Abstract
Objective: To analyze the impact of combined spinal-epidural anesthesia and epidural analgesia on pain and blood pressure in preeclamptic women undergoing painless delivery. Methods: A retrospective analysis was conducted on the clinical data of 137 preeclamptic women who underwent painless delivery at The Second People’s Hospital of Linhai City between July 2022 and July 2024. Based on the type of analgesia intervention received, the women were divided into a control group (n=68, receiving Doula delivery analgesia) and an observation group (n=69, receiving combined spinal-epidural anesthesia and epidural analgesia). Labor duration, pain (assessed using the Visual Analogue Scale (VAS)), blood pressure [systolic blood pressure (SBP), diastolic blood pressure (DBP)], serum markers [prolactin (PRL), tissue-type plasminogen activator (t-PA)] levels, and maternal and neonatal outcomes were compared between the two groups. Results: ① Labor duration: Significant differences were observed between groups (F=10.279), over time (F=35.794), and in their interaction (F=16.589) (P < 0.05). Within groups: both groups had shorter second and third stages of labor compared to the first stage (P < 0.05). Between groups: no significant difference was observed in the second stage (P > 0.05), but the observation group had shorter first and third stages than the control group (P < 0.05). ② Pain: Significant differences were found between the groups (F=19.785), over time (F=8.637), and in their interaction (F=14.492) (P < 0.05). Within groups: both groups had lower VAS scores in the 1-3 stages of labor compared to before analgesia (P < 0.05). Between groups: no significant difference in VAS scores before analgesia (P > 0.05), but the observation group had lower VAS scores in all stages than the control group (P < 0.05). ③ Blood pressure: Significant differences in SBP were found between the groups (F=5.572), over time (F=10.295), and in their interaction (F=8.149) (P < 0.05); similarly, significant differences in DBP were found between the groups (F=4.915), over time (F=9.761), and in their interaction (F=7.784) (P < 0.05). Within groups: both groups had lower SBP and DBP levels at 10 minutes post-analgesia, during the active phase, and in the second stage of labor compared to before analgesia (P < 0.05). Between groups: no significant difference in SBP and DBP levels before analgesia (P > 0.05), but the observation group had lower SBP and DBP levels at 10 minutes post-analgesia, during the active phase, and in the second stage of labor compared to the control group (P < 0.05). ④ Serum indicators: Both groups showed increased PRL and t-PA levels post-delivery compared to pre-delivery (P < 0.05); the observation group had higher post-delivery PRL and lower t-PA levels compared to the control group (P < 0.05). ⑤ Maternal and neonatal outcomes: The observation group had lower rates of cesarean section, postpartum hemorrhage, postpartum blood loss, and neonatal asphyxia compared to the control group (P < 0.05); no significant difference was found in neonatal Apgar scores between the two groups (P > 0.05). Conclusion: The combined use of spinal-epidural anesthesia and epidural analgesia in preeclamptic women undergoing painless delivery shows significant effectiveness. Compared to Doula delivery analgesia, this method accelerates labor, relieves pain, lowers blood pressure, improves serum indicators, and decreases adverse maternal and neonatal outcomes.
Keywords: Spinal-epidural anesthesia, epidural analgesia, preeclampsia, labor, pain, blood pressure, impact
Introduction
Preeclampsia is a pregnancy-specific complication that typically occurs after 20 weeks of gestation, primarily characterized by hypertension, accompanied by proteinuria and edema. In severe cases, it can progress to eclampsia, resulting in multi-organ dysfunction [1]. This condition poses a significant threat to both maternal and fetal health, with potential adverse effects on fetal development and the delivery process [2]. Epidemiological data [3] indicate an incidence of about 5% to 10%, though its pathogenesis remains complex and not fully understood. Preeclampsia is associated with various factors, including genetics, immune responses, endothelial dysfunction, and inflammation [4,5]. During labor, the unique pathophysiological state of preeclamptic patients often presents additional challenges and risks. First, intense labor pain can over-activate the sympathetic nervous system, further elevating blood pressure, exacerbating maternal hypertension, and raising the risk of maternal and fetal complications [6]. Additionally, vascular dysfunction and coagulopathy in hypertensive states may increase the risk of postpartum hemorrhage [7]. Moreover, impaired placental function in preeclamptic patients also increases the likelihood of fetal distress, intrauterine growth restriction, preterm birth, and neonatal asphyxia [8]. Therefore, effectively managing pain and blood pressure in preeclamptic patients during labor is a significant challenge for obstetricians.
