Abstract
Advances in early tumor detection have led to an increase in the reported incidence of multiple primary cancers. However, the concurrent occurrence of gastric adenocarcinoma and hepatocellular carcinoma remains rare. We present the case of a 68-year-old male patient with chronic hepatitis B who was diagnosed with stage Ib liver cancer (T1bN0M0). Gastroscopy revealed a mucosal lesion in the posterior wall of the gastric body, and biopsy confirmed moderately to poorly differentiated adenocarcinoma. The patient underwent laparoscopic gastrectomy combined with partial liver resection, followed by three cycles of S-1 plus oxaliplatin (SOX) chemotherapy. Given the lack of standardized treatment protocols for managing multiple primary cancers, developing a treatment plan for these two synchronous cancers is challenging. This case underscores the importance of early screening for multiple primary cancers and the need for a multidisciplinary approach to formulate an optimal treatment strategy. Furthermore, we conducted a literature review of reports on the simultaneous occurrence of gastric and hepatic cancers over recent decades. Unlike previous studies, we also examined postoperative adjuvant chemotherapy regimens suitable for both gastric adenocarcinoma and hepatocellular carcinoma patients.
Keywords: Gastric adenocarcinoma, hepatocellular carcinoma, multidisciplinary team, surgery, synchronous multiple primary cancers
Introduction
A previous study involving 96,174 cancer patients reported a 2.3% incidence of multiple primary cancers [1]. In recent years, advances in the early detection of tumors have led to an increase in the number of patients diagnosed with synchronous primary cancers [2]. In China, the incidence of gastric cancer and primary liver cancer ranks third and fourth, respectively, in terms of the incidence of malignant tumors and third and second, respectively, in terms of mortality [3]. While gastric adenocarcinoma and hepatocellular carcinoma are common cancers, their simultaneous occurrence is exceedingly rare. The pathogenesis of multiple primary carcinomas is unclear but may involve factors such as carcinogen exposure, chemoradiotherapy, genetic susceptibility, and genetic instability [4]. Synchronous tumors complicate treatment strategies, particularly when both tumors are surgically resectable. Surgical treatment is preferred in cases where tumors in both organs can be surgically resected [5]. However, there is no standardized protocol for postoperative adjuvant chemotherapy for synchronous tumors. We present the case of a patient with synchronous gastric adenocarcinoma and small hepatocellular carcinoma who received S-1+oxaliplatin (SOX) regimen adjuvant chemotherapy after simultaneous hepatectomy and gastrectomy. The co-occurrence of gastric adenocarcinoma and hepatocellular carcinoma is exceptionally rare, presenting unique challenges for clinical diagnosis and treatment. This case underscores the importance of early diagnosis and the role of multidisciplinary approaches in treating multiple primary malignancies. The reporting of this study adheres to the CARE guidelines [6].
Case description
A 68-year-old man with a major complaint of pain in the right upper quadrant for 4 days, accompanied by yellow staining of the skin and sclera, was admitted to our hospital in April 2021. During the course of the disease, the patient vomited once, and the vomit contained undigested food. Other symptoms included fatigue, poor appetite, and weight loss of 1.5 kg. His medical history included chronic viral hepatitis B infection, with irregular treatment for more than 20 years. He had no prior surgeries. The laboratory data were positive for hepatitis B surface antigen (HBsAg), hepatitis B e antibody (HBeAb), and hepatitis B core antibody (HBcAb). His plasma ammonia concentration was 52 µmol/L. Routine blood and biochemical parameters; coagulation function; and the levels of tumor markers, including AFP, CEA, CA199, CA125, and CA724, were normal.
