Abstract
The detrimental effects of prenatal alcohol exposure on the development of humans are well understood and include fetal alcohol spectrum disorders (FASD), a broad set of conditions referring to the adverse physical and behavioral health impairments associated with exposure to alcohol in utero. Using a case-control study design, the purpose of this study was to better understand the complex comorbidity patterns associated with FASD (N = 3,248) and to examine how they differ with the general patient population (N = 16,240) and a cohort of behavioral health controls (N = 16,240). Employing a novel unsupervised machine learning algorithm applied to a nationally representative hospital discharge database, we found 57 distinct comorbidities that frequently occurred among FASD cases, in addition to a set of 144 complex overlapping comorbidity patterns. The identified comorbidities were generally more likely to occur in the FASD cases compared to the general patient population control group, while differences with behavioral health controls were less readily apparent. This study adds to a small but growing body of research on comorbidities experienced by individuals with FASD. We discuss the implications of the identified comorbidity patterns on the ongoing identification, treatment, and surveillance of FASD in the US.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-13366-9.
Keywords: Fetal alcohol spectrum disorders, Fetal alcohol syndrome, Comorbidities, Diagnoses, Machine learning, National inpatient sample
Subject terms: Medical research, Epidemiology, Comorbidities
Introduction
Worldwide, it is estimated that approximately 10% of women consume alcohol during pregnancy1,2. There are currently no known safe levels of alcohol consumption during pregnancy, and the impact of prenatal alcohol exposure (PAE) on the health and development of newborns is well documented3. Perhaps most notably, PAE is known to produce fetal alcohol spectrum disorders (FASD), an umbrella term that encompasses the broad spectrum of adverse physical, mental, cognitive, and behavioral outcomes resulting from exposure to alcohol during pregnancy4,5. While symptoms vary from individual to individual, FASD is known to result in abnormal facial features, growth delays and developmental deficiencies, and substantial impacts on social and emotional development and interactions6–8. These issues impact individuals with FASD across the lifespan, with recent studies finding high levels of healthcare utilization9,10difficulties in a variety of social and living circumstances11 and detrimental physical and behavioral health outcomes that persist into adulthood12,13.
Recently, one survey of more than 500 individuals living with FASD found notable prevalences of a variety of physical and behavioral comorbidities across nearly every body system14. Yet, despite the call for FASD to be recognized as a “whole body” diagnosis14 research specifically focused on understanding comorbidity patterns among those with FASD is lacking7,15. For example, one systematic review and meta-analysis found only twelve studies in the United States (US) that reported on the frequency of at least one disease condition among individuals with FASD16. A separate systematic review specifically focused on mental disorders associated with FASD found an additional ten studies17. Taken together, this small but growing body of literature has yielded important insights about the unique health challenges of FASD, reporting frequently on comorbidities such as attention-deficit/hyperactivity disorder (ADHD)18–22; speech, hearing, language, and vision deficits19,21,23–25 mood and behavioral disorders22,26–28; and a range of other physical health outcomes, including growth and developmental delays29–31.
While these studies highlight the complexity and breadth of the adverse health outcomes associated with FASD, continued investigation of the comorbidities associated with the condition is warranted for several reasons. First, many of the studies19,22–26,28,30–32 were conducted on small, convenience or clinic-based samples ranging in size from ten to 50 participants, and therefore lack generalizability to a larger population of individuals with FASD. Second, with notable exception13,14,26 nearly all studies to date have reported on comorbidities associated with FASD in children, either missing or intentionally excluding adults with FASD. This limitation is especially important as individuals who were first diagnosed with fetal alcohol syndrome (FAS, the most severe form of FASD) in the mid 1970 s are now aging into middle-adulthood, a critical developmental milestone where the onset of age-specific comorbidities or chronic conditions may surface7. Finally, because many of the studies have utilized purposive and limited samples, they lacked direct comparison groups. As a consequence, comparisons of the prevalence of comorbidities experienced by those with FASD to other populations have instead relied on indirect comparisons to other published estimates14–16 that may not be directly comparable to the FASD population.
The purpose of this study is to improve our understanding of comorbidities associated with FASD in the US. Using a diverse sample from a nationally representative hospital discharge database, we make several important and necessary contributions. First, we utilize an unsupervised machine learning algorithm to identify frequently occurring comorbidities among individuals diagnosed with FASD, with special emphasis placed on exploring patterns of overlapping comorbidities. Second, using a case-control study design, we compare the odds of experiencing these comorbidity patterns to the general patient population (individuals without FASD) and a population of individuals with behavioral health related diagnoses (also without FASD). We discuss the implications of the identified comorbidity patterns on the ongoing identification, treatment, and surveillance of FASD in the US.
Methods
This study utilized hospital discharge data obtained from the National Inpatient Sample (NIS), which is a component of the Healthcare Cost and Utilization Project (HCUP) collected and maintained by the Agency for Healthcare Research and Quality. The NIS is the largest publicly available all-payer inpatient database, representing more than 35 million hospitalizations annually occurring at more than 4,500 acute care hospitals in the US. The NIS is nationally representative, with a complex multi-stage sampling frame that covers 98% of the U.S. population and 97% of all hospital discharges in the US33. We conducted a retrospective case-control study comparing the prevalence of comorbidities among discharges associated with FASD to the general patient population and to behavioral health-related discharges following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for case-control studies34. This study was considered exempt from institutional review board oversight because data from the NIS are deidentified.
Selection procedures
Case-control selection procedures are displayed visually in Fig. 1. There are currently no validated algorithms for the identification of FASD in administrative databases like the NIS35 and existing approaches in prior studies widely differ10,19,36–38. For our analyses, we identified FASD cases as those with any inpatient discharge record containing the International Classification of Diseases, Tenth Revision (ICD-10) code Q86.0, fetal alcohol syndrome, occurring in either the primary or secondary discharge diagnosis fields available within the NIS. Controls were first assigned to the general patient population group if their discharge record did not contain the Q86.0 code as either a primary or secondary diagnosis. From the pool of these general patient population controls, we further identified behavioral health discharges using Clinical Classification Software Refined (CCSR) applied to the NIS database. We considered the pool of behavioral health discharges those containing CCSR diagnosis codes from 14 categories of the mental, behavioral, and neurodevelopmental disorders (MBD) CCSR code set occurring in either the primary or secondary NIS discharge diagnosis fields. Complete information for all codes utilized is provided in Supplemental Material 1.
Fig. 1.
Case-control selection procedures. White boxes represent sample sizes and relevant exclusion criteria applied to the National Inpatient Sample. Blue boxes represent final sample sizes for FASD cases, the general patient population control group, and the behavioral health control group.
Three exclusion criteria were applied in our selection procedures. Given that the focus of our study was to identify and compare comorbidity patterns between cases and controls, we first limited the sample selection to years 2016 through 2020 of the NIS. Prior years of the NIS were specifically excluded to eliminate known measurement bias due to changes in diagnostic coding practices associated with the switch from ICD-9 to ICD-10, which occurred in October 201539,40. Prior to selection of cases and controls, we further excluded the NIS database to remove any records that contained the P04.3 ICD-10 code for “newborn affected by maternal use of alcohol.” These records were excluded due to potential measurement error from changes in the P04.3 code during the study window; prior to October 2016, this code reflected any “newborn (suspected to be) affected by maternal use of alcohol.” Finally, we excluded any record in 2020 containing an ICD-10 code for COVID-19 to remove the potential influence of the pandemic-specific issues on FASD comorbidity patterns. FASD cases that met inclusion criteria were matched to the general patient population and behavioral health control groups meeting inclusion criteria at a ratio of one to five, matching on patient age, sex, and race.