Painless delivery is a crucial technique in modern obstetrics, aimed at reducing pain during labor through pharmacological or non-pharmacological means, thus improving the childbirth experience, and mitigating stress responses induced by pain [9]. Among various techniques, Doula delivery analgesia is widely used due to its relatively low complication rate [10]. However, Doula delivery analgesia alone may not fully meet the special needs of preeclamptic patients, particularly in terms of pain and blood pressure control [11]. In recent years, the combined use of spinal-epidural anesthesia and epidural analgesia has gained attention. Spinal-epidural anesthesia effectively relieves labor pain by blocking the transmission of spinal nerve roots [12], while epidural analgesia prolongs the analgesic effect, helping to stabilize blood pressure [13]. This combined analgesic approach is promising for improving the childbirth experience in preeclamptic patients.
Although studies [14] have explored the effects of combined spinal-epidural anesthesia and epidural analgesia in general obstetric patients, research specifically focusing on preeclamptic patients remains limited. The innovation of this study lies in its investigation of the combined spinal-epidural anesthesia and epidural analgesia approach specifically for preeclamptic women undergoing painless delivery, a group that may not respond to conventional analgesia techniques in the same manner. This study not only evaluates the effectiveness of this combined anesthetic technique in managing labor pain and blood pressure but also provides new insights into its impact on maternal and neonatal outcomes. By comparing this approach with Doula delivery analgesia, this study offers evidence on how the combined technique can enhance labor progression, pain relief, and blood pressure regulation in preeclamptic patients. These findings may significantly contribute to the development of more tailored and effective management strategies for this high-risk population, offering a promising alternative to conventional pain management techniques.
Materials and methods
Basic information
A retrospective analysis was conducted on the clinical data of 137 pregnant women with hypertensive disorders who underwent painless delivery at The Second People’s Hospital of Linhai City from July 2022 to July 2024. Inclusion criteria: ① Meeting the clinical diagnostic criteria for hypertensive disorders of pregnancy [15]; ② SBP > 90 mmHg, DBP > 140 mmHg; ③ Singleton pregnancy with gestational age > 28 weeks; ④ American Society of Anesthesiologists (ASA) [16] classification I-II; ⑤ Complete clinical data, comprehensive prenatal examinations, no significant cephalopelvic disproportion, and delivery at our hospital. Exclusion criteria: ① Multiple pregnancies; ② Combined with ectopic pregnancy, gestational diabetes, placental abruption, severe preeclampsia, etc.; ③ Presence of birth canal abnormalities and/or oligohydramnios (< 400 mL); ④ Presence of umbilical cord around the neck for more than 2 weeks; ⑤ Severe organ dysfunction; ⑥ Presence of immune system, hematological diseases, and/or severe infections; ⑦ Accompanied by cognitive dysfunction and/or psychiatric disorders; ⑧ Allergic reactions or contraindications to the treatment or interventions used in this study. The patients were divided into a control group (n=68, receiving Doula delivery analgesia) and an observation group (n=69, receiving combined spinal-epidural anesthesia and epidural analgesia) based on the analgesic interventions received. This study was approved by the Medical Ethics Committee of The Second People’s Hospital of Linhai City. The study was conducted in accordance with the Declaration of Helsinki.
Sample size calculation
The sample size for this study was determined through a statistical power analysis. Based on prior research and clinical experience, we anticipated a medium effect size of 0.5 (Cohen’s d), a significance level (α) of 0.05, and a statistical power (1-β) of 0.8. According to these parameters, the power analysis indicated that a minimum of 63 participants per group were required to detect statistically significant differences. To account for possible dropout and incomplete data, a total of 137 participants were included in this study (68 in the control group and 69 in the observation group), exceeding the calculated minimum of 126 participants. This ensures adequate statistical power for the study’s analyses and enhances the reliability of the results. The power analysis was performed using standard statistical methods and confirmed that the sample size is sufficient to detect differences in labor duration, pain relief, blood pressure control, and maternal and neonatal outcomes.
Methods
During the preoperative preparation stage, the pregnant women were instructed to fast for 12 hours and refrain from drinking water for 4 hours. Routine oxygen therapy was administered, and their vital signs were closely monitored. Before the start of the surgery, an intravenous line was established in the upper limb, and the patients were positioned in the left lateral decubitus position to facilitate the procedure. During the latent phase of the first stage of labor, a vaginal examination was conducted every 4 hours, and fetal heart monitoring was performed to assess labor progress. Upon entering the active phase, vaginal examinations and fetal heart monitoring were performed every 2 hours. In the second stage of labor, continuous fetal heart monitoring was performed, and patients were guided to undergo natural delivery. In case of any emergency, forceps or episiotomy were used to assist delivery as needed. If these methods were ineffective, a cesarean section was promptly performed.
