Abstract
OBJECTIVE: The authors' aim was to investigate whether antecedent nutritional routes influence immune responses after surgical insult. SUMMARY BACKGROUND DATA: Total parenteral nutrition (TPN) may influence host responses to infection. To the best of the authors' knowledge, however, no study has focused on the mechanisms underlying the influence of nutritional route on local, systemic, and remote organ (lung) responses after surgical insult. METHODS: Sixty-eight rats were divided into TPN and total enteral nutrition (TEN) groups. The two groups received identical nutrients for 7 days and were then challenged intraperitoneally with 3 x 10(8) Escherichia coli. In the first experiment, the rats were observed for survival. In the second experiment, the rats were killed before (0 hours) challenge or 2 or 6 hours after challenge. Peritoneal exudative cells (PEC) and bronchoalveolar cells (BALC) were harvested and cultured in vitro. Colony-forming units of bacteria in the peritoneal lavage fluid (PLF) were determined. Tumor necrosis factor (TNF), interleukin-1 alpha (IL-1 alpha), interferon-gamma (IFN-gamma) levels in serum, PLF, bronchoalveolar lavage fluid (BALF), and cell culture supernatants were measured. RESULTS: The 48-hour survival rate was higher in TEN than in TPN rats. Local immunity was depressed in the TPN group. Bacterial colony counts in PLF were significantly higher in the TPN group than in the TEN group after challenge. The number of PECs was significantly lower, and at 2 hours, local cytokine (TNF and IL-1 alpha) responses were diminished in the TPN group compared with the TEN group at 2 hours. The number of PECs showed a significant positive correlation with levels of local cytokines in the TEN group but not in the TPN group. Elevation of local IFN-gamma was significant from 0 to 6 hours in the TEN group but not in the TPN group. In vitro production of TNF by PEC was impaired in the TPN rats before challenge. Remote organ (lung) responses were suppressed in the TPN group. The number of BALCs and the TNF levels in BALF declined significantly between 0 and 2 hours in the TEN group but not