Abstract
Abstract
Objective
Patients with non-ST segment elevation acute coronary syndrome (NSTEACS) admitted into emergency department are usually combined with a high number of comorbidities. Charlson Comorbidity Index (CCI) is the most commonly used measure to assess comorbidity in clinical practice. However, the impact of CCI on the clinical outcomes of patients with NSTEACS are still unclear.
Design
A multicenter retrospective cohort study.
Setting
We used data from the Chongqing Medical University Medical Data Science Academy in Chongqing, China, which contains data from seven tertiary hospitals.
Participants
Data from 3308 consecutive patients aged over 18 diagnosed with NSTEACS admitted to emergency departments of seven hospitals from August 2012 to March 2023 were retrospectively analysed.
Methods
Patients were divided into two groups based on CCI: CCI <3 as low CCI, and CCI ≥3 as high CCI. A propensity score matching (PSM) analysis using the 1:1 nearest neighbour matching method with a calliper value of 0.02 was adopted to control for differences between the comparison cohorts. Univariate and multivariate logistic regression analyses were carried out to produce ORs with 95% CIs to identify whether the CCI is a potential independent predictor of in-hospital outcomes in the matched cohort.
Primary and secondary outcome measures
In-hospital mortality rate, major adverse cardiovascular events (MACEs), length of stay and readmission rate.
Results
876 and 2432 patients belonged to the high CCI group (CCI ≥3) and the low CCI group (CCI <3). After PSM, 618 pairs were matched. There were significant differences in sociodemographic, clinical characteristics and laboratory tests between the two groups before PSM. The results were balanced and comparable after PSM (p>0.05). In patients with high CCI, in-hospital mortality, the incidence of MACEs, length of stay (LOS) and readmission rate were significantly higher compared with those with low CCI. Univariate analysis revealed that a higher CCI was associated with an increased incidence of MACEs, prolonged LOS and a higher readmission rate. Multivariate analysis demonstrated that even after adjusting for various confounding factors, a higher CCI remained an independent risk factor for an increased incidence of MACEs, prolonged LOS and higher readmission rate.
Conclusion
A high CCI not only increases the risk of in-hospital MACEs but also prolongs the length of stay and increases the readmission rate. We recommend that the CCI be used as a crucial risk indicator for clinical practitioners to identify and manage patients with a poor prognosis.
Keywords: Multimorbidity, Coronary heart disease, Prognosis
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a systematic analysis that used a multicenter, large sample data from the Chongqing Medical University Medical Data Science Academy in Chongqing, China with a time span of 10 years.
This study eliminated the effects of relevant factors between the high Charlson Comorbidity Index (CCI) and low CCI groups by using propensity score matching.
The effect of selection bias could not be excluded entirely due to limitations of retrospective studies.
This study only focused on outcomes during the patients’ hospital stay and did not follow-up with the patients or consider their long-term outcomes.
Introduction
Non-ST segment elevation acute coronary syndrome (NSTEACS) encompasses a spectrum of clinical presentations related to acute myocardial ischemia, with or without acute ischaemic changes on the ECG, including unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI). Although these conditions present similarly, they differ in severity.1 Currently, ACS is a leading cause of mortality in the Asia-Pacific region, accounting for approximately half of the global burden.2 With rising life expectancy and the increasing prevalence of coronary artery disease (CAD) with age, the general incidence of ACS, especially NSTEMI, is growing.3,5 It is estimated that more than 7 million people worldwide are diagnosed with ACS each year, with NSTEACS accounting for approximately 70%.6 Patients with ACS are often accompanied by multimorbidity.7 Compared with ACS, NSTEACS patients are typically older and have more comorbidities, such as hypertension and diabetes, which directly or indirectly hinder optimal recovery. They also have a higher incidence of invasive therapy and major adverse cardiovascular events (MACEs).8 9 There is a high prevalence (>50%) of three or more additional comorbidities for the six most frequently managed conditions in cardiovascular medicine (heart failure, stroke, hyperlipidaemia, atrial fibrillation, ischaemic heart disease and hypertension).10 Mortality rates from ACS have declined in recent decades, and life expectancy has increased. Therefore, a highly heterogeneous group of complex multimorbid patients now requires more attention.11 A comorbidity is defined as a clinical condition that exists at the time of the onset of the event and is likely to influence the outcome under study.12 Greater use of evidence-based therapies and methods has improved clinical outcomes for ACS patients in recent years. Consequently, more ACS patients are surviving longer, generally beyond 12 months. Long-term outcomes have not improved in NSTEACS patients at the same rate as in ACS patients, possibly reflecting the more complex clinical phenotype of NSTEACS patients, including older age, greater burden of comorbidities, and a higher likelihood of previous myocardial infarction.13 Currently, the most commonly used measure to assess comorbidity in clinical practice is the Charlson Comorbidity Index (CCI). The CCI, proposed by Charlson in 1987, accounts for multiple comorbidities by creating a sum score weighted according to the presence of various conditions, and is commonly used in outcome and mortality studies.14 It has been shown to predict long-term mortality in different clinical populations, including internal medical, surgical, intensive care unit (ICU), trauma and cancer patients.14 Several studies have reported that CCI is correlated with increased length of stay (LOS), lower survival and higher hospital readmission in NSTEACS patients.15 16 As an indicator to comprehensively evaluate the coexistence of multiple chronic diseases in patients, CCI has received extensive attention in recent years. The CCI has been validated in cardiovascular research. Notably, Radovanovic et al demonstrated its prognostic validity in over 29 000 ACS patients through the nationwide AMIS Plus Registry.17 However, its role in patients with NSTEACS has not been fully explored. Limited studies have been conducted to evaluate the extent and impact of CCI on the clinical outcomes (in-hospital mortality, MACEs, LOS and readmission) of NSTEACS patients in China. In this study, we hypothesise that more significant comorbidities measured by CCI are related to an incremental incidence of adverse clinical outcomes. Therefore, we aim to clarify the extent and impact of multimorbidity on in-hospital outcomes in patients presenting with NSTEACS in a multicentre retrospective cohort. This will help clinical practitioners better understand and assess this condition and inform decision-making about the care delivered to such patients.
