Summary
Introduction
Patients living with obesity present specific challenges for airway management. They have been overrepresented repeatedly in studies looking at complications associated with airway management. Whilst generic airway guidelines offer assistance, there are no evidence‐based guidelines specific to this group of patients to support safe and effective airway management.
Methods
An expert multidisciplinary working party was convened on behalf of the Society of Obesity and Bariatric Anaesthesia to conduct this work. A systematic review was performed and was followed by a three‐round Delphi process.
Results
Forty‐three recommendations are made which cover a number of key areas of practice including: pre‐assessment; planning; peroxygenation; tracheal intubation; supraglottic airway devices; tracheal extubation; training; and organisational responsibilities.
Discussion
It is hoped the recommendations made will improve the safety and efficacy of airway management in patients living with obesity. They are not intended to replace current airway management guidelines.
Keywords: airway, guidelines, obesity, tracheal intubation
Plain Language Summary
People who are very overweight (obese) can have special problems when doctors need to help them breathe during surgery or emergencies. These problems happen more often in people with obesity than in others. There are general guidelines to help doctors, but there aren't any special guidelines just for people with obesity. A group of experts from different areas came together to help fix this problem. They looked at lots of research and then worked together through a process called Delphi (which means they asked for expert opinions in three rounds) to make a set of suggestions. The team made 43 suggestions. These suggestions cover important parts of helping people breathe safely, like: checking the person before surgery; making a plan; giving oxygen; putting in and taking out breathing tubes; using special breathing masks; training doctors and nurses; and making sure hospitals have the right processes. The team hopes these ideas will help doctors and nurses keep people with obesity safer when they need help with breathing.
Recommendations
The detailed airway management strategy, including rescue strategies in the event of difficulty, should be discussed by the anaesthetic team before induction of anaesthesia.
Patients should be pre‐oxygenated in a ramped, ≥ 30° head‐up position (preferably in the operating theatre), with a high inspiratory fraction of oxygen.
Consider use of high‐flow nasal oxygen for peroxygenation.
Tracheal intubation should take place in the operating theatre on the operating table rather than the anaesthetic room.
A videolaryngoscope should be used as the first‐line technique.
Before tracheal extubation, patients should be pre‐oxygenated with a high inspiratory fraction of oxygen, positioned in a head‐up position, with adequate reversal of neuromuscular blocking drugs confirmed using quantitative neuromuscular monitoring.
Organisations should ensure provision of suitable equipment to guarantee safe and effective management of patients living with obesity.
Why were these guidelines developed?
The prevalence of obesity in society is increasing and there is evidence of increased incidence of airway complications in this patient cohort [1, 2, 3, 4, 5, 6]. These recommendations have been developed by the Society for Obesity and Bariatric Anaesthesia (SOBA) UK to improve the safety of patients living with obesity who require airway management as part of their care.
What guidelines currently exist?
There are multiple guidelines available for airway management worldwide [7, 8, 9, 10, 11, 12, 13, 14, 15]. Additionally, there are peri‐operative guidelines available on the management of patients living with obesity [16]. However, in all existing guidelines, recommendations relating specifically to airway management in patients living with obesity are an addendum or subsection. To our knowledge there are no other recommendations specific to the airway management of patients living with obesity.
How do these guidelines differ from existing guidelines?
These guidelines are specific to the management of patients living with obesity.
Introduction
The prevalence of obesity in society is increasing, and the 7th National Audit Report indicated that in the last decade the median BMI in patients having surgery has increased substantially [1, 2, 4, 17]. Classically a BMI > 30 kg.m‐2 has been used to define levels of obesity, but this has changed recently. The Lancet Commission on Obesity has redefined clinical obesity as “a chronic, systemic illness characterised by alterations in the function of tissues, organs, the entire individual, or a combination thereof, due to excess adiposity”, and suggested that in patients with BMI > 40 kg.m‐2 excess adiposity can be assumed [18]. Approximately 59% of surgical patients being treated by anaesthetists are classed as ‘overweight’ or ‘obese’ by standard BMI criteria [2, 4]. It is no longer acceptable to assume that management of patients living with obesity is the sole responsibility of bariatric anaesthetists. Patients living with severe obesity are now often encountered presenting for all types of surgery, in elective and emergency settings [17]. As a result, all anaesthetists must be competent and able to provide safe care for these patients.
While airway management is a single component of this overall provision of safe care, it is a vital one. The ultimate aim is to provide ongoing oxygenation and prevent desaturation and subsequent hypoxia in patients undergoing general anaesthesia. Despite classic teaching that tracheal intubation itself is no more difficult in patients living with obesity, this cohort of patients is consistently overrepresented in the poor outcome groups from airway studies [5, 6, 19]. The exact reasons have not been elucidated fully. However, it is clear that airway management in patients living with obesity represents a specific challenge, and that best practice guidance for clinicians and departments is likely to be helpful.
Methods
These consensus recommendations were developed by an eight‐member working group on behalf of SOBA. The group comprised consultant anaesthetists (including SOBA representatives and advanced airway experts); two patient representatives; an operating department practitioner; and a resident anaesthetist. Formulation of these consensus recommendations conformed to recognised methodology and adhered to the AGREE 2.0 reporting checklist [20].
To inform recommendations, a systematic review was performed in accordance with the PRISMA reporting checklist and prospectively registered with PROSPERO [21, 22]. Details of the methodology are shown in Fig. 1, with data extraction in online Supporting Information Appendix S1 and risk of bias assessments in online Supporting Information Tables S1 and S2. The results of the systematic review were used to inform a three‐round Delphi process to formulate recommendations [23, 24]. An initial long list of 50 recommendations were proposed by the Working Group, with each recommendation reviewed and rated for content and clarity (online Supporting Information Appendix S2). The proposed recommendations were based on several factors including: strength of supporting evidence; applicability to clinical practice; multidisciplinary team involvement; and practical utility of the recommendations. Each recommendation was voted on anonymously using a structured Microsoft Excel sheet (Microsoft, Inc., Redmond, WA, USA) by all members of the Working Group as either ‘include’, ‘exclude’ or ‘revise’, as well as whether each recommendation should be made a top recommendation. All members participated in all rounds. Recommendations supported by ≥ 75% of the group at the end of round 2 were included in this final document. Seven recommendations with 50–74% acceptance proceeded to a third and final round of discussions. This took place in the form of a virtual round‐table discussion. Individuals were asked to vote to include or exclude the outstanding recommendations following group discussion, and the wording of all included recommendations was ratified.