in the TPN group. Interferon-gamma levels in BALF were higher in the TEN group than in the TPN group at 2 hours. Systemic cytokine responses were disturbed in the TPN group. Production of systemic TNF was greater, but the IFN-gamma response was diminished in the TPN group compared with the TEN group after intraperitoneal bacterial challenge. CONCLUSION: Local, systemic, and remote organ (lung) immune responses to intraperitoneal bacterial challenge are suppressed in TPN-treated animals, leading to poor survival after challenge. Enteral nutrition before surgical insult may enhance host immune responses after the insult as compared to parenteral nutrition.
Full text
PDF


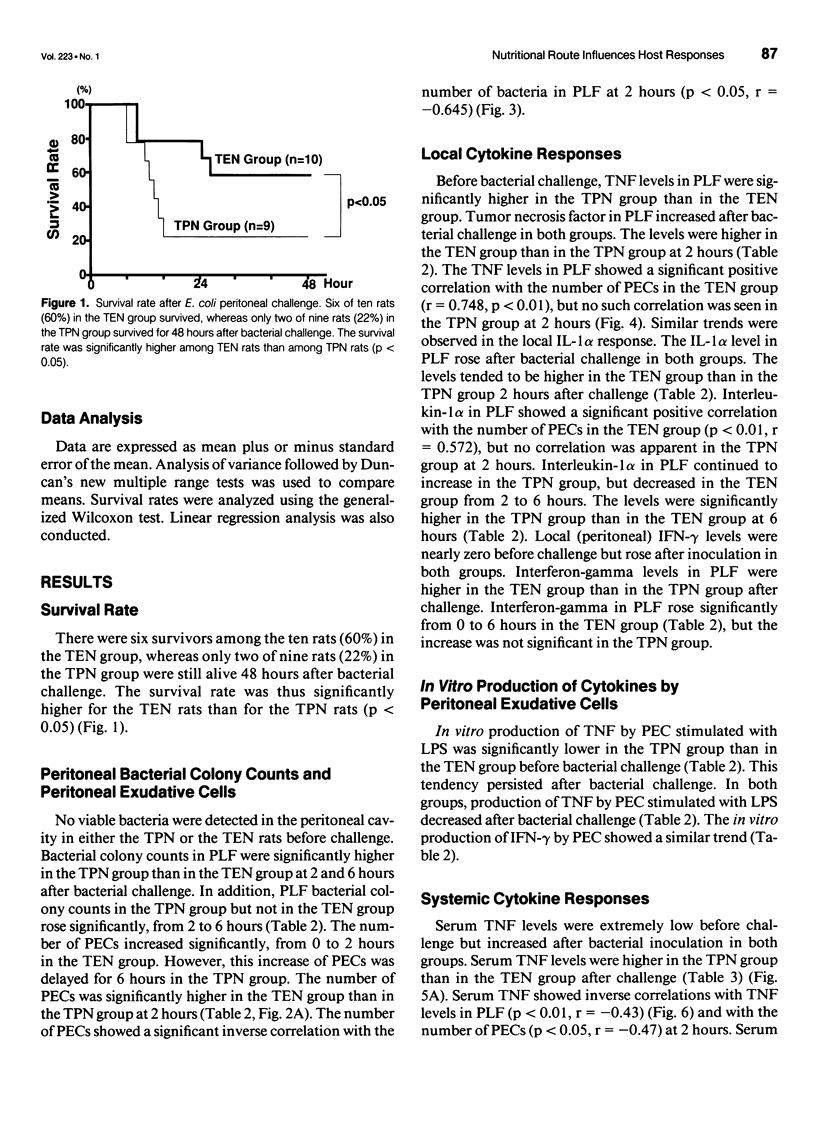
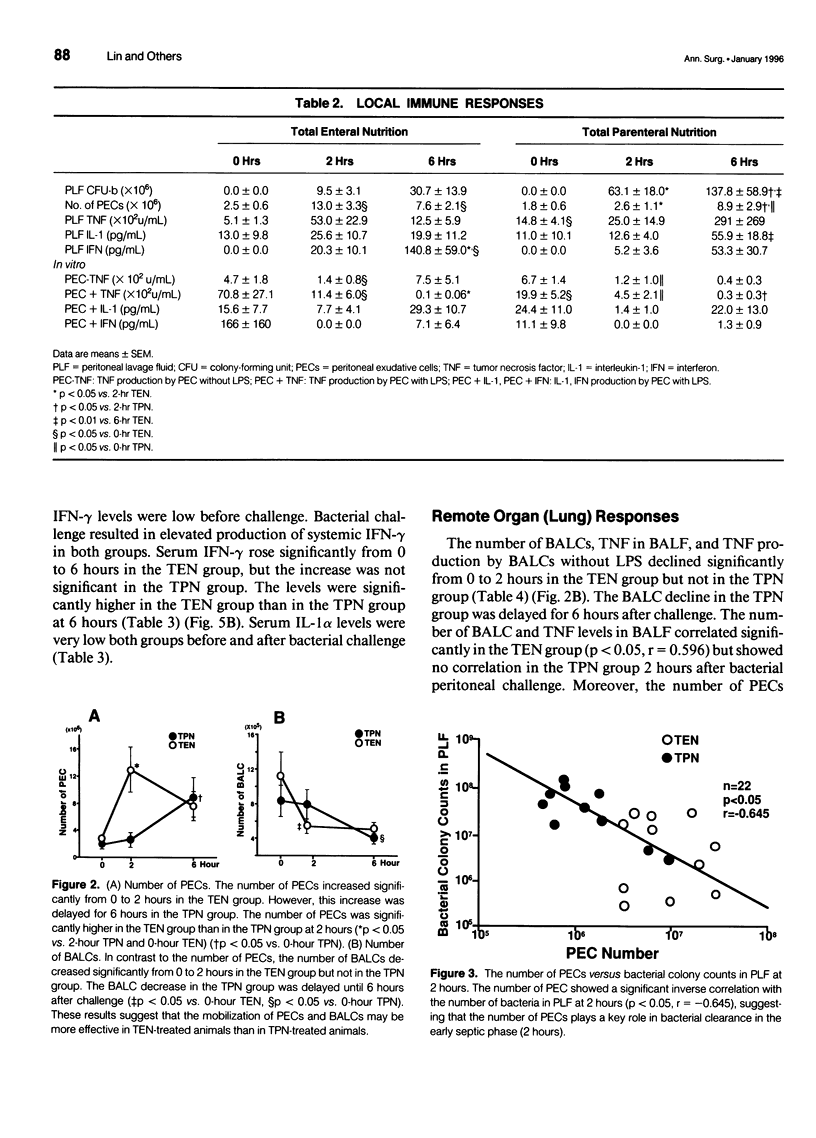
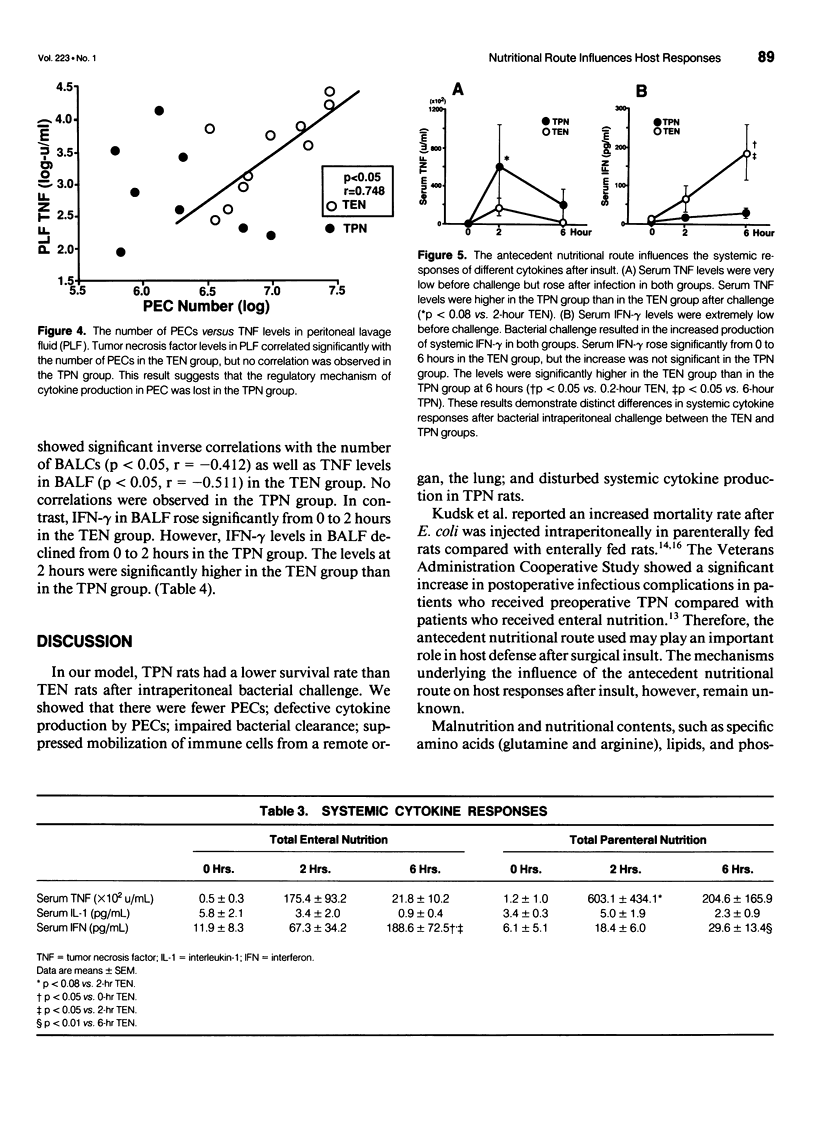
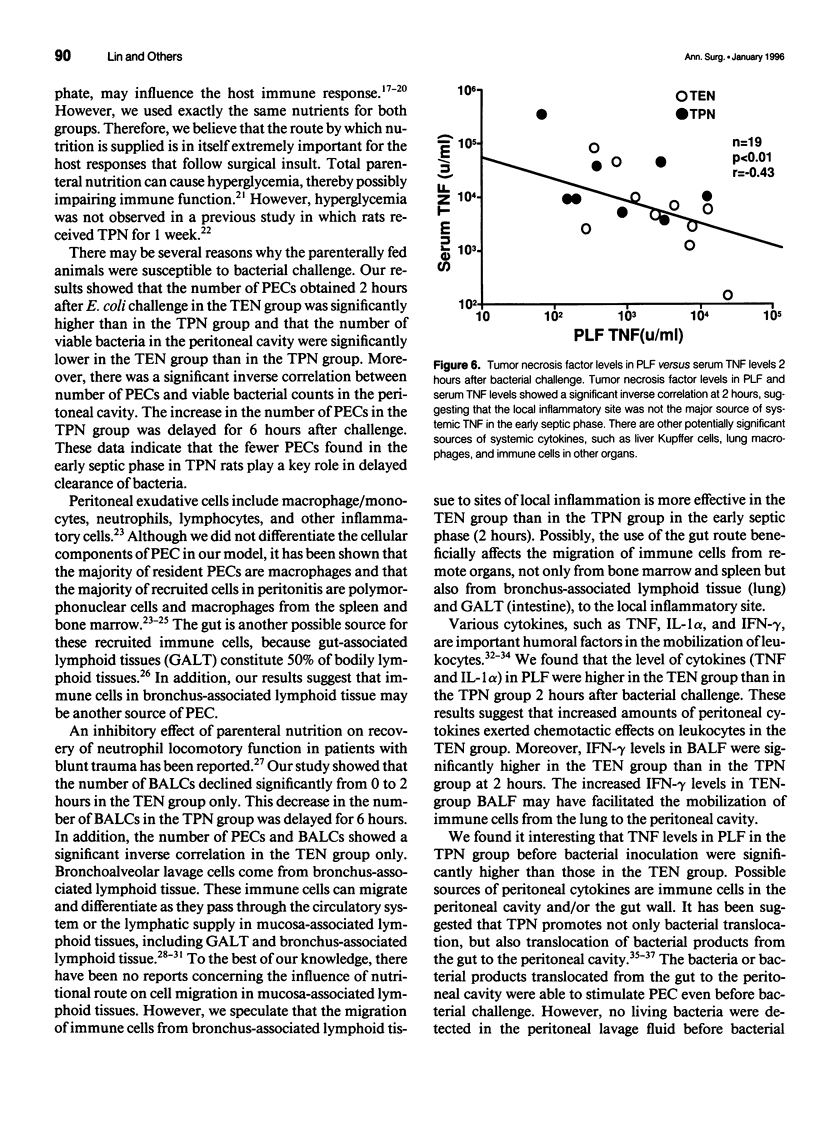


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alverdy J. C., Aoys E., Moss G. S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988 Aug;104(2):185–190. [PubMed] [Google Scholar]
- Alverdy J., Chi H. S., Sheldon G. F. The effect of parenteral nutrition on gastrointestinal immunity. The importance of enteral stimulation. Ann Surg. 1985 Dec;202(6):681–684. doi: 10.1097/00000658-198512000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhahn R. H., Renk C. M. Immune response and leucine oxidation in oral and intravenous fed rats. Am J Clin Nutr. 1984 Jan;39(1):45–53. doi: 10.1093/ajcn/39.1.45. [DOI] [PubMed] [Google Scholar]
- Byerley L. O., Alcock N. W., Starnes H. F., Jr Sepsis-induced cascade of cytokine mRNA expression: correlation with metabolic changes. Am J Physiol. 1992 May;262(5 Pt 1):E728–E735. doi: 10.1152/ajpendo.1992.262.5.E728. [DOI] [PubMed] [Google Scholar]
- Clinchy B., Elenström C., Möller G. The effect of T cell-derived cytokines on B cell motility in vitro. Cell Immunol. 1993 Jan;146(1):62–70. doi: 10.1006/cimm.1993.1006. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Yawata Y., VanSanten L., Gilberstadt S., Silvis S., Jacob H. S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med. 1974 Jun 20;290(25):1403–1407. doi: 10.1056/NEJM197406202902504. [DOI] [PubMed] [Google Scholar]