Method
Study design and database description
This multicenter retrospective study used data from the Chongqing Medical University Medical Data Science Academy (MDSA) in Chongqing, China. The MDSA is a medical big data platform containing over 50 million medical records of outpatients, emergency and hospitalised patients, including demographic data, course records, laboratory indicators, examination results, treatment regimens and outcomes from seven tertiary hospitals since 1998. To ensure the data were representative, we selected patients admitted to the hospital during the 10-year period from August 2012 to March 2023.
Study population
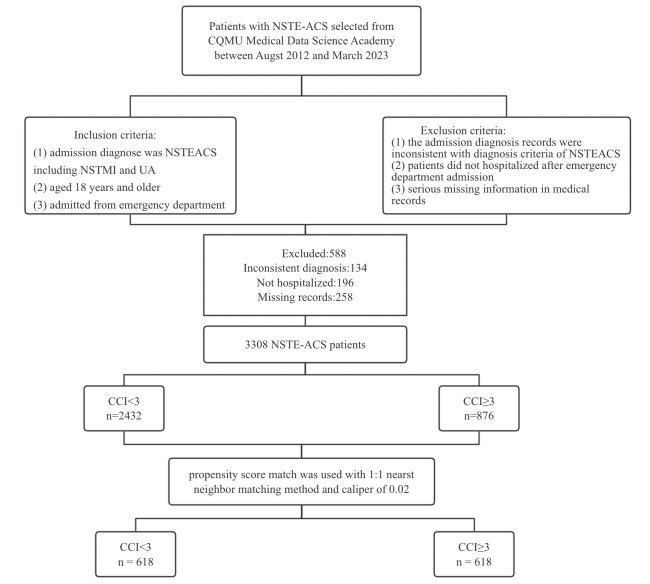
Patients with a principal diagnosis of NSTEACS, defined by the International Classification of Diseases, Ninth Edition, Clinical Modification code, were included in this study. The relevant NSTEACS codes were 410.7× for NSTEMI and 411.1 for UA. The diagnosis criteria were based on the ‘Guideline and consensus for the management of patients with non ST segment elevation acute coronary syndrome (2016)’.18 Patients were eligible for inclusion if at least one of the above codes was listed as a main reason or diagnosis for their admission. Additionally, patients were included if they met the following criteria: (1)patients aged 18 years and older; (2)admitted from the emergency department; and (3) the admission period was from August 2012 to March 2023. The exclusion criteria were: (1) the admission diagnosis records were inconsistent with the diagnosis criteria of NSTEACS; (2) patients were not hospitalised after emergency department admission; and (3) serious missing information in medical records (>40% missing data for one patient). Figure 1 shows the flow diagram of patient screening. Following ED triage, patients were stratified to ICU, semi-intensive monitoring or general wards based on GRACE score and haemodynamic status. ICU admission mandated at least one high-risk feature (eg, systolic blood pressure (BP) <90 mm Hg). Care escalation protocols followed the European Society of Cardiology (ESC) Guidelines for NSTEACS.19 Treatments were protocolised according to GRACE risk strata. High-risk patients received urgent angiography (<24 hours) with revascularisation as appropriate. Medical therapy followed American College of Cardiology (ACC)/American College of Cardiology (AHA) Class I recommendations,20 with dose adjustments for renal/hepatic dysfunction.
Figure 1. The flow diagram of patient screening. CCI, Charlson Comorbidity Index; NSTEACS, non-ST segment elevation acute coronary syndrome; UA, unstable angina. CQMU, Chongqing Medical University; NSTMI, Non ST Elevation Miocardial Infarction.
This study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University (approval no. K2023-142). The requirement for informed consent was waived due to the retrospective and anonymised nature of the data analysis. All patient information was pre-existing, de-identified to ensure confidentiality.
Study variables
From the MDSA platform, we extracted patients’ demographic characteristics (age, sex, job), clinical characteristics (smoking, drinking, hyperlipidaemia, hypertension, coronary heart disease, diabetes mellitus, prior stroke, deficiency anaemia, atrial fibrillation, arrhythmia, renal insufficiency, respiratory system diseases, hypoalbuminaemia), Killip classification, lesion vessels (anterior descending artery, right coronary artery, left circumflex artery, left main artery and other), decreased left ventricular ejection fraction (LVEF), coronary angiography, revascularisation procedures (Percutaneous Coronary Intervention,PCI), clinical measures (GRACE 2.0 score) and laboratory index including cardiac troponin T cTnT, creatine kinase isoenzyme MB(CK-MB), N-terminal pro-brain natriuretic peptide (NTProBNP), glucose, creatinine, white blood cell, neutrophil, estimated glomerular filteration rate (eGFR), Aspartate Aminotransferase(AST), Alanine Aminotransferase (ALT). Comorbidity was assessed by our research team using the CCI, which incorporated 19 different comorbidities. A full list of included comorbidities and assigned weights is provided in table 1.
