Abstract
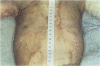
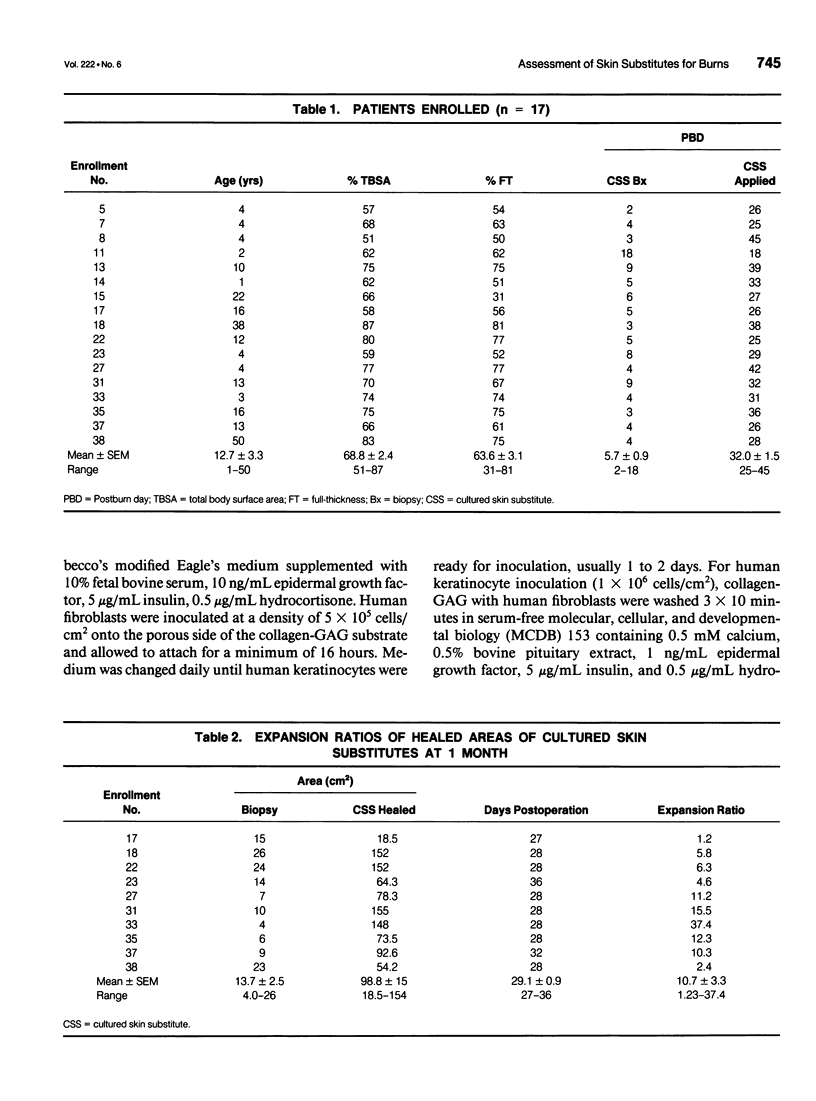
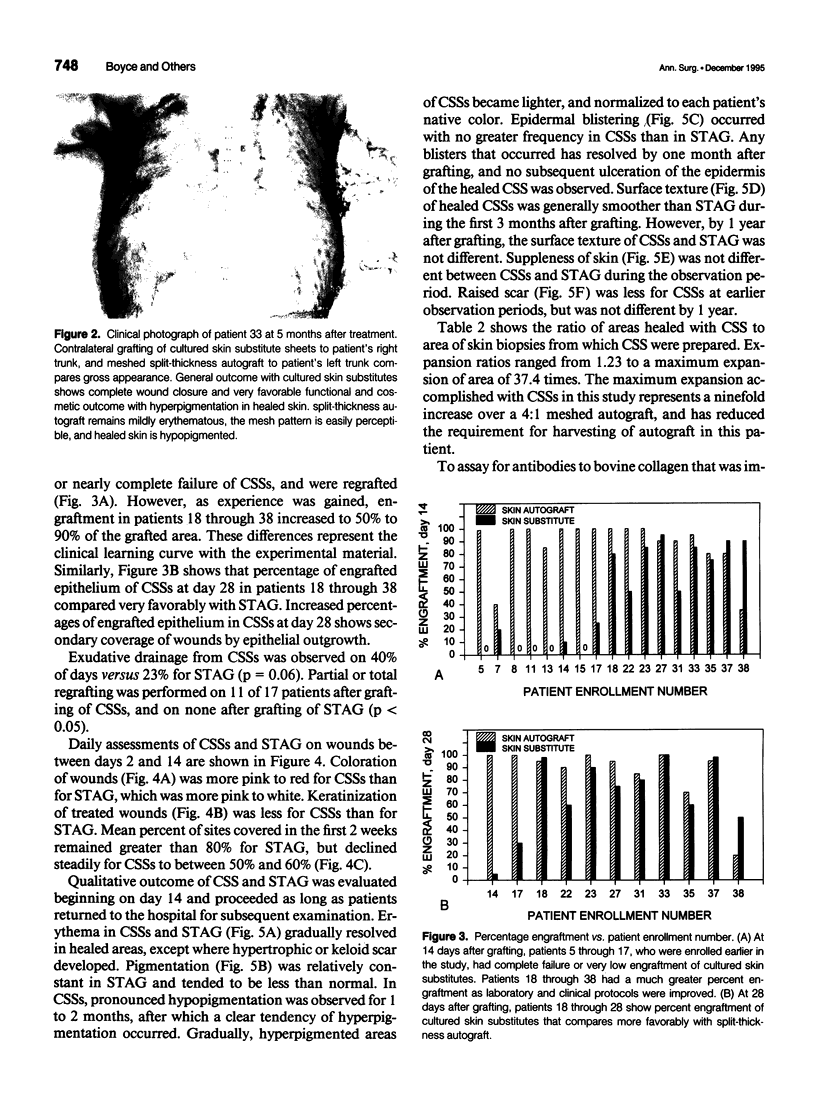
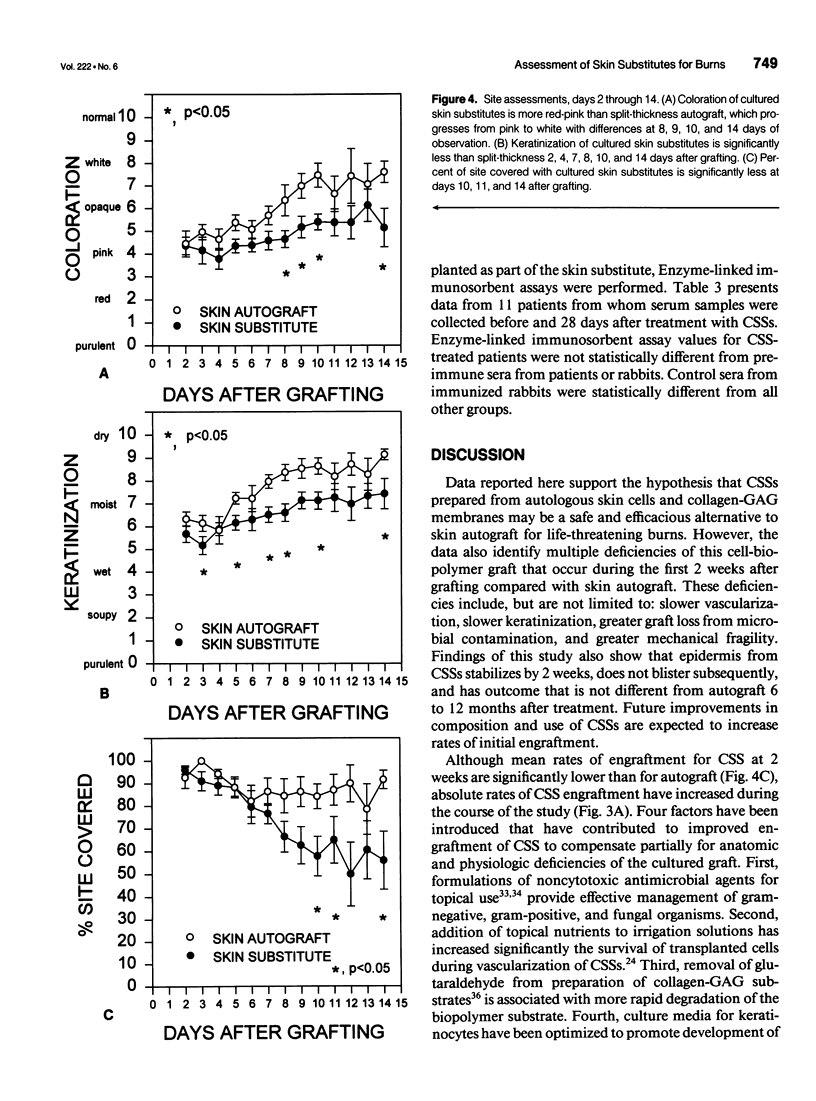
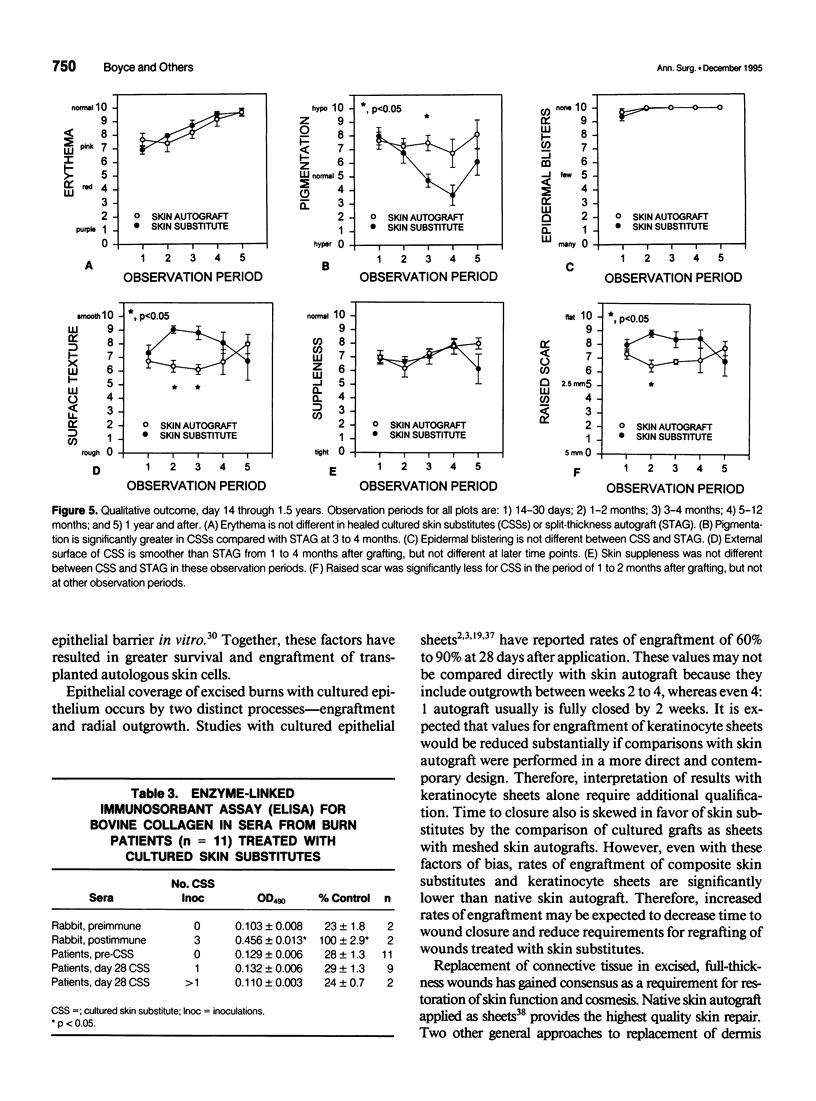
OBJECTIVE: Comparison of cultured skin substitutes (CSSs) and split-thickness autograft (STAG) was performed to assess whether the requirement for autologous skin grafts may be reduced in the treatment of massive burns. SUMMARY BACKGROUND DATA: Cultured skin substitutes consisting of collagen-glycosaminoglycan substrates populated with autologous fibroblasts and keratinocytes have been demonstrated to close full-thickness skin wounds in athymic mice and to express normal skin antigens after closure of excised wounds in burn patients. METHODS: Data were collected from 17 patients between days 2 and 14 to determine incidence of exudate, incidence of regrafting, coloration, keratinization, and percentage of site covered by graft (n = 17). Outcome was evaluated on an ordinal scale (0 = worst; 10 = best) beginning at day 14, with primary analyses at 28 days (n = 10) and 1 year (n = 4) for erythema, pigmentation, epithelial blistering, surface roughness, skin suppleness, and raised scar. RESULTS: Sites treated with CSSs had increased incidence of exudate (p = 0.06) and decreased percentage of engraftment (p < 0.05) compared with STAG. Outcome parameters during the first year showed no differences in erythema, blistering, or suppleness. Pigmentation was greater, scar was less raised, but regrafting was more frequent in CSS sites than STAG. No differences in qualitative outcomes were found after 1 year, and antibodies to bovine collagen were not detected in patient sera. CONCLUSIONS: These results suggest that outcome of engrafted CSSs is not different from STAG and that increased incidence of regrafting is related to decreased percentage of initial engraftment. Increased rates of engraftment of CSSs may lead to improved outcome for closure of burn wounds, allow greater availability of materials for grafting, and reduce requirements for donor skin autograft.