Abstract
Background
N6-methyladenosine (m6A) modification is a prevalent RNA modification in epigenetics. METTL3, acting as the principal methyltransferase responsible for catalysing m6A, is regarded as a master regulator of this RNA modification. Nonetheless, the complex roles and underlying mechanisms of m6A in relation to intervertebral disc degeneration (IDD) are yet to be fully elucidated. In light of this, this study aimed to explore the intricate functions and mechanisms of METTL3-mediated m6A modification in IDD.
Methods
Our previous batch of RNA sequencing data (GSE167199) and public single-cell data (GSE165722) were utilized to probe the relationship between m6A-related genes and IDD. m6A quantification, RNA m6A immunoblotting, quantitative real-time PCR, western blot and immunofluorescent staining were used to validate the levels of m6A modification and expression of m6A-related genes in nucleus pulposus (NP) tissues and cells. Moreover, gain- and loss-of-function experiments in NP cells were conducted to explore the impact of METTL3 on IDD. In vivo, the effects of METTL3 inhibition and miR-338-3p suppression on IDD progression were assessed.
Results
A significant association between METTL3-mediated m6A modification and IDD was identified. Overexpressing METTL3 induced apoptosis, accelerated senescence and inhibited matrix synthesis in NP cells. Additionally, METTL3-mediated m6A modification could expedite the production and maturation of pri-miR-338-3p in NP cells via DGCR8. In vivo, inhibiting METTL3 mitigated IDD progression, while suppressing miR-338-3p notably alleviated IDD during METTL3 overexpression.
Conclusions
This study reveals that targeting METTL3 attenuates IDD progression through the METTL3-m6A-miR-338-3p axis, thereby highlighting the therapeutic potential of METTL3 inhibition for IDD. Future studies should prioritize the development of biomaterial delivery systems for METTL3 inhibitors to ensure both therapeutic protection and sustained, site-specific drug release.
Keywords: Intervertebral disc degeneration, N6-methyladenosine, METTL3, miR-338-3p
1. Introduction
Low back pain is a prevalent musculoskeletal disorder that has become an increasingly significant global burden of disease [1]. Importantly, intervertebral disc degeneration (IDD) is identified as one of the chief factors contributing to low back pain [2,3]. Epidemiology indicates that IDD affects 18% to 30% of the general population, and it is rising with continued population ageing [4,5]. Currently, IDD is primarily treated through drug therapy or surgery [6]. These treatments mainly focus on alleviating pain and inflammation, rather than addressing the root causes of disc degeneration. Therefore, clarifying the molecular mechanisms of IDD is imperative to develop effective prevention and treatment methods, which represent significant scientific challenges.
IDD is mainly characterized by degeneration and protrusion of the nucleus pulposus (NP) in the intervertebral disc, as well as loss of disc height, calcification of the cartilage endplate and degeneration of the spinal facet joints [7]. At the molecular level, there are several manifestations associated with IDD, including accelerated senescence of NP cells, decreased NP cell proliferation, increased NP cell apoptosis and an imbalance in extracellular matrix synthesis and degradation [8–10]. microRNAs (miRNAs), which are single-stranded noncoding RNAs with a length of 18–24 nucleotide sequences, regulate the activity of up to 50% of coding genes in mammals [11]. Emerging studies demonstrated that miRNAs play a crucial role in the pathogenesis of IDD through posttranscriptional regulatory mechanisms [12–17]. Recently, our previous study found that miR-338-3p is upregulated in the NP tissues of IDD patients. miR-338-3p has been identified as targeting SIRT6, which exerts considerable influence on regulating the MAPK/ERK signalling pathway involved in the pathological process of IDD. More importantly, in vivo, silencing of miR-338-3p has been shown to effectively inhibit IDD [18]. However, factors responsible for the elevated expression of miR-338-3p in degenerated NP cells still remain unclear. Given this, it is crucial to identify the regulatory molecules that enable this excessive expression and provide a new avenue for treating IDD.
RNA modifications are fundamental epigenetic regulators of eukaryotic gene expression. Key modifications including N6-methyladenosine (m6A), 5-methylcytosine (m5C), pseudouridine (Ψ) and A-to-I editing collectively influence cellular processes through RNA stability control, translational regulation and inflammatory modulation [19]. m6A methylation is a modification that replaces a hydrogen atom attached to the 6th nitrogen atom on adenine with a methyl group [20]. It is the RNA modification mode with the highest endogenous abundance, accounting for approximately 0.1%–0.4% of the total adenine (m6A/A, or approximately 3-5 m6A sites per transcript) and is highly conserved across multiple species [21,22]. In vivo, m6A methylation, a reversible RNA modification, is catalysed by the coordinated actions of RNA methylation transferase and demethylase. The methyltransferase enzyme consists of at least three proteins (METTL3, METTL14 and WTAP). On the other hand, demethylases mainly include FTO and ALKBH5 [21]. Ubiquitous in eukaryotic cells, m6A modification governs multiple non-coding RNAs such as miRNAs, long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), ultimately directing their metabolic and functional fates [23]. Mature miRNAs are produced through a series of shear processing from primary miRNAs (pri-miRNAs) and precursor miRNAs (pre-miRNAs) [24]. Recent studies have shown that modifying the m6A of pri-miRNAs in mammalian cells assists DGCR8 in recognizing and processing pri-miRNAs. This modification significantly impacts the maturation process of pri-miRNAs, giving rise to the differential expression of miRNAs in various biological processes [24–26]. Emerging evidence indicates that m6A modification is closely implicated in the pathological processes of skeletal disorders, including osteoarthritis, osteoporosis and particularly IDD [27].
Single-cell analytical technology offers a more comprehensive way to explore m6A modification mechanisms in IDD. This study analyses the role of m6A modification in IDD using single-cell RNA sequencing (scRNA-seq) and bulk RNA sequencing (bulk RNA-seq) data, revealing a significant correlation between METTL3 and the IDD pathology. Accumulating evidence indicates that METTL3 dysregulation drives pathologies such as cancer [28] and neurodegenerative disorders [29]. Fang et al. demonstrated that METTL3-mediated m6A methylation of SIAH1 mRNA promotes the progression of IDD [30]. Moreover, METTL3 enhances miR-143-3p expression, which suppresses the protective role of SOX5 in nucleus pulposus cells by virtue of impairing their viability and metabolism and ultimately causes IDD [31]. However, the precise mechanisms linking METTL3-mediated m6A modification to IDD progression require further elucidation. Targeting this gap, we hypothesize that METTL3-mediated m6A hypermethylation promotes pri-miR-338-3p processing, thereby accelerating IDD progression. In this study, the mechanism through which METTL3-mediated m6A modification promotes the processing and maturation of pri-miR-338-3p was explored in IDD. These findings might shed light on the epigenetic molecular mechanism of IDD and offer a theoretical foundation for developing targeted drugs that focus on the METTL3-m6A-miRNA signalling axis.
2. Materials and methods
2.1. Bulk RNA-seq analysis
The mRNA expression matrix of human NP tissues was extracted from our previously published dataset GSE167199 [32]. The dataset GSE167199 includes three samples derived from IDD patients and three from controls with spinal cord injury. Access it at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE167199. To identify differentially expressed genes (DEGs), a differential expression analysis of mRNA was performed using the R package ‘limma’ [33]. DEGs with a fold change of 2.0 or greater and an adjusted p value less than .05 were considered statistically significant. To investigate the role of m6A methylation, a search was conducted on GeneCards-the human gene database [34] (https://www.genecards.org/. Accessed 5 June 2023) using the keyword ‘m6A methylation’, which yielded a total of 863 m6A genes with a relevance score of 8 or above. Higher relevance scores indicate stronger gene-disease associations, with the ≥8 cutoff chosen based on prior studies [35,36]. By intersecting these 863 m6A genes with the DEGs, the m6A methylation DEGs in IDD were obtained. To conduct the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses, the following methods were employed: The latest annotations for KEGG pathways were obtained from the KEGG rest API [37]. To retrieve GO annotations for the genes, the R package ‘org.Hs.eg.db’ (version 3.1.0) was utilized [38]. Based on the m6A DEGs, we made use of the R package ‘clusterProfiler’ (version 3.14.3) to perform GO and KEGG enrichment analyses [39]. Furthermore, we constructed a protein-protein interaction (PPI) network by means of the STRING website [40] (https://cn.string-db.org/. Accessed 20 June 2023). The network was then optimized and visualized via Cytoscape software (version 3.10) [41].