Table 1. Comorbidity conditions among NSTEACS patients with different CCI.
| Condition | Assign weights for each condition | Total number | CCI <3 n=2432 (%) |
CCI ≥3 n=876 (%) |
P value* | ||
|---|---|---|---|---|---|---|---|
| Myocardial infarction | 1 | 619 | 355 | 14.6 | 264 | 30.1 | <0.001 |
| Congestive heart failure | 1 | 156 | 72 | 3 | 84 | 9.6 | <0.001 |
| Peripheral vascular disease | 1 | 72 | 36 | 1.5 | 36 | 4.1 | <0.001 |
| Cerebrovascular disease | 1 | 736 | 440 | 18.1 | 296 | 33.8 | <0.001 |
| Dementia | 1 | 27 | 12 | 0.5 | 15 | 1.7 | =0.001 |
| Chronic pulmonary disease | 1 | 523 | 315 | 13 | 208 | 23.7 | <0.001 |
| Connective tissue disease | 1 | 15 | 5 | 0.2 | 10 | 1.1 | <0.001 |
| Ulcer disease | 1 | 95 | 52 | 2.1 | 43 | 4.9 | <0.001 |
| Mild liver disease | 1 | 373 | 207 | 8.5 | 166 | 18.9 | <0.001 |
| Diabetes | 1 | 828 | 567 | 23.3 | 262 | 29.8 | <0.001 |
| Hemiplegia | 2 | 107 | 0 | 0 | 107 | 12.2 | <0.001 |
| Moderate or severe renal disease | 2 | 347 | 23 | 0.9 | 324 | 37 | <0.001 |
| Diabetes with end organ damage | 2 | 241 | 33 | 1.4 | 208 | 23.7 | <0.001 |
| Any tumour without metastasis | 2 | 59 | 7 | 0.3 | 52 | 5.9 | <0.001 |
| Leukaemia | 2 | 103 | 11 | 0.5 | 92 | 10.5 | <0.001 |
| Lymphoma | 2 | 159 | 8 | 0.3 | 151 | 17.2 | <0.001 |
| Moderate or severe liver disease | 3 | 113 | 0 | 0 | 113 | 12.9 | <0.001 |
| Metastatic solid tumour | 6 | 6 | 0 | 0 | 6 | 0.7 | <0.001 |
| AIDS | 6 | 4 | 0 | 0 | 4 | 0.5 | =0.001 |
Statistically significant results (p<0.05) are displayed in bold.
CCI, Charlson Comorbidity Index; NSTEACS, non-ST segment elevation acute coronary syndrome.
In our study, the original CCI scoring system was used, which assigns points solely based on the presence and severity of comorbidities (eg, diabetes, heart failure, renal disease) without incorporating age as a scoring component. The age-adjusted CCI, which adds 1 point per decade over 40 years (eg, +1 for 50–59 years, +2 for 60–69 years, etc.), was not applied in our analysis. Based on the histories of patients obtained from the HIS standardised case report forms, the CCI was calculated based on documented comorbidities, excluding age-related points, to assess the independent association of comorbidity burden with outcomes. We extracted the comorbidities of each patient and calculated the total score. There is no universally accepted cut-off point for the measurement of multimorbidity. Previous studies predominately included patients using a CCI cut-off point of three to dichotomise (with a minimum included CCI of 0).4 21 Therefore, we decided to refer to the previous literature and adopt a CCI of 3 as the cut-off point.
Study outcomes
The primary outcomes of this study are all-cause in-hospital mortality rate and the incidence rate of MACEs. The secondary outcomes are LOS and readmission rate. In-hospital mortality rate was calculated as the ratio of the number of patients who died (from any cause) to the total number of cases. There are various definitions regarding MACEs. We adopt the definition used in the previous study, which includes a combination of the following: all-cause death; malignant arrhythmia; development of or hospitalisation for heart failure; and cardiogenic shock.22 LOS was calculated as the period from the time of admission to the time of discharge. Unplanned readmissions were defined as cardiovascular-related rehospitalisations within 30 days of discharge, excluding scheduled procedures. Events were captured across regional hospitals via integrated EHR and validated by an independent committee. Using the current admission as a reference, we defined a readmission as cardiovascular-related rehospitalisations within 30 days of discharge, excluding scheduled procedures. Events were captured across regional hospitals via integrated EHR and validated by an independent committee. Outcome validation was performed through dual independent verification of electronic medical records, with adjudication by a third reviewer in cases of discordance. A 10% random sample underwent additional manual source document verification. Outcome assessors were blinded to CCI scores, which were calculated independently by a separate member in our team. Propensity score matching (PSM) was used to control for differences in the comparison cohorts.
Statistical analysis
Descriptive statistics were used to summarise baseline characteristics. The distribution of variables was assessed using the Shapiro-Wilk test, with normality defined as p >0.05. Normally distributed variables are presented as mean and SD and were compared using the independent samples t test. Non-normally distributed variables are presented as median with IQR and compared using the Wilcoxon rank-sum test. Categorised variables were presented as frequencies and percentages and were compared using the χ2 test.