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell E., Ehrlich H. P., Buttle D. J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981 Mar 6;211(4486):1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Beumer G. J., van Blitterswijk C. A., Bakker D., Ponec M. Cell-seeding and in vitro biocompatibility evaluation of polymeric matrices of PEO/PBT copolymers and PLLA. Biomaterials. 1993 Jul;14(8):598–604. doi: 10.1016/0142-9612(93)90178-5. [DOI] [PubMed] [Google Scholar]
- Beumer G. J., van Blitterswijk C. A., Ponec M. Biocompatibility of a biodegradable matrix used as a skin substitute: an in vivo evaluation. J Biomed Mater Res. 1994 May;28(5):545–552. doi: 10.1002/jbm.820280504. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Christianson D. J., Hansbrough J. F. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988 Oct;22(10):939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- Boyce S. T. Epidermis as a secretory tissue. J Invest Dermatol. 1994 Jan;102(1):8–10. doi: 10.1111/1523-1747.ep12371721. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Foreman T. J., English K. B., Stayner N., Cooper M. L., Sakabu S., Hansbrough J. F. Skin wound closure in athymic mice with cultured human cells, biopolymers, and growth factors. Surgery. 1991 Nov;110(5):866–876. [PubMed] [Google Scholar]
- Boyce S. T., Greenhalgh D. G., Kagan R. J., Housinger T., Sorrell J. M., Childress C. P., Rieman M., Warden G. D. Skin anatomy and antigen expression after burn wound closure with composite grafts of cultured skin cells and biopolymers. Plast Reconstr Surg. 1993 Apr;91(4):632–641. doi: 10.1097/00006534-199304000-00010. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Hansbrough J. F. Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery. 1988 Apr;103(4):421–431. [PubMed] [Google Scholar]
