Abstract
Maternal uniparental disomy of chromosome 7 (matUPD7), the inheritance of both chromosomes from only the mother, is observed in ∼10% of patients with Silver-Russell syndrome (SRS). It has been suggested that at least one imprinted gene that regulates growth and development resides on human chromosome 7. To date, three imprinted genes—PEG1/MEST, γ2-COP, and GRB10—have been identified on chromosome 7, but their role in the etiology of SRS remains uncertain. In a systematic screening with microsatellite markers, for matUPD7 cases among patients with SRS, we identified a patient who had a small segment of matUPD7 and biparental inheritance of the remainder of chromosome 7. Such a pattern may be explained by somatic recombination in the zygote. The matUPD7 segment at 7q31-qter extends for 35 Mb and includes the imprinted gene cluster of PEG1/MEST and γ2-COP at 7q32. GRB10 at 7p11.2-p12 is located within a region of biparental inheritance. Although partial UPD has previously been reported for chromosomes 6, 11, 14, and 15, this is the first report of a patient with SRS who has segmental matUPD7. Our findings delimit a candidate imprinted region sufficient to cause SRS.
Uniparental disomy (UPD) is the inheritance of both homologous chromosomes of a chromosome pair from only one parent (Engel 1980). “Isodisomy” refers to the inheritance of two copies of a single chromosome—and “heterodisomy” refers to the inheritance of both chromosomes of a pair—from only one parent. Mechanisms leading to UPD include gamete complementation (fertilization by a disomic and nullisomic gamete); trisomy rescue (extraction of the supernumerary chromosome, which, in one-third of cases, leads to UPD); monosomy duplication (duplication of the single chromosome present); and postfertilization errors (gene conversion and mitotic recombinations) (Spence et al. 1988; Engel 1993). UPD can disrupt the balance between imprinted genes and, thereby, can lead to phenotypic manifestations (Cattanach and Beechey 1990). Genomic imprints are set differently during oogenesis and spermatogenesis, and the imprinted genes are expressed by either the maternal or the paternal allele (Surani et al. 1984).
More than 20 cases of maternal UPD for chromosome 7 (matUPD7) have been reported (Spence et al. 1988; Voss et al. 1989; Spotila et al. 1992; Eggerding et al. 1994; Kotzot et al. 1995; Langlois et al. 1995; Eggermann et al. 1997; Preece et al. 1997; Price et al. 1999; Bernard et al. 1999). All patients have presented with severe growth retardation, and ⩾14 patients had Silver-Russell syndrome (SRS [MIM 180860]). SRS is a syndrome of severe pre- and postnatal growth retardation with some typical dysmorphic features, including asymmetry and/or hemihypertrophy of trunk, limbs, and face; clino- and brachydactyly of the fifth fingers; a triangular face with a broad and prominent forehead; a small lower jaw; and downturned mouth corners (Silver et al. 1953; Russell 1954) (table 1). Most cases of SRS are sporadic, but recessive, dominant, and X-linked modes of inheritance have all been suggested (Partington 1986; Duncan et al. 1990; Teebi 1992). Chromosomal aberrations have also been reported in patients with SRS. Approximately 10% of patients with SRS exhibit matUPD7, but the normal growth and development observed in patients with paternal UPD7 (Höglund et al. 1994) suggest that imprinted genes play a role in the etiology of SRS (Kotzot et al. 1995; Eggermann et al. 1997; Preece et al. 1997). It has been suggested that the matUPD7 phenotype is caused either by a lack of a paternally expressed growth-promoting gene or by an excess of a maternally expressed growth-suppressing gene. To date, three imprinted genes have been identified on human chromosome 7: PEG1/MEST (Riesewijk et al. 1997) and γ2-COP (Blagitko et al. 1999) are located at 7q32, and GRB10 is located at 7p11.2-p12 (Blagitko et al. 2000; Yoshihashi et al. 2000). However, their roles in the molecular etiology of SRS remain unclear.
Table 1.
