Abstract
OBJECTIVE: The authors' aim was to determine the requirement for an active interleukin (IL)-1 receptor during the development and progression of acute pancreatitis. SUMMARY OF BACKGROUND DATA: Interleukin-1 is a pro- inflammatory cytokine that has been shown to be produced during acute pancreatitis. Earlier animal studies of moderate and severe pancreatitis have shown that blockade of this powerful mediator is associated with attenuated pancreatic destruction and dramatic increases in survival. The exact role played by IL-1 and the requirement for activation of its receptor in the initiation and progression of pancreatitis is unknown. METHODS: Conventional and IL-1 receptor ¿knockout¿ animals were used in parallel experiments of acute pancreatitis induced by intraperitoneal injection of cerulean (50 microg/kg every 1 hour X 4). The conventional mouse strain had the IL-1 receptor blocked prophylactically by means of a recombinant IL-1 receptor antagonist (10 mg/kg injected intraperitoneally every 2 hours). The second mouse strain was genetically engineered by means of gene targeting in murine embryonic stem cells to be devoid of type 1 IL-1 receptor (IL-1 receptor knockout). Animals were killed at 0, 0.5, 1, 2, 4, and 8 hours, with the severity of pancreatitis determined by serum amylase, lipase, and IL-6 levels and blind histologic grading. Strain-specific controls were used for comparison. RESULTS: The genetic absence of the IL-1 receptor or its pharmacologic blockade resulted in significantly attenuated pancreatic vacuolization, edema, necrosis, inflammation, and enzyme release. Serum IL-6, a marker of inflammation severity, was dramatically decreased in both groups. CONCLUSIONS: Activation of the IL-1 receptor is not required for the development of pancreatitis but apparently is necessary for the maximal propagation of pancreatic injury and its associated inflammation.
Full text
