Abstract
Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), marked by persistent symptoms lasting weeks to months after acute SARS-CoV-2 infection, affects multiple organ systems including the respiratory, cardiovascular, neurological, gastrointestinal, and renal systems. These prolonged effects stem from chronic inflammation, immune dysregulation, and direct viral injury. MicroRNAs (miRNAs)—small non-coding RNAs involved in gene regulation—play a pivotal role in this process by modulating immune responses, inflammation, and cellular stress. Altered miRNA expression patterns during and after infection contribute to the pathogenesis of Long COVID. While conventional miRNA detection techniques have been valuable, they face limitations in sensitivity, throughput, and detecting RNA modifications. This review highlights Oxford Nanopore Sequencing (ONS) as a promising alternative, offering real-time, long-read, amplification-free RNA sequencing that preserves native modifications. ONS enables direct sequencing of full-length miRNAs and their precursors, providing novel insights into miRNA processing and regulatory roles. Despite current challenges with short-read accuracy, ongoing technical advances are improving ONS performance. Its integration in miRNA profiling holds significant potential for uncovering novel regulatory interactions and advancing clinical biomarker discovery in Long COVID and other conditions.
Keywords: Immune dysregulation, Noncoding RNA, Nanopore sequencing, Inflammation, Biomarker discovery
Introduction
Gene expression in higher organisms is subject to a multitude of regulating factors, from genetic heritage to epigenetic alterations or protein modifications. In this myriads of factors, microRNAs (miRNA) appear to play an extensive role, as outlined in an increasing amount of literature. MiRNA are small non-coding RNAs in length of up to 24 nucleotides with the main role in post-transcriptional gene expression regulation, binding to a complementary sequence in messenger RNA (mRNA). MiRNA were first discovered in the nematode Caenorhabditis elegans, where the LIN-14 protein is downregulated in the progression from the first to the second larval stage and appears lin-4 that give rise to two small RNAs of 21–61 nucleotides in length. Later, another small RNA, let-7, was discovered in the later larval stage in C. elegans and homologues of this gene in many other organisms, including humans [1].
MiRNA are found at cellular level, but some of them can be exported through different mechanisms from the cell. Biosynthesis begins in the nucleus with the transcriptions of miRNA by ARN polymerase II and III and continue with the processing of pri-miRNA and pre-miRNA by Drosha and Dicer enzymes, respectively, until obtaining mature miRNA. At the cytoplasmatic level, mature miRNA can act as post-transcriptional regulators with Argonaute proteins (Ago2) and induced silencing complex (RISC) [2]. Once reaching the extracellular environment, miRNA have high stability and persist in high level, even after cell death. This unusual stability despite the existence of RNases and the possibility of detection in human fluids make miRNAs potential biomarkers for some pathologies detection, prediction of evolution of the disease, such as cancer diagnosis [3], brain and liver injury detection [4, 5], and evaluation of some cardiovascular diseases, namely cardiac fibrosis, hypertrophy, angiogenesis, and heart failure [6]. Also, miRNAs have been identified as both diagnostic markers but also as treatment avenues for respiratory diseases. For example, miR-29 and miR-200 decrease and miR-155 increases in pulmonary fibrosis [7–9], mir-17–92 plays role in lung development, overexpression being correlated with the proliferation of epithelial cells, and in lung cancer, being an oncogene [10], and miR-15b was reported as increased in chronic obstructive pulmonary disease [11]. MiR-146a, dysregulated in chronic obstructive pulmonary disease, has in important role in pathogenesis through controlling the abnormal immune response. MiR-146 may have a therapeutic role, because it targets cyclooxygenase-2 (COX-2), causing downregulation of production of prostaglandin E2, an inflammatory mediator, and nuclear factor kappa B (NF-kB), repressing NF-kB transducers interleukin-1 receptor-associated kinase and TNF receptor-associated factor 6 [10]. Some factors are considered when using miRNAs as biomarkers, for example age, sex and smoking, among them aging having a significant impact on the miRNA level [12, 13]. Physical exercises have a limited short-effect on the miRNAs blood levels, but training impacts the miRNA signature [14, 15]. At the same time, miRNAs are key players in the epigenetic regulatory network. These small non-coding RNAs modulate gene expression post-transcriptionally, influencing various biological processes and immune responses. As epigenetic modulators, miRNAs regulate gene expression without altering the DNA sequence. They are regulated by mechanisms such as DNA methylation and histone modification. The interaction between miRNAs and the epigenetic pathway forms a feedback loop that is crucial for maintaining gene expression balance. Disruptions in this loop can lead to pathological conditions [16].
While miRNAs are crucial in the regulation of gene expression in COVID-19 and long COVID, their role is part of a broader epigenetic regulatory network that includes other factors such as DNA methylation and histone modification. The interplay between these elements contributes to the complexity of the disease and its varied clinical manifestations. Understanding the full scope of miRNA interactions and their regulatory mechanisms could lead to more effective therapeutic strategies and improve our ability to predict disease outcomes.
Regarding the diagnostic rate of long COVID, estimates range from 10 to 30% of those infected with SARS-CoV-2 experiencing persistent symptoms [17]. The complexity of Long COVID, including its wide range of symptoms and lack of standardized diagnostic criteria, contributes to the underdiagnosis and underreporting of the condition globally. The prevalence of Long COVID varies significantly across regions, with estimates of 31% in North America, 44% in Europe, and 51% in Asia [18] and a higher prevalence of Long COVID in high-income countries (HICs) compared to low- and middle-income countries (LMICs), with 69% of individuals in HICs reporting Long COVID symptoms compared to 45.3% in LMICs [19]. The lack of standardized diagnostic criteria and biomarkers complicates the clinical recognition and diagnosis of Long COVID, leading to potential underdiagnosis [17]. Thus, the persistent nature of Long COVID symptoms poses a significant burden on healthcare systems, necessitating multidisciplinary care approaches [20] and the absence of a global consensus on the definition and diagnostic approaches further poses the challenge of accurately diagnosing and managing the condition [21].
This review synthesizes current evidence on the potential of ONS for the detection and profiling of microRNAs with particular emphasis on their involvement in Long COVID. It integrated recent findings into miRNA dysregulation across multiple organ systems affected by post-acute COVID-19 syndrome and critically evaluates ONS as a next-generation sequencing platform capable of addressing key limitations of conventional miRNA detection methods. The review’s novelty lies in highlighting the intersection between third-generation sequencing technology and the growing need for accurate, multiplexed biomarker detection in complex multisystemic conditions such as Long COVID.
MicroRNAs in COVID-19
Dysregulation of miRNAs level appears as a result of a disorder in the organism. Similar to other pathologies, COVID-19 is associated with abnormal synthesis or secretion of miRNA in blood or cell. During the SARS-CoV-2 infection, the virus acts on different levels, and the anti-inflammatory response of the organisms depends on the stage of infection. Moreover, in severe cases, the cytokines storm, including interleukin-6 (IL-6), IL-2 and IL-7, damages organs, with long-term effects [22]. When the cells perceive changes due to SARS-CoV-2 infection, they respond by triggering signalling events dominated by proteins, that are controlled by specific miRNA. The level of specific miRNAs changes due to the bind with SARS-CoV-2, affecting infection or viral replication, but also to the viral genome that adsorb host functional miRNAs. Similarly, viral miRNAs interact with own genome leading to viral replication and infection affection, or bind host miRNAs and genes, repressing their expression and triggering intracellular signalling pathways [22].
The assessment of the changes of microRNA expression in COVID-19 indicate that some miRNAs are biomarkers for the presence of the virus in organism, with altered levels in COVID-19 cases compared to healthy controls [23]. Beside this, dysregulation of miRNA levels exhibit potential for screening, up- or downregulation being associated with severity degree or mortality. Srivastava et al. [24]showed microRNAs with a distinct signature pattern in dead, severe and moderate COVID-19 patients, of which 7 upregulated and 24 downregulated. Moreover, miRNAs level, such as hsa-miR-32-5p and hsa-miR-1246, can distinguish between critically ill patients and asymptomatic cases, especially when are correlated with interfering factors such as obesity [25].
Common MicroRNA profiles of COVID-19 patients, as opposed to healthy controls, analysed through different techniques in samples such as plasma, serum or cells, have shown different levels of regulation (Table 1).
Table 1.
Regulation of specific miRNAs in COVID-19 and associated detection techniques
| microRNA | Sample | Detection method | Instrument model/analysis kit | Regulation | References |
|---|---|---|---|---|---|
| miR-27a-3p | Plasma | RT-PCR | QuantStudio 7 Flex Real-Time PCR System (Applied Biosystem)/ miRCURY LNA miRNA Custom Panels (Qiagen) | Up | [26] |
| miR-27b-3p | Up | ||||
| miR-148a-3p | Up | ||||
| miR-199a-5p | Up | ||||
| miR-491-5p | Up | ||||
| miR-16-5p | Down | ||||
| miR-92a-3p | Down | ||||
| miR-150-5p | Down | ||||
| miR-451a | Down | ||||
| miR-486-5p | Down | ||||
| let-7i-5p | Peripheral blood mononuclear cells | RT-PCR | Rotor-Gene Real-Time PCR (Qiagen)/ miScript SYBR Green PCR Kit (Qiagen) | Up | [27] |
| miR-29a-3p | Up | ||||
| miR-146a-3p | Up | ||||
| miR-155 | Plasma | RT-PCR | Max3005P QPCR system; Stratagene, (Agilent Biotechnology)/ MiScript SYBR Green PCR kit (Qiagen) | Up | [28] |
| miR-19b-3p | Plasma | RT-PCR | N/A/miRCURY LNA miRNA miRNome qPCR Panel I (Qiagen) | Up | [29] |
| miR-19a-3p | Up | ||||
| miR-92a-3p | Up | ||||
| miR-15a-5p | Up | ||||
| miR-23a-3p | Up | ||||
| miR-320a | Up | ||||
| miR-17-5p | Down | ||||
| miR-142-5p | Down | ||||
| miR-200c-3p | Saliva | RT-PCR | ABI 7500 Fast Real-Time PCR System (Applied Biosystems)/ HOT FIREPol Probe Universal qPCR Mix (Solis BioDyne) | Up | [30] |
| miR-21-5p | Plasma | RT-PCR | StepOne Real-Time PCR System (Thermo Scientific)/ TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems) | Up | [31] |
| miR-145-5p | Peripheral venous blood | Microarray | GCS 3000 scanner(Affymetrix)/ GeneChip miRNA 4.0 array (Affymetrix) | Up | [32] |
| miR-6780b-3p | Plasma | Sequencing | Illumina HiSeq 2500 platform/ QIAseq miRNA Library Kit (Qiagen) | Up | [33] |
| miR-6883-3p | Up | ||||
| miR-4769-5p | Up | ||||
| miR-6873-3p | Up | ||||
| miR-320b | Up | ||||
| miR-7111-3p | Up | ||||
| miR-4755-3p | Up | ||||
| miR-320c | Up | ||||
| miR-6511a-3p | Up | ||||
| miR-320d | Up | ||||
| miR-5187-3p | Up | ||||
| miR-4508 | Up | ||||
| miR-4659a-5p | Up | ||||
| miR-4433b-5p | Down | ||||
| miR-16-2-3p | Down | ||||
| miR-126-3p | Down | ||||
| miR-150-5p | Down | ||||
| miR-224-5p | Down | ||||
| miR-16-2-3p | Peripheral blood | Sequencing | Illumina HiseqX Ten/ N/A | Up | [23] |
| miR-6501-5p | Up | ||||
| miR-618 | Up | ||||
| miR-183-5p | Down | ||||
| miR-627-5p | Down | ||||
| miR-144-3p | Down | ||||
| miR-98-3p | Serum | RT-PCR | CFX Connect PCR System (Bio-Rad) /miRCURY LNA miRNA Serum/Plasma Focus PCR Panels (QIAGEN) | Up | [34] |
| miR-423-3p | Up | ||||
| miR-1246 | Up | ||||
| miR-146-5p | Erythrocytes | Sequencing | Illumina Hiseq 4000 | Down | [33] |
| miR-21-5p | Down | ||||
| miR-142-3p | Down | ||||
| miR-3605-3p | Up | ||||
| miR-146a-5p | Serum | Sequencing | NextSeq 550 System (Illumina)/ NextSeq 500/550 High Output Kit (Illumina) | Down | [35] |
| miR-195-5p | Plasma | RT-PCR |
N/A /Taqman microRNA assays (Applied Biosystems) |
Down | [36] |
| let-7b-5p | Peripheral blood mononuclear cells | Sequencing |
Illumina Hiseq 2500/NEBNext Multiplex Small RNA Library Prep Set for Illumina (NEB) |
Down | [37] |
| miR-103a-2-5p | Down | ||||
| miR-200c-3p | Down | ||||
| miR-2115-3p | Down |
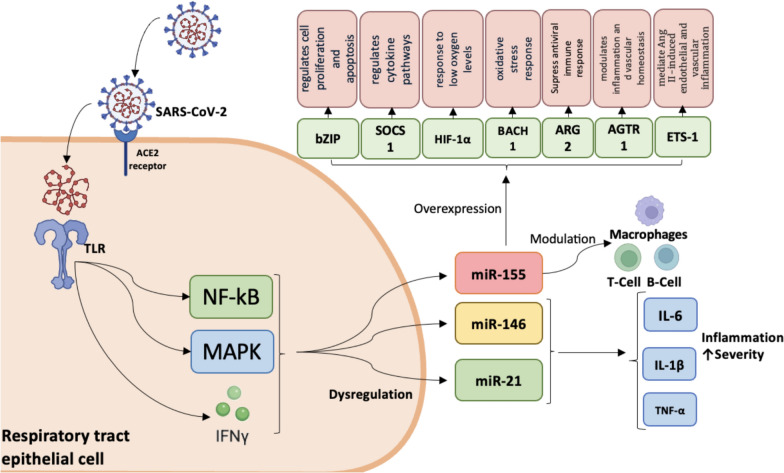
Dysregulation of miRNA levels in COVID-19 is associated with factors that appear in infection. For example, miRNAs can regulate approximatively 8000 genes, each miRNA supressing multiple genes and also a single mRNA being targeted by multiple miRNAs [38]. MiRNAs in SARS-CoV-2 infection exhibit significant differential expression, with some miRNAs being upregulated and others downregulated. Their levels change from the first line of defence of the body against infection, when the epithelial cells of the upper respiratory tract recognize the virus through the Toll-like receptors (TLR). TLR signalling leads to the activation of the transcription NF-kB, mitogen-activated protein kinases (MAPK), and members of the regulation of interferon, all of these being associated with dysregulation of some miRNAs. Three miRNAs, miR-155, miR-146a and miR-21, were reported to be induced following TLR activation [39], with their dysregulated expression also observed in COVID-19 cases (Table 1) (Fig. 1).