2.2. Single-cell RNA-seq analysis
Using the terms ‘intervertebral disc degeneration (IDD)’, ‘degenerative disease’ and ‘nucleus pulposus tissue’, a search was conducted on the NCBI Gene database [42] (https://www.ncbi.nlm.nih.gov/geo/. Accessed 26 June 2023). Subsequently, the scRNA-seq transcriptome datasets (GSE165722) were acquired. The R package Seurat [43] for scRNA-seq was employed for the analysis. It enabled the detection of highly variable genes, implementation of data normalization, performance of principal component analysis (PCA) for dimensionality reduction, cell clustering and conduction of Uniform Manifold Approximation and Projection (UMAP) for nonlinear dimensionality reduction. Finally, differential genes were identified and the cells were annotated.
2.3. Human NP samples
This study was approved by the Research Ethics Committee of The First Affiliated Hospital of Guangxi Medical University on 27 February 2023 (Approval No. 2023-S297-01; Date: 27 February 2023). All experimental protocols and methods were executed in strict accordance with relevant guidelines and regulations and adhered to the principles outlined in the Declaration of Helsinki. Written informed consent was achieved from each adult participant. IDD NP tissues were obtained from 35 patients (mean age: 42.3 ± 5.8 years) undergoing lumbar microdiscectomy, while normal NP tissues were collected from 35 trauma patients (mean age: 43.7 ± 3.6 years). All participants were recruited from the Department of Spine Surgery at the First Affiliated Hospital of Guangxi Medical University, with data collection spanning from January and June 2024. They were diagnosed using MRI technology and physical examination. According to the Pfirrmann classification, all IDD patients had severe intervertebral disc degeneration (Pfirrmann’s grade IV or V). These patients with trauma had no prior history of low back pain or IDD and their preoperative MRI scans implied no significant degeneration (Pfirrmann grades I-II).
2.4. Cell culture
Primary NP cells were isolated and identified following the previously published method [17,18,44]. Samples were transferred to a sterile cell culture chamber. The annulus fibrosus and cartilage components were carefully removed, and the remaining tissue was washed three times with phosphate-buffered saline (PBS). The tissue was then cut into 1mm3 pieces. Afterward, the dissociated tissues were initially digested with 0.25% trypsin (Thermo Fisher Scientific, MA, USA) for 30 min at 37 °C, followed by digestion with 0.2% collagenase type II (Invitrogen, CA, USA) for 4 h at 37 °C. A complete medium (Gibco, NY, USA) containing 10% foetal bovine serum (FBS) (Invitrogen, CA, USA) and 1% penicillin/streptomycin (Biological Industries, Israel) was added to stop the digestion process and obtain the primary NP cells. All NP cells were cultured and maintained in DMEM, 1% penicillin-streptomycin and 10% FBS at 37 °C with 5% CO2. NP cells at passages 3–4 were harvested for subsequent experiments. After 48 h of culture, the medium was replaced with fresh medium, and the NP cells were treated with IL-1β at a final concentration of 10 ng/ml to simulate IDD [45]. Subsequently, the cells were then cultured for an additional 48 h before undergoing follow-up detection.
2.5. Cell transfection
shRNA-METTL3 or negative control (NC), miR-338-3p mimics or inhibitors and their negative controls were constructed by Vazyme (Nanjing, China). pcDNA3.1-METTL3 and an empty pcDNA3.1 control vector were obtained from Cyagen Biosciences (Suzhou, China). For METTL3 knockdown, cells were transduced with lentiviral vectors expressing METTL3-targeting shRNA. For overexpression studies, parallel experiments utilized pcDNA3.1-METTL3 plasmid transfection. NP cells at 60–70% confluence were transfected using Lipofectamine 3000 (Invitrogen, CA, USA) in accordance with the manufacturer’s protocol, with subsequent experiments conducted 48 h post-transfection. Following transduction, cells were subjected to G418 (BioSharp, Hefei, China) selection to generate stable transformants. The efficiency of METTL3 knockdown and overexpression was validated by quantitative real-time PCR (qRT-PCR) and Western blot analyses.
2.6. RNA m6A immunoblotting
Total RNA was extracted and treated with deoxyribonuclease I (Sigma-Aldrich, MO, USA). Subsequently, the RNA was subjected to further processing employing the GenElute messenger RNA Miniprep Kit (Sigma-Aldrich, MO, USA) and the RiboMinus Transcriptome Isolation Kit (Thermo Fisher Scientific, MA, USA). Then, Poly(A)+ RNA (500 ng) and ribosomal RNA (rRNA)-free poly(A)+RNA (200 ng) were mixed in a 1:1 ratio with glyoxal loading dye (Ambion, TX, USA), followed by denaturation at 65 °C for 15 min. According to the instructions for the NorthernMax Kit (Ambion, TX, USA), the samples were separated in a 1% denatured agarose gel for 1 h at 70 V and transferred to a nylon membrane (GE Healthcare Life Biosciences, MA, USA) at room temperature for 2.5 h. Next, the membranes were ultraviolet-crosslinked and blocked-in blocking buffer (5% nonfat dry milk in 0.1% PBST; 0.1% Tween-20 in 1× PBS, pH 7.4) for 1 h and then incubated with rabbit anti-m6A antibody (ab208198, Abcam) diluted 1:1000 in 0.1% PBST overnight at 4 °C. Following washing three times with 0.1% PBST, HRP-conjugated goat anti-rabbit IgG (ab6721, Abcam) was diluted 1:2500 and incubated with the membranes at room temperature for 1 h. Finally, the membranes were washed thoroughly with 0.1% PBST and detected using a 3,3′-diaminobenzidine peroxidase substrate kit (Yease, Shanghai, China). Subsequently, equal amounts of total RNA were separated in a 1% denaturing agarose gel at 70 V for 1 h and then visualized through ethidium bromide staining.
2.7. RNA m6A quantification
Total RNA was extracted from NP tissues or ex vivo NP cells using TRIzol reagent (Invitrogen, CA, USA) and quantified using Nanodrop 2000 (Thermo Fisher Scientific, MA, USA). The m6A content of the total RNA was determined using the m6A RNA methylation quantification kit (ab185912, Abcam). In the assay procedure, RNAs were initially coated onto the wells and incubated at 37 °C for 90 min. Next, the capture antibody (1:1000), detection antibody (1:2000) and enhancer solution (1:5000) were sequentially incubated as per the manufacturer’s instructions. The m6A levels were then determined through calorimetric measurement by reading the absorbance of each well at a wavelength of 450 nm and calculating the concentrations based on a standard curve.
2.8. RNA extraction and qRT-PCR
Total RNA was extracted from ex vivo NP cells or tissues using TRIzol reagent (Invitrogen, CA, USA). Briefly, cells were washed and lysed in 1 mL TRIzol on ice, while tissues were homogenized in liquid nitrogen prior to TRIzol addition. After chloroform separation and isopropanol precipitation, RNA was washed with 75% ethanol, air-dried, dissolved in DEPC-water for immediate use or long-term storage at −80 °C and quantified using Nanodrop 2000. The RNA samples were then reverse-transcribed to cDNA using random primers through PrimeScript RT reagent kit (TaKaRa, Dalian, China). For miRNA reverse transcription, the miRNA First - Strand cDNA Synthesis Kit (Tailing Reaction; Sangon Biotech, Shanghai, China) was employed as per the manufacturer’s instructions. qRT-PCR was performed using the SYBR Premix Ex Taq Kit (TaKaRa, Dalian, China) on the LightCycler®96 system (LIGHTCYCLER, Mannheim, Germany) under the following conditions: initial denaturation at 95 °C for 30 sec, followed by 40 cycles of 95 °C for 5 sec and 60 °C for 30 sec, with a melting curve analysis to verify specificity. Each reaction included positive controls (cDNA samples with confirmed target expression) and negative controls (no-template controls and no-reverse transcription controls), with β-actin or U6 serving as endogenous controls. The sequences of primers for METTL3, METTL14, WTAP, METTL4, FTO, ALKBH5, YTHDF2, MMP13, collagen II, pri-miR-338-3p, pre-miR-338-3p, miR-338-3p, β-actin and U6 are indicated in Supplemental File 1: Table S1.
