Abstract
Objective: To evaluate the efficacy of therapeutic hypothermia in enhancing cognitive and motor outcomes six months post-TBI. Methods: A retrospective cohort analysis was conducted on data from the Medical Information Mart for Intensive Care (MIMIC) database. A total of 259 TBI patients were divided into a Routine group (n = 135) and a Hypothermia Therapy group (n = 124). The hypothermia group received core temperature reduction to 33-35°C for 3-5 days. Cognitive and motor functions were assessed using the Functional Independence Measure (FIM), Fugl-Meyer Motor Assessment (FMA), and Glasgow Coma Scale (GCS) at baseline and six months post-treatment. Patients’ satisfaction was also evaluated. Results: After six months of treatment, the hypothermia group showed significantly higher FIM motor scores, and FIM cognitive scores than the routine group (P < 0.01). A higher proportion of patients in the hypothermia group achieved mild impairment on the FMA assessment than in the routine group (P = 0.003). The hypothermia group demonstrated better consciousness levels (P = 0.006) and reported significantly higher patient satisfaction rate (P = 0.004). Conclusion: Hypothermia therapy significantly improved cognitive and motor recovery in TBI patients, enhanced consciousness levels, and increased patient satisfaction rate.
Keywords: Traumatic brain injury, hypothermia therapy, neuroprotection, cognitive recovery, motor function, patient outcomes
Introduction
Traumatic brain injury (TBI) represents a major global public health concern, contributing significantly to morbidity and mortality worldwide [1]. Beyond the immediate neurological deficits, TBI results in persistent cognitive and motor impairments, profoundly compromising survivors’ functional independence and long-term quality of life. Although advancements in acute trauma management have improved outcomes, the treatment of TBI remains challenging due to the intricate cascade of secondary injury mechanisms that unfold following the initial trauma [2]. These secondary injuries, characterized by neuroinflammation, excitotoxicity, oxidative stress, and programmed cell death, exacerbate neuronal damage.
Current treatment strategies focus on mitigating these secondary effects to preserve neurological function and enhance recovery [3]. Among emerging interventions, therapeutic hypothermia has garnered considerable attention as a neuroprotective strategy to attenuate secondary brain damage [4]. By lowering core body temperature, therapeutic hypothermia reduces metabolic rate and energy demand, thereby limiting lactate production and free radical generation, which helps minimize oxidative stress [5]. Additionally, hypothermia suppresses pro-inflammatory cytokine release and microglial activation, further protecting neurons from additional injury. Preclinical studies and early clinical trials have shown that hypothermia can stabilize the blood-brain barrier, regulate cellular apoptosis, and enhance autophagy mechanisms, collectively contributing to its neuroprotective effects [6].
Despite these promising preclinical findings, the translation of hypothermia therapy into routine clinical practice for TBI patients remains controversial. Current evidence demonstrates considerable heterogeneity in treatment outcomes, largely attributable to variations in patient selection criteria, cooling methodologies (including target temperatures, duration, and rewarming rates), and endpoint assessments [7]. Previous research has primarily focused on the immediate benefits of hypothermia, such as reductions in intracranial pressure and cerebral edema, with limited longitudinal investigations into the effect on functional recovery and long-term outcomes. Consequently, there remains a critical need for comprehensive studies that evaluate the sustained effects of hypothermia on cognitive and motor recovery following TBI [8].
The lack of detailed longitudinal studies examining the long-term cognitive and motor outcomes of hypothermia therapy in TBI patients highlights a significant gap in current research. Such investigations are essential for establishing evidence-based protocols and optimizing therapeutic strategies. Our study was specifically designed to address this critical research gap by comprehensive evaluation of hypothermia’s effects on functional outcomes at six-month follow-up. We hope to provide valuable insight into the sustained neuroprotective effects of hypothermia as a more effective treatment strategy for TBI patients.
Materials and methods
Case selection
Data of 259 TBI patients were collected from Medical Information Mart for Intensive Care (MIMIC; https://mimic.mit.edu) database. According to the treatment strategy, they were divided into a Routine group (n = 135) and a Hypothermia Therapy group (n = 124). This study has been approved by the Medical Ethics Committee of Guangxi Normal University.
Inclusion criteria: (1) patients aged 18 years or older; (2) a confirmed history of trauma [9]; (3) fulfillment of diagnostic criteria for TBI [10]; (4) admission to the hospital within 6 hours following the TBI [11]; (5) availability of complete clinical data; and (6) completed follow-up at 6 months post-TBI.
Exclusion criteria: (1) presence of multiple organ injuries, such as pulmonary contusion, laceration, or visceral rupture [12]; (2) brain injuries resulting from indirect or non-direct violence; (3) physical disabilities; (4) abnormal coagulation function; (5) a history of lower limb fracture surgery; (6) concurrent severe systemic infection or organ failure; and (7) a diagnosis of mental illness.
