Abstract
Hypertension and lung cancer are two of the most prevalent chronic diseases worldwide, each contributing significantly to public health burdens. While both diseases have been extensively studied individually, their comorbidity remains an underexplored area of research. Recent studies suggest that genetic susceptibility plays a crucial role in the coexistence of these conditions, with overlapping genetic variants influencing both vascular homeostasis and tumorigenesis. The relationship between hypertension and lung cancer is complex, with shared risk factors and common pathogenic mechanisms, including inflammation, oxidative stress, and metabolic dysregulation. Environmental exposures—such as air pollution, tobacco smoke, and heavy metals—can trigger these genetic and epigenetic alterations, thereby increasing susceptibility to both conditions. Beyond genetic predisposition, epigenetic modifications significantly contribute to disease pathogenesis, including DNA methylation, histone modifications, and microRNA (miRNA) regulation. In hypertension, aberrant DNA methylation affects genes involved in vascular remodeling, such as At1b and Scnn1a, influencing blood pressure regulation. Similarly, in lung cancer, tumor suppressor genes such as p16, RASSF1A, and KCNK3 undergo methylation-induced silencing, promoting tumor progression. Histone modifications, particularly histone deacetylase (HDAC) activity, play a key role in both diseases, with HDAC inhibitors like valproic acid showing therapeutic potential in lowering blood pressure and inhibiting lung cancer cell proliferation. Understanding the shared genetic and epigenetic mechanisms between hypertension and lung cancer offers new opportunities for risk prediction, early intervention, and targeted therapies. Future research should focus on integrating genetic screening with environmental risk assessment to develop precision medicine strategies for individuals at high risk of both conditions.
Keywords: Hypertension, Lung cancer, Inheritance, Environment, Interactions
Introduction
Hypertension is a major global health issue. According to epidemiological data, hypertension prevalence has doubled in the past 40 years, mainly as a result of an aging population. It is estimated that nearly 1.4 billion adults worldwide (over the age of 18, with 20% of women and 25% of men) have hypertension, with a significant portion of cases undiagnosed [1]. Hypertension is a major risk factor for cardiovascular diseases and the leading cause of mortality globally, resulting in approximately 7.6 million deaths annually [2]. Substantial evidence indicates that environmental exposure plays a crucial role in the development and progression of hypertension. For instance, exposure to occupational, social, and road traffic noise can impact hearing and is also associated with irritability or sleep disturbances [3]. Cancer remains the top cause of mortality worldwide. It is estimated that approximately 1 in 5 men and women are diagnosed with cancer, while around 1 in 9 men and 1 in 10 women die from it. In 2022, lung cancer was the most common type of cancer, with nearly 2.5 million new cases globally, accounting for 12.4% of all cancer cases. Lung cancer is also the leading cause of cancer-related deaths, with approximately 1.8 million deaths attributed to it (18.7%). The incidence and mortality rates of lung cancer continue to rise, particularly in regions with high smoking rates and severe air pollution [4]. Studies suggest that a substantial proportion of lung cancer cases can be attributed to environmental exposures. For example, the high incidence of lung cancer among Chinese women is believed to be associated with outdoor air pollution and household exposure to solid fuel combustion for heating and cooking [5]. Additionally, exposure to wildfires in the U.S. has been linked to poorer overall survival in patients with non-small cell lung cancer (NSCLC) who underwent surgical resection, highlighting the increased health risks faced by lung cancer patients in the context of wildfire exposure. This underscores the need to prioritize health risks related to wildfires in climate adaptation efforts [6].
Hypertension is known to have a strong genetic component, with heritability in the general population estimated at 25–60%. Extensive preclinical and clinical data suggest that elevated blood pressure and its associated cardiovascular risks are largely due to the interaction between genetic and environmental factors [7–11]. There is growing evidence that monogenic syndromes related to either hypertension or hypotension have substantial genetic contributions. Moreover, the identification rate of validated single nucleotide polymorphisms (SNPs) related to blood pressure in genome-wide association studies (GWAS) has increased exponentially. Over the past decade, GWAS and whole-exome sequencing have uncovered numerous potential blood pressure-related pathways, however consistent associations with hypertension remain uncommon or rare [12, 13]. These findings are not unique to hypertension; similar trends are observed in other complex chronic diseases, such as anemia and diabetes, as well as in lung cancer, which has reported similar results [14, 15]. While it is widely recognized that many lung cancer cases are triggered by smoking and other behavioral or environmental risk factors, there is also an acknowledged genetic risk element [16]. Genetic and environmental influences on various phenotypes and disease risks have cross-generational heritability. Extensive epidemiological and experimental evidence in model organisms and humans suggests that the effects of environmental exposure and genetic variation can be transmitted across multiple generations following exposure.
Cancer patients bear a substantial burden of cardiovascular disease, with hypertension being the most common cardiovascular condition among all cancer patients (10.8%), followed by diabetes (5.3%) and dyslipidemia (1.2%) [17]. Similarly, hypertension is one of the most common cardiovascular comorbidities among lung cancer patients [17, 18]. In lung cancer, hypertension is independently associated with mortality, with studies showing that patients with both lung cancer and hypertension have significantly poorer outcomes compared to those without comorbidities [19]. Additionally, in a cohort of 101,776 lung cancer cases, hypertension significantly increased mortality risk at 1-year (47.9%), 5-year (30.5%), and 10-year (28.2%) intervals compared to other non-pulmonary comorbidities [20]. Lung cancer is associated with high blood pressure levels, with every 10-mmHg increase in blood pressure increasing lung cancer risk by 10% [21]. There are many factors contributing to hypertension and lung cancer, and their incidence rates rise with age, which negatively impacts patients’ quality of life and places a heavy economic load on the global healthcare system. Experts have developed the concept of “tumor hypertension,” underscoring the close connection between cancer and hypertension [2]. Despite the fact that high blood pressure and lung cancer seem to belong to different domains, there is evidence that they may share some genetic and biological mechanisms. Consequently, exploring the genetic susceptibility of hypertension and lung cancer not only helps to reveal the common pathogenesis of these two diseases, yet al.so provides a scientific basis for the formulation of comprehensive prevention and treatment strategies as well as personalized medicine.
This review aims to comprehensively summarize and analyze existing research findings to explore the genetic susceptibility and potential shared mechanisms underlying hypertension and lung cancer. By systematically assessing studies on genetic polymorphisms, epigenetic modifications, and gene-environment interactions, we hope to provide valuable insights for researchers and clinicians in related fields. Additionally, we offer suggestions for future research directions to enhance our understanding of the intersection between these two disease states.
Potential relationship between hypertension and cancer
In cancer patients, hypertension remains an understudied but complex comorbidity, and current research shows inconsistent results in relation to hypertension and various cancer types. Although the causal relationship between cancer and hypertension is unclear, some studies have reported correlations between the two. Our understanding of the link between hypertension and cancer risk is evolving. For instance, hypertension has been observed to influence cancer risk, with studies reporting a statistically significant association between hypertension and an increased risk of breast cancer. In subgroup analyses, hypertension was positively correlated with breast cancer incidence in postmenopausal women [22]. Hypertension is also linked to an elevated overall risk of endometrial cancer [23]. Moreover, a meta-analysis of 86 prospective studies evaluating the association between hypertension and various cancer types indicated that hypertension may be associated with multiple types of cancer [24]. Researchers found that patients with a history of cancer are at a higher risk of hypertension, regardless of whether they receive active antitumor treatment. Numerous sensitivity analyses have confirmed a robust association between cancer and hypertension incidence. Patients with certain types of cancer are more likely to develop hypertension than those without cancer, with levels of risk varying according to cancer type [25]. Furthermore, there is a complex pattern to cancer risk among hypertensive patients. For example, a retrospective study found that, among men, diastolic blood pressure was associated with an increased risk of cancer, particularly in those smoking more than 10 cigarettes per day. Among men using antihypertensive drugs at baseline, functional stages of hypertension (Stage I: hypertension without end-organ damage; Stage II: hypertension with left ventricular hypertrophy; Stage III: hypertension with extracardiac organ damage) were significantly associated with increased cancer risk. In women who were not using antihypertensive drugs at baseline, diastolic blood pressure was linked to an increased risk of cancer [26].
Hypertension is a common side effect of many cancer therapies. Notably, certain anticancer treatments can cause a rapid rise in blood pressure, potentially worsening pre-existing cardiovascular conditions and, in severe cases, leading to acute hypertension-related complications [27]. Additionally, studies have shown that hypertension, whether treated or untreated, is modestly associated with increased cancer incidence and mortality [28]. Approximately 30% of cancer patients undergoing treatment also suffer from hypertension, and critical chemotherapy may be interrupted due to severe, newly onset, or worsening hypertension. For this patient group, timely diagnosis and optimal management of hypertension are crucial, as hypertension is a recognized risk factor for cardiotoxicity induced by chemotherapy. Without prompt treatment, it may alter cancer therapy, resulting in reduced or terminated doses of anticancer agents and potentially life-threatening end-organ damage [29]. Several classes of antineoplastic drugs, including vascular endothelial growth factor inhibitors, proteasome inhibitors, platinum derivatives, corticosteroids, and radiotherapy, are consistently associated with an increased risk of new-onset or uncontrolled hypertension in previously stabilized patients. Furthermore, hypertension is a primary risk factor for chemotherapy-induced cardiotoxicity, one of the most severe cardiovascular adverse effects of anticancer treatments. A growing body of evidence suggests that multiple antineoplastic agents accelerate the progression of hypertension [30].
The risk of cancer has also been linked to certain blood pressure medications. Calcium channel blockers (CCBs) are divided into dihydropyridine and non-dihydropyridine classes. Dihydropyridines include nifedipine, nicardipine, felodipine, and amlodipine, while non-dihydropyridines include diltiazem and verapamil. CCBs are widely used to control blood pressure and treat symptoms of angina [31]. For instance, diltiazem and verapamil have been shown to slow tumor growth in murine xenograft models of meningioma [32]. Amlodipine, a Ca²⁺ channel blocker commonly used for hypertension and angina, has been reported to inhibit the PI3K/Akt and Raf/MEK/ERK pathways via the epidermal growth factor receptor (EGFR) and regulate cell cycle-related proteins, such as cyclin D1, p-Rb, p27, and p21. Consequently, the combination of amlodipine and gefitinib achieves a synergistic effect in inhibiting cell proliferation by blocking the cell cycle. In xenograft models of A549 lung cancer, this combination effectively inhibits tumor growth compared to monotherapy, showing promising therapeutic potential [33].
Angiotensin receptor blockers (ARBs) are a class of drugs approved for the treatment of various common conditions, such as hypertension and heart failure [34, 35]. In 2003, Pfeffer et al. in a trial first observed that ARBs might increase in cancer risk [36]. A 2010 meta-analysis indicated a significantly increased risk of new cancer occurrence in patients randomized to ARB therapy. Among the specific cancers examined, only lung cancer incidence was significantly higher in patients treated with ARBs. While some mechanisms by which anticancer drugs induce hypertension have been identified, further preclinical and clinical studies are needed to elucidate the exact pathophysiology of hypertension associated with anticancer therapy and to optimize its management [37]. Other studies have reported that angiotensin II (Ang II) can accelerate cancer cell progression and metastasis. The renin-angiotensin system (RAS) plays an essential role in blood pressure regulation, and Ang II is a well-known pressor peptide associated with RAS. Ang II promotes hematogenous lung metastasis of melanoma cells by upregulating E-selectin in pulmonary vascular endothelial cells [38].
Family history and genetic susceptibility
The genetic susceptibility plays a critical role in the pathogenesis of both lung cancer and hypertension. Most individuals inherit susceptibility genes from their parents, although a few cases arise from new mutations not inherited from either parent. In an autosomal dominant inheritance pattern, if one parent carries a pathogenic cancer susceptibility gene alteration, their offspring have a 50% chance of inheriting the defective gene. Previous studies on familial risk of lung cancer have shown that individuals with a first-degree relative suffering from lung cancer, multiple family members suffering from lung cancer, or relatives with early-onset lung cancer have an increased risk of developing the disease themselves. Specifically, individuals with a family history of lung cancer among first-degree relatives (parents, siblings, or offspring) face approximately a 50% higher risk of lung cancer compared to those without such a history [39]. A recent meta-analysis also found that the risk of lung cancer is associated with having an affected father, mother, brother, or sister [40]. Moreover, another prospective study indicated that having a mother or maternal relatives with lung cancer is a risk factor for the disease [41]. In summary, family history serves as a straightforward indicator of genetic risk, influenced by shared and individual environmental exposures. Even after adjusting for smoking, the association between lung cancer and family history remains significant. In clinical practice, family history assessment is readily available and helps identify high-risk individuals. A positive family history of lung cancer should potentially be considered a variable when selecting individuals for lung cancer screening [42].
Similarly, family history is a valuable tool in identifying high-risk individuals for hypertension. Studies have shown that males with both parents diagnosed with hypertension exhibit elevated systolic blood pressure (SBP) and diastolic blood pressure (DBP) during both day and night [43]. Family studies also contribute to in validating genetic susceptibility models. By assessing the predictive power of polygenic models in high-risk individuals with a family history of hypertension, researchers can evaluate these models’ accuracy and practical application. For instance, a family history of cardiovascular disease can clinically help identify individuals at high risk of non-stroke cardiovascular events, irrespective of ethnicity, and those at increased risk of stroke among African Surinamese [44]. Moreover, predictive models have been developed for both SBP and DBP. The SBP model incorporates six environmental factors—age, BMI, waist circumference, weekly exercise frequency, and a parental history of hypertension (either one or both)—along with a single SNP (rs7305099). The DBP model includes six environmental factors (weight, alcohol intake, weekly exercise frequency, triglycerides, parental history of hypertension) and three SNPs (rs5193, rs7305099, rs3889728). The area under the curve (AUC) values for these models were 0.673 for SBP and 0.817 for DBP [45]. Then, specific findings from genetic polymorphism research have enhanced our understanding of how these genetic factors may regulate disease risk at the genetic level. Numerous polymorphic loci have been identified with significant associations with lung cancer and hypertension risk, offering a potential genetic basis for the comorbidity mechanisms of these two diseases. For example, a GWAS study in European populations identified that the 15q25.1 locus variation in the CHRNA5 gene (such as rs55781567) significantly increases lung cancer risk, but only in individuals who have smoked previously. Additionally, the LUAD GWAS in East Asian (EA) populations identified 12 novel variants, while a trans-ancestry meta-analysis between EA and European (EUR) populations discovered 4 new variants. In a dataset with ancestry-matched lung tissue, colocalization and analysis identified genes that may serve alveolar function. Notably, most variants identified in the EA GWAS had no evidence of association in EUR populations [46, 47].
Recent genetic studies have identified overlapping susceptibility loci that may underlie the comorbidity between hypertension and lung cancer. One such gene is MALAT1, a long non-coding RNA located on chromosome 11q13.1 initially discovered in NSCLC, which also plays a regulatory role in vascular biology. Previous studies have shown that MALAT1 plays a crucial regulatory role in lung cancer cell growth, metastasis, and invasion, and it is closely related to lung cancer prognosis [48, 49]. Concurrently, the rs664589 G allele of MALAT1 has been associated with a 1.33-fold increased risk of hypertension, particularly among individuals with obesity, male sex, or a history of smoking and alcohol use. Additionally, plasma MALAT1 levels are significantly lower in G allele carriers than in C allele carriers, suggesting that this polymorphism may influence hypertension development through lncRNA expression regulation [50]. These results suggesting its function spans both oncogenic and cardiovascular pathways. Another gene of particular interest is ALDH2, which encodes aldehyde dehydrogenase 2, a key enzyme involved in alcohol metabolism [51]. The rs671 polymorphism of ALDH2 has been linked to elevated lung cancer risk among drinkers and is also associated with increased carotid intima-media thickness and blood pressure in hypertensive patients. The ALDH2 polymorphism rs671 has been linked to elevated lung cancer risk among drinkers and is also associated with increased carotid intima-media thickness and blood pressure in hypertensive patients. Notably, the effects of this variant are modulated by alcohol consumption, highlighting a gene–environment interaction. Within specific drinking subgroups, rs671 has been associated with variations in systolic blood pressure and may modulate the tumor microenvironment (TME). Some studies have suggested that rs671 may influence the clinical response to PD-1/PD-L1 immunotherapy in thoracic malignancies, although its role as a predictive biomarker remains to be fully validated [52–54]. Another epigenetic regulator of interest is JMJD3 (also known as KDM6B), a histone H3K27 demethylase that plays crucial roles in both vascular and cancer biology. Genome-wide association studies have identified the rs62059712 T allele at the KDM6B locus as significantly associated with elevated SBP. Mechanistically, this variant disrupts SP1 transcription factor binding to the JMJD3 promoter, reducing its expression in vascular smooth muscle cells (SMCs). The resulting downregulation of JMJD3 leads to suppressed EDNRB expression and compensatory upregulation of EDNRA, promoting endothelin-mediated vasoconstriction, ERK pathway activation, and vascular remodeling—all key features of hypertension [55–57]. Beyond its role in vascular biology, JMJD3 also contributes to lung cancer progression through epigenetic reprogramming of metastasis-associated signaling pathways. Elevated JMJD3 expression has been consistently observed in human lung tumors and is particularly enriched in Ras-activated lung cancer cells. Mechanistic studies reveal that JMJD3 facilitates TGF-β/Smad signaling and epithelial–mesenchymal transition (EMT) by regulating syntenin, a scaffolding protein that enhances TGF-β signaling. Moreover, interferon-gamma (IFNγ) increases the expression of ZEB1 in a STAT1-JMJD3-dependent manner, thereby promoting the invasiveness of lung cancer cells [58–60]. In addition to its tumor-intrinsic functions, JMJD3 also modulates the tumor immune microenvironment. Recent studies using metastatic breast cancer models have demonstrated that cancer cell–derived exosomal miR-138-5p can be transferred to macrophages, where it suppresses JMJD3 expression, inhibits M1 polarization, and promotes M2-like immunosuppressive phenotypes. These reprogrammed macrophages contribute to enhanced lung metastasis, and circulating levels of exosomal miR-138-5p have been positively associated with disease progression in patients [61]. Although these findings were initially observed in breast cancer, the lung microenvironment appears to serve as a key site of immune modulation, suggesting broader implications for lung cancer as both a primary and metastatic target.