Identification of comorbidity patterns
We identified comorbidity patterns associated with FASD by employing a novel unsupervised machine learning technique, association rule mining (ARM), as described fully in Supplemental Material 2. ARM belongs to a growing class of machine learning models used to detect, examine, and describe comorbidity patterns apparent in structured administrative healthcare databases like the NIS41. It has been used in prior studies to detect the presence of comorbidity patterns in conditions such as cancer42rheumatoid arthritis43and mood disorders44. ARM identifies comorbidity patterns by deploying an a priori search algorithm to find frequently occurring patterns that exist in comorbidity item sets established by user-defined thresholds (see Supplemental Material 2 for more explanation).
For the current study, we utilized ARM to inductively identify all comorbidity patterns that occurred in at least 3% of FASD cases. Because ARM can determine patterns of co-occurring diagnoses, we introduce specific terminology to frame our results. We refer to first-order comorbidities as single comorbidities found by the algorithm (for example, diabetes). We refer to second-order comorbidities as any pair of comorbidities that occurred together (for example, diabetes and cancer). Similarly, we refer to third-order comorbidities as triplets of co-occurring comorbidities (for example, diabetes, cancer, and anxiety disorders). In the present study, no higher-order comorbidities (i.e., four or more comorbidities) were identified by the ARM a priori algorithm. All comorbidities identified by the algorithm were subject to review by two clinicians with subject matter expertise in the diagnosis and treatment of FASD. Their two-round review process evaluated the clinical relevance of each comorbidity, and their input determined which comorbidities to keep, which to remove, or which to collapse into a broader comorbidity category (see Supplemental Material 3). Following prior studies, we visualized the first-order comorbidities using an ordered bar chart and the second- and third-order comorbidities by projecting the patterns onto network diagrams44,45. Given the high number of second-order comorbidities identified (see Results section), to aid in visualization of the network diagram we utilized a fast greedy clustering algorithm46 to group frequently co-occurring comorbidities into three comorbidity clusters.
Statistical analyses
For FASD cases and both control groups, we described demographic characteristics associated with the inpatient hospitalizations, including age, sex, race, expected primary payer, length of stay in days, discharge disposition, hospital census region, and severity of illness at presentation to the hospital (using all patient refined diagnosis related groups)47. Nationally representative estimates for each characteristic were calculated using PROC SURVEYFREQ in SAS version 9.448. Differences in the demographic characteristics between cases and controls were examined using a Rao-Scott Chi-square test, which accounts for the complex survey design of the NIS data49.
For each first-, second-, and third-order comorbidity pattern identified using ARM, we calculated nationally representative estimates to quantify the percentage of hospitalizations for both cases and controls where the comorbidity pattern was present, also using PROC SURVEYFREQ in SAS 9.448. We then examined differences in the likelihood of having each comorbidity pattern between cases and controls using iterated augmented generalized estimating equations (augGEE)50. This approach was necessary given the rarity of certain comorbidity patterns in the control groups, which resulted in these models failing convergence in traditional survey-weighted logistic regression due to issues of separation51. For each comorbidity pattern identified, we estimated the unadjusted and adjusted odds of having that comorbidity at time of hospitalization, controlling for the eight demographic characteristics described above. The augGEE models accounted for the complex sampling design of the NIS, and results were made nationally representative by incorporating sampling weights using the method described by Rader et al.52.
For the univariate and multivariable analyses, missing data were assumed missing completely at random and were addressed using a hot deck imputation procedure prior to calculating nationally representative estimates49. We selected hot deck imputation for two reasons. First, it has been used in prior studies with HCUP data by AHRQ53 and other researchers54,55. Second, we preferred hot deck imputation to more complex approaches such as multiple imputation given the computationally intense estimation procedures required to produce the augGEE model estimates. Simple hot deck imputation allowed for the augGEE models to estimate given available computational resources. Additionally, given the high number of models estimated, we reduced the probability of making type 1 errors by considering statistically significant results as those with P values  0.001. For comparisons between cases and controls, we report in-text for the top ten most frequently occurring first-, second-, and third-order comorbidities. Complete results are reported in Supplemental Material 4–9. Finally, we performed one sensitivity analysis to ensure robust findings. Given that a higher percentage of FASD cases were enrolled in Medicaid compared to the two control groups (see results section below), we calculated nationally representative estimates of each comorbidity pattern in the subset of discharges whose primary payer was Medicaid only.
0.001. For comparisons between cases and controls, we report in-text for the top ten most frequently occurring first-, second-, and third-order comorbidities. Complete results are reported in Supplemental Material 4–9. Finally, we performed one sensitivity analysis to ensure robust findings. Given that a higher percentage of FASD cases were enrolled in Medicaid compared to the two control groups (see results section below), we calculated nationally representative estimates of each comorbidity pattern in the subset of discharges whose primary payer was Medicaid only.
Results
The final sample size (see Fig. 1) following application of exclusion criteria was 3,248 FASD discharges (weighted N = 16,240), 16,240 general patient discharges (weighted N = 81,200), and 16,240 behavioral health discharges (weighted N = 81,200). Demographic characteristics of the cases and controls are displayed in Table 1. Approximately half (51.38%) of the FASD cases were between 15 and 34 years of age, with more men (53.76%) than women (46.24%), and a greater percentage of white individuals (60.50%) compared to other races. Public insurance sources such as Medicare and Medicaid (78.82%) covered most of the hospitalizations for FASD cases, with about one-fourth (24.69%) of hospitalizations lasting nine or more days, and most (79.40%) resulting in a routine discharge from the hospital. These hospitalizations for FASD cases were geographically dispersed, with the highest percentage of hospitalizations occurring in the West North Central region of the US (21.92%). Regarding severity of illness, slightly more than half of the FASD hospitalizations were associated with a moderate loss of function (51.33%).
Table 1.
Demographic characteristics of inpatient hospitalizations.