In addition, we conducted a PSM analysis in the high CCI (CCI ≥3) and low CCI (CCI <3) groups to control for differences in the comparison cohorts. The 1:1 nearest neighbour matching method was adopted with a calliper value of 0.02, using the low CCI group was used as the benchmark for matching. Propensity scores were used to match NSTEACS patients with high and low CCI. Standardised mean differences (SMD) were used to assess the balance of baseline data between the two groups, with SMD <0.1 indicating a good balance, which can be considered a small difference between the two groups. Univariate and multivariate logistic regression analyses were carried out to produce ORs with 95% CIs to identify whether the CCI is a potential independent predictor for the primary and secondary outcomes in the matched cohort. Model 1 is an unadjusted univariate analysis. Model 2 was a multivariate analysis adjusted for age, sex, smoking and drinking. Model 3 was a multivariate analysis adjusted for age, sex, smoking, drinking, PCI, coronary angiography and Killip classification. Model 4 was a multivariate analysis adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification and GRACE 2.0 score. Analyses were performed using SPSS Statistics 26 (IBM Corporation) along with R V.4.3.0, with a 2-sided P value of <0.05 for statistical significance. All statistical analyses, including PSM, were reviewed and validated by an independent biostatistician to ensure methodological rigour and adherence to causal inference principles.
Patient and public involvement
None.
Result
Clinical characteristics of non-ST segment elevation acute coronary syndrome (ACS) patients before and after propensity score matching (PSM)
A total of 3308 patients admitted with NSTEACS in the MDSA between 2012 and 2023. Among these, 2432 patients had a low CCI (CCI <3), while 876 patients had a high CCI (CCI ≥3). Prior to PSM, the proportion of patients aged 65 and older was 69.07% (2285/3308). Among individuals with CCI ≥3, 65 years and older accounted for 80.48% of the population cohort (705/876), whereas in the group with CCI <3, this proportion constituted 64.97% of the study population (1580/2432). Patients with a high CCI exhibited a higher prevalence of hypertension, stroke, anaemia, atrial fibrillation, heart failure, diabetes mellitus, arrhythmias, renal insufficiency and respiratory system diseases. They also had higher Killip classification scores, a greater incidence of hypoalbuminaemia and a lower left ventricular ejection fraction. These differences were statistically significant (p<0.05) (online supplemental material). Following PSM, 618 pairs of low and high CCI patients were matched, with no differences observed between the groups in sociodemographic, clinical characteristics and laboratory-related variables, as all SMD were<0.1, indicating balance and comparability between the groups (online supplemental material 2, figure 2).
Figure 2. analysis of standardised mean differences before and after PSM. LVEF, left ventricular ejection fraction; eGFR, estimated Glomerular Filtration Rate; CHD, Coronary Heart Disease; PCI,Percutaneous Coronary Intervention; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CK,Creatine Kinase; CK-MB, Creatine Kinase-MB; WBC, White Blood Cell.
Comorbidity conditions among non-ST segment elevation acute coronary syndrome (ACS) patients with different Charlson Comorbidity Index (CCI)
Among the 19 comorbidities included in the CCI, differences were observed in NSTEACS patients with low CCI and high CCI. The top three comorbidities are diabetes, cerebrovascular disease and myocardial infarction, respectively. Specifically, 29.8%, 33.8%, 30.1% and 23.7% of patients with high CCI had diabetes, cerebrovascular disease, myocardial infarction and chronic lung disease, respectively. These proportions were significantly higher compared with those with low CCI (23.3% for diabetes, 18.1% for cerebrovascular disease, 14.6% for myocardial infarction and 13% for chronic lung disease). For the remaining comorbidities, the proportion of patients with high CCI was also significantly higher than that in the low CCI group, and these differences were statistically significant. (table 1).
Clinical outcomes analysis of non-ST segment elevation acute coronary syndrome (NSTEACS) patients before and after propensity score matching (PSM)
In-hospital mortality
In-hospital mortality occurred in 51 patients, resulting in a mortality rate of 1.5%. Patients with a CCI ≥3 had a significantly higher incidence of in-hospital mortality compared with those with a CCI <3 (0.9% vs 3.2%, p<0.001). After PSM, there were no significant differences between patients with CCI ≥3 and those with CCI <3 (1.9% vs 1.8%, p=0.83) (table 2). Univariate and multivariate logistic regression analyses did not reveal any statistically significant association between CCI and in-hospital mortality (table 3).
Table 2. In-hospital outcomes stratified by CCI score before and after PSM.
| Variable n (%) |
Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n=3308) | CCI <3 (n=2432) | CCI ≥3 (n=876) | P value | Total (n=1236) | CCI <3 (n=618) | CCI ≥3 (n=618) | P value* | |
| In-hospital mortality | 51 (1.5) | 23 (0.9) | 28 (3.2) | <0.000 | 23 (1.9) | 12 (1.9) | 11 (1.8) | 0.83 |
| MACEs | 395 (11.9) | 134 (5.5) | 261 (29.8) | <0.000 | 217 (17.6) | 61 (9.9) | 156 (25.2) | <0.000 |
| LOS | 8.69±5.71 | 7.99±4.75 | 10.64±7.43 | <0.000 | 9.38±5.99 | 8.7±5.34 | 10.6±6.52 | <0.000 |
| Readmission | 1142 (34.5) | 779(32) | 363 (41.4) | <0.000 | 466 (37.7) | 214 (34.6) | 252 (40.8) | 0.026 |
Statistically significant results (p<0.05) are displayed in bold.
CCI, Charlson Comorbidity Index; LOS, length of stay; MACES, major adverse cardiovascular events; PSM, propensity score matching.
