Abstract
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a significant global health issue characterized by a complex etiology and high rates of recurrence. IBD increases the risk of acquiring colorectal cancer (CRC). CRC, the third leading cause of cancer worldwide, is becoming increasingly prevalent each year. Treatments targeting IBD and associated CRC do not yield effective results. One of the cell death processes associated with IBD pathogenesis is apoptosis. Although apoptosis helps maintain the intestinal barrier of the gut, excessive apoptosis is linked to the development of IBD and contributes to the growth and progression of CRC. In IBD, pro-apoptotic molecules are elevated, while they decrease in CRC. Therefore, therapies that inhibit pro-apoptotic molecules in IBD and enhance apoptosis in CRC may help prevent IBD and the associated CRC. This article reviews the mechanisms of apoptosis and its involvement in IBD and CRC. It also examines possible therapies, including gene and combination therapies that target apoptosis molecules and their signaling pathways in IBD and CRC.
Keywords: Apoptosis, inflammatory bowel disease, colorectal cancer, therapy
Introduction
Apoptosis is an inherently programmed phenomenon that regulates cell death and clears malfunctioning cells. In 1972, Kerr et al. first used the term “apoptosis” to describe “physiological phenomena of broad significance in tissue dynamics” [1]. Cells undergoing apoptosis are characterized by chromatin condensation, nuclear fragmentation, internucleosome deoxyribonucleic acid (DNA) fragmentation, plasma membrane vacuole formation, corn kernelization (the appearance of plasma membrane boiling, which usually occurs within minutes), budding of cell debris (apoptotic bodies), preservation of organelle stability, and eventual phagocytosis [2]. Apoptosis is a form of programmed cell death in which specific molecular mechanisms guide cells to change their effect on the environment. The most in-depth research is currently on apoptosis, necroptosis, and pyroptosis. Of these, apoptosis is thought to be immunologically inactive (silent) and well coordinated [3] to avoid the occurrence of inflammatory responses around apoptotic cells.
Homeostasis and transformation are critical to human health, and apoptosis is crucial in growth, immune surveillance, and neoplastic development [4]. Intestinal epithelial cells (IECs) act as an interface between organisms and gastrointestinal content, emphasizing the importance of maintaining cell viability under the onslaught of pathogens, toxins, and cytokines, and maintaining an equilibrium between cell growth and apoptosis [5]. It has been found that the loss of gut cells due to apoptosis largely offsets the number of cells needed to produce the intestinal epithelium, which is regulated by this process [6].
Inflammatory bowel disease (IBD) is a collection of persistent, nonspecific gut inflammatory illnesses caused by immunological, genetic, environmental, and other factors, with the major hallmarks being abdominal pain, weight loss, and diarrhea. IBD, including ulcerative colitis (UC) and Crohn’s disease (CD), has significantly increased in both developed and developing countries, with an increasing age of onset that was previously affecting children and young adults [7]. IBD is an important risk factor for colorectal cancer (CRC) [8]. Therefore, researching treatment methods and their effects on IBD is crucial to reducing the occurrence of IBD and CRC. Lately, the pathogenesis of apoptosis in IBD has gradually been highlighted [9]. The development of IBD is triggered by excessive IEC apoptosis, which compromises the gut barrier, permits ‘dangerous’ bacteria to colonize, and initiates chronic inflammation [10]. Additionally, the pathophysiology of IBD-developed CRC, sometimes referred to as colitis-associated cancer (CAC), includes apoptosis [11]. This article examines the mechanisms of apoptosis and their role in IBD and CRC. This review will also examine effective treatment strategies for CRC and IBD through the regulation of apoptosis.
Mechanisms and pathways of apoptosis
Cellular mechanisms
Cytochrome c’s release from mitochondria is a crucial part of apoptosis, which is controlled by an equilibrium between pro- and anti-apoptotic B-cell lymphoma-2 (Bcl-2) family proteins, initiator caspases (caspase-8, -9, and -10), and effector caspases (caspase-3, -6, and -7) [3]. Based on the activating chemical, apoptosis can be classified as extrinsic or intrinsic.
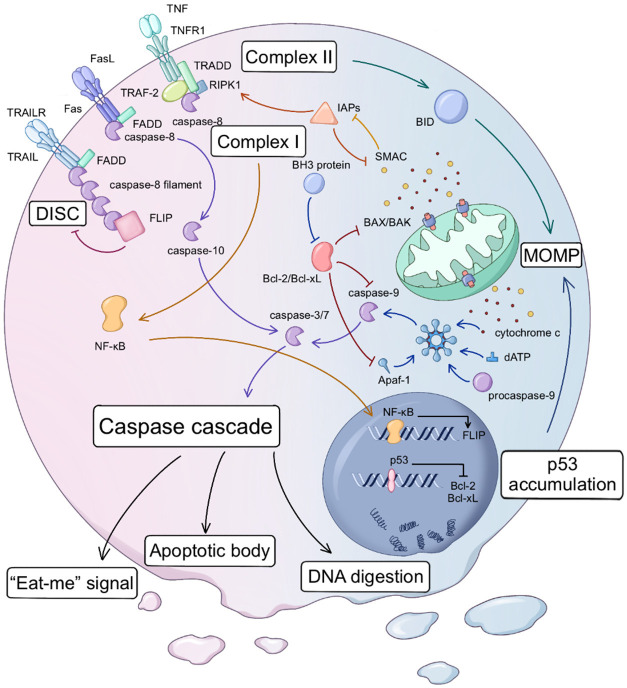
Dysregulation of intracellular homeostasis leads to mitochondrial membrane permeabilization (MOMP), causing the intrinsic route to release cytochrome c into the cytoplasm through the mitochondrial membrane gap (Figure 1). MOMP is due to the creation of pores in the outer mitochondrial membrane by oligomerized Bcl-2 pro-apoptotic factors (Bcl-2-associated X protein (BAX), Bcl-2-killer (BAK), etc.) [12]. When MOMP releases cytochrome c, it causes apoptosomes to develop and caspase-3 to become active [13]. Cytochrome c, apoptosis protease-activator factor 1 (Apaf-1), 2’deoxyadenosine 5’-triphosphate (dATP), and procaspase-9 are all found in the enormous apoptosome. The apoptosome stimulates the apoptotic initiator caspase-9, which subsequently breaks down the precursors of caspase-3 and caspase-7, leading to the execution of apoptosis by caspase-3 and caspase-7 [14,15]. Once activated, caspase-3 and caspase-7 activate several other procaspases downstream, resulting in an apoptosis-amplifying cascade [15-17].
Figure 1.
Apoptosis is caused by two key routes known as the intrinsic and extrinsic routes. MOMP induces the intrinsic route. BAK/BAX oligomers generate holes in the mitochondrial outer membrane, releasing cytochrome c and SMAC/DIABLO into the cytosol. Pro-apoptotic or anti-apoptotic Bcl-2 family proteins modulate BAK/BAX activity. Cytochrome c interacts with Apaf-1, incorporating procaspase-9 and forming the apoptosome. Auto-proteolytic cleavage within the apoptosome activates caspase-9, commencing the caspase-processing cascade. P53 can inhibit the expression of Bcl-2/Bcl-xL, and P53 accumulation can also activate BAK/BAX. The extrinsic route is initiated by death receptors located on the cell surface. When death receptors connect with their ligands, the extrinsic route is activated. Fas and TRAIL attract FADD from the cell’s death domain (DD) and combine it, forming a group. Subsequently, they recruit caspase-8 via the death effector domain (DED) to facilitate its oligomerization. The oligomerized caspase-8 exposes DED to connect more caspase-8 to form a caspase-8 filament, further forming DISC. The self-activated caspase-8/10 activates the downstream effect caspase-3/7, which in turn causes the caspase cascade amplification effect. After TNFR binds to its ligand, it can combine with TRAF-2 and TRADD while simultaneously recruiting receptor-interacting protein kinase (RIPK1) and FLIP to form complex I in the early stage of apoptosis. This complex prevents the apoptotic function of caspase-8. Subsequently, the relevant protein is internalized to form complex II, which induces mitochondrial MOMP through BID regulation in an intrinsic manner. FLIP, NF-κB, and IAP family members can inhibit the occurrence of apoptosis. The caspase cascade is the common endpoint of the intrinsic and extrinsic pathways. Afterward, nuclear fragmentation occurs, DNA is digested, and apoptotic bodies form, releasing “eat-me” signals to attract phagocytes.
In contrast to the intrinsic route, cell surface death receptors trigger the extrinsic route. In other words, the signal is derived from the outside of the cell (Figure 1). When death receptors are activated by their ligands, they oligomerize and attract adaptor proteins such as Fas-associated protein with death domain (FADD) or tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) associated via death domain (TRADD). This process activates caspase-8 and caspase-10 simultaneously, forming a death-inducing signaling complex (DISC) [18-24]. In most cases, respiratory chain complexes (RCC) I and II work in conjunction with DISC [25], culminating in the activation of caspase and the occurrence of a caspase cascade. The intrinsic and the extrinsic pathways are not entirely independent. When caspase-8/10 is activated, RCC II in the extrinsic pathway can also generate feedback into the intrinsic pathway and promote MOMP indirectly through the Bcl-2 family’s homology domain 3 (BH3) interacting domain death agonist (BID) [26].
Regulators of apoptosis
During apoptosis, the pro-apoptotic and anti-apoptotic teams struggle to pull the rope in the “death game” to maintain a delicate balance of apoptosis. The following are the key proteins responsible for regulating apoptosis.
Bcl-2 family
The interplay of anti- and pro-apoptotic proteins in the Bcl-2 family initiates the intrinsic route. The Bcl-2 family is grouped by four preserved Bcl-2 homology domains (BH1-4) [27]. Three subgroups of the Bcl-2 family have been identified according to functional and structural traits [28]. The first group includes BH3 proteins such as Bcl-2 interacting mediator of cell death (BID), P53 up-regulated modulator of apoptosis (PUMA), and Bcl-2 associated agonist of cell death (BAD). They detect cell damage and activate the second group of members in either a direct or indirect manner. The second group of members is the apoptosis executor, which consists of BAK, BAX, and Bcl-2-related ovarian killer (BOX), and is responsible for punching holes within the mitochondrial membrane so that the mitochondrial contents leak. The third group consists of anti-apoptotic proteins like Bcl-2 and Bcl-xL, which hinder the work of the first or second groups of proteins [29].
In normal cells, anti-apoptotic proteins (e.g., Bcl-xL) and pro-apoptotic proteins (e.g., BAX, BAK) undergo heterodimerization by binding to their BH3 motifs, thereby blocking their action [30]. However, when the cell is damaged or stops receiving survival signals, the BH3-only protein frees BAX and BAK by sequentially inhibiting Bcl-2 and Bcl-xL. Therefore, BH3 may be the site of pro-apoptotic inhibitor action. Clinically, there have been studies on the combination of BH3-mimetic drugs and STING agonists to treat TP53-mutant blood cancer [31]. Bcl-xL also prevents the formation of apoptosomes by interacting with the cell death abnormality gene 4 (CED-4)-like moiety of Apaf-1, and caspase-9 assembles with its NH2-terminal caspase recruitment domain [32]. Targeting the anti-apoptotic Bcl-2 protein is an appealing cancer therapeutic strategy since malignancy is characterized by Bcl-2 protein-mediated resistance to intrinsic apoptosis [33].