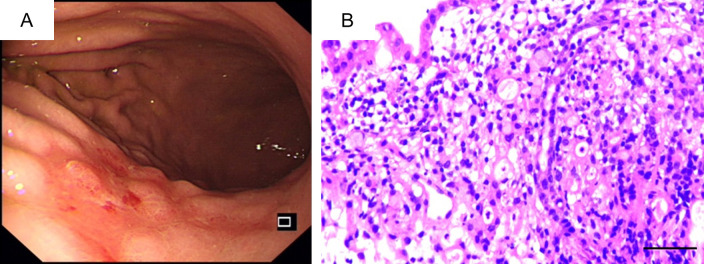
Abdominal contrast-enhanced computed tomography (CT) revealed a space-occupying lesion in liver segment VI and no abnormalities in the gastric wall (Figure 1A). Dynamic magnetic resonance imaging enhanced with gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) revealed a lesion in segment VI of the liver, and the possibility of liver cancer was considered (Figure 1B). The patient was diagnosed with liver cancer stage Ib (T1bN0M0) according to the American Joint Committee on Cancer Tumor, Node, Metastasis (TNM) system. Given the patient’s history of vomiting, gastroscopy was routinely performed prior to surgery. A gastroscopic examination revealed chronic atrophic gastritis, bile reflux, and gastric cancer (Figure 2A). Pathology results suggested moderately poorly differentiated adenocarcinoma of the posterior gastric body wall (Figure 2B). Based on these findings, a multidisciplinary team (MDT), comprising surgeons, a gastroenterologist, a radiologist, a hepatologist, and an oncologist, was included in the treatment of this case. The MDT discussed whether the patient had primary liver cancer or metastatic liver cancer. Primary liver cancer typically shows low-density lesions on non-contrast CT scans and high-density enhancement on contrast-enhanced CT. In contrast, metastatic liver cancer shows low density on plain CT scans, and annular enhancement appears on enhanced CT, with low density in the center of the lesion and high-density enhancement at the edge. On MR images, primary liver cancer shows low and high signals on T1-weighted images and T2-weighted images, respectively, whereas metastatic liver cancer usually shows high signals on T2-weighted images. Additionally, AFP levels tend to increase in patients with primary liver cancer, whereas patients with metastatic liver cancer may have elevated levels of markers specific to the primary tumor. However, the elevation of tumor markers cannot be used as the sole diagnostic criterion for either primary or metastatic liver cancer. In this case, the levels of AFP and other tumor markers in the patient were within normal limits, and gastroscopic biopsy indicated moderately poorly differentiated adenocarcinoma. While the possibility of metastasis cannot be entirely excluded, the hepatic space-occupying lesion may be concurrently resected with the gastric lesion. Following the MDT discussion, laparoscopy-assisted distal subtotal gastrectomy with D2 lymphadenectomy and segment VI hepatectomy were simultaneously performed. During the surgical procedure, the liver demonstrated a firm consistency with an irregular surface morphology, and a space-occupying lesion was detected on the hepatic surface in segment VI. To achieve adequate resection margins and ensure tumor-free edges, segmental hepatectomy on segment VI was performed rather than opting for local resection. The gastric wall appeared unremarkable; however, enlarged lymph nodes were noted along the lesser curvature of the stomach. To prevent postoperative lymph node metastasis in patients with gastric cancer, lymphadenectomy was performed at both the first and second stations of the regional lymph nodes. Pathological examination of the surgical specimen revealed moderately differentiated adenocarcinoma in the posterior gastric body wall, some of which were poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma (Figure 3A). It was diagnosed as an adenocarcinoma measuring 4 × 3 cm, with invasion of the muscularis mucosa, no lymphatic or perineural involvement, and stage pT1aN1Mx. Immunohistochemical staining revealed CK8/18 (+), C-erbB-2 (+), AFP (-), glypican-3 (-), SALL4 (-), Syn (+), CgA (-), and Ki-67 (≥80%). Histological examination of the liver segment revealed moderately differentiated hepatocellular carcinoma (HCC) without vascular invasion; the tumor measured 4.7 × 3 × 2 cm with necrosis (Figure 3B). Immunohistochemical staining revealed glypican-3 (-), hepatocytes (+), AFP (+), CK19 (-), CK20 (-), Arg-1 (-), CD34 (+), and Ki-67 (≥70%). These immunohistochemical results supported the diagnosis of gastric adenocarcinoma and hepatocellular carcinoma. Ki-67 is highly expressed in both gastric and liver cancer samples, indicating a high risk of tumor invasion and recurrence. For such patients, more aggressive treatment measures should be implemented post surgery to control tumor growth and spread.
Figure 1.

A. Contrast-enhanced computed tomography showed a circular low-density shadow in liver segment VI with uneven density inside the lesion. B. Coronal view of dynamic Magnetic Resonance Imaging (MRI) enhanced with gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) showed low signal intensity in the hepatobiliary phase of the liver SVI segment.
Figure 2.
A. A patchy mucosal lesion is observed in the posterior wall of the gastric body, exhibiting a rough and red appearance with nodular hyperplasia, accompanied by clustering of the surrounding mucosa towards the center. B. Gastroscopic biopsy revealed that the cancer cells presented an irregular adenoid, string-like, diffuse and infiltrating pattern, with enlarged nuclei, atypia, and deep staining (Magnification 40 × 10, bar = 50 μm).
