Abstract
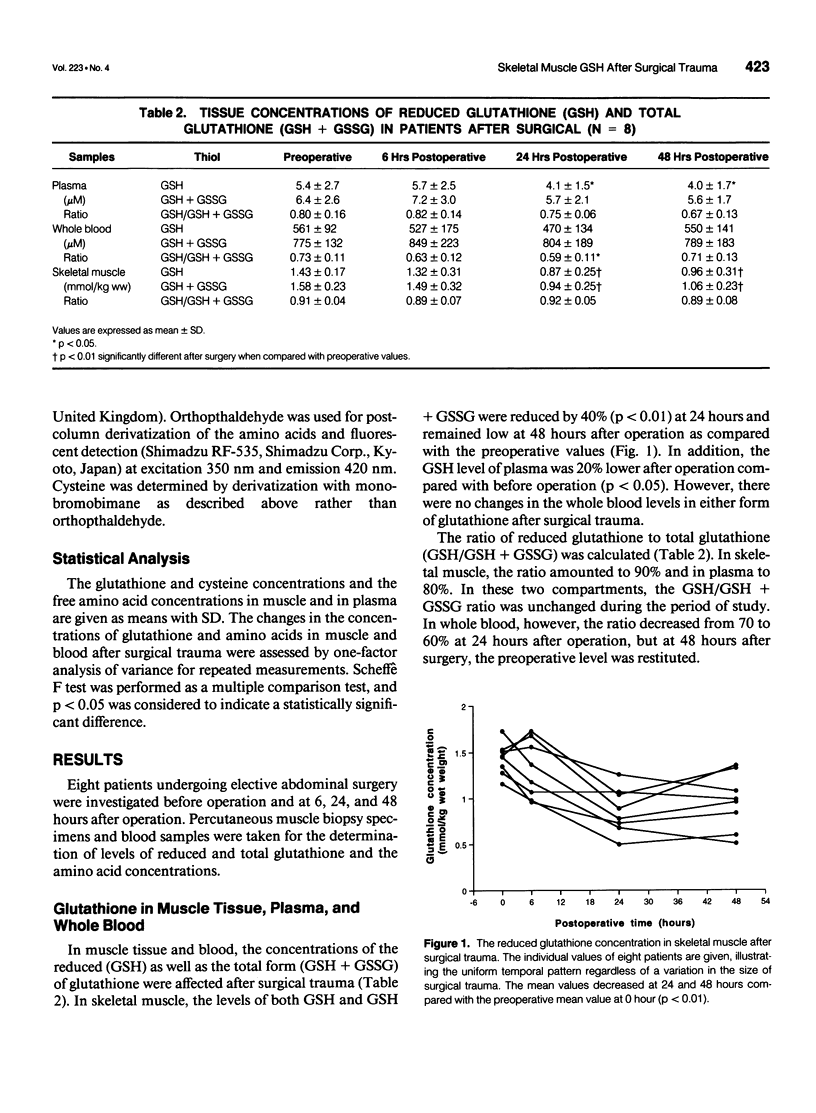
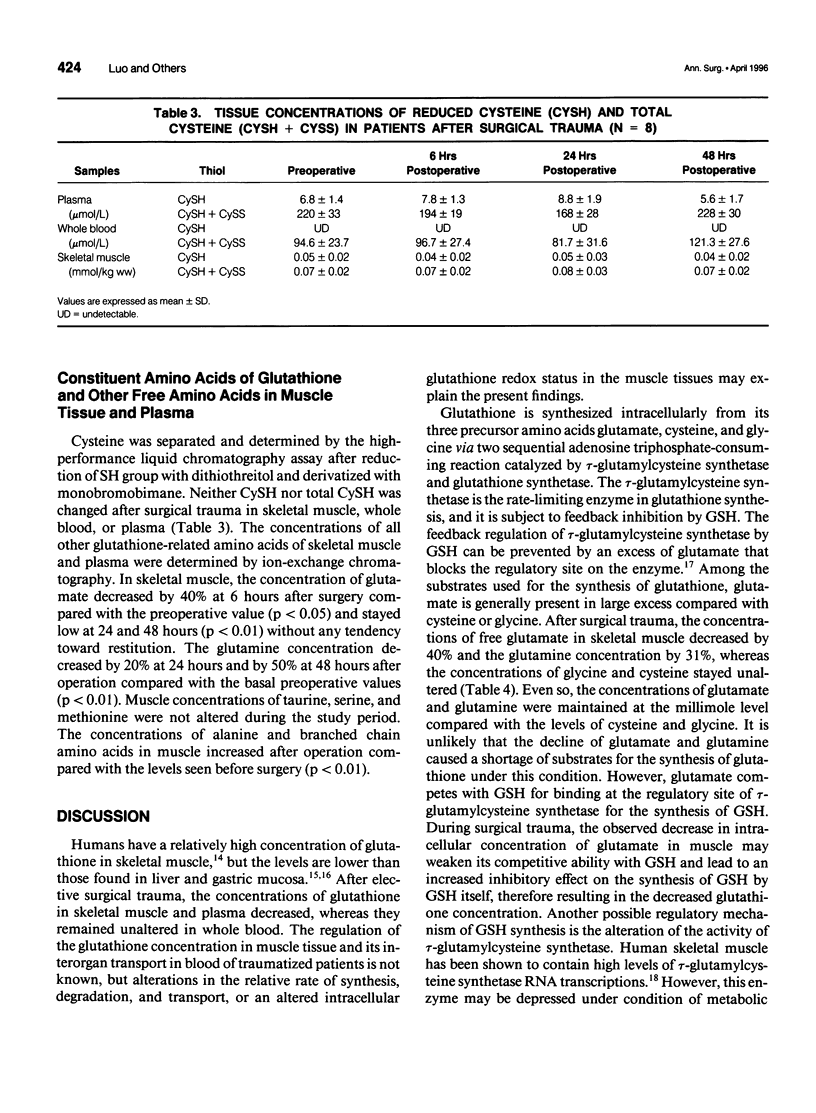
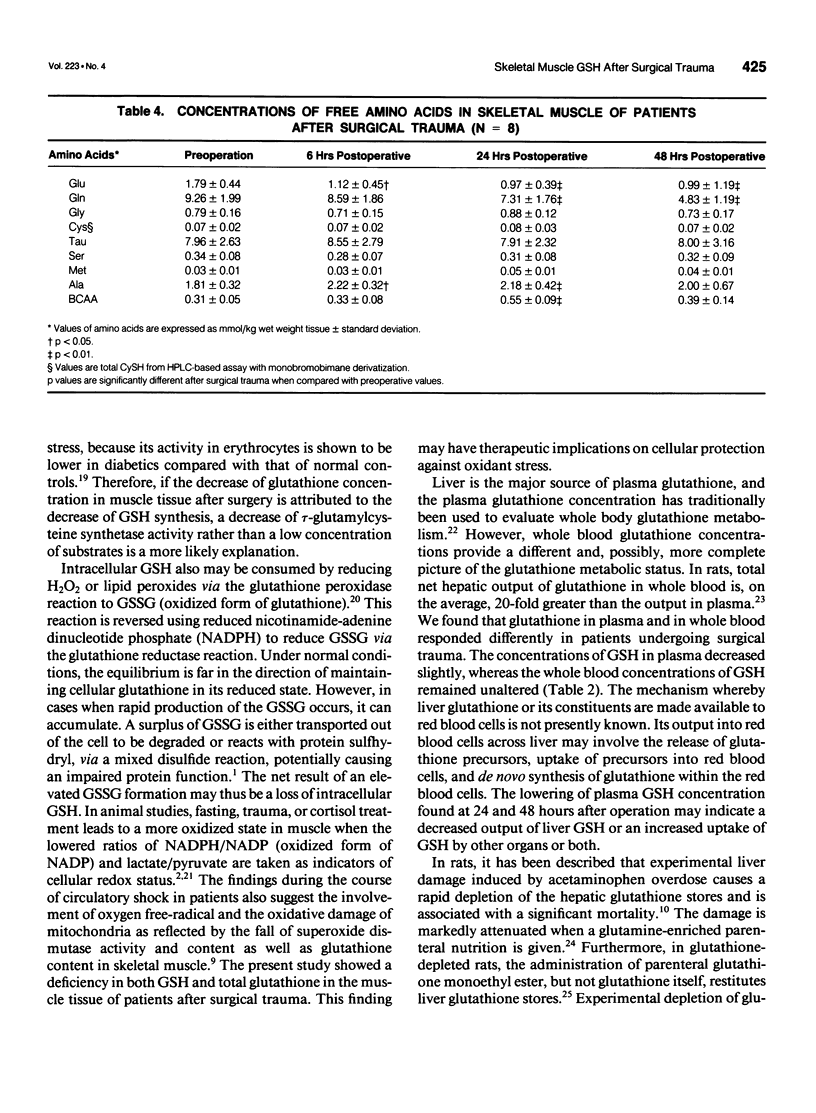
OBJECTIVE: The authors investigate the effect of surgical trauma on skeletal muscle concentrations of glutathione in patients undergoing selective abdominal surgery. SUMMARY BACKGROUND DATA: The posttraumatic state is accompanied by characteristic changes in the pattern of free amino acids and a decline of protein synthesis in human skeletal muscle. Glutathione has multiple metabolic functions that are involved in cellular homeostasis. It is unknown how surgical trauma affects the glutathione metabolism of skeletal muscle in surgical patients. METHODS: Eight patients undergoing elective abdominal surgery were investigated. Percutaneous muscle biopsies and blood samples were taken before operation and at 6, 24, and 48 hours after operation. The concentrations of glutathione were determined in muscle tissue, plasma, and whole blood, as well as the concentrations of the related amino acids in muscle and plasma. RESULTS: In skeletal muscle, the levels of both reduced and total glutathione decreased by 40% (p<0.01) at 24 hours and remained low at 48 hours after operation compared with the preoperative values. The glutathione concentration in plasma was 20% lower after operation compared with the concentration before operation (p<0.05). There were no changes at the whole blood levels of glutathione. Tissue glutamate and glutamine decreased significantly after operation (p<0.001), whereas intracellular cysteine and glycine remained unchanged. CONCLUSIONS: Skeletal muscle glutathione deficiency occurs after surgical trauma. This may lead to an increase in the susceptibility to intracellular oxidative injury.