- Boyce S. T., Holder I. A. Selection of topical antimicrobial agents for cultured skin for burns by combined assessment of cellular cytotoxicity and antimicrobial activity. Plast Reconstr Surg. 1993 Sep;92(3):493–500. doi: 10.1097/00006534-199309000-00018. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Medrano E. E., Abdel-Malek Z., Supp A. P., Dodick J. M., Nordlund J. J., Warden G. D. Pigmentation and inhibition of wound contraction by cultured skin substitutes with adult melanocytes after transplantation to athymic mice. J Invest Dermatol. 1993 Apr;100(4):360–365. doi: 10.1111/1523-1747.ep12471822. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Supp A. P., Harriger M. D., Greenhalgh D. G., Warden G. D. Topical nutrients promote engraftment and inhibit wound contraction of cultured skin substitutes in athymic mice. J Invest Dermatol. 1995 Mar;104(3):345–349. doi: 10.1111/1523-1747.ep12665374. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Warden G. D., Holder I. A. Noncytotoxic combinations of topical antimicrobial agents for use with cultured skin substitutes. Antimicrob Agents Chemother. 1995 Jun;39(6):1324–1328. doi: 10.1128/aac.39.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S. T., Williams M. L. Lipid supplemented medium induces lamellar bodies and precursors of barrier lipids in cultured analogues of human skin. J Invest Dermatol. 1993 Aug;101(2):180–184. doi: 10.1111/1523-1747.ep12363678. [DOI] [PubMed] [Google Scholar]
- Boyce S., Michel S., Reichert U., Shroot B., Schmidt R. Reconstructed skin from cultured human keratinocytes and fibroblasts on a collagen-glycosaminoglycan biopolymer substrate. Skin Pharmacol. 1990;3(2):136–143. doi: 10.1159/000210860. [DOI] [PubMed] [Google Scholar]
- Carver N., Leigh I. M. Keratinocyte grafts and skin equivalents. Int J Dermatol. 1991 Aug;30(8):540–551. doi: 10.1111/j.1365-4362.1991.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Compton C. C., Gill J. M., Bradford D. A., Regauer S., Gallico G. G., O'Connor N. E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989 May;60(5):600–612. [PubMed] [Google Scholar]