Treatment approach
All patients initially received an intravenous loading dose of 18 mg/kg of phenytoin (Sodium phenytoin for injection, Shanghai Xinya Pharmaceutical Co., Ltd, National Medical Products Administration Approval Number H31021717), followed by a daily maintenance dose of 300 mg for seven days. For pain management, each patient was administered an intravenous dose of 5 to 10 mg of morphine per hour (Hydrobromic acid allyl morphine injection, Qinghai Pharmaceutical Factory Co., Ltd, National Medical Products Administration Approval No. H63020164) for at least 72 hours. In terms of respiratory management, patients in the routine group received intermittent intravenous vecuronium (Vecuronium Bromide for Injection, Nanjing Xinbai Pharmaceutical Co., Ltd, National Medical Products Administration Approval Number H20067267) as needed, whereas those in the hypothermia therapy group received continuous vecuronium infusion for 72 hours to prevent shivering. Intracranial pressure (ICP) was monitored in all patients. Elevated intracranial pressure (exceeding 20 mmHg) was managed with a stepwise approach involving intravenous vecuronium, ventricular drainage, hyperventilation (maintaining arterial carbon dioxide pressure above 30 mmHg), and administration of mannitol (Injectio Mannitou, Lanxiha Sanlian Pharmaceutical Co., Ltd, National Medical Products Administration Approval Number H23020609) until serum osmolality reached 315 mOsm/kg.
Patients in the hypothermia therapy group underwent hypothermia treatment, which involved wrapping ice packs with towels and placing them on the head and major blood vessels, in addition to using an ice cap and ice blanket. Temperature was closely monitored by measuring anal temperature every 30 minutes until it stabilized between 33-35°C. This treatment continued for 3 to 5 days. For rewarming, cooling measures were ceased, and patients were covered with warm materials such as blankets to allow slow rewarming. The rate of rewarming was controlled to increase by 1°C every 4 hours, returning to a temperature of 36-37°C within 12 hours. During hypothermia, potassium was supplemented as needed to maintain normal serum levels. Glucose-containing fluids were provided exclusively for parenteral nutrition.
Nutritional support, whether enteral or parenteral, began 48 hours after injury for the routine group and 72 hours after injury for the hypothermia therapy group.
Outcome measures
Intracranial pressure and cerebral hemodynamics detection
Six months post-treatment, patients received head Doppler ultrasound examination. Patients were positioned supine. ICP, cerebral perfusion pressure (CPP), and mean blood flow velocity (MBFV) were monitored using the transcranial Doppler blood flow analyzer (EME Companion, TC2021-III, Germany) (Figure 1).
Figure 1.
Head Doppler ultrasound examination six months post-treatment.
The calculation of ICP used the Aaslid formula:
CPP was calculated as the mean arterial pressure (MAP) minus ICP (CPP = MAP - ICP).
MBFV represents the average speed of blood flow through specific vessels (indicated as ‘mean’ in the Figure 1). Higher MBFV generally indicates better cerebral perfusion, whereas lower MBFV may suggest inadequate cerebral perfusion or issues such as vascular stenosis.
Functional independence measure (FIM)
FIM was used to evaluate a broad range of activities of daily living (ADLs), providing a comprehensive assessment of patient independence and functional status. The FIM score comprises 18 items, categorized into motor and cognitive functions [13]. Each item was rated on a scale from 1 to 7 based on the level of assistance the individual needs to complete the activity. A score of 7 indicates complete independence, meaning the activity can be performed entirely without help. A score of 6 signifies conditional independence, where the individual may require auxiliary equipment or additional time but no direct assistance from others. Scores ranging from 5 to 2 represent varying degrees of dependence, with higher scores indicating less need for supervision, preparation, guidance, or physical assistance. A score of 1 denotes complete dependence, meaning the individual was almost entirely unable to participate in activities and requires significant or total assistance from others. The overall FIM score was calculated by summing the scores of each individual item [14].
Fugl-Meyer motor assessment (FMA)
Six months post-treatment, the FMA was employed to evaluate the patient’s motor function. This assessment includes 50 items that measure coordinated extension and flexion of muscles, reflex activity, hyperreflexia, and coordination ability. Specifically, 23 items assess the upper limbs, and 27 items assess the lower limbs. The scoring system ranges from 0 to 2 for each item, with a total possible score of 100 points. Scores between 96 and 100 indicate mild impairment, scores from 86 to 95 signify significant obstacles, scores from 50 to 85 represent moderate impairment, and scores below 50 were classified as severe impairment [15].
Glasgow coma scale (GCS)
Six months post-treatment, the GCS was used to evaluate the patient’s level of consciousness by assessing their eye-opening, verbal, and motor responses [16]. The scale has a total score of 15 points, with lower scores indicating more severe levels of coma. Eye-opening responses were scored from 4 points for spontaneous eye opening to 1 point for no response. Verbal responses range from 5 points for normal conversation to 1 point for no verbal response. Motor responses were scored from 6 points for following instructions to 1 point for no movement response. A total score of 15 indicates full wakefulness. A GCS score of 13 or higher signifies mild consciousness impairment; scores between 9 and 12 indicate moderate impairment; and scores of 8 or lower represent severe consciousness impairment [17].
