Abstract
Chronic kidney disease (CKD) can progress to an advanced stage, eventually developing into end-stage renal disease (ESRD). Currently, the only effective treatment for ESRD is renal replacement therapy, with maintenance hemodialysis (MHD) being the most widely used modality, accounting for approximately 90% of all dialysis patients. However, the perioperative risk of surgery and anesthesia in these patients remains extremely high. Therefore, careful selection of surgical timing, appropriate anesthetic agents, and suitable anesthetic techniques is crucial. This report describes the anesthetic management of a 62-year-old female patient who had been hospitalized in the orthopedic ward for nine months due to left toe necrosis secondary to diabetic foot, in the setting of CKD requiring MHD. She was later admitted to Xingtai Central Hospital with progressive necrosis involving the left second and third toes and extending to the dorsum and plantar aspect of the foot. The patient was diagnosed with left-sided diabetic foot necrosis, stage 5 CKD, type 2 diabetes mellitus with multiple complications, grade 3 hypertension (high risk), and chronic hepatitis B infection. An elective transtibial (below-knee) amputation of the left lower limb was planned. Anesthesia was provided using color Doppler ultrasound-guided left iliac fascia block, left lateral femoral cutaneous nerve block, and left sciatic nerve block. Intravenous isoproterenol infusion, dexmedetomidine, and dizocine injection were administered for intraoperative sedation and analgesia. The patient’s intraoperative vital signs remained stable. Postoperatively, the patient was awake, in good general condition, and was transferred to the ward with regular monitoring.
Keywords: Renal failure, maintenance hemodialysis, surgery, anesthesia management
Introduction
Chronic kidney disease (CKD) refers to chronic structural or functional renal abnormalities due to various causes, with or without decreased glomerular filtration rate (GFR). It is characterized by abnormal renal pathology, markers of kidney damage, or an unexplained decline in GFR to below 60 ml/(min·1.73 m2) for more than three months [1]. CKD is classified into stages 1-5 based on the degree of GFR reduction, with stage 5 representing end-stage renal disease (ESRD), also known as the uremic stage [1]. As a typical chronic disease, ESRD has a prolonged course, high medical costs, and significant mortality, posing a serious global public health burden [2,3].
The main life-sustaining treatment for ESRD is renal replacement therapy (RRT), which includes hemodialysis, peritoneal dialysis, and kidney transplantation [4]. Due to donor shortages and the practicality of dialysis, maintenance hemodialysis (MHD) remains the predominant treatment for ESRD [5]. Dialysis effectively removes toxic metabolites from the blood, alleviating symptoms of renal failure and prolonging patient survival [6]. However, patients dependent on long-term dialysis often develop comorbid conditions that may require surgical intervention [7].
Diabetes mellitus (DM) is a group of metabolic disorders characterized by chronic hyperglycemia. The prevalence of diabetes-related complications continues to rise, with diabetic foot (DF) becoming increasingly common. Among its clinical manifestations, diabetic foot ulcer (DFU) is the most frequent. Without timely and appropriate treatment, amputation may be required, significantly reducing patients’ quality of life. In China, the five-year mortality rate following DF amputation reaches approximately 40% [8]. According to the Chinese Diabetic Foot Prevention and Treatment Guidelines, DF refers to ulceration and tissue destruction distal to the ankle in diabetic patients, often complicated by infection and varying degrees of lower extremity arterial occlusion, and can severely involve muscle and bone [9]. Surgical intervention, including amputation in severe cases, remains common clinical practice.
Renal dysfunction, comorbidities, and pathophysiological changes secondary to dialysis significantly affect perioperative management [10]. Therefore, the anesthetic management of these patients is highly challenging and requires thorough preoperative evaluation and optimization, careful selection of anesthetic agents, and appropriate anesthetic techniques to preserve residual renal function, minimize complications, and enhance perioperative safety. Diabetic nephropathy is among the most severe complications of diabetes and commonly progresses to renal failure. Once on MHD, patients are more susceptible to infections due to compromised immunity [11]. In this report, we describe a patient with DF who was undergoing MHD and underwent successful below-knee amputation due to severe foot ulcer infection, with a favorable postoperative outcome.