Control group
The control group received Doula delivery analgesia. When cervical dilation reached 2 cm or more, the Doula delivery analgesia device (manufactured by Shanghai Huanxi Medical Device Co., Ltd., model YX-101) was used for pain relief. The device was operated strictly according to the manufacturer’s instructions, and patients were guided to cooperate actively to complete the entire delivery process smoothly.
Observation group
The observation group received combined spinal-epidural anesthesia and epidural analgesia. The procedures were as follows: First, epidural puncture was performed at the L2-L3 intervertebral space, and a 25 G spinal anesthesia needle was inserted into the subarachnoid space. A slow injection of 10-15 mg of 1% ropivacaine hydrochloride (Jiangsu Hengrui Medicine Co., Ltd., National Medicine Standard H20060137) and 5-10 μg of fentanyl citrate (Yichang Humanwell Pharmaceutical Co., Ltd., National Medicine Standard H42022076) was administered. Then, an epidural catheter was placed and connected to a patient-controlled analgesia micro-pump (Henan Tuoren Medical Device Group Co., Ltd., model TR-10-275). A mixture of 50 μg/mL of fentanyl and 0.1% ropivacaine hydrochloride was injected through the micro-pump, with a total volume of 100 mL. The dosage for each administration was 5 mL, the infusion rate was maintained at 6 mL/h, and the time interval between doses was 15 minutes. After the procedure, combined epidural analgesia was implemented with an injection of 0.4 μg sufentanil citrate and 0.1% ropivacaine hydrochloride. The loading dose was 5 mL, and the maintenance dose was also 6 mL/h, with a time interval of 30 minutes between doses.
Observation indicators
(1) Time spent in each stage of labor: The duration of the first, second, and third stages of labor was uniformly recorded by the medical staff at our hospital.
(2) Pain assessment: Pain levels were evaluated using the Visual Analog Scale (VAS) [17] at four time points: pre-analgesia, during the first, second, and third stages of labor. The VAS scores range from 0 to 10, with higher scores indicating greater pain.
(3) Blood pressure assessment: Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automatic blood pressure monitor before analgesia, 10 minutes after analgesia, during the active phase, and during the second stage of labor.
(4) Serum biomarker levels: Before and after delivery, 6 mL of venous blood was drawn from the patients, centrifuged routinely to obtain the supernatant. Prolactin (PRL) levels were measured using chemiluminescence, and tissue-type plasminogen activator (t-PA) levels were measured using enzyme-linked immunosorbent assay (ELISA). The ELISA kit for t-PA was purchased from Wuhan Saipei Biotechnology Co., Ltd. (Lot No. SP11580).
(5) Maternal and neonatal outcomes: Cesarean section rate, postpartum hemorrhage rate, postpartum hemorrhage volume, neonatal asphyxia rate, and neonatal Apgar score [18] were recorded. Apgar scores range from 0 to 10, with higher scores indicating better neonatal condition.
Statistical analysis
GraphPad Prism 8 software was used for graphing, and SPSS 22.0 software was used for data processing. Count data were expressed as (n, %) and analyzed by χ2 test; measurement data were expressed as (x̅±s), with independent sample t-test used for comparisons between the two groups and paired t-test used for intra-group comparisons. For data collected at multiple time points, repeated measures analysis of variance (ANOVA) was conducted to assess differences across time points, between groups, and their interactions. Post hoc pairwise comparisons were performed using Bonferroni correction where appropriate. P < 0.05 was considered statistically significant.
Results
Comparison of general data
The general data of the two groups was comparable, with no statistically significant differences (P > 0.05), as shown in Table 1.
Table 1.
Comparison of general data between the two groups [x̅±s, n (%)]
| Group | Age (years) | Gestational Age (weeks) | Parity | ASA Classification | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Primipara | Multipara | Grade I | Grade II | |||
| Control group (n=68) | 25.37±2.46 | 39.17±0.34 | 28 (41.18) | 40 (58.82) | 19 (27.94) | 49 (72.06) |
| Observation group (n=69) | 25.84±2.32 | 39.09±0.38 | 33 (47.83) | 36 (52.17) | 16 (23.19) | 53 (76.81) |
| t/x2 | 1.15 | 1.298 | 0.613 | 0.406 | ||
| P | 0.251 | 0.196 | 0.433 | 0.523 | ||
ASA Classification: American Society of Anesthesiologists Physical Status Classification System.