Satisfaction
Patient satisfaction in both groups was assessed using the Press Ganey Patient Satisfaction Survey [18]. The questionnaire primarily evaluated patients’ satisfaction with the treatment process, outcome, and medical procedures. Scores ranged from 0 to 100, with higher scores indicating greater satisfaction. Scores of 90-100 were classified as very satisfied, 60-89 as generally satisfied, and below 60 as dissatisfied. Overall satisfaction was calculated as the percentage of patients who were either very satisfied or generally satisfied divided by the total number of cases, multiplied by 100%.
Statistical analysis
All statistical analyses were carried out using SPSS software version 28.0 (SPSS Inc., Chicago, IL, USA). Measured data that followed a normal distribution were presented as mean ± standard deviation and compared using student t test. Categorical data were expressed as frequency and percentage and analyzed by chi-square test. Statistical significance was set at P < 0.05.
Results
Demographic characteristics
There was no significant differences in the mean age, body mass index (BMI), sex distribution, smoking or drinking history, hypertension, diabetes, educational level, marital status, ethnicity, injury type or damage type, motor cortex injury, cerebral hernia, intracranial hematoma, or skull fracture frequency between the routine group and the hypothermia group (all P > 0.05) (Table 1). These findings indicate a well-matched sample population for the subsequent evaluation of cognitive and motor outcomes at 6 months post-injury.
Table 1.
Comparison of demographic characteristics between groups
| Routine group (n = 135) | Hypothermia therapy group (n = 124) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 46.63 ± 15.62 | 47.56 ± 15.61 | 0.478 | 0.633 |
| Body Mass Index (kg/m2) | 23.54 ± 1.55 | 23.61 ± 1.42 | 0.411 | 0.681 |
| Female/Male | 72 (53.33%)/63 (46.67%) | 68 (54.84%)/56 (45.16%) | 0.059 | 0.808 |
| Smoking history (Yes/No) | 31 (22.96%)/104 (77.04%) | 30 (24.19%)/94 (75.81%) | 0.054 | 0.816 |
| Drinking history (Yes/No) | 35 (25.93%)/100 (74.07%) | 32 (25.81%)/92 (74.19%) | 0 | 0.983 |
| Hypertension (Yes/No) | 21 (15.56%)/114 (84.44%) | 16 (12.9%)/108 (87.1%) | 0.371 | 0.542 |
| Diabetes (Yes/No) | 16 (11.85%)/119 (88.15%) | 17 (13.71%)/107 (86.29%) | 0.201 | 0.654 |
| Educational level (Junior college graduate or lower/College graduate or higher) | 69 (51.11%)/66 (48.89%) | 74 (59.68%)/50 (40.32%) | 1.918 | 0.166 |
| Marital Status (Married/Unmarried) | 102 (75.56%)/33 (24.44%) | 95 (76.61%)/29 (23.39%) | 0.040 | 0.842 |
| Ethnicity (Han/Other) | 127 (94.07%)/8 (5.93%) | 116 (93.55%)/8 (6.45%) | 0.031 | 0.861 |
| Injury type | 0.294 | 0.961 | ||
| Car accident injury | 64 (47.41%) | 62 (50%) | ||
| Falling injury | 38 (28.15%) | 35 (28.23%) | ||
| Smash injury | 18 (13.33%) | 15 (12.1%) | ||
| Other | 15 (11.11%) | 12 (9.68%) | ||
| Damage type | 0.228 | 0.633 | ||
| Simple traumatic brain injury | 44 (32.59%) | 37 (29.84%) | ||
| Multiple injuries | 91 (67.41%) | 87 (70.16%) | ||
| Motor Cortex Injury (Yes/No) | 23 (17.04%)/112 (82.96%) | 21 (16.94%)/103 (83.06%) | 0 | 0.983 |
| Cerebral hernia (Yes/No) | 14 (10.37%)/121 (89.63%) | 11 (8.87%)/113 (91.13%) | 0.167 | 0.683 |
| Intracranial hematoma (Yes/No) | 18 (13.33%)/117 (86.67%) | 15 (12.1%)/109 (87.9%) | 0.089 | 0.766 |
| Skull fracture (Yes/No) | 6 (4.44%)/129 (95.56%) | 5 (4.03%)/119 (95.97%) | 0.027 | 0.869 |
Comparison of treatment-related indicators
Both groups received similar amount of morphine (P = 0.809) and potassium (P = 0.807). However, the hypothermia group required significantly higher doses of vecuronium (P = 0.002) and demonstrated a more favorable cumulative fluid balance during the first 96 hours of admission compared to the routine group (P = 0.003). Although the hypothermia group showed lower food intake by day six than the routine group, which was not statistically significant (P = 0.087). There was no significant difference observed in the administration of mannitol between the two groups (P = 0.216). These findings suggest that while hypothermia therapy may require adjustments in concurrent medication administration, it may contribute to improved fluid management in the acute phase post-injury (Table 2).