Figure 3.

Hematoxylin and eosin staining of pathological sections after surgery. A. Gastric mucosal epithelial dysplasia was manifested by irregular glandular tubular and sieve-like arrangement, along with infiltrative growth (Magnification 10 × 10, bar = 200 μm). B. The normal structure of liver tissue was disrupted, with atypical cells identified within it. These cells were arrayed in beam, acinar and sheet-like patterns and grew in an infiltrative manner (Magnification 20 × 10, bar = 100 μm).
One month after surgery, the patient started receiving three cycles of the SOX regimen (oxaliplatin 130 mg/m2 on day 1 plus oral S-1 40 mg/m2 twice daily on days 1-14 every 3 weeks). The patient was unable to continue subsequent chemotherapy. However, there was no evidence of recurrence eight months after surgery, with no positive tumor markers and no detection of cancer from abdominal CT. Currently, the patient is alive and under follow-up. We recommend regular imaging and tumor marker testing every six months and suggest genetic testing to better understand the molecular characteristics of tumors. Psychological support is also provided during follow-up to maintain a positive mindset.
Ethics approval
The study was conducted in accordance with the 1975 Declaration of Helsinki (revised in 2013). All details about the patient was removed to protect privacy, and written informed consent was obtained from the patient prior to treatment. Written informed consent was not required for publication because all patient details had been deidentified. In addition, medical ethics committee approval was not required due to the case-report nature of this article.
Literature review
We conducted a literature review on synchronous gastric cancer (GC) and hepatocellular carcinoma (HCC). A PubMed search using the keywords ‘gastric adenocarcinoma’, ‘hepatocellular carcinoma’, and ‘synchronous cancer’, along with their synonyms, yielded nine relevant studies. These included one retrospective study and eight individual case reports. We retrospectively evaluated indicators such as age, sex, diagnosis, medical history, clinicopathological diagnosis, intervention, complications, and prognosis (Table 1) [4-13].
Table 1.
Literature regarding synchronous GC and HCC
| Study | Case | Age | Gender | Diagnosis | Medical history | Pathology | Therapy | Complications | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Kim YI et al (1989) [6] | 1 | 66 | Female | GC+HCC | Hepatitis B | P/D AC; HCC | B-I gastrectomy + left lobectomy | Bile bleak | HCC recurrence after 10 mos postoperatively |
| Uenishi T et al (2003) [5] | 13 | 48-76 | Male | GC+HCC | Chronic hepatic disease | 5 P/D, 4 M/D and 4 W/D AC; HCC | STG/TG/WR + right lobectomy/Segmentectomy/limited resection | 1 died of hepatic failure, 4 massive ascites, 1 liver abscess and 1 intra-abdominal bleeding | 6 died during 7 mos to 49 mos, 3 HCC recurrence, 3 alive at the end of the study |
| Chang JY et al (2003) [7] | 1 | 74 | Male | GC+HCC+CC | No chronic illness | W/D AC; W/D HCC; M/D CC | STG + left lobectomy + Right hepatic WR | Uneventful | Under follow-up |
| Ewertsen C et al (2009) [8] | 1 | 71 | Male | GNEC+HCC | Type 2 diabetes; chronic heart failure; chronic obstructive lung disease; adiposities | P/D NEC; HCC | Local resection for GNEC + sorafenib for HCC | Uneventful | Normal in the follow-up after 6 mos |
| Ramakrishna B et al (2012) [9] | 1 | 60 | Male | GC+HCC | Hypertensive; right hydropneumothorax | P/D AC; W-M/D HCC | DG+LLS | Uneventful | No follow-up |
| Wang Y et al (2014) [4] | 1 | 58 | Male | GC+HCC | Hepatitis B | P/D AC; HCC | DG + Partial hepatectomy | Uneventful | Normal in the follow-up after one year |
| Ferreira E Mora H et al (2015) [10] | 1 | 76 | Male | GIST+HCC | Minor depression | Gastrointestinal stromal tumors; HCC | Atypical gastrectomy + left lobectomy | Uneventful | Normal in the follow-up after 30 mos |
| Wang C et al (2018) [11] | 1 | 68 | Male | GC+HCC | Tuberculosis; hepatitis B | P/D AC; M-P/D HCC | DG + Segmentectomy + RHVT | Uneventful | Normal in the follow-up after 9 mos |
| Hammami A et al (2020) [12] | 1 | 50 | Male | GC+HCC | No chronic illness | P/D AC; HCC | TG+TACE | Uneventful | Normal in the follow-up after 2 years |
Abbreviations: P/D, poorly differentiated; M-P/D, moderately-to-poorly differentiated; W-M/D, well-to-moderately differentiated; M/D, moderately differentiated; W/D, well differentiated; AC, adenocarcinoma; DG, distal gastrectomy; STG, Subtotal gastrectomy; TG, total gastrectomy; LLS, left lateral sectionectomy; WR, wedge resection; mos, months; RHVT, right hepatic vein thrombectomy; CC, cholangiocarcinoma; GNEC, gastric neuroendocrine carcinoma.