Comparison of labor duration
ANOVA revealed significant differences in labor duration between groups (F=10.279, P < 0.05), across time points (F=35.794, P < 0.05), and in the interaction between group and time (F=16.589, P < 0.05). Within groups: The duration of the second and third stages of labor was significantly shorter than that of the first stage in both groups (P < 0.05). Between groups: The duration of the second stage labor showed no significant difference (P > 0.05), while the first and third stages of labor were significantly shorter in the observation group compared to the control group (P < 0.05), as shown in Figure 1.
Figure 1.

Comparison of labor duration between the two groups (x̅±s, min). Note: *P < 0.05, compared with the control group at the same time point; #P < 0.05, compared with the first stage of labor in the same group; ΔP < 0.05, compared with the second stage of labor in the same group.
Comparison of pain levels
ANOVA showed significant differences in VAS scores between groups (F=19.785, P < 0.05), across time points (F=8.637, P < 0.05), and in the interaction between group and time (F=14.492, P < 0.05). Within groups: In both groups, the VAS scores during the first, second, and third stages of labor were significantly lower than those before analgesia (P < 0.05). Between groups: There was no significant difference in VAS scores before analgesia between the two groups (P > 0.05). However, during the first, second, and third stages of labor, the VAS scores were significantly lower in the observation group compared to the control group (P < 0.05), as shown in Figure 2.
Figure 2.

Comparison of pain levels between the two groups during various periods (x̅±s, points). VAS score: Pain assessed using the Visual Analogue Scale (VAS) score. Note: *P < 0.05, compared with the control group at the same time point; #P < 0.05, compared with the first stage of labor in the same group; ΔP < 0.05, compared with the second stage of labor in the same group; ▲P < 0.05, compared with before analgesia in the same group.
Comparison of blood pressure
ANOVA revealed significant differences in SBP between groups (F=5.572, P < 0.05), across time points (F=10.295, P < 0.05), and in the interaction between group and time (F=8.149, P < 0.05). Similarly, significant differences were observed for DBP by group (F=4.915, P < 0.05), time (F=9.761, P < 0.05), and interaction (F=7.784, P < 0.05). Within groups: In both groups, the SBP and DBP levels at 10 minutes after analgesia, during the active phase, and during the second stage of labor were significantly lower than those before analgesia (P < 0.05). Between groups: There was no significant difference in SBP and DBP levels before analgesia between the two groups (P > 0.05). However, 10 minutes after analgesia, during the active phase, and during the second stage of labor, the SBP and DBP levels were significantly lower in the observation group than in the control group (P < 0.05), as shown in Figure 3.
Figure 3.
Comparison of blood pressure between two groups at various time points (x̅±s, mmHg). Note: A: Systolic Blood Pressure (SBP) levels; B: Diastolic Blood Pressure (DBP) levels. *P < 0.05, compared with the control group at the same time point; #P < 0.05, compared with 10 minutes after analgesia in the same group; ΔP < 0.05, compared with the active phase in the same group; ▲P < 0.05, compared with before analgesia in the same group.
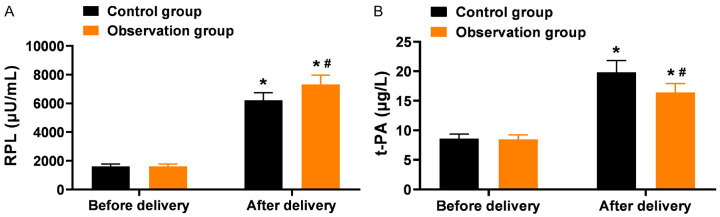
Comparison of serum biomarkers
The PRL and t-PA levels increased after delivery compared to before delivery in both groups (P < 0.05). The observation group had higher PRL levels and lower t-PA levels compared to the control group after delivery (P < 0.05), as shown in Figure 4.
Figure 4.
Comparison of serum biomarkers between the two groups before and after delivery (x̅±s). A: Serum levels of prolactin (PRL) levels; B: Tissue-type plasminogen activator (t-PA) levels. Note: *P < 0.05, compared with before delivery within the same group; #P < 0.05, compared with the control group after delivery.