Figure 1.
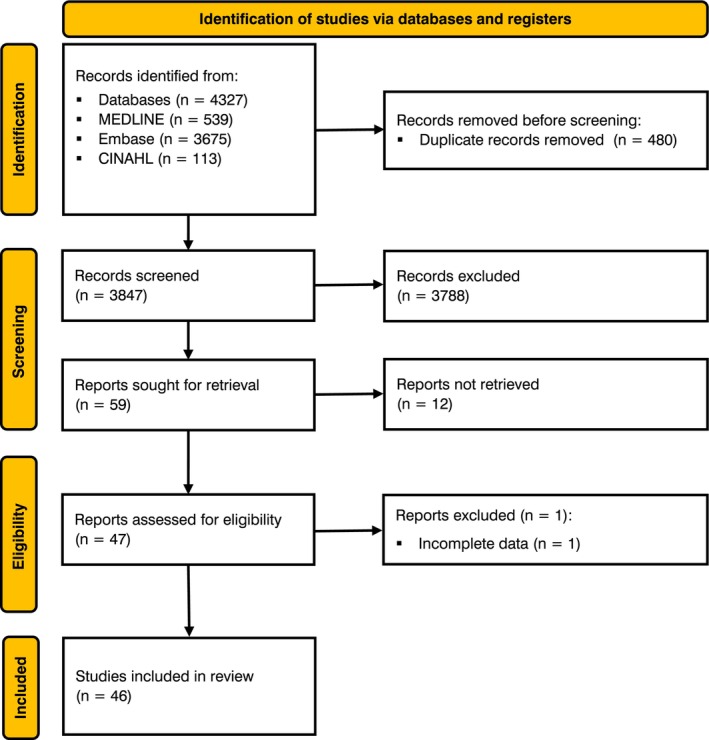
Systematic review methodology.
The strength of evidence supporting the recommendations was graded using a modified version of the system developed by the Centre for Evidence‐based Medicine [25], as used for previous airway guideline recommendations [8, 13, 26]. Recommendations were graded A to D according to the strength of the available evidence (Table 1), and the judgements on the strength of recommendations were made based on analysis of the evidence, consensus voting and discussion through the Delphi process: strong recommendation ≥ 80% consensus; moderate recommendation 65–79% consensus; and weak recommendation 50–64% consensus. During development, drafting and finalising of these consensus recommendations the Working Group met remotely six times over 24 months. A draft of the document and supporting information was then circulated to SOBA committee members and advanced airway experts for comment. The comments and feedback received were used to further refine and inform the final recommendations and document. The working group agreed unanimously to include four additional recommendations and edit two recommendations based on the feedback from reviewers. This resulted in a total of 43 recommendations being included in the document.
Table 1.
Grading of recommendations based on the level of evidence available.
| Grade | Level of evidence available |
|---|---|
| A | Consistent systematic reviews of RCTs, single RCTs or all‐or‐none studies |
| B | Consistent systematic reviews of low‐quality RCTs or cohort studies, individual cohort study or epidemiological outcome studies |
| Consistent systematic reviews of case–control studies, individual case–control studies | |
| Extrapolations from systematic reviews of RCTs, single RCTs or all‐or‐none studies | |
| C | Case series, case reports |
| Extrapolations from systematic reviews of low‐quality RCTs, cohort studies or case–control studies, individual cohort study, epidemiological outcome studies, individual case–control studies | |
| Extrapolations from systematic reviews of case–control studies | |
| D | Expert opinion or ideas based on theory, on bench studies or first principles alone |
| Troublingly inconsistent or inconclusive studies of any level |
RCT, randomised controlled trial.
Results
Pre‐operative assessment
Pre‐operative assessment is a critically important aspect of anaesthetic care and is the starting point for a process of optimisation and prehabilitation [27]. It ensures each patient attending for surgery is in the best health possible and offers an opportunity to identify risk factors for surgery and discuss the various anaesthesia management options. This facilitates adaptation of clinical approaches by enabling availability of specific staff or equipment according to individual patient requirements [27, 28]. Whilst acknowledging variation between institutions in process and limitations for emergent surgery, this is of particular importance in patients living with obesity, as risk factors for difficult airway management may be identified in advance and acted on accordingly [28]. Whilst obesity is not directly linked to difficult tracheal intubation, it is linked to difficult facemask ventilation, which is associated with composite airway management failure and a 10‐fold increase in the risk of failed tracheal intubation [5, 29, 30]. Patients living with obesity are at higher risk of complications, including (but not limited to) airway complications [5]. Thus, shared decision‐making discussions, using patient‐appropriate language in advance of surgery, are encouraged when possible [31]. Obstructive sleep apnoea (OSA) is common in this patient cohort, with some patients already established on home continuous positive airway pressure (CPAP) before surgery [32]. However, service limitations mean that undiagnosed OSA is also a potential risk factor in these patients, and pre‐operative assessment is an opportunity to identify those who may be at risk. In addition, patients living with obesity are more likely to be taking glucagon‐like peptide‐1 agonists and sodium‐glucose co‐transporter‐2 inhibitors, which can increase the risk of pulmonary aspiration and should be factored into a patient's individual risk assessment and considered when determining an appropriate airway strategy [33].
All patients living with obesity should have their airway pre‐assessed formally in advance of surgery (Grade D, strong recommendation).
Patients should be consented for obesity‐related complications including (but not limited to) airway difficulties (Grade D, strong recommendation).
Patients may benefit from a face‐to‐face appointment with an anaesthetist in advance of elective surgery (Grade D, strong recommendation).
Pre‐assessment discussions should use patient‐first language, minimising the use of medical jargon (Grade D, strong recommendation).
Patients on CPAP/non‐invasive ventilation should be instructed to bring their machine on the day of surgery (Grade C, strong recommendation).
Planning
Previous studies have shown that failure to modify anaesthetic techniques for patients living with obesity is linked to poorer outcomes [3, 19]. It is recognised that ‘one size does not fit all’, and adaptations of techniques and equipment choice should be tailored to the requirements of individual patients. These changes may increase the anaesthetic time due to the gathering and set up of equipment and/or in the conduct of anaesthesia. The primary focus is patient safety, necessitating careful curation of operating theatre lists, with patients living with obesity highlighted in advance to allow extra anaesthetic time.