Table 2.
Comparison of post hospitalization treatment between groups
| Routine group (n = 135) | Hypothermia therapy group (n = 124) | t | P | |
|---|---|---|---|---|
| Morphine (mg) | 8.21 ± 3.05 | 8.3 ± 3.27 | 0.241 | 0.809 |
| Vecuronium (mg) | 6.91 ± 2.84 | 8.21 ± 3.81 | 3.101 | 0.002 |
| Mannitol (g) | 43.15 ± 17.65 | 45.95 ± 18.62 | 1.239 | 0.216 |
| Potassium (mmol) | 26.62 ± 11.24 | 26.95 ± 10.32 | 0.244 | 0.807 |
| Food intake by day 6 (kcal/day) | 1598.65 ± 549.65 | 1482.67 ± 534.97 | 1.718 | 0.087 |
| Cumulative fluid balance during first 96 hours (ml) | 3061.85 ± 1146.54 | 2656.32 ± 1056.61 | 2.952 | 0.003 |
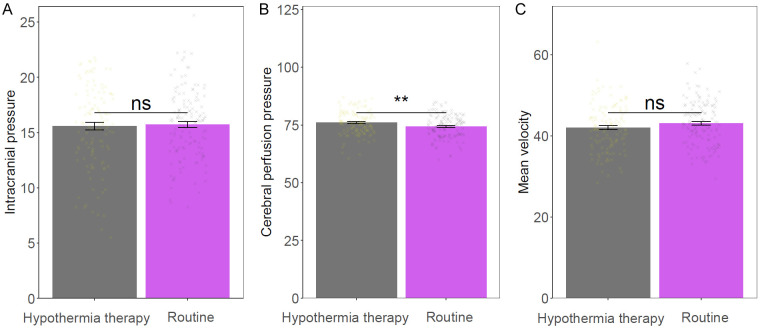
Intracranial pressure and cerebral hemodynamics comparison
No significant difference in intracranial pressure (P = 0.758) and Mean velocity measurements (P = 0.145) between the two groups. However, the hypothermia group had significant increase in cerebral perfusion pressure than the routine group (P = 0.009). These findings imply that hypothermia therapy might enhance cerebral perfusion pressure while maintaining adequate control of intracranial pressure and similar blood flow velocities following TBI (Figure 2).
Figure 2.
Comparison of intracranial pressure and cerebral hemodynamics between the two groups. A: Intracranial pressure; B: Cerebral perfusion pressure; C: Mean velocity. ns: no significant difference; **: P < 0.01.
FIM comparison
No significant differences were observed in motor function (P = 0.633) or cognitive function (P = 0.112) between the groups. However, at the six-month follow-up, the hypothermia therapy group exhibited a significant improvement in both motor function (P = 0.008) and cognitive function scores (P = 0.003). These findings suggest that hypothermia therapy enhanced both motor and cognitive recovery in patients with TBI, highlighting its utility in promoting functional independence post-injury (Figure 3).
Figure 3.
Comparison of FIM score between the two groups. A: Cognitive function before treatment; B: Cognitive function 6 months after treatment; C: Motor function before treatment; D: Motor function 6 months after treatment. FIM: Functional Independence Measure. ns: no significant difference; **: P < 0.01.
Motion state comparison
Compared to the routine therapy group, the hypothermia group exhibited a higher proportion of patients with mild impairment (27.42% vs. 17.78%) and a lower proportion with severe impairment (9.68% vs. 14.81%). Additionally, moderate impairment was less frequent in the hypothermia group (15.32% vs. 31.85%), whereas obvious obstacles were more prevalent (47.58% vs. 35.56%). These findings indicate that hypothermia therapy may contribute to better motor function recovery, resulting in a higher likelihood of achieving mild motor impairment post-TBI (P = 0.003; Table 3).
Table 3.
Comparison of Fugl Meyer Motor Assessment between groups
| Routine group (n = 135) | Hypothermia therapy group (n = 124) | χ2 | P | |
|---|---|---|---|---|
| Mild impairment | 24 (17.78%) | 34 (27.42%) | 13.703 | 0.003 |
| Obvious obstacles | 48 (35.56%) | 59 (47.58%) | ||
| Moderate impairment | 43 (31.85%) | 19 (15.32%) | ||
| Severe impairment | 20 (14.81%) | 12 (9.68%) |
GCS comparison
The hypothermia therapy group exhibited a significantly higher proportion of patients with mild consciousness impairment compared to the routine treatment group (71.77% vs. 52.59%). In contrast, moderate impairment was more common in the routine group (38.52% vs. 22.58%), while severe impairment showed a similar trend (8.89% vs. 5.65%). These results suggest that hypothermia therapy was associated with an improved consciousness level, as evidenced by higher rates of mild impairment and lower incidences of moderate and severe impairments, highlighting a benefit in the management of TBI (P = 0.006; Table 4).