In these studies, the median age of the patients was 66 years (range 48-76), and one patient was female. Most patients had chronic disease, with 16 cases of hepatitis B, hepatitis C, or liver cirrhosis. Subtotal gastrectomy was performed in 14 patients with gastric body or antrum cancer. Total gastrectomy was performed in four patients with a proximal stomach or infiltrating gastric cancer, and wedge resection of the stomach was performed in three patients with gastrointestinal stromal tumors (GISTs), early gastric cancer, or recurrent anemia of type IV gastric neuroendocrine carcinoma. Five major and 14 minor hepatectomies were performed, and two patients were excluded from resection for vascular invasion or multiple lesions. Histopathological findings of gastric surgical samples revealed that most cases were tubular adenocarcinomas of different degrees, with only one case of a GIST and one case of poorly differentiated neuroendocrine carcinoma. During liver tissue resection, one patient was confirmed to have simultaneous HCC and cholangiocarcinoma. Although postoperative complications occurred in eight of the 21 patients in these studies, only one patient died from postoperative liver failure. In the follow-up of all patients, except for one patient without follow-up data, nine patients experienced tumor recurrence, six died 7-49 months after surgery, and 11 patients were still in good health at the end of these studies. Based on the results of the above studies, for synchronous tumors, considering the safety of surgery, an active surgical treatment strategy may improve the overall survival of patients.
Discussion
Multiple primary cancers (MPCs) refer to the occurrence of two or more malignant neoplasms with different histologies in the same individual [14]. These malignancies may be confined to one organ or involve different organs. The reported frequency of multiple primary cancers ranges from 2% to 17% [15]. The occurrence of MPCs is correlated with a high frequency of germline pathogenic variants [16]. MPCs occur in patients with high-risk breast and ovarian cancers and may be closely associated with BRCA1 and BRCA2 mutations [17]. Primary head and neck cancers accompanied by primary upper gastrointestinal cancers are often associated with p53 mutations [18]. Additionally, smoking, as an independent risk factor, was associated with a 9.7% greater risk of developing MPCs than individuals who had never smoked [19]. MPCs in patients with head and neck cancer, especially those located in the esophagus and hypopharynx, are associated with alcohol consumption and polymorphisms in genes involved in alcohol metabolism [20]. The concept of field cancerization provides a theoretical framework for MPC development, suggesting that exposure to regional carcinogens induces irreversible genetic mutations, ultimately leading to MPCs [21]. In our case, the patient did not exhibit any deleterious habits, such as smoking or alcohol consumption. The appearance of small HCC in this patient may be attributed to a history of hepatitis B, suggesting that the hepatitis B virus is a primary etiological factor for HCC in the Chinese population. Synchronous GC may be associated with mutations in tumor susceptibility genes and compromised immune function resulting from chronic hepatitis B infection. Therefore, long-term and regular follow-up is imperative for patients afflicted with chronic disorders such as hepatitis B. For follow-up evaluations, abdominal CT and gastroscopy are recommended. Abdominal CT should be performed once a year, and gastroscopy should be conducted once every 1-2 years. This follow-up protocol not only facilitates surveillance of disease advancement but also enables timely modification of the therapeutic plan, thereby guaranteeing the most favorable treatment outcome and quality of life for patients.
Currently, no standardized treatment protocols or guidelines are available for managing synchronous MPCs. The management of synchronous MPCs poses a clinical challenge, requiring a combination of modalities such as surgery, chemotherapy, radiotherapy, and localized therapies. Dyer reported a case in which a female patient presented with a pancreatic neuroendocrine tumor, lymphoma, and colorectal cancer [22]. The patient underwent R-CHOP (rituximab, doxorubicin, vincristine, cyclophosphamide, and prednisolone) chemotherapy, endoscopic rectal tumor resection, and laparoscopic left pancreatectomy for the respective tumors [23]. In another case of synchronous breast, kidney, and thyroid cancers, radical resection of the three tumors was performed sequentially with a maximum interval of six months between surgical procedures, and no recurrence or metastasis was observed during postoperative follow-up [23].