Comparison of maternal and neonatal outcomes
The observation group had lower cesarean section rate, postpartum hemorrhage rate, postpartum hemorrhage volume, and neonatal asphyxia rate compared to the control group (P < 0.05). There was no significant difference in neonatal Apgar scores between the two groups (P > 0.05), as shown in Table 2.
Table 2.
Comparison of maternal and neonatal outcomes between the two groups [x̅±s, n (%)]
| Cesarean Section Rate | Postpartum Hemorrhage Rate | Postpartum Hemorrhage Volume (mL) | Neonatal Asphyxia Rate | Neonatal Apgar Score (points) | |
|---|---|---|---|---|---|
| Control group (n=68) | 17 (25.00) | 13 (19.12) | 243.86±12.79 | 8 (11.76) | 9.26±0.78 |
| Observation group (n=69) | 6 (8.70) | 3 (4.35) | 223.57±10.34 | 1 (1.45) | 9.32±0.75 |
| t/x2 | 6.517 | 7.243 | 10.218 | 4.375 | 0.459 |
| P | 0.01 | 0.007 | < 0.001 | 0.036 | 0.647 |
Discussion
The intense pain experienced during childbirth is considered one of the most severe pain experiences in a woman’s life, and patients with gestational hypertension are particularly vulnerable to the negative impacts of pain due to the nature of their condition [19]. As pain intensifies, women may experience adverse emotions such as fear, anxiety, and tension. These emotional responses further exacerbate the body’s stress reaction, leading to a significant secretion of stress hormones like adrenaline and norepinephrine. The increase in these endogenous and exogenous stress substances not only disrupts the autonomic nervous system function but may also cause a series of negative effects, including a sharp rise in blood pressure, uterine contractions disturbances, poor cervical dilation, and ineffective contractions [20]. These physiological changes can directly affect the progression of labor, potentially leading to prolonged labor and increasing the risks of severe complications such as postpartum hemorrhage, neonatal asphyxia, and fetal distress. Additionally, prolonged pain and stress responses may also weaken the mother’s physical strength, further increasing the risk of cesarean section. Therefore, effective pain relief during labor for patients with gestational hypertension is critical for ensuring maternal and neonatal safety. In recent years, with advancements in perinatal medicine, clinical attention to labor pain has grown. Numerous studies [21,22] have demonstrated that appropriate pain management not only alleviates maternal suffering but also improves labor outcomes, shortens labor duration, and reduces the incidence of maternal and neonatal complications. For patients with gestational hypertension, selecting the appropriate analgesic method is crucial, not only to relieve pain but also to stabilize blood pressure and facilitate smooth delivery, thereby ensuring maternal and infant health. Therefore, pain management for patients with gestational hypertension is a crucial measure for improving labor quality and reducing labor risks, ultimately contributing to maternal and neonatal safety.
Labor analgesia refers to various techniques used to alleviate or eliminate pain during childbirth, effectively reducing stress responses and minimizing the occurrence of adverse events. Among the available methods, labor analgesia using a parturition guide has gained significant attention in recent years as a non-pharmacological approach. This technique involves the continuous delivery of low-frequency D-T pulse waves to provide gentle and sustained stimulation to specific peripheral nerves of the laboring woman. This stimulation promotes the natural production of endogenous opioids (pain-relieving substances similar to morphine) within the body, activating the pain relief system and enhancing the release of analgesic neurotransmitters to reduce pain [23]. However, despite avoiding the medical risks associated with pharmacological analgesia, a study [24] has shown that the overall analgesic effect of labor analgesia using a parturition guide often falls short of clinical expectations, particularly during intense pain during childbirth, where its effectiveness appears relatively limited. Therefore, there is a need to explore more effective analgesic methods in clinical practice.
Epidural anesthesia is a common method of spinal block anesthesia, which achieves regional anesthesia by blocking the sensory nerves that innervate the uterus, thereby effectively reducing pain during labor [25]. Ropivacaine hydrochloride, a long-acting amide-type local anesthetic, is commonly used in epidural anesthesia. It has unique nerve-blocking properties, selectively blocking sensory nerves while having minimal effect on motor nerves, thus providing pain relief without significantly affecting uterine contractions. Additionally, its minimal impact on visceral function, blood pressure, and normal intestinal motility makes it a safer option in obstetric anesthesia [26]. Despite its significant advantages in alleviating labor pain, epidural anesthesia also has some limitations. Previous study [27] has shown that while ropivacaine hydrochloride effectively blocks sensitive sensory nerves and maintains the mother’s consciousness, its muscle relaxant effect is relatively weak, which may lead to intraoperative injuries and potentially affect wound healing.