| Characteristic | FASD Cases | General patient controls | Behavioral health controls | FASD vs. GP | FASD vs. BH |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |||
| Age (in Years) | Matched | Matched | |||
| Less than 1 | 1,300 (8.00%) | 6,500 (8.00%) | 6,500 (8.00%) | ||
| 1–4 | 550 (3.39%) | 2,750 (3.39%) | 2,750 (3.39%) | ||
| 5–14 | 2,825 (17.40%) | 14,125 (17.40%) | 14,125 (17.40%) | ||
| 15–24 | 5,030 (30.97%) | 25,150 (30.97%) | 25,150 (30.97%) | ||
| 25–34 | 3,315 (20.41%) | 16,575 (20.41%) | 16,575 (20.41%) | ||
| 35–44 | 1,495 (9.21%) | 7,475 (9.21%) | 7,475 (9.21%) | ||
| 45–54 | 935 (5.76%) | 4,675 (5.76%) | 4,675 (5.76%) | ||
| 55–64 | 595 (3.66%) | 2,975 (3.66%) | 2,975 (3.66%) | ||
| 65–74 | 130 (0.80%) | 650 (0.80%) | 650 (0.80%) | ||
| 75 + | 65 (0.40%) | 325 (0.40%) | 325 (0.40%) | ||
| Sex | Matched | Matched | |||
| Male | 8,730 (53.76%) | 43,650 (53.76%) | 43,650 (53.76%) | ||
| Female | 7,510 (46.24%) | 37,550 (46.24%) | 37,550 (46.24%) | ||
| Race | Matched | Matched | |||
| White | 9,825 (60.50%) | 49,125 (60.50%) | 49,125 (60.50%) | ||
| Black | 3,135 (19.30%) | 15,675 (19.30%) | 15,675 (19.30%) | ||
| Hispanic | 1,120 (6.90%) | 5,600 (6.90%) | 5,600 (6.90%) | ||
| Asian or Pacific Islander | 310 (1.91%) | 1,550 (1.91%) | 1,550 (1.91%) | ||
| Native American or Other | 1,850 (11.39%) | 9,250 (11.39%) | 9,250 (11.39%) | ||
| Expected primary payer | < 0.0001 | < 0.0001 | |||
| Medicare | 3,760 (23.15%) | 5,240 (6.45%) | 7,650 (9.42%) | ||
| Medicaid | 9,040 (55.67%) | 33,520 (41.28%) | 37,140 (45.74%) | ||
| Private Insurance | 2,705 (16.66%) | 33,635 (41.42%) | 28,200 (34.73) | ||
| Self-Pay | 305 (1.88%) | 5,025 (6.19%) | 4,555 (5.61%) | ||
| No Charge or Other | 430 (2.65%) | 3,780 (4.66%) | 3,655 (4.50%) | ||
| Length of stay (in days) | < 0.0001 | < 0.0001 | |||
| Less than 2 | 4,225 (26.01%) | 38,445 (47.35%) | 27,050 (33.31%) | ||
| 3–5 | 5,130 (31.59%) | 27,475 (33.84%) | 28,805 (35.47%) | ||
| 6–8 | 2,875 (17.70%) | 7,470 (9.20%) | 12,255 (15.09%) | ||
| 9 + | 4,010 (24.69%) | 7,810 (9.62%) | 13,090 (16.12%) | ||
| Disposition | < 0.0001 | < 0.0001 | |||
| Routine | 12,895 (79.4%) | 70,555 (86.89%) | 13,478 (82.99%) | ||
| Transfer: Short-Term Hosp. | 345 (2.12%) | 1,235 (1.52%) | 295 (1.82%) | ||
| Transfer: SNF, ICF, or Other | 1,835 (11.30%) | 3,460 (4.26%) | 6,005 (7.39%) | ||
| Home Health Care | 825 (5.08%) | 3,660 (4.51%) | 3,850 (4.74%) | ||
| Against Medical Advice | 240 (1.48%) | 1,735 (2.14%) | 1,990 (2.45%) | ||
| Died | 100 (0.62%) | 555 (0.68%) | 490 (0.60%) | ||
| Hospital census region | < 0.0001 | < 0.0001 | |||
| New England | 665 (4.09%) | 3,385 (4.17%) | 3,525 (4.34%) | ||
| Middle Atlantic | 1,710 (10.53%) | 12,925 (15.92%) | 11,570 (14.25%) | ||
| East North Central | 2,735 (16.84%) | 11,050 (13.61%) | 13,155 (16.20%) | ||
| West North Central | 3,560 (21.92%) | 6,085 (7.49%) | 8,475 (10.44%) | ||
| South Atlantic | 2,160 (13.30%) | 17,255 (21.25%) | 17,030 (20.97%) | ||
| East South Central | 585 (3.60%) | 5,840 (7.19%) | 5,530 (6.81%) | ||
| West South Central | 780 (4.80%) | 8,770 (10.80%) | 7,975 (9.82%) | ||
| Mountain | 1,630 (10.04%) | 5,630 (6.93%) | 4,995 (6.15%) | ||
| Pacific | 2,415 (14.87%) | 10,260 (12.64%) | 8,945 (11.02%) | ||
| Severity of illness (APR-DRG) | < 0.0001 | < 0.0001 | |||
| No class specified | 65 (0.40%) | 70 (0.09%) | 100 (0.12%) | ||
| Minor loss of function | 3,015 (18.57%) | 33,075 (40.73%) | 22,745 (28.01%) | ||
| Moderate loss of function | 8,335 (51.33%) | 31,510 (38.81%) | 36,715 (45.22%) | ||
| Major loss of function | 3,810 (23.46%) | 12,565 (15.47%) | 15,965 (19.66%) | ||
| Extreme loss of function | 1,015 (6.25%) | 3,980 (4.90%) | 5,675 (6.99%) |
Note. FASD = fetal alcohol spectrum disorder cases. GP = general patient controls. BH = behavioral health controls. SNF = skilled nursing facility. ICF = intermediate care facility.
Statistically significant differences existed between FASD cases and both control groups for all demographic characteristics, excluding the demographic variables used for matching. Compared to the general patient population and individuals with other behavioral health diagnoses, hospitalizations for FASD were more frequently covered by public insurance sources (P <.0001) and more frequently resulted in longer lengths of stay (P <.0001). Compared to the two control groups, hospitalizations for FASD also exhibited a lower frequency of routine discharges from the hospital, instead experiencing transfers to skilled nursing facilities and intermediate care facilities at a higher level than the general patient population and those with other behavioral health diagnoses (P <.0001). Regarding geographic location, the FASD hospitalizations occurred more frequently in the West North Central region of the US (P <.0001). Regarding severity of illness, FASD cases experienced moderate and major losses of function at a higher frequency than the general patient population (P <.0001), whereas the behavioral health control group exhibited higher levels of minor loss of function compared to the FASD cases (P <.0001).
Identified comorbidity patterns associated with FASD
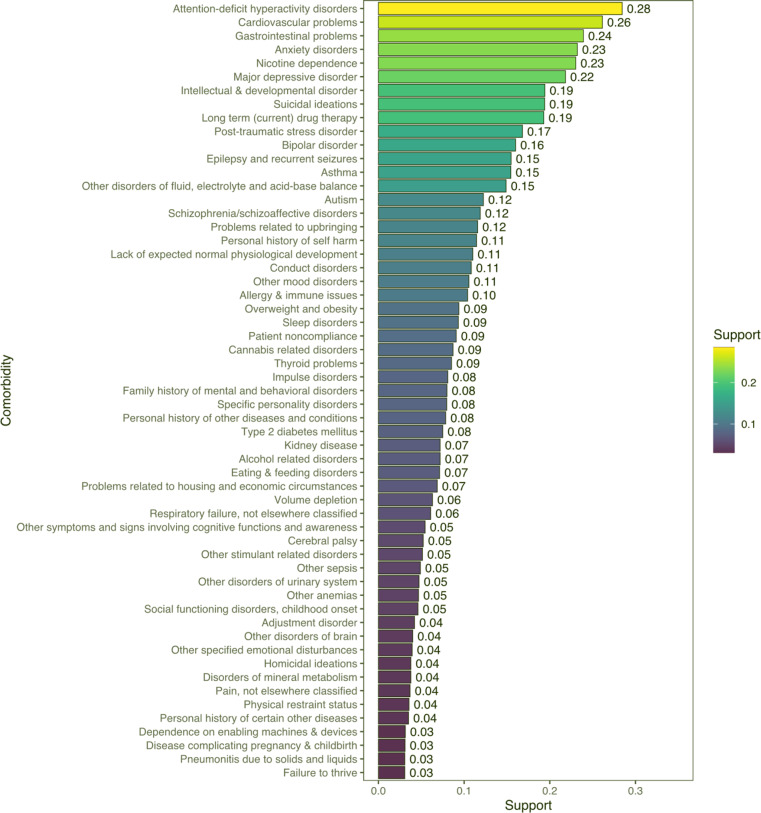
Following subject matter expert review, 57 unique first-order comorbidities associated with FASD were identified, as displayed in Fig. 2. Many of the most frequently occurring comorbidities associated with FASD (see Table 2) encompassed a wide range of physical, behavioral, and substance use related diagnoses, including: ADHD (28.4%), cardiovascular problems (26.1%), gastrointestinal problems (23.9%), anxiety disorders (23.2%), nicotine dependence (23.0%), major depressive disorder (MDD, 21.8%), intellectual and developmental disorders (19.4%), suicidal ideations, (19.4%), long-term drug therapy use (19.3%), and post-traumatic stress disorder (16.8%). Additional noteworthy physical comorbidities included epilepsy and recurrent seizures (15.5%), allergy and immune issues (10.4%), thyroid problems (8.5%), kidney disease (7.2%), and cerebral palsy (5.2%). Of interest within the first-order comorbidities identified were several conditions reflecting the unique social and personal circumstances among FASD cases, including issues such as: problems related to upbringing (11.6%), personal history of self-harm (11.5%), problems related to housing and economic circumstances (6.9%), and homicidal ideations (3.8%). A complete list of nationally representative first-order comorbidity estimates is provided in Supplemental Material 4.