Time and thought must be put into the logistics of the anaesthetic, namely where it should take place and what equipment is required [34]. The safety of patients and staff is of paramount importance, and devices to aid manual handling, positioning and securing of patients can be incredibly useful [16]. Patients living with obesity are at higher risk of desaturation [35]. This is multifactorial but mainly due to a decreased functional residual capacity and an increased basal metabolic oxygen demand [36, 37]. Thus, minimising circuit disconnections and maintaining positive pressure ventilation with positive end‐expiratory pressure is preferred. This may necessitate anaesthetising patients on an appropriate operating table in the location where the procedure is due to be performed [34].
Although difficult tracheal intubation is not linked to increased BMI, difficult facemask ventilation is [30]. The importance of considering difficulties with facemask ventilation as part of the airway strategy cannot be overstated, particularly given its association with composite airway failure [30]. In a ‘cannot intubate, cannot oxygenate’ situation, performing an emergency front‐of‐neck airway (plan D) is likely to be more difficult in patients living with obesity [38]. Ultrasound is a useful adjunct in these patients to help identify the cricothyroid membrane and cricoid cartilage [38]. However, it does not guarantee success and there is a significant risk of inability to perform an emergency front‐of‐neck airway in these patients.
Operating theatre lists should flag patients with a BMI > 40 kg.m‐2 (Grade D, strong recommendation).
Additional time should be allocated for patients living with obesity when operating theatre list planning (Grade D, strong recommendation).
Location of anaesthesia should be considered, particularly in emergencies. Appropriate equipment and personnel should be made available where out‐of‐theatre tracheal intubation is required (Grade D, strong recommendation).
The BMI of patients and any anticipated difficulties should be discussed at the team brief (Grade D, strong recommendation).
The detailed airway management strategy, including rescue strategies in the event of difficulty, should be discussed by the anaesthetic team before induction of anaesthesia (Grade D, strong recommendation).
If there is any suggestion that your airway strategy may end up at plan D (emergency front‐of‐neck airway) alternative techniques including awake tracheal intubation and regional anaesthesia must be considered, and a risk–benefit decision on proceeding should be made on an individual patient basis (Grade D, strong recommendation).
Ergonomics, including operating theatre and equipment layout, should be considered (Grade D, strong recommendation).
Equipment necessary for anaesthetic modification should be discussed at the latest at the team brief (Grade D, strong recommendation).
Devices to assist manual handling should be considered (e.g. hover mattress/slide sheets) (Grade D, strong recommendation).
Devices to assist patient positioning should be considered (e.g. Oxford HELLP pillow) (Grade C, strong recommendation).
Equipment to secure patients on the operating table safely should be available and appropriate (Grade D, strong recommendation).
The operating table should be checked for weight limitations before the team brief (Grade D, strong recommendation).
Ultrasound‐guided identification and marking of the cricothyroid membrane, or cricoid cartilage if using cricoid force, for rapid sequence induction and tracheal intubation may be considered before induction of anaesthesia (Grade C, strong recommendation).
Facemask ventilation and peroxygenation
Pre‐oxygenation is a key technique in anaesthetic practice, and of particular importance in the management of patients living with obesity. By increasing oxygen stores, desaturation should be delayed extending the safe apnoeic time. The relevant physiology is well‐described and it is considered integral to safe anaesthetic practice, such that in 2015 the Difficult Airway Society Guidelines state “All patients should be pre‐oxygenated before the induction of general anaesthesia” [10, 39].
Oxygen delivery via facemask is the standard technique used for pre‐oxygenation transitioning to facemask ventilation following apnoea on induction of anaesthesia [10, 19]. A BMI > 30 kg.m‐2 is an independent risk factor for difficult facemask ventilation, which is in turn associated with composite airway failure [30, 40]. Use of airway adjuncts, supraglottic airway devices (SADs) or two‐handed/person techniques can be used to maximise ventilation efficiency and thus oxygen delivery.
The importance of peroxygenation planning and ramped, head‐up positioning during pre‐oxygenation cannot be overstated. A minimum of 30° head‐up tilt is recommended, where feasible. The benefits of head‐up positioning in patients living with obesity have been shown clearly by several studies [41, 42, 43, 44]. Several methods have been described to achieve adequate pre‐oxygenation, although variations in patient populations and individual physiology may impact their effectiveness [43, 44, 45, 46, 47]. Patients living with obesity have differing physiology, with decreased functional residual capacity; lower tidal volumes; decreased lung compliance; higher oxygen demand; and a higher risk of atelectasis. Collectively, these factors result in more rapid desaturation following induction of anaesthesia, and a greater risk of hypoxia compared with patients not living with obesity [48].
Anatomical differences (e.g. large neck circumference, increased adipose tissue limiting neck movement and causing airway obstruction, etc.) may contribute to difficult facemask ventilation and necessitate alternative approaches. In these patients, ventilation via a SAD before tracheal intubation (or between tracheal intubation attempts) to maintain oxygenation is a safe and effective technique that is not well‐described in the literature but is used commonly in practice. The priority is to ensure effective oxygen delivery to prevent desaturation, prolong the safe apnoea time and prevent hypoxia during tracheal intubation.
Peroxygenation encompasses pre‐oxygenation and apnoeic oxygenation and ventilation [49]. Several techniques for peroxygenation are described in the literature, the most familiar and widely practiced being the use of high‐flow nasal oxygen (HFNO) [47, 49, 50, 51, 52, 53]. High‐flow nasal oxygen for peroxygenation in patients living with obesity has become a well‐recognised method that is commonly used. The Society of Obesity and Bariatric Anaesthesia has long suggested this technique to be considered in this patient population [34]. The evidence base for its effectiveness in this cohort of patients is growing and newer devices offer the ability to alternate seamlessly between facemask ventilation and delivery of HFNO for apnoeic oxygenation [47, 49, 54, 55].
Patients should be pre‐oxygenated in a ramped, ≥ 30° head‐up position, preferably in the operating theatre (Grade A, strong recommendation).
Pre‐oxygenation should start as soon as is practically possible (Grade D, strong recommendation).
Facemask ventilation in patients with a high BMI is more likely to be difficult. The use of adjuncts, two‐person techniques and SADs may be helpful (Grade C, strong recommendation).
The addition of oxygenation techniques via nasal cannula (high‐ or low‐flow) alongside facemask may confer benefits and delay desaturation (Grade A, strong recommendation).