Case report
A 62-year-old female was admitted to the orthopedic ward with necrosis of her left toe persisting for nine months, accompanied by worsening pain over the preceding 20 days. Nine months prior, she had developed blackening and necrosis of the left first toe without an obvious trigger, accompanied by mild pain. She was diagnosed with DF at a local clinic but was discharged without targeted treatment. Over time, the necrosis extended to the second and third toes as well as the dorsum and sole of the left foot. Pain in the left foot progressively worsened during the last 20 days, necessitating oral tramadol for pain control. She was admitted to hospital on November 8, 2021, for further diagnosis and management.
Her medical history included hypertension for over 30 years, managed with nifedipine extended-release tablets 10 mg once daily and Betaloc (metoprolol) 12.5 mg twice daily, though blood pressure control remained unstable. She had a 15-year history of type 2 diabetes mellitus, currently treated with insulin. She also had chronic renal failure for seven years and was receiving MHD three times per week (Monday, Thursday, and Saturday). Additionally, she had a 15-year history of chronic hepatitis B without antiviral treatment. She denied any history of surgery, trauma, blood transfusion, or known drug or food allergies.
The admission diagnoses were: 1. DF necrosis of the left foot; 2. Stage 5 chronic kidney disease; 3. Type 2 DM with multiple complications; 4. Grade 3 hypertension (very high risk); 5. Chronic hepatitis B virus infection.
An elective transtibial (below-knee) amputation of the left lower limb was scheduled for November 11.
General condition: The patient was conscious, bedridden, and breathing steadily. She had dry skin and mucous membranes, hearing loss, and blurred vision. Bedside vital signs were: Blood pressure (BP): 197/84 mmHg, hear rate (HR): 76 beats/min, respiratory rate: 18 breaths/min, body temperature: 36.7°C. She was 150 cm tall, weighed 45 kg, with a BMI of 20.0 kg/m2. Head and neck mobility were normal. She had multiple missing teeth, receding gums, and was classified as Mallampati grade II.
Laboratory tests from November 9: Routine blood and coagulation tests were largely normal: WBC 7.98 × 109/L, Hb 115 g/L, PLT 180 × 109/L, APTT 28.9 s, PT 11.8 s, and fibrinogen concentration (FIB) 6.48 g/L. White blood cell count: 7.98 × 109/L, hemoglobin: 115 g/L, platelet count: 180 × 109/L, activated partial thromboplastin time: 28.9 s, prothrombin time: 11.8 s, and fibrinogen: 6.48 g/L. Liver and kidney function: potassium 4.59 mmol/L, sodium 136.0 mmol/L↓, chlorine 94.8 mmol/L↓, creatinine 324.3 μmol/L↑, urea 14.9 mmol/L↑, β2-micron Globulin 20.23 mg/L↑, albumin 31.2 g/L↓, glucose 10.45 mmol/L↑. Blood gas analysis: oxygen partial pressure 81.8 mmHg↓. The rest of the test results were normal.
Respiratory system: No recent symptoms of upper respiratory tract infection.
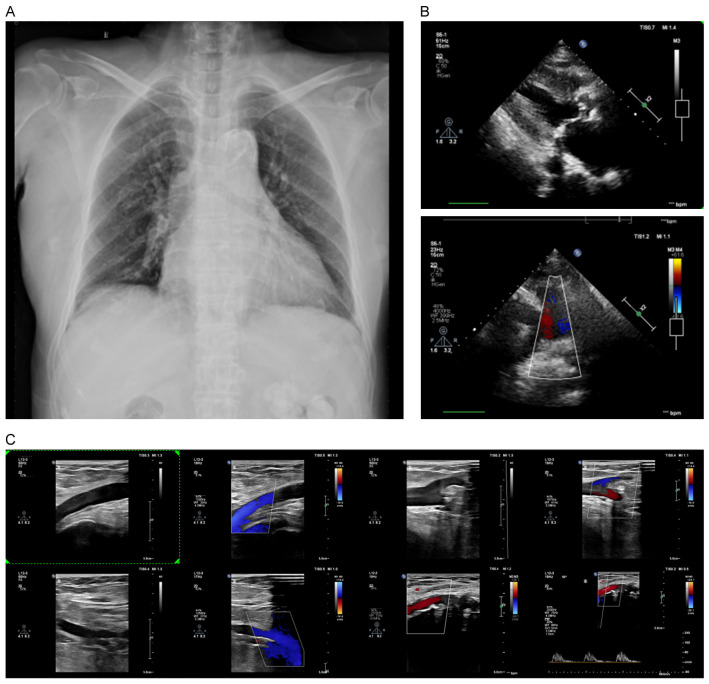
Chest X-ray: 1) Cardiomegaly (cardiothoracic ratio ~0.55); 2) Aortic sclerosis (Figure 1A); mild pneumonia.