- Compton C. C., Hickerson W., Nadire K., Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil. 1993 Nov-Dec;14(6):653–662. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- Cooper M. L., Boyce S. T., Hansbrough J. F., Foreman T. J., Frank D. H. Cytotoxicity to cultured human keratinocytes of topical antimicrobial agents. J Surg Res. 1990 Mar;48(3):190–195. doi: 10.1016/0022-4804(90)90212-k. [DOI] [PubMed] [Google Scholar]
- Cuono C. B., Langdon R., Birchall N., Barttelbort S., McGuire J. Composite autologous-allogeneic skin replacement: development and clinical application. Plast Reconstr Surg. 1987 Oct;80(4):626–637. doi: 10.1097/00006534-198710000-00029. [DOI] [PubMed] [Google Scholar]
- Desai M. H., Mlakar J. M., McCauley R. L., Abdullah K. M., Rutan R. L., Waymack J. P., Robson M. C., Herndon D. N. Lack of long-term durability of cultured keratinocyte burn-wound coverage: a case report. J Burn Care Rehabil. 1991 Nov-Dec;12(6):540–545. doi: 10.1097/00004630-199111000-00009. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 Aug 16;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Remensnyder J. P., Kehinde O., Green H. Cultured epithelial autografts for giant congenital nevi. Plast Reconstr Surg. 1989 Jul;84(1):1–9. doi: 10.1097/00006534-198907000-00001. [DOI] [PubMed] [Google Scholar]
- Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981 Jan 10;1(8211):75–78. [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbrough J. F., Boyce S. T., Cooper M. L., Foreman T. J. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. JAMA. 1989 Oct 20;262(15):2125–2130. [PubMed] [Google Scholar]
- Hansbrough J. F., Doré C., Hansbrough W. B. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. J Burn Care Rehabil. 1992 Sep-Oct;13(5):519–529. doi: 10.1097/00004630-199209000-00004. [DOI] [PubMed] [Google Scholar]