Consider use of HFNO as a first‐line method for peroxygenation (pre‐oxygenation and apnoeic oxygenation) (Grade D, strong recommendation).
If not using an apnoeic oxygenation technique, the use of a SAD to facilitate ventilation between facemask pre‐oxygenation and tracheal intubation attempts is safe and effective (Grade D, strong recommendation).
Basal collapse atelectasis occurs early and should be anticipated (Grade C, strong recommendation).
Tracheal intubation
It has long been suggested that tracheal intubation itself is no more difficult in patients living with obesity than those not [56]. This is the opinion of SOBA and others, but there is evidence to suggest the opposite [57]. What is clear is that patients living with obesity are overrepresented in the poor outcome and airway complication groups of multiple airway studies. The 4th National Audit Project concluded that “obese patients are at increased risk of an adverse airway event” and commented on the physiological issues contributing to desaturation and risk of aspiration [19].
Anticipated difficult facemask ventilation and/or tracheal intubation supports the consideration of an awake tracheal intubation technique [8]. Awake tracheal intubation can be a safe and effective technique in patients living with obesity, but obesity alone is rarely an indication for its use. Use of videolaryngoscopy as a first‐line technique is proven to have benefits over direct laryngoscopy, as is clear from the Cochrane review on this topic [58]. This is further supported by a recent meta‐analysis specific to patients living with obesity [59]. The specific choice of use of laryngoscope blade is currently based on user preference, unless otherwise indicated. However, there is some evidence that hyperangulated blades may confer additional benefits compared with Macintosh blades in patients living with obesity, but it is important to note that a suitably trained operator is required for this [58, 60]. Passage of the tracheal tube might be aided by the use of adjuncts such as bougies or stylets. Flow dynamics support the use of larger internal diameter tracheal tubes where feasible. However, tracheal tube selection is multifactorial, accounting for both patient and procedural factors, and an individualised approach should be taken for each patient.
Appropriate dosing of neuromuscular blocking drugs before tracheal intubation is of critical importance. Use of quantitative neuromuscular monitors can help ensure paralysis before attempts at tracheal intubation [61]. The type of body weight used for dosing calculations will depend on the type of neuromuscular blocking drug used (e.g. for non‐depolarising muscle relaxants use lean body weight; for depolarising muscle relaxants use total body weight). There are multiple resources available to support dosing calculations, including the SOBA mobile phone application.
Optimising human factors and ergonomics to maximise the chance of a successful first‐pass tracheal intubation attempt should be considered. Maintaining the ramped position during tracheal intubation may be facilitated by simple interventions such as the intubator using a step. Similarly, tracheal intubation in the operating theatre on the operating table can minimise manual handling, limit circuit disconnections, help maintain positive end‐expiratory pressure and prevent de‐recruitment.
A videolaryngoscope should be used as a first‐line technique (Grade A, strong recommendation), preferably with a hyperangulated blade where the user is sufficiently trained (Grade C, strong recommendation).
Ensure adequate neuromuscular blockade before tracheal intubation (Grade B, strong recommendation).
Consider use of appropriate airway adjuncts (such as a stylet or bougie) and rescue techniques where difficulty during tracheal intubation is encountered (Grade B, strong recommendation).
Awake tracheal intubation techniques are safe and effective in patients living with obesity but may be more challenging due to the physiological and anatomical changes. While obesity alone (in the absence of other factors) is rarely an indication for awake tracheal intubation, we recommend maintaining a low threshold for awake tracheal intubation in this group of patients (Grade D, strong recommendation).
Tracheal intubation in the operating theatre on the operating table rather than the anaesthetic room is recommended as this minimises the need for manual handling, allows correct positioning and negates the need for ventilator disconnection and the transfer from anaesthetic room to operating theatre (Grade D, strong recommendation).
Use of a step during tracheal intubation should be considered in order to maintain the ramped patient position (Grade D, strong recommendation).
Supraglottic airway devices
Supraglottic airway devices can be an effective method for maintaining oxygenation in patients living with obesity. Use of a SAD may be appropriate as the primary airway management plan; however, there should be a very low threshold for tracheal intubation in this cohort of patients. Use of SADs in this patient population has been associated with increased airway events, with patients of BMI > 40 kg.m‐2 appearing to have close to four times higher odds of conversion to tracheal intubation [5, 62]. Physiological differences specific to this patient cohort increase their risk of gastric aspiration and make conversion to tracheal intubation more challenging should difficulties arise [33]. Spontaneous ventilation can lead to atelectasis; therefore, positive pressure ventilation modes are preferred [63]. This applies even when the planned surgical time is short.
Supraglottic airway devices may be used to provide ventilation before tracheal intubation or between attempts at tracheal intubation, to maintain oxygenation when facemask ventilation is difficult (Grade D, moderate recommendation).
If planning to use a SAD as the primary airway device, a second‐generation device should be used, ensuring head‐up positioning of the patient throughout and a plan for tracheal intubation should complications arise (Grade B, strong recommendation). Use of a controlled ventilation mode may also be considered (Grade D, moderate recommendation).
Tracheal extubation
Tracheal extubation is an elective procedure which should be planned for appropriately. The importance of having a tracheal extubation strategy cannot be overstated. A plan for re‐intubation of the trachea must be included as part of the tracheal extubation strategy. Patients living with obesity may require additional risk mitigation compared with other patient groups. The physiological issues which result in the need for a specific approach to peroxygenation and tracheal intubation still exist at the point of tracheal extubation [39]. Studies suggest patients living with obesity are at greater risk of airway issues in the post‐anaesthesia care unit [64]. Whilst these events might not be attributable specifically to tracheal extubation, they suggest the immediate postoperative period is a critical stage in the management of these patients [64].
The head‐up position improves lung ventilation which, combined with pre‐oxygenation with a high inspiratory fraction of oxygen before tracheal extubation, maximises functional residual capacity and oxygen reserve [34, 39]. Complete reversal of neuromuscular blockade is essential and the routine use of sugammadex in this cohort of patients confers benefits [61]. Spontaneous ventilation via a tracheal tube in patients living with obesity can lead to atelectasis; therefore, controlled ventilation is preferred [63]. Standard approaches to tracheal extubation, such as ensuring that adequate tidal volumes are achieved and that the patient is awake and co‐operative, increase the likelihood of successful tracheal extubation. Whilst the tracheal re‐intubation rate is generally low, it remains higher than that seen in the non‐obese population, necessitating appropriate planning [5].
