Abstract
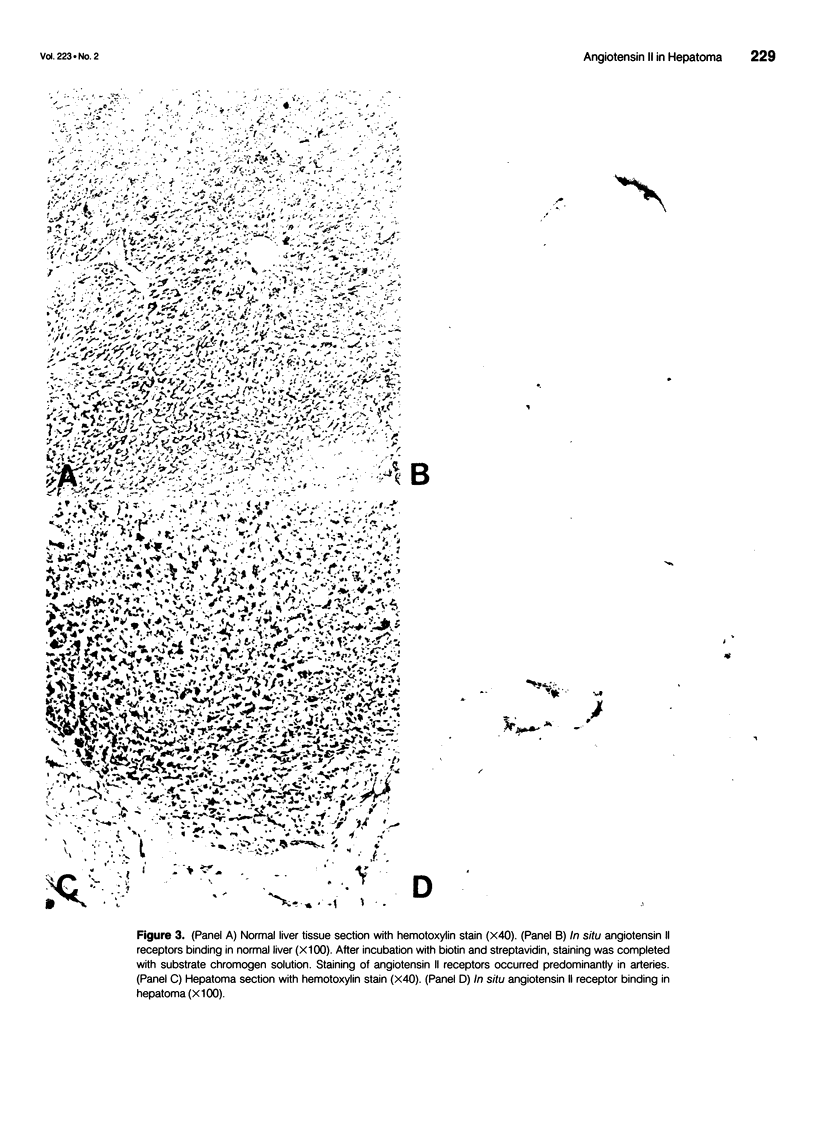
OBJECTIVE: The authors' objective was to determine the origin of the diminished pressor responsiveness of angiotensin II infusion in hepatoma by evaluating angiotensin II receptor status in normal liver, hepatoma tumor, and cultured hepatocytes and H4IIE cells. SUMMARY BACKGROUND DATA: Hepatocellular cancer is a highly vascular tumor, where the neovasculature is unique in that it arises only from the hepatic arterial circulation, whereas normal liver has both hepatic arterial and portal venous blood supply. The tumor neovasculature is also characterized by an abnormal vascular reactivity to vasoconstrictors, including the response to angiotensin II. The altered response of tumor vasculature to angiotensin II offers a potential therapeutic opportunity for modulation of tumor blood flow. However, the origin of the decreased vascular response is unknown. METHODS: The authors evaluated the hepatic vascular response to angiotensin II infusion by determining hepatic arterial blood flow to normal liver and to tumor by means of radioactive microspheres. The angiotensin II receptor status in the normal liver, hepatoma tumor, and cultured hepatocytes and H4IIE cells was determined br radioligand binding analysis and in cryostat sections derived from normal liver and hepatoma tumor by means of in situ binding analysis with biotinylated angiotensin II. RESULTS: Angiotensin II infusion decreased the hepatic arterial flow to normal liver and increased hepatoma to liver flow ratio. The number of angiotensin II receptors in normal liver was significantly higher than that in hepatoma (239 +/- 20 fmol/mg protein in normal liver vs. 162 +/- 15 fmol/mg protein in hepatoma) without a change in the affinity (4.4 +/- 0.8 nM in normal liver vs. 4.7 +/- 1.2 nM in hepatoma). H4IIE cells and primary hepatocytes had low receptor density. In situ binding analysis revealed that angiotensin II receptors were mainly on the smooth muscle cells of the neovasculature. CONCLUSIONS: The data suggests that the diminished vascular response to angiotensin II hepatoma may relate a loss of angiotensin II receptor on tumor neovasculature.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berssenbrugge A. D., Goodfriend T. L., Ball D. L., Rankin J. H. The effect of pregnancy on the angiotensin II pressor response in the rabbit. Am J Obstet Gynecol. 1980 Mar 15;136(6):762–767. doi: 10.1016/0002-9378(80)90453-6. [DOI] [PubMed] [Google Scholar]
- Bouscarel B., Blackmore P. F., Exton J. H. Characterization of the angiotensin II receptor in primary cultures of rat hepatocytes. Evidence that a single population is coupled to two different responses. J Biol Chem. 1988 Oct 15;263(29):14913–14919. [PubMed] [Google Scholar]
- Corriu C., André P., Schott C., Michel M., Stoclet J. C. ANG II receptor expression and function during phenotypic modulation of rat aortic smooth muscle cells. Am J Physiol. 1994 Feb;266(2 Pt 2):H631–H636. doi: 10.1152/ajpheart.1994.266.2.H631. [DOI] [PubMed] [Google Scholar]