Fig. 1.
Main signalling pathways in COVID-19 immunological response with participation of miRNAs after SARS-CoV-2’s binding by angiotensin-converting enzyme 2 (ACE2). TLR detect pathogen-associated molecular patterns and initiate inflammatory cytokines production via miRNAs; alternatively, TLR may directly bind SARS-CoV-2 spike protein. Abbreviations: ACE2, Angiotensin-converting enzyme 2; TLR, Toll like receptor; NF-kB, nuclear factor-kappa B; MAPK, mitogen-activated protein kinases; IFN γ, Interferon gamma; IL, interleukin; TNF-α, Tumor Necrosis Factor-alpha; IL-1β, Interleukin 1β, IL-6, Interleukin 6; BACH1, BTB and CNC homology 1; bZIP, basic leucine zipper transcription factor 1; SOCS1, suppressor of cytokine signalling 1; HIF-1α, hypoxia-inducible factor 1-alpha; ARG2, Arginase 2; ETS-1, Transformation-specific Sequence 1 factor; AGTR1, angiotensin II receptor type 1
MiR-155 also modulates the activity of immune cells such as macrophages, T cells, and B cells, thereby influencing the effectiveness of the antiviral response. Their overexpression is due to the targeting genes associated with host response against SARS-CoV-2, such as BTB and CNC homology 1 (BACH1), basic leucine zipper transcription factor 1 (bZIP), suppressor of cytokine signalling 1 (SOCS1), hypoxia-inducible factor 1-alpha (HIF-1α), Arginase-2 (ARG2), E26 Transformation-specific Sequence 1 (ETS-1) factor, and angiotensin II receptor type 1 (AGTR1) that encodes angiotensin type 1 receptor (AT1R) [40]. Overexpression of miR-155 suppresses the hypertrophic effects induced by angiotensin II by silencing AGTR1 and subsequently inhibiting downstream calcium signalling pathways involving calcineurin and nuclear factor of activated T-cells (NFAT-4), suggesting that miR-155 attenuates angiotensin II-induced cardiac hypertrophy mainly through direct repression of AGTR1 [41]. MiR-146a and miR-21 also have role in severe forms, when the immune response is deregulated and inflammation occurs with overproduction of proinflammatory cytokines. Their altered levels during infection lead to the excessive production of IL-6, IL-1β and tumor necrosis factor α (TNF-α), contributing to the severity of the disease [42].
In addition to the effect on the immune response, miRNAs target the SARS-CoV-2 genome and inhibit virus replication. MiR-29 family have 11 binding sites and miR-21 has 4 binding sites on the virus genome [43]. Another mechanism of deregulation of microRNA expression is related to the ability of SARS-CoV-2 virus to manipulate the host miRNAs expression, for example miR-2392, facilitating its own replication [44]. Moreover, miRNAs target ACE2 receptor and modulate and play significant role in their regulation. The overexpression of miR-200c (Table 1) in COVID-19 is due to their ability to inhibit both ACE2 mRNA and ACE2 protein level in cardiomyocytes [45]. Due to their ability to influence multiple aspects of the immune response and viral replication, miRNAs can be a promising area in the research and development of therapeutics for COVID-19. Approaches aimed at inhibiting viral replication, modulating the inflammatory response, stimulating antiviral immunity, preventing cardiovascular complications, and using miRNAs in diagnosis and vaccine development can significantly contribute to the management and treatment of COVID-19. SARS-CoV-2 attaches to the cell membrane and the spike protein is cleaved by the transmembrane serine protease 2 (TMPRSS2). Next, the virus utilizes ACE2 for host cell entry. Targeting the miRNA involved in TMPRSS2 or ACE2 expression leads to the inhibition of virus entry in host cell. MiR-98-5p directly targets the three prime untranslated region (3′UTR) of TMPRSS2 and regulated their expression in endothelial cells [46]. Other 29 miRNAs were reported having potential binding against TMPRSS2 gene and 31 miRNAs, for example miR-106b-5p, miR-130a-3p, miR-141-3p, miR-200 family and others, have strong binding potential against ACE2, revealing a potential target for therapeutic strategy for SARS-CoV-2 infection [47]. Beyond viral entry, the regulation of miRNAs involved in the host inflammatory response may play a critical role in mitigating disease severity. MiR-21, in particular, is known to regulate inflammation and cardiovascular function. Therapeutic inhibition of miR-21 (e.g., using anti-miR-21 strategies) may reduce COVID-19 severity by preventing the cytokine storm and associated cardiovascular complications. For example, Tocilizumab, an IL-6 receptor antagonist, has been shown to reduce miR-21 expression, offering cardioprotective effects in patients with early-diagnosed COVID-19-related myocarditis.
MicroRNAs and long COVID
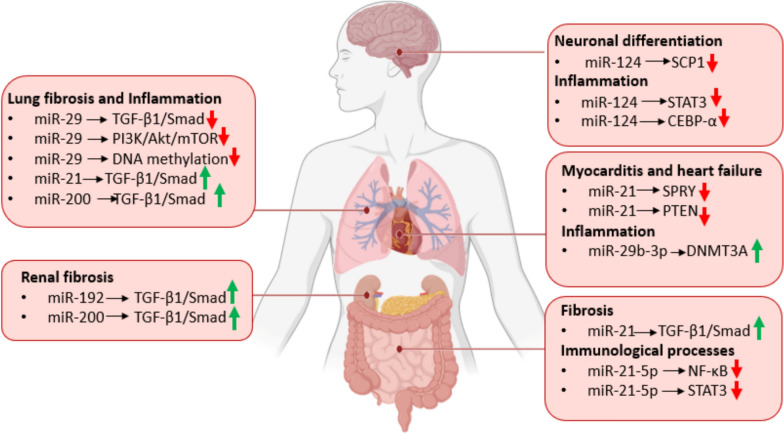
Multiple studies have documented a wide range of persistent symptoms following acute SARS-CoV-2 infection, a condition now formally recognized as Long COVID. Long COVID affect the full spectrum of COVID-19 patients, from those with mild to the most severe forms. Similar to the acute phase, Long COVID involve multiple organs and systems including the respiratory, cardiovascular, neurological, gastrointestinal, and musculoskeletal systems [48]. Recent research has identified distinct circulating miRNA profiles associated with both short-term SARS-CoV-2 infection and the long-term cognitive and systemic impairments observed in Long COVID. Altered miRNA expression in Long COVID is linked to persistent inflammation, central nervous system dysfunction, and other chronic complications such as cardiovascular abnormalities and pulmonary fibrosis (Fig. 2). Many of the miRNAs identified as dysregulated in Long COVID (Table 2) are also altered during the acute phase of COVID-19 (Table 1), which shows that altered miRNAs levels in Long COVID are closely related to inflammation, a hallmark of Long COVID.
Fig. 2.
Specific miRNAs involvement in Long COVID occurrence and development in (i) the brain, where miR-124 promotes neuronal differentiation by repressing SCP1 and limits neuroinflammation via Signal Transducer and Activator of Transcription 3 (STAT3) and C/EBP-α; (ii) the lung, where miR-29 downregulates TGF-β1/Smad, PI3K/Akt/mTOR, and DNA methylation pathways, thus exerting anti-fibrotic and anti-inflammatory effects; miR-21 and miR-200 upregulate TGF-β1/Smad signaling, promoting pulmonary fibrosis and chronic inflammation; (iii) the heart, where upregulated miR-21 inhibits SPRY and PTEN and increased miR-29b-3p upregulates DNA Methyltransferase 3 Alpha (DNMT3A), collectively exacerbating myocarditis and heart failure; (iv) the kidney, where miR-192 and miR-200 upregulate TGF-β/Smad promoting renal fibrosis; and (v) the intestine, where miR-21 drives fibrotic extracellular-matrix production, miR-21-5p downregulate NF-κB/STAT3 to orchestrate immune responses. Abbreviations: TGF-β, Transforming Growth Factor Beta; Smad, Intracellular proteins mediating TGF-β signaling; PI3K, Phosphoinositide 3-Kinase; Akt, Protein Kinase B; mTOR, Mechanistic Target of Rapamycin; STAT3, Signal Transducer and Activator of Transcription 3; NF-κB, Nuclear Factor kappa-light-chain-enhancer of Activated B cells; CEBP-α, CCAAT/Enhancer-Binding Protein Alpha; SCP1, Small C-terminal domain Phosphatase 1; SPRY, Sprouty proteins (receptor tyrosine kinase signaling inhibitors); PTEN, Phosphatase and Tensin Homolog; DNMT3A, DNA Methyltransferase 3 Alpha
Table 2.
Regulation patterns of specific miRNAs in Long COVID
| microRNA | Sample type | Regulation | References |
|---|---|---|---|
| miR-29a-3p | Peripheral blood mononuclear cells | up | [27] |
| miR-146a-3p | up | ||
| let-7b-3p | up | ||
| miR-9-5p | Plasma | up | [49] |
| miR-486-5p | up | ||
| miR-122-5p | up | ||
| miR-199a-5p | up | ||
| miR-214-3p | up | ||
| miR-146a-5p | Plasma | up | [50] |
| miR-17-5p | Plasma | down | [51] |
| miR-223-3p | Exhaled breath condensate | down | [52] |
| miR-146a-5p | up | ||
| miR-126-3p | down | ||
| miR-221-3p | Plasma | down | [53] |
| miR-16-5p | down | ||
| miR-9-5p | down | ||
| miR-34a | Plasma | up | [54] |
MiR-146a, overexpressed in both COVID-19 and Long COVID [27, 52], regulates inflammatory responses by targeting mRNAs encoding interleukin-1 Receptor-Associated Kinase 1 (IRAK-1) and tumor necrosis factor receptor-associated factor 6 (TRAF6), preventing macrophage hyperactivation and systemic inflammation. A deficiency in miR-146a can lead to an exaggerated response to endotoxins and predispose individuals to autoimmune conditions. In vivo studies have shown that miR-146a is crucial for preventing sepsis-induced NF-kB signalling and cardiac dysfunction by inhibiting IRAK and TRAF6 expression. Additionally, miR-146a maintains the integrity of tight junctions and suppresses the synthesis of proinflammatory cytokines such as IL-8, chemokine (C–C motif) ligand 20 (CCL20), and TNF-α in response to TLR stimulation [55]. This regulatory role makes miR-146a essential for controlling inflammation and may influence the persistent inflammatory symptoms observed in Long COVID.
Neurological symptoms and associated microRNAs in long COVID
Neurological symptoms are among the most commonly reported and debilitating aspects of Long COVID, including "mental fog", headache, cognitive impairment, sleep, mood, smell, or taste disorders, myalgias, dysautonomia [56]. SARS-CoV-2 can invade the central nervous system through the olfactory bulb or via hematogenous spread, crossing the blood–brain barrier and can infect neurons and glial cell, leading to direct neuronal damage [57]. Evidence suggests also that viral RNA can persist in the central nervous system for extended periods, potentially causing chronic inflammation and ongoing neurological symptoms [58].
Moreover, the immune response to SARS-CoV-2 infection can lead to immune-mediated damage in the nervous system. For example, elevated levels of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β can cross the blood–brain barrier and cause neuroinflammation, leading to neuronal damage and dysfunction [59]. Several miRNAs have been implicated in neurological disorders as important regulators of gene expression, influencing neuronal function and inflammatory responses in the brain. For example, miR-9 is involved in the regulation of neurogenic inflammation and may play a role in the neurological symptoms associated with Long COVID. By modulating proinflammatory cytokines, interacting with inflammatory signalling pathways, and influencing the processes of apoptosis and neurogenesis, miR-9 contributes to protecting the central nervous system from inflammatory damage [60].