PDF


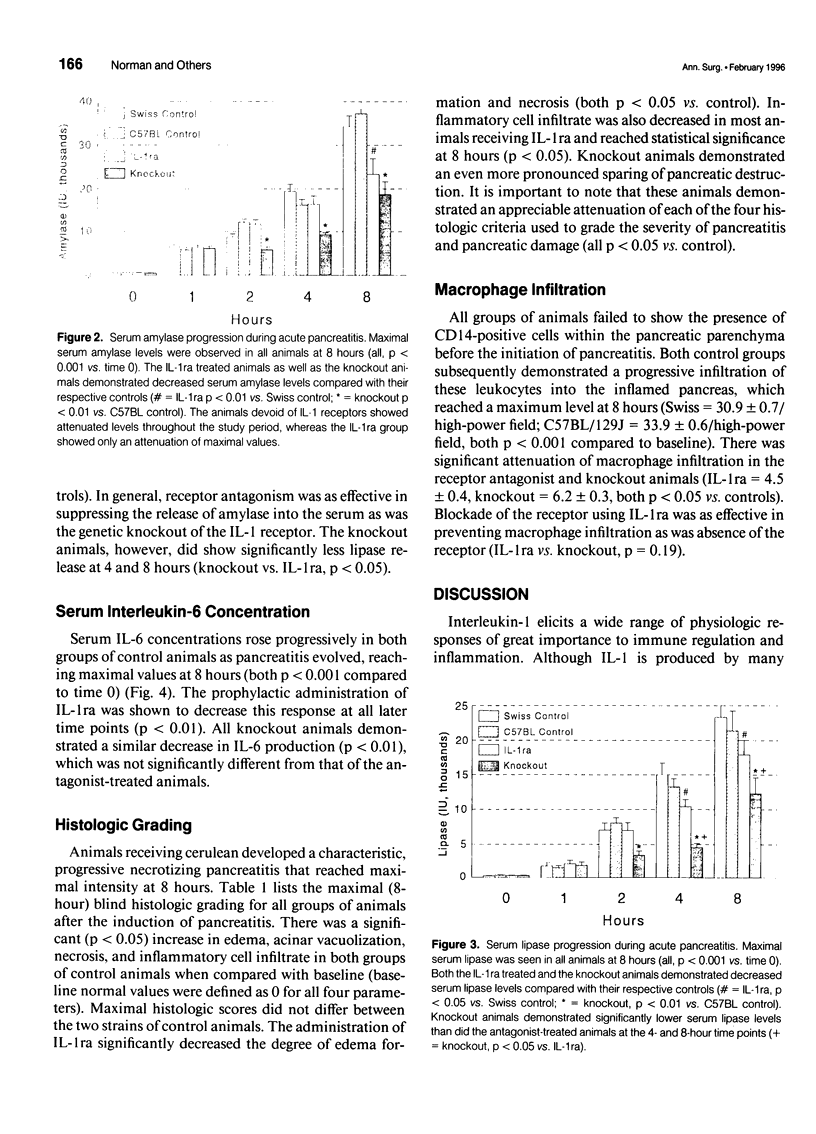
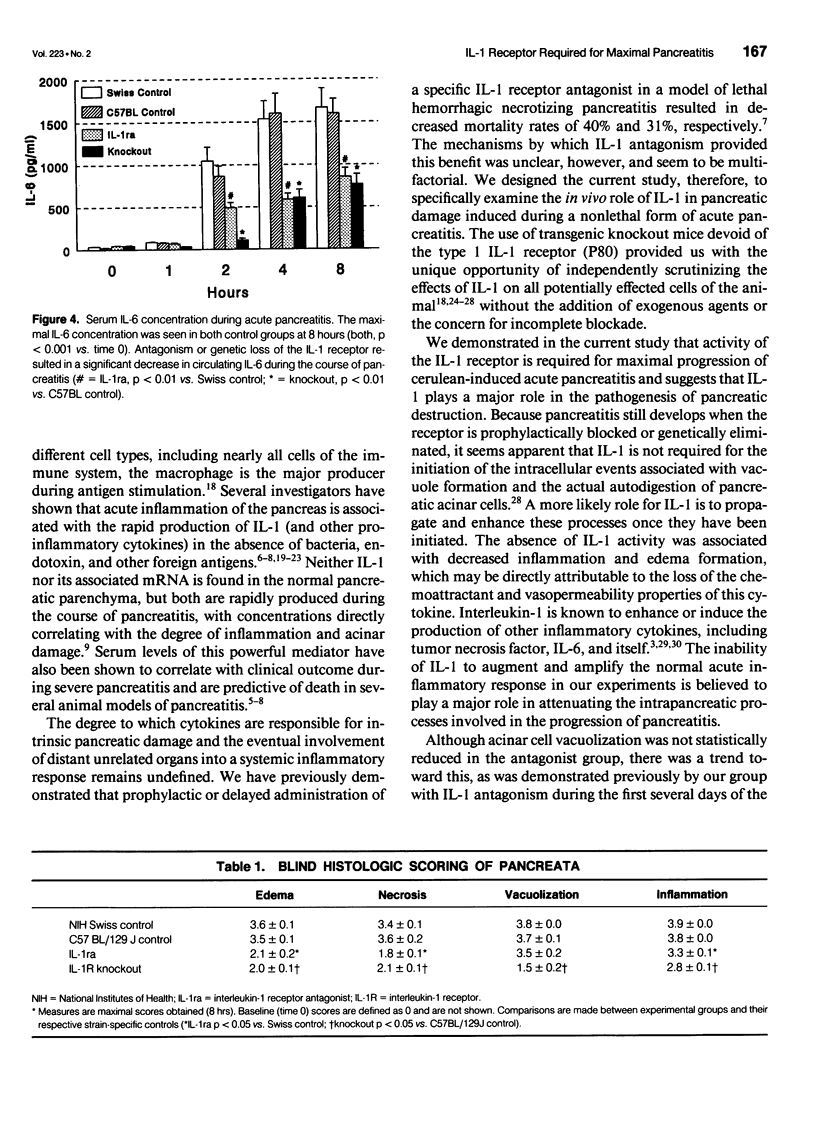


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basgen J. M., Nevins T. E., Michael A. F. Quantitation of antigen in tissue by immunofluorescence image analysis. J Immunol Methods. 1989 Nov 13;124(1):77–83. doi: 10.1016/0022-1759(89)90188-9. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. The interleukin-1 family: 10 years of discovery. FASEB J. 1994 Dec;8(15):1314–1325. [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Granowitz E. V., Clark B. D., Vannier E., Callahan M. V., Dinarello C. A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood. 1992 May 1;79(9):2356–2363. [PubMed] [Google Scholar]
- Grewal H. P., Kotb M., el Din A. M., Ohman M., Salem A., Gaber L., Gaber A. O. Induction of tumor necrosis factor in severe acute pancreatitis and its subsequent reduction after hepatic passage. Surgery. 1994 Feb;115(2):213–221. [PubMed] [Google Scholar]
- Gross V., Leser H. G., Heinisch A., Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993 Dec;40(6):522–530. [PubMed] [Google Scholar]
- Heath D. I., Cruickshank A., Gudgeon M., Jehanli A., Shenkin A., Imrie C. W. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993 Jan;34(1):41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi E. D., Remick D. G. Monocytes are the major producers of interleukin-1 beta in an ex vivo model of local cytokine production. J Interferon Cytokine Res. 1995 Jan;15(1):89–94. doi: 10.1089/jir.1995.15.89. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., Ghezzi P., van der Meer J. W., Dinarello C. A. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990 Jul;162(1):215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- Leach S. D., Modlin I. M., Scheele G. A., Gorelick F. S. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991 Jan;87(1):362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry S. F. Cytokine mediators of immunity and inflammation. Arch Surg. 1993 Nov;128(11):1235–1241. doi: 10.1001/archsurg.1993.01420230063010. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Nagano I., Nakamura S., Yoshioka M., Onodera J., Kogure K., Itoyama Y. Expression of cytokines in brain lesions in subacute sclerosing panencephalitis. Neurology. 1994 Apr;44(4):710–715. doi: 10.1212/wnl.44.4.710. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G. W., Franz M. G. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995 Sep;130(9):966–970. doi: 10.1001/archsurg.1995.01430090052018. [DOI] [PubMed] [Google Scholar]
- Rosen S. D. Robert Feulgen Lecture 1993. L-selectin and its biological ligands. Histochemistry. 1993 Sep;100(3):185–191. doi: 10.1007/BF00269091. [DOI] [PubMed] [Google Scholar]
- Tani S., Otsuki M., Itoh H., Fujii M., Nakamura T., Oka T., Baba S. Histologic and biochemical alterations in experimental acute pancreatitis induced by supramaximal caerulein stimulation. Int J Pancreatol. 1987 Oct-Dec;2(5-6):337–348. doi: 10.1007/BF02788433. [DOI] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. Expression of pancreatic enzymes (alpha-amylase, trypsinogen, and lipase) during human liver development and maturation. Gastroenterology. 1995 Apr;108(4):1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- Uhl J., Newton R. C., Giri J. G., Sandlin G., Horuk R. Identification of IL-1 receptors on human monocytes. J Immunol. 1989 Mar 1;142(5):1576–1581. [PubMed] [Google Scholar]
- Waldman A. S. Targeted homologous recombination in mammalian cells. Crit Rev Oncol Hematol. 1992 Jan;12(1):49–64. doi: 10.1016/1040-8428(92)90064-w. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Penninger J., Mak T. W. Genetically modified animals and immunodeficiency. Curr Opin Immunol. 1993 Aug;5(4):585–594. doi: 10.1016/0952-7915(93)90042-q. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]