2.9. Western blot analysis
Total protein was extracted from cells and tissues using RIPA buffer (Beyotime, Shanghai, China), and protein concentration was measured via BCA assay kit (Beyotime, Shanghai, China). The total protein was dissociated using 10% sodium dodecyl sulphate-poly-acrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Sigma, Darmstadt, Germany). The membranes were blocked with 5% nonfat milk for 2 h and incubated overnight at 4 °C with the following primary antibodies: anti-METTL3 (ab195352, Abcam, 1:1000), anti-METTL14 (ab300104, Abcam, 1:1000), anti-WTAP (ab195380, Abcam, 1:1000), anti-METTL4 (PA5-23881, Thermo Fisher Scientific, 1:1000), anti-FTO (ab126605, Abcam, 1:1000), anti-ALKBH5 (ab195377, Abcam, 1:1000), anti-YTHDF2 (ab220163, Abcam, 1:1000), anti-MMP13 (ab39012, Abcam, 1:1000), anti-collagen II (ab307675, Abcam, 1:1000), anti-GAPDH (ab9485, Abcam, 1:5000) and anti-β-actin (ab115777, Abcam, 1:5000). After being washed, the membranes were incubated with a secondary HRP-conjugated antibody (ab205718, Abcam, 1:5000) and finally scanned by an Odyssey Infrared Imaging System (LI-COR Biosciences, NE, USA).
2.10. Immunofluorescence staining
To investigate the expression of METTL3, collagen II and MMP13 on NP cells, immunofluorescence staining was performed. After transfection, the cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min and permeabilized for 10 min using Triton®100 (Solarbio, Beijing, China). Cells were blocked with goat serum (Boster, Wuhan, China) at 37 °C for 1 h. Subsequently, they were incubated at 37 °C for 2 h with primary antibodies and 45 min with the corresponding secondary antibody conjugated to Alexa Fluor 488 or Alexa Fluor 594. The primary antibodies utilized included: anti-METTL3 (ab195352, Abcam), anti-collagen II (ab307675, Abcam) and anti-MMP13 (ab39012, Abcam). Finally, nuclei were marked with DAPI. Images were obtained using a confocal laser scanning microscope (Olympus, Tokyo, Japan).
2.11. Flow cytometry, senescence assay, cell counting kit-8 (CCK-8) and EdU assay
At 48 h transfection, the apoptotic ratio of NP cells was determined using the Apoptosis Detection Kit of fluorescein isothiocyanate (FITC) (BestBio, Shanghai, China). The suspended cells were collected by centrifugation and washed twice with cold PBS. Subsequently, the cells were collected by centrifugation for 5 min and then resuspended in 400 μL of Annexin V binding solution. Cells were then stained with FITC Annexin V (5 μL) and incubated at 2–8 °C for 15 min in the dark, followed by co-staining with PI (10 μL) and incubation for an additional 5 min under the same conditions. Finally, the samples were analysed via flow cytometry (Bio-Rad ZE5, Bio-Rad Laboratories, CA, USA). Meanwhile, Senescence-Associated β-gal Assay Kit (Beyotime, Shanghai, China) was used to evaluate the senescence of NP cells after transfection. For NP cells, cultures were fixed for 15 min at room temperature, rinsed twice with PBS and stained with freshly prepared SA-β-gal solution at 37 °C for 16–18 h in a low-CO2, light-protected environment. Cellular proliferation was assessed with the CCK-8 assay (Biosharp, Hefei, China). At 24, 48 and 72 h post-transfection, CCK-8 was added to the cells, which were then incubated for 3 h before measuring absorbance at 450 nm. Cellular proliferation was further evaluated using the EdU assay. The transfected NP cells were incubated with EdU medium (Beyotime, Shanghai, China), collected and stained with Hoechst 33258.
2.12. Coimmunoprecipitation (Co-IP)
The transfected NP cells were lysed with nondenatured cell lysate buffer (Solarbio, Beijing, China). The total protein was quantified using BCA assay kit (Beyotime, Shanghai, China) with a portion of each sample being reserved as input. For western blot analyses, ∼500 µg of protein was incubated overnight at 4 °C with anti-DGCR8 (ab191875, Abcam), anti-METTL3 (ab195352, Abcam), or control IgG (ab172730, Abcam). The mixtures were then incubated with protein A/G magnetic beads (MedChemExpress, NJ, USA) for 2–4 h at 4 °C. The protein lysates from A/G magnetic beads were collected by centrifugation (1000 g, 5 min, 4 °C), washed three times with PBS buffer and resuspended in SDS-PAGE sample loading buffer (Beyotime, Shanghai, China). The immunoprecipitated complexes were treated with either RNase or RNase inhibitor (Sigma Aldrich, MO, USA) for 5 min at 37 °C. The proteins in the supernatants were then analysed by SDS-PAGE and western blot using specific antibodies, containing anti-METTL3 (ab195352, Abcam) and anti-DGCR8 (ab191875, Abcam). The groups included input, IgG (negative control) and IP.
2.13. m6A RNA immunoprecipitation assay (MeRIP) and bioinformatic analysis
Following the manufacturer’s protocol, RIP experiments were conducted using the EZMagna RIP kit (Millipore, MA, USA). The transfected NP cells were lysed in RIP buffer with protease and RNase inhibitors at 4 °C. The cell lysate was incubated overnight at 4 °C with magnetic beads pre-coated with anti-DGCR8 (ab191875, Abcam), anti-m6A (ab208198, Abcam), or control IgG (ab172730, Abcam). Finally, the immunoprecipitated RNAs underwent purification using a standard phenol/chloroform procedure, and qRT-PCR analysis was performed on the purified RNAs, with total RNA serving as the input control. In addition, we utilized the SRAMP website [46] (http://www.cuilab.cn/sramp/. Accessed 16 June 2024) to analyse potential m6A targeting positions.
2.14. Animal experiments
The animal experiments in this study were approved by the Medical Laboratory Animal Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Approval No. 2023-D043-01; Date: 2 March 2023). All animal experiments were conducted between July and October 2024. The research was conducted and reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. The male SD rats (SPF, aged 12 weeks) from The Guangxi Medical University Animal Center were randomly divided into the following groups: healthy (n = 10), IDD (n = 10), shNC (n = 10), shMETTL3 (n = 10), METTL3 + antagomiR-NC (n = 10) and METTL3 + antagomiR-338-3p (n = 10). As detailed previously, the injury-induced IDD rat model was created using the needle puncture method [47]. In short, all these rats were anesthetized with pentobarbital, administered via intraperitoneal injection and sterilized. In the IDD, shNC and shMETTL3 groups, after full exposure of the coccygeal discs (Co7/Co8 and Co8/Co9), a needle attached to a 21 G syringe was inserted into the NP centre, aligned parallel to the endplates and rotated 180° for 10 s. The AAV9-shMETTL3 and AAV9-METTL3 vectors, including their controls, were produced by Vazyme (Nanjing, China). The antagomiR-NC and antagomiR-338-3p were obtained from RiboBio (Guangzhou, China). One week after IDD surgery, the shNC and shMETTL3 groups were administered with AAV9 - shNC and AAV9-shMETTL3 respectively, via injection at the centre of the intervertebral disc. Concurrently, rats in the IDD groups received intra-discal injections of an equal volume of normal saline. The METTL3 + antagomiR-NC and METTL3 + antagomiR-338-3p groups were first injected with AAV9-METTL3 into the intervertebral disc. One week later, they received additional injections of antagomiR-NC and antagomiR-338-3p, respectively. Based on previous IDD animal studies [48,49], X-ray and MRI examinations were conducted at 12 weeks post-puncture to observe the long-term progression of IDD. This was followed by further analysis utilizing the disc height index (DHI) and relative water content. The rats were euthanized 12 weeks post-surgery, and disc samples were gathered for histological and molecular analysis. The samples were first fixed in 4% paraformaldehyde, decalcified and photographed for macroscopic examination. Subsequently, they underwent dehydration, embedding and paraffin sectioning, followed by staining with H&E and Safranin O/Fast Green (S&O) for histological analysis.
2.15. Statistical analysis
Sample size estimation was performed using G*Power 3.1.9.2 with the following parameters: α = 0.05, effect size (d) = 0.8 and statistical power (1 − β) = 0.90. All data analyses were conducted utilizing SPSS, version 20.0. The Kolmogorov-Smirnov test was employed to assess the normality of data distribution. Results are expressed as means ± SD or as medians (with interquartile ranges from the 25th to 75th percentiles), as specified in the figure legends. The statistical differences between groups were evaluated using Student’s t-test or one-way ANOVA, followed by Tukey’s honestly significant difference (HSD) post hoc test for multiple comparisons when ANOVA results were significant (p < .05). For nonparametric data, the Mann-Whitney U test was employed to compare the two independent samples. Statistical significance was set as follows: *p < .05, **p < .01, ***p < .001 and ****p < .0001.