Characteristics of SRS in the Proband[Note]
| Characteristic of SRS | Status in Probanda | Occurrence in Patients with SRS (%) |
| Diagnostic: | ||
| Born small for gestational age | + | 94 |
| Postnatal growth retardation | + | 100 |
| Relative macrocephaly | + | 64 |
| Triangular face | + | 83 |
| Bossing forehead | + | 65 |
| Downturned mouth corners | + | 74 |
| Micrognathia | − | 33 |
| Clino- and/or brachydactyly | + | 80 |
| Asymmetry | − | 74 |
| Confirmatory: | ||
| Feeding difficulties | + | 56 |
| Low-set ears/ear anomalies | + | 53 |
| High-pitched voice | + | 22 |
| Delayed closure of anterior fontanelle | + | 18 |
Note.— Diagnosis of SRS was made on the basis of the most typical characteristics listed. The frequencies of characteristics noted in SRS are from reports by Escobar et al. (1978), Wollmann et al. (1995), and Price et al. (1999). Features vary extensively between patients, and no unambiguous criteria have been set for the diagnosis.
+ = Present; − = absent.
Abnormal inheritance of small genomic regions containing imprinted genes causes both Angelman syndrome (AS [MIM 105830]) and Prader-Willi syndrome (PWS [MIM 176270]), at 15q11-q13 (Nicholls et al. 1998), as well as Beckwith-Wiedemann syndrome (BWS [MIM 130650]), at 11p15.5 (Reik and Maher 1997). Similarly, it is likely that only a small region on chromosome 7 is responsible for the SRS phenotype. Although the finding of imprinted genes on chromosome 7 has focused interest on these loci, a distinct candidate region remains to be identified. So far, all but one matUPD7 case have had matUPD7 for the whole chromosome; that patient had paternal isodisomy of 7p and maternal isodisomy of 7q, compatible with the involvement of a 7q gene (Eggerding et al. 1994). One patient with a maternal duplication of 7p12.1-p13 and one with a maternal duplication of 7p11-p13 (both including duplication of the imprinted GRB10 gene) have been reported, evoking further interest in this region as a possible carrier of an SRS-causing gene (Joyce et al. 1999; Monk et al. 2000). The presence of a patient with SRS who had both a paternally derived ring chromosome for 7p12-q11 and matUPD7 for the remainder of chromosome 7 indicated that 7p12-q11 may be excluded (Miyoshi et al. 1999). We report here the first case of segmental matUPD7 in a patient with SRS, which narrows the candidate region for a SRS gene.
In screening for matUPD7 among patients with SRS, we have studied DNA samples from 33 patients and their parents. DNA was isolated from blood samples by standard procedures (Lahiri et al. 1991). Initial screening for cases of matUPD7 was performed, by genotyping the patients and their parents with 14 chromosome 7–specific fluorescent tetra- and dinucleotide repeat microsatellite markers. PCRs were performed in 10-μl reactions containing 20 ng of DNA, 1×AmpliTaq Buffer II, 2.0 μM MgCl2, 100 μM each dNTP, 4.0 μM each primer, and 5 U of AmpliTaq Gold polymerase (PE Biosystems). Amplification was done in an initial denaturation of 10 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 59°C, and 30 s at 72°C, with a final extension at 72°C for 10 min. For the 14 screening markers, the products were resolved by an automated sequencer (ABI), and analysis of genotyping data was performed with GENOTYPER software (PE Biosystems). When one or more markers suggested irregular inheritance, as many as 76 additional microsatellite markers were genotyped. The PCR products for additional markers were resolved on 6% polyacrylamide gels by electrophoresis and were silver stained; the results were read visually. Paternity was verified by the genotyping of six microsatellite markers from chromosomes 4, 6, and 11, in a way similar to that described above. The study has been approved by the Ethical Review Board of the Hospital for Children and Adolescents, Helsinki University Central Hospital. A written consent to participate in this study was given by the patients' parents.