The correct dose of sugammadex for routine reversal is a complex issue that remains unresolved. Initially based on total body weight, this was later investigated by Lancker et al., who concluded that ideal body weight plus 40% was appropriate [65]; this is the scale that features in the SOBA drug dosing application. However, others including Carron et al. have suggested that total body weight should be used [66]. Undoubtedly, the safest approach is to rely on quantitative measurement of neuromuscular blockade and adjust the dose accordingly.
There is a high risk of desaturation and/or airway obstruction in patients living with obesity at the point of tracheal extubation. Use of HFNO or CPAP may confer benefits, and electively planning tracheal extubation directly onto such devices may help prevent basal collapse. Such postoperative oxygenation strategies can be continued in post‐anaesthesia care units and following discharge from recovery. This is particularly true in patients with OSA who use home CPAP devices [67].
Consideration of safe postoperative destinations is a key aspect of the tracheal extubation strategy, necessitating consideration at the earliest opportunity (ideally pre‐operatively), enabling advance planning and bed availability before anaesthesia (particularly where level 2 postoperative care is required), whilst enhancing patient understanding regarding risk.
Tracheal extubation is an elective procedure and must be planned for appropriately. This includes a plan for re‐intubation of the trachea when required (Grade D, strong recommendation).
Before tracheal extubation, patients should be pre‐oxygenated with a high inspiratory fraction of oxygen, positioned in a head‐up position, with adequate reversal of neuromuscular blocking drugs confirmed using quantitative neuromuscular monitoring (Grade C, strong recommendation).
Consider tracheal extubation directly onto HFNO/CPAP in patients who are deemed at increased risk of desaturation (Grade B, strong recommendation).
Ensure appropriate equipment and personnel are available for re‐intubation of the trachea, if required (Grade D, strong recommendation).
Postoperative destination should be considered at the earliest opportunity to enable advanced planning, specifically suitability and need for level 2 support, if the patient is known to have, or there is a high suspicion of, OSA (Grade D, strong recommendation).
Organisational responsibilities and training
Organisations have a responsibility to ensure patients living with obesity receive appropriate and safe care during their peri‐operative journey, by supporting staff training and provision of specialist equipment. Obesity leads play a key role in their departments by assessing local needs and working with management teams to ensure access to appropriate equipment and specific training for staff. They are recommended by SOBA as personnel responsible for overseeing and implementing best practice care for patients living with obesity presenting to anaesthetic services. Guidelines from the Association of Anaesthetists and SOBA recommend a minimum standard for training, which includes knowledge (and training) in the use and maintenance of obesity‐specific equipment [16]. The Royal College of Anaesthetists recognises the importance of training specific to the management of patients living with obesity within its 2021 curriculum, placing the onus on anaesthetists in training (resident anaesthetists) and their trainers to ensure anaesthetic competencies pertinent to this patient cohort are met before Certificate of Completion of Training [68].
Staff should be supported to attain and maintain competencies to manage patients living with obesity (Grade D, strong recommendation).
Organisations should have guidelines in place for the management of patients with BMI > 40 kg.m‐2 (Grade D, strong recommendation).
Organisations should have a departmental obesity lead (Grade D, strong recommendation).
Organisations should ensure provision of suitable equipment to ensure safe and effective management of patients living with obesity (Grade D, strong recommendation).
Resident anaesthetists should receive specific training in anaesthesia for patients living with obesity. They should be supervised appropriately, according to their level of training and clinical competency (Grade D, strong recommendation).
Special circumstances
There are particular circumstances for which specific airway management guidelines already exist (e.g. patients having obstetric surgery, who are critically unwell or have a cervical spine injury) [7, 8, 10, 12, 26]. These recommendations serve to support those guidelines and are not intended to replace them. Where differences in recommendations exist, a pragmatic approach based on each individual patient's risk assessment should be taken.
Rapid sequence induction and tracheal intubation are indicated in patients identified at increased risk of pulmonary aspiration following induction of anaesthesia. The ultimate aim of airway management in this instance is to secure a safe airway with a tracheal tube as quickly and as safely possible following rapid onset of anaesthesia. There is a spectrum of risk, and individual patient risk assessment should be used to inform the rapid sequence induction and tracheal intubation strategy. These are high‐risk tracheal intubations, and patients may be critically unwell, presenting with a physiologically difficult airway requiring additional considerations.
The principles supporting the recommendations on pre‐assessment, planning, positioning, peroxygenation and tracheal intubation still apply, but there are a few specific features of rapid sequence induction and tracheal intubation. Delivery of neuromuscular blocking drugs following induction of anaesthesia should be immediate and consist of a dose that will ensure rapid paralysis (e.g. rocuronium 1.2 mg.kg‐1, lean body weight). Depth of anaesthesia monitoring gives a delayed reading and should not dictate when neuromuscular blocking drugs are given [69]. Calibration and use of quantitative neuromuscular monitors in these circumstances is impractical and adds unnecessary delays, so should not be used at induction of anaesthesia. Instead, priority should be given to ensuring adequate dosing of the neuromuscular blocking drug in the first instance. Suction should be on and immediately available in all instances.
Discussion
Patients living with obesity present a number of well‐described challenges for the anaesthetist, ranging from the physiological and pharmacological to the anatomical and logistical [16, 17, 31, 34, 70, 71]. The aim of this expert group was not to repeat or reword current general airway guidance for all patients, but rather to create practical, pragmatic and clinically relevant recommendations for this specific cohort of patients. The repeated overrepresentation of this patient cohort in poor outcome and complication groups in airway studies is a significant concern [3, 5, 6]. A standard approach to their airway management is inappropriate, and careful planning is required to achieve a safe outcome.
The Delphi process used followed best practice methodology and has resulted in a list of recommendations which the authors believe are clinically important. Limitations include the paucity of high‐quality airway research specific to patients living with obesity. Many airway papers attempt to make sub‐group analyses in patients living with obesity, but the included numbers are often too small to be generalisable [59, 72]. In order to increase sample size, a BMI > 30 kg.m‐2 is often selected to define ‘obesity’. Whilst historically this number may represent a degree of obesity, it is difficult to extrapolate such findings to the airway encountered in patients with a BMI of ≥ 40 kg.m‐2. This is an issue not limited to airway research and is the case for many areas of obesity research for years. A recent Lancet Commission on obesity aims to change that with new diagnostic criteria and definitions of obesity which are likely to change the approach of obesity medicine, obesity management and obesity research [18]. Other limitations included qualitative data synthesis and the inability to prevent ‘group‐think’ in the final round of the Delphi study.