- Echtenacher B., Falk W., Männel D. N., Krammer P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990 Dec 1;145(11):3762–3766. [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N., Parker P., Greene H. L., Ghishan F. K. Intravenous lipid emulsions and human neutrophil function. J Pediatr. 1981 Dec;99(6):913–916. doi: 10.1016/s0022-3476(81)80019-4. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fan J., Shimokama T., Haraoka S., Tokunaga O., Watanabe T. Monocyte-endothelial cell interactions in vitro, with reference to the influence of interleukin-1 and tumor necrosis factor. Biol Cell. 1993;79(1):17–26. doi: 10.1016/0248-4900(93)90258-g. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Fletcher J. P., Little J. M. A comparison of parenteral nutrition and early postoperative enteral feeding on the nitrogen balance after major surgery. Surgery. 1986 Jul;100(1):21–24. [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Barber A., He W., Moldawer L. L., Bushman E. D., Coyle S. M., Shires G. T., Lowry S. F. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann Surg. 1989 Oct;210(4):449–457. doi: 10.1097/00000658-198910000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Shires G. T., Lowry S. F. The biologic characteristics of cytokines and their implication in surgical injury. Surg Gynecol Obstet. 1990 Apr;170(4):363–378. [PubMed] [Google Scholar]
- Gennari R., Alexander J. W., Eaves-Pyles T. IFN-gamma decreases translocation and improves survival following transfusion and thermal injury. J Surg Res. 1994 Jun;56(6):530–536. doi: 10.1006/jsre.1994.1085. [DOI] [PubMed] [Google Scholar]
- Gianotti L., Alexander J. W., Pyles T., Fukushima R. Arginine-supplemented diets improve survival in gut-derived sepsis and peritonitis by modulating bacterial clearance. The role of nitric oxide. Ann Surg. 1993 Jun;217(6):644–654. doi: 10.1097/00000658-199306000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Andrew D. P., Jablonski-Westrich D., Holzmann B., Butcher E. C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994 Apr 1;152(7):3282–3293. [PubMed] [Google Scholar]
