiration, slow selection for the wild type is present. This selection is not likely to be caused by active selection at the mtDNA level but merely by the fitness of the cell to divide. Our data show that DNA diagnosis of MELAS in elderly individuals should be based on analysis of tissues in which the levels of mutant mtDNA are likely to be high, such as muscle or probably hair follicles. In this and in many other mtDNA diseases, there is a danger of false-negative results when DNA from leukocytes is used.
Acknowledgments
We are grateful to Drs. Sarah Bundey, Paul Smith, John Tolmie, David Goudie, and Ian Holt, for providing patient samples; and to Ms. Kate Hall of the Neonatal Screening laboratory in Birmingham and Mr. R. Kennedy of the Neonatal Screening laboratory in Glasgow, for locating Guthrie cards. Dr. R. Dunbar, from the Institute of Molecular Medicine, Oxford, and Ritva Timonen, from the National Public Health Institute of Helsinki, kindly provided technical assistance. We thank the European Neuromuscular Centre for financial support and their Mitochondrial Consortium (particularly the late Professor Anita Harding) for inspiration for this study. J. P. is a Royal Society University Research Fellow.
Electronic-Database Information
Accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for MELAS [MIM 540000])
References
- Ciafaloni E, Ricci E, Shanske S, Moraes CT, Silvestri G, Hirano M, Simonetti S, Angelini C, Donati MA, Garcia C, Martinuzzi A, Mosewich R, Servidei S, Zammarchi E, Bonilla E, De Vivo D, Rowland LP, Schon EA, Di Mauro S (1992) MELAS: clinical features, biochemistry, and molecular genetics. Ann Neurol 31:391–398 [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Fahy E, Bodis-Wollner I, Sherman J, Howell N (1996) Longitudinal study of a heteroplasmic 3460 Leber hereditary optic neuropathy family by multiplexed primer-extension analysis and nucleotide sequencing. Am J Hum Genet 58:325–334 [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Nonaka I, Horai S (1990) A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348:651–653 [DOI] [PubMed] [Google Scholar]
- Hammans SR, Sweeney MG, Brockington M, Morgan Hughes JA, Harding AE (1991) Mitochondrial encephalopathies: molecular genetic diagnosis from blood samples. Lancet 337:1311–1313 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Momoi MY, Tominaga K, Momoi T, Nihei K, Yanagisawa M, Kagawa Y, Ohta S (1990) A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun 173:816–822 [DOI] [PubMed] [Google Scholar]
- Koga Y, Nonaka I, Kobayashi M, Tojyo M, Nihei K (1988) Findings in muscle in complex I (NADH coenzyme Q reductase) deficiency. Ann Neurol 24:749–756 [DOI] [PubMed] [Google Scholar]
- Larsson NG, Holme E, Kristiansson B, Oldfors A, Tulinius M (1990) Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res 28:131–136 [DOI] [PubMed] [Google Scholar]
- Larsson NG, Tulinius MH, Holme E, Oldfors A, Andersen O, Wahlstrom J, Aasly J (1992) Segregation and manifestations of the mtDNA tRNA(Lys) A→G(8344) mutation of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet 51:1201–1212 [PMC free article] [PubMed]
- Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Karppa M, Majamaa-Voltti KA, Rusanen H, Sorri M, Peuhkurinen KJ, Hassinen IE (1998) Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski GS, Davis EL, Aslanzadeh J, Hopfer SM (1995) Enhanced direct amplification of Guthrie card DNA following selective elution of PCR inhibitors. Nucleic Acids Res 23:3788–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton J, Morten K (1993) Noninvasive diagnosis of the MELAS syndrome from blood DNA. Ann Neurol 34:116 [DOI] [PubMed] [Google Scholar]
- Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G (1996) Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods 196:137–151 [DOI] [PubMed] [Google Scholar]
- Sue CM, Quigley A, Katsabanis S, Kapsa R, Crimmins DS, Byrne E, Morris JG (1998) Detection of MELAS A3243G point mutation in muscle, blood and hair follicles. J Neurol Sci 161:36–39 [DOI] [PubMed] [Google Scholar]
- Suomalainen A, Majander A, Pihko H, Peltonen L, Syvanen AC (1993) Quantification of tRNA3243(Leu) point mutation of mitochondrial DNA in MELAS patients and its effects on mitochondrial transcription. Hum Mol Genet 2:525–534 [DOI] [PubMed] [Google Scholar]
- Syvänen A-C, Aalto-Setälä K, Harju L, Kontula K, Söderlund H (1990) A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics 8:684–692 [DOI] [PubMed] [Google Scholar]
- t' Hart LM, Jansen JJ, Lemkes HH, de Knijff P, Maassen JA (1996) Heteroplasmy levels of a mitochondrial gene mutation associated with diabetes mellitus decrease in leukocyte DNA upon aging. Hum Mutat 7:193–197 [DOI] [PubMed] [Google Scholar]
- van den Ouweland JM, Lemkes HH, Trembath RC, Ross R, Velho G, Cohen D, Froguel P, Maassen JA (1994) Maternally inherited diabetes and deafness is a distinct subtype of diabetes and associates with a single point mutation in the mitochondrial tRNA(Leu(UUR)) gene. Diabetes 43:746–751 [DOI] [PubMed] [Google Scholar]
- Weber K, Wilson JN, Taylor L, Brierley E, Johnson MA, Turnbull DM, Bindoff LA (1997) A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet 60:373–380 [PMC free article] [PubMed] [Google Scholar]