Caspase
Caspase is the executor of apoptosis, and the caspase cascade is the common endpoint of both extrinsic and intrinsic pathways. All caspases are present in cells as dormant zymogens and are proteolytically stimulated to participate in apoptosis. The initiator is auto-activated, triggering a chain reaction of subsequent caspases. Once stimulated, the caspase effector hydrolyzes the broad-spectrum cellular target, leading to cell death, ensuring tightly regulated activation [34]. Caspases are regulated through transcriptional mechanisms and post-translational modifications. Additionally, caspase activity is influenced by various molecules, such as the inhibitors of apoptosis proteins (IAP) family and the second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low PI (SMAC/DIABLO), among others (see section Death receptors and dependence receptors below). Understanding the mechanisms of these sites can provide reference targets for IBD and cancer treatment.
Death receptors and dependence receptors
The extrinsic route is triggered by two kinds of receptors found in plasma membranes: death receptors, which are triggered by the interaction of similar ligands, and dependency receptors, which become active when their particular ligand levels drop below a predetermined threshold [35,36].
The death receptors involved in apoptosis include TNF receptor 1 (TNFR1), Fas (CD95), and TNF-related apoptosis-inducible receptor (TRAILR). Their ligands include TNF, FasL (CD95-ligand), and TNF-related apoptosis-inducing ligand (TRAIL) [37]. TRAIL and Fas activate apoptosis through similar mechanisms. Neither the death receptors nor their ligands are already trimers before binding, which has often been misunderstood in the past [38]. When the receptor and ligand bind, a higher-order complex is formed, which exposes a “death domain (DD)” inside the cell. Conformational changes in intracellular DD can bind FADD, a small cytosolic protein that also has DD. Subsequently, they are assembled into groups through DD-DD interactions, exposing the death effector domain (DED), which attracts and oligomerizes caspase-8, further forming DISC. TNF-α or interferon can upregulate Fas, increasing cellular sensitivity to FasL, leading to apoptosis [39]. Unlike Fas and TRAIL, TNFR1 binds to the ligand, exposing DD, which binds to TNF-receptor-associated factor-2 (TRAF-2) and TRADD rather than FADD. TRADD can enhance the binding of TRAF-2 while attracting other compounds, such as receptor-interacting protein kinase (RIPK1) and cIAP. RIPK1 induces the formation of different complexes and regulates apoptosis via multiple signaling pathways.
The extrinsic pathway can also be activated by dependence receptors that detect the withdrawal and absence of their ligands. More than 20 dependence receptors have been discovered, and some have been shown to inhibit tumor development by inducing apoptosis. Under normal physiological conditions, these receptors promote cell proliferation, survival, and differentiation, but when their ligand availability decreases to a certain threshold, they activate the caspase cascade, which promotes apoptosis [35].
Other apoptosis molecules
Numerous signaling molecules indirectly influence cell death by modulating Bcl-2 family and caspase expression, activation, and function. In addition, some molecules affect the apoptosis mechanism by modulating the expression of related genes and binding to death receptors.
The protein FLICE-like inhibitory protein (FLIP) regulates the extrinsic apoptotic pathway. It can also bind to FADD in DISC through the DED-DED connection, which prevents the production of the caspase-8 filament, thus regulating apoptosis [40]. RIPK1 is another protein that regulates the extrinsic apoptotic pathway. During the early phases of stimulation, the recruitment of TRADD, ubiquitinated RIPK1, caspase-8, and FLIP to DISC inhibits the pro-apoptotic activity of caspase-8, forming what is called complex I, a pro-survival complex. Additionally, RIPK1 promotes the transcription of some pro-survival genes, such as FLIP, through the nuclear factor (NF)-kappaB essential modulator (NF-κB) pathway. When TNFR1 activates NF-κB, cells avoid apoptosis and instead produce inflammatory cytokines. Eventually, NF-κB may be internalized or detached from the receptor. If NF-κB is either ineffective or has a minimal impact, apoptosis may be reinitiated [19].
In mammals, the IAP family regulates apoptosis negatively and is characterized by a baculoviral IAP repeat domain (BIR) [41]. The most researched X-linked inhibitor of apoptosis protein (XIAP), IAP1/2, comprises three distinct domains, each serving specific functions [42]. XIAP inhibits initiator caspase-9 through the binding of four amino acids on its BIR3 domain to the N-terminal tetrapeptide of the caspase-9 small subunit [43], and the connection region between BIR1 and BIR2 targets effector caspase-3 and caspase-7 specifically. Research has shown that XIAP can enter the inner mitochondrial compartment and inhibit the activation of SMAC on caspase through its E3 ligase activity [44]. In addition, IAP proteins, including IAP1 and IAP2, adversely control apoptosis via their processes, including their Ub-E3 ligase activity, which ubiquitinates RIPK1 and subsequently stimulates the transcription factor NF-κB [45]. The mitochondrial contents SMAC/DIABLO, released alongside cytochrome c, can compete with caspase-9 for binding to XIAP. This action allows caspase-9 to be released through its similar IAP-binding tetrapeptide motif, alleviating XIAP’s inhibition of apoptosis [46]. SMAC can also be combined with IAP to relieve the blocking of caspases 3 and 7, although the mechanism is currently unclear. IAP’s anti-apoptotic action helps tumor cells evade apoptosis, and SMAC can function as an intrinsic IAP antagonist [47]. In the past few years, several IAP protein inhibitors have been designed to mimic the endogenous IAP antagonist, but no IAP inhibitors have been approved for marketing worldwide. Previously, xevinapant has been awarded a breakthrough therapy designation by the US Food and Drug Administration (FDA). According to the phase II clinical data, xevinapant has the potential to significantly enhance the standard therapy for patients with head and neck cancer, which is expected to be approved as an innovative therapy for cancer patients [48].
P53 is a pro-apoptotic regulator. Over half of human cancers have P53 mutations [49]. It attacks the promoter domains of many pro-apoptotic Bcl-2 proteins that regulate their production and inhibit transcription of pro-survival Bcl-2 genes [50,51]. Moreover, P53 also functions in the cytosol and mitochondria in a transcription-independent manner. Stress-induced accumulation of P53 results in the direct activation of BAX and BAK [52].
Role of apoptosis in immune response and inflammation
Unlike necroptosis and pyroptosis, which amplify the immune response, apoptosis is often conserved, immune, “inert”, and associated with mechanisms that minimize the immune response. The conventional processes of necroptosis and pyroptosis involve cell membrane rupture through a lytic mechanism, which releases intracellular pro-inflammatory substances. These substances are then converted into damage-associated molecular patterns (DAMPs), triggering an inflammatory response. In contrast, apoptosis sends signals to phagocytic cells to gather at the site of cell death. This process creates membrane-coated fragments called “apoptotic bodies”, which carry an “eat me” signal. This signal attracts phagocytic cells, helping to suppress the immune response. At present, the most deeply studied “eat me” signal is phosphatidylserine (PtdSer, PS) exposure. PtdSer in healthy cells is always confined to the cytoplasmic leaflet by “flippase”, and apoptosis activates “scramblase” and inactivates “flippase”, which leads to PS exposure and “eat me” signals, and both conditions are indispensable, ultimately promoting the recognition, phagocytosis, and digestion of phagocytic cells. The Adenosine Triphosphatase (ATPase) Phospholipid Transporting 11C gene (ATP11C) and the cell division cycle protein 50A (CDC50A), both belonging to the P4-ATPase family, are necessary for the flipping of phosphatidylserine (PS) to the outer layer of the plasma membrane [53,54]. Rapid apoptotic cell removal by phagocytic cells inhibits the discharge of pro-inflammatory cell contents [55]. Other signals about phagocytosis on the surface of apoptotic cells are shown in Table 1. Apoptotic cells, in addition to actively promoting autophagocytosis, also inhibit inflammation and help to repair surrounding tissues. Studies have found that cells inhibit inflammation, cell proliferation, and wound healing during apoptosis by secreting metabolites and using them in surrounding healthy cells [56]. This suggests that apoptotic cells are not inert cells waiting to be removed, but positive workers who actively repair surrounding tissues before death. However, if tissues do not effectively remove apoptotic cells, such as when nerve injury-induced protein (NINJ) induces secondary membrane rupture during apoptosis, it can also cause an inflammatory response [57].
Table 1.
Phagocytosis-related signals on the surface of apoptotic cells
| Types of signal | Molecules | Type of specimen | Mechanism | Reference |
|---|---|---|---|---|
| “Find me” signal | LPC | MCF-7 breast cancer cells | IPLA2, upon cleavage by caspase, facilitates the release of LPCs in dying cells, promoting monocyte recruitment. | [155] |
| S1P | Jurkat and U937 leukemia cells | Apoptosis stimulation leads to SphK1 elevation, which is linked to S1P production, which recruits macrophages to the site of apoptotic cells. | [156] | |
| CX3CL1 | Burkitt Lymphoma cells | The soluble chemokine fragment of fractalkine, which is typically located on the plasma membrane and acts as an intercellular adhesion molecule, is released as a 60 kDa fragment during apoptosis and can act as a chemoattractant. | [157] | |
| The nucleotides ATP and UTP | Jurkat cells, primary thymocytes, MCF-7 cells, lung epithelial cells | Early apoptotic cells release the nucleotides ATP and UTP via PANX1, which are then induced by P2Y2 on monocytes, resulting in a “find me” signal that attracts monocytes. | [158] | |
| “Eat me” signal | PtdSer | Many different cell types | The two recognition modes of PtdSer include direct recognition by phagocytic receptors (e.g., TIM-4) and indirect recognition, in which bridging molecules (e.g., MFG-E8) bind to PtdSer, which is recognized by membrane proteins. Thus, PtdSer mediates the structural rearrangement of phagocytic cells’ cytoskeleton to make cadaveric uptake easier. | [159] |
| ICAM-1 | Many different cell types | Alteration in ICAM-1 epitopes on the cell surface leads to the release of an “eat me” signal. | [160] | |
| CRT | Murine CT26 colon cancer cells | When cells die due to ER stress, eIF2α is phosphorylated by activated protein kinase RNA-like ER kinase, causing CRT to translocate from the ER to the cell surface. The phagocytes’ low-density lipoprotein receptor-related protein (CD91) detects this and absorbs the cells. | [161,162] |
Abbreviations: LPC, Lipid lysophosphatidylcholine; MCF-7, Michigan cancer foundaion-7; iPLA2, Calcium-independent phospholipase A2; S1P, Sphingosine 1-phosphate; SphK1, S1P kinase 1; CX3CL1, Fractalkine; ATP, Adenosine triphosphate; UTP, Uridine triphosphate; PANX1, Pannexin 1; PtdSer, Phosphatidylserine; TIM-4, T cell immunoglobulin and mucin-domain-containing molecule 4; MFG-E8, Milk fat globule-epidermal growth factor (EGF) factor 8; ICAM-1, Intercellular adhesion molecule-1; CRT, Calreticulin; ER, Endoplasmic reticulum.