Figure 1.
Radiographic image of the patient. A: Chest X-ray; B: Cardiac ultrasound; C: Arteriovenous ultrasound.
Circulatory system: ECG showed sinus rhythm, left ventricular hypertrophy with ST-T changes, QT prolongation, and other abnormalities. Echocardiography (Figure 1B) showed valvular degeneration with aortic stenosis, left ventricular hypertrophy, left atrial enlargement, trace pericardial effusion, and a left ventricular ejection fraction of 57%.
Metabolic equivalents (METs): 1 MET (long-term bed rest, unable to perform self-care); NYHA functional class II; Revised Cardiac Risk Index score: 2 points (1 for insulin-dependent diabetes, 1 for preoperative creatinine >2.0 mg/dL), corresponding to a class 3 cardiac risk with an estimated cardiac complication rate of 7%.
Other systems: Duplex ultrasonography of both lower limbs (Figure 1C) showed heterogeneous intima-media thickening with multiple plaques, arterial stenosis, and occlusion.
Essen stroke risk score: 4 points (hypertension, diabetes, peripheral vascular disease, other cardiovascular disease), indicating moderate risk with an annual stroke risk of 7-9%.
A multidisciplinary preoperative consultation with nephrology, cardiology, and endocrinology was conducted. Recommendations included increasing insulin and antihypertensive dosages and maintaining blood pressure at the higher end of the acceptable range.
Anesthesia process
The patient entered the operating room at 08:50 a.m. on November 11. Routine monitoring was initiated: HR: 79 beats/min, BP: 210/100 mmHg, oxygen saturation (SpO2): 98%. At 09:25, a color Doppler ultrasound-guided left fascia iliaca block was performed using 20 ml of a mixture of 0.5% ropivacaine and 1% lidocaine. At 09:30, a left lateral femoral cutaneous nerve block was administered with 3 ml of the same mixture. At 09:35, a left sciatic nerve block was performed using 10 ml of the same mixture.
By 09:44, surgical site pinprick testing confirmed adequate analgesia. The operation commenced at 09:45. From 09:40 to 10:30, propofol was infused intravenously at 100 mg/h; from 09:40 to 10:15, dexmedetomidine was infused at 10 µg/h to achieve light sedation and anxiolysis. At 10:26, 1 mg of dezocine was administered IV to alleviate discomfort from traction. The operation was completed at 10:50. The patient received supplemental oxygen via a facemask throughout the procedure.
Intraoperatively, SpO2 remained ≥98%, BP fluctuated between 165-210/80-100 mmHg, mean arterial pressure was approximately 170 mmHg, HR varied from 65-80 bpm, and point-of-care glucose was 5.6 mmol/L. Total blood loss was about 150 ml, with no urine output. Total fluid replacement was 150 ml of crystalloid.
The patient was awake, in stable condition, and returned to the ward for regular postoperative monitoring. Recovery was uneventful, and she was discharged on December 11. She continued on a diabetic diet and oral hypoglycemic agents. Routine postoperative follow-up visits confirmed satisfactory recovery.
Discussion
Among DF complications, DFU is one of the most common clinical manifestations. Without timely and standardized treatment, amputation is often required, which significantly reduces patients’ quality of life [12]. MHD is the main treatment for ESRD in China, with over 80% of patients with uremia receiving this modality [13].
It is generally recommended that patients on MHD undergo surgery on non-dialysis days [14]. Preoperative hemodialysis is essential to correct electrolyte imbalances and optimize fluid status [15]. Long-term dialysis patients have an increased risk of perioperative bleeding and transfusion requirements, so adequate dialysis before surgery is necessary. Effective preoperative dialysis has been shown to improve platelet function [16]. From a pharmacokinetic and pharmacodynamic perspective, the kidney is a major organ for drug metabolism and excretion; therefore, impaired renal function can significantly affect drug actions [17]. Careful anesthetic selection is thus critical in this population.