Table 3. Univariate and multivariate logistic regression models for the association between CCI score and incidence of the in-hospital primary and secondary outcomes (CCI <3 used as reference).
| The impact of CCI on in-hospital outcomes | OR (95%CI) | P value* |
|---|---|---|
| The impact of CCI on in-hospital mortality | ||
| Model 1: unadjusted | 0.83 (0.36 to 1.94) | 0.67 |
| Model 2: adjusted for age, sex, smoking, drinking | 0.81 (0.34 to 1.90) | 0.62 |
| Model 3: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification | 0.72 (0.29 to 1.81) | 0.48 |
| Model 4: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification, GRACE score | 0.8 (0.33 to 1.96) | 0.63 |
| The impact of CCI on in-hospital MACEs | ||
| Model 1: unadjusted | 3.06 (2.22 to 4.22) | <0.001 |
| Model 2: adjusted for age, sex, smoking, drinking | 3.1 (2.25 to 4.28) | <0.001 |
| Model 3: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification | 3.84 (2.69 to 5.49) | <0.001 |
| Model 4: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification, GRACE score | 3.89 (2.71 to 5.57) | <0.001 |
| The impact of CCI on length of stay | ||
| Model 1: unadjusted | 1.36 (1.07 to 2.03) | <0.001 |
| Model 2: adjusted for age, sex, smoking, drinking | 1.41 (0.75 to 2.07) | <0.001 |
| Model 3: adjusted for age, sex, smoking, drinking, PCI, Coronary angiography, Killip classification | 1.39 (0.73 to 2.04) | <0.001 |
| Model 4: adjusted for age, sex, smoking, drinking, PCI, Coronary angiography, Killip classification, GRACE score | 1.34 (0.68 to 1.99) | <0.001 |
| The impact of CCI on readmission | ||
| Model 1: unadjusted | 1.29 (1.03 to 1.63) | 0.028 |
| Model 2: adjusted for age, sex, smoking, drinking | 1.28 (1.02 to 1.62) | 0.035 |
| Model 3: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification | 1.29 (1.02 to 1.63) | 0.035 |
| Model 4: adjusted for age, sex, smoking, drinking, PCI, coronary angiography, Killip classification, GRACE score | 1.28 (1.01 to 1.62) | 0.041 |
Statistically significant results (p<0.05) are displayed in bold.
CCI, Charlson Comorbidity Index; MACEs, major adverse cardiovascular events.
In-hospital major adverse cardiovascular events (MACEs)
There were 395 patients who experienced in-hospital MACEs, resulting in an incidence of 11.9%. The incidence of MACEs was significantly higher in patients with a high CCI compared with those with a low CCI, and this difference persisted even after PSM (table 2). Both univariate and multivariate logistic regression analyses were employed to assess the impact of CCI on the occurrence of MACEs. On average, patients with high CCI had twice the risk of MACEs compared with those CCI<3 (OR 2.09, 95% CI 2.22 to 4.22, p<0.001). This association remained significant when adjusted for age, sex, smoking and drinking (OR 3.1, 95% CI 2.25 to 4.28, p<0.001), as well as when further adjusted for PCI, Coronary angiography, Killip classification (OR 3.84, 95% CI 2.69 to 5.49, p<0.001) and GRACE 2.0 (OR 3.89, 95% CI 2.71 to 5.57, p<0.001) (table 3).
Length of stay (LOS)
The mean hospital LOS for patients with low CCI was 7.99±4.75 days, while for patients with high CCI, it was 10.64±7.43 days (p<0.001). Even after PSM, patients with high CCI still had a significantly longer hospital LOS compared with those with low CCI, with the difference being statistically significant (10.6±6.52 days vs 8.7±5.34 days, p<0.001) (table 2). The model results, without adjusting for confounding factors, indicated a statistically significant effect of CCI on hospital LOS (OR 1.36, 95% CI 1.07 to 2.03, p<0.001). This effect persisted even after adjusting for confounding factors including age, sex, smoking and drinking (OR 1.41, 95% CI 2.25 to 4.28, p<0.001), as well as further adjusting for PCI, coronary angiography, Killip classification (OR 1.39, 95% CI 2.69 to 5.49, p<0.001) and GRACE 2.0 score (OR 1.34, 95% CI 2.71 to 5.57, p<0.001) (table 3).
Readmission
The overall readmission rate was 34.5%, with a readmission rate of 32% for patients with low CCI and 41.4% for patients with high CCI, showing a statistically significant difference. After PSM, the readmission rates were 34.6% for low CCI patients and 40.8% for high CCI patients, again showing a statistically significant difference (table 2). The impact of high CCI on patient readmission rates was statistically significant without controlling for confounding factors. This effect persisted even after adjusting for confounding factors such as age, gender, smoking and drinking (OR 1.41, 95% CI 2.25 to 4.28, p<0.001), as well as when further adjusting for PCI, coronary angiography, Killip classification (OR 1.39, 95% CI 2.69 to 5.49, p<0.001) and GRACE 2.0 score (OR 1.34, 95% CI 2.7 to 5.57, p<0.001) (table 3).
Discussion
This study investigated the clinical characteristics of patients with NSTEACS and revealed the relationship between CCI, an indicator of comorbidity burden, and clinical outcomes during hospitalisation based on multicentre, large-sample data. Our study found that NSTEACS patients with a high CCI not only increases the risk of in-hospital MACEs but also prolongs the LOS and increases the readmission rate. ACS has emerged as a major cardiovascular condition, with NSTEACS accounting for a significant proportion. Patients with NSTEACS often present with one or more comorbidities, which elevates their mortality risk.23 While previous studies have analysed the effect of CCI on clinical outcomes of patients with NSTEACS, these studies were generally single-centre and involved small sizes.4 16 In contrast, our studies employed PSM to reduce bias and control for confounding factors.