Apoptosis in the gastrointestinal tract
The intestinal epithelium comprises a single layer of IECs depressed inward, forming crypts and villous structures (Figure 2). IECs are made up of different cell types, each performing its function. The general epithelial cell lineage consists of differentiated stem cells, proliferative progenitor cells located in crypts, and terminally differentiated cells growing along the crypt-villus axis [58,59]. Differentiated cells can be further divided into secretory and absorptive cells according to their specific functions. Absorptive cells are the predominant IECs in the intestine; they are closely linked to create a physical barrier and significantly increase the absorptive surface area through the microvilli on their surfaces [58-60]. Secretory cells include Paneth cells, goblet cells, and enteroendocrine cells. Paneth cells confined to crypts express antimicrobial peptides such as defensins, lysozyme, or phospholipase A, which help the host defend against broad-spectrum fungi, bacteria, and some viruses [61,62]. Goblet cells, found in the small intestine’s crypt-villous axis and the large intestine’s crypts, help protect against physical and chemical damage by releasing mucus (mostly mucin2 (MUC2)) into the intestinal space [63]. Enteroendocrine cells can be found in the upper part of the crypt-villous axis, which coordinates intestinal function by secreting specific gut hormones [64]. The intestinal epithelium possesses an extraordinary capacity for regeneration, accomplishing a renewal cycle of the intestine in about 4-5 days. Epithelial cells proliferate, differentiate, and migrate along the crypt-villous axis to the apex of the villi as they mature, eventually falling off into the lumen [65]. In addition to differentiating stem cells and proliferating progenitor cells, Paneth cells also evade this migration and settle on villi [66]. Exfoliated epithelium is often “squeezed out” by other cells, and tight junction proteins underneath the exfoliated cells ensure that adjacent cells can come together to fill the gaps created by the exfoliated cells [67], so that a single exfoliated cell does not affect the integrity of the intestinal barrier (Figure 2). Apoptosis is a normal part of the natural renewal of the intestinal epithelium, and the integrity of the intestinal structure and barrier is inseparable from the strict control of epithelial cell proliferation and death.
Figure 2.
Changes in the intestinal environment between healthy individuals and those with IBD. Healthy humans have an intact intestinal barrier, which includes the integrity of the mucosal membrane and a dense arrangement of IECs that can withstand physical and chemical harm. The intestinal epithelium of IBD patients exhibits morphological alterations, including decreased tight junctions and increased apoptosis. Furthermore, the colonic mucus layer is significantly thin, allowing bacteria to colonize the intestine and infiltrate intestinal tissue via intercellular gaps, resulting in an inflammatory response marked by an increase in pro-inflammatory T cells and a decrease in anti-inflammatory T cells.
Large-scale IEC apoptosis, leading to villous atrophy and barrier disruption, plays a central role in inflammatory diseases of the gastrointestinal tract, including IBD. In individuals suffering from IBD, elevated apoptosis is found at the site of acute inflammation along the crypt-villous axis [68,69]. Under pathophysiological conditions, excessive IEC apoptosis leads to inflammation, which subsequently transforms into necroptotic mechanisms, generating more pathological features than apoptosis and potentially triggering additional cell death mechanisms, like pyroptosis and ferroptosis [70]. Overall, increased IEC mortality and loss of barrier integrity will promote additional inflammation, more damage to the intestinal epithelium, and perhaps dysbiosis, creating a vicious cycle.
Apoptosis in IBD
Role of apoptosis in IBD pathophysiology
IBD is a persistent inflammation of the intestines, including CD and UC. IBD pathology is complicated and encompasses at least three interacting factors: genetic predisposing factors, gut microbiota, and immune-mediated tissue damage [5].
IEC death is a common pathogenic characteristic of IBD-induced inflammation that disrupts the intestinal barrier’s integrity. Nevertheless, little is known about the molecular mechanisms of apoptosis in the gut inflammatory response. Increased IEC apoptosis and disruption of intestinal mucosal integrity and barrier function were detected in the inflammatory sites/tissues of patients with UC and CD [71]. More epithelial cells with markers of apoptosis were found in the affected and surrounding uninvolved areas of individuals with untreated active UC compared to healthy colonic crypts. This included indicators such as terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) positivity, expression of Fas (CD95) and Fas ligand (FasL), along with morphological characteristics indicative of DNA laddering and apoptosis [72].
IBD causes increased levels of inflammatory cytokines. TNF-α levels are elevated in systemic and intestinal tissues in patients with IBD [73,74], along with the IBD risk alleles (e.g., RELA, NFKB1, TNFAIP3) associated with TNF signaling identified by genome-wide association studies (GWAS), indicating that TNF-α has an essential function in IBD [75]. Deleting TNF-α AU-rich elements (ARE) in mice increases homeostatic levels of TNF-α mRNA and overproduction [76].
In IBD, an inappropriate immune response leads to continuous stimulation of the immune system, in which immune cells involved in apoptosis play a pivotal role. For example, T cells resist apoptosis by enhancing the expression of anti-apoptotic factors, such as Bcl-2, Bcl-xL, FLIP, etc., so that activated T cells accumulate excessively in the intestine and produce a large number of cytokines, resulting in the persistence and expansion of mucosal inflammation [77]. Intrinsic lymphocytes (LPLs) cause inflammatory cell infiltration in inflamed tissues through a similar mechanism [78]. Over-apoptosis undercompensates regulatory T (Treg) cells, which is thought to result in chronic activation of T cells [79]. Monocytes and M1 macrophages that invade the lamina propria of intestinal tissue directly disrupt the epithelial barrier through dysregulation of tight junction proteins and induce apoptosis of epithelial cells, thereby driving intestinal inflammation in IBD [80].
Dysregulation of apoptosis in IBD
While apoptosis appears to be related to gut structural integrity, it is undeniable that disrupted or elevated levels of apoptosis can cause serious gut disorders. Based on this idea, multiple investigations have shown that phenotypic animals with higher intestinal epithelial apoptosis are more susceptible to spontaneous or induced intestinal inflammation.
Studies have shown that the deletion of caspase-8 or FADD leads to impaired apoptosis and the development of chronic inflammatory diseases such as IBD [81]. Its immunodeficiency may stem from caspase-8’s failure to cleave and inactivate NEDD4 binding protein 1 (N4BP1), a protein that inhibits the expression of pro-inflammatory cytokines and chemokines [82]. Although the specific mechanism is not well understood, TNF-α can upregulate the levels of RIPK3 and cylindromatosis (CYLD) molecules in the necroptotic signaling pathway in the presence of caspase-8 and FADD, resulting in the apoptotic program to necroptosis, which uses its inflammatory properties to cause damage to the intestinal barrier, thereby promoting mucosal ulcer formation [83].
NF-κB promotes IEC survival and intestinal immunological homeostasis, possibly through TNF signaling. Studies have shown that mice specifically deficient in the IEC gene NEMO (an essential modulator of NF-κB) were characterized by the development of chronic colitis shortly after birth [60,84]. Another study has shown that NEMO deficiency leads to excessive TNF-dependent apoptosis within the epithelium, followed by barrier disruption and bacterial translocation into the intestinal wall, which drives inflammation [84]. Inappropriate bacterial colonization can lead to intestinal complications such as fibrosis, particularly due to adherent invasive Escherichia coli (AIEC). A study demonstrated that yersinin produced by AIEC in mouse models of IBD promotes intestinal fibrosis [85]. Blocking TNF or toll-like receptor (TLR) signaling in these mice suppressed colitis formation, indicating a tight relationship between NF-κB activity, epithelial cell apoptosis, bacterial translocation, and gut inflammation. These data suggest that modulating apoptosis can be a promising therapeutic strategy.
Therapeutic implications
Anti-TNF-α antibodies
TNF-α is among the most relevant pro-inflammatory agents in IBD. TNF-α levels in IBD patients are directly related to disease severity. Anti-TNF therapy has been demonstrated to be especially useful for IBD patients, and it represents an important milestone in the treatment of IBD, which was first introduced in the 1990s [86]. Anti-TNF monoclonal antibodies bind and neutralize TNF by inhibiting pro-inflammatory cytokine secretion, inhibiting chemokine secretion, and downregulating the inflammatory response (Figure 3). These effects can be mediated by direct neutralization of soluble TNF and membrane-bound TNF interactions. Infliximab, an anti-TNF-α monoclonal antibody administered intravenously, can produce and sustain moderate to severe active luminal CD and moderate to severe active UC in adults and children [87,88]. In a subgroup of CD patients treated with anti-TNF-α, it was reported to downregulate IEC death and restore the epithelial barrier [89,90]. However, not all patients respond well to treatment and may develop resistance and negative consequences. In recent years, many studies have been conducted on the prediction of TNF-α inhibitor resistance at the transcription and translation levels of genes. For example, high expression of the cytokine oncostatin M (OSM) in human intestinal stromal cells is closely related to the failure of TNF-neutralizing therapy [91]. These studies have helped to better understand the mechanism of IBD resistance to infliximab treatment and help patients make a transition.
Figure 3.
The therapeutic target of IBD drugs. Anti-TNF-α, IL-6/IL-6R mAb, and 5-ASA act on different targets through different pathways to decrease inflammatory factors and relieve apoptosis of IECs. At the same time, they promote apoptosis of CD4+ T cells, reduce inflammatory cell infiltration, and relieve intestinal inflammation.
Targeted treatment of IBD based on the NF-κB pathway
NF-κB is important for IEC survival and immune homeostasis. Therefore, inhibitor kappa B kinase (IKK), a key molecule in the NF-κB pathway, is considered a target for IBD treatment (Figure 3). Clinically, 5-aminosalicylic acid (5-ASA) is the treatment of choice for UC and can inhibit the activity of IKK in IECs [92,93]; however, in some patients, it has moderate efficacy or severe and irreversible side effects (Figure 3). In another study, targeted inhibition of the NF-κB pathway enhanced dextran sodium sulfate (DSS)-induced IBD [94]. Thus, NF-κB may be a target for IBD treatment in one cell type but promote survival in another. This complicates the effects of broadly targeting NF-κB signaling in IBD and requires more in-depth understanding and research [5].
Induction of T lymphocyte apoptosis in targeting IBD
Accumulating evidence suggests that excessive CD4+ T cell activation is the primary pathological pathway implicated in the onset and persistence of inflammatory processes, which impacts treatment. A probable key element in pathogenesis is increased T cell resistance to apoptosis, which disrupts mucosal homeostasis and allows for an unchecked buildup of activated CD4+ T cells, consequently amplifying the inflammatory response [95]. An important regulator of T cell death is signal transducer and activator of transcription 3 (STAT3). The interleukin-6 (IL-6)/sIL-6 R complex interacts with gp130 on the CD4+ T cell membrane, resulting in an elevated expression of STAT3 and nuclear translocation. This induces anti-apoptotic genes, like Bcl-xL, resulting in greater T cell resilience to apoptosis in the lamina propria. Subsequently, T cell expansion contributes to the persistence of chronic intestinal inflammation (Figure 3). Patients with active CD have demonstrated clinical benefit using a recently produced humanized anti-IL-6R monoclonal antibody that triggers intestinal T cell death [96]. These findings elucidate to some extent the pathogenesis implicated in IBD. There is potential for novel therapy approaches based on good pathophysiological principles. Some other strategies for targeting apoptosis for IBD are described in Table 2.
Table 2.