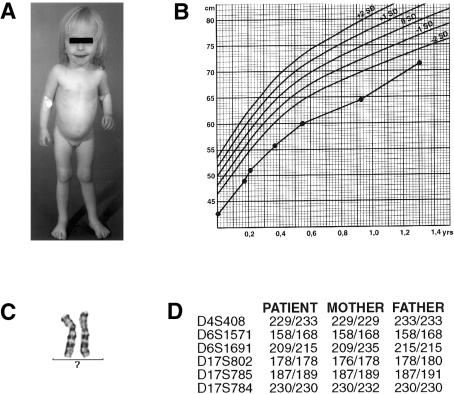
Full chromosome–length matUPD7 was found in four cases of SRS, and segmental matUPD7 was found in one case. This female patient (fig. 1) was born small for gestational age, at 37+5 wk gestation, with a birth weight of 1,510 g (−4.3 SD [standard deviation from the mean weight for age]) and a length of 40 cm (−4.9 SD). Her parents are healthy and of average height: her father's height is 182 cm (+0.4 SD) and her mother's is 168 cm (+0.4 SD), yielding an expected height of +0.4 SD; her two elder siblings have grown normally. At age 1.35 years, her height was 71.5 cm (−2.9 SD), her weight was 6,950 g (−22% relative to median weight for height), and her head circumference was 47 cm (−0.2 SD). She was slender in appearance and had relative macrocephaly, a slightly triangular face, a bossed forehead, slightly downturned mouth corners, slight clinodactyly of the fifth fingers, and dimples on her scapulae. She had a high-pitched voice, and her teeth have erupted irregularly. Her psychomotor development was normal, as was her hearing and eyesight. SRS was diagnosed on the basis of these typical yet mild SRS characteristics (table 1 and fig. 1). The phenotype is consistent with our previous observations of a mild SRS phenotype in patients with matUPD7 (Hannula et al., in press).
Figure 1.
A, Patient at age 1.7 years. B, Growth of patient, according to population-standard charts. The patient’s growth improved steadily but remained ∼3 SD below the mean height for age. Ages are corrected to the preterm birth. C, High-resolution partial karyotype of the patient, excluding a deletion at 7q31-qter. D, Verification of paternity, with six non-chromosome 7–specific markers.
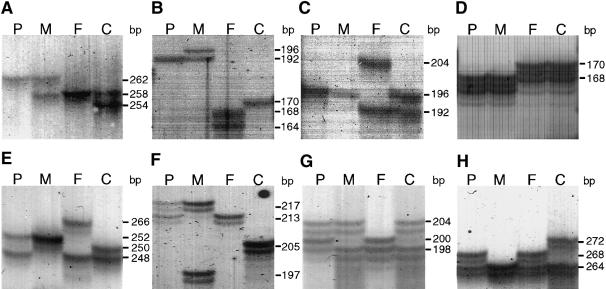
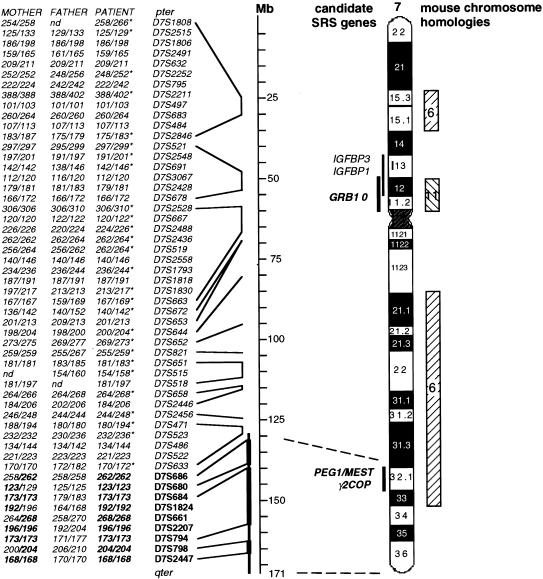
In this patient, irregular inheritance was first observed for marker D7S1824 on 7q on the basis of homozygosity for a maternal allele and lack of paternal alleles (figs. 2 and 3), whereas markers D7S1808, D7S2846, and D7S821 showed biparental inheritance (fig. 3). Other markers gave results compatible with either situation. Further genotyping, with a total of 90 microsatellite markers along chromosome 7, confirmed isodisomic matUPD7 for 7q31-qter and biparental inheritance for the remainder of the chromosome (figs. 2 and 3).