- Hennessey P. J., Black C. T., Andrassy R. J. Nonenzymatic glycosylation of immunoglobulin G impairs complement fixation. JPEN J Parenter Enteral Nutr. 1991 Jan-Feb;15(1):60–64. doi: 10.1177/014860719101500160. [DOI] [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Duijvestijn A. M., Hartwig N., Van der Harten H. J., Pals S. T. The ontogeny of human lymphocyte recirculation: high endothelial cell antigen (HECA-452) and CD44 homing receptor expression in the development of the immune system. Eur J Immunol. 1990 Jul;20(7):1483–1489. doi: 10.1002/eji.1830200712. [DOI] [PubMed] [Google Scholar]
- Illig K. A., Ryan C. K., Hardy D. J., Rhodes J., Locke W., Sax H. C. Total parenteral nutrition-induced changes in gut mucosal function: atrophy alone is not the issue. Surgery. 1992 Oct;112(4):631–637. [PubMed] [Google Scholar]
- Inoue S., Epstein M. D., Alexander J. W., Trocki O., Jacobs P., Gura P. Prevention of yeast translocation across the gut by a single enteral feeding after burn injury. JPEN J Parenter Enteral Nutr. 1989 Nov-Dec;13(6):565–571. doi: 10.1177/0148607189013006565. [DOI] [PubMed] [Google Scholar]
- Jacobs D. L., Rikkers L. F., Forst C. F., Sorrell W. T., Petersen S. R. Effects of enteral vs parenteral nutrition on reticuloendothelial function. J Surg Res. 1987 Jun;42(6):661–664. doi: 10.1016/0022-4804(87)90010-2. [DOI] [PubMed] [Google Scholar]
- James S. P., Kwan W. C., Sneller M. C. T cells in inductive and effector compartments of the intestinal mucosal immune system of nonhuman primates differ in lymphokine mRNA expression, lymphokine utilization, and regulatory function. J Immunol. 1990 Feb 15;144(4):1251–1256. [PubMed] [Google Scholar]
- Kudsk K. A., Carpenter G., Petersen S., Sheldon G. F. Effect of enteral and parenteral feeding in malnourished rats with E. coli-hemoglobin adjuvant peritonitis. J Surg Res. 1981 Aug;31(2):105–110. doi: 10.1016/0022-4804(81)90037-8. [DOI] [PubMed] [Google Scholar]