More research is needed in airway management in patients living with obesity. However, the current focus is to ensure patient safety and to prevent patient harm. The Society of Obesity and Bariatric Anaesthesia will continue to promote best practice care in all aspects of anaesthesia for patients living with obesity and will continue to collaborate with all interested groups and societies to achieve these goals.
Supporting information
Appendix S1. Data extraction spreadsheet.
Appendix S2. Delphi Study results.
Table S1. Observational studies for airway management in patients living with obesity.
Table S2. Risk of Bias assessment for included randomised controlled trials clinical studies.
Plain Language Summary
Acknowledgements
These guidelines are not intended to represent a minimum standard of practice, nor are they to be regarded as a substitute for good clinical judgement. They present key principles and suggested strategies for airway management in patients living with obesity. They are primarily intended for adults, but several principles also apply to children. The recommendations put forward are not meant to replace existing airway management guidelines. This document is intended to guide appropriately trained healthcare professionals. This systematic review was prospectively registered with PROSPERO (CRD 42023448279). Thanks to Katie Osbourne and Rhys Whelan from NHS Wales Library services for their help and support with the systematic review. The authors wish to thank: Dr A. McNarry; Dr J. Cousins; Dr Z. Burton; Dr I. Murdoch; Dr M. Thomas; Dr E. Warwick; Dr L. Pengelly; Dr P. Fabb; Dr B. Retnasingham; Dr S. Ho; Dr I. Sharieff; and Dr V. Male for their expert review. AM has previously received honoraria as educational grants from Fisher and Paykel and Verethon Medical. HI is a Fellow of Anaesthesia. BB has received honoraria as educational grants from Fisher and Paykel. IA has previously received honoraria as educational grants from Stortz, Ambu, Fisher and Paykel, Flexicare, Verathon Medical and BioMarin. No data or statistical code were generated.
1 Consultant, Lewisham and Greenwich NHS Trust, London, UK
2 Speciality Trainee, Department of Anaesthesia, Swansea Bay University Health Board, Swansea, UK
3 Speciality Trainee, Health Education and Improvement Wales, Cardiff, UK
4 Consultant, Department of Anaesthesia, University College London Hospitals, London, UK
5 Consultant, Department of Anaesthesia and Perioperative Medicine, Guy's and St Thomas’ NHS Foundation Trust, London, UK
6 Honorary Senior Lecturer, King's College London, London, UK
7 Patient Representative, Dumfries, UK
8 Operating Department Practitioner, University College London Hospitals, London, UK
9 Consultant, The Grange University Hospital, Aneurin Bevan University Health Board, Cwmbran, UK
Plain Language Summary available on PubMed and in the Supporting Information.
References
- 1. UK Government Office for Health Improvement and Disparities . Obesity Profile: short statistical commentary May 2024. 2024. https://www.gov.uk/government/statistics/update‐to‐the‐obesity‐profile‐on‐fingertips/obesity‐profile‐short‐statistical‐commentary‐may‐2024 (accessed 04/02/2025).
- 2. Armstrong RA, Soar J, Kane AD, et al. Peri‐operative cardiac arrest: epidemiology and clinical features of patients analysed in the 7th National Audit Project of the Royal College of Anaesthetists. Anaesthesia 2024; 79: 18–30. 10.1111/anae.16156. [DOI] [PubMed] [Google Scholar]
- 3. Royal College of Anaesthetists , Difficult Airway Society . NAP4: Major Complications of Airway Management in the United Kingdom. 2011. https://www.rcoa.ac.uk/research/research‐projects/national‐audit‐projects‐naps/nap4‐major‐complications‐airway‐management (accessed 02/05/2025).
- 4. Kane AD, Soar J, Armstrong RA, et al. Patient characteristics, anaesthetic workload and techniques in the UK: an analysis from the 7th National Audit Project (NAP7) activity survey. Anaesthesia 2023; 78: 701–711. 10.1111/anae.15989. [DOI] [PubMed] [Google Scholar]
- 5. Shaw M, Waiting J, Barraclough L, Ting K, Jeans J, Black B. Airway events in obese vs. non‐obese elective surgical patients: a cross‐sectional observational study. Anaesthesia 2021; 76: 1585–1592. 10.1111/anae.15513. [DOI] [PubMed] [Google Scholar]
- 6. Cumberworth A, Lewith H, Sud A, Jefferson H, Athanassoglou V, Pandit JJ. Major complications of airway management: a prospective multicentre observational study. Anaesthesia 2022; 77: 640–648. 10.1111/anae.15668. [DOI] [PubMed] [Google Scholar]
- 7. Higgs A, McGrath B, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth 2018; 120: 323–352. 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 8. Ahmad I, El‐Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2020; 75: 509–528. 10.1111/anae.14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022; 136: 31–81. 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 10. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015; 115: 827–848. 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Australian and New Zealand College of Anaesthetists . Guidelines for the management of evolving airway obstruction: transition to the can't intubate can't oxygenate airway emergency. 2017. https://www.anzca.edu.au/getattachment/71f54974‐314a‐4d96‐bef2‐c03f39c8a8e9/PS61‐Guideline‐for‐the‐management‐of‐evolving‐airway‐obstruction‐transition‐to‐the‐Can't‐Intubate‐Can't‐Oxygenate‐airway‐emergency (accessed 20/02/2025).
- 12. Mushambi MC, Kinsella SM, Popat M, et al. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015; 70: 1286–1306. 10.1111/anae.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iliff HA, El‐Boghdadly K, Ahmad I, et al. Management of haematoma after thyroid surgery: systematic review and multidisciplinary consensus guidelines from the Difficult Airway Society, the British Association of Endocrine and Thyroid Surgeons and the British Association of Otorhinolaryngology. Head Neck Surg Anaesth 2022; 77: 82–85. 10.1111/anae.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Law JA, Duggan LV, Asselin M, et al. Canadian Airway Focus Group updated consensus‐based recommendations for management of the difficult airway: part 1. Difficult airway management encountered in an unconscious patient. Can J Anesth 2021; 68: 1373–1404. 10.1007/s12630-021-02007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012; 67: 308–340. 10.1111/j.1365-2044.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- 16. Nightingale CE, Margarson MP, Shearer E, et al. Peri‐operative management of the obese surgical patient. Anaesthesia 2015; 70: 859–876. 10.1111/anae.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royal College of Anaesthetists . NAP7 report on perioperative cardiac arrest. 2023. https://www.rcoa.ac.uk/research/research‐projects/national‐audit‐projects‐naps/nap7‐report (accessed 04/02/2025).