Figure 2.
Microsatellite markers indicating matUPD7 and biparental inheritance in distinct segments of chromosome 7. A–D, matUPD7 revealed by markers D7S686 at 7q31 (A), D7S1824 at 7q32-g34 (B), D7S2207 at 7q32-q34 (C), and D7S2447 at 7q36 (D). E–H, Biparental inheritance with markers D7S2252 at 7p15 (E), D7S1830 at 7q11 (F), D7S644 at 7q21 (G), and D7S658 at 7q31.1 (H). P=proband; M=mother; F=father; C=CEPH 1347-02.
Figure 3.
Location of partial isodisomic matUPD7 segment, and its relation to known imprinted loci. Genotyping with as many as 90 microsatellite markers revealed a segment of matUPD7 in 7q31-qter. The segment of matUPD7 was demonstrated unambiguously by the inheritance of only maternal alleles for markers spanning from 132 Mb to qter (boldface letters and thicker line). Uninformative markers within the segment are not listed (thinner line). The approximate cytogenetic positions of the marker loci are shown (dashed lines). The patient was heterozygous for 44 markers (italics) on the remainder of chromosome 7, indicating biparental inheritance. Markers with unambiguous paternal inheritance are denoted by an asterisk (*); the remaining markers are all compatible with paternal inheritance. Allele sizes are in base pairs. nd=no data. Positions of markers are according to the Genetic Location Database map. Locations of the three known imprinted genes on chromosome 7 (i.e., GRB10, PEG1/MEST, and γ2-COP) and of other genes that are strong candidates for SRS are shown to the left of the ideogram. Homologous regions of mouse chromosomes 6 and 11, which harbor imprinted genes with possible effects on growth, are shown to the right (hatched bars). These regions are good candidates for containing imprinted genes in humans.
Homozygosity for 23 consecutive markers and maternal-only inheritance for nine markers within this segment are indisputable evidence for isodisomic matUPD7 in the patient. The matUPD7 segment spanned a minimum of 35 Mb (from D7S686 to D7S2447) and a maximum of 43 Mb (from D7S633 to qter), a result supported unambiguously by the markers D7S686, D7S680, D7S684, D7S1824, D7S661, D7S2207, D7S794, D7S798, and D7S2447 (fig. 3). Within and adjacent to this segment there were homozygous markers for which the father shared the same alleles as were seen in the proband and mother (i.e., markers D7S677, D7S650, D7S530, D7S649, D7S1804, D7S681, D7S631, D7S2195, D7S676, D7S1826, D7S636, D7S642, D7S559, and D7S2423). Biparental inheritance of alleles from both the mother and father was observed for the rest of chromosome 7, with 27 markers unambiguously informative for the paternal inheritance of alleles (figs. 2 and 3). Paternity was confirmed by genotyping of six non-chromosome 7–specific microsatellite markers, and the possibility of a large deletion on the paternal chromosome was excluded by the presence of a normal female karyotype (fig. 1C).