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Takada A., Kasuga H., Tateishi N. Induction of cystine transport activity in isolated rat hepatocytes by sulfobromophthalein and other electrophilic agents. Hepatology. 1986 Nov-Dec;6(6):1361–1368. doi: 10.1002/hep.1840060624. [DOI] [PubMed] [Google Scholar]
- Bannai S., Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89(1):1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984 Sep 3;779(3):289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Beatty P., Reed D. J. Influence of cysteine upon the glutathione status of isolated rat hepatocytes. Biochem Pharmacol. 1981 Jun 1;30(11):1227–1230. doi: 10.1016/0006-2952(81)90302-6. [DOI] [PubMed] [Google Scholar]
- Cho E. S., Sahyoun N., Stegink L. D. Tissue glutathione as a cyst(e)ine reservoir during fasting and refeeding of rats. J Nutr. 1981 May;111(5):914–922. doi: 10.1093/jn/111.5.914. [DOI] [PubMed] [Google Scholar]
- Corbucci G. G., Gasparetto A., Candiani A., Crimi G., Antonelli M., Bufi M., De Blasi R. A., Cooper M. B., Gohil K. Shock-induced damage to mitochondrial function and some cellular antioxidant mechanisms in humans. Circ Shock. 1985;15(1):15–26. [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J Biochem Biophys Methods. 1986 Nov;13(4-5):231–249. doi: 10.1016/0165-022x(86)90102-8. [DOI] [PubMed] [Google Scholar]
- Dass P. D., Bermes E. W., Jr, Holmes E. W. Renal and hepatic output of glutathione in plasma and whole blood. Biochim Biophys Acta. 1992 Dec 8;1156(1):99–102. doi: 10.1016/0304-4165(92)90102-z. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Baxter D. F., Phelps D. T., Fanburg B. L. Increase in endothelial cell glutathione and precursor amino acid uptake by diethyl maleate and hyperoxia. Am J Physiol. 1989 Oct;257(4 Pt 1):L265–L271. doi: 10.1152/ajplung.1989.257.4.L265. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Regulation of cellular glutathione. Am J Physiol. 1989 Oct;257(4 Pt 1):L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- ERIKSEN L. The nature of the intermediate tetrapyrroles in protoporphyrin and heme biosynthesis. Scand J Clin Lab Invest. 1962;14:11–14. [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- Gipp J. J., Bailey H. H., Mulcahy R. T. Cloning and sequencing of the cDNA for the light subunit of human liver gamma-glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem Biophys Res Commun. 1995 Jan 17;206(2):584–589. doi: 10.1006/bbrc.1995.1083. [DOI] [PubMed] [Google Scholar]
- Hinder R. A., Stein H. J. Oxygen-derived free radicals. Arch Surg. 1991 Jan;126(1):104–105. doi: 10.1001/archsurg.1991.01410250112019. [DOI] [PubMed] [Google Scholar]
- Hong R. W., Rounds J. D., Helton W. S., Robinson M. K., Wilmore D. W. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992 Feb;215(2):114–119. doi: 10.1097/00000658-199202000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenkamps R., Thies E., Younes M., Siegers C. P. Glutathione and GSH-dependent enzymes in the human gastric mucosa. Klin Wochenschr. 1984 Feb 15;62(4):183–186. doi: 10.1007/BF01731642. [DOI] [PubMed] [Google Scholar]
- Khairallah E. A., Bartolone J., Brown P., Bruno M. K., Makowski G., Wood S. Does glutathione play a role in regulating intracellular proteolysis? Prog Clin Biol Res. 1985;180:373–383. [PubMed] [Google Scholar]
- Kobayashi T., Robinson M. K., Robinson V., DeRosa E., Wilmore D. W., Jacobs D. O. Glutathione depletion alters hepatocellular high-energy phosphate metabolism. J Surg Res. 1993 Mar;54(3):189–195. doi: 10.1006/jsre.1993.1030. [DOI] [PubMed] [Google Scholar]