Other examples of targeted apoptosis for the treatment of IBD
| Therapeutic strategies | Type of condition (s) | Targets | Mechanism of action | Reference |
|---|---|---|---|---|
| S1P receptor 1 and 5 agonist (ozanimod) | UC | NF-κB, TNF-α | S1P, a protein similar to TNF-α, activates NF-κB, triggering inflammation and immune responses. Ozanimod reduces inflammation by binding to S1P receptors 1 and 5. | [163] |
| LR | UC | Th17/Treg cells, JAK-STAT signaling pathway | LR ethanol extract fights UC by balancing Th17 and Treg cells. LRWE reduces inflammation and apoptosis by inhibiting the JAK-STAT signaling pathway by lowering p-JAK2, p-STAT3, Bcl-2, and BAX protein expression. | [164] |
| Si Shen Wan | UC | PLC-γ1, PI3K/AKT signaling pathway | SSW inhibits apoptosis in IECs by activating PLC-γ1 and inhibiting AKT phosphorylation, thereby inhibiting the PI3K/AKT signaling pathway. | [165] |
| THP | UC | TNF-α, SOCS1/JAK2/STAT3 signaling pathway | THP downregulates pro-inflammatory cytokines IL-6, TNF-α, and IL-17 and enhances forkhead box protein P3 expression. It also modulates the SOCS1/JAK2/STAT3 pathway and supports the intestinal mucosal barrier. | [166] |
| Probiotics (LP082) | UC | TNF-α, NF-κB pathway | The probiotic LP082 protects the intestinal mucosal barrier, reduces inflammation, and regulates microbial imbalance by inhibiting TNF-α and the NF-κB pathway. | [167] |
| Fuzi-ganjiang herb | UC | MAPK, NF-κB and STAT3 signaling pathway | The fuzi-ganjiang combination shows notable anti-inflammatory effects in DSS-induced UC mice, possibly inhibiting MAPK, NF-κB, and STAT3 signaling pathways. | [168] |
| GQ | UC | IL-6/JAK2/STAT3 signaling, Treg and Th17 cell | GQ alleviated DSS-induced UC by suppressing IL-6/JAK2/STAT3 signaling to restore Treg and Th17 cell homeostasis in colonic tissue. | [169] |
| Daphnetin | UC | JAK2/STAT3 signal, Bcl-2 family proteins, caspase | Daphnetin acts in UC via REG3A-activated JAK2/STAT3 signaling, increasing anti-apoptotic protein Bcl-2 and reducing pro-apoptotic proteins BAX and cleaved caspase 3. | [170] |
| Fufangxiaopi formula (FF) | UC | TLR4/NF-κB and MAPK signaling pathways | FF inhibits pro-inflammatory reactions and reduces TNF-α, iNOS, IL-6, IL-1β, and COX-2 production by blocking TLR4/NF-κB and MAPK signaling in RAW264.7 cells. | [171] |
| MBP | UC | NF-κB | MBP alleviates DSS-induced UC by inhibiting the TLR4/MAPK/NF-κB signaling pathway and modulating the gut microbiota. | [172] |
| Licorice | UC | NF-κB signaling pathway | Licorice reduces inflammation, strengthens the gut mucosal barrier via the TLR4/MyD88/NF-κB regulatory route, and balances T helper cell development. | [173] |
| DZT | UC | NF-κB, STAT3/5 pathway | Mitigates UC by targeting IKKα/β and JAK2 kinases to block the NF-κB and STAT3/5 pathways. | [174] |
| ISO | CD | AMPK/PGC1α signaling pathway, caspase, Bcl-2 protein family | The ISO moiety activates the AMPK/PGC1α pathway, reducing apoptotic IECs by lowering cleaved-caspase-3/caspase-3 and BAX levels while increasing Bcl-2. This alleviates TNBS-induced intestinal barrier dysfunction and CD-like colitis in mice. | [175] |
| Curcumin | UC, CD | NF-κB, TNF-α, p38MAPK | Curcumin effectively inhibits inflammation by blocking TNF-α-mediated NF-κB activity and reducing inflammatory activity by decreasing p38 MAPK activity. | [176,177] |
| MPST | UC, CD | PI3K/AKT pathway | MPST directly interacts with AKT, reducing its phosphorylation and overexpression, decreasing caspase-3 protein levels, increasing BCL-xL protein levels, and inhibiting IEC apoptosis. | [178] |
| The JAK inhibitor (Tofacitinib) | UC, CD | JAK-STAT signaling pathway | Tofacitinib inhibits JAK1 and JAK3 signal transduction pathways, reducing inflammatory responses and preventing apoptosis. | [179] |
| OPEN | UC, CD | P53 | PFT-α, an inhibitor of P53, effectively reduces excessive apoptosis in IECs. | [10] |
Abbreviations: IBD, Inflammatory bowel disease; UC, Ulcerative colitis; CD, Crohn disease; S1PR, Sphingosine 1-phosphate receptor; MAPK, Mitogen-activated protein kinase; MPST, 3-mercaptopyruvate sulfurtransferase; PI3K, Phosphatidylinositol 3-kinase; AKT, Protein kinase B; IEC, Intestinal epithelial cell; JAK, Janus kinase; STAT, Signal transducer and activator of transcription; LR, Linderae Radix; LRWE, LR aqueous extract; PLC-γ1, Phospholipase C gamma1; THP, Tetrastigma hemsleyanum polysaccharide; SOCS1, Suppressor of cytokine signaling1; LP082, Lactobacillus plantarum HNU082; DSS, Dextran sodium sulfate; GQ, Gegen Qinlian decoction; IL-6, Interleukin 6; REG3A, Regenerating protein 3 alpha; TLR, Toll-like receptor; FF, Fufangxiaopi formula; iNOS, Inducible nitric oxide synthase; COX-2, Cyclooxygenase-2; MBP, Mesona chinensis Benth polysaccharides; MyD88, Myeloid differentiation factor-88; DZT, Demethylzeylasteral; IKK, Inhibitor kappa B kinase; ISO, Isongifolene; AMPK, AMP-activated protein kinase; PGC1α, Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; TNBS, 2,4,6-trinitrobenzene sulfonic acid; OPEN, Oral pifithrin-α embedded nanomedicine; PFTα, Pifithrin-α.
Apoptosis in CRC
Cells evade apoptosis in CRC formation by several mechanisms, including blocking intrinsic and extrinsic pathways, regulating the apoptosis execution cascade, and reducing P53 expression. The precise mechanisms are detailed below.
The role of apoptosis in CRC development
CRC is one of the most serious complications of IBD. Patients with long-term IBD have an increased risk of CRC compared to the general population. The mechanism of the carcinogenic process associated with IBD is not well understood, but it is generally thought to be the result of chronic inflammation of the gut. Inhibition of apoptosis is regarded as one of the signatures of tumors, and apoptosis decreases during tumor progression [97]. Tumors can avoid apoptosis by using both intrinsic and extrinsic mechanisms, as well as epigenetic changes.
Escape from extrinsic pathways
Death receptors such as Fas and TRAILR are downregulated or mutated, allowing apoptosis signals to be directly blocked outside the cell. It has been demonstrated that some genetic abnormalities contribute to tumor cells’ resistance to Fas-mediated apoptosis. Fas transcriptional suppression is a frequent carcinogenic factor in epithelial transition [98]. The cytosolic side of the protein TNFR1 has a death site [99]. Expression of TNFR 1/TNF-α has been reported in various tumor forms, including leukemia, breast cancer, and hepatocellular carcinoma, and is adversely linked with illness progression [100-102]. The absence of dependence receptors contributes to the apoptotic evasion of cells. Deleted in colorectal cancer (DCC) is a receptor for Netrin-1 and exists as a dependence receptor. DCC is absent or significantly reduced in approximately 50% of colon cancers [2]. Moreover, abnormal expression of DCC has also been found in other tumors, including gastric and esophageal cancer. It has been found that the deletion of DCC is related to chromosome 18q. In over 70% of primary CRCs, the loss of allele on chromosome 18q (loss of heterozygosity) leads to a decrease in the expression of DCC on the cell surface [103]. However, current studies have shown that mice with DCC knockout have reduced tumor susceptibility, although other genes that suppress tumors are present on 18q, suggesting DCC may not be a typical gene that suppresses tumors [104,105]. Chromosome 18q encodes two important tumor suppressor genes: SMAD2 and SMAD4. The loss of these genes can lead to inhibited apoptosis and disruptions in the cell cycle. However, the deletion of 18q shows little correlation with tumor survival or prognosis. As a result, the current status of 18q deletion does not serve as a valuable prognostic marker, indicating that more in-depth research is needed in this area. Another resistance mechanism reported in several human tumors is the overexpression of the anti-apoptotic protein FLIP recruited at the DISC level, which prevents caspase-8 induction and thus makes cells immune to apoptosis mediated by death receptors [40,106,107].
Escape from intrinsic pathways
Alterations in certain components of intrinsic pathways can play an important role in the development of tumors. Overexpression of anti-apoptotic proteins and/or downregulation of pro-apoptotic proteins drives tumor development, and Bcl-xL elevation has been documented in CRC [108], which prevents MOMP and cytochrome c release by binding to and neutralizing pro-apoptotic proteins. These overexpressed proteins give tumor cells a multidrug resistance phenotype, preventing them from experiencing apoptosis [109]. On the contrary, genetic mutations or epigenetic silencing can lead to loss of pro-apoptotic protein function (e.g., BAX, BID). CRC has been linked to changes in the pro-apoptotic BAX gene, leading to resistance to anticancer treatments [110]. TP53 is the gene encoding P53, and its loss is common in CRC because it bridges the gap between adenoma and cancer. Therefore, TP53 is among the most significant genes that decrease cancer. Its deletion or mutation results in an inability to respond to DNA damage or stress signals, thereby driving tumor development [111]. The in vivo role of non-transcriptional P53-mediated apoptosis is poorly understood; however, it is clear that many important cancer regulators inhibit the development of tumors by modulating the stability of P53. For example, serine beta-lactamase-like protein (LACTB) is expected to become a potential treatment target for CRC [112].
In the apoptosis execution stage, IAP protein expression and/or function are disrupted due to genetic aberrations, increased expression of their mRNA or proteins, or absence of endogenous antagonists like SMAC [47]. SMAC binds to a variety of IAP proteins, including XIAP, c-IAP 1, and c-IAP 2, through its evolutionarily highly conserved amino-terminal amino acid residue (Ala-Val-Pro-Ile), which promotes caspase activation and apoptosis [113-115]. In this regard, several cancer forms have been shown to have high IAP expression levels, which is thought to be a sign of a poor prognosis for patients [116,117].
Epigenetic changes
Epigenetics refers to stable changes in gene expression caused by chromosomal changes without changes in the original gene sequence. DNA methylation, non-coding RNA (ncRNA) dysregulation, histone modifications, and other factors play important roles in cancer epigenetics. MicroRNAs (miRNAs), in the context of ncRNAs, are among the most researched epigenetic components in tumors because of their significance in controlling gene expression. These miRNAs attach to several mRNAs’ 3’UTR region, thereby causing their breakdown or translation inhibition, such as miR-34a, which promotes and inhibits P53 acetylation by targeting sirtuin 1 (SIRT1), formin-like protein 2 (FMNL2), and E2F transcription factor 5 (E2F5) [118,119].
Another focus in the field of ncRNAs is long non-coding RNA (lncRNA), which is commonly associated with cancer and modifies epigenetic marks in the nucleus by activating or inhibiting chromatin-modifying proteins. However, in the cytoplasm, they may function as miRNA bait, regulate translation and splicing, and act as proto-oncogenes or tumor suppressor genes [120]. Therefore, lncRNAs perform a key function in cell differentiation and proliferation, which are directly related to cancer. When it is a proto-oncogene, overexpression upregulates important tumor markers, such as pou5f1p1 (OCT4), increasing the risk of CRC [121]. Under physiological conditions, lncRNAs function as tumor suppressor genes that control the expression of P53-dependent genes, so that when lncRNA expression is decreased, the genes regulated by them are altered, creating resistance to apoptosis, thereby increasing their proliferation.