- 18. Rubino F, Cummings DE, Eckel RH, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol 2025; 13: 221–262. 10.1016/S2213-8587(24)00316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth 2011; 106: 617–631. 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 20. Brouwers MC, Kerkvliet K, Spithof K, Consortium ANS . The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. Br Med J 2016; 352: i1152. 10.1136/bmj.i4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J 2009; 339: b2700. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67: 401–409. 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 24. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011; 6: e20476. 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandit JJ, Popat MT, Cook TM, et al. The difficult airway society ‘ADEPT’ guidance on selecting airway devices: the basis of a strategy for equipment evaluation. Anaesthesia 2011; 66: 726–737. 10.1111/j.1365-2044.2011.06787.x. [DOI] [PubMed] [Google Scholar]
- 26. Wiles MD, Iliff HA, Brooks K, et al. Airway management in patients with suspected or confirmed cervical spine injury. Anaesthesia 2024; 79: 856–868. 10.1111/anae.16290. [DOI] [PubMed] [Google Scholar]
- 27. Centre for Perioperative Care . Preoperative Assessment and Optimisation for Adult Surgery Including Consideration of COVID‐19 and Its Implications. 2021. https://www.cpoc.org.uk/preoperative‐assessment‐and‐optimisation‐adult‐surgery (accessed 02/05/2025).
- 28. Thota B, Jan KM, Oh MW, Moon TS. Airway management in patients with obesity. Saudi J Anaesth 2022; 16: 76–81. 10.4103/sja.sja_351_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cattano D, Katsiampoura A, Corso RM, Killoran PV, Cai C, Hagberg CA. Predictive factors for difficult mask ventilation in the obese surgical population. F1000Res 2014; 3: 239. 10.12688/f1000research.5471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodall NM, Cook TM. National census of airway management techniques used for anaesthesia in the UK: first phase of the fourth National Audit Project at the Royal College of Anaesthetists. Br J Anaesth 2011; 106: 266–271. 10.1093/bja/aeq339. [DOI] [PubMed] [Google Scholar]
- 31. Society for Obesity and Bariatric Anaesthesia . Anaesthesia consent for the patient with obesity. 2021. https://www.sobauk.co.uk/_files/ugd/373d41_77109e5b370e4d189246992392f3688e.pdf (accessed 27/01/2025).
- 32. Cooksey J, Mokhlesi B. Postoperative complications in obesity hypoventilation syndrome and hypercapnic OSA. Chest 2016; 149: 11–13. 10.1016/j.chest.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 33. El‐Boghdadly K, Dhesi J, Fabb P, et al. Elective peri‐operative management of adults taking glucagon‐like peptide‐1 receptor agonists, glucose‐dependent insulinotropic peptide agonists and sodium‐glucose cotransporter‐2 inhibitors: a multidisciplinary consensus statement. Anaesthesia 2025; 80: 412–424. 10.1111/anae.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Society for Obesity and Bariatric Anaesthesia . Anaesthesia for patients living with obesity. 2022. https://www.sobauk.co.uk/_files/ugd/373d41_eebe369c3c6b4021bff6f3da059aa796.pdf (accessed 04/02/2025).
- 35. Jense HG, Dubin SA, Silverstein PI, O'Leary‐Escolas U. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg 1991; 72: 89–93. 10.1213/00000539-199101000-00016. [DOI] [PubMed] [Google Scholar]
- 36. Damia G, Mascheroni D, Croci M, Tarenzi L. Perioperative changes in functional residual capacity in morbidly obese patients. Br J Anaesth 1988; 60: 574–578. 10.1093/bja/60.5.574. [DOI] [PubMed] [Google Scholar]
- 37. Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med 1999; 160: 883–886. 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 38. Price TM, McCoy EP. Emergency front of neck access in airway management. BJA Educ 2019; 19: 246–253. 10.1016/j.bjae.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nunn J. Applied respiratory physiology, 4th edn. Philadelphia: Butterworth‐Heinemann, 1993. [Google Scholar]
- 40. Kheterpal S, Han R, Tremper KK, Shanks A, Tait AR, O'Reilly M, Ludwig TA. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006; 105: 885–891. 10.1097/00000542-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 41. Altermatt FR, Muñoz HR, Delfino AE, Cortínez LI. Pre‐oxygenation in the obese patient: effects of position on tolerance to apnoea. Br J Anaesth 2005; 95: 706–709. 10.1093/bja/aei231. [DOI] [PubMed] [Google Scholar]
- 42. Couture EJ, Provencher S, Somma J, Lellouche F, Marceau S, Bussières JS. Effect of position and positive pressure ventilation on functional residual capacity in morbidly obese patients: a randomized trial. Can J Anesth 2018; 65: 522–528. 10.1007/s12630-018-1050-1. [DOI] [PubMed] [Google Scholar]
- 43. Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25° head‐up position than in the supine position in severely obese patients. Anesthesiology 2005; 102: 1110–1115. 10.1097/00000542-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 44. Ern Hung Tsan S, Viknaswaran NL, Lau J, Cheong CC, Wang CY. Effectiveness of preoxygenation during endotracheal intubation in a head‐elevated position: a systematic review and meta‐analysis of randomized controlled trials. Anaesthesiol Intensive Ther 2022; 54: 413–424. 10.5114/ait.2022.123197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiang T‐L, Tam K‐W, Chen J‐T, Wong CS, Yeh CT, Huang TY, Ong JR. Non‐invasive ventilation for preoxygenation before general anesthesia: a systematic review and meta‐analysis of randomized controlled trials. BMC Anesthesiol 2022; 22: 1–14. 10.1186/s12871-022-01842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baraka A, Salem M, Hagburg C. Preoxygenation. In: Benumof and Hagburg's Airway Management, 3rd edn. Philadelphia: Elsevier, 2012: 657–682. [Google Scholar]
- 47. Crístian de Carvalho C, Iliff HA, Santos Neto JM, Potter T, Alves MB, Blake L, el‐Boghdadly K. Effectiveness of preoxygenation strategies: a systematic review and network meta‐analysis. Br J Anaesth 2024; 133: 152–163. 10.1016/j.bja.2024.02.028. [DOI] [PubMed] [Google Scholar]