Primarily expressed in the brain, miR-124 regulates the expression of various processes involved in neuronal differentiation, function and plasticity. It downregulates genes like small C-terminal domain phosphatase 1 (SCP1), which represses neuronal gene expression, thereby promoting neuronal differentiation [61]. MiR-124 also promotes neurogenesis in the adult brain by enhancing the proliferation and differentiation of neural progenitor cells, contributing to the maintenance and repair of neuronal circuits [62]. Dysregulation of miR-124 has been linked to various neurodegenerative diseases and cognitive dysfunction [63]. MiR-124 exerts anti-inflammatory effects by targeting and downregulating genes involved in the inflammatory response, such as signal transducer and activator of transcription 3 (STAT3) and CCAAT/enhancer binding protein (CEBP-α), reducing neuroinflammation and protecting neurons from inflammation-induced damage [64].
An interesting correlation may be seen between cerebrospinal fluid (CSF) miRNA detection data and peripheral blood in patients with long COVID. miRNAs have been identified in both CSF and blood, and their expression profiles can reflect underlying pathological processes. For instance, in Alzheimer’s and Parkinson’s diseases, miRNAs in CSF and serum have been correlated with disease status and pathology features, indicating that miRNAs can serve as biomarkers for neurodegenerative diseases [65]. In the context of spontaneous intracerebral hemorrhage, miRNA profiles in CSF and plasma have shown significant differences, with some miRNAs being more abundant in CSF, suggesting that CSF miRNAs may better reflect local brain events compared to blood miRNAs [66]. The variability in miRNA detection and expression profiles across different studies may be attributed to technical factors, such as RNA isolation methods and profiling platforms. Optimizing these technical parameters is crucial for reliable miRNA biomarker discovery [67]. The correlation between CSF and blood miRNA profiles can be influenced by the blood–brain barrier and the differential release of miRNAs from brain tissues into the bloodstream. This is evident in conditions like ischemic stroke, where distinct miRNA profiles were observed in CSF and blood [68].
Cardiovascular complication in long COVID and associated microRNAs
Long COVID may include persistent cardiovascular symptoms such as palpitations, chest pain, arrhythmias, pericarditis and myocarditis. Some of them are residual post-inflammatory damage, such as dysregulation of the immune system by cytokines, an inadequate antibody response, and an ongoing underlying viral infection [69]. Other result from endothelial dysfunction, SARS-CoV-2 causing endothelial cell injury, leading to vascular inflammation, thrombosis, and impaired blood flow [70], and from dysregulation of the nervous system, resulting in abnormal heart rate and blood pressure control, contributing to symptoms such as palpitations and orthostatic tolerance [71].
Several miRNAs have been identified as key regulators of cardiovascular function and are implicated in the pathogenesis of cardiovascular complications in Long COVID. MiR-21, promotes fibrosis by targeting and downregulating antifibrotic genes, such as sprouty homolog 1 (SPRY1) and phosphatase and tensin homolog (PTEN), leading to myocarditis and heart failures [72, 73]. However, the mechanisms are different in acute and Long COVID. In acute infections, miR-21 is implicated in the activation of specific signaling pathways that lead to fibrosis, while in Long COVID, the process is more complex, involving immune dysregulation and persistent inflammation. In acute myocardial infarction (AMI), miR-21 promotes cardiac fibrosis through the activation of the TGF-β1/Smad-3 signaling pathway. This pathway is crucial for the progression of fibrosis, as miR-21 expression correlates with increased levels of TGF-β1 and Smad-3, which are key mediators of fibrotic processes [74]. In the context of Long COVID, miR-21 may interact with the renin–angiotensin–aldosterone system (RAAS) and other pathways, such as those involving fibroblast activation, contributing to fibrosis. In chronic viral myocarditis, miR-21 modulates the TGF-β1/Smad7 signaling pathway, which differs from the Smad-3 pathway activated in acute infections. This modulation results in increased collagen deposition and fibrosis, highlighting a different mechanism [75].
MiR-29a plays a significant role in inflammation and cardiac dysfunction associated with Long COVID. Studies have shown that increased levels of miR-29b-3p, a variant of miR-29, in cardiac tissues are associated with inflammation and inflammatory cell death (pyroptosis) in viral myocarditis (VMC). Inhibition of miR-29b-3p reduces inflammation and pyroptosis by upregulating DNMT3A, an essential protein in chromatin remodelling and antiviral immune response. This suggests that miR-29a and its variant miR-29b-3p are involved in inflammatory and cellular damage mechanisms, which may contribute to the persistent cardiovascular symptoms observed in patients with Long COVID [76]. MiR-126, essential for maintaining vascular integrity and angiogenesis, is involved in endothelial cell function. Dysregulation of miR-126 can lead to endothelial dysfunction and contribute to vascular complications [77]. Downregulation of miR-126 in post COVID sequelae [52], may be associated with cardiovascular complication, which appears as a result of angiogenesis and incapacity of maintaining vascular integrity [77]. MicroRNAs can be detected in blood and other body fluids, allowing for non-invasive monitoring of cardiovascular health in Long COVID patients. For example, the expression levels of these miRNAs can provide insights into disease progression and the risk of developing severe cardiovascular complications. Moreover, elevated levels of miR-21, miR-155, and reduced levels of miR-29 can serve as indicators of ongoing fibrosis, inflammation, and cardiac dysfunction [73, 78, 79].
MiRNAs associated with pulmonary complications in long COVID
The altered miRNAs level in Long COVID are also related to the pulmonary complications. Pulmonary complications can be explained by several mechanisms. Chronic immune activation and the release of pro-inflammatory cytokines can lead to sustained lung inflammation and damage [80], and the body’s healing response to severe inflammation and injury leads to the development of fibrous scar tissue in the lungs and finally, to pulmonary fibrosis [81]. SARS-CoV-2 can also persist in the lungs, causing prolonged infection [82]. Moreover, the virus causes endothelial damages and microvascular thrombosis, impairing blood flow and gas exchange in the lungs [83].
Pulmonary conditions in Long COVID are linked to alterations in miRNA expression. MiRNAs like miR-21, miR-29 and miR-200 have been found to regulate major fibrotic pathways such as transforming growth factor beta 1/Smad (TGF-β1/Smad), Phosphoinositide 3-kinase/Protein Kinase B/ mammalian target of rapamycin (PI3K/Akt/mTOR), DNA methylation, influencing the development and progression of fibrosis by modulating extracellular matrix production and epithelial-to-mesenchymal transition [8, 84, 85]. MiR-21 and miR-29, dysregulated in Long COVID, play opposing roles in the regulation of fibrosis. The upregulation of miR-21 and downregulation of miR-29 can lead to an imbalance in fibrotic processes, resulting in increased fibrosis in the lungs [79, 86]. MiR-155 and miR-146a regulate the inflammatory response. miR-155 promotes inflammation [87], while miR-146a acts as a feedback inhibitor to limit excessive inflammation [88]. Dysregulation of these miRNAs in Long COVID can lead to chronic inflammation and tissue damage.
Long COVID could also influence lung cancer risk. For example, persistent inflammatory responses in the lungs can create a microenvironment conducive to the initiation and progression of cancer cells [89]. Another mechanism of cancer development is immune dysregulation, Long COVID leading to prolonged immune system activation or suppression, both affecting the body’s ability to detect and destroy cancer cells, potentially allowing malignant cells to proliferate [80]. miR-21, miR-34a, and let-7, involved in tumorigenesis, cell proliferation, apoptosis, and metastasis in lung cancer [90, 91], are dysregulated in Long COVID and may be relevant in the disease diagnosis.
MiRNAs associated with gastrointestinal symptoms in Long COVID
Long COVID is also characterized by a variety of gastrointestinal persistent symptoms such as diarrhea, abdominal pain, nausea, vomiting and loss of appetite. Their persistence can be explained by several mechanisms. SARS-CoV-2 RNA has been detected in the stool of patients weeks after the respiratory symptoms have resolved, indicating the virus's ability to infect and persist in the gastrointestinal tract [92]. The virus damages intestinal tissue, given the presence of ACE2 in glandular epithelial cells. Moreover, tissue damage is also mediated by inflammation, as evidenced by the presence of infiltrating plasma cells, lymphocytes and interstitial edema in the lamina propria of the stomach, duodenum, and rectum [93]. Gastrointestinal involvement in Long COVID is further exacerbated by immune dysregulation, with elevated levels of pro-inflammatory cytokines like IL-6, TNF-α, and IL-1β contributing to inflammation and disruption of gut barrier function [80]. Given the large microbial load in the gut, the intestinal microbiota plays a key role in regulating host metabolic and immune gene expression. In turn, this ecosystem can be modulated by viral infections, which may stimulate or suppress host immune responses [94]. In this context, understanding the role of miRNAs in Long COVID-associated gastrointestinal symptoms offers important diagnostic and therapeutic implications. MicroRNAs exhibit remarkable stability in fecal matter due to their resistance to RNase degradation and tolerance to harsh gastrointestinal conditions. This enables them to serve as information monitors of gastrointestinal health while also reflecting broader systemic conditions, such as inflammatory diseases [95]. Long term persistence of the virus in the gastrointestinal tract and the presence of the virus RNA in the stool samples, may be associated with alteration in miRNAs level. Consequently, their detection in fecal samples has emerged as a promising non-invasive approach for understanding and diagnosing persistent gastrointestinal complications associated with post acute SARS-CoV-2 infection.
Several miRNAs have been involved in gastrointestinal symptoms and may be relevant in the context of Long COVID. MiR-155 is known for its role in regulating inflammatory responses and is often upregulated in inflammatory conditions, which can contribute to gut inflammation in Long COVID [78]. MiR-21, involved in fibrotic processes, is upregulated in chronic inflammatory diseases and may contribute to tissue remodelling and fibrosis in the gut, potentially leading to motility disorders and chronic gastrointestinal symptoms [96]. MiR-21-5p was also dysregulated in COVID-19 patients and have been associated with immunological processes, being involved in the modulation of interferons, NF-κB and STAT3 [97]. MiR-122, primarily studied in liver disease, is involved in metabolic regulation and inflammation and is also downregulated in severe cases of COVID-19. Altered levels of miR-122 in the gut could affect lipid metabolism and inflammatory pathways, contributing to long-term gastrointestinal symptoms after SARS-CoV-2 infection [98, 99].
Other miRNAs such as miR-223 and miR-31 are important for maintaining intestinal immune balance and epithelial integrity, having a similar contribution in Long COVID-related gastrointestinal dysfunction. MiR-223, for example, helps restrain neutrophilic inflammation and has protective roles in colitis. In miR-223-deficient mice, derepression of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) leads to enhanced inflammasome activation, resulting in increased caspase-1 activation and overproduction of IL-1β, a potent pro-inflammatory cytokine. This hyperactivation of the inflammasome contributes to exaggerated neutrophil recruitment and sustained intestinal inflammation [100]. MiR-31 regulates the stability of the intestinal barrier and increase in inflammatory bowel diseases, by preventing expression of inflammatory cytokine receptors (interleukin 7 receptor and interleukin 17 receptor A) and signaling glycoprotein 130 (GP130) [101]. Additionally, miR-192 and miR-215, which are involved in epithelial homeostasis [102] and miR-124, which regulates neuro-immune signalling in the enteric nervous system [103], may contribute to post-viral gastrointestinal symptoms such as motility disorders and altered gut-brain communication.
Renal dysfunction in long COVID and associated microRNAs
A range of kidney-related complications has been reported in patients with Long COVID, varying from mild functional abnormalities to more severe forms of renal dysfunction, explained by multiple underlying mechanisms. SARS-CoV-2 has been shown to directly infect kidney cells, leading to cytopathic effects and persistent viral presence in renal tissue [104]. Long-term effects can be caused also by endothelial cell injury caused by the binding of the virus to the ACE2 receptor, leading to microvascular damage in the kidneys [70]. Moreover, the virus’s interaction with ACE2 receptors can also disrupt the Renin-Angiotensin System, promoting hypertension and kidney damage [105]. As in other organs, elevated levels of pro-inflammatory cytokines may lead to renal inflammation and structural damage [82]. Persistent inflammation coupled with ongoing viral presence in the renal parenchyma, may result in chronic kidney disease, progressive decline in renal function and renal fibrosis [106]. In the context of Long COVID, dysregulation of miRNAs associated with kidney dysfunction may be relevant, with significant diagnostic and therapeutic implications. In particular, miR-21, is well known for its role in promoting renal fibrosis while miR-29 plays a protective role by inhibiting the expression of profibrotic genes.