In our case, we performed laparoscopic-assisted resection of hepatic and gastric neoplasms and administered three cycles of SOX chemotherapy during postoperative follow-up. SOX serves as the primary regimen for postoperative adjuvant chemotherapy in stage II/III gastric adenocarcinoma, effectively improving disease-free survival [24]. S-1 has potential as an effective pharmaceutical with a favorable safety profile for the treatment of advanced HCC [25]. The results of a phase II clinical trial suggest that the SOX regimen could be a promising alternative for advanced HCC, showing efficacy and safety comparable to that of FOLFOX while being better tolerated by patients [26]. Therefore, SOX was selected as the adjuvant chemotherapy regimen after surgery for patients with synchronous gastric adenocarcinoma and hepatocellular carcinoma.
For patients with synchronous MPCs, developing individualized precision diagnosis and treatment strategies is essential. Physicians must understand each type of tumor, as well as the interactions and possible concurrent effects among the various treatment modalities. Consequently, diagnostic and therapeutic recommendations derived from MDT discussions can provide an optimal treatment course for patients. The treatment of synchronous MPCs should prioritize tumor prognosis. In cases where tumors are localized, simultaneous resection may be a viable option; however, in cases where both tumors are advanced, it is advisable to select chemotherapy that is effective whenever possible [27]. If this is not feasible, the chemotherapy regimen should prioritize treating tumors with a poorer prognosis, which can be combined with localized therapies such as radiotherapy or microwave ablation [15,27].
This study highlights the effective management of a complex cancer case involving synchronous gastric adenocarcinoma and small HCC using a combination of surgical resection and SOX chemotherapy. These findings emphasize the vital role of MDTs in managing MPCs, substantially improving patient outcomes. Future research should focus on elucidating the genetic and molecular mechanisms underlying synchronous malignancies to develop more targeted and effective treatments. Additionally, public health initiatives should prioritize early cancer detection and screening in high-risk populations to prevent the progression of these complex cases.
Acknowledgements
We are grateful to the medical records department for providing the case data. This study was supported by the Natural Science Foundation of Gansu Province (23JRRA1621, 21JR7RA410).
Disclosure of conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Kwon JH, Kim H, Lee JK, Hong YJ, Kang HJ, Jang YJ. Incidence and characteristics of multiple primary cancers: a 20-year retrospective study of a single cancer center in korea. Cancers (Basel) 2024;16:2346. doi: 10.3390/cancers16132346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copur MS, Manapuram S. Multiple primary tumors over a lifetime. Oncology (Williston Park) 2019;33:629384. [PubMed] [Google Scholar]
- 3.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Wu XT. Stomach carcinoma presenting with a synchronous liver cancer: a case report and literature review. Case Rep Gastrointest Med. 2014;2014:970293. doi: 10.1155/2014/970293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uenishi T, Kubo S, Hirohashi K, Osugi H, Shuto T, Tanaka H, Yamamoto T, Ogawa M, Kinoshita H. Surgical management of synchronous hepatocellular carcinoma and gastric cancer. Dig Surg. 2003;20:133–140. doi: 10.1159/000069389. [DOI] [PubMed] [Google Scholar]
- 6.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache. 2013;53:1541–1547. doi: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 7.Kim YI, Tada I, Kuwabara A, Kobayashi M. Double cancer of the liver and stomach with situs inversus totalis--a case report. Jpn J Surg. 1989;19:756–759. doi: 10.1007/BF02471729. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Kim BH, Hong SW, Kim YW, Oh JH. A case report of synchronous double primary liver cancers combined with early gastric cancer. Korean J Intern Med. 2003;18:115–118. doi: 10.3904/kjim.2003.18.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewertsen C, Henriksen BM, Hansen CP, Knigge U. Synchronous gastric neuroendocrine carcinoma and hepatocellular carcinoma: a case report. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.03.2009.1667. bcr03.2009.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishna B, Patel K, Vyas F. Synchronous hepatocellular carcinoma and gastric carcinoma-a case report with review of the literature. J Gastrointest Cancer. 