- Goldberg J. A., Kerr D. J., Wilmott N., McKillop J. H., McArdle C. S. Regional chemotherapy for colorectal liver metastases: a phase II evaluation of targeted hepatic arterial 5-fluorouracil for colorectal liver metastases. Br J Surg. 1990 Nov;77(11):1238–1240. doi: 10.1002/bjs.1800771114. [DOI] [PubMed] [Google Scholar]
- Gunther S., Gimbrone M. A., Jr, Alexander R. W. Identification and characterization of the high affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res. 1980 Aug;47(2):278–286. doi: 10.1161/01.res.47.2.278. [DOI] [PubMed] [Google Scholar]
- Hemingway D. M., Cooke T. G., Chang D., Grime S. J., Jenkins S. A. The effects of intra-arterial vasoconstrictors on the distribution of a radiolabelled low molecular weight marker in an experimental model of liver tumour. Br J Cancer. 1991 Apr;63(4):495–498. doi: 10.1038/bjc.1991.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami T., Iwai N., Sasaki K., Yamamo Y., Bardhan S., Chaki S., Guo D. F., Furuta H. Cloning, expression and regulation of angiotensin II receptors. J Hypertens. 1992 Aug;10(8):713–716. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malik A. B., Kaplan J. E., Saba T. M. Reference sample method for cardiac output and regional blood flow determinations in the rat. J Appl Physiol. 1976 Mar;40(3):472–475. doi: 10.1152/jappl.1976.40.3.472. [DOI] [PubMed] [Google Scholar]
- Onohara S., Kobayashi H., Itoh Y., Shinohara S. Intra-arterial cis-platinum infusion with sodium thiosulfate protection and angiotensin II induced hypertension for treatment of hepatocellular carcinoma. Acta Radiol. 1988 Mar-Apr;29(2):197–202. [PubMed] [Google Scholar]
- Sasaki Y., Imaoka S., Hasegawa Y., Nakano S., Ishikawa O., Ohigashi H., Taniguchi K., Koyama H., Iwanaga T., Terasawa T. Changes in distribution of hepatic blood flow induced by intra-arterial infusion of angiotensin II in human hepatic cancer. Cancer. 1985 Jan 15;55(2):311–316. doi: 10.1002/1097-0142(19850115)55:2<311::aid-cncr2820550202>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sitzmann J. V., Wu Y., Cameron J. L. Altered angiotensin-II receptors in human hepatocellular and hepatic metastatic colon cancers. Ann Surg. 1994 May;219(5):500–507. doi: 10.1097/00000658-199405000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than T. T., Sitzmann J. V., Li S. S., Lin P. W., Grochow L. B. A method for hepatic arterial perfusion studies in the rat. J Surg Res. 1989 Sep;47(3):251–254. doi: 10.1016/0022-4804(89)90116-9. [DOI] [PubMed] [Google Scholar]
- Ullian M. E., Linas S. L. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest. 1989 Sep;84(3):840–846. doi: 10.1172/JCI114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P., Mosselmans R., Ronveaux M. F. Adult rat hepatocytes in primary monolayer culture. Ultrastructural characteristics of intercellular contacts and cell membrane differentiations. J Cell Biol. 1977 Sep;74(3):858–877. doi: 10.1083/jcb.74.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- Wu Y. P., Li S. S., Campbell K. A., Sitzmann J. V. Modulation of splanchnic vascular sensitivity to angiotensin II. Surgery. 1991 Aug;110(2):162–168. [PubMed] [Google Scholar]