Reduced levels of miR-29 allow for the upregulation of these genes, facilitating the accumulation of fibrous connective tissue in the kidneys. TGF-β, a key mediator of extracellular matrix remodelling, during viral infections is elevated in plasma from patients with post-COVID-19 sequelae [107]. TGF-β positively regulates miR-21 expression, thereby exacerbating fibrosis, while negatively regulating miR-29, which otherwise acts as an antifibrotic agent [108]. Importantly, MiR-29 can be detected in various body fluids, including plasma and urine, making it a potential biomarker for renal dysfunction in Long COVID. Changes in plasma miR-29 levels may reflect ongoing renal pathology and fibrosis, providing a non-invasive means of monitoring kidney health. In urine, miR-29 levels can serve as a direct indicator of renal fibrosis and inflammation. Monitoring urinary miR-29 could offer a valuable tool for assessing the extent of renal damage and tracking the effectiveness of therapeutic interventions [109]. Moreover, mir-200 family members, which target SMAD and TGF-β, are involved in epithelial-to-mesenchymal transition [110], a key event in renal fibrosis, and may appear dysregulated in Long COVID, as a result of renal fibrosis. Another one, mir-192, expressed in kidneys, could be implicated as a potential biomarker. Mir-192 promotes fibrosis regulating the TGF-β/Smad3 pathway [111], which is known to be activated in both fibrotic kidney disease and post-viral fibrotic complications, including Long COVID Most of the deregulated miRNAs in patients with renal symptoms in Long COVID are involved in pathways that lead to the development of fibrosis, but research on the mechanisms by which they are increased or decreased, or the target genes, are still scarce.
microRNA detection
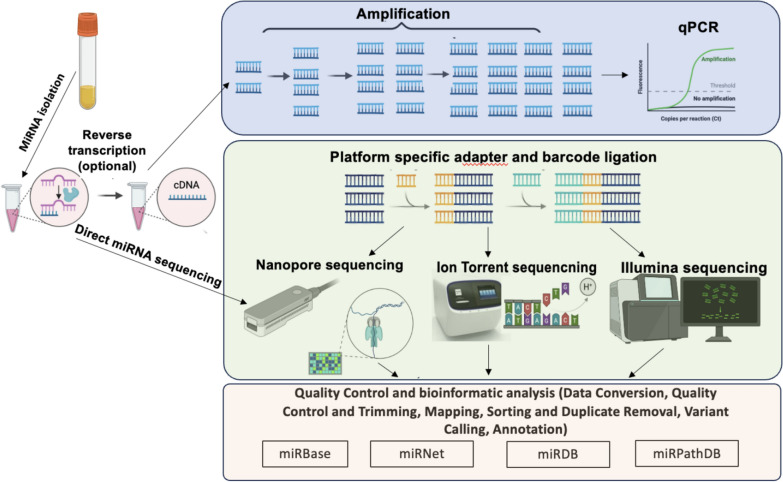
The detection and quantification of miRNAs are essential for understanding their roles in gene regulation and their potential as biomarkers for various diseases. Traditionally, miRNAs are detected using amplification-based techniques, such as polymerase chain reaction (PCR) or Next Generation Sequencing (Fig. 3). Each technique has its own strengths and limitations, and the choice of method depends on the specific requirements of the study, such as sensitivity, throughput, and available resources. Advances in technology continue to improve the accuracy and efficiency of miRNA detection, contributing to our understanding of their biological functions and clinical applications.
Fig. 3.
Typical routes for miRNA detection using molecular analysis platforms (acronyms in the bottom row refer to online miRNA databases)
Amplification-based methods
The most commonly used method for detecting and quantifying miRNAs is quantitative real-time polymerase chain reaction (RT-PCR), which involves reverse transcription of miRNA into complementary DNA, following by amplification and detection using specific primers and probes. PCR provides quantitative results, being highly sensitive and specific and suitable for detecting low abundance miRNAs; however it requires careful primer design and laborious sample preparation [112].
A widely adopted technology for miRNAs profiling is Illumina Sequencing, which employs a sequencing-by-synthesis approach and enables direct sequencing of RNA molecules. This techology generates large volumes of sequencing data, allowing for the identification and profiling of miRNAs accross diverse organisms [113]. The HiSeq and MiSeq platforms are examples of Illumina's sequencing technologies, with the HiSeq platform offering higher throughput and sensitivity, which is particularly beneficial for detecting low-abundance miRNAs [114]. Illumina sequencing has significantly advanced our understanding of miRNA biogenesis, post-transcriptional modifications, and their roles in various biological processes and diseases. It has been instrumental in identifying miRNA biomarkers for conditions such as endometriosis and other pathologies [115, 116]. Furthermore, its capability to detect miRNA editing and isomiRs (miRNA shear isoforms) provides valuable insights into the complexity of miRNA regulation and function [117, 118].
The analysis of miRNA sequencing data involves several computational steps, including alignment of reads to reference genomes, identification of known and novel miRNAs, and quantification of miRNA expression levels [117]. Bioinformatics tools are crucial for handling the large datasets generated by sequencing, allowing for the discovery of miRNA expression patterns and the identification of differentially expressed miRNAs [115, 117]. To ensure accurate interpretation, statistical tools are employed to address the variability in miRNA expression data, including potential biases introduced during library preparation and sequencing [119].
While Illumina sequencing offers numerous advantages for miRNA detection, it is important to acknowledge its potential limitations and challenges. One key concern is the potential discrepancy between miRNA expression in sequencing data and actual miRNA abundance which can affect data interpretation. The library preparation process can be complex and requires careful optimization to ensure efficient capture and amplification of miRNAs. Additional considerations include the reliance on 3′ adaptors, which can introduce bias, and the influence of sample size on the ability to detect moderate changes in miRNA expression [119]. Although sequencing costs have decreased over the years, Illumina sequencing remains relatively expensive compared to other miRNA detection methods, such as qRT-PCR or microarrays. Despite these challenges, ongoing advancements in sequencing technologies and bioinformatics are expected to further enhance the accuracy and applicability of miRNA detection using Illumina platforms.
Less used for microRNA detection, Ion Torrent sequencing operates by measuring pH changes during DNA polymerization, enabling the detection of nucleotide incorporation without the need for optical measurements. This technology is advantageous for its speed and cost-effectiveness but has limitations in terms of sequencing accuracy, particularly in homopolymer regions. The accuracy of Ion Torrent sequencing is lower compared to other platforms like Illumina, with higher error rates, particularly for insertions and deletions [112]. Because the Torrent sensor counts protons, long homopolymers can confuse the base-caller, so cross-platform validation is essential. In a head-to-head comparison of identical breast-cell-line libraries on the Ion personal genome machine (PGM) and on SOLiD 5500XL, Branco et al. recovered 97% of SOLiD-detected miRNAs on the PGM with an expression correlation of R2 = 0.90, the main discrepancy being a systematic over-call of uracil-rich sequences, an error that can be corrected in silico post-processing [120]. The same small-RNA sequencing workflow was applied to peri-operative serum exosomes and found that heightened expression of miR-17–92-cluster members hpredicted colorectal-cancer relapse within two years post surgery [121]. Similarly, the ANDROMEDA nested case–control study, embedded within an Italian mammography screening program, analysed 131 pre-diagnostic plasma libraries using the Ion Torrent PGM platform. A logistic regression model based on seven miRNA expression ratios increased the screening area under the curve (AUC) from 0.66 (mammography alone) to 0.79, demonstrating how semiconductor sequencing can augment population-scale cancer screening [122]. More than 450 miRNAs were recovered using Ion Torrent techniques, notably kidney-enriched miR-192 and miR-194, markers now pursued for diabetic-nephropathy surveillance [123]. In infectious-disease research, Chakrabarty and colleagues combined PGM profiling with qPCR validation to idenify a dual-origin signature comprising host miR-146a-5p/miR-125b-5p plus two Mycobacterium-encoded RNAs. This composite biomarker achieved 85–100% sensitivity in detecting extra-pulmonary tuberculosis [124].
Oxford nanopore sequencing
ONS is a third-generation sequencing technology that offers real-time, long-read sequencing capabilities. The technology is based on the passage of nucleic acid molecules through a nanopore, which generates an electrical signal that is used to identify the sequence of the molecule. Single-stranded nucleic acids are passed through a protein nanopore embedded in a synthetic membrane. As the nucleic acid translocate through the pore, changes in ionic current are measured and translated into nucleotide sequences [125]. While providing solutions for amplicon-based sequencing, ONS allows the direct sequencing of RNA molecules without the need for reverse transcription or amplification, preserving the native RNA modifications [126]. ONS can sequence full-length RNA molecules, being a long-reads techniques, but sequence also their precursor forms, providing comprehensive information about miRNA biogenesis and processing [127]. This technique can distinguish between different miRNA isoforms and detect RNA modifications that may affect miRNA function [128]. In addition to its uniques advantages, Oxford Nanopore Technology (ONT) can be used to profile miRNA expression in various tissues and under different physiological conditions, helping to identify miRNAs involved in specific biological processes or diseases [127]. By sequencing miRNA and their target mRNAs, this technique provides insights into miRNA-mediated gene regulatory networks ad identify novel miRNA-target interactions [126].
Despite its advantages, there are several challenges and considerations associated with ONT for miRNA detection. MiRNAs are typically 20–24 nucleotides long, which poses a challenge for sequencing accuracy and efficiency and requires specialized protocols and data analysis tools to handle these short sequences effectively [126]. Moreover, ONS has a higher error rate compared to other sequencing technologies like Illumina and continuous improvements in sequencing chemistry and data analysis algorithms are needed to enhance accuracy [125]. Thus, efficient extraction and preparation of high-quality RNA samples are critical for successful miRNA sequencing using Oxford Nanopore and protocols need to minimize RNA degradation and loss [127].
Although Oxford Nanopore offers the possibility to sequence microRNA, few studies are focused on using this platform for miRNA-based research or diagnostic. Thus, using inhouse made lipid bilayer nanopore systems, an advanced method for miRNA detection using a nanopore-based DNA computing system was presented [129]. This method utilizes synthetic diagnostic DNA (dgDNA) complexes that are designed to hybridize with specific miRNAs in body fluids. The nanopore-based technique significantly enhances the sensitivity of miRNA detection, achieving the ability to detect miRNAs at subfemtomolar concentrations (as low as 1.3 fM). The methodology involves preparing a nanopore setup where a voltage is applied across a membrane containing a single nanopore. As the dgDNA-miRNA complexes translocate through the nanopore, they cause distinct changes in the ionic current, which are recorded and analysed to identify specific miRNA sequences. This process does not require amplification steps, which reduces the risk of errors and allows for more direct and accurate detection. Using ONT, a study employing multiplexed experiments succesfully detected a mixture of 40 miRNAs and proteins, highlighting the platform capability to accurately detect miRNAs directly from human serum without the need for extraction or amplification. This represents a significant advancement over traditional miRNA detection methods like RT-qPCR, which often require multiple processing steps and are prone to amplification biases. By employing barcoded probes with a unique identifier sequence and using a highly sensitive alignment protocol, the researchers achieved over 95% alignment accuracy in single-probe experiments. Furthermore, the system was able to distinguish between similar miRNAs with a specificity of 99.03% and a sensitivity of 100%, showcasing its potential for precise miRNA profiling in clinical samples, particularly for applications in cardiovascular disease and other conditions where miRNA expression patterns serve as critical biomarkers [130].
Limitations of Oxford nanopore miRNA sequencing
Oxford Nanopore Technologies offers the only portable platform that can, in principle, read individual miRNA molecules end-to-end, an attractive avenue for tracking the dysregulated immune and metabolic pathways now linked to Long COVID. Yet, several technical hurdles still place ONT at a disadvantage when compared with mature platforms such as Illumina’s small-RNA workflows. First, intrinsic accuracy with 22-nt sequences is below short-read standards, but the Nanopore-Induced Phase-Shift Sequencing (NIPSS) approach could differentiate miRNA isoforms, however single-read sequence identity is ≈ 85–88%, requiring heavy consensus polishing for confident base calls [131]. The SR-Cat-Seq protocol improved raw accuracy by converting each miRNA into tandem repeats and then deriving a consensus, explicitly noting that “the short size of sRNAs and high error rate of the nanopore sequencer currently prevent reliable direct calling” [132]. Another approach, direct-miR-seq extends both miRNA termini with custom adaptors; it boosted yield 26-fold and drove the per-base error rate down to 3.4%, which is still an order of magnitude higher than Illumina’s per-base error but a major step forward [133]. By contrast, Illumina HiSeq/NovaSeq small-RNA libraries routinely place > 96% of bases at Q30 (≤ 0.1% error) [134]. The accuracy gap is therefore still approximately one log-unit even after ONT’s newest chemistries.
Second, library-construction trade-offs are noted, as ONT cannot yet thread 20–24 nt RNAs through pores with high fidelity, thus every successful protocol (NIPSS, SR-Cat-Seq, Direct-miR-seq) adds artificial scaffolds, concatemerises cDNA, or uses splint adaptors. The extra steps include sequence-dependent ligation [135] and consume more input RNA, which is problematic for low-biomass matrices such as post-infection plasma. Also, these steps lengthen hands-on time, partially offsetting ONT’s real-time read-out advantage. Third, small-RNA pipelines are almost turnkey when it comes to data-analysis complexity and tool maturity using Illumina, with dozens of aligners and differential-expression suites calibrated on ≥ 99% accurate reads. In contrast, ONT miRNA data still lack a de-facto standard. The availability of only one pipeline dedicated to trimming, error-correcting and mapping single-cell miRNA Nanopore reads, SingmiR, underscores the steep bioinformatic learning curve researchers face [136]. Also, while the ONT platforms such as MinION have a low initial price, the per-flow-cell yield is modest for short RNAs, so multi-flow-cell runs (or a PromethION upgrade) are needed for cohort-scale studies [132]. Illumina still delivers > 10 million miRNA reads in a single lane at a service cost often reported around US $200–300 per sample, making it the economical choice for discovery-scale long-COVID biomarker screens. However, while ONT may be behind other platforms for Long COVID miRNA biomarker discovery, its ability to read native RNA would, in theory, capture viral and host-miRNA base modifications emerging in post-acute sequelae, layers of information lost in Illumina cDNA libraries. Also, a pragmatic strategy is to profile miRNAs on Illumina for differential expression and then deploy Direct-miR-seq or SR-Cat-Seq selectively on the most interesting candidates to confirm sequence variants or RNA edits that may modulate host-virus interactions. As such, for ONT to get to par with its more mature counterparts, a validation and standardization route should be followed, although the endeavours are all but far from simple and, probably, require a centralized approach.