Fig. 2.
First-order comorbidities (n = 57 comorbidities) associated with FASD. Bars and reported values represent the support levels (frequency) for each of the 57 comorbidities identified by association rule mining and subsequent clinical review of discharge records from the FASD cases.
Table 2.
Nationally representative estimates of comorbidity patterns by study group.
| FASD Cases No. (%) |
General Patient Controls No. (%) |
Behavioral Health Controls No. (%) | |
|---|---|---|---|
| First-order comorbidities | |||
| Attention-deficit hyperactivity disorders | 4,620 (28.4%) | 4,320 (5.3%) | 11,045 (13.6%) |
| Cardiovascular problems | 4,245 (26.1%) | 16,050 (19.8%) | 19,990 (24.6%) |
| Gastrointestinal problems | 3,885 (23.9%) | 10,625 (13.1%) | 17,055 (21.0%) |
| Anxiety disorders | 3,770 (23.2%) | 10,480 (12.9%) | 30,005 (37.0%) |
| Nicotine dependence | 3,740 (23.0%) | 17,880 (22.0%) | 23,315 (28.7%) |
| Major depressive disorder | 3,545 (21.8%) | 10,365 (12.8%) | 29,910 (36.8%) |
| Intellectual & developmental disorder | 3,155 (19.4%) | 620 (0.8%) | 1,840 (2.3%) |
| Suicidal ideations | 3,150 (19.4%) | 4,930 (6.1%) | 13,705 (16.9%) |
| Long term (current) drug therapy | 3,135 (19.3%) | 12,490 (15.4%) | 15,645 (19.3%) |
| Post-traumatic stress disorder | 2,730 (16.8%) | 2,385 (2.9%) | 7,090 (8.7%) |
| Second-order comorbidities | |||
| Major depressive disorder & anxiety disorders | 1,605 (9.9%) | 5,005 (6.2%) | 13,950 (17.2%) |
| Gastrointestinal problems & cardiovascular problems | 1,595 (9.8%) | 4,070 (5.0%) | 6,815 (8.4%) |
| Attention-deficit hyperactivity disorders & anxiety disorders | 1,490 (9.2%) | 1,220 (1.5%) | 3,325 (4.1%) |
| Suicidal ideations & major depressive disorder | 1,450 (8.9%) | 2,925 (3.6%) | 8,090 (10.0%) |
| Attention-deficit hyperactivity disorders & suicidal ideations | 1,325 (8.2%) | 950 (1.2%) | 2,480 (3.1%) |
| Nicotine dependence & anxiety disorders | 1,295 (8.0%) | 4,220 (5.2%) | 10,970 (13.5%) |
| Nicotine dependence & major depressive disorder | 1,280 (7.9%) | 3,805 (4.7%) | 10,015 (12.3%) |
| Conduct disorders & Attention-deficit hyperactivity disorders | 1,250 (7.7%) | 745 (0.9%) | 1,985 (2.4%) |
| Suicidal ideations & anxiety disorders | 1,245 (7.7%) | 2,125 (2.6%) | 5,800 (7.1%) |
| Attention-deficit hyperactivity disorders & Major depressive disorder | 1,230 (7.6%) | 1,150 (1.4%) | 3,310 (4.1%) |
| Third-order comorbidities | |||
| Anxiety disorders & suicidal ideations & major depressive disorder | 710 (4.4%) | 1,480 (1.8%) | 4,055 (5.0%) |
| Nicotine dependence & major depressive disorder & anxiety disorders | 620 (3.8%) | 2,005 (2.5%) | 4,950 (6.1%) |
| Attention-deficit hyperactivity disorders & major depressive disorder & anxiety disorders | 600 (3.7%) | 585 (0.7%) | 1,685 (2.1%) |
| Attention-deficit hyperactivity disorders & major depressive disorder & suicidal ideations | 585 (3.6%) | 535 (0.7%) | 1,415 (1.7%) |
| Gastrointestinal problems & long term (current) drug therapy & cardiovascular problems | 560 (3.4%) | 1,630 (2.0%) | 2,500 (3.1%) |
| Nicotine dependence & suicidal ideations & major depressive disorder | 545 (3.4%) | 985 (1.2%) | 2,300 (2.8%) |
| Personal history of self-harm & major depressive disorder & suicidal ideations | 525 (3.2%) | 730 (0.9%) | 1,940 (2.4%) |
| Anxiety disorders & suicidal ideations & attention-deficit hyperactivity disorders | 520 (3.2%) | 435 (0.5%) | 1,085 (1.3%) |
| Major depressive disorder & post-traumatic stress disorder & suicidal ideations | 490 (3.0%) | 440 (0.5%) | 1,565 (1.9%) |
Note. Rows represent the top ten most frequently occurring first- and second-order comorbidities identified. Rows for the.
third-order comorbidities represent all nine patterns identified.
Regarding second-order comorbidities, 135 unique patterns were identified by the unsupervised machine learning process, as displayed in Fig. 3. The most frequently occurring of these patterns (see Table 2) tended to represent overlapping behavioral health diagnoses such as MDD and anxiety disorders (9.9%), ADHD and anxiety disorders (9.2%), and suicidal ideations and MDD (8.9%), though the physical health comorbidities of gastrointestinal problems and cardiovascular problems also occurred in 9.8% of FASD cases. Additionally, in the FASD cases nicotine dependence frequently co-occurred with behavioral health diagnoses (see Table 2 and Supplemental Material 5) such as anxiety disorders (8.0%), MDD (7.9%), cardiovascular problems (7.1%), and suicidal ideations (6.8%). Several noteworthy co-occurring physical health comorbidities were also identified (see Supplemental Material 5), such as disorders of fluid, electrolyte, and acid-base balance and cardiovascular problems (7.3%); epilepsy and recurrent seizures and gastrointestinal problems (5.7%); and asthma and gastrointestinal problems (5.4%).
Fig. 3.
Second-order comorbidity patterns (N = 135 patterns) associated with FASD. Nodes represent a specific comorbidity, while lines between nodes represent the formation of two comorbidities as a unique pattern among FASD cases. Nodes of unique comorbidity clusters are color coded.