Combined spinal-epidural anesthesia is a method that combines epidural anesthesia with spinal anesthesia techniques. Compared to single epidural anesthesia or other anesthesia methods, combined spinal-epidural anesthesia demonstrates more prominent efficacy in terms of onset speed and pain relief. By achieving dual sensory nerve blockade, it reduces the required amount of anesthetic medication, provides better muscle relaxation, facilitates handling of deep tissues during surgery, and minimizes damage to surrounding tissues, thus aiding in the smoother healing of the surgical wound [28]. An additional study [29] indicated that combined spinal-epidural anesthesia, through intrathecal administration, can accelerate the onset of analgesia, quickly lower catecholamine levels, enhance uterine contraction strength, and reduce cervical tension by blocking spinal nerves below T10. This relaxation of vaginal and pelvic muscles reduces labor resistance and promotes a smoother delivery. In this study, we combined epidural analgesia with spinal-epidural anesthesia to meet the analgesic needs of the mother while avoiding adverse events such as excessive medication doses or abnormalities in lower limb motor nerve function caused by local anesthetic accumulation during continuous medication. This approach allows for more precise control of analgesia while enhancing maternal safety and comfort [30].
The results of this study indicate that the observation group showed significantly better performance than the control group in key indicators such as labor duration, pain levels, blood pressure changes, and maternal and infant outcomes. This suggests that the combined use of spinal-epidural anesthesia and epidural analgesia effectively shortens labor duration for women with pregnancy-induced hypertension, reduces pain during labor, and lowers blood pressure levels, thus achieving more favorable delivery outcomes.
Regarding maternal and infant health, milk production and secretion are regulated by various factors, including genetic background, environmental influences, physiological status, and endocrine regulation [31]. Research has confirmed the critical role of prolactin (PRL) in lactation. During labor, intense pain and psychological stress often trigger neuroendocrine responses that lead to metabolic changes, which can inhibit milk production and secretion, adversely affecting early breastfeeding success. This study found that PRL levels in the observation group were significantly higher than in the control group after delivery, suggesting that the combined use of spinal-epidural anesthesia and epidural analgesia helps promote PRL secretion in women with pregnancyinduced hypertension. This improvement may be attributed to the effective pain relief provided by this anesthesia method, which reduces the inhibitory effects of intense pain and stress, thus supporting smoother breastfeeding. The findings align with previous studies indicating that alleviating pain and stress enhances the neuroendocrine environment for lactation in preeclamptic women, who often experience impaired milk production due to hormonal imbalances and the increased physiological strain of pregnancy [32].
Furthermore, trauma and stress responses during labor can lead to tissue hypoxia and ischemia, triggering the release of a large amount of tissue factors that activate fibrinolysis and coagulation processes, potentially resulting in disseminated intravascular coagulation (DIC) and microthrombosis. Tissue plasminogen activator (t-PA), an essential component of the fibrinolytic system, is closely related to endothelial cell damage and stress responses [33]. Elevated t-PA levels, indicating enhanced fibrinolytic activity, are associated with a higher risk of complications such as DIC and postpartum hemorrhage. This study found that t-PA levels were significantly lower in the observation group compared to the control group after delivery, suggesting that combined spinal-epidural anesthesia and epidural analgesia can reduce the trauma experienced by the mother during labor and provide protection against excessive fibrinolysis. The observed decrease in t-PA levels likely reflects a reduction in stress-induced endothelial damage and a more stable coagulation profile, which can significantly reduce the risk of postpartum hemorrhage and other thrombotic complications. This protective effect is particularly crucial for women with pregnancy-induced hypertension, who are at heightened risk of coagulopathy and hemorrhage [34].
In summary, the use of combined spinal-epidural anesthesia and epidural analgesia for pain management in women with pregnancy-induced hypertension undergoing labor is superior to Doula-assisted labor analgesia in accelerating labor, relieving pain, lowering blood pressure, improving relevant serum markers, and reducing the risk of adverse maternal and infant outcomes. However, it is important to note that this study has some limitations: ① Small sample size: The small sample size of this study may affect the reliability and applicability of the results and limits the statistical significance of some findings. ② Study design limitations: This study is a retrospective analysis, which may have potential information bias and treatment selection preferences, making it less persuasive than randomized controlled trials or prospective study designs. ③ Single-center study: This study was conducted at a single hospital, which may limit the external validity of the results and the generalizability of the findings across different healthcare institutions. ④ Lack of mechanistic research: Although this study shows that combined spinal-epidural anesthesia and epidural analgesia is more effective than Doula-assisted labor analgesia in pain management for women with pregnancy-induced hypertension, the specific mechanisms are not fully elucidated. To address these limitations, further research will focus on increasing the sample size, improving the study design, and conducting multi-center, large-sample joint studies to enhance the reliability and comprehensiveness of the results. Additionally, mechanistic research will be conducted to further understand the effects of these treatment methods and optimize clinical management.