- Kudsk K. A., Croce M. A., Fabian T. C., Minard G., Tolley E. A., Poret H. A., Kuhl M. R., Brown R. O. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992 May;215(5):503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk K. A., Stone J. M., Carpenter G., Sheldon G. F. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983 Jul;23(7):605–609. doi: 10.1097/00005373-198307000-00010. [DOI] [PubMed] [Google Scholar]
- Maderazo E. G., Woronick C. L., Quercia R. A., Hickingbotham N., Drezner A. D. The inhibitory effect of parenteral nutrition on recovery of neutrophil locomotory function in blunt trauma. Ann Surg. 1988 Aug;208(2):221–226. doi: 10.1097/00000658-198808000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard G., Kudsk K. A. Is early feeding beneficial? How early is early? New Horiz. 1994 May;2(2):156–163. [PubMed] [Google Scholar]
- Moore F. A., Feliciano D. V., Andrassy R. J., McArdle A. H., Booth F. V., Morgenstein-Wagner T. B., Kellum J. M., Jr, Welling R. E., Moore E. E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992 Aug;216(2):172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oghiso Y., Yamada Y., Shibata Y. Exudation of proliferative macrophages in local inflammation in the peritoneum. J Leukoc Biol. 1992 Oct;52(4):421–424. doi: 10.1002/jlb.52.4.421. [DOI] [PubMed] [Google Scholar]
- Ogle C. K., Ogle J. D., Mao J. X., Simon J., Noel J. G., Li B. G., Alexander J. W. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN J Parenter Enteral Nutr. 1994 Mar-Apr;18(2):128–133. doi: 10.1177/0148607194018002128. [DOI] [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Redmond H. P., Leon P., Lieberman M. D., Hofmann K., Shou J., Reynolds J. V., Goldfine J., Johnston R. B., Jr, Daly J. M. Impaired macrophage function in severe protein-energy malnutrition. Arch Surg. 1991 Feb;126(2):192–196. doi: 10.1001/archsurg.1991.01410260080011. [DOI] [PubMed] [Google Scholar]
- Saito H., Trocki O., Alexander J. W., Kopcha R., Heyd T., Joffe S. N. The effect of route of nutrient administration on the nutritional state, catabolic hormone secretion, and gut mucosal integrity after burn injury. JPEN J Parenter Enteral Nutr. 1987 Jan-Feb;11(1):1–7. doi: 10.1177/014860718701100101. [DOI] [PubMed] [Google Scholar]