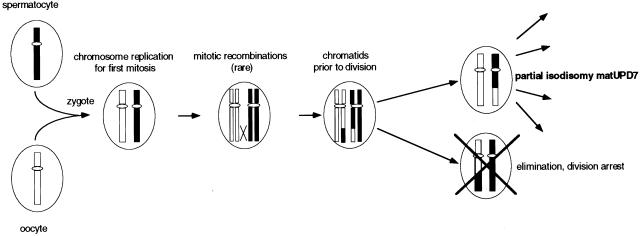
The segmental pattern of isodisomy can be explained by the presence of rare early postzygotic mitotic recombinations. Most likely, there is a single continuous matUPD7 segment, but the data are also consistent with the more complex explanation of three discontinuous isodisomic segments at 7q31-qter. Isodisomic matUPD7 could have resulted, in the simplest case, from a rare mitotic crossing-over occurring during the first zygotic division, with subsequent loss of one daughter cell (fig. 4). Mosaic UPD would result from either the persistence of both daughter cells or somatic recombination during a later cell division. A pattern of mosaicism was not indicated by the results of blood DNA analysis, but it cannot be ruled out in other tissues. Somatic recombination has been suggested as a cause of segmental UPD for patUPD6q24-qter (Das et al. 2000), patUPD11p15 in BWS (Henry et al. 1993), matUPD14q23-q24.2 (Martin et al. 1999), and in PWS (Nicholls et al. 1989; Gregory et al. 1991). Other explanations for the observed segmental matUPD7 are more complex. For example, heterodisomic matUPD7 is, in theory, possible for markers for which the child and mother share the same alleles, either homozygous or heterozygous; but we consider this alternative unlikely, since the presence of both partial heterodisomic and partial isodisomic matUPD7 regions would have had to result from complex meiotic and mitotic errors.
Figure 4.
Mitotic recombinations leading to the partial isodisomic matUPD7 segment. Fertilization between a normal spermatocyte and oocyte leads to a disomic zygote. Mitosis starts with chromosome replication, and unusual recombinations occur between a maternal and paternal chromatid. A daughter cell with partial isodisomic matUPD7 will be the result in 1/4 of the possible chromatid combinations. When one daughter cell has partial isodisomic matUPD7, the other will have partial isodisomic patUPD7. If both daughter cells survive, this will lead to mosaic UPD. However, because only the matUPD7 phenotype was observed in this patient, the patUPD7 daughter cell must have been eliminated. Loss of the patUPD7 cell may be a result of homozygosity for a lethal allele, loss of crucial chromosomal regions on the maternal chromosome, or other factors affecting the viability of the cell.
The cluster of the imprinted genes PEG1/MEST and γ2-COP, at 7q32, is located in the matUPD7 segment. Putative candidate genes for SRS that are located in the observed matUPD7 segment include those near PEG1/MEST and γ2-COP, as well as those that clearly regulate growth and that cause SRS-like phenotypes when mutated. Imprinted genes tend to occur in clusters (Reik and Maher 1997), which makes it possible that genes such as CPA1, CPA2, CATR1, UBE2H, and NRF1 (Hayashida et al. 2000), which are located near PEG1/MEST and γ2-COP, may be imprinted as well. Other interesting genes in the matUPD7 segment include CULLIN 1 and NOS3. CULLIN 1 has a role in the regulation of mammalian G1/S transitions (Yu et al. 1998) and, when mutated in Caenorhabditis elegans, leads to hyperplasia of all tissues and to abnormally small cells (Kipreos et al. 1996). NOS3 synthesizes nitric monoxide, which has a crucial role in the regulation of both vasomotor tone and blood flow through the intrauterine artery to the fetus. Deficiency of nitric monoxide in pregnant rats causes fetal growth retardation (Yallampalli and Garfield 1993).
The third imprinted gene, GRB10, as well as the candidate genes IGFBP1 and IGFBP3, are located in a biallelically inherited region in our patient (fig. 3), suggesting that SRS can arise despite regular inheritance of these genes. However, the two cases of maternal duplication at 7p11-p13 (Joyce et al. 1999; Monk et al. 2000), which is the region that includes GRB10, raised the possibility that a maternally expressed (paternally imprinted) growth-suppressing gene is located within this region. Polymorphisms and mutations in GRB10 have been reported in patients with SRS (Blagitko et al. 2000; Yoshihashi et al. 2000), but the role of GRB10 in SRS remains unknown. IGFBP1, a known regulator of fetal growth, was also included in the duplicated region. IGFBP1 maps to mouse chromosome 11, which causes growth failure in cases of matUPD11 in mice (Cattanach and Beechey 1990) and which thus is a good candidate for the SRS gene. However, IGFBP1 and IGFBP3 are both biallelically expressed in humans (Wakeling et al. 2000).