3. Results
3.1. Analysing the m6A methylation-related genes for IDD in bulk RNA-seq
The mRNA expression matrix of NP tissues was obtained from our previously published dataset GSE167199 [32]. This dataset included a total of six samples, with three samples from the IDD group and three samples from the spinal cord injury (control) group. Differential expression analysis of mRNA identified 5154 DEGs, comprising 4190 upregulated and 964 downregulated (Figure 1A). Intersection analysis between 863 m6A genes and 5154 DEGs showed that 44 m6A genes exhibited differential expression between the IDD and control groups (Figure 1B). Heatmap visualization demonstrated that the majority of m6A DEGs were significantly upregulated in the IDD group (Figure 1C). The PPI network revealed interactions among the 44 m6A DEGs, with METTL3 occupying central positions (Figure 1D). KEGG analysis indicated enrichment of these 44 m6A DEGs in pathways such as the p53 signalling pathway, cellular senescence and cell cycle (Figure 1E). Additionally, GO analysis highlighted METTL3’s crucial role in the biological processes, molecular functions and cellular components associated with IDD (Supplemental Files 2–4: Figures S1–S3).
Figure 1.
Potential association between m6A methylation-related genes and intervertebral disc degeneration (IDD). (A) the volcano plot of gene expression differential analysis: red represents upregulated genes, blue represents downregulated genes, and grey represents genes with no significant differential expression. (B) Venn diagram: Intersection analysis resulted in 44 m6A differentially expressed genes (DEGs). (C) Heatmap visualization of the expression levels of the 44 m6A DEGs: the horizontal axis represents sample names, and the vertical axis represents gene names. Darker red indicates higher gene expression levels, while darker blue indicates lower gene expression levels. (D) Protein-protein interaction (PPI) network of the 44 m6A DEGs: Nodes represent genes, and edges represent interactions between genes. Nodes closer to the centre and with darker colours indicate more interactions with other genes. (E) The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis results of the 44 m6A DEGs.
3.2. Identifying the m6A methylation-related genes for IDD in scRNA-seq
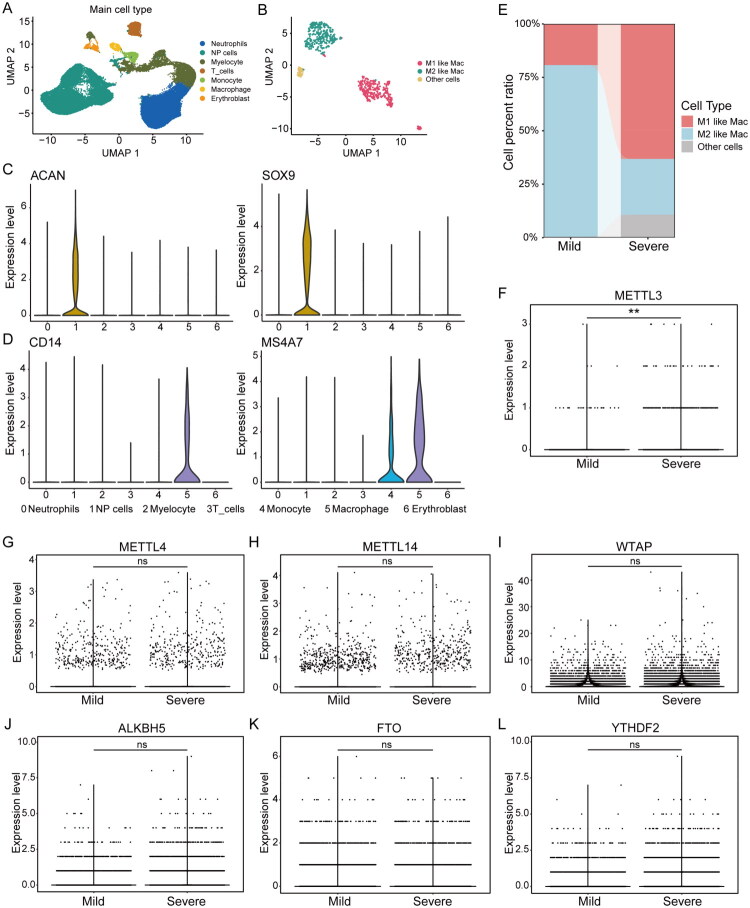
The scRNA-seq data were obtained from the GEO165722 dataset, which consist of eight NP tissue samples. Based on previous original research [50], the samples were categorized into mild (grades II and III) and severe (grades IV and V) IDD groups in accordance with the Pfirrmann grading, with four samples in each group. After initial quality control, a total of 41,548 cell samples were obtained, incorporating 19,594 NP cells and 21,954 other cells. These samples were further analysed after removing low-quality genes, reducing dimensionality and addressing batch effects. NP cells and macrophage cells were identified based on the expression patterns of specific marker genes (Figure 2A–D). Compared with the mild IDD group, patients with severe IDD showed an increased proportion of M1-like macrophages and a decreased proportion of M2-like macrophages (Figure 2E). The m6A modification, a dynamic and reversible process in eukaryotic cells, is tightly regulated by m6A methyltransferases, demethylases and binding proteins, which primarily consist of METTL3, METTL14, METTL4, WTAP, FTO, ALKBH5 and YTHDF2. To investigate the potential role of m6A modification in IDD, we examined the differential gene expression levels of m6A regulators in NP cells at the single-cell level. Patients with severe IDD showed markedly elevated METTL3 levels (p < .01) compared to those with mild IDD (Figure 2F). However, no significant differences were observed in the expression levels of METTL14, METTL4, WTAP, ALKBH5, FTO and YTHDF2 (Figure 2G–L).
Figure 2.
ScRNA-Seq reveals the expression of m6A regulators in NP cells. (A-B) Uniform manifold approximation and projection (UMAP) plot showing the identification of human NP cells and macrophage cells, with different colours indicating different cells. (C,D) Marker genes of human NP cells and macrophages cells. (E) Distribution of M1-like and M2-like macrophages in NP tissues of the mild IDD group and the severe IDD group. (F–L) Expression of m6A regulators in human NP cells of patients with IDD. (F) Compared with the mild IDD group, METTL3 expression was upregulated in the severe IDD group. No significant differences were recognized in the expression levels of METTL14, METTL4, WTAP, ALKBH5, FTO and YTHDF2. Data are presented as the median with interquartile range (25th to 75th percentiles). p Values are from Mann–Whitney U test. **p < .01, ns ‘no significance’.
3.3. m6A modification and METTL3 were upregulated in IDD
To investigate the potential role of m6A modification in IDD, we analysed the levels of m6A in total RNAs from NP tissues. Our colorimetric m6A quantification strategy revealed that elevated m6A modification levels in IDD NP tissues compared to controls (Supplemental File 5: Figure S4A). Additionally, after isolating poly(A)+ RNAs from NP tissues and further depleting rRNA, RNA immunoblotting confirmed increased m6A levels in the rRNA-free poly(A)+ RNAs of IDD NP tissues (Supplemental File 5: Figure S4B-C). Together, these data suggested that m6A levels were upregulated in IDD. Moreover, the expression pattern of m6A regulators at the mRNA and protein levels was detected in NP tissues. The results showed that the levels of METTL3 were significantly upregulated in the IDD group compared with controls, as revealed by qRT-PCR and western blot at both the mRNA and protein levels (Figure 3A–C). However, the levels of METTL14, METTL4, WTAP, FTO, ALKBH5 and YTHDF2 remained no significant change (Figure 3A–C). Additionally, immunofluorescence staining demonstrated increased METTL3 expression in IL-1β-treated NP cells (Figure 3D,E).
Figure 3.
METTL3 Were upregulated in IDD. (A) qRT-PCR analysis showed significantly elevated METTL3 mRNA expression in IDD NP tissues (n = 35) compared to controls (n = 35). Data are means ± SD, and p values are from two-tailed unpaired t test. (B,C) Protein expression level of METTL14, METTL3, FTO, ALKBH5, WTAP, METTL4 and YTHDF2 in IDD NP tissues and controls. β-actin was used as the loading control for METTL14, METTL3, FTO, ALKBH5 and WTAP, while GAPDH served as the loading control for METTL4 and YTHDF2. The grouping of blots cropped from different parts of the same gel. Data represent mean ± SD (n = 3). p Values are from two-tailed unpaired t test. (D,E) Representative immunofluorescence staining of METTL3 and DAPI staining of nuclei in NP cells of control and IDD groups (scale bar, 20 μm). Quantification of relative fluorescent pixel intensity. Data are presented as means ± SD (n = 5), and p values are derived from two-tailed unpaired t test. *p < .05, **p < .01, ***p < .001, ****p < .0001.