- Santos A. A., Rodrick M. L., Jacobs D. O., Dinarello C. A., Wolff S. M., Mannick J. A., Wilmore D. W. Does the route of feeding modify the inflammatory response? Ann Surg. 1994 Aug;220(2):155–163. doi: 10.1097/00000658-199408000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J., Lappin J., Minnard E. A., Daly J. M. Total parenteral nutrition, bacterial translocation, and host immune function. Am J Surg. 1994 Jan;167(1):145–150. doi: 10.1016/0002-9610(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Soesatyo M., Biewenga J., van Rooijen N., Kors N., Sminia T. The in situ immune response of the rat after intraperitoneal depletion of macrophages by liposome-encapsulated dichloromethylene diphosphonate. Res Immunol. 1991 Sep;142(7):533–540. doi: 10.1016/0923-2494(91)90098-4. [DOI] [PubMed] [Google Scholar]
- Soesatyo M., Van den Dobbelsteen G. P., Van Rees E. P., Biewenga J., Sminia T. The in vivo antibody response in rat gut-associated lymphoid tissue (GALT) after immunization with bacterial polysaccharide antigen. Res Immunol. 1993 Feb;144(2):121–128. doi: 10.1016/0923-2494(93)80067-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Miura S., Tashiro H., Serizawa H., Hamada Y., Yoshioka M., Tsuchiya M. Morphological alteration of gut-associated lymphoid tissue after long-term total parenteral nutrition in rats. Cell Tissue Res. 1991 Oct;266(1):29–36. doi: 10.1007/BF00678708. [DOI] [PubMed] [Google Scholar]
- Verweij W. R., Namavar F., Schouten W. F., Kostense P. J., Pellenkoft M., De Graaff J., MacClaren D. M. In-vitro activity of peritoneal cells from rats after intra-abdominal infection with Bacteroides fragilis and Escherichia coli. J Med Microbiol. 1993 Jan;38(1):13–18. doi: 10.1099/00222615-38-1-13. [DOI] [PubMed] [Google Scholar]
- Zanetti G., Heumann D., Gérain J., Kohler J., Abbet P., Barras C., Lucas R., Glauser M. P., Baumgartner J. D. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-alpha and to lipopolysaccharide. J Immunol. 1992 Mar 15;148(6):1890–1897. [PubMed] [Google Scholar]
- Zhang Y., Ramos B. F., Jakschik B. A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992 Dec 18;258(5090):1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]