Regarding third-order comorbidities, nine unique patterns were identified by the unsupervised machine learning process, as displayed in Fig. 4. Each of the nine identified patterns occurred in less than 5% of FASD cases but nonetheless represented serious health concerns. The most frequently occurring third-order comorbidity pattern was anxiety disorders, suicidal ideations, and MDD, occurring in 4.4% of FASD cases (see Table 2 and Supplemental Material 6). Many of the third-order patterns represented overlapping behavioral health diagnoses, for example: nicotine dependence, MDD, and anxiety disorders (3.8%); ADHD, MDD, and anxiety disorders (3.7%); and ADHD, MDD, and suicidal ideations (3.6%). The most notable third-order pattern containing physical health diagnoses was gastrointestinal problems, long term drug therapy, and cardiovascular problems, occurring in 3.4% of FASD cases. Otherwise, third-order comorbidities tended to represent patterns of behavioral health and substance use comorbidities.
Fig. 4.
Third-order comorbidity patterns (N = 9 patterns) associated with FASD. Nodes represent the formation of three comorbidities as a unique pattern among FASD cases.
Differences in comorbidity patterns between cases and controls
The results of the adjusted augGEE models estimating the likelihood of experiencing each of the comorbidity patterns are displayed in Table 3 (for all results, including unadjusted models, see Supplemental Material 7–9). Generally speaking, FASD cases were significantly more likely to experience the identified comorbidity patterns compared to the general patient population. For example, even after controlling for demographic characteristics, the odds of FASD cases experiencing ADHD were 7.97 times higher (OR 7.97, 99.9% CI, 6.67–9.53); PTSD 4.13 times higher (OR 4.13, 99.9% CI 3.34–5.11); conduct disorders and ADHD 6.00 times higher (OR 6.00, 99.9% CI 4.40–8.18); ADHD and anxiety disorders 5.17 times higher (OR 5.17, 99.9% CI 3.87–6.89); ADHD, MDD, and anxiety disorders 3.68 times higher (OR 3.68, 99.9% CI 2.50–5.40); and anxiety disorders, suicidal ideations, and ADHD 3.68 times higher (OR 3.68, 99.9% CI 2.38–5.70) than the general patient population (see Table 3). The most notable difference between FASD cases and the general patient population was for intellectual and developmental disorders (IDD), in which the odds of experiencing IDD were 20.98 times higher for FASD cases compared to the general patient population (OR 20.98, 99.9% CI 15.51–28.39). Similar findings were exhibited for the sensitivity analysis of Medicaid only discharges, where the frequency of the identified comorbidity patterns was greater in FASD cases compared to the general patient population (see Supplemental Material Tables 10 and 11, and 12).
Table 3.
Comparison of comorbidity patterns between cases and controls.
| Adjusted OR, FASD Cases vs. GP Controls | Adjusted OR, FASD Cases vs. BH Controls | |
|---|---|---|
| First-order comorbidities | ||
| Attention-deficit hyperactivity disorders | 7.97 (6.67–9.53)* | 3.10 (2.67–3.59)* |
| Cardiovascular problems | 1.19 (1.02–1.39)* | 0.99 (0.86–1.14) |
| Gastrointestinal problems | 1.64 (1.41–1.9)* | 1.09 (0.95–1.25) |
| Anxiety disorders | 1.59 (1.35–1.86)* | 0.51 (0.45–0.59)* |
| Nicotine dependence | 0.87 (0.74–1.02) | 0.64 (0.55–0.74)* |
| Major depressive disorder | 1.35 (1.15–1.59)* | 0.45 (0.39–0.52)* |
| Intellectual & developmental disorder | 20.98 (15.51–28.39)* | 7.96 (6.44–9.83)* |
| Suicidal ideations | 2.49 (2.07–2.99)* | 1.12 (0.97–1.31) |
| Long term (current) drug therapy | 1.16 (0.98–1.37) | 0.98 (0.85–1.13) |
| Post-traumatic stress disorder | 4.13 (3.34–5.11)* | 1.69 (1.44–1.99)* |
| Second-order comorbidities | ||
| Major depressive disorder & anxiety disorders | 1.19 (0.96–1.49) | 0.51 (0.42–0.61)* |
| Gastrointestinal problems & cardiovascular problems | 1.55 (1.24–1.94)* | 1.12 (0.93–1.36) |
| Attention-deficit hyperactivity disorders & anxiety disorders | 5.17 (3.87–6.89)* | 2.24 (1.80–2.79)* |
| Suicidal ideations & major depressive disorder | 1.70 (1.34–2.17)* | 0.85 (0.69–1.04) |
| Attention-deficit hyperactivity disorders & suicidal ideations | 4.61 (3.38–6.29)* | 2.41 (1.9–3.07)* |
| Nicotine dependence & anxiety disorders | 1.15 (0.90–1.48) | 0.51 (0.42–0.62)* |
| Nicotine dependence & major depressive disorder | 1.22 (0.95–1.58) | 0.59 (0.48–0.73)* |
| Conduct disorders & Attention-deficit hyperactivity disorders | 6.00 (4.40–8.18)* | 3.13 (2.42–4.04)* |
| Suicidal ideations & anxiety disorders | 1.98 (1.52–2.59)* | 0.99 (0.80–1.23) |
| Attention-deficit hyperactivity disorders & Major depressive disorder | 4.03 (3.00–5.41)* | 1.71 (1.37–2.14)* |
| Third-order comorbidities | ||
| Anxiety disorders & suicidal ideations & major depressive disorder | 1.65 (1.18–2.31)* | 0.80 (0.61–1.05) |
| Nicotine dependence & major depressive disorder & anxiety disorders | 1.15 (0.82–1.62) | 0.60 (0.45–0.79)* |
| Attention-deficit hyperactivity disorders & major depressive disorder & anxiety disorders | 3.68 (2.50–5.40)* | 1.54 (1.13–2.12)* |
| Attention-deficit hyperactivity disorders & major depressive disorder & suicidal ideations | 3.14 (2.05–4.79)* | 1.71 (1.23–2.39)* |
| Gastrointestinal problems & long term (current) drug therapy & cardiovascular problems | 1.39 (0.99–1.96) | 1.05 (0.77–1.42) |
| Nicotine dependence & suicidal ideations & major depressive disorder | 1.84 (1.24–2.73)* | 1.18 (0.87–1.60) |
| Personal history of self-harm & major depressive disorder & suicidal ideations | 2.41 (1.58–3.67)* | 1.21 (0.85–1.72) |
| Anxiety disorders & suicidal ideations & attention-deficit hyperactivity disorders | 3.68 (2.38–5.70)* | 1.96 (1.39–2.77)* |
| Major depressive disorder & post-traumatic stress disorder & suicidal ideations | 2.96 (1.84–4.76)* | 1.15 (0.81–1.62) |
Note. FASD = fetal alcohol spectrum disorder, GP = general patient, BH = behavioral health. Rows represent the top ten most frequently occurring first- and second-order comorbidities identified. Rows for the third-order comorbidities represent all nine patterns identified. Adjusted models control for age, sex, race, payer, length of stay, disposition, census region, and severity of illness. * P ≤.001.