Overall, mounting evidence suggests that genetic and epigenetic susceptibility factors play a pivotal role in shaping the risk landscape of both lung cancer and hypertension. Family history serves as a practical proxy for inherited risk, while specific loci—such as MALAT1, ALDH2, and JMJD3—exemplify how shared molecular mechanisms may simultaneously influence oncogenic processes, vascular regulation, and immune responses. These findings not only underscore the biological overlap between the two conditions, but also support the hypothesis that lung cancer and hypertension may share common pathways.
Epigenetics
Epigenetic research involves heritable changes that affect gene expression without altering the DNA sequence, including DNA methylation, histone modifications, microRNA(miRNAs) and Long Non-Coding RNAs (lncRNAs) regulation. In recent years, epigenetics has been extensively studied in hypertension and lung cancer, uncovering the role these modifications play in disease onset and progression [62, 63].
DNA methylation
DNA methylation, a major epigenetic mechanism, involves the addition of methyl groups to DNA—typically at CpG islands—leading to transcriptional repression. Aberrant DNA methylation patterns are implicated in the onset and progression of both hypertension and lung cancer, particularly through the silencing of regulatory genes and modulation of cellular signaling pathways [64]. In hypertensive patients, certain gene loci frequently show abnormal DNA methylation [65, 66]. Genome-wide analyses have identified CpG sites significantly associated with both systolic and diastolic blood pressure, with some methylation changes shown to both predict and be influenced by blood pressure over time [67]. For example, methylation of the At1b gene, which encodes the angiotensin 1b receptor, contributes to salt-sensitive hypertension [68]. Importantly, non-CpG island regions and distal enhancers also participate in blood pressure regulation [69]. Complex interactions between DNA methylation and genetic variations have also been reported, specifically how methylation changes in non-coding regions affect hypertension onset and progression. The hypertension-related variant rs1275988, located in a cell-type-specific enhancer, aggravates hypertension by regulating local DNA methylation, Kcnk3 expression, and subsequent vascular remodeling. In animal models, the rs1275988C/C genotype, combined with a high-salt diet, exacerbates hypertension and induces pronounced vascular remodeling. Importantly, KCNK3 also plays a critical role in lung cancer. Prior studies demonstrated that KCNK3 is markedly downregulated in LUAD tissues and is associated with poor prognosis. Both in vivo and in vitro, KCNK3 overexpression substantially regulates carcinogenesis and glucose metabolism in LUAD. Mechanistic research revealed that KCNK3-mediated differential metabolites are primarily enriched in the AMPK signaling pathway [70]. These findings indicate that KCNK3 serves as a shared epigenetic mediator linking vascular dysfunction in hypertension and metabolic reprogramming in lung cancer. Moreover, classical methylation changes in lung cancer—such as silencing of tumor suppressors p16 and RASSF1A—further illustrate the importance of aberrant DNA methylation in tumorigenesis [71–75].Together, these observations support the notion that shared methylation-regulated pathways, exemplified by KCNK3, may underlie the molecular crosstalk between hypertension and lung cancer.
Histone modification
Histone modifications refer to structural changes in histones through chemical modifications, such as acetylation, methylation, and phosphorylation, which affect DNA packaging and gene expression. These modifications play a crucial role in gene regulation and cellular function. In hypertensive patients, abnormal histone acetylation and methylation are commonly observed, impacting vascular smooth muscle cell proliferation and function [76]. For example, in arterial hypertension, increased reactive oxygen species (ROS) and pro-inflammatory cytokines are regulated by histone deacetylase (HDAC) enzymes, which modulate the gene expression of these hypertension-promoting factors. HDAC inhibitors have been found to help regulate vascular tension and reduce blood pressure [77]. In spontaneously hypertensive rats, the well-studied HDAC inhibitor valproic acid has been shown to lower blood pressure, inflammatory cytokines, hypertrophy markers, and ROS levels [78]. Additionally, in a high-fat diet-induced hypertension mouse model, valproic acid was found to inhibit HDAC1, reducing angiotensin II and its receptor expression, thereby preventing hypertension progression [79]. Elevated HDAC expression and activity are also observed in certain cancers; for example, HDAC1-3 are overexpressed in ovarian cancer and HDAC1 and HDAC3 in lung cancer [80]. Research suggests that HDAC inhibitors could suppress tumor growth through anti-inflammatory and anti-proliferative effects. Valproic acid, for instance, has been reported to induce apoptosis in small cell lung cancer cell lines and enhance the effectiveness of cisplatin combined with etoposide—two standard first-line chemotherapy agents for small cell lung cancer. Valproic acid induces apoptosis in mitochondria and death receptors, and its anti-lung cancer effects can be enhanced by inhibiting cyclin-dependent kinases [81]. Histone modifications frequently observed in lung cancer cells include an increase in H3K27me3, a modification that promotes cancer cell growth and survival by suppressing tumor suppressor gene expression. Aberrant expression of histone-modifying enzymes, such as EZH2, is also associated with increased invasiveness and poor prognosis in lung cancer [82].
MiRNAs
MiRNAs are short, endogenous, non-coding RNAs that regulate gene expression at the post-transcriptional level by binding to the 3′ untranslated regions (UTRs) of their target mRNAs. Approximately half of miRNA genes undergo CpG promoter region hypermethylation, leading to miRNA silencing or downregulation [83, 84]. Substantial evidence supports this mode of action. For instance, CpG island hypermethylation-mediated silencing of miR-124a has been observed in various lung cancer cell lines, including H358, CALU3, A549, A427, H2126, and H209 [83]. Another study found that methylation-mediated downregulation of miR-200c is associated with increased invasive potential in NSCLC cell lines [85].
MiRNAs also play a significant role in hypertension. Hypertension-induced vascular remodeling is characterized by medial thickening, luminal narrowing, and extracellular matrix restructuring within the vascular wall. Vascular smooth muscle cells (VSMCs), as the main component of the vascular media, are crucial for maintaining vascular tone in response to hemodynamic and fluid changes, and play a pivotal role in vascular diseases like hypertension [86, 87]. Over the past decade, the role of miRNAs in VSMC development, phenotypic transformation, and vascular pathology has been widely studied. One of the most thoroughly investigated miRNA clusters in this regard is the miR-143/-145 family, which is enriched in VSMCs and transcriptionally regulated by serum response factor and myocardin [88, 89].
In addition to its impact on hypertension, the miR-143/-145 cluster is also critical in cancer. MiR-143/145 in the TME significantly promotes tumor growth by stimulating endothelial cell proliferation. In vivo, deletion of miR-143/145 results in the derepression of its target CAMK1D (an inhibitory kinase), whose overexpression impedes endothelial cell mitosis. Consequently, tumors in miR-143/145-deficient animals exhibit reduced angiogenesis, increased apoptosis, and restricted expansion due to limitations in their ability to engulf pulmonary vasculature [90]. Moreover, the interaction between the renin-angiotensin-aldosterone system (RAAS) and miRNAs also demonstrates a shared regulatory pattern in both lung cancer and hypertension. Certain genes within the RAAS, such as the vasopressin receptor (AVPR1A), interact with specific miRNA binding sites and are linked to the pathogenesis of hypertension. MiRNAs such as miR-526b and miR-578 regulate the expression of RAAS components, which not only exacerbate hypertension but may also influence tumor cell drug resistance and invasiveness, thereby further promoting the progression of lung cancer [91–94].
LncRNAs
LncRNAs are transcripts longer than 200 nucleotides that do not encode proteins but play essential roles in regulating gene expression at transcriptional, post-transcriptional, and epigenetic levels. Increasing evidence suggests that lncRNAs are critical contributors to both hypertension and lung cancer, serving as potential molecular bridges in their comorbidity [95–98].
Several lncRNAs have been implicated in both hypertension and lung cancer. H19, for instance, is upregulated in the decompensated right ventricle of PAH patients and is associated with right ventricular hypertrophy and fibrosis; it promotes vascular smooth muscle cell proliferation and inflammation in hypertension and is differentially expressed across patient subgroups. In lung cancer, H19 downregulation significantly reduces clonogenicity and anchorage-independent growth, and it acts as a critical downstream effector of c-Myc to promote tumorigenesis [99–101]. Similarly, GAS5 plays a dual protective role in both cardiovascular and cancer contexts. In hypertension, GAS5 is significantly downregulated in endothelial cells (ECs) and VSMCs, and its silencing exacerbates vascular remodeling, increases capillary leakage, and promotes endothelial dysfunction by modulating β-catenin signaling. In lung cancer, particularly NSCLC, reduced GAS5 expression is associated with advanced TNM stage and larger tumor volume. Mechanistically, GAS5 inhibits proliferation and promotes apoptosis through the regulation of E2F1, p21, and p53, and suppresses oncogenic pathways including EGFR, MAPK, and AKT. These findings underscore GAS5 and H19 as pivotal molecular nodes in both hypertension-induced vascular remodeling and lung cancer progression [99, 102–106].
Recent genome-wide association studies have also identified LINC00944 as a novel locus associated with blood pressure regulation. Some studies have found that LINC00944 may serve as a prognostic and immunological biomarker, linking tumor progression and immune features across NSCLC subtypes [107, 108]. Another notable lncRNA, MEG3, is downregulated in both hypertensive and lung cancer tissues and has been shown to modulate the p53 and Wnt signaling pathways. In NSCLC, MEG3 suppresses tumor proliferation and metastasis via the miR-21-5p/PTEN and MDM2/p53 axes, while in pulmonary hypertension, it promotes vascular remodeling by sponging miR-328-3p and upregulating IGF1R, highlighting its dual context-dependent roles in tumor suppression and vascular dysfunction [109–111].
These findings indicate that lncRNAs may represent a class of shared epigenetic regulators linking the pathogenesis of hypertension and lung cancer. Further research is warranted to explore their diagnostic and therapeutic potential in the context of disease comorbidity.
The role of gene-environment interactions in hypertension and lung cancer
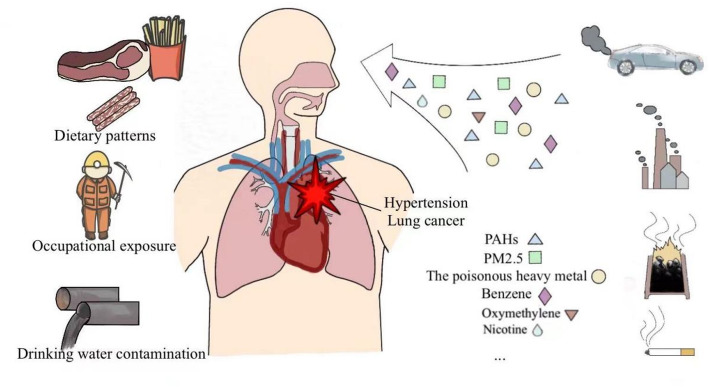
Gene-environment interactions are key factors in understanding the pathogenesis of complex diseases such as hypertension and lung cancer (Fig. 1). Although inheritance genetics plays an important role in the development of these diseases, the influence of environmental factors and lifestyle cannot be ignored (Table 1). Not only can they directly affect the occurrence of disease, but they can also significantly increase an individual’s risk of disease through interaction with the genetic background.
Fig. 1.
Environmental and lifestyle risk factors contributing to hypertension and lung cancer. This figure illustrates the key environmental and lifestyle factors associated with the development of hypertension and lung cancer, including dietary patterns, occupational exposure, drinking water contamination, air pollution, smoking, alcohol consumption, heavy metal, and other exposures. These factors are depicted with corresponding icons and linked to their impact on the human respiratory and cardiovascular systems, highlighting the interplay between genetic susceptibility and environmental triggers
Table 1.
Influence of exposures on hypertension and lung cancer
| Environmental factors/lifestyle | Primary sources | Impact on hypertension | Impact on lung cancer | References |
|---|---|---|---|---|
| Air pollution | Industrial emissions, vehicle exhaust, PM2.5 | PM2.5 induces ROS generation, reduces NO levels, leading to vascular dysfunction. | PM2.5 particles carry carcinogens such as polycyclic aromatic hydrocarbons, causing DNA damage and mutation accumulation. | [282–290] |
| Long-term exposure can activate chronic inflammatory pathways (such as IL-6, TNF-α). | Chronic lung inflammation increases the risk of tumor angiogenesis and invasion. | |||
| Smoking | Tobacco products | Nicotine activates the sympathetic nervous system, causing vasoconstriction and inflammatory responses. | Carcinogens like benzo[α] pyrene in tobacco directly induce DNA mutations and interfere with DNA repair functions, promoting cancer development. | [291–296] |
| Tobacco chemicals promote vascular hardening and arterial inflammation, exacerbating the hypertension process. | Inhibits immune system function, enhancing tumor escape ability. | |||
| Occupational exposure | Heavy metals (lead, cadmium), benzene compounds | Heavy metals activate vascular inflammatory responses through ROS generation and NF-κB pathway activation. | Benzene compounds form adducts with DNA, leading to gene mutations. | [297–303] |
| Cadmium can induce apoptosis of vascular endothelial cells, leading to endothelial dysfunction. | Asbestos fibers stimulate chronic pulmonary inflammation, accelerating cancer development. | |||
| Dietary patterns | High-salt diet, saturated fat intake | A high-salt diet activates the RAAS system, leading to increased blood volume and elevated vascular tone. | A high-fat diet promotes tumor development by regulating the tumor microenvironment metabolism and pro-inflammatory responses, supporting lipid metabolic remodeling and signal molecule release. | [304–308] |
| A high-fat diet supports pro-inflammatory and pathogenic environments, such as promoting the release of trimethylamine N-oxide, thereby accelerating the development of atherosclerosis. | ||||
| Drinking water contamination | Heavy metals (arsenic, lead), organic pollutants | Arsenic contamination is associated with increased blood pressure, possibly through ROS generation leading to vascular dysfunction. | Arsenic increases cancer risk by inducing DNA damage and interfering with DNA repair functions. | [300, 309–313] |
| Arsenic in drinking water causes vascular endothelial dysfunction, manifesting as imbalances in vasodilation and vasoconstriction. | Organic pollutants may be associated with lung cancer through epigenetic regulation. | |||
| Microplastics | Plastic degradation in the natural environment | Microplastics can enter the human cardiovascular system, triggering inflammation, oxidative stress, and other reactions, thereby promoting the development of cardiovascular diseases such as atherosclerosis and myocardial injury. | By physically penetrating cells and chemically inducing reactive oxygen species, microplastics cause cellular dysfunction and genomic instability, thereby promoting lung cancer progression. | [314–325] |
Diet and drinking habits
Dietary habits play a crucial role in the development of hypertension. The intake of high amounts of salt is strongly associated with hypertension, as excessive sodium levels in the body resulting from increased dietary intake or reduced urinary excretion are well-established risks for hypertension. A cross-sectional studies show that populations with higher sodium intake tend to have elevated average blood pressure levels and a higher prevalence of hypertension [112]. In a prospective cohort study, high sodium intake was shown to predict mortality and coronary heart disease risk independently of other cardiovascular risk factors, including blood pressure, providing direct evidence of the harmful effects of high salt intake in adults [113]. Additionally, in the absence of exogenous antioxidants, cancer cells maintain redox homeostasis by expressing endogenous antioxidants. Antioxidants in the diet can reduce reactive oxygen species (ROS) and DNA damage in tumors, suppressing the expression of p53, a tumor suppressor typically activated by DNA damage [113]. For instance, supplementation with antioxidants like N-acetylcysteine (NAC) and vitamin E in the diet can accelerate the progression of primary lung tumors in Kras2 LSL/+ (K) mice [114]. Inorganic arsenic in water and food is a key toxic substance for risk assessment and exposure mitigation. Chronic exposure to arsenic can affect multiple organ systems, leading to various cancers, cardiovascular diseases, and respiratory conditions [115, 116]. Among study participants, a statistically significant association was found between arsenic exposure through drinking water and hypertension, with cumulative lifetime arsenic exposure of 2,188–7,025 µg/L-years and > 7,025 µg/L-years yielding odds ratios for hypertension of 1.12 (95% CI: 0.84, 1.49) and 1.60 (95% CI: 1.20, 2.13), respectively [117]. A 13-year study in southwestern Taiwan also found that diastolic blood pressure increased with higher arsenic intake from drinking water [118]. Arsenic has a documented impact on both hypertension and lung cancer. Between 1950 and 1969, studies found that white men and women living in counties with copper, lead, or zinc smelting and refining industries had a significantly higher average lung cancer mortality rate. Later investigations suggested that inorganic arsenic emissions from these industries likely played a major role [119]. Numerous statistical studies and experiments have since confirmed this association, establishing lung cancer as one of the most fatal cancer types linked to arsenic exposure [120, 121]. Furthermore, among both smokers and nonsmokers, arsenic-induced lung cancers predominantly manifest as squamous cell carcinoma (SqCC) and small cell carcinoma (SCC) [122]. Chronic arsenic exposure may also cause epigenetic changes, such as the arsenic-induced depletion of S-adenosylmethionine (SAM), which can alter CpG methylation in gene promoters, including p53 [123–125]. It has been reported that arsenic exposure modifies histone methylation patterns, particularly in H3K4, H3K9, and H3K27, in both malignant and non-malignant lung cell lines. These changes lead to reduced gene expression through alterations in histone acetylation and DNA methylation [126, 127].