Challenges in using sequencing techniques in clinical diagnosis
Integrating sequencing techniques into clinical diagnostics involves several critical requirements to ensure accuracy, efficiency, and clinical utility. These requirements encompass technical, operational, and regulatory aspects, which are essential for the successful implementation of sequencing technologies such as next-generation sequencing clinical settings. The integration process is complex and requires careful consideration of various factors to optimize patient outcomes and streamline diagnostic workflows. The technical and operational requirements include high sensitivity and specificity that sequencing techniques must achieve in order to accurately detect genetic variants. Also, data analysis and interpretation using advanced bioinformatics tools are necessary since the complexity of data generated by NGS requires robust computational infrastructure and expertise in variant interpretation to ensure clinical relevance [137].
Efficient sample processing and workflow optimization are essential for meeting sample requirements and ensuring a streamlined workflow. For example, the Oncomine Precision Assay was validated for use with various sample types, including formalin-fixed paraffin-embedded samples, to ensure compatibility with clinical workflows [138].
Another aspect where tehcnologies such as Oxford Nanopore may have an advantage is the turnaround time (TAT), vital for timely clinical decision-making. The implementation of automated sequencing platforms can significantly decrease TAT, as demonstrated by a 56% reduction in TAT in a clinical setting [138].Besides purely technical aspects, regulatory and quality control considerations apply, as laboratories must adhere to international standards and obtain necessary accreditations, such as ISO15189, to ensure quality and reliability of sequencing services. Also, establishing robust quality control procedures is essential for maintaining the accuracy and consistency of sequencing results. This includes regular performance evaluations and adherence to established quality metrics [139]. Another fundamental aspect that must be pursued is clinical validation of the sequencing techniques to demonstrate their utility in diagnosing and managing diseases. For instance, RNA sequencing has been used to enhance the detection of disease-associated genes in neurological disorders [140].
While the integration of sequencing techniques into clinical diagnostics offers significant potential for improving patient care, it also presents challenges that must be addressed. These include the need for continuous updates to genomic databases, ongoing reanalysis of sequencing data, and the development of automated systems for variant interpretation. Additionally, ethical and societal considerations, such as informed consent and data privacy, must be carefully managed to ensure responsible use of genomic information in clinical settings. Thus, although Oxford Nanopore sequencing is promising in terms of portability, cost, and speed, its elevated error rates and complex data analysis requirements currently limit its utility in clinical diagnostics [141, 142]. Nanopore sequencing is known for its relatively high error rates, which can range from 5 to 15% depending on the sequencing chemistry and computational tools used [143]. This is particularly problematic for miRNA sequencing, where even small errors can significantly impact the analysis of isoforms and editing events [144].
Studies have shown that deletions are more prevalent than mismatches and insertions in nanopore sequencing, with systematic biases observed across nucleotide sequences. Cytosine/uracil-rich regions are more prone to errors compared to guanine/adenine-rich regions [145, 146]. However, tools like DeChat and NanoReviser have been developed to address these errors. DeChat focuses on repeat- and haplotype-aware error correction, significantly reducing errors without losing read information [140, 147]. NanoReviser uses deep learning to improve basecalling quality, reducing error rates by over 5% in some datasets [148]. Although recent chemistries such as R10 and improved basecallers have reduced error rates, issues persist with indel errors, homopolymer regions, and methylation-induced miscalls [149, 150]. In addition, the raw data from nanopore sequencing is complex, requiring sophisticated preprocessing to convert electrical signals into base calls. This complexity can hinder the accessibility of the technology for general users [151]. In this context, miRNAs present unique challenges due to their short length and the need for high precision in isoform variation analysis. Existing error correction methods often fail to address the specific needs of miRNA sequencing [144]. As such, various computational approaches have been developed to improve read accuracy and facilitate data analysis. These include the use of k-mer lattice structures for error correction and the integration of long-read specific tools with short-read differential expression methods [144, 152].
Multiple studies highlight a knowledge gap in standardized, scalable bioinformatics pipelines capable of reliably distinguishing true variants from sequencing artifacts, especially for low-frequency variants and structural variations [153–155]. Controversies exist regarding the sufficiency of current error correction and variant calling methods, with some advocating deep learning approaches for improved accuracy, while others emphasize the need for hybrid sequencing strategies [148, 156, 157]. Failure to address these gaps risks misdiagnosis and undermines confidence in nanopore-based clinical assays [158]. Despite these challenges, the potential of nanopore sequencing in clinical diagnostics remains significant. The technology's ability to provide long reads and real-time data is unmatched, and ongoing improvements in error correction and data analysis tools continue to enhance its applicability. However, the high error rates and data complexities necessitate further advancements to fully realize its potential in clinical settings.
Standardization of ONS-generated miRNA profiles for enhanced diagnostic accuracy in clinical settings
Standardization of miRNA profiles generated from molecular data is essential for improving diagnostic accuracy and reliability in clinical settings, particularly in cancer diagnostics and genetic disorders. miRNAs have emerged as promising biomarkers due to their stability in biofluids and their role in regulating gene expression. However, the variability in miRNA measurement methodologies has hindered their clinical application.
Preanalytical considerations for miRNA standardization
Preanalytical variables are critical determinants of miRNA measurement accuracy. These variables include sample collection, processing, and storage conditions. For circulating miRNAs, hemolysis and blood cell contamination significantly affect profiles, necessitating careful handling of samples. Studies recommend processing samples within 2 h of collection, using ethylenediaminetetraacetic acid (EDTA) as the preferred anticoagulant and avoiding heparin due to its inhibitory effects on reverse transcription [159, 160]. The stability of microRNAs (miRNAs) under various freezing temperatures is a critical factor for their use as biomarkers in clinical and research settings. This stability is crucial for their potential application in diagnostics and prognostics and miRNAs stored at ultra-low temperatures, such as the vapor phase of liquid nitrogen, have shown no significant degradation over extended periods. For instance, miRNAs remained stable for up to 17 years under these conditions, with only miR-451a showing alterations due to contamination rather than degradation [161, 162].
Studies on the impact of freeze–thaw cycles have shown that most miRNAs remain stable, although some, such as miR-30c-5p, exhibit mild sensitivity [161]. Glinge et al. [163] further demonstrated that miRNAs can withstand up to four freeze–thaw cycles without significant degradation, despite a gradual reduction in concentration with repeated cycling. Additionally, miRNAs stored at room temperature or 4 °C for up to 24 h also maintained stability; however, prolonged exposure led to degradation. These findings suggest that while miRNAs are generally robust, maintaining optimal storage conditions is essential to preserve their integrity. Samples should be filtered to remove cellular debris and can be stored at room temperature or 4 °C for up to 24 h. For long-term preservation, storage at –20 °C or –80 °C maintains miRNA stability for at least one year. In tissue-based miRNA analysis, warm ischemic time should be kept under 1 h, and cold ischemic time (at 4 °C) should not exceed 24 h. Formalin fixation for up to 72 h prior to processing is acceptable, and enrichment for target cell populations is critical to minimize variability [159].
Analytical and postanalytical considerations for miRNA standardization
The analytical phase involves miRNA quantification, typically performed using quantitative reverse transcription (RT-qPCR) or NGS. RT-qPCR is widely used due to its sensitivity and specificity, but its accuracy depends on appropriate normalization to account for technical variations introduced during sample collection, RNA extraction, and amplification. The selection of stable endogenous controls (ECs) is critical for reliable normalization. miR-191 and miR-103 have been identified as stable ECs across normal and cancerous tissues, outperforming commonly used controls like U6 snRNA and 5S rRNA [164, 165]. Algorithms such as NormFinder and GeNorm are recommended for identifying stable ECs. These tools evaluate the stability of candidate miRNAs across samples and datasets, ensuring robust normalization. For example, NormFinder performs optimally with 5–10 candidate genes, while GeNorm is sensitive to correlated genes and requires careful selection of uncorrelated miRNAs [165].
Postanalytical factors include data normalization, imputation of missing data, and statistical analysis. Normalization strategies vary depending on the dataset and platform used. For instance, quantile normalization, such as the Adjusted Quantile Normalization (AQuN) method, has been successfully applied to combine miRNA expression data from multiple studies, increasing statistical power and revealing novel biomarkers [166]. Similarly, the use of within-sample expression ratios of miRNA pairs has been proposed as a robust approach for constructing stable diagnostic models across datasets [167]. Missing data imputation is another critical aspect of postanalytical processing. Single or multiple imputation strategies can significantly impact the selection of stable ECs and subsequent survival analysis. For example, in high-grade serous carcinoma, hsa-miR-23a-3p and hsa-miR-193a-5p have been validated as reliable ECs, while U6-snRNA exhibits variable expression and is not recommended [168].
Despite notable advancements in miRNA standardization, several challenges remain. The lack of consensus on normalization methods and the variability in miRNA quantification across platforms hinder the comparability and reproducibility of studies. For instance, small RNA-seq, FirePlex, EdgeSeq, and nCounter platforms exhibit considerable performance differences in terms of reproducibility, accuracy, and specificity [169]. Additionally, the integration of multi-omics data requires sophisticated bioinformatics tools to manage complex datasets, further complicating the standardization process [170]. Future directions include the development of microfluidics-based detection systems for multiplexed miRNA profiling, which could facilitate rapid point-of-care testing and precision medicine [171]. AI-assisted models for miRNA biomarker panel optimization also hold promise for improving diagnostic accuracy and prognosis in cancer and other diseases [171].
miRNA–multi-omics integration via ONS in long COVID
The integration of miRNA and transcriptomics data has become a powerful approach for identifying molecular pathways and biomarkers in Long COVID. miRNAs as small non-coding RNAs that regulate gene expression post-transcriptionally, have been implicated in various COVID-19-related pathologies due to their dysregulated expression. Transcriptomics, which provide a comprehensive overview of gene expression profile, enables identification of differentially expressed genes (DEGs) and enriched pathways associated with Long COVID. Studies have shown that miRNA-transcriptomics integration can identify pathways such as MAPK signaling, immune response, and inflammation, which are critical in Long COVID [172, 173]. For example, miR-200c-3p and miR-142-3p have been linked to immune dysregulation in Long COVID patients [173]. Also, multi-omics integration has revealed distinct molecular signatures for Long COVID subgroups, such as multisystemic inflammation and neurological manifestations. These signatures are characterized by specific miRNA-mRNA interactions that drive subgroup-specific pathologies [172, 174]. Sex-specific miRNA and transcriptomic differences have also been observed in Long COVID, with males and females showing distinct gene expression patterns. For example, males often show upregulation of genes associated with neuro-cardiovascular disorders, whereas females exhibit more diverse immune and respiratory-related alterations [132, 133]. Epidemiological data indicate that women have a higher risk of developing Long COVID than men, with reported risk ratios ranging from 1.31 to 1.50 across age groups [175, 176]. Immunologically, females with Long COVID tend to exhibit increased frequencies of exhausted T cells and heightened antibody responses to latent viruses, while males demonstrate elevated levels of natural killer (NK) cells and pro-inflammatory cytokines such as IL-8 [177]. Sex-specific immune dysregulation—such as enhanced TGF-β signaling in males and elevated XIST expression in females—appears to contribute to Long COVID pathogenesis [178–180].
Also, women have a higher risk of developing Long COVID compared to men, with studies showing a risk ratio of 1.31–1.50 for females across various age groups [175, 176]. Females with Long COVID exhibit increased frequencies of exhausted T cells and higher antibody reactivity to latent viruses, while males show elevated NK cells and plasma cytokines like IL-8 [177]. Observed sex-specific immune dysregulation, such as increased TGF-β signaling in males and X inactive-specific transcript (XIST) expression in females, contributes to the pathogenesis of Long COVID [178].
As such, the design of biomarker panels for Long COVID should consider sex-specific immune responses and symptom profiles. Tailored therapeutic strategies, including sex-specific rehabilitation programs and pharmacological treatments, are recommended to address the distinct needs of men and women with Long COVID [181].
Proteomics complements transcriptomics by providing information into protein expression and post-translational modifications. Integrating miRNA and proteomics datasets enables identification of protein-level changes and their regulatory miRNAs, thus providing more comprehensive understanding of the molecular mechanisms in Long COVID.
Proteomics have identified proteins involved in inflammation, immune response, and tissue repair in Long COVID. For example, Abhydrolase Domain Containing 17A, Depalmitoylase (ABHD17A) and Casein Kinase 1 Delta (CSNK1D) have been associated with specific Long COVID subgroups, and are regulated by miRNAs such as miR-143-3p [172, 182]. Also, miRNA-proteomics integration has underscored the role of inflammatory pathways, such as NF-κB signaling, in Long COVID. Proteins like IL-10 and NFKB1 are regulated by specific miRNAs, contributing to persistent inflammation [183, 184]. Additionally, serum proteins such as orticotropin-releasing hormone (CRH) and fucose-1-phosphate guanylyltransferase (FPGT) have been identified as subgroup-specific biomarkers in Long COVID, with miRNAs playing a regulatory role in their expression [172, 185].