3.4. Effects of METTL3 on the proliferation, apoptosis and senescence of NP cells
To further investigate METTL3’s role in IDD, shRNA-METTL3 and NC, METTL3 overexpression by pcDNA3.1-METTL3 and empty pcDNA3.1 were used to perform gain- and loss-of-function experiments in NP cells. METTL3 expression was significantly decreased and increased by the action of shRNA1, shRNA2 and pcDNA3.1-METTL3 (Figure 4A). The RNA modification level of m6A also changed correspondingly (Figure 4B). Inhibition of METTL3 significantly stimulated NP cell proliferation, while its overexpression notably suppressed the proliferation, as demonstrated by EdU and CCK-8 assays (Figure 4C–E). In addition, qRT-PCR and immunofluorescence staining analysis of ECM anabolic/catabolic markers revealed that METTL3 overexpression markedly upregulated the expression of MMP13 and reduced the expression of collagen II. In contrast, METTL3 inhibition decreased MMP13 expression and increased collagen II expression (Figure 4F–G, J–M). To rule out potential off-target effects of METTL3 shRNA, METTL3 expression was rescued into knockdown cells (Supplemental File 6: Figure S5). In addition, we assessed cellular senescence using an acidic β-galactosidase activity assay. Overexpression of METTL3 in NP cells resulted in accelerated senescence and enlarged nuclei. Conversely, inhibition of it significantly alleviated senescence (Figure 4H–1). We further performed Annexin V-FITC/PI-labelled flow cytometry which revealed that METTL3-overexpressing NP cells induced apoptosis (Figure 4N). Overall, we confirm that overexpression of METTL3 suppresses proliferation, induces apoptosis, accelerates senescence and inhibits matrix synthesis in NP cells. On the contrary, inhibition of METTL3 stimulates cell proliferation, attenuates apoptosis and senescence and promotes matrix synthesis.
Figure 4.
Effects of METTL3 on the proliferation, apoptosis and senescence of nucleus pulposus (NP) cells. (A) The silencing efficiency of si-METTL3 or the overexpression efficiency of pcDNA-METTL3 assessed by Western blot analysis. β-actin was used as the loading control. The grouping of blots cropped from different parts of the same gel. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (B) In human NP cells, m6A modification decreased after METTL3 silencing but increased after METTL3 overexpression. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (C–E) CCK-8 (C) and EdU assays (D–E) assessing the proliferation levels of human NP cells after transfection with METTL3 overexpression or inhibition (scale bar, 100 μm). Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (F–G) qRT-PCR analysis showed the mRNA expression levels of collagen II and MMP13 in human NP cells after METTL3 silencing or overexpression. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (H–I) Senescence-associated β-galactosidase activity in human NP cells showing senescence reversed after METTL3 silencing, while senescence accelerated after overexpression of METTL3 (scale bar, 100 μm). Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (J–M) Immunofluorescence analysis of collagen II and MMP13 expression levels (scale bar, 100 μm). Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (N) Apoptosis of human NP cells detected by flow cytometry with annexin V-FITC/PI labelling, silencing, or overexpression of METTL3 significantly affected the apoptosis of human NP cells. *p < .05, **p < .01, ***p < .001, ****p < .0001.
3.5. METTL3 bound with DGCR8 to regulate pri-miR-338-3p
Previous studies have shown that METTL3 can bind with DGCR8 to enhance the processing and maturation of pri-miRNAs [26]. Additionally, our team analysed 2000 miRNAs in human NP tissues, finding a significant increase of miR-338-3p in IDD NP tissues. miR-338-3p targets SIRT6 and crucially modulates the MAPK/ERK pathway in IDD pathogenesis [18]. In the current study, we found that in IDD NP tissues and IL-1β-treated NP cells, pre-miR-338-3p and miR-338-3p expression was increased, whereas pri-miR-338-3p expression was decreased compared to controls (Figure 5A,B). Furthermore, the expression changes of pri-miR-338-3p, pre-miR-338-3p and miR-338-3p were examined following the overexpression or inhibition of METTL3 to further confirm whether METTL3 could regulate the generation of miR-338-3p. The results showed that the expression of miR-338-3p and pre-miR-338-3p increased significantly following METTL3 overexpression, while the expression of pri-miR-338-3p decreased significantly (Figure 5C). Conversely, the expression of miR-338-3p decreased significantly after METTL3 inhibition, while the expression of pri-miR-338-3p increased significantly (Figure 5D). In addition, bioinformatic analysis identified m6A methylation sites within pri-miR-338-3p (Figure 5E). For more detailed information, please refer to Supplemental File 7: Figure S6. Furthermore, the Co-IP assay revealed that METTL3 in NP cells could bind to the DGCR8 protein. RNase treatment attenuated the interaction between METTL3 and DGCR8, suggesting that this binding may be partially mediated by miRNAs (Figure 5F). In METTL3 - knockdown NP cells, there was a marked reduction in miRNAs bound to DGCR8 compared to control cells (Figure 5G). Moreover, MeRIP analysis revealed that inhibition of METTL3 significantly decreased the amount of pri-miR-338-3p modified by m6A (Figure 5H). DGCR8 immunoprecipitation displayed a decrease in pri-miR-338-3p bound to DGCR8 in METTL3-knockdown NP cells (Figure 5I). Above all, these results reflected that METTL3-mediated m6A methylation could promote the processing and maturation of pri-miR-338-3p through DGCR8 in NP cells.
Figure 5.
METTL3 Bound with DGCR8 to regulate pri-miR-338-3p. (A,B) qRT-PCR analysis showed the expression of pri-miR-338-3p, pre-miR-338-3p and miR-338-3p in NP tissues and NP cells. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (C,D) qRT-PCR analysis showed the expression of pri-miR-338-3p, pre-miR-338-3p and miR-338-3p in NP cells after METTL3 silencing or overexpression. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (E) m6A methylation modification sites identified on pri-miR-338-3p through SRAMP website. (F) Co-IP assay indicating that METTL3 in NP cells could bind to the DGCR8 protein. The interaction between METTL3 and DGCR8 was attenuated after RNase treatment. The grouping of blots cropped from different parts of the same gel. (G) Immunoprecipitation of DGCR8, METTL3 from control cells or METTL3-knockdown NP cells. (H) MeRIP assays were conducted using anti-m6A antibodies in both METTL3-knockdown and control NP cells. Subsequent qRT-PCR analysis was performed to evaluate the binding of pri-miR-338-3p to m6A. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. (I) Immunoprecipitation of DGCR8-associated RNA from METTL3-knockdown and control NP cells was followed by qRT-PCR analysis to assess the binding of pri-miR-338-3p to DGCR8. Data are means ± SD (n = 3), and p values are from two-tailed unpaired t test. *p < .05, **p < .01, ***p < .001, ****p < .0001.
3.6. miR-338-3p act against the effects of METTL3 knockdown on NP cells
To further validate the regulation of NP cell biological functions by the METTL3/miR-338-3p axis, miR-338-3p inhibitors or mimics were co-transfected into NP cells alongside the inhibition or overexpression of METTL3 expression. CCK-8 and EdU assays demonstrated that the inhibition of miR-338-3p significantly counteracted the inhibitory effect of METTL3 overexpression on NP cell proliferation. Similarly, overexpression of miR-338-3p partially reversed the promotive effect of METTL3 inhibition on NP cell proliferation (Figure 6A–C). Inhibition of miR-338-3p significantly alleviated the senescence acceleration and nuclear enlargement caused by METTL3 overexpression in NP cells. Conversely, miR-338-3p overexpression counteracted the alleviation of NP cell senescence observed after METTL3 inhibition (Figure 6D,E). Additionally, qRT-PCR and immunofluorescence staining revealed that inhibition of miR-338-3p partially reversed the upregulation of MMP13 expression and the downregulation of collagen II expression in NP cells was mediated by METTL3 overexpression. Consistent with these findings, overexpression of miR-338-3p partially reversed the reduction in MMP13 expression and the increase in collagen II expression in NP cells caused by METTL3 inhibition (Figure 6F–K). Furthermore, inhibiting miR-338-3p effectively counteracted the stimulatory impact of METTL3 overexpression on NP cell apoptosis. Conversely, overexpression of miR-338-3p was observed to promote NP cell apoptosis following METTL3 inhibition (Figure 6L). Taken together, these results indicate that METTL3 reverses the effects of miR-338-3p in restraining cell proliferation, promoting apoptosis and senescence and inhibiting matrix synthesis.