This is the first report of a patient with SRS who has a distinct matUPD7 segment and biparental inheritance of the remainder of the chromosome. In previously reported cases, the matUPD7 has constituted the entire chromosome, or at least the long arm in its entirety. Our results significantly narrow the candidate region for imprinted genes that cause SRS and suggest that the 7q31-qter region should be screened carefully, at high marker density, in patients with SRS in whom matUPD7 has not been found.
Acknowledgments
This study was supported by the P. and S. Sohlberg Foundation, the Finnish Medical Foundation, the Sigrid Juselius Foundation, the Foundation for Pediatric Research, the Ulla Hjelt Fund, the Research and Science Foundation of Farmos, the Helsinki Biomedical Graduate School, and the Academy of Finland.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Genetic Location Database, The, http://cedar.genetics.soton.ac.uk/public_html/ldb.html
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SRS [MIM 180860], AS [MIM 105830], PWS [MIM 176270], and BWS [MIM 130650])
References
- Bernard LE, Penaherrera MS, Van Ellen MI, Wang MS, Tong S-L, Gareis F, Langlois S Robinson WP (1999) Clinical and molecular findings in two patients with Russell-Silver syndrome and UPD7: comparison with non-UPD7 cases. Am J Med Genet 87:230–236 [PubMed] [Google Scholar]
- Blagitko N, Mergenthaler S, Schultz U, Wollmann HA, Craigen W, Eggermann T, Ropers H-H, Kalscheuer VM (2000) Human GRB10 is imprinted and expressed from the paternal and maternal allele in a highly tissue- and isoform-specific fashion. Hum Mol Genet 9:1587–1595 [DOI] [PubMed] [Google Scholar]
- Blagitko N, Schultz U, Schinzel AA, Ropers H-H, Kalscheuer VM (1999) γ2-COP, a novel imprinted gene on chromosome 7q32, defines a new imprinting cluster in the human genome. Hum Mol Genet 8:2387–2396 [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Beechey CV (1990) Autosomal and X-chromosome imprinting. Dev Suppl 1990:63–72 [PubMed] [Google Scholar]
- Das S, Lese CM, Song M, Jensen JL, Wells LA, Barnoski BL, Roseberry JA, Camacho JM, Ledbetter DH, Schnur RE (2000) Partial paternal uniparental disomy of chromosome 6 in an infant with neonatal diabetes, macroglossia, and craniofacial abnormalities. Am J Hum Genet 67:1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PA, Hall JG, Shapiro LR, Vibert BK (1990) Three generation dominant transmission of the Silver-Russell syndrome. Am J Med Genet 35:245–250 [DOI] [PubMed] [Google Scholar]
- Eggerding FA, Schonberg SA, Chehab FF, Norton ME, Cox VA, Epstein CJ (1994) Uniparental isodisomy for paternal 7p and maternal 7q in a child with growth retardation. Am J Hum Genet 55:253–265 [PMC free article] [PubMed] [Google Scholar]
- Eggermann T, Wollmann HA, Kuner R, Eggermann K, Enders H, Kaiser P, Ranke MB (1997) Molecular studies in 37 Silver-Russell syndrome patients: frequency and etiology of uniparental disomy. Hum Genet 100:415–419 [DOI] [PubMed] [Google Scholar]
- Engel E (1980) A new genetic concept: uniparental disomy and its potential effect, isodisomy. Am J Med Genet 6:137–143 [DOI] [PubMed] [Google Scholar]
- ——— (1993) Uniparental disomy revisited: the first twelve years. Am J Med Genet 46:670–674 [DOI] [PubMed] [Google Scholar]
- Escobar V, Gleiser S, Weaver DD (1978) Phenotypic and genetic analysis of the Silver-Russell syndrome. Clin Genet 13:278–288 [DOI] [PubMed] [Google Scholar]