Another promising application of ONS is the reconstruction of miRNA–mRNA regulatory networks from the acute to Long COVID stages. This involves capturing the dynamic interactions between microRNAs and their target mRNAs over time, which is essential for elucidating regulatory mechanisms underlying disease progression. To support this, several computational tools have been developed to analyze time-series multi-omics data and reconstruct these networks. For example, the TIMEOR tool integrates time-series RNA-seq data with protein–DNA binding data to uncover temporal regulatory mechanisms, enabling the inference of causal relationships within gene regulatory networks (GRNs) [186, 187]. Similarly, TimiRGeN is tailored for time-series miRNA–mRNA data, identifying interactions that affect signaling pathways and providing a platform for visualization and hypothesis generation [188]. Another approach, mirDREM, uses probabilistic modeling to reconstruct dynamic regulatory networks, accounting for the combined influence of miRNAs and transcription factors on gene expression over time. It has been successfully applied to contexts such as postnatal lung development, revealing both known and novel miRNAs involved in regulatory control [189]. While these tools offer robust frameworks for miRNA–mRNA network reconstruction, several challenges remain. The inherent complexity of biological systems, variability in omics data, and the need for high-quality temporal datasets all impact the accuracy and interpretability of dynamic network models. Integrative approaches and careful experimental design are therefore critical for meaningful application in disease contexts such as Long COVID. The integration of ONS-generated miRNA data with transcriptomics and proteomics hold significant potential for advancing our understanding of Long COVID. While miRNA-transcriptomics integration provides insights into gene regulation and immune response, miRNA-proteomics integration offers protein-level perspectives on inflammation and tissue repair. Both approaches complement each other, offering a more comprehensive view of Long COVID pathophysiology. Future research should focus on longitudinal studies, standardized protocols, and advanced computational tools to fully harness the potential of multi-omics integration in Long COVID research.
Conclusions and perspectives
The prolonged and heterogeneous manifestations of Long COVID pose a significant public health challenge, with substantial gaps in our understanding of its molecular mechanisms. MicroRNAs have emerged as promising biomarkers, offering insights into disease pathophysiology and potential tools for patient stratification and outcome prediction. Clinically, several miRNAs such as miR-200c-3p, miR-142-3p, miR-146a-5p, and miR-125b-5p have been consistently associated with sustained inflammation, immune dysregulation, and impaired tissue repair, all hallmark features of Long COVID. Their dysregulated expression correlates with key pathways, including NF-κB signaling and cytokine imbalance, supporting their relevance for disease monitoring. Importantly, miRNA expression profiles appear to vary by sex and clinical phenotype, suggesting their utility in identifying disease subtypes and forecasting disease progression. Their inherent stability in biofluids and tissue-specific expression patterns make them particularly suitable for non-invasive diagnostics and longitudinal assessments.
On the research front, advances in sequencing technologies have expanded the landscape of miRNA profiling. Illumina remains the gold standard for sensitivity and accuracy, while Ion Torrent offers a faster and more accessible alternative, though it still requires further validation. Oxford Nanopore’s amplification-free, real-time sequencing approach holds unique promise for detecting native miRNAs and their modifications, especially in point-of-care settings, as improvements in accuracy continue. Integrating miRNA data with transcriptomic and proteomic analyses has already revealed sex-specific and subgroup-specific molecular signatures, reinforcing the value of multi-omics strategies in unraveling the complex biology of Long COVID.
To advance the role of miRNA profiling in Long COVID research and clinical care, several priorities should be addressed: (i) standardization of protocols across platforms, harmonized guidelines for sample collection, RNA extraction, normalization (e.g., use of validated endogenous controls like miR-191), and imputation are essential for cross-study comparability; (ii) platform-appropriate validation strategies, ntegrating orthogonal technologies (e.g., validating Illumina findings with ONS or qPCR) will improve confidence in biomarker discovery; (iii) enhanced bioinformatics and AI integration, use machine learning models to help decode the complex interactions between miRNAs, mRNAs, and proteins in Long COVID, supporting biomarker panel refinement and individualized risk prediction; (iv) longitudinal and multi-omics studies tracking miRNA dynamics over time and correlating them with clinical, immunological, and transcriptomic data to better understand disease trajectories and recovery patterns; (v) clinical translation and point-of-care development, use of portable sequencing platforms such as ONS, combined with microfluidics and cloud-based analysis tools, to offer a path toward real-time miRNA diagnostics in outpatient and low-resource settings. Future work should focus on validating candidate miRNAs in large cohorts, refining detection platforms, and translating these insights into clinical practice.
Acknowledgements
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, under the Romania’s National Recovery and Resilience Plan Funded by EU – NextGenerationEU” program, project ”Artificial intelligence-powered personalized health and genomics libraries for the analysis of long-term effects in COVID-19 patients (AI-PHGL-COVID)”; number 760073/23.05.2023, code 285/30.11.2022, within Pillar III, Component C9, Investment 8
Author contribution
N.-E. P. wrote the main sections of the paper and prepared figures; O.-A. C. S. conceptualized and reviewed the paper; A. L. conceptualized, reviewed and wrote specific parts of the text; M. D. prepared figures and wrote specific parts of the text; I.-O. S. critically reviewed the paper; M. C. curated the text and reviewed the pape.
Funding
Ministerul Cercetării, Inovării şi Digitalizării,285/30.11.2022.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olga Adriana Căliman-Sturdza, Email: olga.caliman-sturdza@usm.ro.
Andrei Lobiuc, Email: andrei.lobiuc@usm.ro.
References
- 1.Bhaskaran M, Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol. 2014;51(4):759–74. 10.1177/0300985813502820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier D, Rivera B, Fabian MR, Foulkes WD. miRNA biogenesis and inherited disorders: clinico-molecular insights. Trends Genet. 2023;39(5):401–14. 10.1016/J.TIG.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell PS, et al. Circulating micrornas as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–8. 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redell JB, Moore AN, Ward NH, Hergenroeder GW, Dash PK. Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma. 2010;27(12):2147–56. 10.1089/NEU.2010.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci. 2009;106(11):4402–7. 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta SK, Bang C, Thum T. Circulating micrornas as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3(5):484–8. 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 7.Cushing L et al. miR-29 Is a major regulator of genes associated with pulmonary fibrosis. 2012;45(2): 287–294 10.1165/RCMB.2010-0323OC. [DOI] [PMC free article] [PubMed]
- 8.Yang S, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180(2):484–93. 10.1016/J.AJPATH.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pottier N, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009;4(8): e6718. 10.1371/JOURNAL.PONE.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato T, Baskoro H, Rennard SI, Seyama K, Takahashi K. Micrornas as therapeutic targets in lung disease: prospects and challenges chronic obstructive pulmonary diseases. J COPD Foundation. 2016;3(1):382–8. 10.15326/JCOPDF.3.1.2015.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzie ME, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67(2):122–31. 10.1136/THORAXJNL-2011-200089. [DOI] [PubMed] [Google Scholar]
- 12.Meder B, et al. Influence of the confounding factors age and sex on microRNA profiles from peripheral blood. Clin Chem. 2014;60(9):1200–8. 10.1373/CLINCHEM.2014.224238. [DOI] [PubMed] [Google Scholar]
- 13.Angulo M, Lecuona E, Sznajder JI. Role of micrornas in lung disease. Arch Bronconeumol. 2012;48(9):325–30. 10.1016/J.ARBR.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backes C, et al. Blood born miRNAs signatures that can serve as disease specific biomarkers are not significantly affected by overall fitness and exercise. PLoS One. 2014;9(7): e102183. 10.1371/JOURNAL.PONE.0102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardle SL, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS One. 2015;10(4): e0122107. 10.1371/JOURNAL.PONE.0122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurj A, Zanoaga O, Braicu C, and Berindan-Neagoe I. Epigenetic regulation and microRNA expression. in MicroRNA, Elsevier, (2022) pp. 153–167. 10.1016/B978-0-323-89774-7.00003-0.
- 17.Ozanic K, Watanabe ASA, Machado ABF, da Silva VL, Dias VC, and Diniz CG. Unveiling long COVID: general perceptions and challenges for diagnosis and management. (2025). 10.20944/preprints202501.0164.v1.
- 18.Ramonfaur D, et al. The global clinical studies of long COVID. Int J Infect Dis. 2024;146: 107105. 10.1016/j.ijid.2024.107105. [DOI] [PubMed] [Google Scholar]
- 19.Pazukhina E, et al. Long Covid: a global health issue–a prospective, cohort study set in four continents. BMJ Glob Health. 2024;9(10): e015245. 10.1136/bmjgh-2024-015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris M, et al. PD59 review of the international epidemiology of long COVID. Int J Technol Assess Health Care. 2024;40(S1):S119–20. 10.1017/S0266462324003179. [Google Scholar]
- 21.Loreche AM, Pepito VCF, Dayrit MM. Long covid: a call for global action. Public Health Challenges. 2023. 10.1002/puh2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, et al. The miRNA: a small but powerful RNA for COVID-19. Brief Bioinform. 2021;22(2):1137–49. 10.1093/BIB/BBAB062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Hu X, Li L, Li J. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J Clin Lab Anal. 2020;34(10): e23590. 10.1002/JCLA.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava S, et al. Evaluation of altered miRNA expression pattern to predict COVID-19 severity. Heliyon. 2023. 10.1016/j.heliyon.2023.e13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calderon-Dominguez M, et al. Serum microRNAs targeting ACE2 and RAB14 genes distinguish asymptomatic from critical COVID-19 patients. Mol Ther Nucleic Acids. 2022;29:76–87. 10.1016/j.omtn.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Gonzalo-Calvo D, et al. Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Trans Res. 2021;236:147–59. 10.1016/J.TRSL.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donyavi T, et al. Acute and post-acute phase of COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a–3p, 155–5p, and let-7b-3p in PBMC. Int Immunopharmacol. 2021;97: 107641. 10.1016/J.INTIMP.2021.107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haroun RAH, Osman WH, Amin RE, Hassan AK, Abo-Shanab WS, Eessa AM. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology. 2022;54(1):104–10. 10.1016/J.PATHOL.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fayyad-Kazan M, et al. Circulating miRNAs: potential diagnostic role for coronavirus disease 2019 (COVID-19). Infect Genet Evol. 2021;94: 105020. 10.1016/J.MEEGID.2021.105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimenta R, et al. MiR-200c-3p expression may be associated with worsening of the clinical course of patients with COVID-19. Mol Biol Res Commun. 2021;10(3):141–7. 10.22099/MBRC.2021.40555.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bautista-Becerril B, et al. High expression levels of miR-21-5p in younger hospitalized COVID-19 patients are associated with mortality and critical disease. Int J Mol Sci. 2023;24(12):10112. 10.3390/IJMS241210112/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parray A, et al. Snornas and mirnas networks underlying covid-19 disease severity. Vaccines (Basel). 2021;9(10):1056. 10.3390/VACCINES9101056/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicoletti ADS, et al. Differentially expressed plasmatic microRNAs in Brazilian patients with coronavirus disease 2019 (COVID-19): preliminary results. Mol Biol Rep. 2022;49(7):6931–43. 10.1007/s11033-022-07338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon-Dominguez M, et al. Serum microRNAs targeting ACE2 and RAB14 genes distinguish asymptomatic from critical COVID-19 patients. Mol Ther Nucleic Acids. 2022;29:76–87. 10.1016/J.OMTN.2022.06.006/ATTACHMENT/6A646FC9-20AC-4CF8-9322-65910C104B6C/MMC2.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannella A, et al. Circulating microrna signatures associated with disease severity and outcome in COVID-19 patients. Front Immunol. 2022;13: 968991. 10.3389/FIMMU.2022.968991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moatar AI, et al. Plasma miR-195–5p predicts the severity of Covid-19 in hospitalized patients. Sci Rep. 2023;13(1):1–11. 10.1038/s41598-023-40754-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng HY, et al. Longitudinal transcriptome analyses show robust T cell immunity during recovery from COVID-19. Signal Transd Targeted Therapy. 2021;5(1):1–12. 10.1038/s41392-020-00457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asirvatham AJ, Magner WJ, Tomasi TB. miRNA regulation of cytokine genes. Cytokine. 2009;45(2):58–69. 10.1016/J.CYTO.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn SR, O’Neill LA. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23(7):421–5. 10.1093/INTIMM/DXR034. [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulos KI, Papadopoulou A, Aw TC. Beauty and the beast: host microRNA-155 versus SARS-CoV-2. Human Cell. 2023;36(3):908–22. 10.1007/S13577-023-00867-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Mir-155 functions downstream of angiotensin II receptor subtype 1 and calcineurin to regulate cardiac hypertrophy. Exp Ther Med. 2016;12(3):1556–62. 10.3892/etm.2016.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivieri F, et al. miR-21 and miR-146a: the micrornas of inflammaging and age-related diseases. Ageing Res Rev. 2021;70: 101374. 10.1016/J.ARR.2021.101374. [DOI] [PubMed] [Google Scholar]