DNA methylation is one of the most prevalent epigenetic modifications that regulate gene expression. Alterations in normal DNA methylation patterns include DNA hypomethylation (which pathologically occurs in the normal unmethylated regions of the genome) and DNA hypermethylation (which typically occurs in the CpG islands of gene promoters), and these cancers with hypermethylation of DNA are defined as having a “CpG island methylation phenotype” (or CIMP). There is strong evidence that promoter CpG hypermethylation is associated with transcriptional repression of tumor suppressor genes in cancer cells [122]. This evidence is notably strong in the case of CRC, where this aberrant hypermethylation has been found in the promoter region of important tumor suppressor genes, including cyclin-dependent kinase inhibitor 2A (CDKN2A) and adenomatous polyposis coli (APC). In contrast, hypermethylation outside the CpG island appears to be positively correlated with gene expression [123].
Targeting apoptosis in CRC therapy
CRC is a prevalent cancer of the gastrointestinal tract that poses a significant clinical challenge. Management of CRC has traditionally consisted of surgery, often in combination with chemotherapy. Nonetheless, inoperable CRC (metastatic CRC) is a complicated issue that requires novel therapeutic options. Currently, various treatment options for CRC have been established, including chemotherapy and targeted medicine. However, chemotherapy drugs work by inhibiting rapidly dividing cells but indiscriminately kill all cells (including bone marrow, gastrointestinal mucosa, and hair follicles), resulting in serious side effects such as bone marrow suppression, anemia, hair loss, nausea, and so on [124]. In addition to this, long-term chemotherapy produces specific toxicity to cells, such as ototoxicity, nephrotoxicity, pulmonary toxicity, neurotoxicity, or Raynaud’s syndrome [124]. Some cancer cells develop resistance through gene copy number changes, chromosomal instability, or drug efflux pump mechanisms, resulting in ineffective treatment [125]. Targeted drugs are also helpless against the evolution of drug resistance, and tumors activate escape attacks through target mutations or bypass signals, resulting in reduced efficacy [126]. On the other hand, the cost of targeted drug development and treatment is too high, making it difficult for the public to afford treatment costs.
The limitations of traditional chemotherapy and targeted drugs have driven the exploration of alternative treatments to improve therapy outcomes, reduce side effects, and reduce the likelihood of acquiring secondary tumors. For example, apoptosis inducers and gene therapy are emerging drugs and treatment strategies, and their related clinical evaluation experiments are a major research hotspot.
Emerging therapeutic targets of apoptosis
Pharmacological agents
Restoring the apoptotic pathway through drugs that target apoptotic pathways constitutes a promising anti-cancer treatment (Figure 4).
Figure 4.
The emerging treatment of CRC. The figure shows new technologies for treating CRC, including targeted drugs, gene therapy, and combination therapies that integrate immunotherapy and targeted therapy. Antibody-drug conjugate (ADC) is a promising targeted medication that combines the benefits of monoclonal antibody targeting with chemotherapy cytotoxic drugs. ADCs consist of naked antibodies, cytotoxic payloads, and linkers, which selectively transport cytotoxic drugs to tumors and induce tumor cell apoptosis. The CRISPR/Cas9 system can realize genome editing. It induces cancer cell apoptosis through disrupting oncogene expression or inserting the target gene. Combination therapy combines the characteristics of the two therapies to improve the treatment rate. Targeted treatment causes cell death and apoptosis, thus inducing macrophages and malignant cells to produce cytokines that cause inflammation; however, the accumulation of DNA double strands stimulates the production of type I interferon. Effective innate immunity is induced by type 1 interferon and inflammatory cytokines. Immunotherapy that blocks the programmed cell death protein 1 (PD-1)-PD-L1 route, such as anti-PD-1 antibody, further strengthens anti-CRC T cell immunity. Abbreviations: HDR, homology-directed repair pathway; sgRNA, single guide RNA; PAM sequence, protospacer-adjacent-motif sequence; CRISPR, clustered regularly interspaced short palindromic repeats.
In the extrinsic pathway, metabolic inhibitors that downregulate c-FLIP and interferon to enhance caspase-8 activation are intended to allow tumor-to-death receptor-induced apoptosis. Moreover, stimulation of extrinsic pathways to induce apoptosis may eliminate resistance to therapeutics that work by damaging DNA, as apoptosis that relies on death receptors can happen without the stress response (Figure 4). One example of this treatment technique is the ligand TRAIL, which is known to induce apoptosis in several types of tumor cells [127]. These include recombinant human TRAIL, monoclonal antibodies agonistic to death receptor 4 (DR4) and DR5, and all-trans retinoic acid (ATRA) [128,129]. The structure of tiny molecules targeting and promoting caspase-8 induction of TRAIL-induced cell death in sensitive malignancies is an innovative strategy. Research in various cell lines, including leukemia and prostate cancer cells, has demonstrated that in the presence of TRAIL, the small molecule CaspPro facilitates the activation of caspase-8 and caspase-3 and the cleavage of polyadenosine-diphosphate-ribose polymerase (PARP), ultimately resulting in cell death [130]. Due to its distinct lethality to transformed cells versus normal cells, Apo2/TRAIL offers promise as a possible tumor therapeutic agent.
These anti-cancer strategies focus on targeting intrinsic pathways to develop inhibitors for anti-apoptotic proteins that are often overexpressed in tumor cells. This includes creating agents that act directly on the inner mitochondrial membrane, agents that antagonize the anti-apoptotic members of the Bcl-2 protein family, and agents that enhance the activity of pro-apoptotic members of the Bcl-2 protein family, such as BAX [131] (Figure 4). Arsenic trioxide is a drug that targets the promyelocytic leukemia protein-retinoic acid receptor alpha (PML-RARα) protein in the inner mitochondrial membrane. At low concentrations, it causes differentiation, while at high concentrations, it destroys the membrane sites. However, the mechanism is to degrade PML-RARα protein [132]. The BH3 mimic binds to the hydrophobic groove of the anti-apoptotic protein, mimicking the binding of the BH3-only protein to the pro-survival protein, resulting in the discharge of the BH3-only protein from the complex and induction of BAX and BAK. Currently, multiple BH3 mimetics are being studied as Bcl-2 inhibitors at various stages of human clinical trials, including ABT 199 (venetoclax), ABT-737, GX 15-070 (obatoclax), and TW 37, among others [131]. The study of Bcl-2 family antagonists is evolving, highlighting the relevance of these compounds as effective antitumor agents.
Antibody-drug conjugates (ADCs) are a promising delivery system that combines the targeting ability of monoclonal antibodies with the benefits of cytotoxic chemotherapeutic agents. ADCs comprise antibodies, cytotoxic payloads, and linkers designed to deliver cytotoxic drugs to tumors in a targeted manner. This new anti-tumor therapy acts like a “biological missile” that destroys tumor cells while improving the therapeutic index and reducing systemic toxicity. So far, various ADCs have been tested and approved for several solid tumors, including CRC. One of the most critical factors in ADC design is the selection of target antigens highly expressed on the surface of cancer cells, as ADCs activate apoptotic signals by targeting tumor-specific antigens to deliver pro-apoptotic toxins precisely to cancer cells. HER2 is highly expressed on the surface of many cancer cells, and an ADC drug (Enhertu) targeting HER2 has been successfully used in HER2-positive breast cancer [133]. Cytotoxic medicines’ pro-apoptotic action may be beneficial against HER2-positive CRC, which accounts for 3-5% of all CRC cases.
Gene therapy approaches
Gene therapy is promising because it allows personalized treatments, including gene editing techniques and virus-directed enzyme-prodrug therapy. The concept of addressing mutations in the therapy of CRC has aroused interest in the scientific community. The mutations play a crucial role in the development of cancer, making them specific targets for tumors. Additionally, they are usually expressed in most tumor cells [134]. Due to P53’s powerful apoptotic action, gene therapy can be used in cancer trials that target P53. The common goal of all P53-targeting techniques is to activate P53 in tumor cells expressing wild-type P53 or restore its function in cells expressing the deficient type of P53 [52]. The first gene therapy product, Gendicine (recombinant human P53 adenovirus), was marketed in 2004. Gendicine-transduced cells express the wild-type P53 protein, which suppresses tumors, is activated by cellular stress, and either promotes DNA repair and cell cycle arrest or, depending on the degree of cellular stress, encourages programmed cell death (apoptosis, senescence, and/or autophagy). In brief, by recovering P53 function and therefore “braking” the process of cell division, cancer formation is subsequently prevented [135]. Although Gendicine has been used in clinical treatment for many years, there is still a lack of standard treatment options for P53-targeted gene therapy, which needs further investigation in clinical treatment [136].
Genetic mutation accumulation has a substantial impact on the evolution of CRC. Conventional gene editing techniques have numerous limitations, including gene editing effectiveness (Figure 4). In contrast to other methods, clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-mediated genome editing is simple and effective and can achieve precise editing. The mechanism of CRISPR/Cas9 gene editing is simply a three-step process: the first step is that the host’s CRISPR system precisely detects the protospacer adjacent motifs (PAMs) of the foreign gene and cleaves them into appropriately sized spacer sequences; in the second step, CRISPR sites are transcribed with assistance from RNase III, Cas1/Cas2 complexes, and additional Cas proteins to form effector complexes (crRNP), forming flanking arrays in CRISPR arrays; finally, the infection-specific genetic memory is created in the CRISPR/Cas9 system [137]. This allows specific genes to be edited into certain cells, resulting in precise genome editing (Figure 4). Conventional genetic therapy approaches include gene modification or substitution, virus-directed enzyme-prodrug therapy (VDEPT), immunogenetic therapy, and other treatments [138], but clinical efficacy is poor. CRISPR/Cas9-based genetic treatment is achievable due to the accurate editing of single guide RNA (sgRNA)-guided Cas9 nucleases. It has been found that systemic administration of Cas9 ribonucleoprotein (RNP) to dual-target mutant Apc and Kirsten rat sarcoma viral oncogene (Kras) using HA-decorated phenylboronic dendrimer (HAPD) can substantially limit CRC growth in xenograft mice and considerably decrease CRC-related lung and liver metastasis [137,139]. However, since several gene mutations accumulate to cause cancer, more study is needed to identify additional genes and transport systems that cause minimal systemic harm.
Combination therapies
There is evidence that combination therapies have given relatively satisfactory clinical results in inhibiting tumor progression and improving patient survival compared with monotherapy. Combination therapy has emerged as a novel anti-cancer strategy.
Combination therapies can combine apoptosis regulation with existing treatments. For example, chemotherapy drugs induce apoptosis through DNA damage but are limited by the anti-apoptotic mechanism of tumor cells, such as Bcl-2 overexpression. The combination of chemotherapy and pro-apoptotic drugs can enhance efficacy. Clinical studies have shown that the BH3 analogue navitoclax significantly enhances the killing of TP53 wild-type CRC cells by the chemotherapy drug oxaliplatin by inhibiting Bcl-2/Bcl-xL. In addition, chemotherapeutic multidrug resistance (MDR) and TRAIL resistance are common in CRC cells. Cancer cells evade the effects of chemotherapy by overexpressing chemoefflux pump proteins and genetic mutations, inducing CRC to become resistant to anti-epidermal growth factor receptor (anti-EGFR) therapy [140]. Photodynamic therapy (PDT), on the other hand, can stimulate cells to produce reactive oxygen species (ROS) by triggering photosensitizers in cells with lasers so that excess ROS induces apoptosis by upregulating death receptors and/or downregulating anti-apoptotic proteins to make CRC cells sensitive to TRAIL [141,142]. In the study by She et al., they used the Ze-IR700 photosensitizer with high affinity for epidermal growth factor receptor (EGFR) for tumor cell-targeted PDT, and after that, measured the effect of PDT on gene expression in CRC cells by RNA sequencing, thereby evaluating the synergistic antitumor effects of long-acting TRAIL and PDT in mice bearing chemo-MDR and TRAIL-resistant CRC cell tumor grafts. The study found that pretreatment of CRC cells with PDT targeting tumor cells significantly increased (10-60-fold) the sensitivity of these CRC cells to TRAIL by upregulating death receptors. The combination of long-acting TRAIL and PDT, rather than monotherapy, greatly induced apoptosis in CRC cells, effectively eradicating large (~150 mm3) CRC tumor xenografts in mice [143]. This suggests that the combination of long-acting TRAIL and PDT has the potential to fight chemotherapy MDR and TRAIL-resistant CRC, which may be developed as a new strategy for the precision treatment of refractory CRC.