- 48. Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology 1997; 87: 979–982. 10.1097/00000542-199710000-00034. [DOI] [PubMed] [Google Scholar]
- 49. Patel A, El‐Boghdadly K. Apnoeic oxygenation and ventilation: go with the flow. Anaesthesia 2020; 75: 1002–1005. 10.1111/anae.15066. [DOI] [PubMed] [Google Scholar]
- 50. Moon TS, Tai K, Kim A, et al. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, double‐blinded, controlled trial of nasal cannula oxygen administration. Obes Surg 2019; 29: 3992–3999. 10.1007/s11695-019-04077-y. [DOI] [PubMed] [Google Scholar]
- 51. Baraka AS, Taha SK, Siddik‐Sayyid SM, et al. Supplementation of pre‐oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia 2007; 62: 769–773. 10.1111/j.1365-2044.2007.05104.x. [DOI] [PubMed] [Google Scholar]
- 52. Gaszynski T, McKechnie A. Pre‐oxygenation and apneic oxygenation in patients living with obesity – a review of novel techniques. Saudi J Anaesth 2022; 16: 322–326. 10.4103/sja.sja_351_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel A, Nouraei SAR. Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–329. 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schutzer‐Weissmann J, Wojcikiewicz T, Karmali A, et al. Apnoeic oxygenation in morbid obesity: a randomised controlled trial comparing facemask and high‐flow nasal oxygen delivery. Br J Anaesth 2023; 130: 103–110. 10.1016/j.bja.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wong DT, Dallaire A, Singh KP, et al. High‐flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth Analg 2019; 129: 1130–1136. 10.1213/ANE.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 56. Brodsky JB, Lemmens HJM, Brock‐Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg 2002; 94: 732–736. 10.1097/00000539-200203000-00047. [DOI] [PubMed] [Google Scholar]
- 57. Kristensen MS. Airway management and morbid obesity. Eur J Anaesthesiol 2010; 27: 923–927. 10.1097/EJA.0b013e32833d91aa. [DOI] [PubMed] [Google Scholar]
- 58. Hansel J, Rogers AM, Lewis SR, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation: a Cochrane systematic review and meta‐analysis update. Br J Anaesth 2022; 129: 612–623. 10.1016/j.bja.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chaudery H, Hameed H, Sharif Z, Asinger S, McKechnie A. Comparative efficacy of Videolaryngoscopy and direct laryngoscopy in patients living with obesity: a meta‐analysis. Cureus 2024; 12: e76558. 10.7759/cureus.76558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ott S, Müller‐Wirtz LM, Bustamante S, et al. Learning tracheal intubation with a hyperangulated videolaryngoscopy blade: sub‐analysis of a randomised controlled trial. Anaesthesia 2025; 80: 395–403. 10.1111/anae.16491. [DOI] [PubMed] [Google Scholar]
- 61. Klein AA, Meek T, Allcock E, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: guideline from the Association of Anaesthetists. Anaesthesia 2021; 76: 1212–1223. 10.1111/anae.15501. [DOI] [PubMed] [Google Scholar]
- 62. Hartsuyker P, Kanczuk ME, Lawn D, Beg S, Mengistu TS, Hiskens M. The effect of class 3 obesity on the functionality of supraglottic airway devices: a historical cohort analysis with propensity score matching. Can J Anesth 2023; 70: 1744–1752. 10.1007/s12630-023-02582-4. [DOI] [PubMed] [Google Scholar]
- 63. Fernandez‐Bustamante A, Hashimoto S, Serpa Neto A, Moine P, Vidal Melo MF, Repine JE. Perioperative lung protective ventilation in obese patients. BMC Anesthesiol 2015; 15: 56. 10.1186/s12871-015-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mendonça J, Pereira H, Xará D, Santos A, Abelha FJ. Obese patients: respiratory complications in the post‐anesthesia care unit. Rev Port Pneumol (Engl Ed) 2014; 20: 12–19. 10.1016/j.rppneu.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 65. Van Lancker P, Dillemans B, Bogaert T, Mulier JP, De Kock M, Haspeslagh M. Ideal versus corrected body weight for dosage of sugammadex in morbidly obese patients. Anaesthesia 2011; 66: 721–725. 10.1111/j.1365-2044.2011.06782.x. [DOI] [PubMed] [Google Scholar]
- 66. Carron M, Freo U, Parotto E, Ori C. The correct dosing regimen for sugammadex in morbidly obese patients. Anaesthesia 2012; 67: 298–299. 10.1111/j.1365-2044.2012.07060.x. [DOI] [PubMed] [Google Scholar]
- 67. Li R, Liu L, Wei K, Zheng X, Zeng J, Chen Q. Effect of noninvasive respiratory support after extubation on postoperative pulmonary complications in obese patients: a systematic review and network meta‐analysis. J Clin Anesth 2023; 91: 111280. 10.1016/j.jclinane.2023.111280. [DOI] [PubMed] [Google Scholar]
- 68. Royal College of Anaesthetists . 2021 Curriculum learning syllabus: stage 3 special interest areas. 2021. https://rcoa.ac.uk/documents/2021‐curriculum‐learning‐syllabus‐stage‐3‐special‐interest‐areas/anaesthesia‐bariatric (accessed 04/02/2025).
- 69. Rani D, Harsoor S. Depth of general anaesthesia monitors. Indian J Anaesth 2012; 56: 437. 10.4103/0019-5049.103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Warwick E, McKechnie A. Society for Obesity and Bariatric Anaesthesia UK (SOBA UK) response to the 7th National Audit Project report. Anaesthesia 2024; 79: 441–442. 10.1111/anae.16254. [DOI] [PubMed] [Google Scholar]
- 71. Von Thaer S, McVey J, Shelton J, Johnson Q. Obesity and anesthesia: challenges in the perioperative period. Mo Med 2024; 121: 156–163. [PMC free article] [PubMed] [Google Scholar]
- 72. Wang T, Sun S, Huang S. The association of body mass index with difficult tracheal intubation management by direct laryngoscopy: a meta‐analysis. BMC Anesthesiol 2018; 18: 79. 10.1186/s12871-018-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Data extraction spreadsheet.
Appendix S2. Delphi Study results.
Table S1. Observational studies for airway management in patients living with obesity.
Table S2. Risk of Bias assessment for included randomised controlled trials clinical studies.
Plain Language Summary