Table 4.
Comparison of GCS between two groups
| Routine group (n = 135) | Hypothermia therapy group (n = 124) | χ2 | P | |
|---|---|---|---|---|
| Mild consciousness impairment | 71 (52.59%) | 89 (71.77%) | 10.092 | 0.006 |
| Moderate consciousness impairment | 52 (38.52%) | 28 (22.58%) | ||
| Severe consciousness impairment | 12 (8.89%) | 7 (5.65%) |
GCS: Glasgow Coma Scale.
Patient satisfaction rate comparison
Overall satisfaction was significantly higher in the hypothermia therapy group (91.13%) compared to the routine treatment group (80.74%; P = 0.017; Table 5). These findings suggest that therapeutic hypothermia could enhance both clinical outcomes and patient experience in TBI management.
Table 5.
Comparison of satisfaction between two groups
| Routine group (n = 135) | Hypothermia therapy group (n = 124) | χ2 | P | |
|---|---|---|---|---|
| Dissatisfied | 26 (19.26%) | 11 (8.87%) | ||
| Generally satisfied | 62 (45.93%) | 47 (37.9%) | ||
| Very satisfied | 47 (34.81%) | 66 (53.23%) | ||
| Overall satisfaction | 109 (80.74%) | 113 (91.13%) | 5.696 | 0.017 |
Discussion
The present study aimed to explore the efficacy of hypothermia therapy as a neuroprotective intervention following TBI six months post-treatment. The findings demonstrated that patients who received hypothermia therapy exhibited significantly improved cognitive and motor functions compared to those receiving routine treatment, alongside enhanced satisfaction and consciousness levels. These promising outcomes merit a detailed reflection on the underlying mechanisms that might contribute to the observed therapeutic benefits of hypothermia therapy.
One of the foremost explanations for the benefits observed under hypothermia therapy was its ability to mitigate secondary brain injury processes that commonly follow the primary traumatic insult. After TBI, a cascade of secondary injuries - involving neuroinflammatory responses, excitotoxicity, oxidative stress, and cellular apoptosis - exacerbates brain tissue damage. Hypothermia was hypothesized to attenuate these deleterious processes by diminishing metabolic demands in brain tissue, thereby preserving cellular integrity and function. The reduction of cerebral metabolic rate consequentially leads to decreased production of lactate and free radicals, limiting oxidative stress and safeguarding neuronal structures [19].
A significant finding in this study was the notable improvement in cerebral perfusion pressure, which was higher in the hypothermia-treated group compared to the routine group. This outcome can be attributed to the vasoconstrictive effect of hypothermia, leading to reduced ICP by minimizing cerebral edema and improving microcirculation [20]. By maintaining more stable ICP and enhancing cerebral hemodynamics, hypothermia therapy may promote better delivery of oxygen and nutrients to the brain, further supporting neuronal recovery and function [21]. In support of this hypothesis, Lilla et al. [22] conducted a study on subarachnoid hemorrhage (SAH) in animals and found that hypothermia improved CPP by slightly increasing MAP during the cooling phase and significantly reducing ICP. They observed a trend towards increased cerebral blood flow (CBF) during the first 60 minutes after SAH. Additionally, the rate of injured neurons was significantly lower in hypothermia-treated animals compared with normothermic controls. These experimental results align closely with our clinical observations, suggesting that hypothermia-mediated stabilization of ICP and improvement in CBF represent fundamental mechanisms underlying its neuroprotective efficacy in acute brain injury.
The role of hypothermia in modulating inflammatory responses was another critical mechanism to consider. Temperature reduction was known to suppress the activation of microglia and astrocytes, curtailing the release of pro-inflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α), Interleukin-1 beta (IL-1β), and Interleukin-6 (IL-6). This dampened inflammatory response potentially prevents extensive neuronal damage and cell death. Moreover, by slowing the rate of neuroinflammation, hypothermia provides a conducive environment for neurorestorative processes, facilitating synaptic repair and neurogenesis [23].
Interestingly, while both groups demonstrated comparable baseline intracranial pressure, the hypothermia-treated cohort exhibited significantly improved cerebral perfusion pressure, indicating a distinct hemodynamic advantage. This observed effect may be mediated through hypothermia-induced enhancement of cerebral autoregulation, which appears to optimize vascular responsiveness and dynamically match cerebral blood flow to metabolic demands. Such preserved autoregulatory capacity not only substantiates hypothermia’s neuroprotective properties but also ensures critical maintenance of perfusion to vulnerable brain regions following traumatic injury.