- Gregory CA, Schwartz J, Kirkilionis AJ, Rudd N, Hamerton JL (1991) Somatic recombination rather than uniparental disomy suggested as another mechanism by which genetic imprinting may play a role in the etiology of Prader-Willi syndrome. Hum Genet 88:42–48 [DOI] [PubMed] [Google Scholar]
- Hannula K, Kere J, Pirinen S, Holmberg C, Lipsanen-Nyman M. Do patients with maternal uniparental disomy for chromosome 7 have a distinct mild Silver-Russell phenotype? J Med Genet (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida S, Yamasaki K, Asada Y, Soeda E, Niikawa N, Kishino T (2000) Construction of a physical and transcript map flanking the imprinted MEST1/PEG at 7q32. Genomics 66:221–225 [DOI] [PubMed] [Google Scholar]
- Henry I, Puech A, Riesewijk A, Ahnine L, Mannens M, Beldjord C, Bitoun C, Tournade MF, Landrieu P, Junien C (1993) Somatic mosaicism for partial paternal isodisomy in Wiedemann-Beckwith syndrome: a post-fertilization event. Eur J Hum Genet 1:19–29 [DOI] [PubMed] [Google Scholar]
- Höglund P, Holmberg C, de la Chapelle A, Kere J (1994) Paternal isodisomy for chromosome 7 is compatible with normal growth and development in a patient with congenital chloride diarrhea. Am J Hum Genet 55:747–752 [PMC free article] [PubMed] [Google Scholar]
- Joyce CA, Sharp A, Walker JM, Bullman H, Temple KI (1999) Duplication of 7p12.1-p13, including GRB10 and IGFBP1, in mother and daughter with features of Silver-Russell syndrome. Hum Genet 105:273–280 [DOI] [PubMed] [Google Scholar]
- Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM (1996) cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell 85:829–839 [DOI] [PubMed] [Google Scholar]
- Kotzot D, Schmitt S, Bernasconi F, Robinson WP, Lurie IW, Ilyina H, Mehes K, Hamel BCJ, Otten BJ, Hegersberg M, Werder E, Schoenle E, Schinzel A (1995) Uniparental disomy 7 in Silver-Russell syndrome and primordial growth retardation. Hum Mol Genet 4:583–587 [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI Jr (1991) A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 19:5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Yong SL, Wilson RD, Kwong LC, Kalousek DK (1995) Prenatal and postnatal growth failure associated with maternal heterodisomy for chromosome 7. J Med Genet 32:871–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RA, Sabol DW, Rogan PK (1999) Maternal uniparental disomy of chromosome 14 confined to an interstitial segment (14q23-14q24.2). J Med Genet 36:633–636 [PMC free article] [PubMed] [Google Scholar]
- Miyoshi O, Kondoh T, Taneda H, Otsuka K, Matsumoto T, Niikawa N (1999) 47,XX,UPD(7)mat,+r(7)pat/46,XX,UPD(7)mat mosaicism in a girl with Silver-Russell syndrome (SRS): possible exclusion of the putative SRS gene from a 7p13-p11 region. J Med Genet 36:326–329 [PMC free article] [PubMed] [Google Scholar]
- Monk D, Wakeling EL, Proud V, Hitchins M, Abu-Amero SN, Stanier P, Preece MA, Moore GE (2000) Duplication of 7p11.2-p13, including GRB10, in Silver-Russell syndrome. Am J Hum Genet 66:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R, Knoll J, Butler M, Karam S, Lalande M (1989) Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature 342:281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Saitoh S, Horsthemke B (1998) Imprinting in Prader-Willi and Angelman syndromes. Trends Genet 14:194–200 [DOI] [PubMed] [Google Scholar]