- Heimbach D., Luterman A., Burke J., Cram A., Herndon D., Hunt J., Jordan M., McManus W., Solem L., Warden G. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988 Sep;208(3):313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housinger T. A., Hills J., Warden G. D. Management of pediatric facial burns. J Burn Care Rehabil. 1994 Sep-Oct;15(5):408–411. [PubMed] [Google Scholar]
- Hull B. E., Finley R. K., Miller S. F. Coverage of full-thickness burns with bilayered skin equivalents: a preliminary clinical trial. Surgery. 1990 May;107(5):496–502. [PubMed] [Google Scholar]
- Kaplon R. J., Michler R. E., Xu H., Kwiatkowski P. A., Edwards N. M., Platt J. L. Absence of hyperacute rejection in newborn pig-to-baboon cardiac xenografts. Transplantation. 1995 Jan 15;59(1):1–6. doi: 10.1097/00007890-199501150-00001. [DOI] [PubMed] [Google Scholar]
- Kessler D. A., Siegel J. P., Noguchi P. D., Zoon K. C., Feiden K. L., Woodcock J. Regulation of somatic-cell therapy and gene therapy by the food and drug administration. N Engl J Med. 1993 Oct 14;329(16):1169–1173. doi: 10.1056/NEJM199310143291607. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Cooper D. M., Knighton D. R., Margolis D. J., Pecoraro R. E., Rodeheaver G., Robson M. C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994 Apr;130(4):489–493. [PubMed] [Google Scholar]
- Leenaars P. P., Hendriksen C. F., Angulo A. F., Koedam M. A., Claassen E. Evaluation of several adjuvants as alternatives to the use of Freund's adjuvant in rabbits. Vet Immunol Immunopathol. 1994 Mar;40(3):225–241. doi: 10.1016/0165-2427(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., Herndon D. N., Hollyoak M. A., Atkinson Y. H., Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995 Jul 15;60(1):1–9. [PubMed] [Google Scholar]