Alcohol
Drinking alcohol is a significant environmental factor impacting the development of various chronic diseases, including hypertension and cancer. A growing body of research shows that long-term excessive alcohol consumption not only contributes to the onset of hypertension but also is associated with increased risks of several cancers. Excessive alcohol intake is a notable trigger for hypertension, showing a dose-dependent relationship with elevated blood pressure and the prevalence of hypertension [128]. Studies have found that prolonged alcohol consumption activates the renin-angiotensin system, raising blood pressure and causing vascular damage. Alcohol elevates blood pressure by influencing sodium and water reabsorption and activating the sympathetic nervous system. Moreover, gene-environment interactions may play a critical role in the process by which alcohol induces hypertension [129, 130]. For instance, genetic polymorphisms in genes such as ADH1B and SLC39A8 are closely related to drinking habits and blood pressure regulation in hypertensive individuals. People with these specific genetic variants are more susceptible to blood pressure elevation after alcohol consumption [131]. Additionally, a cohort study of 371,463 individuals provided genetic evidence that varying alcohol intake levels are linked to a persistent, nonlinear increase in the risk of hypertension and coronary artery disease. Moderate alcohol consumption led to a slight increase in risk, while higher intake resulted in a sharp, exponential rise in risk [132]. Even without hypertension, daily alcohol intake can raise blood pressure. A meta-analysis of seven cohort studies involving nearly 20,000 healthy participants from Japan, Korea, and the United States showed that daily alcohol consumption was associated with elevated systolic blood pressure in non-hypertensive individuals. Those who drank about 12 g of alcohol daily (equivalent to less than one beer or five ounces of wine) had systolic blood pressure readings 1.25 mmHg higher than non-drinkers. A dose-response relationship was also observed: a daily intake of 48 g of alcohol resulted in a systolic blood pressure increase of 4.90 mmHg [133]. The link between alcohol consumption and lung cancer is more complex. Although alcohol itself is not a primary cause of lung cancer, the risk of lung cancer significantly increases when combined with smoking. Acetaldehyde, a carcinogenic byproduct of alcohol metabolism, can damage DNA and may exacerbate the carcinogenic effects of tobacco, thereby contributing to the development of lung cancer [134]. In lung cancer, specific genetic polymorphisms, such as the Arg48His polymorphism in the ADH1B gene, are associated with an increased risk of alcohol-related lung cancer. Studies have shown that individuals carrying the Arg48His variant have a more than threefold increased risk of lung cancer when drinking 10 to 29.9 g of alcohol daily [135]. Furthermore, alcohol consumption may alter the TME, enhancing the growth and spread of cancer cells. Interactions between alcohol consumption and genetic background play a critical role in the development of both hypertension and lung cancer. Specifically, polymorphisms in ethanol-metabolizing genes like ADH1B and ALDH2 are closely linked to oxidative stress, DNA damage, and inflammatory responses induced by alcohol consumption. Alcohol increases free radical production, disrupts the body’s antioxidant defenses, induces chronic inflammation, and affects gene expression regulation, ultimately heightening an individual’s disease susceptibility [53, 54, 134].
Smoking
Smoking is one of the major causative factors in multiple diseases, particularly in the development of lung cancer and cardiovascular disease, where it plays a crucial role. Long-term smoking not only elevates the risk of lung cancer but is also closely associated with the incidence of hypertension. Among the more than 60 identified carcinogens in cigarette smoke, at least 20 are credible lung cancer carcinogens. Additionally, numerous pro-inflammatory changes have been observed in the lungs of smokers, with inflammation closely linked to tumor promotion and the activation of factors like NFκB [136–138]. In a case-control study involving smokers with normal lung function, COPD, and lung cancer (COPD subtype), it was found that the GG genotype of the rs1489759 HHIP SNP and the CC genotype of the rs2202507 GYPA SNP exhibited a “protective” effect against both COPD (OR 0.59, p = 0.006 for HHIP and OR = 0.65, p = 0.006 for GYPA) and lung cancer (OR = 0.70, p = 0.05 for HHIP and OR = 0.70, p = 0.02 for GYPA) [139]. Compared to non-smoking-related lung cancer, rare sensitizing and non-sensitizing EGFR mutations are more likely to occur in smoking-related lung cancer cases; however, approximately 60% of rare EGFR exon 20 insertion cases involve patients who have never smoked, with most being female (similar to patients with classical sensitizing EGFR mutations) [140–142]. A 2013 GWAS meta-analysis involving two independent cohorts identified SNPs associated with overall survival in never-smoking European NSCLC individuals. Among the top 25 SNPs, six showed genotype-expression associations in expression quantitative trait locus analysis. These variants were neither in genes previously linked to lung cancer risk in non-smokers nor in those associated with OS in smoking-related lung cancer patients. In the initial consistency meta-analysis, three of these variants reached genome-wide significance (rs7976914, rs4237904, and rs4970833) [143, 144]. Likewise, smoking can induce hypertension-related changes. Blood pressure is a classic complex hereditary trait with heritability estimated at 30–50%. The gene encoding the G(s) protein α-subunit (GNAS1) is a candidate genetic determinant of hypertension. A Japanese study involving 2,000 patients showed a significant interaction between GNAS1 polymorphisms and smoking in the pathogenesis of hypertension (p = 0.0005). This interaction was observed in non-heavy smokers, where a significant association was found between the gene polymorphism and hypertension (odds ratio = 1.52, 95% confidence interval 1.16 to 2.00, p = 0.0028). Among non-heavy smokers, a significant interaction between this gene polymorphism and aging was also noted in the pathogenesis of hypertension [145]. Furthermore, studies have reported a major quantitative trait locus for systolic blood pressure on chromosome 15q in non-smokers, indicating loci that influence blood pressure via gene-smoking interactions [146].
Air pollution
Air pollution is recognized as one of the leading global threats to human health, playing a crucial role in the development of both hypertension and lung cancer. Increasing evidence suggests that long-term exposure to air pollutants, such as nitrogen dioxide (NO2) and ozone, significantly raises the risk of cardiovascular and respiratory diseases [147–150]. Epidemiological data and findings from mouse and human cell models indicate that particulate matter with an aerodynamic diameter smaller than 2.5 μm (PM2.5) promotes lung cancer by stimulating the growth of lung cells with pre-existing carcinogenic mutations. Studies have shown that PM2.5 exposure activates APOBEC3B, leading to gene mutations associated with the APOBEC mutation signature. This mechanism has been validated among lung cancer patients from four distinct geographic regions, suggesting that PM2.5 exposure may trigger lung cancer-related gene mutations through APOBEC3B and DDR (DNA damage response) pathway activation [151]. Research has also linked PM2.5 levels to 32,957 cases of EGFR-driven lung cancer across four countries. The study found that exposure to air pollutants triggers macrophage infiltration into mouse lungs, leading to the release of interleukin-1β, which promotes tumor growth [152]. A study on data from 400,000 individuals across the UK and Asian countries examined the association between lung cancer, particularly EGFR-mutant lung cancer common among non-smokers, and PM2.5 concentrations below 2.5 μm. The findings indicated a positive correlation between higher PM2.5 levels and the incidence of EGFR-mutated lung cancer and other cancers [153]. Indoor air pollution also contributes to lung cancer risk. For instance, primary human airway epithelial cells exposed to smoke extracts from traditional stoves (TCS), improved stoves (ICS), and liquefied petroleum gas (LPG) stoves showed changes in gene expression, DNA methylation, and hydroxymethylation after sulfonic acid conversion. TCS extracts alone caused alterations in 52 genes involved in oxidative stress pathways, while exposure to TCS, ICS, and LPG smoke extracts resulted in significant changes in DNA methylation and hydroxymethylation [154].
Similarly, air pollution is known to impact hypertension. Prior studies have shown that air pollution may be a risk factor for hypertension, as long-term environmental air pollution exposure has been associated with increased cardiovascular mortality. Hypertension is a critical risk factor for cardiovascular disease. Recent findings indicate that individuals exposed to PM2.5 experience increased arterial blood pressure within hours to days after exposure [155]. Another study reported a positive association between hypertension incidence and long-term exposure to PM2.5 and nitrogen oxides [156]. Short-term exposure to sulfur dioxide (SO2), PM2.5, and PM10 showed a significant correlation with hypertension. Long-term exposure to NO2 and PM10 was also significantly associated with hypertension. Exposure to other environmental air pollutants, including short-term exposure to NO2, ozone, and carbon monoxide, as well as long-term exposure to NOx, PM2.5, and SO2, showed positive associations with hypertension, though without statistical significance [157]. Furthermore, epigenetic age acceleration (EAA), an epigenetic biomarker of aging, has been linked to indoor air pollution from coal combustion and associated pulmonary arterial hypertension, particularly 5-methylcytosine, which correlates with the mortality biomarker Grim Age EAA [158].
Radon
Radon is a naturally occurring radioactive gas released from the decay of uranium in rocks and soil, and it can infiltrate indoor environments through building foundations, accumulating especially in poorly ventilated residential spaces. It has long been recognized as the second leading cause of lung cancer globally after tobacco smoking, accounting for an estimated 10–20% of lung cancer cases and 3–20% of lung cancer-related deaths. The risk appears particularly pronounced among never-smokers, with dose–response studies confirming that both the concentration and duration of radon exposure are critical. Inhabitants of homes with indoor radon levels exceeding 300 Bq/m³ are at significantly increased risk, and long-term exposure beyond 40 years further compounds this risk. Recent studies have also indicated sex-specific effects, with males potentially being more susceptible than females [159–163].
Beyond its well-established pulmonary carcinogenicity, growing evidence implicates radon exposure in cardiovascular pathophysiology. A large-scale cohort study from Massachusetts reported a 15% increased risk of hypertensive disorders of pregnancy (HDP) associated with an interquartile-range increase in residential radon levels, independent of traditional risk factors such as age, socioeconomic status, and PM2.5 exposure. The effect was even more pronounced (up to 38%) in women under 20 years of age. This suggests that radon may impair vascular health by promoting endothelial dysfunction, oxidative stress, and systemic inflammation—mechanisms also central to hypertension development [164].
Mechanistically, radon and its radioactive progeny emit alpha particles that can cause direct DNA damage, genomic instability, and persistent low-grade inflammation in lung tissues. These processes not only facilitate oncogenesis but may also contribute to vascular remodeling and stiffness—hallmarks of hypertensive pathology. Moreover, radon exposure has been linked to disrupted redox balance and impaired nitric oxide (NO) signaling, both of which are pivotal in maintaining vascular tone and endothelial function. These shared molecular alterations highlight radon exposure as a potentially modifiable environmental risk factor in the comorbidity of lung cancer and hypertension [165–167]. Given its ubiquitous presence in many regions and its often underestimated health impact, radon warrants further investigation as a dual risk factor in pulmonary and cardiovascular pathology.
Asbestos
Asbestos, a group of naturally occurring fibrous minerals, has long been recognized as a potent environmental carcinogen, particularly associated with lung cancer, mesothelioma, and asbestosis. Epidemiological data suggest that the incidence of asbestos-related lung cancer (ARLC) is approximately six times higher than that of mesothelioma, and all six commercially used types of asbestos fibers are implicated in carcinogenesis. Both occupational and environmental asbestos exposure significantly increase lung cancer risk, even in non-smokers, and there appears to be no safe threshold of exposure. In some regions, particularly those with naturally contaminated soil or historical industrial activity, environmental asbestos exposure contributes to an elevated incidence of lung cancer, often diagnosed at younger ages and in both sexes [168–172].
In addition to its established role in cancer, asbestos exposure is increasingly linked to cardiovascular outcomes, particularly pulmonary hypertension. Asbestosis—a fibrotic lung disease resulting from chronic inhalation of asbestos fibers—impairs gas exchange, increases pulmonary vascular resistance, and can lead to right heart strain and heart failure. A meta-analysis by Yi Rong et al. further confirmed that asbestos exposure is associated with a statistically significant increase in cardiovascular mortality among exposed workers (pooled SMR = 1.11, 95% CI: 1.01–1.22) [173]. Moreover, studies have reported higher rates of ischemic heart disease and cerebrovascular mortality in asbestos-exposed populations [174]. Mechanistically, Chronic inhalation of asbestos fibers induces persistent pulmonary inflammation and fibrosis, which not only impairs gas exchange but also contributes to pulmonary vascular remodeling, ultimately leading to pulmonary hypertension. The sustained elevation of pulmonary arterial pressure increases right ventricular afterload, thereby predisposing individuals to right-sided heart failure [175–177].
Recent Korean cohort studies reinforce this health burden, showing that patients with occupational asbestos exposure—whether diagnosed with mesothelioma or ARLC—have shorter survival times and lower 3- and 5-year survival rates compared to those with environmental exposure. Furthermore, longer exposure duration and closer proximity to asbestos sources were significantly associated with worse prognosis [178]. Taken together, these findings underscore asbestos as a shared environmental risk factor in the comorbidity of lung cancer and hypertension.
Common biological pathways
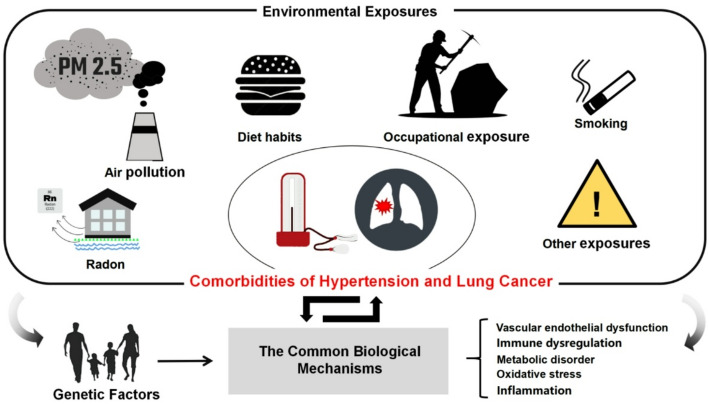
Lung cancer and hypertension, despite being distinct diseases, share several underlying biological mechanisms (Fig. 2).
Fig. 2.