In this case, propofol was administered via continuous intravenous infusion at 100 mg/h during the surgery. Propofol is a non-barbiturate intravenous anesthetic with rapid onset, quick recovery, and minimal side effects, making it widely used for general anesthesia [18]. It is primarily metabolized in the liver through glucuronidation and aromatic hydroxylation; only 0.3% of the unchanged drug is excreted in urine. Propofol clearance depends mainly on hepatic metabolism and is largely unaffected by renal impairment, making it safe for use in patients with renal dysfunction [19]. Additionally, propofol may exert renal protective effects by inhibiting apoptosis, reducing cytokine and inflammatory factor expression, suppressing the release of inflammatory mediators, scavenging oxygen free radicals, and mitigating calcium overload [20]. Because it does not accumulate in patients with normal liver function, propofol is suitable for both induction and maintenance of anesthesia [21]. Therefore, an appropriate propofol induction regimen is particularly important.
Dexmedetomidine was also infused at a constant rate of 10 µg/h to maintain light sedation and reduce perioperative anxiety. Dexmedetomidine is a highly selective α2-adrenergic receptor agonist that acts mainly in the brain and spinal cord to decrease sympathetic outflow, providing sedation, analgesia, and anxiolysis while lowering blood pressure and heart rate with minimal respiratory depression [22]. It is primarily metabolized in the liver into inactive metabolites, 95% of which are excreted by the kidneys and about 4% in feces, with negligible unchanged drug excretion [23]. Because its pharmacokinetics do not differ significantly from those in healthy individuals, dexmedetomidine is considered safe for use in patients with renal disease, although dosage adjustments may be necessary to avoid prolonged sedation.
Additionally, 1 mg of dezocine was administered intravenously to alleviate discomfort caused by surgical traction. Dezocine is a partial agonist of μ- and κ-opioid receptors with potent analgesic effects, reportedly stronger than morphine [24,25]. Adverse effects are rare at typical clinical concentrations (average peak plasma concentration ~45 ng/ml), and compared to full μ-opioid agonists, dezocine has limited physical dependence and minimal respiratory depression [26]. It also has mild sedative properties; studies have shown a dose-dependent sedative effect with a ceiling phenomenon, with significant sedation observed at 5 mg/kg but diminishing effect at higher doses [27]. Dezocine has negligible δ-receptor activity, so side effects such as agitation and anxiety are uncommon. Recent studies also suggest dezocine may inhibit norepinephrine and serotonin reuptake [28]. After intramuscular injection, dezocine reaches peak effect within 10-90 minutes and provides analgesia comparable to morphine, with a similar duration of action. It is mainly metabolized in the liver to glucuronide conjugates, with about two-thirds excreted renally and approximately 1% excreted unchanged; other excretion routes may include the biliary tract [29]. Therefore, dezocine is suitable for analgesia in patients with renal dysfunction.
Regarding anesthetic technique, methods with minimal systemic impact - such as local anesthesia, nerve block anesthesia, or spinal anesthesia - are generally preferred. However, because most hemodialysis regimens use heparin or low-molecular-weight heparin, the risk of neuraxial anesthesia is higher due to potential spinal hematoma, which could lead to catastrophic complications. A large data analysis involving 264,421 patients undergoing general anesthesia and 64,119 patients receiving neuraxial anesthesia, peripheral nerve block, or combined techniques found no significant difference in 30-day mortality between techniques [30]. Moreover, there is no strong evidence that the choice of anesthetic technique affects the incidence of postoperative myocardial infarction, stroke, renal complications, pulmonary embolism, or peripheral nerve injury. Several studies have demonstrated that inhalational anesthetics, propofol, dexmedetomidine, and opioid analgesics may exert renal protective effects through multiple mechanisms [31,32].
In conclusion, this successful amputation case demonstrates that amputation surgery is safe and feasible for DF patients on MHD when using a regimen combining propofol, dexmedetomidine, and dezocine. This experience may provide a useful reference for anesthetic management in similar clinical scenarios.
Disclosure of conflict of interest
None.