The odds of experiencing the identified comorbidity patterns between FASD cases and the behavioral health control group were not as readily apparent. For example, in the identified first-order comorbidities, the odds of FASD cases experiencing IDD (OR 7.96, 99.9% CI 6.44–9.83), ADHD (OR 3.10, 99.9% CI 2.67–3.59)), and PTSD (OR 1.69, 99.9% CI 1.44–1.99) were all higher compared to the behavioral health control group. However, FASD cases were significantly less likely than the behavioral health control to experience MDD (OR 0.45, 99.9% CI 0.39–0.52), anxiety disorders (OR 0.51, 99.9% CI 0.45–0.59), and nicotine dependence (OR 0.64, 99.9% CI 0.55–0.74). Similar findings were exhibited for the second- and third-order comorbidities as well. For example, the odds of FASD cases experiencing conduct disorders and ADHD (OR 3.13, 99.9% CI 2.42–4.04) and ADHD, MDD, and suicidal ideations (OR 1.71, 99.9% CI 1.23–2.39) were significantly higher than the behavioral health controls. But alternatively, the odds of FASD cases experiencing comorbidity patterns like nicotine dependence and anxiety disorders (OR 0.51, 99.9% CI 0.42 − 0.2) and nicotine dependence, MDD, and anxiety disorders (OR 0.60, 99.9% CI 0.45–0.79) were significantly lower than the behavioral health controls. Similar findings were exhibited for the sensitivity analysis of Medicaid only discharges, where the frequency of the identified comorbidity patterns slightly varied in FASD cases compared to the behavioral health control group (see Supplemental Material Tables 10 and 11, and 12).
Discussion
The purpose of this study was to better understand the comorbidity patterns among individuals living with FASD. Using a nationally representative database of inpatient hospitalizations in the United States, we employed an unsupervised machine learning algorithm to inductively identify comorbidity patterns associated with FASD. The results of the analysis identified a distinct set of 57 comorbidities, including physical and behavioral health conditions, in addition to several substance use diagnoses. These comorbidities represented 135 unique patterns of two overlapping, “second-order” comorbidities, and nine patterns of three overlapping, “third-order” comorbidities. Compared to the general patient population, the odds of having these comorbidity patterns tended to be higher in FASD cases, while there were less noticeable differences between FASD cases and the behavioral health control group.
The results of our analyses highlight the complexity of diagnoses associated with FASD and have several implications for identification of the condition. The comorbidities identified in this study add to a growing body of literature12–14,16 demonstrating the negative impact of PAE on both the brain and the body of individuals living with FASD and provide insight into the convergence of disease processes associated with PAE. While many of the behavioral health conditions identified in this study (for example, ADHD, anxiety disorders, and major depressive disorder) are well documented as frequently occurring among individuals with FASD18,26,56,57the additional findings of physical health conditions such as cardiovascular problems, gastrointestinal problems, and epilepsy/recurrent seizures may aid in the identification of FASD when considered in totality during the diagnostic evaluation of FASD candidates. Stated alternatively, the comorbidity patterns identified in this study may lay the foundation for continued research on better conceptualizing FASD as a syndrome that includes both mental and physical health problems. One additional finding of interest was that compared to the general patient population and behavioral health controls, the odds of experiencing nicotine dependence was lower in the FASD cases. This finding may in part be explained by well-documented higher rates of smoking and nicotine dependence in adults with behavioral health diagnoses compared to the general patient population58,59. Additionally, when examining the prevalence of second- and third-order comorbidities, it appears that nicotine dependence tended to co-occur with other behavioral health diagnoses more frequently in the FASD cases, which further supports the notion that nicotine dependence more frequently occurs in those with mental health issues.
Our analysis comparing overlapping comorbidity patterns between FASD cases and behavioral health controls may also be of particular interest to healthcare providers and researchers working to improve identification of FASD. In addition to a lack of reliable information on PAE, one of the persistent challenges in identification of individuals with FASD is that their behavioral health conditions frequently overshadow or take precedence over their FASD diagnosis, especially in childhood60. The overlapping behavioral health comorbidity patterns found in this study may prove useful as a distinctive diagnostic feature in helping to identify individuals with FASD apart from individuals solely faced with a behavioral health diagnosis alone. For example, the finding that FASD cases in this study were 13.3 times more likely to experience ADHD with an intellectual and developmental disorder and 3.9 times more likely to experience ADHD and autism compared to behavioral health controls may point toward distinct behavioral health patterns that can alert clinicians to the need of referral for FASD evaluation and diagnosis.
For providers of care, the findings of our study also highlight the importance of comprehensively considering the diagnosis of FASD and its potential comorbidities in providing care and identifying problems early on. Given the wide variety of comorbidities identified in this study, it is likely that individuals with FASD will need robust and well-integrated care for appropriate treatment. Individuals with FASD may present to the healthcare system with wide-spanning healthcare challenges, and therefore our findings add to a growing consensus that appropriate care for FASD should involve team-based approaches among medical and non-medical providers61. This is especially true for the different physical and behavioral health conditions found in this study that may exacerbate one another. For example, in the general population it is well established that gastrointestinal problems frequently co-occur and may worsen or be worsened by behavioral health conditions like anxiety and depression62,63. Therefore, comprehensive treatment of these issues among individuals with FASD should ideally include GI specialists and healthcare providers trained to diagnosis and treat behavioral health conditions. More generally, education of health care providers about the potential for a range of comorbidities associated with FASD may help in the early identification and prevention of some of these negative outcomes.
The findings of this study also have important implications for population-based surveillance of FASD. Because there is no biological test for FASD, traditional methods such as active case reporting from newborn screening programs are not possible for FASD surveillance. One alternative approach gaining in popularity for surveillance of rare conditions is case ascertainment using integrated and deduplicated administrative healthcare data. Hospital discharge data, like the NIS data used in this study, may be a useful potential source for administrative data that could be used in such a surveillance system for FASD. Specifically, the comorbidity patterns identified in our results could form the basis to create an algorithm to identify individuals with FASD in administrative healthcare data. Given the lack of research on the accuracy of ICD codes for identifying FASD in administrative data35creation of a validated algorithm is indeed a crucial next step for population-based surveillance of FASD.
The descriptive findings of this study also set realistic expectations for the limited number of FASD cases to be found in single-source administrative data, particularly when relying on ICD diagnosis codes alone. In the nearly 35 million hospital discharge records used in our analysis, only 3,248 records contained an FASD ICD-10 code. Despite the high prevalence of alcohol consumption in the community, FASD is known to be underdiagnosed64and data sources like the NIS likely under-identify individuals with FASD, especially as they are treated in the hospital where the focus may be on broader medical issues. Therefore, it is likely that multiple sources of integrated administrative data would be necessary to comprehensively identify and surveil individuals with FASD. Approaches that first identify an individual with FASD (for example, from clinic or other registry data), then link to other administrative databases using personal identifiers, would be particularly useful for surveillance efforts.
There are several strengths of the current study that warrant discussion. To the best of our knowledge, this is the first study to systematically examine overlapping comorbidity patterns associated with FASD, rather than solely focusing on single comorbidities in isolation. Furthermore, we employed a novel unsupervised machine learning algorithm to identify the comorbidity patterns analyzed. This method likely improves on prior studies by reducing potential recall bias associated with studies that relied on self-reported comorbidities from participants. Additionally, our use of a case-control study design allowed direct comparison to not only the general patient population, but also to a cohort of behavioral health controls. These comparisons may lay the foundation for further exploration of distinct comorbidity profiles to be used in enhanced identification and treatment of FASD. Taken together, these strengths also highlight the utility of utilizing administrative data sources for research in the field of FASD.
At the same time, there are important limitations of the current study. While the NIS data are nationally representative, each record is indicative of a deidentified inpatient discharge from the hospital. Therefore, it is possible that the same individual(s) could have contributed multiple hospitalizations to the analysis, potentially resulting in some degree of overestimation of the comorbidities across all study groups. The data use agreement for the NIS does not allow for re-identification of discharge records to the person level, and therefore we were unable to quantify the extent of these comorbidities at the person-level instead of the discharge-level. However, one prior population-based retrospective cohort study of adults with fetal alcohol syndrome in Canada found that during a four-year follow up period, 28% of adults with FAS were hospitalized at least one additional time beyond their index hospitalization65which may serve as an approximation of rehospitalizations in the current study.