Our study yielded several key findings. We observed a high prevalence of multimorbidity, with a CCI ≥3 representing 26% of the study population. The proportion of patients aged 65 and older was 69.07%, with 80.48% of these patients having a high CCI of ≥3. Turner reported that 49.9% of patients had multimorbidity and noted non-linear relationships between age and increasing comorbidities.24
Clinical guidelines recommend a holistic, patient-centred method when managing patients with NSTEACS.8 Given the rise in multimorbidity, clinicians need to integrate the assessment of coexisting conditions into decision-making processes and provide comprehensive information about the associated risks of adverse clinical outcomes to patients and their families. Multimorbidity is associated with many adverse health outcomes in the general older adult population, as well as in specific populations (eg, cancer, health failure, diabetes). These outcomes include diminished physical and cognitive functioning, reduced quality of life, as well as increased healthcare utilisation and mortality.25
In our study, there were 51 deaths, with a mortality rate of 1.5%. Patients with a high CCI experienced a higher mortality rate during hospitalisation. After adjusting for confounding factors using PSM, both univariate and multivariate logistic regression analyses showed that CCI did not have a statistically significant impact on in-hospital mortality. Asenso reported an in-hospital mortality rate of 6.1% for NSTEACS patients,16 which is slightly higher than our study. In contrast, Beska et al4 reported a mortality rate of 31%, significantly higher than our finding. This discrepancy may be due to the extended 5 year follow-up period in Beska et al’s study. In our study, the mortality rate among high CCI patients was significantly higher compared with low CCI patients. Although the impact of CCI on patient mortality did not reach statistical significance, this does not diminish the clinical relevance of CCI as a prognostic indicator. The lack of statistical significance may stem from the focus of our study solely on in-hospital mortality without long-term follow-up, which could be a contributing factor to this outcome. Future research should consider extending the follow-up period to further investigate these outcomes.
Despite the significant decrease in in-hospital mortality, the incidence of MACEs remained high. In this study, the incidence of MACE was 11.9%. Patients with a high CCI had an incidence of MACEs of 29.8%, significantly higher than the 5.5% observed in patients with a low CCI. This residual risk is likely due to inflammatory, prothrombotic and metabolic pathways that current therapies do not effectively address and is influenced by concomitant comorbidities.26 27 In our study, patients with CCI ≥3 had a threefold increased risk of the incidence of MACEs during hospitalisation compared with those with CCI <3. This impact persisted even after adjusting the confounding factors. Turner reported that approximately 7.8% of patients experienced MACEs, slightly lower than the incidence observed in our study. They also found multimorbidity was associated with increased MACEs.24 Gouda et al reported that, compared with ACS patients, NSTEACS patients have a higher incidence rate of MACEs. Each incremental pre-existing comorbidity was associated with a higher incidence rate of MACEs.28 Related studies have reported that comorbidities such as renal insufficiency and hypertension are independent risk factors for MACEs in NSTEACS patients.29,31 Currently, the impact of comorbidity on the occurrence of MACEs in NSTEACS patients has not been fully elucidated. These gaps can be partially attributed to the fact that comorbidities such as diabetes and chronic kidney disease contribute to systemic inflammation and stress responses, which can exacerbate atherosclerosis progression.32 This heightened inflammatory state increases the risk of MACEs in NSTEACS patients. Further studies are warranted to delineate the underlying pathophysiology of comorbidities for MACEs in NSTEACS patients.
Comorbidity not only exacerbates the clinical outcomes of patients with NSTEACS, but also extends the LOS and increases healthcare utilisation. Patients with high CCI typically have multiple chronic conditions, which can lead to an increase in complications, necessitating more complex and diverse treatment regimens. They also tend to recover more slowly, requiring longer periods of inpatient care and observation. The average length of hospital stay for NSTEACS patients was 8.69±5.71 days, with 7.99±4.45 days for patients with low CCI and 10.64±7.43 days for those with high CCI. Breen et al also reported multimorbidity was associated with increased LOS, with median LOS days in multimorbid patients ranging from 5 to 9 days compared with 3 to 4 days in non-multimorbid patients.7 In our study, CCI was significantly associated with prolonged length of hospital stay in NSTEACS patients. NSTEACS Patients with multimorbidity visit their primary care providers and specialists more often and are more likely to be admitted to the hospital and experience a longer hospital LOS.33 They are also more likely to require long-term care services.34 Consequently, multimorbidity contributes to higher healthcare costs and a significant economic burden.
Despite improvements in acute care and survival after NSTEACS hospitalisation, early readmissions remain common and have significant clinical and financial impacts. Patients with NSTEACS may encounter readmission due to procedure-related complications, recurrence of myocardial infarction or heart failure, severity of the patients’ medical condition, socioeconomic status and poor access to outpatient care.35 In our study, the readmission rate was 34.5%, with NSTEACS patients with CCI ≥3 having a significantly higher readmission rate than those with CCI <3. The risk of readmission was approximately 1.28 times higher in patients with high CCI than in those with low CCI, and this difference persisted after adjustment for multiple confounders. Lemor et al36 showed the 30-day readmission rate was 13.4%, which was lower than our study. They also identified predictors of 30-day readmissions after NSTEACS, finding that pre-existing kidney disease, prior coronary artery bypass grafting, age over 75 years and the presence of significant comorbidities defined as CCI ≥3 were independently associated with a greater readmission rate, which was in accordance with our study.