On the motor side, the FMA indicated improved motor recovery in patients undergoing hypothermia therapy, with a higher proportion of these patients demonstrating mild impairments compared to those receiving routine treatment. This motor recovery might be related to the reduced extent of injury and preservation of motor cortex functionality due to hypothermia’s neuroprotective effects. David et al. [24] conducted a study on the intraparenchymal implantation of SB623 cells in patients with chronic motor deficits after TBI. Their results showed that SB623 cell therapy significantly improved motor status at 24 weeks, with continued improvement in function and ADL at 48 weeks. These findings suggest that cell therapy can modify chronic neurological deficits after TBI, providing additional evidence for the potential benefits of neuroprotective interventions. Additionally, hypothermia-induced reduction in axonal injury should not be overlooked as it potentially contributes to the retention and restoration of motor pathways.
The enhanced consciousness and overall patient satisfaction observed in the hypothermia group align with the physiologic benefits and clinical efficacy demonstrated in this study. An improved level of consciousness, associated with a higher prevalence of mild consciousness impairment, signifies less neurocognitive burden and aligns with the proposed mechanisms of hypothermia attenuating secondary brain insults. Patient satisfaction being higher in the hypothermia group might reflect perceived improvement in functional recovery and quality of life, providing additional support for the applicability of hypothermia in clinical settings.
Notably, hypothermia therapy required modest adjustments in concurrent medication administration, as seen in the increased doses of vecuronium required to prevent shivering [25]. Shivering counteracts the cooling effects and increases metabolic demand, negating some benefits of hypothermia; hence, adequate neuromuscular blockade was essential to maintain the targeted therapeutic range of lowered core body temperature.
This study had several limitations that should be considered alongside its promising findings. The retrospective cohort design carries inherent risks of selection bias and limited control over confounding variables, while the lack of randomization means baseline differences between groups may have influenced outcomes. Additionally, the reliance on historical data might have affected the completeness and accuracy of the recorded outcomes and treatment details. The study’s focus on a single center may limit the generalizability of the results to different clinical settings or populations. Furthermore, the absence of standardization in the implementation of hypothermia protocols, including the duration and degree of cooling, calls for caution in interpreting the data. Future multicenter prospective randomized trials with standardized protocols and diverse populations are needed to validate these findings and enhance external validity [26]. Extended follow-up beyond six months would better assess long-term neuroprotective effects and quality of life impacts. Further investigation into the effects of therapeutic hypothermia on specific biomarkers, such as inflammatory factors, oxidative stress markers, and neuron-specific proteins, could provide insights into its mechanisms of action and offer a basis for personalized treatment. Additionally, investigating combination therapies with antioxidants, anti-inflammatories, or stem cells may further optimize TBI management.
Conclusion
Hypothermia therapy emerged as a favorable intervention improving both cognitive and motor outcomes following TBI. This study adds to the growing body of evidence supporting the adoption of hypothermia as a neuroprotective strategy, highlighting its capacity to modulate pathophysiologic processes such as neuroinflammation and cerebral perfusion. These findings might help refine therapeutic approaches for individuals suffering from traumatic brain injuries.
Acknowledgements
This study was supported by Guangxi Key Laboratory of Rare and Endangered Animal Ecology, Guangxi Normal University (Guikeneng 22-A-02-03).
Disclosure of conflict of interest
None.
References