It is also important to consider that the comorbidities identified in this study were captured at time of hospitalization. The reported comorbidities should accordingly be considered in light of being identified at an acute healthcare encounter requiring hospitalization and may have differed had the study cohort been drawn from a community-based sample of individuals living with FASD. Finally, reliance on a single ICD code (Q86.0, fetal alcohol syndrome) for the identification of the FASD study cohort captured the most clinically severe FASD cases. Because there currently exist no ICD codes for other forms of FASD, we were unable to discern differences in comorbidity patterns by severity of FASD (for example, by including those with partial fetal alcohol syndrome or alcohol-related neurodevelopmental disorder). This limitation is especially important because FASD is known to be misdiagnosed and underdiagnosed21,66and it is highly possible that more individuals with FASD were present in the NIS data than we could identify by relying on the Q86.0 ICD code alone. Such misdiagnosis and underdiagnosis could also mean that individuals with FASD were potentially incorrectly assigned to the general patient population or behavioral health control groups, which could result in overestimated differences of the odds of experiencing the comorbidities identified. Future research could address these limitations by drawing on a more diverse set of individuals with FASD to conduct a prospective study of comorbidity patterns across the lifespan.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This publication was supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award totaling $1,163,850 with 100% funded by CDC/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.
Author contributions
BKA conceived the study, accessed the data, and assumed full responsibility for all analyses conducted. CC and JK provided clinical subject matter expertise and participated in clinical review of comorbidities identified by the machine learning algorithm. All authors equally contributed to the study design and read, reviewed, and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the National Inpatient Sample (NIS) from the Healthcare Cost and Utilization Project (HCUP), which is maintained by the Agency for Healthcare Research and Quality (AHRQ). While all HCUP data are deidentified, access to the NIS database is contingent upon execution of a data use agreement with AHRQ and purchase of the data. Individual users are prohibited by AHRQ from releasing the data for public use, and therefore we cannot openly share the data used in this study. Those interested in accessing the NIS data are directed to the HCUP website: https://hcup-us.ahrq.gov. Individuals may also contact the corresponding author, Brandon K. Attell at battell1@gsu.edu.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popova, S., Lange, S., Probst, C., Gmel, G. & Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 5, e290–e299 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Popova, S., Lange, S., Probst, C., Gmel, G. & Rehm, J. Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder. Biochem. Cell. Biol.96, 237–240 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Popova, S. et al. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primer. 9, 11 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Hoyme, H. E. et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics138, e20154256 (2016). [DOI] [PMC free article] [PubMed]
- 5.Maya-Enero, S., Ramis-Fernández, S. M., Astals-Vizcaino, M. & García-Algar, Ó. Neurocognitive and behavioral profile of fetal alcohol spectrum disorder. Pediatría Engl. Ed.95, 208–e1 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Skorka, K., McBryde, C., Copley, J., Meredith, P. J. & Reid, N. Experiences of children with fetal alcohol spectrum disorder and their families: a critical review. Alcohol Clin. Exp. Res.44, 1175–1188 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Moritz, K. M., Akison, L. K., Hayes, N. & Reid, N. Physical and mental health in FASD. In Fetal Alcohol Spectrum Disorders: A Multidisciplinary Approach (eds Abdul-Rahman, O. A. & Petrenko, C. L. M.) 241–267 (Springer International Publishing, 2023). 10.1007/978-3-031-32386-7_12. [Google Scholar]
- 8.Mattson, S. N., Crocker, N. & Nguyen, T. T. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol. Rev.21, 81–101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popova, S., Lange, S., Burd, L. & Rehm, J. Health care burden and cost associated with fetal alcohol syndrome: based on official Canadian data. (2012). [DOI] [PMC free article] [PubMed]
- 10.Oh, S. S. et al. Hospitalizations and mortality among patients with fetal alcohol spectrum disorders: a prospective study. Sci. Rep.10, 19512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLachlan, K., Flannigan, K., Temple, V., Unsworth, K. & Cook, J. L. Difficulties in daily living experienced by adolescents, transition-aged youth, and adults with fetal alcohol spectrum disorder. Alcohol Clin. Exp. Res.44, 1609–1624 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Moore, E. M. & Riley, E. P. What happens when children with fetal alcohol spectrum disorders become adults? Curr. Dev. Disord Rep.2, 219–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coles, C. D. et al. Prenatal alcohol exposure and health at midlife: Self-reported health outcomes in two cohorts. Alcohol Clin. Exp. Res.48, 2045–2059 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Himmelreich, M., Lutke, C. & Hargrove, E. T. Routledge,. The lay of the land: Fetal alcohol spectrum disorder (FASD) as a whole-body diagnosis. in The Routledge handbook of social work and addictive behaviors 191–215 (2020).
- 15.Reid, N., Hayes, N., Young, S. B., Akison, L. K. & Moritz, K. M. Caregiver-reported physical health status of children and young people with fetal alcohol spectrum disorder. J. Dev. Orig Health Dis.12, 420–427 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Popova, S. et al. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet387, 978–987 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Weyrauch, D., Schwartz, M., Hart, B., Klug, M. G. & Burd, L. Comorbid mental disorders in fetal alcohol spectrum disorders: A systematic review. J. Dev. Behav. Pediatr.38, 283–291 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Burd, L., Klug, M. G., Martsolf, J. T. & Kerbeshian, J. Fetal alcohol syndrome: neuropsychiatric phenomics. Four State Fetal Alcohol Consort Clin. Epidemiol. Find.25, 697–705 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Kvigne, V. L., Leonardson, G. R., Borzelleca, J., Neff-Smith, M. & Welty, T. K. Hospitalizations of children who have fetal alcohol syndrome or incomplete fetal alcohol syndrome. S D Med62, 97–103 (2009). [PubMed]
- 20.Kambeitz, C., Klug, M. G., Greenmyer, J., Popova, S. & Burd, L. Association of adverse childhood experiences and neurodevelopmental disorders in people with fetal alcohol spectrum disorders (FASD) and non-FASD controls. BMC Pediatr.19, 498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chasnoff, I. J., Wells, A. M. & King, L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics135, 264–270 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Herman, L. E., Acosta, M. C. & Chang, P. N. Gender and attention deficits in children diagnosed with a fetal alcohol spectrum disorder. J Popul. Ther. Clin. Pharmacol15(3), e411–419 (2008). [PubMed]
- 23.Church, M. W., Eldis, F., Blakley, B. W., Bawle, E. V. & Hearing Language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol Clin. Exp. Res.21, 227–237 (1997). [PubMed] [Google Scholar]
- 24.Hug, T. E., Fitzgerald, K. M. & Cibis, G. W. Clinical and electroretinographic findings in fetal alcohol syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus. 4, 200–204 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Kvigne, V. L. et al. Characteristics of children who have full or incomplete fetal alcohol syndrome. J. Pediatr.145, 635–640 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Famy, C., Streissguth, A. P. & Unis, A. S. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry. 155, 552–554 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Astley, S. J. Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington state fetal alcohol syndrome diagnostic & prevention network. Can. J. Clin. Pharmacol.17, e132–e164 (2010). [PubMed] [Google Scholar]