Figure 6.
miR-338-3p counteracts the effects of METTL3 knockdown on NP cells. miR-338-3p inhibitors or mimics were co-transfected into human NP cells along with the inhibition or overexpression of METTL3 expression. (A–C) CCK-8 (A) and EdU assays (B–C) assessing the proliferation levels of human NP cells. Data are means ± SD (n = 3), and p values are from one-way ANOVA test. (D–E) Senescence-associated β-galactosidase activity in human NP cells, assessing senescence (scale bar, 100 μm). Data are means ± SD (n = 3), and p values are from one-way ANOVA test. (F–G) qRT-PCR analysis showed the mRNA expression levels of MMP13 and collagen II in human NP cells. Data are means ± SD (n = 3), and p values are from one-way ANOVA test. (H–K) Immunofluorescence analysis of collagen II and MMP13 expression levels in human NP cells (scale bar, 100 μm). Data are means ± SD (n = 3), and p values are from one-way ANOVA test. (L) Apoptosis of human NP cells detected by flow cytometry with Annexin V-FITC/PI labelling. *p < .05, **p < .01, *** p < .001, ****p < .0001.
3.7. METTL3 ameliorates the progression of IDD in rat
To investigate the influence of METTL3 on IDD in vivo, rats with injury-induced IDD were intradiscally injected with AAV9-shNC and AAV9-shMETTL3 (Figure 7A). DHI was assessed by measuring the distance between adjacent vertebral bodies on X-ray images, serving as a direct indicator of IDD. X-ray results demonstrated significant disc collapse in the IDD group compared to healthy controls, which was significantly improved with METTL3 inhibition (Figure 7B,C). DHI is inherently linked to disc water content. T2-weighted MRI imaging showed clear disc structures and high-water content in the healthy group, while the IDD group exhibited blurred disc boundaries and reduced water content. Greyscale values at disc centres indicated that the shMETTL3 group had higher disc water content than the IDD and shNC groups (Figure 7D,E). Western blot analysis revealed a significant increase in METTL3 levels in the NP tissues of IDD rats compared to healthy rats. Treatment with AAV9-shMETTL3 notably reduced METTL3 expression in the NP tissues of IDD rats. Moreover, silencing METTL3 significantly suppressed MMP13 expression and increased collagen II expression (Figure 7F–G). In addition, a colorimetric m6A quantification strategy revealed higher m6A modifications in the NP tissues of IDD rats than in the healthy group. Treatment with AAV9-shMETTL3 significantly reduced m6A modifications in the NP tissues of IDD rats (Figure 7(H)). Furthermore, qRT-PCR analysis demonstrated that METTL3 inhibition significantly reduced the expression of both pre-miR-338-3p and mature miR-338-3p in the NP tissues of IDD rats, while simultaneously increasing expression of pri-miR-338-3p (Figure 7I–K). Moreover, the gross morphology in Figure 7(L) illustrates significant improvement in the shMETTL3 group compared to the IDD and shNC groups, which almost resembles the healthy group. Histological staining with H&E and S&O indicated severe degeneration in the IDD and shNC groups, characterized by NP loss and annulus fibrosus disorganization. However, METTL3 inhibition notably reversed these changes (Figure 7L,M). The concordance between histological analysis and radiological findings further corroborates the effect of METTL3 inhibition on alleviating IDD. To confirm the impact of the METTL3/miR-338-3p axis on IDD in vivo, antagomiR-338-3p or antagomiR-NC were co-transfected into intervertebral discs along with METTL3 overexpression. X-ray results demonstrated significant disc collapse in the METTL3 + antagomiR-NC group compared to the METTL3 + antagomiR-338-3p group (Figure 7N,O). T2-weighted MRI imaging and greyscale values at the disc centres indicated that the METTL3 + antagomiR-338-3p group exhibited higher disc water content compared to the METTL3 + antagomiR-NC group (Figure 7(P,Q). Moreover, gross morphology and histological staining with H&E and S&O revealed severe degeneration in the METTL3 + antagomiR-NC group (Figure 7R,S). Inhibition of miR-338-3p significantly alleviated IDD in vivo following METTL3 overexpression. Overall, the METTL3-mediated m6A modification accelerates the production and maturation of pri-miR-338-3p, thereby promoting IDD progression (Figure 8). These results suggest that inhibiting METTL3 could attenuate IDD progression by impeding pri-miR-338-3p maturation, thus highlighting METTL3 as a potential therapeutic target for IDD.
Figure 7.
METTL3 Ameliorates the progression of IDD in rat. (A) The framework for IDD modelling and its treatments. (B,C) Representative X-rays of rat tails from the healthy (n = 10), IDD (n = 10), shNC (n = 10) and shMETTL3 (n = 10) groups, showing quantitative assessment of disc height. Data are means ± SD, and p values are from two-tailed unpaired t test. (D,E) Typical T2-weighted MRI images of rat tails from the healthy, IDD, shNC and shMETTL3 groups, with quantitative assessment of water content in NP tissues. Data are means ± SD (n = 10), and p values are from two-tailed unpaired t test. (F,G) Western blot analysis was performed to evaluate the protein expression of METTL3, MMP13 and collagen II in the NP tissues of IDD rats with or without AAV-shMETTL3 treatment. β-actin was used as the loading control. The grouping of blots cropped from different parts of the same gel. Data are presented as means and displayed in a heatmap format (n = 3), and p values are from two-tailed unpaired t test. (H) m6A modification in total RNA extracted from the NP tissues of IDD rats with or without AAV-shMETTL3 treatment. Data are presented as means and displayed in a heatmap format (n = 10), and p values are from two-tailed unpaired t test. (I–K) The expression of pri-miR-338-3p, pre-miR-338-3p and miR-338-3p in the NP tissues of IDD rats with or without AAV-shMETTL3 treatment. Data are presented as means and displayed in a heatmap format (n = 3), and p values are from two-tailed unpaired t test. (L,M) Gross morphological changes (scale bar, 1 mm) and histological evaluation of the intervertebral discs from IDD rats with or without AAV-shMETTL3 treatment, as assessed by H&E and S&O staining (scale bar, 100 μm). Data are presented as means and displayed in a heatmap format (n = 10), and p values are from two-tailed unpaired t test. (N–O) Representative X-rays of rat tails from the METTL3 + antagomiR-NC and METTL3 + antagomiR-338-3p groups, demonstrating quantitative assessment of disc height. Data are presented as means and displayed in a heatmap format (n = 10), and p values are from two-tailed unpaired t test. (P,Q) Typical T2-weighted MRI images of rat tails from the METTL3 + antagomiR-NC and METTL3 + antagomiR-338-3p groups, with quantitative assessment of water content in NP tissues. Data are presented as means and displayed in a heatmap format (n = 10), and p values are from two-tailed unpaired t test. (R,S) Gross morphological changes (scale bar, 1 mm) and histological evaluation of the intervertebral discs from rats in the METTL3 + antagomiR-NC and METTL3 + antagomiR-338-3p groups, as assessed by H&E and S&O staining (scale bar, 100 μm). Data are presented as means and displayed in a heatmap format (n = 10), and p values are from two-tailed unpaired t test. *p < .05, **p < .01, ***p < .001 and **** p < .0001 denote statistical significance compared to the shNC group. # p < .05, ## p < .01, ### p < .001 and #### p < .0001 denote statistical significance compared to the healthy control group.
Figure 8.
Model of the METTL3/pri-miR-338-3p/miR-338-3p signalling axis in IDD. Factors such as ageing, inflammation and injury contribute to the elevation of METTL3 expression. METTL3-mediated m6A methylation promotes the processing and maturation of pri-miR-338-3p via DGCR8 in NP cells, which aggravates the progression of IDD.