The common biological mechanisms between hypertension and lung cancer. This figure highlights the shared biological mechanisms underlying hypertension and lung cancer, focusing on five core processes: oxidative stress, metabolic dysregulation, immune dysfunction, inflammation, and vascular endothelial dysfunction. Oxidative stress, driven by the accumulation of reactive ROS, reduces NO bioavailability, induces DNA damage, and triggers the release of pro-inflammatory cytokines, leading to endothelial dysfunction, vascular remodeling, and TME alterations. Metabolic dysregulation manifests in hypertension as mitochondrial dysfunction and lipid metabolism abnormalities, contributing to insulin resistance and vascular damage, while in lung cancer, metabolic reprogramming, including the Warburg effect and lipid metabolism alterations, supports tumor growth and invasion. Chronic inflammation is a hallmark of both diseases, where cytokines such as IL-6 and TNF-α drive vascular inflammation and remodeling in hypertension and induce DNA damage, promote angiogenesis, and facilitate immune escape in lung cancer. Immune dysfunction, characterized by immune cell infiltration and cytokine release, exacerbates vascular damage in hypertension, whereas in lung cancer, immune escape mechanisms such as PD-L1 upregulation and immunosuppressive microenvironments enable tumor progression. Finally, vascular endothelial dysfunction is central to both diseases, presenting as vascular remodeling and endothelial gap widening in hypertension and as abnormal angiogenesis and endothelial barrier breakdown in lung cancer, facilitating tumor growth and metastasis
The vascular endothelial dysfunction
Endothelial cells line the inner layer of blood vessels and play critical roles in regulating vascular tone, maintaining blood flow, and providing anticoagulant properties. Under normal conditions, endothelial cells secrete various vasoactive substances, such as NO and prostaglandins, which facilitate vasodilation to maintain balanced blood pressure. However, in hypertensive patients, endothelial function is often compromised, leading to what is known as “endothelial dysfunction.” Typically, these cells modulate blood vessel dilation and contraction by releasing factors like NO, prostaglandins, and endothelin, thereby stabilizing blood pressure. During endothelial dysfunction, structural and functional abnormalities arise in the endothelial cells, impairing their ability to regulate vasodilation, thrombosis, and vascular wall repair. This dysfunction manifests as reduced vascular response to dilators (particularly to NO and prostacyclin), heightened pro-inflammatory responses leading to vascular wall inflammation, increased oxidative stress contributing to vascular damage, and pathological changes. For instance, NO, a critical factor for vasodilation, is synthesized by endothelial NO synthase (eNOS) within endothelial cells [179–183]. In hypertensive conditions, NO production is reduced, or its bioavailability decreases, resulting in enhanced vasoconstriction. Oxidative stress plays a key role in lowering NO bioavailability. In hypertensive individuals, elevated ROS levels combine with NO to form harmful peroxynitrite, effectively lowering active NO levels [184]. Oxidative stress, marked by an excess of ROS relative to antioxidant systems, is a key pathological mechanism in hypertension. ROS not only directly damage endothelial cells but also inhibit eNOS activity, further reducing NO production and aggravating vasoconstriction. The interplay between oxidative stress and diminished NO forms a vicious cycle, worsening hypertension [182, 185]. Inflammation and oxidative stress from endothelial dysfunction stimulate vascular smooth muscle cell proliferation, leading to vascular wall thickening and luminal narrowing. This vascular remodeling decreases elasticity, heightening peripheral resistance and blood pressure [186, 187].
In cancers such as lung cancer, endothelial dysfunction can promote tumor growth and metastasis through various mechanisms [188–190]. Lung cancer cells secrete multiple pro-angiogenic factors like vascular endothelial growth factor (VEGF), which stimulate endothelial cell proliferation and new blood vessel formation, supplying the tumor with blood and essential nutrients [191, 192]. Under conditions of endothelial dysfunction, dysregulated angiogenesis creates an abnormal vascular network [193]. Unlike normal vessels, tumor-associated blood vessels tend to be fragile and highly permeable, facilitating cancer cell dissemination to distant organs through the bloodstream [194]. VEGF overexpression is especially critical, as tumor cells use it to drive angiogenesis around them, securing essential oxygen and nutrients for growth and metastasis [195]. Endothelial dysfunction increases vascular wall permeability, facilitating the infiltration of inflammatory cells such as macrophages and lymphocytes into the TME. These cells release various pro-inflammatory factors, which further drive cancer cell proliferation and metastasis [196–198]. Endothelial dysfunction is not only a key pathological feature of hypertension but also an early warning sign of cardiovascular disease. Additionally, it plays a significant role in the progression of lung cancer. Many anti-angiogenic therapies, such as VEGF inhibitors, suppress tumor growth and metastasis by blocking angiogenesis and repairing abnormal endothelial cells [199, 200]. In conclusion, treatments that improve endothelial function may help reduce the combined risk in patients with both hypertension and lung cancer.
Inflammation
Inflammation is a protective response to injury or infection, yet chronic systemic inflammation plays a central role in the pathogenesis of hypertension and cancer [201, 202]. Individuals with inflammation-mediated diseases are more susceptible to hypertension. For example, compared to individuals without these inflammatory conditions, patients with psoriatic arthritis have a 90% higher risk of hypertension [203], those with rheumatoid arthritis have a 50% higher risk [204], and individuals with periodontitis have a 22% increased risk of hypertension [205]. Even in patients without classic inflammation-mediated diseases, inflammatory dysregulation has been observed in primary hypertension. As blood pressure increases, plasma levels of C-reactive protein (CRP) [206, 207]and cytokines (particularly IL-6, tumor necrosis factor (TNF) [208, 209], and IL-1β, IL-18, and CC chemokine ligand 2 (CCL2) also elevate [210–213]. Various cytokines can also impact vascular function and structure, as well as renal sodium transport, ultimately resulting in increased systemic vascular resistance, elevated blood pressure, and sodium and volume retention [214, 215]. The lipopolysaccharide (LPS) endotoxin produced by gram-negative bacteria can trigger a strong immune response, and intraperitoneal injection of LPS in rodents serves as a classic model of systemic inflammation. In rats, LPS-induced elevations in plasma CRP, tumor necrosis factor-alpha (TNF-α), and IL-1β levels are associated with increases in blood pressure. In LPS-treated rats, cyclooxygenase-2 inhibition can prevent blood pressure elevation, suggesting that LPS-induced inflammation contributes to the hypertensive effect [216]. While GWAS studies have identified SNPs in gene loci that play roles in classical hypertension mechanisms (such as vasoconstriction, sodium reabsorption, and sympathetic nervous system activity), the identified gene loci also relate to inflammation and immunity. A recent meta-analysis revealed that among 97 genes from various GWAS containing SNPs associated with hypertension, 81 had direct or indirect roles in inflammation and/or immunity [217].
Chronic inflammatory responses also contribute to tumor promotion [218]. Tumor-associated inflammation involves complex interactions between epithelial and stromal cells, which can sometimes result in epigenetic changes that drive malignancy or even initiate tumorigenesis. Generally, chronic inflammation leads to the production of growth factors that support the development of nascent tumors, making them resemble “wounds that do not heal” [218, 219]. Cancer biology is shifting from focusing solely on cancer cells to a broader view. This new perspective places cancer cells within a network of stromal cells, including fibroblasts, vascular cells, and immune cells, all of which together form the TME. Inflammation, whether occurring within the context of chronic inflammatory disease or tumor-induced inflammation, significantly influences TME composition, particularly the plasticity of tumor and stromal cells [218]. A hallmark of cancer is the loss of cell-intrinsic tumor suppressive functions. One of the most frequently mutated tumor suppressors is Tp53, which encodes the p53 protein. The p53 protein has various functions in regulating cellular homeostasis, one of which includes transcriptional antagonism of NF-κB, a key positive regulator of inflammation [220, 221]. Due to the persistent NF-κB activation signaling present within the TME and even normal tissues, the loss of functional p53 leads to increased NF-κB-dependent inflammatory gene expression. In cancer, this inflammatory profile promotes tumor progression and metastasis [221–223].
Immune dysregulation
For decades, immune cells and cytokines have been associated with human hypertension, but recent advances in immunological tools and animal models have allowed detailed examination of their roles in various experimental models. New data from both human and rodent models suggest that immune cells are not passive bystanders but play an essential role in hypertension and cancer [221, 224–227]. Immune responses can occur in the intima, media, and adventitia of blood vessels. The endothelium and microvascular endothelial cells in these layers are critical for the recruitment and activation of leukocytes, which commonly contribute to the pathophysiological processes of hypertension and target organ damage. Current research indicates that both innate and adaptive immunity are involved in the pathogenesis of hypertension [228–230]. Studies have shown that mice with mutations in the colony-stimulating factor 1 gene, which causes monocyte/macrophage dysfunction, have reduced blood pressure elevation and vascular injury when exposed to Ang II or DOCA (deoxycorticosterone acetate) and salt [231, 232]. Animal studies have also demonstrated that Rag1 knockout mice, which lack mature T and B cells, show reduced Ang II-induced salt-sensitive hypertension [229, 233]. A study on the effect of Ang II on humanized mouse models found that Ang II treatment increased total CD3 + and CD4 + T helper cells, as well as CD45RO + memory T cells in the kidneys. This effect was eliminated by preventing hypertension with hydrochlorothiazide and hydralazine [234]. Numerous immune cells infiltrate the vascular wall, releasing cytokines like TNF-α, IL-6, and interferon-gamma (IFN-γ), which damage endothelial cells, promote vascular remodeling, and increase vascular resistance, ultimately leading to hypertension [225, 226]. Furthermore, genetic polymorphisms associated with immune response have been found to influence susceptibility to hypertension. A Mendelian randomization study reported a consistent, positive, and potentially causal association between lymphocyte count and systolic and diastolic blood pressure [235].
The development and progression of lung cancer are closely linked to immune escape. Immune cells in the TME, such as macrophages and T cells, can be reprogrammed by cancer cells to support tumor growth rather than suppress it. Cancer cells evade antitumor immune responses by secreting immunosuppressive factors like transforming growth factor-beta (TGF-β) and PD-L1, allowing the tumor to proliferate and metastasize [236, 237]. T lymphocytes with deletion variants exhibit lower caspase-8 activity and experience activation-induced cell death when stimulated by cancer cell antigens. A case-control study involving 4,995 cancer patients and 4,972 controls from a Chinese population found that a variant in the Caspase-8 gene was associated with reduced susceptibility to several cancers, including lung, esophageal, gastric, colorectal, cervical, and breast cancers. The effect was dose-dependent, with the degree of susceptibility linked to the number of risk alleles [238]. Studies have shown that the rs822336 SNP in the PD-L1 promoter/enhancer region affects PD-L1 expression by competing for binding sites with the transcription factors C/EBPβ (CCAAT-enhancer-binding protein beta) and NFIC (nuclear factor I C). This interaction helps predict how advanced NSCLC patients will respond to anti-PD-1/PD-L1 immunotherapy, offering a new predictive biomarker [239]. These findings suggest that genetic variants modulating immune function may contribute to susceptibility to both cancer and hypertension.
Oxidative stress
Oxidative stress plays a critical role in hypertension due to the fundamental roles of ROS and redox signaling in molecular, cellular, and systemic processes that contribute to endothelial injury, vascular dysfunction, cardiovascular remodeling, renal impairment, sympathetic nervous system excitation, immune cell activation, and systemic inflammation—key factors in the pathophysiology of hypertension [240, 241]. ROS are among the many molecular contributors to hypertension progression. ROS, small molecules derived from molecular oxygen through redox reactions or electron excitation, include free radicals such as superoxide anion and non-radicals such as hydrogen peroxide. Due to their highly reactive nature, ROS promote the oxidation of proteins, DNA, and lipids, ultimately disrupting cellular function and viability [242]. In fact, studies have demonstrated that oxidative stress plays a causal role in the pathogenesis of hypertension across various animal models [243]. Evidence from experimental models indicates that oxidative stress and hypertension have a causal relationship [244, 245]. ROS are closely associated with hypertension’s major pathogenic features, including endothelial dysfunction, vascular hyperreactivity, vascular injury, arterial remodeling, renal impairment, sympathetic nervous system activation, inflammation, and immune cell activation [244, 246]. Moreover, treatment with antioxidants and ROS scavengers can sustainably reduce blood pressure in many experimental models of hypertension [245]. Oxidative stress is also linked to human hypertension. Population studies indicate increased systemic oxidative stress biomarkers in hypertensive patients, correlating oxidative stress with a higher risk of hypertension even in normotensive individuals [247]. A cross-sectional study involving 1,793 individuals with normal and hypertensive blood pressure found that the rs7770619 C > T polymorphism in the PPARD gene was closely associated with the oxidative stress biomarker malondialdehyde (MDA) in plasma, a risk factor for hypertension. Individuals with the CT genotype had lower hypertension risk, systolic blood pressure, blood glucose, and plasma MDA levels than those with the CC genotype, suggesting that the PPARD rs7770619 SNP could be a potential candidate gene for hypertension [248].
Most cancer risks, including lung cancer, arise from uncontrolled factors, such as environmental pollution, toxins, and free radicals, that lead to oxidative stress—often induced and accumulated by smoking. These factors, combined with environmental pollutants and toxins, contribute to genetic alterations [249]. Cigarette smoke is a primary cause of inflammatory cell recruitment, leading to alterations in inflammatory cytokine secretion, making individuals susceptible to lung cancer. A key factor in this process is the excessive accumulation of ROS caused by smoking. These radicals and ROS may result from moderate leakage in the mitochondrial electron transport chain, endoplasmic reticulum, and chloroplasts. The imbalance between ROS and the antioxidant defense system leads to oxidative stress within cells, oxidizing functional biomolecules, damaging cells, and causing tissue injury [249, 250]. Studies have identified three SNPs (rs1695, rs2333227, rs699512) in oxidative stress-related genes that are significantly associated with overall survival in advanced NSCLC patients treated with EGFR TKIs. These genetic variations in SNPs affect patient response to treatment, exhibiting a gene-dosage effect [251]. Additionally, the rs662 polymorphism in the PON1 gene shows a significant interaction between smoking, lung cancer risk, and oxidative stress. Individuals with the rs662 AA genotype have a lower lung cancer risk in non-smokers and lower levels of the oxidative stress marker 8-OHdG, suggesting that PON1 gene polymorphisms co-regulate lung cancer occurrence and oxidative stress levels in conjunction with environmental factors [252]. These findings indicate that oxidative stress-related genetic variations play an important role in lung cancer prognosis and hypertension.
Metabolic disorder
Metabolic dysregulation is a key mechanism in both hypertension and lung cancer, particularly involving energy metabolism, lipid metabolism, and glucose metabolism [253–258]. These metabolic pathways not only drive the progression of each disease independently but are also interconnected through shared biological mechanisms. In hypertension, patients often exhibit abnormalities in energy metabolism, particularly in vascular smooth muscle and endothelial cells. Mitochondrial dysfunction leads to insufficient ATP (adenosine triphosphate) production, affecting the balance of vascular contraction and relaxation. Energy deficits result in impaired smooth muscle cell contractility, reducing vascular regulation and leading to sustained vascular constriction and elevated blood pressure. Dysregulated glucose metabolism can also contribute to hypertension by promoting insulin resistance, which activates the sympathetic nervous system and increases norepinephrine secretion, further inducing vasoconstriction and raising blood pressure [258–260]. Moreover, lipid metabolism disorders are closely associated with hypertension, particularly elevations in cholesterol, triglycerides, and low-density lipoprotein. These lipids deposit on vascular walls, facilitating atherosclerotic plaque formation and increasing vascular resistance. Atherosclerosis reduces vascular elasticity, impairs blood flow, and contributes significantly to the development and progression of hypertension [261]. Metabolic imbalances, especially in lipid and glucose metabolism, are often accompanied by increased ROS, leading to oxidative stress that further damages the vascular endothelium, inhibits NO production, and impairs vascular regulation [262, 263]. For example, adipose tissue is closely linked to metabolic hypertension, and in obesity, excess free fatty acids and inflammatory adipokines from fat tissue damage the vascular endothelium, increasing blood pressure [264]. Obesity also promotes renal sodium reabsorption and activates the sympathetic and renin-angiotensin systems, raising blood pressure [265].
In lung cancer and other malignancies, cellular metabolism undergoes significant alterations, with the “Warburg effect” being the most prominent. Cancer cells rely on glycolysis for energy production, even in the presence of oxygen, rather than oxidative phosphorylation. This metabolic reprogramming supports rapid cancer cell proliferation, as glycolysis not only supplies energy but also provides metabolic intermediates necessary for cellular growth. This phenomenon enhances cancer cell proliferation and promotes tumor growth [266–268]. Lipid metabolism dysregulation also plays an important role in lung cancer. Cancer cells require large amounts of fatty acids to support cell membrane synthesis and signaling. The activation of lipid synthesis pathways accelerates cancer cell proliferation, while lipid peroxidation in metabolic processes can increase oxidative stress, promoting cancer progression [269, 270]. In addition to directly impacting cancer cells, metabolic changes affect the surrounding microenvironment. With the advent of new cancer therapies targeting the immune microenvironment, these metabolic changes may influence both cancer progression and therapeutic responses [271]. In small cell lung cancer (SCLC) patients, genetic variations in glutathione metabolism and DNA repair pathway genes have been significantly associated with survival. SNPs in genes such as GSS, ABCC2, and XRCC1 affect metabolic and DNA damage repair capacities, modulating patient response to treatment and impacting prognosis. Metabolic dysregulation plays a shared pathogenic role in hypertension and lung cancer, highlighting the potential for targeted metabolic interventions [272]. Interventions aimed at correcting metabolic imbalances, such as the use of antioxidants and metabolic regulators, may help improve outcomes in both diseases.
Critical synthesis of genetic, epigenetic, and environmental evidence
The comorbidity of hypertension and lung cancer emerges from a complex interplay of genetic predisposition, epigenetic regulation, and environmental exposures. While each of these domains has been extensively discussed individually, it is their convergence that underlies the shared pathophysiological mechanisms observed in clinical and molecular studies.