- 43.Jafarinejad-Farsangi S, Jazi MM, Rostamzadeh F, Hadizadeh M. High affinity of host human microRNAs to SARS-CoV-2 genome: an in silico analysis. Noncoding RNA Res. 2020;5(4):222–31. 10.1016/J.NCRNA.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyson McDonald J, et al. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep. 2021. 10.1016/j.celrep.2021.109839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu D, et al. Micrornas targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J Mol Cell Cardiol. 2020;148:46–9. 10.1016/J.YJMCC.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matarese A, Gambardella J, Sardu C, Santulli G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8(11):462. 10.3390/BIOMEDICINES8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukhopadhyay D, Mussa BM. Identification of novel hypothalamic MicroRNAs as promising therapeutics for SARS-CoV-2 by regulating ACE2 and TMPRSS2 expression: an in silico analysis. Brain Sci. 2020;10(10):666. 10.3390/BRAINSCI10100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021. 10.1136/BMJ.N1648. [DOI] [PubMed] [Google Scholar]
- 49.García-Hidalgo MC, et al. MicroRNA-guided drug discovery for mitigating persistent pulmonary complications in critical COVID-19 survivors: a longitudinal pilot study. Br J Pharmacol. 2024. 10.1111/BPH.16330. [DOI] [PubMed] [Google Scholar]
- 50.Curcio R, et al. Exosomal miR-17–5p, miR-146a-3p, and miR-223–3p correlate with radiologic sequelae in survivors of COVID-19-related acute respiratory distress syndrome. Int J Mol Sci. 2023;24:13037. 10.3390/IJMS241713037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García-Hidalgo MC, et al. Identification of circulating microrna profiles associated with pulmonary function and radiologic features in survivors of SARS-CoV-2-induced ARDS. Emerg Microbes Infect. 2022;11(1):1537–49. 10.1080/22221751.2022.2081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paris D, et al. The biomarkers’ landscape of post-COVID-19 patients can suggest selective clinical interventions. Sci Rep. 2023;13(1):1–15. 10.1038/s41598-023-49601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Hidalgo MC, et al. Identification of circulating microRNA profiles associated with pulmonary function and radiologic features in survivors of SARS-CoV-2-induced ARDS. Emerg Microbes Infect. 2022;11(1):1537–49. 10.1080/22221751.2022.2081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mone P, et al. Endothelial extracellular vesicles enriched in microRNA-34a predict new-onset diabetes in Coronavirus disease 2019 (COVID-19) patients: novel insights for long COVID metabolic sequelae. J Pharmacol Exp Ther. 2024;389(1):34–9. 10.1124/JPET.122.001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HM, Kim TS, Jo EK. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49(6):311. 10.5483/BMBREP.2016.49.6.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stefanou M-I, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. 2022. 10.1177/20406223221076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–8. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 58.Song E, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exper Med. 2021. 10.1084/JEM.20202135/211674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12(1):1–3. 10.1186/S13195-020-00640-3/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuva-Aydemir Y, Simkin A, Gascon E, Gao F-B. MicroRNA-9. RNA Biol. 2011;8(4):557–64. 10.4161/RNA.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21(7):744–9. 10.1101/GAD.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, et al. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced Hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res. 2019;44(4):811–28. 10.1007/s11064-018-02714-z. [DOI] [PubMed] [Google Scholar]
- 63.Sun Y, Luo Z-M, Guo X-M, Su D-F, Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 2015. 10.3389/fncel.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU.1 pathway. Nat Med. 2010;17(1):64–70. 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burgos K, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS One. 2014;9(5): e94839. 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwuchukwu I, Nguyen D, Sulaiman W. Micro RNA profile in cerebrospinal fluid and plasma of patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. 2016;22(12):1015–8. 10.1111/cns.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W-X, Fardo DW, Jicha GA, Nelson PT. A customized quantitative PCR microRNA panel provides a technically robust context for studying neurodegenerative disease biomarkers and indicates a high correlation between cerebrospinal fluid and choroid plexus microRNA expression. Mol Neurobiol. 2017;54(10):8191–202. 10.1007/s12035-016-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sørensen SS, Nygaard A-B, Nielsen M-Y, Jensen K, Christensen T. Mirna expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res. 2014;5(6):711–8. 10.1007/s12975-014-0364-8. [DOI] [PubMed] [Google Scholar]
- 69.Shrestha AB, et al. Long COVID syndrome and cardiovascular manifestations: a systematic review and meta-analysis. Diagnostics. 2023;13(3):491. 10.3390/DIAGNOSTICS13030491/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raj SR, et al. Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res. 2021;31(3):365–8. 10.1007/S10286-021-00798-2/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4. 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 73.Surina, Fontanella RA, Scisciola L, Marfella R, Paolisso G, Barbieri M. miR-21 in human cardiomyopathies. Front Cardiovasc Med. 2021;8:767064. 10.3389/FCVM.2021.767064/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, et al. MiR-208b/miR-21 promotes the progression of cardiac fibrosis through the activation of the TGF-β1/Smad-3 signaling pathway: an in vitro and in vivo study. Front Cardiovasc Med. 2022. 10.3389/fcvm.2022.924629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue YM, Chen MG, Chen DV, Wu WF, Liu YL, and Lin FH. The effect of microRNA-21 on myocardial fibrosis in mice with chronic viral myocarditis,” Zhonghua Xin Xue Guan Bing Za Zhi. 2018; pp. 450–457 [DOI] [PubMed]
- 76.Wang Y, Zhang Z, Li H, Wang M, Qiu Y, Lu L. miR-29b-3p regulates cardiomyocytes pyroptosis in CVB3-induced myocarditis through targeting DNMT3A. Cell Mol Biol Lett. 2024;29(1):1–20. 10.1186/S11658-024-00576-8/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang S, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. 10.1016/J.DEVCEL.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Connell RM, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33(4):607–19. 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13027–32. 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nalbandian A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.George PM, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–16. 10.1136/THORAXJNL-2020-215314. [DOI] [PubMed] [Google Scholar]
- 82.Gupta A, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–32. 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M, et al. The role of MiR-29 in the mechanism of fibrosis. Mini-Rev Med Chem. 2023;23(19):1846–58. 10.2174/1389557523666230328125031. [DOI] [PubMed] [Google Scholar]
- 85.Hirawat R, Jain N, Saifi MA, Rachamalla M, Godugu C. Lung fibrosis: post-COVID-19 complications and evidences. Int Immunopharmacol. 2023;116: 109418. 10.1016/J.INTIMP.2022.109418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu G, et al. MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–97. 10.1084/JEM.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahesh G, Biswas R. MicroRNA-155: a master regulator of inflammation. J Interferon Cytokine Res. 2019;39(6):321–30. 10.1089/JIR.2018.0155/ASSET/IMAGES/LARGE/FIGURE3.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng Z, et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp Lung Res. 2013;39(7):275–82. 10.3109/01902148.2013.808285. [DOI] [PubMed] [Google Scholar]
- 89.Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Rep Pract Oncol Radiother. 2020;25(3):422–7. 10.1016/J.RPOR.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slabáková E, Culig Z, Remšík J, Souček K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Disease. 2017;8(10):e3100–e3100. 10.1038/cddis.2017.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang G, et al. Exosomal miR-21/Let-7a ratio distinguishes non-small cell lung cancer from benign pulmonary diseases. Asia Pac J Clin Oncol. 2020;16(4):280–6. 10.1111/AJCO.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mao R, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–78. 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831-1833.e3. 10.1053/J.GASTRO.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li N, Ma WT, Pang M, Fan QL, Hua JL. The commensal microbiota and viral infection: a comprehensive review. Front Immunol. 2019;10:1551. 10.3389/FIMMU.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duran-Sanchon S, et al. Identification and validation of MicroRNA profiles in fecal samples for detection of colorectal cancer. Gastroenterology. 2020;158(4):947-957.e4. 10.1053/J.GASTRO.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen HT, et al. Mir-21 in the cancers of the digestive system and its potential role as a diagnostic, predictive, and therapeutic biomarker. Biology. 2021. 10.3390/BIOLOGY10050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reyes-Long S, et al. Role of the MicroRNAs in the pathogenic mechanism of painful symptoms in long COVID: systematic review. Int J Mol Sci. 2023;24:3574. 10.3390/IJMS24043574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gutmann C, et al. Association of cardiometabolic microRNAs with COVID-19 severity and mortality. Cardiovasc Res. 2022;118(2):461–74. 10.1093/CVR/CVAB338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang XW, Heegaard NHH, Orum H. Micrornas in liver disease. Gastroenterology. 2012;142(7):1431–43. 10.1053/J.GASTRO.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neudecker V, et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214(6):1737–52. 10.1084/jem.20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian Y, et al. Microrna-31 reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterology. 2019;156(8):2281-2296.e6. 10.1053/j.gastro.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 102.Runtsch MC, Round JL, and Oâ€TMConnell RM. MicroRNAs and the regulation of intestinal homeostasis. Front Genet. 2014;5 10.3389/fgene.2014.00347. [DOI] [PMC free article] [PubMed]
- 103.Chen H, et al. Mir-124 mediates the effects of gut microbial dysbiosis on brain function in chronic stressed mice. Behav Brain Res. 2025;476: 115262. 10.1016/j.bbr.2024.115262. [DOI] [PubMed] [Google Scholar]
- 104.Puelles VG, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–2. 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. 10.1016/J.EJIM.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su H, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–27. 10.1016/J.KINT.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramírez-Martínez G, et al. Possible role of matrix metalloproteinases and TGF-β in COVID-19 severity and sequelae. J Interferon Cytokine Res. 2022;42(8):352–68. 10.1089/JIR.2021.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chung ACK, Lan HY. MicroRNAs in renal fibrosis. Front Physiol. 2015. 10.3389/fphys.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang G, Kwan BCH, Lai FMM, Chow KM, Li PKT, Szeto CC. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol. 2012;36(5):412–8. 10.1159/000343452. [DOI] [PubMed] [Google Scholar]
- 110.Li JY, Yong TY, Michael MZ, Gleadle JM. The role of microRNAs in kidney disease. Nephrology. 2010;15(6):599–608. 10.1111/j.1440-1797.2010.01363.x. [DOI] [PubMed] [Google Scholar]
- 111.Chung ACK, Huang XR, Meng X, Lan HY. Mir-192 mediates TGF-β/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21(8):1317–25. 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bragg LM, Stone G, Butler MK, Hugenholtz P, Tyson GW. Shining a light on dark sequencing: characterising errors in Ion Torrent PGM data. PLoS Comput Biol. 2013;9(4):1003031. 10.1371/JOURNAL.PCBI.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo S MicroRNA expression analysis using the illumina MicroRNA-Seq platform. 2012; pp. 183–188. 10.1007/978-1-61779-427-8_12. [DOI] [PubMed]
- 114.Cherchi R, et al. Next generation sequencing for miRNA detection on the exhaled breath condensate: a pilot study. Epigenet Insights. 2023. 10.1177/25168657231160985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bendifallah S, et al. Endometriosis associated-miRNome analysis of blood samples: a prospective study. Diagnostics. 2022;12(5): 1150. 10.3390/diagnostics12051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coenen-Stass AML, et al. Evaluation of methodologies for microRNA biomarker detection by next generation sequencing. RNA Biol. 2018. 10.1080/15476286.2018.1514236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gunaratne PH, Coarfa C, Soibam B, and Tandon A miRNA data analysis: next-gen sequencing. 2012; pp. 273–288. 10.1007/978-1-61779-427-8_19. [DOI] [PubMed]
- 118.Tang H-M, Chen H, Zhang J, Ren J-Y, Xu N. Application of next generation sequencing in microRNA detection. Hereditas (Beijing). 2012;34(6):784–92. 10.3724/SP.J.1005.2012.00784. [DOI] [PubMed] [Google Scholar]
- 119.Baroin-Tourancheau A, Benigni X, Doubi-Kadmiri S, Taouis M, Amar L. Lessons from microRNA sequencing using illumina technology. Adv Biosci Biotechnol. 2016;07(07):319–28. 10.4236/abb.2016.77030. [Google Scholar]
- 120.Branco GP, et al. A comparison between SOLiD 5500XL and Ion Torrent PGM-derived miRNA expression profiles in two breast cell lines. Genet Mol Biol. 2020. 10.1590/1678-4685-gmb-2018-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsumura T, et al. Exosomal microrna in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113(2):275–81. 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiorino G, et al. Plasma microRNA ratios associated with breast cancer detection in a nested case–control study from a mammography screening cohort. Sci Rep. 2023;13(1): 12040. 10.1038/s41598-023-38886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014;86(2):433–44. 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- 124.Chakraborty C, Bhattacharya M, Alshammari A, Albekairi TH. Blueprint of differentially expressed genes reveals the dynamic gene expression landscape and the gender biases in long COVID. J Infect Public Health. 2024;17(5):748–66. 10.1016/j.jiph.2024.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Jain M, Olsen HE, Paten B, Akeson M. The Oxford Nanopore MinION: delivery of nanopore sequencing to the genomics community. Genome Biol. 2016;17(1):1–11. 10.1186/S13059-016-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garalde DR, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15(3):201–6. 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 127.Workman RE, et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat Methods. 2019;16(12):1297–305. 10.1038/s41592-019-0617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davidsen K, Sullivan LB. A robust method for measuring aminoacylation through tRNA-seq. Elife. 2024. 10.7554/ELIFE.91554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takeuchi N, Hiratani M, Kawano R. Pattern recognition of microRNA expression in body fluids using nanopore decoding at subfemtomolar concentrations. JACS Au. 2022;2(8):1829–38. 10.1021/jacsau.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koch C et al. Highly multiplexed detection of microRNAs, proteins and small molecules using barcoded molecular probes and nanopore sequencing. bioRxiv. 2022; p. 2022.12.13.520243 10.1101/2022.12.13.520243.