- Partington MW (1986) X-linked short stature with skin pigmentation: evidence for heterogeneity of the Russell-Silver syndrome. Clin Genet 29:151–156 [DOI] [PubMed] [Google Scholar]
- Preece MA, Price SM, Davies V, Clough L, Stanier P, Trembath RC, Moore GE (1997) Maternal uniparental disomy in Silver-Russell syndrome. J Med Genet 34:6–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SM, Stanhope R, Garrett C, Preece MA, Trembath RC (1999) The spectrum of Silver-Russell syndrome: a clinical and molecular genetic study and new diagnostic criteria. J Med Genet 36:837–842 [PMC free article] [PubMed] [Google Scholar]
- Reik W, Maher ER (1997) Imprinting in clusters: lessons from Beckwith-Wiedemann syndrome. Trends Genet 13:330–334 [DOI] [PubMed] [Google Scholar]
- Riesewijk AM, Hu L, Schulz U, Tariverdian G, Höglund P, Kere J, Ropers H-H, Kalscheuer VM (1997) Monoallelic expression of human PEG1/MEST is paralleled by parent-specific methylation in fetuses. Genomics 42:236–244 [DOI] [PubMed] [Google Scholar]
- Russell A (1954) A syndrome of “Intra-uterine” dwarfism recognizable at birth with cranio-facial dysostosis, disproportionately short arms, and other anomalies (5 examples). Proc R Soc Med 47:1040–1044 [PubMed] [Google Scholar]
- Silver HK, Kiyasu W, George J, Dreamer WC (1953) Syndrome of congenital hemihypertrophy, shortness of stature, and elevated urinary gonadotropins. Pediatrics 12:368–375 [PubMed] [Google Scholar]
- Spence JE, Perciaccante RG, Greig GM, Willard HF, Ledbetter DH, Hejtmancik JF, Pollack MS, O’Brien WE, Beaudet AL (1988) Uniparental disomy as a mechanism for human genetic disease. Am J Hum Genet 42:217–226 [PMC free article] [PubMed] [Google Scholar]
- Spotila LD, Sereda L, Prockop DJ (1992) Partial isodisomy for maternal chromosome 7 and short stature in an individual with a mutation at the COLIA2 locus. Am J Hum Genet 51:1396–1405 [PMC free article] [PubMed] [Google Scholar]
- Surani MAH, Barton SC, Norris ML (1984) Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308:548–550 [DOI] [PubMed] [Google Scholar]
- Teebi AS (1992) Autosomal recessive Silver-Russell syndrome. Clin Dysmorphol 1:151–156 [PubMed] [Google Scholar]
- Voss R, Ben-Simon E, Avital A, Godfrey S, Zlotogora J, Dagan J, Tikochinski Y, Hillel J. (1989) Isodisomy of chromosome 7 in a patient with cystic fibrosis: could uniparental disomy be common in humans? Am J Hum Genet 45:373–380 [PMC free article] [PubMed] [Google Scholar]
- Wakeling EL, Hitchins M, Abu-Amero SN, Stanier P, Moore GE, Preece MA (2000) Biallelic expression of IGFBP1 and IGFBP3, two candidate genes for the Silver-Russell syndrome. J Med Genet 37:65–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann HA, Kirchner T, Enders H, Preece MA, Ranke MB (1995) Growth and symptoms in Silver-Russell syndrome: review on the basis of 386 patients. Eur J Pediatr 154:958–968 [DOI] [PubMed] [Google Scholar]
- Yallampalli C, Garfield RE (1993) Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol 169:1316–1320 [DOI] [PubMed] [Google Scholar]
- Yoshihashi H, Maeyama K, Kosaki R, Ogata T, Tsukahara M, Goto Y, Hata J, Matsuo N, Smith RJ, Kosaki K (2000) Imprinting of human GRB10 and its mutations in two patients with Russell-Silver syndrome. Am J Hum Genet 67:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z-K, Gervais JLM, Zhang H (1998) Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc Natl Acad Sci USA 95:11324–11329 [DOI] [PMC free article] [PubMed] [Google Scholar]