Disclosure of conflict of interest
None.
References
- 1.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 2.Jung E, Romero R, Yeo L, Gomez-Lopez N, Chaemsaithong P, Jaovisidha A, Gotsch F, Erez O. The etiology of preeclampsia. Am J Obstet Gynecol. 2022;226:S844–S866. doi: 10.1016/j.ajog.2021.11.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1690–1702. doi: 10.1016/j.jacc.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z, Deng C, Xiang X. Blood glucose related to pregnancy induced hypertension syndrome. Am J Transl Res. 2021;13:5301–5307. [PMC free article] [PubMed] [Google Scholar]
- 5.Pan JW, Zhao G. Analysis of factors related to postpartum depression in pregnancy-induced hypertension syndrome patients and construction and evaluation of nomograms. World J Psychiatry. 2023;13:654–664. doi: 10.5498/wjp.v13.i9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauspurg A, Countouris ME, Catov JM. Hypertensive disorders of pregnancy and future maternal health: how can the evidence guide postpartum management? Curr Hypertens Rep. 2019;21:96. doi: 10.1007/s11906-019-0999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SW, Yoon SH, Kim M, Seo YS, Yuk JS. Risk of gestational diabetes and pregnancy-induced hypertension with a history of polycystic ovary syndrome: a nationwide population-based cohort study. J Clin Med. 2023;12:1738. doi: 10.3390/jcm12051738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao H, Gu X, Cai Y, Xiong H, Huang L, Shen W, Ma F, Xiao X, Li S. The influence of pregnancy-induced hypertension syndrome on the metabolism of newborns. Transl Pediatr. 2021;10:296–305. doi: 10.21037/tp-20-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Chen Z, Dang X, Jiang N, Cui K, Su S. Different doses of ropivacaine either with sufentanil or with dexmedetomidine for labor epidural anesthesia regarding painless childbirth: a retrospective, multicenter study. Pharmacology. 2022;107:386–397. doi: 10.1159/000524304. [DOI] [PubMed] [Google Scholar]
- 10.Sobczak A, Taylor L, Solomon S, Ho J, Kemper S, Phillips B, Jacobson K, Castellano C, Ring A, Castellano B, Jacobs RJ. The effect of doulas on maternal and birth outcomes: a scoping review. Cureus. 2023;15:e39451. doi: 10.7759/cureus.39451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramey-Collier K, Jackson M, Malloy A, McMillan C, Scraders-Pyatt A, Wheeler SM. Doula care: a review of outcomes and impact on birth experience. Obstet Gynecol Surv. 2023;78:124–127. doi: 10.1097/OGX.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 12.Halliday L, Nelson SM, Kearns RJ. Epidural analgesia in labor: a narrative review. Int J Gynaecol Obstet. 2022;159:356–364. doi: 10.1002/ijgo.14175. [DOI] [PubMed] [Google Scholar]
- 13.Sharma H. A randomized study comparing the efficacy of ultrasound guided lumbar plexus block and epidural anesthesia for postoperative analgesia in patients undergoing total hip replacement. Asian J Anesthesiol. 2020;58:131–137. doi: 10.6859/aja.202012_58(4).0003. [DOI] [PubMed] [Google Scholar]
- 14.Braga ADFDA, Carvalho VH, Braga FSDS, Pereira RIC. Combined spinal-epidural block for labor analgesia. Comparative study with continuous epidural block. Braz J Anesthesiol (Engl Ed) 2019;69:7–12. doi: 10.1016/j.bjane.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S International Society for the Study of Hypertension in Pregnancy (ISSHP) The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Irlbeck T, Zwißler B, Bauer A. ASA classification: transition in the course of time and depiction in the literature. Anaesthesist. 2017;66:5–10. doi: 10.1007/s00101-016-0246-4. [DOI] [PubMed] [Google Scholar]
- 17.Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. 2021;27:282–285. doi: 10.1097/RHU.0000000000001320. [DOI] [PubMed] [Google Scholar]
- 18.Chen HY. Chauhan SApgar score at 10 minutes and adverse outcomes among low-risk pregnancies. J Matern Fetal Neonatal Med. 2022;35:7109–7118. doi: 10.1080/14767058.2021.1943659. [DOI] [PubMed] [Google Scholar]