Immunotherapy can also have a synergistic effect with targeted therapy, which has become the mainstay of treatment in these situations. It can tailor interventions to specific molecular properties. Immunotherapy has transformed cancer treatment by using the immune system to combat cancerous cells (Figure 4). Targeted cancer treatments specifically target the genetic mechanisms that lead to tumor growth, such as modifications that facilitate cellular proliferation or prevent the death of cells. Nevertheless, tumor cells may stimulate different signaling routes to escape the restricted proliferative routes attacked by these medicines. This is commonly termed adaptive resistance. Immunotherapy is the most promising method for preventing cancer and its recurrence since it stimulates the body’s immunological cells to attack tumors. Nonetheless, cancer also becomes resistant to immunotherapy by downregulating antigens recognized by immunological cells, decreasing the immune system’s ability to detect it [144]. Furthermore, immunological checkpoint molecules like programmed cell death ligand 1 (PD-L1) and immunosuppressive cells like Treg cells are important contributors to the suppression of the tumor microenvironment. These chemicals and cells actively suppress the activity of natural killer (NK) and cytotoxic T cells, hinder the immune system’s capacity to mount a successful anti-tumor response, and ultimately lessen the efficacy of immunotherapy [145,146]. Consequently, the justification for integrating immunotherapies and targeted treatments in cancer therapy stems from their potential for synergistic interactions and complementary modes of action [147]. In one study, inhibiting both programmed cell death protein 1 (PD-1) and the vascular endothelial growth factor receptor (VEGFR) 2 simultaneously decreased the growth of colorectal tumors [148]. Another study found that VEGFR 2 suppression dramatically reduced tumor neovascularization in a mouse model of CRC [149]. On the other hand, tumor angiogenesis was unaffected by PD-1 inhibition. Nevertheless, PD-1 inhibition causes an increase in numerous pro-inflammatory cytokines, which enhances the immune reaction. This hybrid pathway, which targets the immunological system and neoplastic vasculature, synergistically regulates the tumor microenvironment, showing potential applications in CRC treatment [150]. Therefore, integrating specific therapy and immunotherapy may improve efficacy compared to either treatment alone.
Many clinical trials have shown that combination therapy for CRC is effective and potentially beneficial; however, it may come at the expense of higher toxicity and cost. It has the potential to lead to worse outcomes, emphasizing the importance of randomized clinical trials and appropriate patient selection. When integrating these medicines, clinicians want to leverage their advantages while reducing their shortcomings, eventually generating a stronger and more successful treatment method. Cancer is projected to progress from “controllable” to “cured” in the future due to transdisciplinary, interdisciplinary, and technological innovations. A summary of drug categories and targets is shown in Table 3.
Table 3.
Summary of CRC drug categories and targets
| Categories | Targets | Drugs | References |
|---|---|---|---|
| Pharmacological | FLIP-inhibitor | recombinant human TRAIL, monoclonal antibodies agonistic to DR4 and DR5, and ATRA | [128,129] |
| Agents | PML-RARα protein | Arsenic trioxide | [132] |
| BH3-mimetic | ABT 199, ABT-737, GX 15-070, TW 37 | [131] | |
| HER2 | Enhertu (ADC drug) | [133] | |
| Gene Therapies | P53 | Genedicine | [135] |
| Mutated gene | CRISPR/Cas9-mediated genome edition | [137,139] | |
| Combination Therapies | Chemotherapy + BH3-mimetic | Chemotherapy + navitoclax | [140] |
| PDT + ligand TRAIL | Ze-IR 700 + ligand TRAIL | [143] | |
| Immunotherapy + target therapy | PD-1 inhibition + VEGFR2 | [149] |
Abbreviations: ATRA, all-trans retinoic acid; ADC, anti-drug conjugates; BH3, homology domain3; CRC, colorectal cancer; CRISPR, clustered regularly interspaced short palindromic repeats; DR, death receptor; FLIP, FLICE-like inhibitory protein; PML-RARα, promyelocytic leukemia protein-retinoic acid receptor alpha; PDT, photodynamic therapy; PD-1, programmed cell death protein 1; TRAIL, TNF-related apoptosis-inducing ligand; VEGFR, vascular endothelial growth factor receptor.
Challenges and limitations
Targeted apoptosis is currently a hot topic in the treatment of CRC and IBD, but its clinical application still faces many challenges. The first is treatment safety, where drugs targeting Bcl-2 family proteins or IAPs may affect normal cells that rely on these proteins. For example, the Bcl-2 inhibitor venetoclax has significant efficacy in hematologic tumors but may cause cytopenias (such as thrombocytopenia, neutropenia, etc.) [151,152]. Moreover, it can be speculated that the high proliferation of normal IECs may exacerbate toxicity in CRC. However, in the treatment of IBD, over-inhibition of apoptosis may promote the development of chronic diseases into tumors. The second is the variability of the response. Considering the heterogeneity in heredity and phenotype among patients, it has become challenging to anticipate useful biomarkers for targeted apoptotic therapies in individuals. Since targeted medications rely heavily on the target, they are only effective in patients with specific molecular markers, resulting in a limited number of people who can benefit from them. For example, about 15% of individuals diagnosed with lung carcinoma (non-small cell) carry EGFR defects, so EGFR inhibitors are only effective in these 15% of patients, and the treatment of patients who respond is quite constrained [153]. Finally, the signaling pathways and molecular mechanisms of chronic inflammation in the gut and apoptosis in cancer require further investigation. The complex interaction between apoptosis, immunity, and inflammatory responses remains unknown, necessitating more in-depth research to uncover its mysteries.
Future directions
Based on our current understanding of the mechanism linking IBD, CRC, and apoptosis, we can make recommendations for future areas of inquiry. Treatment can be tailored based on patient-specific biomarkers to avoid recovery delays due to ineffective treatments. Biomarkers can be used to identify patients who may benefit from targeted apoptosis therapy. At the same time, developing liquid biopsy technologies can be utilized to track tumor dynamics in real time and guide the treatment regimen adjustments [154]. In addition, the synergistic effect of integrating multiple therapeutic approaches, such as chemotherapy, targeted therapy, immunotherapy, and radiotherapy, can also prevent patients from developing treatment resistance. However, some signaling pathways, such as the downstream signaling and mechanism of TNF, are still unclear, and more in-depth research and clinical trials are needed to explore new, valuable therapeutic targets in the apoptosis pathway. In addition to traditional monodisciplinary study and treatment, strengthening collaboration among oncology, gastroenterology, and immunology generates new ideas for treating gastrointestinal disorders in patients. This allows people to promote innovative research in the context of IBD and CRC. In conclusion, there is no doubt that drugs that target dysregulated apoptotic pathways have a wide range of applications in cancer therapy. The recent intensive efforts dedicated to targeting the apoptotic pathway are optimistic and support the creation of new strategies for medication discovery and therapeutics.
Conclusion
Apoptosis is essential for growth, immune surveillance, and neoplastic development. In the intestine, the imbalance of apoptosis regulation leads to IBD and even CRC. The mechanisms and signaling pathways of the apoptosis program and the associated anti-apoptotic and pro-apoptotic molecules provide targets for therapeutics. Many studies have shown that inhibition of apoptosis in IECs can improve IBD, while activation of apoptosis in cancer cells can treat CRC. However, traditional therapies for the treatment of IBD and CRC have many limitations, and there is still a lot of room for improvement in research on targeted apoptosis for IBD and CRC treatment. More emerging treatment options, such as pharmacological agents, gene therapy, and combination therapies that target apoptosis, are being explored. In future studies, specific therapeutics targeting receptors and apoptotic chemicals, should be investigated further in large preclinical and clinical trials.
Acknowledgements
This study was sponsored by the Henan Provincial Natural Science Foundation Project (No. 252300420143), the Science and Technology Development Project of Henan Province in 2024 (No. 242102310081), the open topic project of Shangqiu Medical College in 2023 (No. KFKT23005), the Zhenjiang Science and Technology Plan (Social Development) (No: SH2024047), the key research and development (social development) projects of the Innovation Special Fund of Danyang (No: SSF202304) and the Undergraduate Innovation and Entrepreneurship Training Program (No: 202410299094Z).