References
- 1.Yang L, He Y, Li X. Physical function and all-cause mortality in patients with chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2023;55:1219–1228. doi: 10.1007/s11255-022-03397-w. [DOI] [PubMed] [Google Scholar]
- 2.Lin MY, Liu MF, Hsu LF, Tsai PS. Effects of self-management on chronic kidney disease: a meta-analysis. Int J Nurs Stud. 2017;74:128–137. doi: 10.1016/j.ijnurstu.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Yu IC, Tsai YF, Fang JT, Yeh MM, Fang JY, Liu CY. Effects of mouthwash interventions on xerostomia and unstimulated whole saliva flow rate among hemodialysis patients: a randomized controlled study. Int J Nurs Stud. 2016;63:9–17. doi: 10.1016/j.ijnurstu.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Wald R, Beaubien-Souligny W, Chanchlani R, Clark EG, Neyra JA, Ostermann M, Silver SA, Vaara S, Zarbock A, Bagshaw SM. Delivering optimal renal replacement therapy to critically ill patients with acute kidney injury. Intensive Care Med. 2022;48:1368–1381. doi: 10.1007/s00134-022-06851-6. [DOI] [PubMed] [Google Scholar]
- 5.Wong SPY, Rubenzik T, Zelnick L, Davison SN, Louden D, Oestreich T, Jennerich AL. Long-term outcomes among patients with advanced kidney disease who forgo maintenance dialysis: a systematic review. JAMA Netw Open. 2022;5:e222255. doi: 10.1001/jamanetworkopen.2022.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang YS, Tsai YC, Chen TW, Li SY. Artificial kidney engineering: the development of dialysis membranes for blood purification. Membranes (Basel) 2022;12:177. doi: 10.3390/membranes12020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Zhang X, Hu F, Yuan H, Gao Z, He L, Zou S. Impact of enhanced recovery after surgery program for hungry bone syndrome in patients on maintenance hemodialysis undergoing parathyroidectomy for secondary hyperparathyroidism. Ann Surg Treat Res. 2022;103:264–270. doi: 10.4174/astr.2022.103.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023;25:36–45. doi: 10.1111/dom.14840. [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Lv G, Cheng X, Ma X, Wang W, Gui J, Hu J, Lu M, Chu G, Chen J, Zhang H, Jiang Y, Chen Y, Yang W, Jiang L, Geng H, Zheng R, Li Y, Feng W, Johnson B, Wang W, Zhu D, Hu Y. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition) Burns Trauma. 2020;8:tkaa017. doi: 10.1093/burnst/tkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury SR, McLure HA. Chronic kidney disease and anaesthesia. BJA Educ. 2022;22:321–328. doi: 10.1016/j.bjae.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair MR, Souli M, Ruffin F, Park LP, Dagher M, Eichenberger EM, Maskarinec SA, Thaden JT, Mohnasky M, Wyatt CM, Fowler VG Jr. Staphylococcus aureus bacteremia among patients receiving maintenance hemodialysis: trends in clinical characteristics and outcomes. Am J Kidney Dis. 2022;79:393–403. e391. doi: 10.1053/j.ajkd.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaka AS, Landsteiner A, Ensrud KE, Logan B, Sowerby C, Ullman K, Yoon P, Wilt TJ, Sultan S. Risk prediction models for diabetic foot ulcer development or amputation: a review of reviews. J Foot Ankle Res. 2023;16:13. doi: 10.1186/s13047-023-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu F, Zhuang B, Wang Z, Wu H, Hui X, Peng H, Bian X, Ye H. Knowledge, attitude, and practice of patients receiving maintenance hemodialysis regarding hemodialysis and its complications: a single-center, cross-sectional study in Nanjing. BMC Nephrol. 2023;24:275. doi: 10.1186/s12882-023-03320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murea M, Moossavi S, Fletcher AJ, Jones DN, Sheikh HI, Russell G, Kalantar-Zadeh K. Renal replacement treatment initiation with twice-weekly versus thrice-weekly haemodialysis in patients with incident dialysis-dependent kidney disease: rationale and design of the TWOPLUS pilot clinical trial. BMJ Open. 2021;11:e047596. doi: 10.1136/bmjopen-2020-047596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segar JL, Chock VY, Harer MW, Selewski DT, Askenazi DJ. Fluid management, electrolytes imbalance and renal management in neonates with neonatal encephalopathy treated with hypothermia. Semin Fetal Neonatal Med. 2021;26:101261. doi: 10.1016/j.siny.2021.101261. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Karaboyas A, Gan L, Hou FF, Liang X, Chen X, Chen Y, Ni Z, Pecoits-Filho R, Zuo L. Platelet count has a U-shaped association with mortality in hemodialysis patients. Sci Rep. 2024;14:26572. doi: 10.1038/s41598-024-77718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taavo M, Rundgren M, Frykholm P, Larsson A, Franzén S, Vargmar K, Valarcher JF, DiBona GF, Frithiof R. Role of renal sympathetic nerve activity in volatile Anesthesia’s effect on renal excretory function. Function (Oxf) 2021;2:zqab042. doi: 10.1093/function/zqab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enlund M, Berglund A, Enlund A, Bergkvist L. Volatile versus propofol general anesthesia and long-term survival after breast cancer surgery: a national registry retrospective cohort study. Anesthesiology. 2022;137:315–326. doi: 10.1097/ALN.0000000000004309. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Anjankar AP. Propofol-related infusion syndrome: a clinical review. Cureus. 2022;14:e30383. doi: 10.7759/cureus.30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akdeniz S, Ulu MB, Bakirtas M, Erdemir F. The renal protective efficacy of ketamine vs. propofol vs. ketofol against renal ischemia/reperfusion injury: an experimental rat study. Medicine Science. 2022;11:1317. [Google Scholar]
- 21.Dinis-Oliveira RJ. Metabolic profiles of propofol and fospropofol: clinical and forensic interpretative aspects. Biomed Res Int. 2018;2018:6852857. doi: 10.1155/2018/6852857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan D, Liu Z, Kaindl J, Maeda S, Zhao J, Sun X, Xu J, Gmeiner P, Wang HW, Kobilka BK. Activation of the α(2B) adrenoceptor by the sedative sympatholytic dexmedetomidine. Nat Chem Biol. 2020;16:507–512. doi: 10.1038/s41589-020-0492-2. [DOI] [PubMed] [Google Scholar]
- 23.Ferland CE, Vega E, Ingelmo PM. Acute pain management in children: challenges and recent improvements. Curr Opin Anaesthesiol. 2018;31:327–332. doi: 10.1097/ACO.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 24.Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37:1105–1121. doi: 10.1002/phar.1986. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Chai JR, Xu XJ, Ye RF, Zan GY, Liu GY, Long JD, Ma Y, Huang X, Xiao ZC, Dong H, Wang YJ. Pharmacological characterization of dezocine, a potent analgesic acting as a κ partial agonist and μ partial agonist. Sci Rep. 2018;8:14087. doi: 10.1038/s41598-018-32568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malis JL, Rosenthale ME, Gluckman MI. Animal pharmacology of Wy-16,225, a new analgesic agent. J Pharmacol Exp Ther. 1975;194:488–498. [PubMed] [Google Scholar]
- 27.Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120:714–723. doi: 10.1097/ALN.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandit UA, Kothary SP, Pandit SK. Intravenous dezocine for postoperative pain: a double-blind, placebo-controlled comparison with morphine. J Clin Pharmacol. 1986;26:275–280. doi: 10.1002/j.1552-4604.1986.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 29.Gan W, Yang X, Chen J, Lyu H, Yan A, Chen G, Li S, Zhang Y, Dan L, Huang H, Duan G. Role of daytime variation in pharmaceutical effects of sufentanil, dezocine, and tramadol: a matched observational study. Front Pharmacol. 2022;13:993506. doi: 10.3389/fphar.2022.993506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saied NN, Helwani MA, Weavind LM, Shi Y, Shotwell MS, Pandharipande PP. Effect of anaesthesia type on postoperative mortality and morbidities: a matched analysis of the NSQIP database. Br J Anaesth. 2017;118:105–111. doi: 10.1093/bja/aew383. [DOI] [PubMed] [Google Scholar]
- 31.Motayagheni N, Phan S, Eshraghi C, Nozari A, Atala A. A review of anesthetic effects on renal function: potential organ protection. Am J Nephrol. 2017;46:380–389. doi: 10.1159/000482014. [DOI] [PubMed] [Google Scholar]
- 32.Zeng B, Liu Y, Xu J, Niu L, Wu Y, Zhang D, Tang X, Zhu Z, Chen Y, Hu L, Yu S, Yu P, Zhang J, Wang W. Future directions in optimizing anesthesia to reduce perioperative acute kidney injury. Am J Nephrol. 2023;54:434–450. doi: 10.1159/000533534. [DOI] [PubMed] [Google Scholar]