- 19.Filipek A, Jurewicz E. Preeclampsia - a disease of pregnant women. Postepy Biochem. 2018;64:232–229. doi: 10.18388/pb.2018_146. [DOI] [PubMed] [Google Scholar]
- 20.Bonapace J, Gagné GP, Chaillet N, Gagnon R, Hébert E, Buckley S. No. 355-physiologic basis of pain in labour and delivery: an evidence-based approach to its management. J Obstet Gynaecol Can. 2018;40:227–245. doi: 10.1016/j.jogc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Smith A, Laflamme E, Komanecky C. Pain management in labor. Am Fam Physician. 2021;103:355–364. [PubMed] [Google Scholar]
- 22.Jin J, Son M. Pain management during vaginal childbirth. JAMA. 2021;326:450. doi: 10.1001/jama.2021.10702. [DOI] [PubMed] [Google Scholar]
- 23.Zuarez-Easton S, Erez O, Zafran N, Carmeli J, Garmi G, Salim R. Pharmacologic and nonpharmacologic options for pain relief during labor: an expert review. Am J Obstet Gynecol. 2023;228:S1246–S1259. doi: 10.1016/j.ajog.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Sun B, Ren J, Yang Y, Yang C. Effects of a doula instrument combined with auricular acupuncture and acupoint application on numerical rating scale scores, labour time of puerperae with a natural delivery and apgar scores of the newborns. Pak J Med Sci. 2023;39:1793–1797. doi: 10.12669/pjms.39.6.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Flores M. Epidural and spinal anesthesia. Vet Clin North Am Small Anim Pract. 2019;49:1095–1108. doi: 10.1016/j.cvsm.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Wu S. Feasibility of epidural injection of ropivacaine and dexamethasone for labor analgesia in women with preeclampsia. Am J Transl Res. 2021;13:7921–7927. [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo RH, Dang JJ, Zhuang JW, Chen QM, Zhang JY, Zheng HW, Wang Z. The incidence of breakthrough pain associated with programmed intermittent bolus volumes for labor epidural analgesia: a randomized controlled trial. Int J Obstet Anesth. 2022;51:103571. doi: 10.1016/j.ijoa.2022.103571. [DOI] [PubMed] [Google Scholar]
- 28.Ando H, Makino S, Takeda J, Maruyama Y, Nojiri S, Sumikura H, Itakura A. Comparison of the labor curves with and without combined spinal-epidural analgesia in nulliparous women- a retrospective study. BMC Pregnancy Childbirth. 2020;20:467. doi: 10.1186/s12884-020-03161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazzari C, Raffaelli R, D’Alessandro R, Simonetto C, Bosco M, Zorzato PC, Uccella S, Taddei F, Franchi M, Garzon S. Effects of neuraxial analgesia technique on labor and maternal-fetal outcomes: a retrospective study. Arch Gynecol Obstet. 2023;307:1233–1241. doi: 10.1007/s00404-022-06600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guasch E, Brogly N, Gilsanz F. Combined spinal epidural for labour analgesia and caesarean section: indications and recommendations. Curr Opin Anaesthesiol. 2020;33:284–290. doi: 10.1097/ACO.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 31.Truchet S, Honvo-Houéto E. Physiology of milk secretion. Best Pract Res Clin Endocrinol Metab. 2017;31:367–384. doi: 10.1016/j.beem.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Faron-Górecka A, Latocha K, Pabian P, Kolasa M, Sobczyk-Krupiarz I, Dziedzicka-Wasylewska M. The involvement of prolactin in stress-related disorders. Int J Environ Res Public Health. 2023;20:3257. doi: 10.3390/ijerph20043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godtfredsen AC, Sidelmann JJ, Dolleris BB, Jørgensen JS, Johansen EKJ, Pedersen MFB, Palarasah Y, Gram JB. Fibrinolytic changes in women with preeclampsia. Clin Appl Thromb Hemost. 2022;28:10760296221126172. doi: 10.1177/10760296221126172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alyas S. Lower vitamin D and sex hormone binding globulin levels and higher progesterone, cortisol and t-PA levels in early second trimester are associated with higher risk of developing gestational diabetes mellitus. J Biol Regul Homeost Agents. 2020;34 doi: 10.23812/20-35-L. [DOI] [PubMed] [Google Scholar]