Disclosure of conflict of interest
None.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev. 2004;84:411–30. doi: 10.1152/physrev.00027.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu H, Huang Y, Liu F, Wang Q, Yao Y. How aging affects bone health via the intestinal micro-environment. Biocell. 2024;48:353–362. [Google Scholar]
- 5.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–24. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 6.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol J. 2022;10:1113–1120. doi: 10.1002/ueg2.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, Xu W, Mao F. The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 2020;2020:7819824. doi: 10.1155/2020/7819824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armacki M, Trugenberger AK, Ellwanger AK, Eiseler T, Schwerdt C, Bettac L, Langgartner D, Azoitei N, Halbgebauer R, Groß R, Barth T, Lechel A, Walter BM, Kraus JM, Wiegreffe C, Grimm J, Scheffold A, Schneider MR, Peuker K, Zeißig S, Britsch S, Rose-John S, Vettorazzi S, Wolf E, Tannapfel A, Steinestel K, Reber SO, Walther P, Kestler HA, Radermacher P, Barth TF, Huber-Lang M, Kleger A, Seufferlein T. Thirty-eight-negative kinase 1 mediates trauma-induced intestinal injury and multi-organ failure. J Clin Invest. 2018;128:5056–5072. doi: 10.1172/JCI97912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Huang Q, Liu M, Zhao T, Song X, Chen Q, Yang Y, Nan Y, Liu Z, Zhang Y, Wu W, Ai K. Precisely inhibiting excessive intestinal epithelial cell apoptosis to efficiently treat inflammatory bowel disease with oral pifithrin-α embedded nanomedicine (OPEN) Adv Mater. 2023;35:e2309370. doi: 10.1002/adma.202309370. [DOI] [PubMed] [Google Scholar]
- 11.Tian R, Liu X, Luo Y, Jiang S, Liu H, You F, Zheng C, Wu J. Apoptosis exerts a vital role in the treatment of colitis-associated cancer by herbal medicine. Front Pharmacol. 2020;11:438. doi: 10.3389/fphar.2020.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 15.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 17.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–4. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, Ding J. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955. e20. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat Commun. 2020;11:4561. doi: 10.1038/s41467-020-18443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–9. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 21.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 22.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–8. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–3. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–9. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 25.Lemarie A, Grimm S. Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer? Oncogene. 2011;30:3985–4003. doi: 10.1038/onc.2011.167. [DOI] [PubMed] [Google Scholar]
- 26.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 27.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lossi L. The concept of intrinsic versus extrinsic apoptosis. Biochem J. 2022;479:357–384. doi: 10.1042/BCJ20210854. [DOI] [PubMed] [Google Scholar]
- 30.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 31.Diepstraten ST, Yuan Y, La Marca JE, Young S, Chang C, Whelan L, Ross AM, Fischer KC, Pomilio G, Morris R, Georgiou A, Litalien V, Brown FC, Roberts AW, Strasser A, Wei AH, Kelly GL. Putting the STING back into BH3-mimetic drugs for TP53-mutant blood cancers. Cancer Cell. 2024;42:850–868. e9. doi: 10.1016/j.ccell.2024.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Pan G, O’Rourke K, Dixit VM. Dixit, Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 33.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16:273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 34.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 35.Gibert B, Mehlen P. Dependence receptors and cancer: addiction to trophic ligands. Cancer Res. 2015;75:5171–5. doi: 10.1158/0008-5472.CAN-14-3652. [DOI] [PubMed] [Google Scholar]
- 36.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 37.Green DR. The death receptor pathway of apoptosis. Cold Spring Harb Perspect Biol. 2022;14:a041053. doi: 10.1101/cshperspect.a041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banner DW, D’Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–45. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 39.Martin DA, Siegel RM, Zheng L, Lenardo MJ. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. J Biol Chem. 1998;273:4345–9. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- 40.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 41.Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, Zhang H, Wu W, Xu N, Ng SC, Fesik SW. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–22. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–70. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 43.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–27. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 44.Hamacher-Brady A, Brady NR. Bax/Bak-dependent, Drp1-independent targeting of X-linked inhibitor of apoptosis protein (XIAP) into Inner mitochondrial compartments counteracts smac/DIABLO-dependent effector caspase activation. J Biol Chem. 2015;290:22005–18. doi: 10.1074/jbc.M115.643064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–9. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–6. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 47.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–24. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 48.Zhao JT, Zheng X, Rao GW, Zheng Q. Research progress in the use of small molecule smac mimetics in combination therapy of cancer. Curr Med Chem. 2024 doi: 10.2174/0109298673283734240206020150. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Qin JJ, Li X, Hunt C, Wang W, Wang H, Zhang R. Natural products targeting the p53-MDM2 pathway and mutant p53: Recent advances and implications in cancer medicine. Genes Dis. 2018;5:204–219. doi: 10.1016/j.gendis.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 51.Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006;13:951–61. doi: 10.1038/sj.cdd.4401916. [DOI] [PubMed] [Google Scholar]
- 52.Speidel D. Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol. 2010;20:14–24. doi: 10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–8. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288:13305–16. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 56.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS, Lorenz U, Ravichandran KS. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–135. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–1080. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 58.van der Flier LG, Clevers H. Clevers, stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 59.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–71. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 61.Salzman NH. Paneth cell defensins and the regulation of the microbiome: détente at mucosal surfaces. Gut Microbes. 2010;1:401–6. doi: 10.4161/gmic.1.6.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 63.Qiao Y, He C, Xia Y, Ocansey DKW, Mao F. Intestinal mucus barrier: a potential therapeutic target for IBD. Autoimmun Rev. 2025;24:103717. doi: 10.1016/j.autrev.2024.103717. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez JG, Enriquez JR, Wells JM. Enteroendocrine cell differentiation and function in the intestine. Curr Opin Endocrinol Diabetes Obes. 2022;29:169–176. doi: 10.1097/MED.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–30. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–84. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 68.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis Colon Rectum. 2003;46:1498–507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 69.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J Gastroenterol Hepatol. 2002;17:758–64. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 70.Subramanian S, Geng H, Tan XD. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao. 2020;72:308–324. [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–32. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–9. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 73.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 74.Leppkes M, Roulis M, Neurath MF, Kollias G, Becker C. Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation. Int Immunol. 2014;26:509–15. doi: 10.1093/intimm/dxu051. [DOI] [PubMed] [Google Scholar]
- 75.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H International IBD Genetics Consortium (IIBDGC) Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 77.Monteleone G, Caprioli F. T-cell-directed therapies in inflammatory bowel diseases. Clin Sci (Lond) 2010;118:707–15. doi: 10.1042/CS20100027. [DOI] [PubMed] [Google Scholar]
- 78.Eder P, Łykowska-Szuber L, Krela-Kaźmierczak I, Stawczyk-Eder K, Iwanik K, Majewski P, Sterzyńska K, Zabel M, Linke K. Disturbances in apoptosis of lamina propria lymphocytes in Crohn’s disease. Arch Med Sci. 2015;11:1279–85. doi: 10.5114/aoms.2015.54203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veltkamp C, Anstaett M, Wahl K, Möller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W, Manns MP, Schulze-Osthoff K, Bantel H. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut. 2011;60:1345–53. doi: 10.1136/gut.2010.217117. [DOI] [PubMed] [Google Scholar]
- 80.Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–305. doi: 10.1097/MIB.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lehle AS, Farin HF, Marquardt B, Michels BE, Magg T, Li Y, Liu Y, Ghalandary M, Lammens K, Hollizeck S, Rohlfs M, Hauck F, Conca R, Walz C, Weiss B, Lev A, Simon AJ, Groß O, Gaidt MM, Hornung V, Clevers H, Yazbeck N, Hanna-Wakim R, Shouval DS, Warner N, Somech R, Muise AM, Snapper SB, Bufler P, Koletzko S, Klein C, Kotlarz D. Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology. 2019;156:275–278. doi: 10.1053/j.gastro.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 82.Gitlin AD, Heger K, Schubert AF, Reja R, Yan D, Pham VC, Suto E, Zhang J, Kwon YC, Freund EC, Kang J, Pham A, Caothien R, Bacarro N, Hinkle T, Xu M, McKenzie BS, Haley B, Lee WP, Lill JR, Roose-Girma M, Dohse M, Webster JD, Newton K, Dixit VM. Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature. 2020;587:275–280. doi: 10.1038/s41586-020-2796-5. [DOI] [PubMed] [Google Scholar]
- 83.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–4. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 84.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 85.Ahn JH, da Silva Pedrosa M, Lopez LR, Tibbs TN, Jeyachandran JN, Vignieri EE, Rothemich A, Cumming I, Irmscher AD, Haswell CJ, Zamboni WC, Yu YA, Ellermann M, Denson LA, Arthur JC. Intestinal E. coli-produced yersiniabactin promotes profibrotic macrophages in Crohn’s disease. Cell Host Microbe. 2025;33:71–88. e9. doi: 10.1016/j.chom.2024.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schölmerich J, Huber G. Biological therapy in IBD. Anti-tumor necrosis factor-alpha and others. Dig Dis. 2003;21:180–91. doi: 10.1159/000073249. [DOI] [PubMed] [Google Scholar]
- 87.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s disease cA2 study group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 88.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 89.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–4. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 91.West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Görtz D, This S, Stockenhuber K, Pott J, Friedrich M, Ryzhakov G, Baribaud F, Brodmerkel C, Cieluch C, Rahman N, Müller-Newen G, Owens RJ, Kühl AA, Maloy KJ, Plevy SE Oxford IBD Cohort Investigators. Keshav S, Travis SPL, Powrie F. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602–9. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan F, Polk DB. Aminosalicylic acid inhibits IkappaB kinase alpha phosphorylation of IkappaBalpha in mouse intestinal epithelial cells. J Biol Chem. 1999;274:36631–6. doi: 10.1074/jbc.274.51.36631. [DOI] [PubMed] [Google Scholar]
- 94.Mukherjee T, Kumar N, Chawla M, Philpott DJ, Basak S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci Signal. 2024;17:eadh1641. doi: 10.1126/scisignal.adh1641. [DOI] [PubMed] [Google Scholar]
- 95.Rutgeerts P, Van Deventer S, Schreiber S. Review article: the expanding role of biological agents in the treatment of inflammatory bowel disease - focus on selective adhesion molecule inhibition. Aliment Pharmacol Ther. 2003;17:1435–50. doi: 10.1046/j.1365-2036.2003.01603.x. [DOI] [PubMed] [Google Scholar]
- 96.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 97.Singh G, Sharma S, Mishra N, Soni A, Kumari M, Singh S. Targeting cell cycle regulators: a new paradigm in cancer therapeutics. Biocell. 2024;48:1639–1666. [Google Scholar]
- 98.Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603–19. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan M, Thorburn J, Pandolfi PP, Thorburn A. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J Cell Biol. 2002;157:975–84. doi: 10.1083/jcb.200204039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hausmann LD, de Almeida BS, de Souza IR, Drehmer MN, Fernandes BL, Wilkens RS, Vieira DSC, Lofgren SE, Lindenau JD, de Toledo E Silva G, Muniz YCN. Association of TNFRSF1A and IFNLR1 gene polymorphisms with the risk of developing breast cancer and clinical pathologic features. Biochem Genet. 2021;59:1233–1246. doi: 10.1007/s10528-021-10060-z. [DOI] [PubMed] [Google Scholar]
- 101.Zou X, Zhang D, Song Y, Liu S, Long Q, Yao L, Li W, Duan Z, Wu D, Liu L. HRG switches TNFR1-mediated cell survival to apoptosis in Hepatocellular Carcinoma. Theranostics. 2020;10:10434–10447. doi: 10.7150/thno.47286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brouwer RE, Hoefnagel J, Borger van Der Burg B, Jedema I, Zwinderman KH, Starrenburg IC, Kluin-Nelemans HC, Barge RM, Willemze R, Falkenburg JH. Expression of co-stimulatory and adhesion molecules and chemokine or apoptosis receptors on acute myeloid leukaemia: high CD40 and CD11a expression correlates with poor prognosis. Br J Haematol. 2001;115:298–308. doi: 10.1046/j.1365-2141.2001.03085.x. [DOI] [PubMed] [Google Scholar]