- Luo J. L., Hammarqvist F., Cotgreave I. A., Lind C., Andersson K., Wernerman J. Determination of intracellular glutathione in human skeletal muscle by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1995 Aug 4;670(1):29–36. doi: 10.1016/0378-4347(95)00137-8. [DOI] [PubMed] [Google Scholar]
- McCord J. M. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983 Sep;94(3):412–414. [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Murakami K., Kondo T., Ohtsuka Y., Fujiwara Y., Shimada M., Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989 Aug;38(8):753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. K., Ahn M. S., Rounds J. D., Cook J. A., Jacobs D. O., Wilmore D. W. Parenteral glutathione monoester enhances tissue antioxidant stores. JPEN J Parenter Enteral Nutr. 1992 Sep-Oct;16(5):413–418. doi: 10.1177/0148607192016005413. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Rounds J. D., Hong R. W., Jacobs D. O., Wilmore D. W. Glutathione deficiency increases organ dysfunction after hemorrhagic shock. Surgery. 1992 Aug;112(2):140–149. [PubMed] [Google Scholar]
- Shi E. C., Fisher R., McEvoy M., Vantol R., Rose M., Ham J. M. Factors influencing hepatic glutathione concentrations: a study in surgical patients. Clin Sci (Lond) 1982 Mar;62(3):279–283. doi: 10.1042/cs0620279. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Fagan J. M., Allen D. Relationship of the redox state to muscle protein degradation. Prog Clin Biol Res. 1985;180:363–372. [PubMed] [Google Scholar]
- Vinnars E., Bergstöm J., Fürst P. Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Ann Surg. 1975 Dec;182(6):665–671. doi: 10.1097/00000658-197512000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T. The possible role of glutathione in protein biosynthesis. Isr J Med Sci. 1973 Feb;9(2):129–135. [PubMed] [Google Scholar]