2012;43(Suppl 1):S56–59. doi: 10.1007/s12029-011-9323-8. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira EMH, Pinto de Sousa J, Devesa V, Barbosa J, Costa J, Portugal R, Costa Maia J. Gastrointestinal stromal tumor of the stomach and hepatocellular carcinoma: an unusual association. Int J Surg Case Rep. 2015;12:75–77. doi: 10.1016/j.ijscr.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Luo X, Dong SL, Leng C, Zhang BX, Zhang BH. Small hepatocellular carcinoma suppressed by chemotherapy for synchronous gastric carcinoma after laparoscopy-assisted radical distal gastrectomy: a case report and literature review. Medicine (Baltimore) 2018;97:e13190. doi: 10.1097/MD.0000000000013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammami A, Elleuch N, Jaziri H, Ben Cheikh Y, Braham A, Ajmi S, Ben Slama A, Ksiaa M, Jmaa A. Synchronous hepatocellular carcinoma in a patient with primary gastric cancer: an exceptional association. Case Rep Gastroenterol. 2020;14:299–305. doi: 10.1159/000506188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripodi D, Cannistra C, Gagliardi F, Casella G, Lauro A, De Luca A, Amabile MI, Palumbo P, Pironi D, Mascagni D, D’Andrea V, Vergine M, Sorrenti S. Coincidental or causal? Concurrence of colorectal carcinoma with primary breast cancer. Dig Dis Sci. 2022;67:437–444. doi: 10.1007/s10620-021-07296-5. [DOI] [PubMed] [Google Scholar]
- 15.Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. doi: 10.1136/esmoopen-2017-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bychkovsky BL, Lo MT, Yussuf A, Horton C, Richardson M, LaDuca H, Garber JE, Rana HQ. Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer. 2022;128:1275–1283. doi: 10.1002/cncr.34056. [DOI] [PubMed] [Google Scholar]
- 17.Shih HA, Nathanson KL, Seal S, Collins N, Stratton MR, Rebbeck TR, Weber BL. BRCA1 and BRCA2 mutations in breast cancer families with multiple primary cancers. Clin Cancer Res. 2000;6:4259–4264. [PubMed] [Google Scholar]
- 18.Chung KY, Mukhopadhyay T, Kim J, Casson A, Ro JY, Goepfert H, Hong WK, Roth JA. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res. 1993;53:1676–1683. [PubMed] [Google Scholar]
- 19.Okeke F, Nriagu VC, Nwaneki CM, Magacha HM, Omenuko NJ, Anazor S. Factors that determine multiple primary cancers in the adult population in the united states. Cureus. 2023;15:e44993. doi: 10.7759/cureus.44993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien HT, Young CK, Chen TP, Liao CT, Wang HM, Cheng SD, Huang SF. Alcohol-metabolizing enzymes’ gene polymorphisms and susceptibility to multiple head and neck cancers. Cancer Prev Res (Phila) 2019;12:247–254. doi: 10.1158/1940-6207.CAPR-18-0449. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Ren W, Du X, Yang L, Tan B. Research progress of multiple primary malignancies associated with esophageal cancer. Cancer Control. 2023;30:10732748231176641. doi: 10.1177/10732748231176641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dayer N, Fasquelle F, Salati E, Dietrich G. Multiple primary malignancies: synchronous lymphoma, pancreatic neuroendocrine tumour and colorectal cancer. BMJ Case Rep. 2021;14:e241938. doi: 10.1136/bcr-2021-241938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia MM, Yang B, Ding C, Yao YR, Guo J, Yang HB. Synchronous multiple primary malignant neoplasms in breast, kidney, and bilateral thyroid: a case report. World J Clin Cases. 2023;11:1513–1520. doi: 10.12998/wjcc.v11.i7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita K, Hosoda K, Niihara M, Hiki N. History and emerging trends in chemotherapy for gastric cancer. Ann Gastroenterol Surg. 2021;5:446–456. doi: 10.1002/ags3.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang WK, You LN, Yang SF, Liu DY, Liu M, Fan XW. S-1 for treatment of advanced hepatocellular carcinoma: a systematic review of the literature. Contemp Oncol (Pozn) 2017;21:16–20. doi: 10.5114/wo.2017.66653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DW, Lee KH, Kim HJ, Kim TY, Kim JS, Han SW, Oh DY, Kim JH, Im SA, Kim TY. A phase II trial of S-1 and oxaliplatin in patients with advanced hepatocellular carcinoma. BMC Cancer. 2018;18:252. doi: 10.1186/s12885-018-4039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pu X, Xu T, Ge C, He Y, Yang X, Chang P. A case of durvalumab-treated double primary cancers of the colon and lung. Ann Palliat Med. 2020;9:3614–3622. doi: 10.21037/apm-20-1086. [DOI] [PubMed] [Google Scholar]