Genetic susceptibility plays a foundational role, with shared polymorphisms and risk loci—such as MALAT1, ALDH2, and JMJD3—implicated in both vascular and oncogenic pathways. These genes affect cellular functions ranging from vascular remodeling and immune modulation to metabolic reprogramming and tumor progression. Importantly, the expression and impact of these genetic factors are further modulated by epigenetic mechanisms such as DNA methylation and histone modifications [49, 53–55, 59, 273]. For instance, genes like KCNK3 and GAS5 demonstrate dual functionality in both blood pressure regulation and tumor suppression, highlighting epigenetic plasticity as a mediator of comorbid disease risk [69, 70, 274–276]. Environmental exposures such as PM2.5, smoking, and alcohol consumption act as external stressors that synergize with genetic and epigenetic mechanisms to amplify disease susceptibility. Many of these agents induce oxidative stress, inflammation, and immune dysregulation, thereby acting as convergent drivers of both hypertension and lung cancer. For instance, PM2.5 has been shown to contribute to endothelial dysfunction and DNA methylation changes, linking vascular injury with tumorigenic processes.
This convergence is further supported by overlapping biological pathways such as chronic inflammation, endothelial dysfunction, oxidative damage, and immune escape—pathways that are reinforced by both intrinsic (genetic/epigenetic) and extrinsic (environmental) inputs. The gene–environment–epigenome axis thus provides a compelling framework for understanding the molecular underpinnings of this comorbidity [12, 277–281]. This relationship is visually represented in Fig. 3. These findings highlight the synergistic effects of genetic and environmental factors, which can guide targeted clinical approaches. From a clinical perspective, individuals with high genetic risk profiles—particularly those exhibiting susceptibility to chronic inflammation or endothelial dysfunction—who are exposed to adverse environmental conditions should be considered priority candidates for early screening and preventive strategies. Such stratification may significantly improve outcomes in patients susceptible to both hypertension and lung cancer.
Fig. 3.
Schematic summary of environmental exposures and shared mechanisms between hypertension and lung cancer. This figure provides a schematic overview of the comorbidities of hypertension and lung cancer, illustrating the interplay of genetic factors and environmental exposures in driving shared biological mechanisms. Environmental exposures, including air pollution (e.g., PM2.5), radon, diet habits, occupational exposure, smoking, and other exposures, are depicted with corresponding icons and linked to their influence on disease development. Genetic factors contribute to susceptibility, while common biological mechanisms—such as vascular endothelial dysfunction, immune dysregulation, metabolic disorder, oxidative stress, and inflammation—form the central pathway connecting the two conditions. The diagram emphasizes the synergistic role of genetic, epigenetic, and environmental factors in the pathogenesis of this comorbidity
Conclusion
Hypertension and lung cancer are two of the most common and burdensome chronic diseases globally. Their coexistence poses complex challenges in both biomedical research and clinical practice, with mounting evidence suggesting shared pathogenic mechanisms. This review synthesizes current knowledge, highlighting shared genetic, epigenetic, and environmental factors as the underlying basis of these diseases. Mechanisms such as inflammation, oxidative stress, vascular dysfunction, and metabolic dysregulation are central to this comorbidity. These shared pathways not only elucidate the intricate interplay between hypertension and lung cancer but also provide potential avenues for integrated therapeutic strategies.
While some important scientific questions have been addressed, several remain unanswered. For example, the precise role of gene-environment interactions in the comorbidity of hypertension and lung cancer is not yet fully understood. Additionally, how external exposures such as smoking, air pollution, and dietary habits influence disease progression through molecular and cellular pathways remain poorly understood. In clinical terms, the comorbidity of hypertension and lung cancer significantly complicates disease management. Hypertension not only adds complexity to the pharmacological treatment of lung cancer but also contributes to poorer outcomes for affected patients. Personalized medicine approaches, driven by biomarkers and genetic risk profiles, may help optimize treatment strategies for this high-risk population.
The study of comorbidity between hypertension and lung cancer faces several challenges. First, the pathological mechanisms of these two diseases are markedly different. Hypertension primarily involves vascular dysfunction, chronic inflammation, and metabolic dysregulation, while lung cancer is driven by processes such as cell proliferation, genetic mutations, and immune evasion. Effectively integrating these disparate mechanisms and exploring their interactions in the context of comorbidity remains a significant research challenge. Secondly, there is considerable individual variability among patients, especially in those with both hypertension and lung cancer, leading to significant differences in disease presentation, progression rates, and treatment responses. This heterogeneity complicates clinical studies, making data analysis and the generalizability of results more difficult. Moreover, current animal models are limited in their ability to fully replicate the complex pathological state of hypertension and lung cancer comorbidity, which hampers experimental design and interpretation. Finally, the lack of long-term clinical follow-up data means that effective interventions and treatment strategies for managing the comorbidity of hypertension and lung cancer have not yet been fully validated. Addressing these challenges will require interdisciplinary collaboration and the development of more robust experimental and clinical research frameworks to advance our understanding and treatment of this comorbidity.
Future research should focus on integrating polygenic risk scores with environmental exposure metrics—such as long-term PM2.5 levels, radon, and occupational chemicals—to quantify cumulative disease risk. Additionally, identifying dual-function molecular regulators using large-scale population-based multi-omics datasets could enable the discovery of shared biomarkers and therapeutic targets. A second pressing need lies in developing robust in vivo and in vitro models to accurately simulate the comorbid disease state. Novel approaches such as organoids, vascularized tumor-on-chip models, and genetically engineered mouse models with inducible vascular and oncogenic mutations may better replicate the interplay between tumor biology and cardiovascular stress. Clinically, prospective cohort studies with detailed phenotyping and longitudinal follow-up are essential to evaluate how coexisting hypertension alters lung cancer progression, response to therapy (e.g., immunotherapy resistance), and overall prognosis. These studies should incorporate stratification by smoking status, sex, and treatment regimens to disentangle confounding effects. From a translational perspective, integrative biomarker panels combining genetic, epigenetic, and exposure data could enable early risk prediction and patient stratification. Artificial intelligence and machine learning models may facilitate the development of personalized screening and treatment strategies tailored to individuals with dual risk profiles. Public health efforts should also emphasize targeted education, early screening, and environmental control policies in high-risk regions—particularly those affected by combined air pollution and occupational exposures.
In conclusion, the association between hypertension and lung cancer is not only a critical focus of scientific research but also an urgent challenge for clinical practice. By advancing our understanding of the mechanisms underpinning their comorbidity, leveraging innovations in precision medicine, and fostering interdisciplinary collaboration, we can improve survival rates and quality of life for affected patients while mitigating the global health impact of these diseases.
Author contributions
Jingtong Zeng, Ying Chen conceived and designed this manuscript, Ying Chen obtained the funding. Wenxun Dong, Daqian He collected the data and prepared the figures. Difang Shi, Zhenghong Yang participated the discussion and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82060426; No. 82272980; No. 82360562); The “Key R&D Program” (Project No. 202403AC100015); The “First-Class Discipline Team Construction Project” of Kunming Medical University for the Environmental, Genetic, and Lung Cancer Pathogenesis Research Team (Project No. 2024KKTDPY07); First-Class Discipline Team of Kunming Medical University (2024XKTDYS07); Yunnan Provincial Chest Tumor Prevention and Treatment Research Innovation Team (No. 202405AS350015); Development of Precision Prevention and Full-Cycle Intelligent Management System for Regionally High-Incidence Lung Cancer in Yunnan (No. 202303AC100203).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingtong Zeng and Difang Shi have contributed equally to this work.
References
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: A systematic analysis of Population-Based studies from 90 countries. Circulation. 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidoguchi S, Sugano N, Tokudome G, Yokoo T, Yano Y, Hatake K, Nishiyama A. New concept of Onco-Hypertension and future perspectives. Hypertension. 2021;77:16–27. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Fu W, Shi O, Li D, Jiang Q, Wang T, Zhou X, Lu Z, Cao S. Impact of exposure to noise on the risk of hypertension: A systematic review and meta-analysis of cohort studies. Environ Res. 2021;195:110813. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. [DOI] [PubMed] [Google Scholar]
- 5.Barone-Adesi F, Chapman RS, Silverman DT, He X, Hu W, Vermeulen R, Ning B, Fraumeni JF Jr., Rothman N, Lan Q. Risk of lung cancer associated with domestic use of coal in xuanwei, china: retrospective cohort study. BMJ. 2012;345:e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Xi Y, Boffa DJ, Liu Y, Nogueira LM. Association of wildfire exposure while recovering from lung cancer surgery with overall survival. JAMA Oncol. 2023;9:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. [DOI] [PubMed] [Google Scholar]
- 8.Hollenberg NK. Genes, hypertension, and intermediate phenotypes. Curr Opin Cardiol. 1996;11:457–63. [DOI] [PubMed] [Google Scholar]
- 9.Hollenberg NK. A genome-wide search for susceptibility loci to human essential hypertension. Curr Hypertens Rep. 2001;3:7–8. [PubMed] [Google Scholar]
- 10.Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, de Geus EJ. Heritability and stability of resting blood pressure. Twin Res Hum Genet. 2005;8:499–508. [DOI] [PubMed] [Google Scholar]
- 11.Williams GH, Fisher ND. Genetic approach to diagnostic and therapeutic decisions in human hypertension. Curr Opin Nephrol Hypertens. 1997;6:199–204. [DOI] [PubMed] [Google Scholar]
- 12.Padmanabhan S, Joe B. Towards precision medicine for hypertension: A review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev. 2017;97:1469–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, Thorleifsson G, Luan J, Donnelly LA, Kanoni S, et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet. 2016;48:1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Sun Y, Kong X, Luan C, Yu Y, Chen F, Chen P. Association between a single nucleotide polymorphism in the 3’-UTR of ARHGEF18 and the risk of nonidiopathic pulmonary arterial hypertension in Chinese population. Dis Markers. 2018;2018:2461845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr SR, Akerley W, Cannon-Albright LA. Genetic contribution to nonsquamous, Non-Small cell lung cancer in nonsmokers. J Thorac Oncol. 2018;13:938–45. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Ma Z, Yang J, Zhao M, Ao H, Zheng X, Wen Q, Yang Y, You J, Qiao S, Yuan J. Prevalence and prognosis significance of cardiovascular disease in cancer patients: a population-based study. Aging. 2019;11:7948–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CD, Stechuchak KM, Zullig LL, Provenzale D, Kelley MJ. Influence of comorbidity on Racial differences in receipt of surgery among US veterans with early-stage non-small-cell lung cancer. J Clin Oncol. 2013;31:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiu W, Huang Y, Li Y, Yu M, Gong Y. Comorbidities and mortality risk among extensive-stage small-cell lung cancer patients in Mainland china: impacts of hypertension, type 2 diabetes mellitus, and chronic hepatitis B virus infection. Anticancer Drugs. 2022;33:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dima S, Chen KH, Wang KJ, Wang KM, Teng NC. Effect of Comorbidity on Lung Cancer Diagnosis Timing and Mortality: A Nationwide Population-Based Cohort Study in Taiwan. Biomed Res Int 2018, 2018:1252897. [DOI] [PMC free article] [PubMed]
- 21.Lindgren A, Pukkala E, Nissinen A, Tuomilehto J. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North karelia, Finland. Am J Epidemiol. 2003;158:442–7. [DOI] [PubMed] [Google Scholar]
- 22.Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, He J. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7:44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drab A, Kanadys W, Malm M, Wdowiak K, Dolar-Szczasny J, Barczyński B. Association of endometrial cancer risk with hypertension- an updated meta-analysis of observational studies. Sci Rep. 2024;14:24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox NS, Amit U, Reibel JB, Berlin E, Howell K, Ky B. Cardiovascular disease and cancer: shared risk factors and mechanisms. Nat Rev Cardiol. 2024;21:617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasawa H, Kaneko H, Suzuki Y, Okada A, Fujiu K, Takeda N, Morita H, Nishiyama A, Yano Y, Node K, et al. Association of cancer with the risk of developing hypertension. Eur Heart J Qual Care Clin Outcomes. 2024;10:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindgren A, Pukkala E, Nissinen A, Kataja V, Notkola IL, Tuomilehto J. Cancer incidence in hypertensive patients in North karelia, Finland. Hypertension. 2001;37:1251–5. [DOI] [PubMed] [Google Scholar]
- 27.van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ, Lang NN. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128:1040–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding JL, Sooriyakumaran M, Anstey KJ, Adams R, Balkau B, Brennan-Olsen S, Briffa T, Davis TM, Davis WA, Dobson A, et al. Hypertension, antihypertensive treatment and cancer incidence and mortality: a pooled collaborative analysis of 12 Australian and new Zealand cohorts. J Hypertens. 2016;34:149–55. [DOI] [PubMed] [Google Scholar]
- 29.Abi Aad S, Pierce M, Barmaimon G, Farhat FS, Benjo A, Mouhayar E. Hypertension induced by chemotherapeutic and immunosuppresive agents: a new challenge. Crit Rev Oncol Hematol. 2015;93:28–35. [DOI] [PubMed] [Google Scholar]
- 30.Katsi V, Magkas N, Georgiopoulos G, Athanasiadi E, Virdis A, Masi S, Kliridis P, Hatziyanni A, Tsioufis C, Tousoulis D. Arterial hypertension in patients under antineoplastic therapy: a systematic review. J Hypertens. 2019;37:884–901. [DOI] [PubMed] [Google Scholar]
- 31.Silvestry FE, St John Sutton MG. Sustained-release calcium channel antagonists in cardiovascular disease: Pharmacology and current therapeutic use. Eur Heart J 1998, 19 Suppl I:I8–14. [PubMed]
- 32.Eisenberg MJ, Brox A, Bestawros AN. Calcium channel blockers: an update. Am J Med. 2004;116:35–43. [DOI] [PubMed] [Google Scholar]
- 33.Fu B, Dou X, Zou M, Lu H, Wang K, Liu Q, Liu Y, Wang W, Jin M, Kong D. Anticancer effects of amlodipine alone or in combination with gefitinib in Non-Small cell lung cancer. Front Pharmacol. 2022;13:902305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey RM, Whelton PK. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American college of cardiology/american heart association hypertension guideline. Ann Intern Med. 2018;168:351–8. [DOI] [PubMed] [Google Scholar]
- 35.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, et al. 2016 ACC/AHA/HFSA focused update on new Pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2016;134:e282–293.27208050 [Google Scholar]
- 36.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of Candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. [DOI] [PubMed] [Google Scholar]
- 37.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor Blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikane S, Hosoda H, Nojiri T, Tokudome T, Mizutani T, Miura K, Akitake Y, Kimura T, Imamichi Y, Kawabe S, et al. Angiotensin II promotes pulmonary metastasis of melanoma through the activation of adhesion molecules in vascular endothelial cells. Biochem Pharmacol. 2018;154:136–47. [DOI] [PubMed] [Google Scholar]