- 103.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 104.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 105.Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–6. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 106.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–3. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 107.Shirley S, Micheau O. Targeting c-FLIP in cancer. Cancer Lett. 2013;332:141–50. doi: 10.1016/j.canlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 108.Foreman KE, Wrone-Smith T, Boise LH, Thompson CB, Polverini PJ, Simonian PL, Nunez G, Nickoloff BJ. Kaposi’s sarcoma tumor cells preferentially express Bcl-xL. Am J Pathol. 1996;149:795–803. [PMC free article] [PubMed] [Google Scholar]
- 109.Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903–10. [PubMed] [Google Scholar]
- 110.Miquel C, Borrini F, Grandjouan S, Aupérin A, Viguier J, Velasco V, Duvillard P, Praz F, Sabourin JC. Role of bax mutations in apoptosis in colorectal cancers with microsatellite instability. Am J Clin Pathol. 2005;123:562–70. doi: 10.1309/JQ2X-3RV3-L8F9-TGYW. [DOI] [PubMed] [Google Scholar]
- 111.Hasbullah HH, Musa M. Gene therapy targeting p53 and KRAS for colorectal cancer treatment: a myth or the way forward? Int J Mol Sci. 2021;22:11941. doi: 10.3390/ijms222111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeng K, Chen X, Hu X, Liu X, Xu T, Sun H, Pan Y, He B, Wang S. LACTB, a novel epigenetic silenced tumor suppressor, inhibits colorectal cancer progression by attenuating MDM2-mediated p53 ubiquitination and degradation. Oncogene. 2018;37:5534–5551. doi: 10.1038/s41388-018-0352-7. [DOI] [PubMed] [Google Scholar]
- 113.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–62. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 114.Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–8. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 115.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 116.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–90. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 117.Fulda S. Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28:1414–22. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 118.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu G, Sun Y, An S, Xin S, Ren X, Zhang D, Wu P, Liao W, Ding Y, Liang L. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99:173–9. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 120.Liu C, Li X, Wu Y, Yang J, Wang M, Ma Y. LncRNAs unraveling their sponge role in glioblastoma and potential therapeutic applications. Biocell. 2024;48:387–401. [Google Scholar]
- 121.Ahadi A. Functional roles of lncRNAs in the pathogenesis and progression of cancer. Genes Dis. 2021;8:424–437. doi: 10.1016/j.gendis.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci. 2015;16:2472–96. doi: 10.3390/ijms16022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–90. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zraik IM, Heß-Busch Y. Management of chemotherapy side effects and their long-term sequelae. Urologe A. 2021;60:862–871. doi: 10.1007/s00120-021-01569-7. [DOI] [PubMed] [Google Scholar]
- 125.Ippolito MR, Martis V, Martin S, Tijhuis AE, Hong C, Wardenaar R, Dumont M, Zerbib J, Spierings DCJ, Fachinetti D, Ben-David U, Foijer F, Santaguida S. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev Cell. 2021;56:2440–2454. e6. doi: 10.1016/j.devcel.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 126.Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 128.Tolcher AW. Regulators of apoptosis as anticancer targets. Hematol Oncol Clin North Am. 2002;16:1255–67. doi: 10.1016/s0889-8588(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 129.Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: target of cancer therapy. Clin Cancer Res. 2002;8:2024–34. [PubMed] [Google Scholar]
- 130.Bucur O, Gaidos G, Yatawara A, Pennarun B, Rupasinghe C, Roux J, Andrei S, Guo B, Panaitiu A, Pellegrini M, Mierke DF, Khosravi-Far R. A novel caspase 8 selective small molecule potentiates TRAIL-induced cell death. Sci Rep. 2015;5:9893. doi: 10.1038/srep09893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 132.Chou WC, Dang CV. Acute promyelocytic leukemia: recent advances in therapy and molecular basis of response to arsenic therapies. Curr Opin Hematol. 2005;12:1–6. doi: 10.1097/01.moh.0000148552.93303.45. [DOI] [PubMed] [Google Scholar]
- 133.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 134.McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7:283ra54. doi: 10.1126/scitranslmed.aaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang WW, Li L, Li D, Liu J, Li X, Li W, Xu X, Zhang MJ, Chandler LA, Lin H, Hu A, Xu W, Lam DM. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29:160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 136.Xia Y, Li X, Sun W. Applications of recombinant adenovirus-p53 gene therapy for cancers in the clinic in China. Curr Gene Ther. 2020;20:127–141. doi: 10.2174/1566523220999200731003206. [DOI] [PubMed] [Google Scholar]
- 137.Hu Y, Liu L, Jiang Q, Fang W, Chen Y, Hong Y, Zhai X. CRISPR/Cas9: a powerful tool in colorectal cancer research. J Exp Clin Cancer Res. 2023;42:308. doi: 10.1186/s13046-023-02901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo L, Hu C, Liu Y, Chen X, Song D, Shen R, Liu Z, Jia X, Zhang Q, Gao Y, Deng Z, Zuo T, Hu J, Zhu W, Cai J, Yan G, Liang J, Lin Y. Directed natural evolution generates a next-generation oncolytic virus with a high potency and safety profile. Nat Commun. 2023;14:3410. doi: 10.1038/s41467-023-39156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wan T, Pan Q, Liu C, Guo J, Li B, Yan X, Cheng Y, Ping Y. A duplex CRISPR-Cas9 ribonucleoprotein nanomedicine for colorectal cancer gene therapy. Nano Lett. 2021;21:9761–9771. doi: 10.1021/acs.nanolett.1c03708. [DOI] [PubMed] [Google Scholar]
- 140.Micsik T, Lőrincz A, Mersich T, Baranyai Z, Besznyák I Jr, Dede K, Zaránd A, Jakab F, Szöllösi LK, Kéri G, Schwab R, Peták I. Decreased functional activity of multidrug resistance protein in primary colorectal cancer. Diagn Pathol. 2015;10:26. doi: 10.1186/s13000-015-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin S, Li Y, Zamyatnin AA Jr, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018;233:5119–5132. doi: 10.1002/jcp.26356. [DOI] [PubMed] [Google Scholar]
- 142.Kessel D, Oleinick NL. Cell death pathways associated with photodynamic therapy: an update. Photochem Photobiol. 2018;94:213–218. doi: 10.1111/php.12857. [DOI] [PubMed] [Google Scholar]
- 143.She T, Shi Q, Li Z, Feng Y, Yang H, Tao Z, Li H, Chen J, Wang S, Liang Y, Cheng J, Lu X. Combination of long-acting TRAIL and tumor cell-targeted photodynamic therapy as a novel strategy to overcome chemotherapeutic multidrug resistance and TRAIL resistance of colorectal cancer. Theranostics. 2021;11:4281–4297. doi: 10.7150/thno.51193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kallingal A, Olszewski M, Maciejewska N, Brankiewicz W, Baginski M. Cancer immune escape: the role of antigen presentation machinery. J Cancer Res Clin Oncol. 2023;149:8131–8141. doi: 10.1007/s00432-023-04737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang J, Gong R, Zhao C, Lei K, Sun X, Ren H. Human FOXP3 and tumour microenvironment. Immunology. 2023;168:248–255. doi: 10.1111/imm.13520. [DOI] [PubMed] [Google Scholar]
- 147.Singh M, Morris VK, Bandey IN, Hong DS, Kopetz S. Advancements in combining targeted therapy and immunotherapy for colorectal cancer. Trends Cancer. 2024;10:598–609. doi: 10.1016/j.trecan.2024.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res. 2019;11:7707–7719. doi: 10.2147/CMAR.S212238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiao J, Wu Y, Wu S, Jiang J. Enhancing colorectal cancer treatment through VEGF/VEGFR inhibitors and immunotherapy. Curr Treat Options Oncol. 2025;26:213–225. doi: 10.1007/s11864-025-01306-8. [DOI] [PubMed] [Google Scholar]
- 150.Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, Yagita H, Nakajima Y. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172:500–6. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hájek R, Porkka K, Illés Á, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 152.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and biological correlates of response in a phase ii study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016:CD010383. doi: 10.1002/14651858.CD010383.pub2. [DOI] [PubMed] [Google Scholar]
- 154.Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32:579–86. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lauber K, Bohn E, Kröber SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 156.Weigert A, Cremer S, Schmidt MV, von Knethen A, Angioni C, Geisslinger G, Brüne B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–40. doi: 10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- 157.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–36. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 158.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–56. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–87. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 161.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 163.Wollny T, Wątek M, Durnaś B, Niemirowicz K, Piktel E, Żendzian-Piotrowska M, Góźdź S, Bucki R. Sphingosine-1-phosphate metabolism and its role in the development of inflammatory bowel disease. Int J Mol Sci. 2017;18:741. doi: 10.3390/ijms18040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Y, Lai W, Zheng X, Li K, Zhang Y, Pang X, Gao J, Lou Z. Linderae Radix extract attenuates ulcerative colitis by inhibiting the JAK/STAT signaling pathway. Phytomedicine. 2024;132:155868. doi: 10.1016/j.phymed.2024.155868. [DOI] [PubMed] [Google Scholar]
- 165.Liu DY, Xu R, Huang MF, Huang HY, Wang X, Zou Y, Yue HY, Zhao HM. Si Shen Wan regulates phospholipase Cγ-1 and PI3K/Akt signal in colonic mucosa from rats with colitis. Evid Based Complement Alternat Med. 2015;2015:392405. doi: 10.1155/2015/392405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bao X, Tang Y, Lv Y, Fu S, Yang L, Chen Y, Zhou M, Zhu B, Ding Z, Zhou F. Tetrastigma hemsleyanum polysaccharide ameliorated ulcerative colitis by remodeling intestinal mucosal barrier function via regulating the SOCS1/JAK2/STAT3 pathway. Int Immunopharmacol. 2024;137:112404. doi: 10.1016/j.intimp.2024.112404. [DOI] [PubMed] [Google Scholar]
- 167.Wu Y, Jha R, Li A, Liu H, Zhang Z, Zhang C, Zhai Q, Zhang J. Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol Spectr. 2022;10:e0165122. doi: 10.1128/spectrum.01651-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang C, Dong J, Jin X, Ma H, Zhang D, Wang F, Cheng L, Feng Y, Xiong X, Jiang J, Hu L, Lei M, Wu B, Zhang G. Intestinal anti-inflammatory effects of fuzi-ganjiang herb pair against DSS-induced ulcerative colitis in mice. J Ethnopharmacol. 2020;261:112951. doi: 10.1016/j.jep.2020.112951. [DOI] [PubMed] [Google Scholar]
- 169.Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang Y, Li R. Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine. 2021;84:153519. doi: 10.1016/j.phymed.2021.153519. [DOI] [PubMed] [Google Scholar]
- 170.He Z, Liu J, Liu Y. Daphnetin attenuates intestinal inflammation, oxidative stress, and apoptosis in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3 signaling pathway. Environ Toxicol. 2023;38:2132–2142. doi: 10.1002/tox.23837. [DOI] [PubMed] [Google Scholar]
- 171.Liu K, Shi C, Yan C, Yin Y, Qiu L, He S, Chen W, Li G. Fufangxiaopi formula alleviates DSS-induced colitis in mice by inhibiting inflammatory reaction, protecting intestinal barrier and regulating intestinal microecology. J Ethnopharmacol. 2024;319:117365. doi: 10.1016/j.jep.2023.117365. [DOI] [PubMed] [Google Scholar]
- 172.Lu H, Shen M, Chen T, Yu Y, Chen Y, Yu Q, Chen X, Xie J. Mesona chinensis benth polysaccharides alleviate DSS-induced ulcerative colitis via inhibiting of TLR4/MAPK/NF-κB signaling pathways and modulating intestinal microbiota. Mol Nutr Food Res. 2022;66:e2200047. doi: 10.1002/mnfr.202200047. [DOI] [PubMed] [Google Scholar]
- 173.Shi G, Kong J, Wang Y, Xuan Z, Xu F. Glycyrrhiza uralensis Fisch. alleviates dextran sulfate sodium-induced colitis in mice through inhibiting of NF-κB signaling pathways and modulating intestinal microbiota. J Ethnopharmacol. 2022;298:115640. doi: 10.1016/j.jep.2022.115640. [DOI] [PubMed] [Google Scholar]
- 174.Wen T, Liu T, Chen H, Liu Q, Shen X, Hu Q. Demethylzeylasteral alleviates inflammation and colitis via dual suppression of NF-κB and STAT3/5 by targeting IKKα/β and JAK2. Int Immunopharmacol. 2024;142:113260. doi: 10.1016/j.intimp.2024.113260. [DOI] [PubMed] [Google Scholar]
- 175.Duan T, Geng Z, Yang J, Yin L, Sun M, Wang S, Zhang X, Li J, Hu J, Lu G. Isongifolene improves crohn’s disease-like colitis in mice by reducing apoptosis of intestinal epithelial cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024;55:1175–1185. doi: 10.12182/20240960204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rahimi HR, Nedaeinia R, Sepehri Shamloo A, Nikdoust S, Kazemi Oskuee R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J Phytomed. 2016;6:383–98. [PMC free article] [PubMed] [Google Scholar]
- 177.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang J, Cen L, Zhang X, Tang C, Chen Y, Zhang Y, Yu M, Lu C, Li M, Li S, Lin B, Zhang T, Song X, Yu C, Wu H, Shen Z. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 2022;56:102469. doi: 10.1016/j.redox.2022.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155–62. doi: 10.1152/ajpgi.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]