- Munster A. M., Weiner S. H., Spence R. J. Cultured epidermis for the coverage of massive burn wounds. A single center experience. Ann Surg. 1990 Jun;211(6):676–680. doi: 10.1097/00000658-199006000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanchahal J., Otto W. R., Dover R., Dhital S. K. Cultured composite skin grafts: biological skin equivalents permitting massive expansion. Lancet. 1989 Jul 22;2(8656):191–193. doi: 10.1016/s0140-6736(89)90374-7. [DOI] [PubMed] [Google Scholar]
- Odessey R. Addendum: multicenter experience with cultured epidermal autograft for treatment of burns. J Burn Care Rehabil. 1992 Jan-Feb;13(1):174–180. [PubMed] [Google Scholar]
- Phillips T. J., Kehinde O., Green H., Gilchrest B. A. Treatment of skin ulcers with cultured epidermal allografts. J Am Acad Dermatol. 1989 Aug;21(2 Pt 1):191–199. doi: 10.1016/s0190-9622(89)70160-2. [DOI] [PubMed] [Google Scholar]
- Rouabhia M., Germain L., Bergeron J., Auger F. A. Allogeneic-syngeneic cultured epithelia. A successful therapeutic option for skin regeneration. Transplantation. 1995 May 15;59(9):1229–1235. [PubMed] [Google Scholar]
- Slivka S. R., Landeen L. K., Zeigler F., Zimber M. P., Bartel R. L. Characterization, barrier function, and drug metabolism of an in vitro skin model. J Invest Dermatol. 1993 Jan;100(1):40–46. doi: 10.1111/1523-1747.ep12354098. [DOI] [PubMed] [Google Scholar]
- Sullivan T., Smith J., Kermode J., McIver E., Courtemanche D. J. Rating the burn scar. J Burn Care Rehabil. 1990 May-Jun;11(3):256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- Warden G. D., Saffle J. R., Kravitz M. A two-stage technique for excision and grafting of burn wounds. J Trauma. 1982 Feb;22(2):98–103. doi: 10.1097/00005373-198202000-00004. [DOI] [PubMed] [Google Scholar]
- Yannas I. V., Burke J. F. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980 Jan;14(1):65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- Yannas I. V., Burke J. F., Gordon P. L., Huang C., Rubenstein R. H. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980 Mar;14(2):107–132. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]