4. Discussion
In the current study, we identified a significant correlation between the methylation regulatory molecules METTL3 and IDD. Clinical sample studies indicated that RNA m6A levels were upregulated in IDD, with METTL3 being the primary factor in the aberrant m6A modification in IDD. Inhibiting METTL3 could attenuate IDD progression in the IDD model. Building upon our previous research [18], we discovered that METTL3 plays a critical role in accelerating pri-miR-338-3p maturation in NP cells through m6A modification. This process could promote NP cell senescence and apoptosis while inhibiting extracellular matrix synthesis. Notably, suppressing miR-338-3p significantly alleviated IDD in vivo during METTL3 overexpression.
m6A modification is a prevalent RNA modification in epigenetics and has a marked impact on the pathophysiological processes of musculoskeletal diseases, including cell senescence, proliferation and apoptosis [51–53]. Our study found a notable elevation in m6A modification in degenerated NP tissues and IL-1β-treated NP cells compared with controls, which was associated with a significant increase in METTL3 expression. This is consistent with some recent studies. Zhu et al. established an IDD mouse model and observed that m6A methylation and METTL3 expression levels were significantly increased in the IDD group [54]. An additional study confirmed a noteworthy rise in the levels of m6A methylation and METTL3 expression in degenerative human endplate cartilage [55]. Gao’s study revealed a significant increase in METTL3 expression in degenerative human NP tissues [31]. Using clinical samples and database analysis, Fang et al. identified that METTL3-mediated m6A could methylate SIAH1 mRNA and ultimately promote the pathological process of IDD [30]. Besides, another study discovered that WTAP is upregulated in ageing NP cells and interacts with METTL3 and METTL14 to enhance the formation of methyltransferase complexes involved in the ageing of NP cells and the development of IDD pathology [56]. Current research, utilizing RNA-seq and integrated scRNA-seq data and further substantiated by experiments such as qRT-PCR, western blot and immunofluorescence, has revealed that METTL3-mediated m6A modification substantially impacts IDD. To recapitulate, the alteration of METTL3-mediated m6A modification is a crucial factor in the pathological progression of IDD.
Previous studies have shown that miRNAs significantly influence the modulation of NP cell apoptosis and extracellular matrix degradation through various signalling pathways [12,13,17,57,58]. Unravelling the mechanisms that trigger miRNA overexpression in degenerative NP tissues and cells could pave the way for novel therapeutic avenues for IDD treatment. The presence of m6A marks on transcripts at pri-miRNA sequences triggers a comprehensive cotranscriptional program that involves the microprocessor machinery in the engagement and processing of pri-miRNAs [26,59]. Furthermore, m6A modification is deemed to affect various cellular processes and disease progression [60,61]. METTL3 has been identified as a key player in the methylation process of pri-miRNAs, which assists DGCR8 in recognizing and processing pri-miRNAs [26]. Our previous study established that miR-338-3p aggravated IDD progression via SIRT6/MAPK/ERK pathway based on in vivo and in vitro studies [18]. In this study, we identified that in IDD tissues and IL-1β-treated NP cells, pre-miR-338-3p and miR-338-3p expression was increased, while pri-miR-338-3p expression was decreased compared to controls. We subsequently assessed the expression of pri-miR-338-3p, pre-miR-338-3p and mature miR-338-3p in NP cells with METTL3 knockdown or overexpression. As expected, METTL3 knockdown significantly increased pri-miR-338-3p levels while decreasing the expression of pre-miR-338-3p and mature miR-338-3p. Conversely, METTL3 overexpression induced the opposite effects. Furthermore, Co-IP assay indicated that METTL3 could bind to DGCR8 in NP cells. Significant reductions in miRNAs bound to DGCR8 were also observed in METTL3-knockdown NP cells compared to controls. Moreover, MeRIP analysis revealed that inhibition of METTL3 significantly decreased the amount of pri-miR-338-3p modified by m6A. DGCR8 immunoprecipitation further showed a reduced level of pri-miR-338-3p bound to DGCR8 in METTL3 - knockdown NP cells. Besides, in NP cells with METTL3 knockdown or overexpression, transfection of miR-338-3p mimics or inhibitors, respectively, revealed that modulating miR-338-3p could rescue the proliferative effects induced by METTL3 manipulation. This study implied that METTL3-mediated m6A modification could accelerate the processing and maturation of pri-miR-338-3p through DGCR8 in IDD. The METTL3-m6A-miR-338-3p axis was identified as a critical mediator in alleviating IDD upon METTL3 inhibition, offering novel insights into potential therapeutic strategies for this condition. The expression levels of METTL3 and miR-338-3p may serve as potential diagnostic and prognostic biomarkers for IDD. Future research could prioritize biomaterial-based delivery systems targeting the METTL3-m6A-miR-338-3p axis to provide therapeutic protection and achieve sustained, site-directed treatment.
This study has several limitations. First, some results are derived from the comprehensive scRNA-seq and RNA-seq data. The relatively small sample sizes in high-throughput sequencing might introduce data bias necessitating larger sample sizes or further clinical sample analysis. Second, the METTL3-m6A-miR-338-3p signalling axis mechanisms in IDD procession demand more detailed elucidation. Third, as this study is based on in human NP cells and rat IDD models, its generalizability to human patients still requires further validation in large mammalian models and preclinical studies. Fourth, given METTL3’s role in global RNA methylation, its inhibition may affect biological processes beyond miR-338-3p regulation. Thus, comprehensive off-target assessments and tissue-specific delivery systems are imperative prior to clinical translation.
5. Conclusion
METTL3-mediated m6A modification crucially accelerates the production and maturation of pri-miR-338-3p, which could promote IDD progression. METTL3 inhibition attenuates IDD progression by modulating the METTL3-m6A-miR-338-3p regulatory axis, demonstrating its therapeutic potential for IDD treatment. Future studies should focus on developing biomaterial delivery systems for METTL3 inhibitors to ensure both therapeutic protection and sustained, site-specific drug release.
Supplementary Material
Funding Statement
This work was supported by National Natural Science Foundation of China (Grant No.82360438), Guangxi Natural Science Foundation (Grant No.2023GXNSFAA026339/2021GXNSFAA075007) and Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (Grant No.2024GXNSFDA010043).
Ethical approval and consent form
This study was approved by the Research Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Approval No. 2023-S297-01; Date: 27 February 2023). The clinical sample study was conducted in full compliance with the Declaration of Helsinki. The animal experiments in this study were approved by the Medical Laboratory Animal Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Approval No. 2023-D043-01; 2 March 2023) and were conducted and reported in accordance with the ARRIVE guidelines. Publicly available de-identified datasets (GEO accession numbers: GSE167199, GSE165722) were analysed in compliance with GEO database policies and the Declaration of Helsinki.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chou R. Low back pain. Ann Intern Med. 2021;174(8):ITC113–ITC128. doi: 10.7326/AITC202108170. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Isa IL, Teoh SL, Mohd Nor NH, et al. Discogenic low back pain: anatomy, pathophysiology and treatments of intervertebral disc degeneration. Int J Mol Sci. 2022;24(1):208. doi: 10.3390/ijms24010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staszkiewicz R, Gładysz D, Gralewski M, et al. Pathomechanism of the IVDs degeneration and the role of neurotrophic factors and concentration of selected elements in genesis of low back pain. Curr Pharm Biotechnol. 2023;24(9):1164–1177. doi: 10.2174/1389201024666221021142904. [DOI] [PubMed] [Google Scholar]
- 4.Knezevic NN, Candido KD, Vlaeyen JWS, et al. Low back pain. Lancet. 2021;398(10294):78–92. doi: 10.1016/s0140-6736(21)00733-9. [DOI] [PubMed] [Google Scholar]
- 5.Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52. doi: 10.1038/s41572-018-0052-1. [DOI] [PubMed] [Google Scholar]
- 6.Xin J, Wang Y, Zheng Z, et al. Treatment of intervertebral disc degeneration. Orthop Surg. 2022;14(7):1271–1280. doi: 10.1111/os.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin DB, Tavakoli J, Freeman BJC, et al. Mechanisms of failure following simulated repetitive lifting: a clinically relevant biomechanical cadaveric study. Spine. 2020;45(6):357–367. doi: 10.1097/BRS.0000000000003270. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Cai F, Shi R, et al. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr Cartil. 2016;24(3):398–408. doi: 10.1016/j.joca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Kirnaz S, Capadona C, Wong T, et al. Fundamentals of intervertebral disc degeneration. World Neurosurg. 2022;157:264–273. doi: 10.1016/j.wneu.2021.09.066. [DOI] [PubMed] [Google Scholar]