Our findings, when considered alongside the 2023 ESC Guidelines for acute coronary syndromes management,37 suggest potential value in a more individualised approach for NSTEACS patients with high CCI scores (CCI ≥3).
The first is risk stratification. While ESC guidelines emphasise GRACE scores for invasive strategy decisions,37 our observational data indicate that comorbidity status might be incorporated when evaluating risks of interventions in high-CCI patients. The second is therapy optimisation. Particular attention appears warranted for drug-disease interactions (eg, anti-thrombotic dosing in Chronic Kidney Disease CKD/malignancy) and polypharmacy review. The third is multidisciplinary coordination. The guideline-recommended assessment of frailty/cognitive status37 could potentially be extended through collaboration with geriatricians/pharmacists for multimorbid patients. The last one is early discharge planning and structured follow-up could be considered for high CCI patients. Our observed association between high CCI and readmission rates implies possible benefits of structured postdischarge monitoring and comorbidity-tailored rehabilitation.
It is important to note that, given the retrospective nature of our study, these suggestions should not be interpreted as definitive clinical recommendations. These observational insights, while aligned with guideline principles, require prospective validation before clinical implementation. The complex needs of NSTEACS patients with high CCI highlight an area warranting further investigation.
There are several limitations in our study. First, as a retrospective study, data collection relied on medical records, which may introduce the possibility of incomplete information and recall bias. Second, the retrospective nature of our study limits the complete randomisation of the exposure. We relied on PSM and multivariate regression models to control for confounders. Although the results obtained from both methods were similar, reducing the likelihood of confounding, residual confounding may still exist. Third, the data for our study were collected from seven different hospitals. The diagnostic validity of comorbidities might be influenced by heterogeneity in physician documentation and code assignment accuracy across different sites. Fourth, our analysis focused on the initial presentation and management within the emergency department (ED). Consequently, data regarding the specific level of in-hospital care received after ED disposition (eg, admission to general wards, ICUs or cardiac care units) were not routinely documented in the accessible EMR fields pertinent to the ED phase of care and were therefore not collected for this study. This limits our ability to describe the spectrum of inpatient resource utilisation or correlate ED findings with subsequent admission acuity levels. Similarly, detailed data on pharmacotherapy administered during the subsequent inpatient admission were not systematically captured within the ED-focused data extraction framework of this study. This precludes a comprehensive analysis of in-hospital medical management strategies beyond the initial ED phase. However, it is important to emphasise that the primary aim of this study was to investigate the impact of CCI on clinical outcomes of patients with NSTEACS at the point of emergency department presentation. The unavailability of detailed post-ED disposition and in-hospital medication data does not invalidate the core findings related to the ED-based aspects of care under investigation. Finally, we only focused on outcomes during the patients’ hospital stay and did not follow-up with the patients or consider their long-term outcomes. Therefore, future research should include prospective studies to examine the impact of the CCI on the long-term clinical outcomes of NSTEACS patients.
Conclusion
The current study investigated clinical characteristics and analysed the impact of CCI on the clinical outcomes of NSTEACS patients. Our study found that patients with a high CCI are older, have more comorbidities and experience a higher incidence of adverse clinical outcomes compared with those with a low CCI. A high CCI not only increases the risk of in-hospital MACEs but also prolongs the length of hospital stay and increases the readmission rate. However, the CCI was not significantly associated with in-hospital mortality in this cohort of NSTEACS patients. We recommend that the CCI be used as a crucial risk indicator for clinical practitioners to identify and manage patients with a poor prognosis.
Supplementary material
Footnotes
Funding: This study was supported by 2024 First-class Disciplines - Nursing Discipline Construction Fund, the First Affiliated Hospital of Chongqing Medical University; Project Code: 03010205040301. 2024 Nursing Research Innovation Project; Project Code:HLPY2024-14. The funder did not influence the results/outcomes of the study despite author affiliations with the funder.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-097359).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Ethics approval: This study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University. Reference number: K2023-142.
Data availability statement
Data are available upon reasonable request.