- 39.Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M, Franklin W, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004;75:460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang L, Chan CPY, Yau WP, Seow WJ. Association between family history of lung cancer and lung cancer risk: a systematic review and meta-analysis. Lung Cancer. 2020;148:129–37. [DOI] [PubMed] [Google Scholar]
- 41.Wang CL, Hsu KH, Chang YH, Ho CC, Chiang CJ, Chen KC, Cheung YC, Huang PC, Chen YR, Chen CY, et al. Low-Dose computed tomography screening in relatives with a family history of lung cancer. J Thorac Oncol. 2023;18:1492–503. [DOI] [PubMed] [Google Scholar]
- 42.Coté ML, Liu M, Bonassi S, Neri M, Schwartz AG, Christiani DC, Spitz MR, Muscat JE, Rennert G, Aben KK, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the international lung cancer consortium. Eur J Cancer. 2012;48:1957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein IB, Shapiro D, Guthrie D. Ambulatory blood pressure and family history of hypertension in healthy men and women. Am J Hypertens. 2006;19:486–91. [DOI] [PubMed] [Google Scholar]
- 44.Valerio L, Peters RJ, Zwinderman AH, Pinto-Sietsma SJ. Association of family history with cardiovascular disease in hypertensive individuals in a multiethnic population. J Am Heart Assoc 2016, 5. [DOI] [PMC free article] [PubMed]
- 45.Li C, Sun D, Liu J, Li M, Zhang B, Liu Y, Wang Z, Wen S, Zhou J. A prediction model of essential hypertension based on genetic and environmental risk factors in Northern Han Chinese. Int J Med Sci. 2019;16:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of Tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz AG, Cote ML, Wenzlaff AS, Land S, Amos CI. Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. J Thorac Oncol. 2009;4:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer. 2017;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mou X, Wang J, Wang L, Wang S. Correlation between single nucleotide polymorphisms of the rs664589 locus in the Long-Chain noncoding RNA lung adenocarcinoma Metastasis-Associated gene 1, hypertension, and its mechanism. Genet Test Mol Biomarkers. 2020;24:120–30. [DOI] [PubMed] [Google Scholar]
- 51.Minegishi Y, Tsukino H, Muto M, Goto K, Gemma A, Tsugane S, Kudoh S, Nishiwaki Y, Esumi H. Susceptibility to lung cancer and genetic polymorphisms in the alcohol metabolite-related enzymes alcohol dehydrogenase 3, aldehyde dehydrogenase 2, and cytochrome P450 2E1 in the Japanese population. Cancer. 2007;110:353–62. [DOI] [PubMed] [Google Scholar]
- 52.Ma XX, Zheng SZ, Shu Y, Wang Y, Chen XP. Association between carotid Intima-media thickness and aldehyde dehydrogenase 2 Glu504Lys polymorphism in Chinese Han with essential hypertension. Chin Med J (Engl). 2016;129:1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuchihashi-Makaya M, Serizawa M, Yanai K, Katsuya T, Takeuchi F, Fujioka A, Yamori Y, Ogihara T, Kato N. Gene-environmental interaction regarding alcohol-metabolizing enzymes in the Japanese general population. Hypertens Res. 2009;32:207–13. [DOI] [PubMed] [Google Scholar]
- 54.Ota M, Hisada A, Lu X, Nakashita C, Masuda S, Katoh T. Associations between aldehyde dehydrogenase 2 (ALDH2) genetic polymorphisms, drinking status, and hypertension risk in Japanese adult male workers: a case-control study. Environ Health Prev Med. 2016;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangum KD, Li Q, Hartmann K, Bauer TM, Wolf SJ, Shadiow J, Moon JY, Barrett E, Joshi A, de Saldana G et al. Epigenetic alteration of smooth muscle cells regulates endothelin-dependent blood pressure and hypertensive arterial remodeling. J Clin Invest 2025. [DOI] [PMC free article] [PubMed]
- 56.Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, Torstenson ES, Kovesdy CP, Sun YV, Wilson OD, et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meor Azlan NF, Koeners MP, Zhang J. Regulatory control of the Na-Cl co-transporter NCC and its therapeutic potential for hypertension. Acta Pharm Sin B. 2021;11:1117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SH, Kim O, Kim HJ, Hwangbo C, Lee JH. Epigenetic regulation of TGF-beta-induced EMT by JMJD3/KDM6B histone H3K27 demethylase. Oncogenesis. 2021;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez A, Houfaf Khoufaf FZ, Idrissou M, Penault-Llorca F, Bignon YJ, Guy L, Bernard-Gallon D. The functions of the demethylase JMJD3 in cancer. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed]
- 60.Yang J, Wang X, Huang B, Liu R, Xiong H, Ye F, Zeng C, Fu X, Li L. An IFNgamma/STAT1/JMJD3 axis induces ZEB1 expression and promotes aggressiveness in lung adenocarcinoma. Mol Cancer Res. 2021;19:1234–46. [DOI] [PubMed] [Google Scholar]
- 61.Xun J, Du L, Gao R, Shen L, Wang D, Kang L, Chen C, Zhang Z, Zhang Y, Yue S, et al. Cancer-derived Exosomal miR-138-5p modulates polarization of tumor-associated macrophages through Inhibition of KDM6B. Theranostics. 2021;11:6847–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776–800. [DOI] [PubMed] [Google Scholar]
- 63.van der Harst P, de Windt LJ, Chambers JC. Translational perspective on epigenetics in cardiovascular disease. J Am Coll Cardiol. 2017;70:590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kresovich JK, Sandler DP, Taylor JA. Methylation-Based biological age and hypertension prevalence and incidence. Hypertension. 2023;80:1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang M. Epigenetic mechanisms and hypertension. Hypertension. 2018;72:1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong X, Miao K, Cao W, Lv J, Yu C, Huang T, Sun D, Liao C, Pang Y, Pang Z, et al. Association between DNA methylation and blood pressure: A 5-Year longitudinal twin study. Hypertension. 2023;80:169–81. [DOI] [PubMed] [Google Scholar]
- 68.Pandey KN. Genetic and epigenetic mechanisms regulating blood pressure and kidney dysfunction. Hypertension. 2024;81:1424–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang D, Shang W, Xu M, Wan Q, Zhang J, Tang X, Shen Y, Wang Y, Yu Y. Genome-Wide methylation analysis reveals a KCNK3-Prominent causal cascade on hypertension. Circ Res. 2024;135:e76–93. [DOI] [PubMed] [Google Scholar]
- 70.Lin G, Lin L, Lin H, Chen W, Chen L, Chen X, Chen S, Lin Q, Xu Y, Zeng Y. KCNK3 inhibits proliferation and glucose metabolism of lung adenocarcinoma via activation of AMPK-TXNIP pathway. Cell Death Discov. 2022;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DH, Kim JS, Ji YI, Shim YM, Kim H, Han J, Park J. Hypermethylation of RASSF1A promoter is associated with the age at starting smoking and a poor prognosis in primary non-small cell lung cancer. Cancer Res. 2003;63:3743–6. [PubMed] [Google Scholar]
- 72.Schmidt ML, Hobbing KR, Donninger H, Clark GJ. RASSF1A deficiency enhances RAS-Driven lung tumorigenesis. Cancer Res. 2018;78:2614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JS, Kim JW, Han J, Shim YM, Park J, Kim DH. Cohypermethylation of p16 and FHIT promoters as a prognostic factor of recurrence in surgically resected stage I non-small cell lung cancer. Cancer Res. 2006;66:4049–54. [DOI] [PubMed] [Google Scholar]
- 74.Toyooka S, Matsuo K, Gazdar AF. DNA methylation in lung cancer. N Engl J Med. 2008;358:2513. author reply 2514. [DOI] [PubMed] [Google Scholar]
- 75.Welch CL, Chung WK. Channelopathy genes in pulmonary arterial hypertension. Biomolecules 2022, 12. [DOI] [PMC free article] [PubMed]
- 76.Pedro Ferreira J, Pitt B, Zannad F. Histone deacetylase inhibitors for cardiovascular conditions and healthy longevity. Lancet Healthy Longev. 2021;2:e371–9. [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Wang Z, Wu J, Liu M, Li M, Sun Y, Huang W, Li Y, Zhang Y, Tang W, et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 signaling. Circ Res. 2019;124:1448–61. [DOI] [PubMed] [Google Scholar]
- 78.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. HDAC Inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi J, Park S, Kwon TK, Sohn SI, Park KM, Kim JI. Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via Inhibition of HDAC1/angiotensin II axis. Int J Obes (Lond). 2017;41:1702–9. [DOI] [PubMed] [Google Scholar]
- 80.Shetty MG, Pai P, Deaver RE, Satyamoorthy K, Babitha KS. Histone deacetylase 2 selective inhibitors: A versatile therapeutic strategy as next generation drug target in cancer therapy. Pharmacol Res. 2021;170:105695. [DOI] [PubMed] [Google Scholar]
- 81.Shirsath N, Rathos M, Chaudhari U, Sivaramakrishnan H, Joshi K. Potentiation of anticancer effect of valproic acid, an antiepileptic agent with histone deacetylase inhibitory activity, by the cyclin-dependent kinase inhibitor P276-00 in human non-small-cell lung cancer cell lines. Lung Cancer. 2013;82:214–21. [DOI] [PubMed] [Google Scholar]
- 82.Frankel AE, Liu X, Minna JD. Developing EZH2-Targeted therapy for lung cancer. Cancer Discov. 2016;6:949–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rusek AM, Abba M, Eljaszewicz A, Moniuszko M, Niklinski J, Allgayer H. MicroRNA modulators of epigenetic regulation, the tumor microenvironment and the immune system in lung cancer. Mol Cancer. 2015;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weber B, Stresemann C, Brueckner B, Lyko F. Methylation of human MicroRNA genes in normal and neoplastic cells. Cell Cycle. 2007;6:1001–5. [DOI] [PubMed] [Google Scholar]
- 85.Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M, Allgayer H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res. 2010;8:1207–16. [DOI] [PubMed] [Google Scholar]
- 86.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiao X, Yu H, Du Z, Li L, Hu C, Du Y, Zhang J, Zhang X, Lv Q, Li F, et al. Vascular smooth muscle cells specific deletion of angiopoietin-like protein 8 prevents angiotensin II-promoted hypertension and cardiovascular hypertrophy. Cardiovasc Res. 2023;119:1856–68. [DOI] [PubMed] [Google Scholar]
- 88.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. [DOI] [PubMed] [Google Scholar]
- 89.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117:870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dimitrova N, Gocheva V, Bhutkar A, Resnick R, Jong RM, Miller KM, Bendor J, Jacks T. Stromal expression of miR-143/145 promotes neoangiogenesis in lung cancer development. Cancer Discov. 2016;6:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bátkai S, Thum T. MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep. 2012;14:79–87. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, Li C, Liu H, Wang J. Circ_0001821 knockdown suppresses growth, metastasis, and TAX resistance of non-small-cell lung cancer cells by regulating the miR-526b-5p/GRK5 axis. Pharmacol Res Perspect. 2021;9:e00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang B, Yao L, Yang L, Zhao F, Zhou W. Inhibition of miR-578 through SOCS2-dependent manner reverses gefitinib resistance in NSCLC cells. Environ Toxicol. 2024;39:1283–93. [DOI] [PubMed] [Google Scholar]
- 94.Yang B, Zhao F, Yao L, Zong Z, Xiao L. CircRNA circ_0006677 inhibits the progression and Glycolysis in Non-Small-Cell lung cancer by sponging miR-578 and regulating SOCS2 expression. Front Pharmacol. 2021;12:657053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui S, Peng Q, Ma Q, Xu X, Zhang W, Jiang X, Tan S, Yang W, Han Y, Oyang L, et al. Crosstalk between RNA-binding proteins and non-coding RNAs in tumors: molecular mechanisms, and clinical significance. Int J Biol Sci. 2025;21:2991–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360–73. [DOI] [PubMed] [Google Scholar]
- 97.Yu B, Wang S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics. 2018;8:3654–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zahid KR, Raza U, Chen J, Raj UJ, Gou D. Pathobiology of pulmonary artery hypertension: role of long non-coding RNAs. Cardiovasc Res. 2020;116:1937–47. [DOI] [PubMed] [Google Scholar]
- 99.Cao Z, Oyang L, Luo X, Xia L, Hu J, Lin J, Tan S, Tang Y, Zhou Y, Cao D, Liao Q. The roles of long non-coding RNAs in lung cancer. J Cancer. 2022;13:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Omura J, Habbout K, Shimauchi T, Wu WH, Breuils-Bonnet S, Tremblay E, Martineau S, Nadeau V, Gagnon K, Mazoyer F, et al. Identification of long noncoding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020;142:1464–84. [DOI] [PubMed] [Google Scholar]
- 101.Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, Tsao MS, Penn LZ. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006;66:5330–7. [DOI] [PubMed] [Google Scholar]
- 102.Wang YN, Shan K, Yao MD, Yao J, Wang JJ, Li X, Liu B, Zhang YY, Ji Y, Jiang Q, Yan B. Long noncoding RNA-GAS5: A novel regulator of Hypertension-Induced vascular remodeling. Hypertension. 2016;68:736–48. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Y, Dai X, Yang T, Zhang N, Liu Z, Jiang Y. Low long noncoding RNA growth Arrest-Specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J Oncol. 2019;2019:2476175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lambrou GI, Hatziagapiou K, Zaravinos A. The Non-Coding RNA GAS5 and its role in tumor Therapy-Induced resistance. Int J Mol Sci 2020, 21. [DOI] [PMC free article] [PubMed]
- 105.Ghafouri-Fard S, Shirvani-Farsani Z, Hussen BM, Taheri M, Samsami M. The key roles of non-coding RNAs in the pathophysiology of hypertension. Eur J Pharmacol. 2022;931:175220. [DOI] [PubMed] [Google Scholar]
- 106.Wu Y, Lyu H, Liu H, Shi X, Song Y, Liu B. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci Rep. 2016;6:31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang W, Zhu X, Bo J, Ma J. Screening of Immune-related LncRNAs in lung adenocarcinoma and Establishing a survival prognostic risk prediction model. Comb Chem High Throughput Screen. 2024;27:1175–90. [DOI] [PubMed] [Google Scholar]
- 108.Xue Q, Wang Y, Zheng Q, Chen L, Jin Y, Shen X, Li Y. Construction of a prognostic immune-related LncRNA model and identification of the immune microenvironment in middle- or advanced-stage lung squamous carcinoma patients. Heliyon. 2022;8:e09521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan C, Zhang H, Yu D, Hu Y, Wang P, Wang D, Fa J, Ran H, Zhang X, Chen Y, et al. A genome-wide association study identifies novel association between genetic variants in GGT7 and LINC00944 and hypertension. Clin Transl Med. 2021;11:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li K, Gong Q, Xiang XD, Guo G, Liu J, Zhao L, Li J, Chen N, Li H, Zhang LJ, et al. HNRNPA2B1-mediated m(6)A modification of LncRNA MEG3 facilitates tumorigenesis and metastasis of non-small cell lung cancer by regulating miR-21-5p/PTEN axis. J Transl Med. 2023;21:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xing Y, Zheng X, Fu Y, Qi J, Li M, Ma M, Wang S, Li S, Zhu D. Long noncoding RNA-Maternally expressed gene 3 contributes to hypoxic pulmonary hypertension. Mol Ther. 2019;27:2166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zou J, Li Y, Yan CH, Wei FF, Zhang L, Wang JG. Blood pressure in relation to interactions between sodium dietary intake and renal handling. Hypertension. 2013;62:719–25. [DOI] [PubMed] [Google Scholar]
- 113.Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–e345322. [DOI] [PubMed] [Google Scholar]
- 114.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra215. [DOI] [PubMed] [Google Scholar]
- 115.Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, Bangalore S, Newman JD, Ahmed A, Islam T, et al. Association between arsenic exposure from drinking water and longitudinal change in blood pressure among HEALS cohort participants. Environ Health Perspect. 2015;123:806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environ Health Perspect. 2017;125:087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hall EM, Acevedo J, López FG, Cortés S, Ferreccio C, Smith AH, Steinmaus CM. Hypertension among adults exposed to drinking water arsenic in Northern Chile. Environ Res. 2017;153:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang SL, Li WF, Chen CJ, Huang YL, Chen JW, Chang KH, Tsai LY, Chou KM. Hypertension incidence after tap-water implementation: a 13-year follow-up study in the arseniasis-endemic area of Southwestern Taiwan. Sci Total Environ. 2011;409:4528–35. [DOI] [PubMed] [Google Scholar]
- 119.Blot WJ, Fraumeni JF Jr. Arsenical air pollution and lung cancer. Lancet. 1975;2:142–4. [DOI] [PubMed] [Google Scholar]
- 120.Mead MN. Arsenic: in search of an antidote to a global poison. Environ Health Perspect. 2005;113:A378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo HR, Wang NS, Hu H, Monson RR. Cell type specificity of lung cancer associated with arsenic ingestion. Cancer Epidemiol Biomarkers Prev. 2004;13:638–43. [PubMed] [Google Scholar]
- 123.Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci. 2006;91:372–81. [DOI] [PubMed] [Google Scholar]
- 124.Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386:263–77. [DOI] [PubMed] [Google Scholar]
- 125.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94:10907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jensen TJ, Novak P, Eblin KE, Gandolfi AJ, Futscher BW. Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis. 2008;29:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaplan NM. Alcohol and hypertension. Lancet. 1995;345:1588–9. [DOI] [PubMed] [Google Scholar]