- 28.Fryer, S. L., McGee, C. L., Matt, G. E., Riley, E. P. & Mattson, S. N. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics119, e733–e741 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Jones, K. L. et al. Fetal alcohol spectrum disorders: extending the range of structural defects. Am. J. Med. Genet. A. 152A, 2731–2735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalberg, W. O. et al. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin. Exp. Res.30, 2037–2045 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Hanson, J. W., Jones, K. L. & Smith, D. W. Fetal alcohol syndrome: experience with 41 patients. JAMA235, 1458–1460 (1976). [PubMed] [Google Scholar]
- 32.Swayze, V. W. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics99, 232–240 (1997). [DOI] [PubMed] [Google Scholar]
- 33.AHRQ. Introduction to the HCUP National Impatient Sample. (2022). https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2020.pdf
- 34.von Elm, E. et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ335, 806 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell, S., Palmeter, S., Laverty, M. & Lagacé, C. Accuracy of administrative database algorithms for autism spectrum disorder, attention-deficit/hyperactivity disorder and fetal alcohol spectrum disorder case ascertainment: a systematic review. Health Promot Chronic Dis. Prev. Can.42, 355–383 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senturias, Y., Ali, M. M. & West, K. Psychotropic medication utilization among children diagnosed with fetal alcohol spectrum disorder. Pediatrics150, e2022056797 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Broccia, M. et al. Heavy prenatal alcohol exposure and overall morbidities: a Danish nationwide cohort study from 1996 to 2018. Lancet Public. Health. 8, e36–e46 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Deputy, N. P. et al. Administratively reported fetal alcohol spectrum disorders in Commercially-and Medicaid-Insured samples of children in the united states, 2015–2021. Drug Alcohol Depend263, 112420 (2024). [DOI] [PMC free article] [PubMed]
- 39.Sanusi, R. A. et al. Transitions between versions of the international classification of diseases and chronic disease prevalence estimates from administrative health data: a population-based study. BMC Public. Health. 22, 701 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khera, R., Dorsey, K. B. & Krumholz, H. M. Transition to the ICD-10 in the united states: an emerging data chasm. JAMA320, 133–134 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Hassaine, A., Salimi-Khorshidi, G., Canoy, D. & Rahimi, K. Untangling the complexity of Multimorbidity with machine learning. Mech. Ageing Dev.190, 111325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, Y. & Xu, R. Mining Cancer-Specific disease comorbidities from a large observational health database. Cancer Inform13s1, 37–44 (2014). CIN.S13893. [DOI] [PMC free article] [PubMed]
- 43.Crowson, C. S. et al. Using unsupervised machine learning methods to cluster comorbidities in a Population-Based cohort of patients with rheumatoid arthritis. Arthritis Care Res.75, 210–219 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cha, S. & Kim, S. S. Comorbidity patterns of mood disorders in adult inpatients: applying association rule mining. Healthcare9, 1155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cha, S. & Kim, S. S. Discovery of association rules patterns and prevalence of comorbidities in adult patients hospitalized with mental and behavioral disorders. Healthcare9, 636 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clauset, A., Newman, M. E. & Moore, C. Finding community structure in very large networks. Phys. Rev. E—Statistical Nonlinear Soft Matter Phys.70, 066111 (2004). [DOI] [PubMed] [Google Scholar]
- 47.3M. 3 M All Patient Refined Diagnosis Related Groups (APR DRG) Methodology Overview. https://3 (2023). mhis-customersupport.s3.amazonaws.com/aws/docs/Groupers/All_Patient_Refined_DRG/Methodology_overview_GRP041/grp041_aprdrg_methodology_overview.pdf?AWSAccessKeyId=AKIAQW57BTWSS65LXO6Y&Expires=1724777071&Signature=DC%2BhOizajA0jrpfqEoia%2BZb2AN4%3D
- 48.SAS Institute. SAS Version 9.4 (SAS Institute Inc, 2022).
- 49.Lewis, T. Complex Survey Data Analysis with SAS (CRC, 2017).
- 50.Geroldinger, A., Blagus, R., Ogden, H. & Heinze, G. An investigation of penalization and data augmentation to improve convergence of generalized estimating equations for clustered binary outcomes. BMC Med. Res. Methodol.22, 168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansournia, M. A., Geroldinger, A., Greenland, S. & Heinze, G. Separation in logistic regression: causes, consequences, and control. Am. J. Epidemiol.187, 864–870 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Rader, K. A. et al. Bias-corrected estimates for logistic regression models for complex surveys with application to the united states’ nationwide inpatient sample. Stat. Methods Med. Res.26, 2257–2269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett, M., McCarty, J., Kenney, T. & Liang, L. Methods Applying AHRQ Quality Indicators to Healthcare Cost and Utilization Project (HCUP) Data for the 2021 National Healthcare Quality and Disparities Report (NHQDR). 73 (2021). www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- 54.Marshall, A. L. et al. Sex-based disparities in venous thromboembolism outcomes: A National inpatient sample (NIS)-based analysis. Vasc Med.22, 121–127 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Xu, F., Wheaton, A. G., Liu, Y. & Greenlund, K. J. Major ambulatory surgery among US adults with inflammatory bowel disease, 2017. PLOS ONE. 17, e0264372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geier, D. A. & Geier, M. R. Fetal alcohol syndrome and the risk of neurodevelopmental disorders: A longitudinal cohort study. Brain Dev.44, 706–714 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Lange, S., Rehm, J., Anagnostou, E. & Popova, S. Prevalence of externalizing disorders and autism spectrum disorders among children with fetal alcohol spectrum disorder: systematic review and meta-analysis. Biochem. Cell. Biol.96, 241–251 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Cook, B. L. et al. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA311, 172–182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glasheen, C., Hedden, S. L., Forman-Hoffman, V. L. & Colpe, L. J. Cigarette smoking behaviors among adults with serious mental illness in a nationally representative sample. Ann. Epidemiol.24, 776–780 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Chudley, A. E., Kilgour, A. R., Cranston, M. & Edwards, M. Challenges of diagnosis in fetal alcohol syndrome and fetal alcohol spectrum disorder in the adult. Am. J. Med. Genet. C Semin Med. Genet.145C, 261–272 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Masotti, P. et al. Integrating care for individuals with FASD: results from a multi-stakeholder symposium. BMC Health Serv. Res. 15, 457 (2015). [DOI] [PMC free article] [PubMed]
- 62.Mussell, M. et al. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J. Psychosom. Res.64, 605–612 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Haug, T. T., Mykletun, A. & Dahl, A. A. Are anxiety and depression related to Gastrointestinal symptoms in the general population?? Scand. J. Gastroenterol.37, 294–298 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Wozniak, J. R., Riley, E. P. & Charness, M. E. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol.18, 760–770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dozet, D., de Oliveira & Popova, S. Claire, Lunsky, Yona, Calzavara, Andrewand Healthcare utilisation and characteristics of adults with fetal alcohol syndrome: a descriptive population-based cohort study in Ontario, Canada. J. Intellect. Dev. Disabil. 1–14 10.3109/13668250.2025.2449677 [DOI] [PubMed]
- 66.May, P. A. et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA319, 474–482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the National Inpatient Sample (NIS) from the Healthcare Cost and Utilization Project (HCUP), which is maintained by the Agency for Healthcare Research and Quality (AHRQ). While all HCUP data are deidentified, access to the NIS database is contingent upon execution of a data use agreement with AHRQ and purchase of the data. Individual users are prohibited by AHRQ from releasing the data for public use, and therefore we cannot openly share the data used in this study. Those interested in accessing the NIS data are directed to the HCUP website: https://hcup-us.ahrq.gov. Individuals may also contact the corresponding author, Brandon K. Attell at battell1@gsu.edu.