- 131.Zhang J, et al. Direct microrna sequencing using nanopore-induced phase-shift sequencing. iScience. 2020;23(3): 100916. 10.1016/j.isci.2020.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maguire S, Guan S. Rolling circle reverse transcription enables high fidelity nanopore sequencing of small RNA. PLoS One. 2022;17(10): e0275471. 10.1371/journal.pone.0275471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi C, et al. Quantitative and multiplexing analysis of microRNAs by direct full-length sequencing in nanopores. J Am Chem Soc. 2025;147(18):15614–24. 10.1021/jacs.5c02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu M, et al. Small RNA-Seq analysis reveals miRNA expression of short distance transportation stress in beef cattle blood. Animals. 2021;11(10): 2850. 10.3390/ani11102850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baran-Gale J, et al. Addressing bias in small RNA library preparation for sequencing: a new protocol recovers microRNAs that evade capture by current methods. Front Genet. 2015. 10.3389/fgene.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Engel A, et al. SingmiR: a single-cell miRNA alignment and analysis tool. Nucleic Acids Res. 2024;52(W1):W374–80. 10.1093/nar/gkae225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sarmady M, Abou Tayoun A. Need for automated interactive genomic interpretation and ongoing reanalysis. JAMA Pediatr. 2018;172(12):1113. 10.1001/jamapediatrics.2018.2675. [DOI] [PubMed] [Google Scholar]
- 138.Werner R, Connolly A, Bennett M, Hand CK, Burke L. Implementation of an ISO15189 accredited next-generation sequencing service with the fully automated Ion Torrent Genexus: the experience of a clinical diagnostic laboratory. J Clin Pathol. 2024;77(4):278–83. 10.1136/jcp-2022-208625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mendez-Rios JD. Guía de procedimientos operacionales y recomendaciones en la Implementación de secuenciación de nueva generación para diagnóstico clínico. Revista Médica de Panamá. 2019;39(1):2412–642. 10.37980/im.journal.rmdp.2019778. [Google Scholar]
- 140.Li S, et al. The clinical utility and diagnostic implementation of human subject cell transdifferentiation followed by RNA sequencing. Am J Hum Genet. 2024;111(5):841–62. 10.1016/j.ajhg.2024.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arres J et al. Assessing the readiness of Oxford nanopore sequencing for clinical genomics applications. 2024. 10.1101/2024.10.30.24316253.
- 142.Petersen LM, Martin IW, Moschetti WE, Kershaw CM, Tsongalis GJ. Third-generation sequencing in the clinical laboratory: exploring the advantages and challenges of nanopore sequencing. J Clin Microbiol. 2019. 10.1128/JCM.01315-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rang FJ, Kloosterman WP, de Ridder J. From squiggle to basepair: computational approaches for improving nanopore sequencing read accuracy. Genome Biol. 2018;19(1):90. 10.1186/s13059-018-1462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X, Ping P, Hutvagner G, Blumenstein M, Li J. Aberration-corrected ultrafine analysis of miRNA reads at single-base resolution: a k -mer lattice approach. Nucleic Acids Res. 2021;49(18):e106–e106. 10.1093/nar/gkab610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu-Wei W, van der Toorn W, Bohn P, Hölzer M, Smyth R, and von Kleist M. Sequencing accuracy and systematic errors of nanopore direct RNA sequencing. 2023 10.1101/2023.03.29.534691. [DOI] [PMC free article] [PubMed]
- 146.Liu-Wei W, van der Toorn W, Bohn P, Hölzer M, Smyth RP, von Kleist M. Sequencing accuracy and systematic errors of nanopore direct RNA sequencing. BMC Genom. 2024;25(1): 528. 10.1186/s12864-024-10440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, et al. Repeat and haplotype aware error correction in nanopore sequencing reads with DeChat. Commun Biol. 2024;7(1): 1678. 10.1038/s42003-024-07376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L, Qu L, Yang L, Wang Y, and Zhu H. An error correction method of nanopore sequencing data using deep learning. in 2020 13th international congress on image and signal processing, biomedical engineering and informatics (CISP-BMEI), IEEE, Oct. 2020; pp. 23–28. 10.1109/CISP-BMEI51763.2020.9263622.
- 149.Biggel M, Cernela N, Horlbog JA, Stephan R. Oxford Nanopore’s 2024 sequencing technology for Listeria monocytogenes outbreak detection and source attribution: progress and clone-specific challenges. J Clin Microbiol. 2024. 10.1128/jcm.01083-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ratcliff JD et al. Improved resolution of highly pathogenic avian influenza virus haemagglutinin cleavage site using oxford nanopore R10 sequencing chemistry. 2023. 10.1101/2023.09.30.560331.
- 151.Cozzuto L, et al. Masterofpores: a workflow for the analysis of Oxford Nanopore direct RNA sequencing datasets. Front Genet. 2020. 10.3389/fgene.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong X, et al. The long and the short of it: unlocking nanopore long-read RNA sequencing data with short-read differential expression analysis tools. NAR Genomics Bioinform. 2021. 10.1093/nargab/lqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou D. Characterizing nanopore sequencing artifacts with deep learning. San Jose State Univ. 2024. 10.31979/etd.3jm8-bx3d. [Google Scholar]
- 154.Liu Y, Kearney J, Mahmoud M, Kille B, Sedlazeck FJ, Treangen TJ. Rescuing low frequency variants within intra-host viral populations directly from Oxford Nanopore sequencing data. Nat Commun. 2022;13(1): 1321. 10.1038/s41467-022-28852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shafin K, et al. Haplotype-aware variant calling with PEPPER-margin-deepvariant enables high accuracy in nanopore long-reads. Nat Methods. 2021;18(11):1322–32. 10.1038/s41592-021-01299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weigel D eLife Assessment: benchmarking reveals superiority of deep learning variant callers on bacterial nanopore sequence data. 2024 10.7554/eLife.98300.1.sa3. [DOI] [PMC free article] [PubMed]
- 157.Chen Y, et al. Efficient assembly of nanopore reads via highly accurate and intact error correction. Nat Commun. 2021;12(1): 60. 10.1038/s41467-020-20236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harris PNA, et al. Rapid nanopore sequencing and predictive susceptibility testing of positive blood cultures from intensive care patients with sepsis. Microbiol Spectr. 2024. 10.1128/spectrum.03065-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khan J, Lieberman JA, Lockwood CM. Variability in, variability out: best practice recommendations to standardize pre-analytical variables in the detection of circulating and tissue microRNAs. Clin Chem Lab Med (CCLM). 2017. 10.1515/cclm-2016-0471. [DOI] [PubMed] [Google Scholar]
- 160.Marzi MJ, et al. Optimization and standardization of circulating MicroRNA detection for clinical application: the miR-test case. Clin Chem. 2016;62(5):743–54. 10.1373/clinchem.2015.251942. [DOI] [PubMed] [Google Scholar]
- 161.Matias-Garcia PR, et al. Impact of long-term storage and freeze-thawing on eight circulating microRNAs in plasma samples. PLoS One. 2020;15(1): e0227648. 10.1371/journal.pone.0227648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Balzano F, et al. MiRNA stability in frozen plasma samples. Molecules. 2015;20(10):19030–40. 10.3390/molecules201019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Glinge C, et al. Stability of circulating blood-based microRNAs–pre-analytic methodological considerations. PLoS One. 2017;12(2): e0167969. 10.1371/journal.pone.0167969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peltier HJ, Latham GJ. Normalization of microrna expression levels in quantitative rt-pcr assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14(5):844–52. 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Treleaven H, Ludwig L, Viloria-Petit A, Wood RD, Ali A, Wood GA. Abstract 2350: comparison of normalization methods for RT-qPCR microRNA expression in cancer datasets. Cancer Res. 2024;84(6_Supplement):2350–2350. 10.1158/1538-7445.AM2024-2350. [Google Scholar]
- 166.Ben-Elazar S, et al. MiRNA normalization enables joint analysis of several datasets to increase sensitivity and to reveal novel miRNAs differentially expressed in breast cancer. PLoS Comput Biol. 2021;17(2): e1008608. 10.1371/journal.pcbi.1008608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma C, et al. In search of the ratio of miRNA expression as robust biomarkers for constructing stable diagnostic models among multi-center data. Front Genet. 2024. 10.3389/fgene.2024.1381917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lopacinska-Jørgensen J, Petersen PHD, Oliveira DVNP, Høgdall CK, Høgdall EV. Strategies for data normalization and missing data imputation and consequences for potential diagnostic microRNA biomarkers in epithelial ovarian cancer. PLoS One. 2023;18(5): e0282576. 10.1371/journal.pone.0282576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Godoy PM et al. Comparison of miRNA profiling methods using synthetic miRNA pools and standardized exRNA samples reveals substantial performance differences. 2019. 10.1101/645762.
- 170.Ou Y, Ren Z. The role of miRNAs as biomarkers in cancer. Asia-Pacific J Oncol. 2024. 10.32948/ajo.2024.10.18. [Google Scholar]
- 171.Muthamilselvan S, Ramasami Sundhar Baabu P, Palaniappan A. Microfluidics for profiling miRNA biomarker panels in AI-assisted cancer diagnosis and prognosis. Technol Cancer Res Treat. 2023. 10.1177/15330338231185284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ai J, et al. Integrated multi-omics characterization across clinically relevant subgroups of long COVID. Natl Sci Rev. 2024. 10.1093/nsr/nwae410.39764508 [Google Scholar]
- 173.Timofeeva AM, Nikitin AO, Nevinsky GA. Circulating miRNAs in the plasma of post-COVID-19 patients with typical recovery and those with long-COVID symptoms: regulation of immune response-associated pathways. Non-Coding RNA. 2024;10(5): 48. 10.3390/ncrna10050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paris D, et al. The biomarkers’ landscape of post-COVID-19 patients can suggest selective clinical interventions. Sci Rep. 2023;13(1):22496. 10.1038/s41598-023-49601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shah DP, et al. Sex differences in long COVID. JAMA Netw Open. 2025;8(1): e2455430. 10.1001/jamanetworkopen.2024.55430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sylvester SV, Rusu R, Chan B, Bellows M, O’Keefe C, Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin. 2022;38(8):1391–9. 10.1080/03007995.2022.2081454. [DOI] [PubMed] [Google Scholar]
- 177.Silva J et al. Sex differences in symptomatology and immune profiles of long COVID. 2024. 10.1101/2024.02.29.24303568.
- 178.Hamlin RE, et al. Sex differences and immune correlates of long covid development, symptom persistence, and resolution. Sci Transl Med. 2024;16:773. 10.1126/scitranslmed.adr1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rusu EC, et al. Towards understanding post-COVID-19 condition: a systematic meta-analysis of transcriptomic alterations with sex-specific insights. Comput Biol Med. 2024;175: 108507. 10.1016/j.compbiomed.2024.108507. [DOI] [PubMed] [Google Scholar]
- 180.Chakraborty C, Bhattacharya M, Alshammari A, Albekairi TH. Blueprint of differentially expressed genes reveals the dynamic gene expression landscape and the gender biases in long COVID. J Infect Public Health. 2024;17(5):748–66. 10.1016/j.jiph.2024.02.018. [DOI] [PubMed] [Google Scholar]
- 181.Delfino C, et al. Post-COVID-19 condition: a sex-based analysis of clinical and laboratory trends. Front Med (Lausanne). 2024. 10.3389/fmed.2024.1376030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gjorgjieva T, et al. Systems genetics identifies miRNA-mediated regulation of host response in COVID-19. Hum Genomics. 2023;17(1): 49. 10.1186/s40246-023-00494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Agamah FE, Ederveen THA, Skelton M, Martin DP, Chimusa ER, ’t Hoen PAC. Network-based integrative multi-omics approach reveals biosignatures specific to COVID-19 disease phases. Front Mol Biosci. 2024. 10.3389/fmolb.2024.1393240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hashemi Sheikhshabani S, et al. In silico identification of potential miRNAs -mRNA inflammatory networks implicated in the pathogenesis of COVID-19. Hum Gene. 2023;36: 201172. 10.1016/j.humgen.2023.201172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gao L, et al. Circulating miRNA profiles in COVID-19 patients and meta-analysis: implications for disease progression and prognosis. Sci Rep. 2023;13(1): 21656. 10.1038/s41598-023-48227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Conard AM, et al. TIMEOR: a web-based tool to uncover temporal regulatory mechanisms from multi-omics data. Nucleic Acids Res. 2021;49(W1):W641–53. 10.1093/nar/gkab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Conard AM et al. TIMEOR: a web-based tool to uncover temporal regulatory mechanisms from multi-omics data. 2020. 10.1101/2020.09.14.296418. [DOI] [PMC free article] [PubMed]
- 188.Patel K, Chandrasegaran S, Clark IM, Proctor CJ, Young DA, Shanley DP. TimiRGeN: R/Bioconductor package for time series microRNA–mRNA integration and analysis. Bioinformatics. 2021;37(20):3604–9. 10.1093/bioinformatics/btab377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schulz MH, Pandit KV, Lino Cardenas CL, Ambalavanan N, Kaminski N, Bar-Joseph Z. Reconstructing dynamic microRNA-regulated interaction networks. Proc National Acad Sci. 2013;110(39):15686–91. 10.1073/pnas.1303236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.