- 129.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–24. [DOI] [PubMed] [Google Scholar]
- 130.Wallace RB, Lynch CF, Pomrehn PR, Criqui MH, Heiss G. Alcohol and hypertension: epidemiologic and experimental considerations. Lipid Res Clin Program Circulation. 1981;64:Iii41–47. [PubMed] [Google Scholar]
- 131.Thompson A, Cook J, Choquet H, Jorgenson E, Yin J, Kinnunen T, Barclay J, Morris AP, Pirmohamed M. Functional validity, role, and implications of heavy alcohol consumption genetic loci. Sci Adv. 2020;6:eaay5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biddinger KJ, Emdin CA, Haas ME, Wang M, Hindy G, Ellinor PT, Kathiresan S, Khera AV, Aragam KG. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5:e223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harris E. Even without hypertension, daily alcohol May increase blood pressure. JAMA. 2023;330:797. [DOI] [PubMed] [Google Scholar]
- 134.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. [DOI] [PubMed] [Google Scholar]
- 135.Álvarez-Avellón SM, Fernández-Somoano A, Navarrete-Muñoz EM, Vioque J, Tardón A. Effect of alcohol and its metabolites in lung cancer: CAPUA study. Med Clin (Barc). 2017;148:531–8. [DOI] [PubMed] [Google Scholar]
- 136.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008;66:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18:667–77. [DOI] [PubMed] [Google Scholar]
- 139.Young RP, Whittington CF, Hopkins RJ, Hay BA, Epton MJ, Black PN, Gamble GD. Chromosome 4q31 locus in COPD is also associated with lung cancer. Eur Respir J. 2010;36:1375–82. [DOI] [PubMed] [Google Scholar]
- 140.Beau-Faller M, Prim N, Ruppert AM, Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet JL, Rouquette I, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burnett H, Emich H, Carroll C, Stapleton N, Mahadevia P, Li T. Epidemiological and clinical burden of EGFR exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS ONE. 2021;16:e0247620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, Jackman DM, Johnson BE, Jänne PA. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.LoPiccolo J, Gusev A, Christiani DC, Jänne PA. Lung cancer in patients who have never smoked - an emerging disease. Nat Rev Clin Oncol. 2024;21:121–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu X, Wang L, Ye Y, Aakre JA, Pu X, Chang GC, Yang PC, Roth JA, Marks RS, Lippman SM, et al. Genome-wide association study of genetic predictors of overall survival for non-small cell lung cancer in never smokers. Cancer Res. 2013;73:4028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abe M, Nakura J, Yamamoto M, Jin JJ, Wu Z, Tabara Y, Yamamoto Y, Igase M, Kohara K, Miki T. Association of GNAS1 gene variant with hypertension depending on smoking status. Hypertension. 2002;40:261–5. [DOI] [PubMed] [Google Scholar]
- 146.Montasser ME, Shimmin LC, Hanis CL, Boerwinkle E, Hixson JE. Gene by smoking interaction in hypertension: identification of a major quantitative trait locus on chromosome 15q for systolic blood pressure in Mexican-Americans. J Hypertens. 2009;27:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–42. [DOI] [PubMed] [Google Scholar]
- 148.Cheng I, Yang J, Tseng C, Wu J, Shariff-Marco S, Park SL, Conroy SM, Inamdar PP, Fruin S, Larson T, et al. Traffic-related air pollution and lung cancer incidence: the California multiethnic cohort study. Am J Respir Crit Care Med. 2022;206:1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang Y, Zhu M, Ji M, Fan J, Xie J, Wei X, Jiang X, Xu J, Chen L, Yin R, et al. Air pollution, genetic factors, and the risk of lung cancer: A prospective study in the UK biobank. Am J Respir Crit Care Med. 2021;204:817–25. [DOI] [PubMed] [Google Scholar]
- 150.Schwarz M, Peters A, Stafoggia M, de’Donato F, Sera F, Bell ML, Guo Y, Honda Y, Huber V, Jaakkola JJK, et al. Temporal variations in the short-term effects of ambient air pollution on cardiovascular and respiratory mortality: a pooled analysis of 380 urban areas over a 22-year period. Lancet Planet Health. 2024;8:e657–65. [DOI] [PubMed] [Google Scholar]
- 151.Fan R, Xu L, Cui B, Li D, Sun X, Qi Y, Rao J, Wang K, Wang C, Zhao K, et al. Genomic characterization revealed PM(2.5)-Associated mutational signatures in lung cancer including activation of APOBEC3B. Environ Sci Technol. 2023;57:6854–64. [DOI] [PubMed] [Google Scholar]
- 152.Harris E. Research shows air pollution promotes lung cancer. JAMA. 2023;329:1543. [DOI] [PubMed] [Google Scholar]
- 153.Gourd E. New evidence that air pollution contributes substantially to lung cancer. Lancet Oncol. 2022;23:e448. [DOI] [PubMed] [Google Scholar]
- 154.Rajasekar P, Hall RJ, Binaya KC, Mahapatra PS, Puppala SP, Thakker D, MacIsaac JL, Lin D, Kobor M, Bolton CE, et al. Nepalese indoor cookstove smoke extracts alter human airway epithelial gene expression, DNA methylation and hydroxymethylation. Environ Pollut. 2023;337:122561. [DOI] [PubMed] [Google Scholar]
- 155.Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, Jamner L, Sioutas C, Longhurst J. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, Zeng W, Li X, Tao J, Yang Z, et al. Associations of Short-Term and Long-Term exposure to ambient air pollutants with hypertension: A systematic review and Meta-Analysis. Hypertension. 2016;68:62–70. [DOI] [PubMed] [Google Scholar]
- 158.Blechter B, Cardenas A, Shi J, Wong JYY, Hu W, Rahman ML, Breeze C, Downward GS, Portengen L, Zhang Y, et al. Household air pollution and epigenetic aging in xuanwei, China. Environ Int. 2023;178:108041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shan X, Tian X, Wang B, He L, Zhang L, Xue B, Liu C, Zheng L, Yu Y, Luo B. A global burden assessment of lung cancer attributed to residential radon exposure during 1990–2019. Indoor Air. 2022;32:e13120. [DOI] [PubMed] [Google Scholar]
- 160.Richardson DB, Rage E, Demers PA, Do MT, Fenske N, Deffner V, Kreuzer M, Samet J, Bertke SJ, Kelly-Reif K, et al. Lung cancer and radon: pooled analysis of uranium miners hired in 1960 or later. Environ Health Perspect. 2022;130:57010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martin-Gisbert L, Ruano-Ravina A, Garcia G, Pineiro-Lamas M, Garcia-Talavera M, Teijeiro A, Candal-Pedreira C. Duration versus intensity of exposure on the risk of lung cancer due to radon exposure in the general population. Sci Total Environ. 2025;981:179569. [DOI] [PubMed] [Google Scholar]
- 162.Lee MS, Eum KD, Li L, Iafrate J, Lanuti M, Koutrakis P, Christiani DC. Ambient beta particle radioactivity and lung cancer survival: results from the Boston lung cancer study. Environ Res. 2025;264:120307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou K, Sun P, Huang H, Zhuo H, Qie R, Xie Y, Luo J, Li N, Li J, He J, et al. Etiology of lung cancer: evidence from epidemiologic studies. J Natl Cancer Cent. 2022;2:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Papatheodorou S, Yao W, Vieira CLZ, Li L, Wylie BJ, Schwartz J, Koutrakis P. Residential radon exposure and hypertensive disorders of pregnancy in massachusetts, USA: A cohort study. Environ Int. 2021;146:106285. [DOI] [PubMed] [Google Scholar]
- 165.Liu Y, Xu Y, Xu W, He Z, Fu C, Du F. Radon and lung cancer: current status and future prospects. Crit Rev Oncol Hematol. 2024;198:104363. [DOI] [PubMed] [Google Scholar]
- 166.Riudavets M, Garcia de Herreros M, Besse B, Mezquita L. Radon and lung cancer: current trends and future perspectives. Cancers (Basel) 2022, 14. [DOI] [PMC free article] [PubMed]
- 167.Angley M, Zhang Y, Koutrakis P, Kahe K. Exposure to radon and ambient particle radioactivity during pregnancy and adverse maternal, fetal and perinatal outcomes: the current literature and potential mechanisms. Environ Res. 2024;263:120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fitzgerald SM. Resolving asbestos and ultrafine particulate definitions with carcinogenicity. Lung Cancer. 2024;189:107478. [DOI] [PubMed] [Google Scholar]
- 169.Frank AL, van Zandwijk N. Asbestos history and use. Lung Cancer. 2024;193:107828. [DOI] [PubMed] [Google Scholar]
- 170.Metintas M, Ak G, Metintas S. Environmental asbestos exposure and lung cancer. Lung Cancer. 2024;194:107850. [DOI] [PubMed] [Google Scholar]
- 171.Klebe S, Rathi V, Russell PA. Lung cancer caused by asbestos: what a reporting pathologist needs to know. Lung Cancer. 2024;195:107849. [DOI] [PubMed] [Google Scholar]
- 172.Jarvholm B, Burdorf A. Asbestos and disease - a public health success story? Scand J Work Environ Health. 2024;50:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rong Y, Luo X, Zhang Z, Cui X, Liu Y, Chen W. Occupational exposure to asbestos and cardiovascular related diseases: A meta-analysis. Prev Med Rep. 2015;2:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harding AH, Darnton A, Osman J. Cardiovascular disease mortality among British asbestos workers (1971–2005). Occup Environ Med. 2012;69:417–21. [DOI] [PubMed] [Google Scholar]
- 175.Toren K, Bergdahl IA, Nilsson T, Jarvholm B. Occupational exposure to particulate air pollution and mortality due to ischaemic heart disease and cerebrovascular disease. Occup Environ Med. 2007;64:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boor P, Casper S, Celec P, Hurbankova M, Beno M, Heidland A, Amann K, Sebekova K. Renal, vascular and cardiac fibrosis in rats exposed to passive smoking and industrial dust fibre Amosite. J Cell Mol Med. 2009;13:4484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, Kleeberger SR. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part Fibre Toxicol. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kang MS, Chae WR, Lee YJ, Moon KW. Occupational and environmental asbestos exposure and survival of patients with asbestos-Related cancer: A Follow-Up study on patients with malignant mesothelioma and asbestos-Related lung cancer in Korea. Toxics 2023, 12. [DOI] [PMC free article] [PubMed]
- 179.Ferro CJ, Webb DJ. Endothelial dysfunction and hypertension. Drugs. 1997;53(Suppl 1):30–41. [DOI] [PubMed] [Google Scholar]
- 180.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–46. [DOI] [PubMed] [Google Scholar]
- 181.Lüscher TF. Heterogeneity of endothelial dysfunction in hypertension. Eur Heart J. 1992;13(Suppl D):50–5. [DOI] [PubMed] [Google Scholar]
- 182.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol. 1999;31:61–74. [DOI] [PubMed] [Google Scholar]
- 183.Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–26. [DOI] [PubMed] [Google Scholar]
- 184.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aboukhater D, Morad B, Nasrallah N, Nasser SA, Sahebkar A, Kobeissy F, Boudaka A, Eid AH. Inflammation and hypertension: underlying mechanisms and emerging Understandings. J Cell Physiol. 2023;238:1148–59. [DOI] [PubMed] [Google Scholar]
- 187.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–88. [DOI] [PubMed] [Google Scholar]
- 188.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74. [DOI] [PubMed] [Google Scholar]
- 189.Houle F, Huot J. Dysregulation of the endothelial cellular response to oxidative stress in cancer. Mol Carcinog. 2006;45:362–7. [DOI] [PubMed] [Google Scholar]
- 190.Hsu T, Nguyen-Tran HH, Trojanowska M. Active roles of dysfunctional vascular endothelium in fibrosis and cancer. J Biomed Sci. 2019;26:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Duffy JP, Eibl G, Reber HA, Hines OJ. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer. 2003;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–73. [DOI] [PubMed] [Google Scholar]
- 193.Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. [DOI] [PubMed] [Google Scholar]
- 195.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- 196.Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–41. [DOI] [PubMed] [Google Scholar]
- 197.Dua RS, Gui GP, Isacke CM. Endothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systems. Eur J Surg Oncol. 2005;31:824–32. [DOI] [PubMed] [Google Scholar]
- 198.Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–7. [DOI] [PubMed] [Google Scholar]
- 200.Blazejczyk A, Papiernik D, Porshneva K, Sadowska J, Wietrzyk J. Endothelium and cancer metastasis: perspectives for antimetastatic therapy. Pharmacol Rep. 2015;67:711–8. [DOI] [PubMed] [Google Scholar]
- 201.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. [DOI] [PubMed] [Google Scholar]
- 202.Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. 2024;21:396–416. [DOI] [PubMed] [Google Scholar]
- 203.Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477–82. [DOI] [PubMed] [Google Scholar]
- 205.Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, Guzik TJ, Hingorani AD, Nart J, D’Aiuto F. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. [DOI] [PubMed] [Google Scholar]
- 206.Abramson JL, Lewis C, Murrah NV, Anderson GT, Vaccarino V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability in healthy adults. Am J Cardiol. 2006;98:649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Abramson JL, Weintraub WS, Vaccarino V. Association between pulse pressure and C-reactive protein among apparently healthy US adults. Hypertension. 2002;39:197–202. [DOI] [PubMed] [Google Scholar]
- 208.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–54. [DOI] [PubMed] [Google Scholar]
- 209.Sesso HD, Jiménez MC, Wang L, Ridker PM, Buring JE, Gaziano JM. Plasma inflammatory markers and the risk of developing hypertension in men. J Am Heart Assoc. 2015;4:e001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171:5589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rabkin SW. The role of Interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med. 2009;6:192–9. [DOI] [PubMed] [Google Scholar]
- 212.Thomas JM, Ling YH, Huuskes B, Jelinic M, Sharma P, Saini N, Ferens DM, Diep H, Krishnan SM, Kemp-Harper BK, et al. IL-18 (Interleukin-18) produced by renal tubular epithelial cells promotes renal inflammation and injury during Deoxycorticosterone/Salt-Induced hypertension in mice. Hypertension. 2021;78:1296–309. [DOI] [PubMed] [Google Scholar]
- 213.Zhang H, Yang K, Chen F, Liu Q, Ni J, Cao W, Hua Y, He F, Liu Z, Li L, Fan G. Role of the CCL2-CCR2 axis in cardiovascular disease: pathogenesis and clinical implications. Front Immunol. 2022;13:975367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hevia D, Araos P, Prado C, Fuentes Luppichini E, Rojas M, Alzamora R, Cifuentes-Araneda F, Gonzalez AA, Amador CA, Pacheco R, Michea L. Myeloid CD11c(+) Antigen-Presenting cells ablation prevents hypertension in response to angiotensin II plus High-Salt diet. Hypertension. 2018;71:709–18. [DOI] [PubMed] [Google Scholar]
- 215.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-Induced hypertension. Hypertension. 2016;68:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rodriguez-Iturbe B, Johnson RJ. Genetic polymorphisms in hypertension: are we missing the immune connection?? Am J Hypertens. 2019;32:113–22. [DOI] [PubMed] [Google Scholar]
- 218.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, et al. p53 is a suppressor of inflammatory response in mice. Faseb J. 2005;19:1030–2. [DOI] [PubMed] [Google Scholar]
- 221.Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R, Slotta-Huspenina J, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93–106. [DOI] [PubMed] [Google Scholar]
- 222.Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I, Haffner-Krausz R, Jung S, et al. CKIα ablation highlights a critical role for p53 in invasiveness control. Nature. 2011;470:409–13. [DOI] [PubMed] [Google Scholar]
- 223.Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. 2013;24:242–56. [DOI] [PubMed] [Google Scholar]
- 224.Hou J, Tian L, Wei Y. Cancer immunotherapy of targeting angiogenesis. Cell Mol Immunol. 2004;1:161–6. [PubMed] [Google Scholar]
- 225.Idris-Khodja N, Mian MO, Paradis P, Schiffrin EL. Dual opposing roles of adaptive immunity in hypertension. Eur Heart J. 2014;35:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nguyen BA, Alexander MR, Harrison DG. Immune mechanisms in the pathophysiology of hypertension. Nat Rev Nephrol. 2024;20:530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ward EM, Flowers CR, Gansler T, Omer SB, Bednarczyk RA. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J Clin. 2017;67:398–410. [DOI] [PubMed] [Google Scholar]
- 228.Abais-Battad JM, Dasinger JH, Fehrenbach DJ, Mattson DL. Novel adaptive and innate immunity targets in hypertension. Pharmacol Res. 2017;120:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol. 2019;176:1818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tellides G, Pober JS. Inflammatory and immune responses in the arterial media. Circ Res. 2015;116:312–22. [DOI] [PubMed] [Google Scholar]
- 231.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–13. [DOI] [PubMed] [Google Scholar]
- 232.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292:H1789–1795. [DOI] [PubMed] [Google Scholar]