- 1.Cox CS Jr, Notrica DM, Juranek J, Miller JH, Triolo F, Kosmach S, Savitz SI, Adelson PD, Pedroza C, Olson SD, Scott MC, Kumar A, Aertker BM, Caplan HW, Jackson ML, Gill BS, Hetz RA, Lavoie MS, Ewing-Cobbs L. Autologous bone marrow mononuclear cells to treat severe traumatic brain injury in children. Brain. 2024;147:1914–1925. doi: 10.1093/brain/awae005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman JM, Curran M, Barber J, Lucas S, Fann JR, Zumsteg JM. Collaborative care for chronic pain after traumatic brain injury: a randomized clinical trial. JAMA Netw Open. 2024;7:e2413459. doi: 10.1001/jamanetworkopen.2024.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karns CM, Wade SL, Slocumb J, Keating T, Gau JM, Slomine BS, Suskauer SJ, Glang A. Traumatic brain injury positive strategies for families: a pilot randomized controlled trial of an online parent-training program. Arch Phys Med Rehabil. 2023;104:1026–1034. doi: 10.1016/j.apmr.2023.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montaldo P, Cirillo M, Burgod C, Caredda E, Ascione S, Carpentieri M, Puzone S, D’Amico A, Garegrat R, Lanza M, Moreno Morales M, Atreja G, Shivamurthappa V, Kariholu U, Aladangady N, Fleming P, Mathews A, Palanisami B, Windrow J, Harvey K, Soe A, Pattnayak S, Sashikumar P, Harigopal S, Pressler R, Wilson M, De Vita E, Shankaran S, Thayyil S. Whole-body hypothermia vs targeted normothermia for neonates with mild encephalopathy: a multicenter pilot randomized clinical trial. JAMA Netw Open. 2024;7:e249119. doi: 10.1001/jamanetworkopen.2024.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerc C, Talebian Nia M, Giesbrecht GG. Heat transfer capabilities of surface cooling systems for inducing therapeutic hypothermia. Ther Hypothermia Temp Manag. 2023;13:149–158. doi: 10.1089/ther.2023.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Huang C, Tian R, Yang X. Target temperature management and therapeutic hypothermia in sever neuroprotection for traumatic brain injury: clinic value and effect on oxidative stress. Medicine (Baltimore) 2023;102:e32921. doi: 10.1097/MD.0000000000032921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MA, McNinch NL, Chaney D, Shauver L, Murray T, Kline P, Lesak A, Franco-MacKendrick L, Scott L, Logan K, Ichesco IK, Liebig C, Congeni J. Reduced concussion symptom burden in early adolescent athletes using a head-neck cooling device. Clin J Sport Med. 2024;34:247–255. doi: 10.1097/JSM.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita M, Oda Y, Kaneda K, Kaneko T, Suehiro E, Dohi K, Kuroda Y, Kobata H, Tsuruta R, Maekawa T. Temperature difference between jugular bulb and pulmonary artery is associated with neurological outcome in patients with severe traumatic brain injury: a post hoc analysis of a brain hypothermia study. PLoS One. 2023;18:e0285525. doi: 10.1371/journal.pone.0285525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willer-Hansen RS, Olsen MH, Hauerberg J, Johansen HK, Andersen ÅB, Møller K. Diagnostic criteria of CNS infection in patients with external ventricular drainage after traumatic brain injury: a pilot study. Acta Anaesthesiol Scand. 2022;66:507–515. doi: 10.1111/aas.14036. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg ND, Iverson GL, Cogan A, Dams OCK, Delmonico R, Graf MJP, Iaccarino MA, Kajankova M, Kamins J, McCulloch KL, McKinney G, Nagele D, Panenka WJ, Rabinowitz AR, Reed N, Wethe JV, Whitehair V, Anderson V, Arciniegas DB, Bayley MT, Bazarian JJ, Bell KR, Broglio SP, Cifu D, Davis GA, Dvorak J, Echemendia RJ, Gioia GA, Giza CC, Hinds SR 2nd, Katz DI, Kurowski BG, Leddy JJ, Sage NL, Lumba-Brown A, Maas AI, Manley GT, McCrea M, Menon DK, Ponsford J, Putukian M, Suskauer SJ, van der Naalt J, Walker WC, Yeates KO, Zafonte R, Zasler ND, Zemek R. The American congress of rehabilitation medicine diagnostic criteria for mild traumatic brain injury. Arch Phys Med Rehabil. 2023;104:1343–1355. doi: 10.1016/j.apmr.2023.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Shinoda J, Asano Y. Disorder of executive function of the brain after head injury and mild traumatic brain injury - neuroimaging and diagnostic criteria for implementation of administrative support in Japan. Neurol Med Chir (Tokyo) 2017;57:199–209. doi: 10.2176/nmc.ra.2016-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docherty A, Emelifeonwu J, Andrews PJD. Hypothermia after traumatic brain injury. JAMA. 2018;320:2204–2206. doi: 10.1001/jama.2018.17121. [DOI] [PubMed] [Google Scholar]
- 13.Maas AIR, Menon DK, Manley GT, Abrams M, Åkerlund C, Andelic N, Aries M, Bashford T, Bell MJ, Bodien YG, Brett BL, Büki A, Chesnut RM, Citerio G, Clark D, Clasby B, Cooper DJ, Czeiter E, Czosnyka M, Dams-O’Connor K, De Keyser V, Diaz-Arrastia R, Ercole A, van Essen TA, Falvey É, Ferguson AR, Figaji A, Fitzgerald M, Foreman B, Gantner D, Gao G, Giacino J, Gravesteijn B, Guiza F, Gupta D, Gurnell M, Haagsma JA, Hammond FM, Hawryluk G, Hutchinson P, van der Jagt M, Jain S, Jain S, Jiang JY, Kent H, Kolias A, Kompanje EJO, Lecky F, Lingsma HF, Maegele M, Majdan M, Markowitz A, McCrea M, Meyfroidt G, Mikolić A, Mondello S, Mukherjee P, Nelson D, Nelson LD, Newcombe V, Okonkwo D, Orešič M, Peul W, Pisică D, Polinder S, Ponsford J, Puybasset L, Raj R, Robba C, Røe C, Rosand J, Schueler P, Sharp DJ, Smielewski P, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Temkin N, Tenovuo O, Theadom A, Thomas I, Espin AT, Turgeon AF, Unterberg A, Van Praag D, van Veen E, Verheyden J, Vyvere TV, Wang KKW, Wiegers EJA, Williams WH, Wilson L, Wisniewski SR, Younsi A, Yue JK, Yuh EL, Zeiler FA, Zeldovich M, Zemek R InTBIR Participants and Investigators. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21:1004–1060. doi: 10.1016/S1474-4422(22)00309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalski RG, Hammond FM, Weintraub AH, Nakase-Richardson R, Zafonte RD, Whyte J, Giacino JT. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021;78:548–557. doi: 10.1001/jamaneurol.2021.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha LSO, Gama GCB, Rocha RSB, Rocha LB, Dias CP, Santos LLS, Santos MCS, Montebelo MIL, Teodori RM. Constraint induced movement therapy increases functionality and quality of life after stroke. J Stroke Cerebrovasc Dis. 2021;30:105774. doi: 10.1016/j.jstrokecerebrovasdis.2021.105774. [DOI] [PubMed] [Google Scholar]
- 16.Bodien YG, Barra A, Temkin NR, Barber J, Foreman B, Vassar M, Robertson C, Taylor SR, Markowitz AJ, Manley GT, Giacino JT, Edlow BL. Diagnosing level of consciousness: the limits of the Glasgow coma scale total score. J Neurotrauma. 2021;38:3295–3305. doi: 10.1089/neu.2021.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook NF. The Glasgow coma scale: a European and global perspective on enhancing practice. Crit Care Nurs Clin North Am. 2021;33:89–99. doi: 10.1016/j.cnc.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Tyser AR, Abtahi AM, McFadden M, Presson AP. Evidence of non-response bias in the Press-Ganey patient satisfaction survey. BMC Health Serv Res. 2016;16:350. doi: 10.1186/s12913-016-1595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Ding YL, Gao L, Zhu Y, Lin YY, Lin XZ. Efficacy of therapeutic hypothermia on mild neonatal hypoxic-ischemic encephalopathy: a prospective randomized controlled study. Zhongguo Dang Dai Er Ke Za Zhi. 2024;26:803–810. doi: 10.7499/j.issn.1008-8830.2401031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel C, Rüdiger M, Benders M, van Bel F, Allegaert K, Naulaers G, Bassler D, Klebermaß-Schrehof K, Vento M, Vilan A, Falck M, Mauro I, Metsäranta M, Vanhatalo S, Mazela J, Metsvaht T, van der Vlught R, Franz AR. Detailed statistical analysis plan for ALBINO: effect of Allopurinol in addition to hypothermia for hypoxic-ischemic Brain Injury on Neurocognitive Outcome - a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III) Trials. 2024;25:81. doi: 10.1186/s13063-023-07828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintermark P, Lapointe A, Steinhorn R, Rampakakis E, Burhenne J, Meid AD, Bajraktari-Sylejmani G, Khairy M, Altit G, Adamo MT, Poccia A, Gilbert G, Saint-Martin C, Toffoli D, Vachon J, Hailu E, Colin P, Haefeli WE. Feasibility and safety of sildenafil to repair brain injury secondary to birth asphyxia (SANE-01): a randomized, double-blind, placebo-controlled phase Ib clinical trial. J Pediatr. 2024;266:113879. doi: 10.1016/j.jpeds.2023.113879. [DOI] [PubMed] [Google Scholar]
- 22.Lilla N, Rinne C, Weiland J, Linsenmann T, Ernestus RI, Westermaier T. Early transient mild hypothermia attenuates neurologic deficits and brain damage after experimental subarachnoid hemorrhage in rats. World Neurosurg. 2018;109:e88–e98. doi: 10.1016/j.wneu.2017.09.109. [DOI] [PubMed] [Google Scholar]
- 23.Montaldo P, Burgod C, Herberg JA, Kaforou M, Cunnington AJ, Mejias A, Cirillo G, Miraglia Del Giudice E, Capristo C, Bandiya P, Kamalaratnam CN, Chandramohan R, Manerkar S, Rodrigo R, Sumanasena S, Krishnan V, Pant S, Shankaran S, Thayyil S. Whole-blood gene expression profile after hypoxic-ischemic encephalopathy. JAMA Netw Open. 2024;7:e2354433. doi: 10.1001/jamanetworkopen.2023.54433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okonkwo DO, McAllister P, Achrol AS, Karasawa Y, Kawabori M, Cramer SC, Lai A, Kesari S, Frishberg BM, Groysman LI, Kim AS, Schwartz NE, Chen JW, Imai H, Yasuhara T, Chida D, Nejadnik B, Bates D, Stonehouse AH, Richardson RM, Steinberg GK, Poggio EC, Weintraub AH. Mesenchymal stromal cell implants for chronic motor deficits after traumatic brain injury: post hoc analysis of a randomized trial. Neurology. 2024;103:e209797. doi: 10.1212/WNL.0000000000209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takebe S, Taguchi S, Obata N, Mizobuchi S. Effect of intravenous lidocaine on rocuronium: a randomized controlled trial. Kobe J Med Sci. 2025;70:E143–E151. doi: 10.24546/0100493127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helland AM, Mydske S, Assmus J, Brattebø G, Wiggen Ø, Kvidaland HK, Thomassen Ø. Experimental hypothermia by cold air: a randomized, double-blind, placebo-controlled crossover trial. Scand J Trauma Resusc Emerg Med. 2025;33:16. doi: 10.1186/s13049-025-01331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]