- 10.Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci. 2017;1410(1):68–84. doi: 10.1111/nyas.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol J, Loedige I, Filipowicz W.. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 12.Ji ML, Lu J, Shi PL, et al. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signaling pathway in human intervertebral disc degeneration. J Bone Miner Res. 2016;31(4):900–909. doi: 10.1002/jbmr.2753. [DOI] [PubMed] [Google Scholar]
- 13.Ji ML, Zhang XJ, Shi PL, et al. Downregulation of microRNA-193a-3p is involved in invertebral disc degeneration by targeting MMP14. J Mol Med. 2016;94(4):457–468. doi: 10.1007/s00109-015-1371-2. [DOI] [PubMed] [Google Scholar]
- 14.Divi SN, Markova DZ, Fang T, et al. Circulating miR-155-5p as a novel biomarker of lumbar degenerative disc disease. Spine. 2020;45(9):E499–E507. doi: 10.1097/brs.0000000000003322. [DOI] [PubMed] [Google Scholar]
- 15.Wang HQ, Yu XD, Liu ZH, et al. Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J Pathol. 2011;225(2):232–242. doi: 10.1002/path.2931. [DOI] [PubMed] [Google Scholar]
- 16.Dong W, Liu J, Lv Y, et al. miR-640 aggravates intervertebral disc degeneration via NF-κB and WNT signalling pathway. Cell Prolif. 2019;52(5):e12664. doi: 10.1111/cpr.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng D, Chen W, Pan C, et al. Exploration of microRNA-106b-5p as a therapeutic target in intervertebral disc degeneration: a preclinical study. Apoptosis. 2023;28(1-2):199–209. doi: 10.1007/s10495-022-01773-6. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Moro A, Wang J, et al. MicroRNA-338-3p as a novel therapeutic target for intervertebral disc degeneration. Exp Mol Med. 2021;53(9):1356–1365. doi: 10.1038/s12276-021-00662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu WW, Zheng SQ, Li T, et al. RNA modifications in cellular metabolism: implications for metabolism-targeted therapy and immunotherapy. Signal Transduct Target Ther. 2024;9(1):70. doi: 10.1038/s41392-024-01777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiener D, Schwartz S.. The epitranscriptome beyond m(6)A. Nat Rev Genet. 2021;22(2):119–131. doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Choe J, Park OH, et al. Molecular mechanisms driving mRNA degradation by m(6)A modification. Trends Genet. 2020;36(3):177–188. doi: 10.1016/j.tig.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Ling S, Feng H, et al. Recent advances in the mutual regulation of m6A modification and non-coding RNAs in atherosclerosis. Int J Gen Med. 2025;18:1047–1073. doi: 10.2147/ijgm.S508197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebert LFR, MacRae IJ.. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alarcón CR, Goodarzi H, Lee H, et al. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alarcón CR, Lee H, Goodarzi H, et al. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, Kong H, Wang X, et al. Novel insights into the interaction between N6-methyladenosine methylation and noncoding RNAs in musculoskeletal disorders. Cell Prolif. 2022;55(10):e13294. doi: 10.1111/cpr.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, He X, Lu X, et al. METTL3 acetylation impedes cancer metastasis via fine-tuning its nuclear and cytosolic functions. Nat Commun. 2022;13(1):6350. doi: 10.1038/s41467-022-34209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TB, Miramontes R, Chillon-Marinas C, et al. Aberrant splicing in Huntington’s disease accompanies disrupted TDP-43 activity and altered m6A RNA modification. Nat Neurosci. 2025;28(2):280–292. doi: 10.1038/s41593-024-01850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang S, Zeng F, Chen R, et al. SIAH1 promotes senescence and apoptosis of nucleus pulposus cells to exacerbate disc degeneration through ubiquitinating XIAP. Tissue Cell. 2022;76:101820. doi: 10.1016/j.tice.2022.101820. [DOI] [PubMed] [Google Scholar]
- 31.Gao D, Hu B, Ding B, et al. N6-methyladenosine-induced miR-143-3p promotes intervertebral disc degeneration by regulating SOX5. Bone. 2022;163:116503. doi: 10.1016/j.bone.2022.116503. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Sun Y, He M, et al. Differentially-expressed mRNAs, microRNAs and long noncoding RNAs in intervertebral disc degeneration identified by RNA-sequencing. Bioengineered. 2021;12(1):1026–1039. doi: 10.1080/21655979.2021.1899533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47–e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GeneCards – the human gene database; [accessed 2023 Jun 5]. Available from: https://www.genecards.org/
- 35.Li L, Liu Z.. SRF facilitates transcriptional inhibition of gem expression by m6A methyltransferase METTL3 to suppress neuronal damage in epilepsy. Mol Neurobiol. 2025;62(3):2903–2925. doi: 10.1007/s12035-024-04396-x. [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Wang H, Yang X, et al. Network pharmacology analysis reveals potential targets and mechanisms of proton pump inhibitors in breast cancer with diabetes. Sci Rep. 2023;13(1):7623. doi: 10.1038/s41598-023-34524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S.. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gene Ontology Consortium . Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:d 1049–d 1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest; [accessed 2023 Jun 20]. Available from: https://cn.string-db.org/ [DOI] [PMC free article] [PubMed]
- 41.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The National Center for Biotechnology Information Gene database ; [accessed 2023 Jun 26]. Available from: https://www.ncbi.nlm.nih.gov/gene/
- 43.Hao Y, Stuart T, Kowalski MH, et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol. 2024;42(2):293–304. doi: 10.1038/s41587-023-01767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karaarslan N, Yilmaz I, Ozbek H, et al. Are specific gene expressions of extracellular matrix and nucleus pulposus affected by primary cell cultures prepared from intact or degenerative intervertebral disc tissues? Turk Neurosurg. 2019;29(1):43–52. doi: 10.5137/1019-5149.Jtn.22210-17.2. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Che M, Xin J, et al. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 46.SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features; [accessed 2024 Jun 16]. Available from: http://www.cuilab.cn/sramp/ [DOI] [PMC free article] [PubMed]
- 47.Wei Q, Liu D, Chu G, et al. TGF-β1-supplemented decellularized annulus fibrosus matrix hydrogels promote annulus fibrosus repair. Bioact Mater. 2023;19:581–593. doi: 10.1016/j.bioactmat.2022.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Zheng D, Chen H, et al. Circadian clock regulation via biomaterials for nucleus pulposus. Adv Mater. 2023;35(32):e2301037. doi: 10.1002/adma.202301037. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Cui W, Kalala JP, et al. To investigate the effect of osteoporosis and intervertebral disc degeneration on the endplate cartilage injury in rats. Asian Pac J Trop Med. 2014;7(10):796–800. doi: 10.1016/s1995-7645(14)60139-5. [DOI] [PubMed] [Google Scholar]
- 50.Tu J, Li W, Yang S, et al. Single-cell transcriptome profiling reveals multicellular ecosystem of nucleus pulposus during degeneration progression. Adv Sci. 2022;9(3):e2103631. doi: 10.1002/advs.202103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Gong W, Shao X, et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis. 2022;81(1):87–99. doi: 10.1136/annrheumdis-2021-221091. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Li M, Jiang L, et al. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019;516(1):22–27. doi: 10.1016/j.bbrc.2019.05.168. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Chen M, Ma L, et al. piRNA-36741 regulates BMP2-mediated osteoblast differentiation via METTL3 controlled m6A modification. Aging. 2021;13(19):23361–23375. doi: 10.18632/aging.203630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu B, Chen HX, Li S, et al. Comprehensive analysis of N6-methyladenosine (m(6)A) modification during the degeneration of lumbar intervertebral disc in mice. J Orthop Translat. 2021;31:126–138. doi: 10.1016/j.jot.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao L, Hu B, Ding B, et al. N(6)-methyladenosine RNA methyltransferase like 3 inhibits extracellular matrix synthesis of endplate chondrocytes by downregulating sex-determining region Y-Box transcription factor 9 expression under tension. Osteoarthr Cartil. 2022;30(4):613–625. doi: 10.1016/j.joca.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Li G, Ma L, He S, et al. WTAP-mediated m(6)A modification of lncRNA NORAD promotes intervertebral disc degeneration. Nat Commun. 2022;13(1):1469. doi: 10.1038/s41467-022-28990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji ML, Jiang H, Zhang XJ, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. doi: 10.1038/s41467-018-07360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng X, Zhang L, Zhang K, et al. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis. 2018;77(5):770–779. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Deng Q, Lv Z, et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18(1):181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 61.Li XC, Jin F, Wang BY, et al. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics. 2019;9(13):3853–3865. doi: 10.7150/thno.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.