- 233.Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97:1127–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Itani HA, McMaster WG Jr., Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Siedlinski M, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel RB, Welsh P, Maffia P, Erdmann J, Tomaszewski M, et al. White blood cells and blood pressure: A Mendelian randomization study. Circulation. 2020;141:1307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bradbury PA, Shepherd FA. Immunotherapy for lung cancer. J Thorac Oncol. 2008;3:S164–170. [DOI] [PubMed] [Google Scholar]
- 237.Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J, Guo Y, Yang M, Zhang X, Zhang Q, et al. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat Genet. 2007;39:605–13. [DOI] [PubMed] [Google Scholar]
- 239.Polcaro G, Liguori L, Manzo V, Chianese A, Donadio G, Caputo A, Scognamiglio G, Dell’Annunziata F, Langella M, Corbi G, et al. rs822336 binding to C/EBPβ and NFIC modulates induction of PD-L1 expression and predicts anti-PD-1/PD-L1 therapy in advanced NSCLC. Mol Cancer. 2024;23:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–7. [DOI] [PubMed] [Google Scholar]
- 241.Lopes RA, Neves KB, Tostes RC, Montezano AC, Touyz RM. Downregulation of nuclear factor erythroid 2-Related factor and associated antioxidant genes contributes to Redox-Sensitive vascular dysfunction in hypertension. Hypertension. 2015;66:1240–50. [DOI] [PubMed] [Google Scholar]
- 242.Camargo LL, Rios FJ, Montezano AC, Touyz RM. Reactive oxygen species in hypertension. Nat Rev Cardiol 2024. [DOI] [PubMed]
- 243.Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;31(Suppl 2):S185–189. [DOI] [PubMed] [Google Scholar]
- 244.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pinheiro LC, Oliveira-Paula GH. Sources and effects of oxidative stress in hypertension. Curr Hypertens Rev. 2020;16:166–80. [DOI] [PubMed] [Google Scholar]
- 246.Griendling KK, Camargo LL, Rios FJ, Alves-Lopes R, Montezano AC, Touyz RM. Oxidative stress and hypertension. Circ Res. 2021;128:993–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pawluk H, Pawluk R, Robaczewska J, Kędziora-Kornatowska K, Kędziora J. Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep. 2017;22:542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim M, Kim M, Yoo HJ, Shon J, Lee JH. Associations between hypertension and the peroxisome proliferator-activated receptor-δ (PPARD) gene rs7770619 C > T polymorphism in a Korean population. Hum Genomics. 2018;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ahmad S, Khan MY, Rafi Z, Khan H, Siddiqui Z, Rehman S, Shahab U, Khan MS, Saeed M, Alouffi S, Khan MS. Oxidation, glycation and glycoxidation-The vicious cycle and lung cancer. Semin Cancer Biol. 2018;49:29–36. [DOI] [PubMed] [Google Scholar]
- 250.Ahmad S, Khan H, Shahab U, Rehman S, Rafi Z, Khan MY, Ansari A, Siddiqui Z, Ashraf JM, Abdullah SM, et al. Protein oxidation: an overview of metabolism of sulphur containing amino acid, cysteine. Front Biosci (Schol Ed). 2017;9:71–87. [DOI] [PubMed] [Google Scholar]
- 251.Xu Y, Pan Q, Wang C, He C, Su Z, Guo X, Zhang J, Kong M, Ke S, Zhang J, et al. Genetic polymorphisms in oxidative stress-related genes are associated with clinical outcome in patients with advanced non-small cell lung cancer receiving tyrosine kinase inhibitors. Am J Cancer Res. 2014;4:934–42. [PMC free article] [PubMed] [Google Scholar]
- 252.Eom SY, Yim DH, Lee CH, Choe KH, An JY, Lee KY, Kim YD, Kim H. Interactions between paraoxonase 1 genetic polymorphisms and smoking and their effects on oxidative stress and lung cancer risk in a Korean population. PLoS ONE. 2015;10:e0119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chakraborty S, Mandal J, Yang T, Cheng X, Yeo JY, McCarthy CG, Wenceslau CF, Koch LG, Hill JW, Vijay-Kumar M, Joe B. Metabolites and hypertension: insights into hypertension as a metabolic disorder: 2019 Harriet Dustan award. Hypertension. 2020;75:1386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen M, Li H, Li Y, Luo Y, He Y, Shui X, Lei W. Glycolysis modulation: new therapeutic strategies to improve pulmonary hypertension (Review). Int J Mol Med 2024, 54. [DOI] [PMC free article] [PubMed]
- 255.Dey P, Kimmelman AC, DePinho RA. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021;11:1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s achilles’ heel. Cancer Cell. 2008;13:472–82. [DOI] [PubMed] [Google Scholar]
- 257.Moshfegh CM, Case AJ. The Redox-Metabolic couple of T lymphocytes: potential consequences for hypertension. Antioxid Redox Signal. 2021;34:915–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Redon J, Cífková R. The metabolic syndrome in hypertension: diagnostic and therapeutic implications. Curr Hypertens Rep. 2007;9:305–13. [DOI] [PubMed] [Google Scholar]
- 259.Dickinson CJ. Cerebral oxidative metabolism in hypertension. Clin Sci (Lond). 1996;91:539–50. [DOI] [PubMed] [Google Scholar]
- 260.Haspula D, Clark MA. Neuroinflammation and sympathetic overactivity: mechanisms and implications in hypertension. Auton Neurosci. 2018;210:10–7. [DOI] [PubMed] [Google Scholar]
- 261.Hurtubise J, McLellan K, Durr K, Onasanya O, Nwabuko D, Ndisang JF. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep. 2016;18:82. [DOI] [PubMed] [Google Scholar]
- 262.Akhigbe R, Ajayi A. The impact of reactive oxygen species in the development of cardiometabolic disorders: a review. Lipids Health Dis. 2021;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ansari RA, Husain K, Rizvi SA. Role of transcription factors in steatohepatitis and hypertension after ethanol: the epicenter of metabolism. Biomolecules 2016, 6. [DOI] [PMC free article] [PubMed]
- 264.Wang H, Li Q, Zhu Y, Zhang X. Omega-3 polyunsaturated fatty acids: versatile roles in blood pressure regulation. Antioxid Redox Signal. 2021;34:800–10. [DOI] [PubMed] [Google Scholar]
- 265.Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13:17–26. [DOI] [PubMed] [Google Scholar]
- 266.Fendt SM. 100 years of the Warburg effect: A cancer metabolism endeavor. Cell. 2024;187:3824–8. [DOI] [PubMed] [Google Scholar]
- 267.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Schwartz L, Seyfried T, Alfarouk KO, Da Veiga Moreira J, Fais S. Out of Warburg effect: an effective cancer treatment targeting the tumor specific metabolism and dysregulated pH. Semin Cancer Biol. 2017;43:134–8. [DOI] [PubMed] [Google Scholar]
- 269.Mukherjee A, Bilecz AJ, Lengyel E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022;41:575–87. [DOI] [PubMed] [Google Scholar]
- 270.Zhang L, Zhu B, Zeng Y, Shen H, Zhang J, Wang X. Clinical lipidomics in Understanding of lung cancer: opportunity and challenge. Cancer Lett. 2020;470:75–83. [DOI] [PubMed] [Google Scholar]
- 271.Fahrmann JF, Vykoukal JV, Ostrin EJ. Amino acid oncometabolism and Immunomodulation of the tumor microenvironment in lung cancer. Front Oncol. 2020;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sun Z, Chen J, Aakre J, Marks RS, Garces YY, Jiang R, Idowu O, Cunningham JM, Liu Y, Pankratz VS, Yang P. Genetic variation in glutathione metabolism and DNA repair genes predicts survival of small-cell lung cancer patients. Ann Oncol. 2010;21:2011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fang C, Wu W, Ni Z, Liu Y, Luo J, Zhou Y, Gong C, Hu D, Yao C, Chen X, et al. Ailanthone inhibits non-small cell lung cancer growth and metastasis through targeting UPF1/GAS5/ULK1 signaling pathway. Phytomedicine. 2024;128:155333. [DOI] [PubMed] [Google Scholar]
- 275.Nguyen LNT, Pyburn JS, Nguyen NL, Schank MB, Zhao J, Wang L, Leshaodo TO, El Gazzar M, Moorman JP, Yao ZQ. Epigenetic regulation by LncRNA GAS5/miRNA/mRNA network in human diseases. Int J Mol Sci 2025, 26. [DOI] [PMC free article] [PubMed]
- 276.Wang J, Ma R, Ma W, Chen J, Yang J, Xi Y, Cui Q. LncDisease: a sequence based bioinformatics tool for predicting lncRNA-disease associations. Nucleic Acids Res. 2016;44:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Keating ST, El-Osta A. Metaboloepigenetics in cancer, immunity, and cardiovascular disease. Cardiovasc Res. 2023;119:357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yu G, Wu L, Su Q, Ji X, Zhou J, Wu S, Tang Y, Li H. Neurotoxic effects of heavy metal pollutants in the environment: focusing on epigenetic mechanisms. Environ Pollut. 2024;345:123563. [DOI] [PubMed] [Google Scholar]
- 279.Zhang H, Pang Y, Yi L, Wang X, Wei P, Wang H, Lin S. Epigenetic regulators combined with tumour immunotherapy: current status and perspectives. Clin Epigenetics. 2025;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Corella D, Ordovas JM. Aging and cardiovascular diseases: the role of gene-diet interactions. Ageing Res Rev. 2014;18:53–73. [DOI] [PubMed] [Google Scholar]
- 281.Suglia SF, Hidalgo B, Baccarelli AA, Cardenas A, Damrauer S, Johnson A, Key K, Liang M, Magnani JW, Pate B, et al. Improving cardiovascular health through the consideration of social factors in genetics and genomics research: A scientific statement from the American heart association. Circ Cardiovasc Qual Outcomes. 2025;18:e000138. [DOI] [PubMed] [Google Scholar]
- 282.Abdul-Rahman T, Roy P, Bliss ZSB, Mohammad A, Corriero AC, Patel NT, Wireko AA, Shaikh R, Faith OE, Arevalo-Rios ECE, et al. The impact of air quality on cardiovascular health: A state of the Art review. Curr Probl Cardiol. 2024;49:102174. [DOI] [PubMed] [Google Scholar]
- 283.Babisch W, Wolf K, Petz M, Heinrich J, Cyrys J, Peters A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: the KORA study. Environ Health Perspect. 2014;122:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fajersztajn L, Veras M, Barrozo LV, Saldiva P. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer. 2013;13:674–8. [DOI] [PubMed] [Google Scholar]
- 285.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA air (Multi-Ethnic study of atherosclerosis and air Pollution). J Am Coll Cardiol. 2012;60:2158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Münzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J. 2018;39:3543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024–36. [DOI] [PubMed] [Google Scholar]
- 288.Swinnen K, Bijnens E, Casas L, Nawrot TS, Delcroix M, Quarck R, Belge C. Health effects of exposure to residential air pollution in patients with pulmonary arterial hypertension: a cohort study in Belgium. Eur Respir J 2022, 60. [DOI] [PubMed]
- 289.Xue Y, Wang L, Zhang Y, Zhao Y, Liu Y. Air pollution: A culprit of lung cancer. J Hazard Mater. 2022;434:128937. [DOI] [PubMed] [Google Scholar]
- 290.Zhang Y, Chen S, Wei J, Jiang J, Lin X, Wang Y, Hao C, Wu W, Yuan Z, Sun J, et al. Long-term PM(1) exposure and hypertension hospitalization: A causal inference study on a large community-based cohort in South China. Sci Bull (Beijing). 2024;69:1313–22. [DOI] [PubMed] [Google Scholar]
- 291.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. [DOI] [PubMed] [Google Scholar]
- 292.Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer. 2022;22:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Smoking is associated with chronic sympathetic activation in hypertension. Blood Press. 2010;19:152–5. [DOI] [PubMed] [Google Scholar]
- 294.Keil U. Coronary artery disease: the role of lipids, hypertension and smoking. Basic Res Cardiol. 2000;95(Suppl 1):I52–58. [DOI] [PubMed] [Google Scholar]
- 295.Lynch J, Jin L, Richardson A, Conklin DJ. Tobacco smoke and endothelial dysfunction: role of aldehydes?? Curr Hypertens Rep. 2020;22:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pardell H, Armario P, Hernández R. [Pathogenesis and epidemiology of arterial hypertension]. Drugs. 1998;56(Suppl 2):1–10. [DOI] [PubMed] [Google Scholar]
- 297.Cheng YY, Rath EM, Linton A, Yuen ML, Takahashi K, Lee K. The current Understanding of Asbestos-Induced epigenetic changes associated with lung cancer. Lung Cancer (Auckl). 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.da Cunha Martins A Jr., Carneiro MFH, Grotto D, Adeyemi JA, Barbosa F Jr. Arsenic, cadmium, and mercury-induced hypertension: mechanisms and epidemiological findings. J Toxicol Environ Health B Crit Rev. 2018;21:61–82. [DOI] [PubMed] [Google Scholar]
- 299.Fernández-Navarro P, García-Pérez J, Ramis R, Boldo E, López-Abente G. Industrial pollution and cancer in spain: an important public health issue. Environ Res. 2017;159:555–63. [DOI] [PubMed] [Google Scholar]
- 300.Pan Z, Gong T, Liang P. Heavy metal exposure and cardiovascular disease. Circ Res. 2024;134:1160–78. [DOI] [PubMed] [Google Scholar]
- 301.Paul P, Malakar AK, Chakraborty S. The significance of gene mutations across eight major cancer types. Mutat Res Rev Mutat Res. 2019;781:88–99. [DOI] [PubMed] [Google Scholar]
- 302.Thives LP, Ghisi E, Thives Júnior JJ, Vieira AS. Is asbestos still a problem in the world? A current review. J Environ Manage. 2022;319:115716. [DOI] [PubMed] [Google Scholar]
- 303.Wu W, Jiang S, Zhao Q, Zhang K, Wei X, Zhou T, Liu D, Zhou H, Zhong R, Zeng Q, et al. Associations of environmental exposure to metals with the risk of hypertension in China. Sci Total Environ. 2018;622–623:184–91. [DOI] [PubMed] [Google Scholar]
- 304.Jama HA, Beale A, Shihata WA, Marques FZ. The effect of diet on hypertensive pathology: is there a link via gut microbiota-driven immunometabolism? Cardiovasc Res. 2019;115:1435–47. [DOI] [PubMed] [Google Scholar]
- 305.Kobets T, Smith BPC, Williams GM. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11. [DOI] [PMC free article] [PubMed]
- 306.Peck B, Schulze A. Lipid metabolism at the nexus of diet and tumor microenvironment. Trends Cancer. 2019;5:693–703. [DOI] [PubMed] [Google Scholar]
- 307.Titze J, Luft FC. Speculations on salt and the genesis of arterial hypertension. Kidney Int. 2017;91:1324–35. [DOI] [PubMed] [Google Scholar]
- 308.Vishwakarma M, Piddini E. Outcompeting cancer. Nat Rev Cancer. 2020;20:187–98. [DOI] [PubMed] [Google Scholar]
- 309.Balarastaghi S, Rezaee R, Hayes AW, Yarmohammadi F, Karimi G. Mechanisms of arsenic Exposure-Induced hypertension and atherosclerosis: an updated overview. Biol Trace Elem Res. 2023;201:98–113. [DOI] [PubMed] [Google Scholar]
- 310.Li L, Bi Z, Wadgaonkar P, Lu Y, Zhang Q, Fu Y, Thakur C, Wang L, Chen F. Metabolic and epigenetic reprogramming in the arsenic-induced cancer stem cells. Semin Cancer Biol. 2019;57:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Minatel BC, Sage AP, Anderson C, Hubaux R, Marshall EA, Lam WL, Martinez VD. Environmental arsenic exposure: from genetic susceptibility to pathogenesis. Environ Int. 2018;112:183–97. [DOI] [PubMed] [Google Scholar]
- 312.Saintilnord WN, Fondufe-Mittendorf Y. Arsenic-induced epigenetic changes in cancer development. Semin Cancer Biol. 2021;76:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhou X, Speer RM, Volk L, Hudson LG, Liu KJ. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin Cancer Biol. 2021;76:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Blackburn K, Green D. The potential effects of microplastics on human health: what is known and what is unknown. Ambio. 2022;51:518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 2020, 17. [DOI] [PMC free article] [PubMed]
- 316.Kadac-Czapska K, Ośko J, Knez E, Grembecka M. Microplastics and oxidative Stress-Current problems and prospects. Antioxid (Basel) 2024, 13. [DOI] [PMC free article] [PubMed]
- 317.Kim EH, Choi S, Kim D, Park HJ, Bian Y, Choi SH, Chung HY, Bae ON. Amine-modified nanoplastics promote the procoagulant activation of isolated human red blood cells and thrombus formation in rats. Part Fibre Toxicol. 2022;19:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lee HS, Amarakoon D, Wei CI, Choi KY, Smolensky D, Lee SH. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem Toxicol. 2021;154:112356. [DOI] [PubMed] [Google Scholar]
- 319.Lu YY, Li H, Ren H, Zhang X, Huang F, Zhang D, Huang Q, Zhang X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J Hazard Mater. 2022;421:126770. [DOI] [PubMed] [Google Scholar]
- 320.Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, D’Onofrio N, Scisciola L, La Grotta R, Frigé C, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 2024;390:900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Płuciennik K, Sicińska P, Misztal W, Bukowska B. Important factors affecting induction of cell death, oxidative stress and DNA damage by Nano- and microplastic particles in vitro. Cells 2024, 13. [DOI] [PMC free article] [PubMed]
- 322.Vlacil AK, Bänfer S, Jacob R, Trippel N, Kuzu I, Schieffer B, Grote K. Polystyrene microplastic particles induce endothelial activation. PLoS ONE. 2021;16:e0260181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wade MJ, Bucci K, Rochman CM, Meek MH. Microplastic exposure is associated with epigenomic effects in the model organism pimephales Promelas (fathead minnow). J Hered 2024. [DOI] [PMC free article] [PubMed]
- 324.Wang L, Xu M, Chen J, Zhang X, Wang Q, Wang Y, Cui J, Zhang S. Distinct adverse outcomes and lipid profiles of erythrocytes upon single and combined exposure to cadmium and microplastics. Chemosphere. 2022;307:135942. [DOI] [PubMed] [Google Scholar]
- 325.Xuan L, Xiao L, Huang R. The geno-toxicological impacts of microplastic (MP) exposure on health: mechanistic pathways and research trends from a Chinese perspective. Environ Sci Process Impacts. 2023;25:26–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.