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 2.Ohira T, Iso H. Cardiovascular Disease Epidemiology in Asia. Circ J . 2013;77:1646–52. doi: 10.1253/circj.CJ-13-0702. [DOI] [PubMed] [Google Scholar]
- 3.Qayyum S, Rossington JA, Chelliah R, et al. Prospective cohort study of elderly patients with coronary artery disease: impact of frailty on quality of life and outcome. Open Heart. 2020;7:e001314. doi: 10.1136/openhrt-2020-001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beska B, Mills GB, Ratcovich H, et al. Impact of multimorbidity on long-term outcomes in older adults with non-ST elevation acute coronary syndrome in the North East of England: a multi-centre cohort study of patients undergoing invasive care. BMJ Open. 2022;12:e061830. doi: 10.1136/bmjopen-2022-061830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiménez-Méndez C, Díez-Villanueva P, Alfonso F. Non-ST segment elevation myocardial infarction in the elderly. Rev Cardiovasc Med. 2021;22:779–86. doi: 10.31083/j.rcm2203084. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Lopes RD, Harrington RA. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA. 2022;327:662–75. doi: 10.1001/jama.2022.0358. [DOI] [PubMed] [Google Scholar]
- 7.Breen K, Finnegan L, Vuckovic K, et al. Multimorbidity in Patients With Acute Coronary Syndrome Is Associated With Greater Mortality, Higher Readmission Rates, and Increased Length of Stay. J Cardiovasc Nurs. 2020;35:E99–110. doi: 10.1097/JCN.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collet J-P, Thiele H. The “Ten Commandments” for the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;41:3495–7. doi: 10.1093/eurheartj/ehaa624. [DOI] [PubMed] [Google Scholar]
- 9.Takeji Y, Shiomi H, Morimoto T, et al. Differences in mortality and causes of death between STEMI and NSTEMI in the early and late phases after acute myocardial infarction. PLoS One. 2021;16:e0259268. doi: 10.1371/journal.pone.0259268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation. 2014;130:1662–7. doi: 10.1161/CIR.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall M, Dondo TB, Yan AT, et al. Association of Clinical Factors and Therapeutic Strategies With Improvements in Survival Following Non-ST-Elevation Myocardial Infarction, 2003-2013. JAMA. 2016;316:1073–82. doi: 10.1001/jama.2016.10766. [DOI] [PubMed] [Google Scholar]
- 12.Powell H, Lim LL, Heller RF. Accuracy of administrative data to assess comorbidity in patients with heart disease. an Australian perspective. J Clin Epidemiol . 2001;54:687–93. doi: 10.1016/s0895-4356(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 13.Cohen M. Long-term outcomes in high-risk patients with non-ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2016;41:464–74. doi: 10.1007/s11239-015-1227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Carrozzino D, Guidi J, et al. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother Psychosom. 2022;91:8–35. doi: 10.1159/000521288. [DOI] [PubMed] [Google Scholar]
- 15.Tisminetzky M, Gurwitz JH, Miozzo R, et al. Impact of cardiac- and noncardiac-related conditions on adverse outcomes in patients hospitalized with acute myocardial infarction. J Comorb . 2019;9:2235042X19852499. doi: 10.1177/2235042X19852499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ofori-Asenso R, Zomer E, Chin KL, et al. Prevalence and impact of non-cardiovascular comorbidities among older adults hospitalized for non-ST segment elevation acute coronary syndrome. Cardiovasc Diagn Ther. 2019;9:250–61. doi: 10.21037/cdt.2019.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart . 2014;100:288–94. doi: 10.1136/heartjnl-2013-304588. [DOI] [PubMed] [Google Scholar]
- 18.Xin Z, Guan X, Zhi BZ. Guideline and consensus for the management of patients with non-ST-elevation acute coronary syndrome. Chinese Soc Cardiol Chinese Med Assoc Edit Board Chinese J Cardiol. 2017;45:359–76. doi: 10.3760/cma.j.issn.0253-3758.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Buske M, Feistritzer H-J, Jobs A, et al. Management of acute coronary syndrome : ESC guidelines 2023. Herz. 2024;49:5–14. doi: 10.1007/s00059-023-05222-1. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 21.Ekerstad N, Pettersson S, Alexander K, et al. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: A follow-up after more than 5 years. Eur J Prev Cardiol. 2018;25:1813–21. doi: 10.1177/2047487318799438. [DOI] [PubMed] [Google Scholar]
- 22.Kelshiker MA, Seligman H, Howard JP, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022;43:1582–93. doi: 10.1093/eurheartj/ehab775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canivell S, Muller O, Gencer B, et al. Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PLoS One. 2018;13:e0195174. doi: 10.1371/journal.pone.0195174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner RM, de Koning EM, Fontana V, et al. Multimorbidity, polypharmacy, and drug-drug-gene interactions following a non-ST elevation acute coronary syndrome: analysis of a multicentre observational study. BMC Med. 2020;18:367. doi: 10.1186/s12916-020-01827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadambi S, Abdallah M, Loh KPM. Function and Cognition in Aging. Clin Geriatr Med. 2020;36:569. doi: 10.1016/j.cger.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin F-J, Tseng W-K, Yin W-H, et al. Residual Risk Factors to Predict Major Adverse Cardiovascular Events in Atherosclerotic Cardiovascular Disease Patients with and without Diabetes Mellitus. Sci Rep. 2017;7:9179. doi: 10.1038/s41598-017-08741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani P, Puri R, Schwartz GG, et al. Association of Initial and Serial C-Reactive Protein Levels With Adverse Cardiovascular Events and Death After Acute Coronary Syndrome: A Secondary Analysis of the VISTA-16 Trial. JAMA Cardiol. 2019;4:314–20. doi: 10.1001/jamacardio.2019.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouda P, Savu A, Bainey KR, et al. Long-term risk of death and recurrent cardiovascular events following acute coronary syndromes. PLoS One. 2021;16:e0254008. doi: 10.1371/journal.pone.0254008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok CS, Hulme W, Olier I, et al. Review of early hospitalisation after percutaneous coronary intervention. Int J Cardiol. 2017;227:370–7. doi: 10.1016/j.ijcard.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 30.Omstedt Å, Höijer J, Djärv T, et al. Hypertension predicts major adverse cardiac events after discharge from the emergency department with unspecified chest pain. Eur Heart J Acute Cardiovasc Care. 2016;5:441–8. doi: 10.1177/2048872615626654. [DOI] [PubMed] [Google Scholar]
- 31.Tsai I-T, Wang C-P, Lu Y-C, et al. The burden of major adverse cardiac events in patients with coronary artery disease. BMC Cardiovasc Disord. 2017;17:1. doi: 10.1186/s12872-016-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature New Biol. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Frølich A, Ghith N, Schiøtz M, et al. Multimorbidity, healthcare utilization and socioeconomic status: A register-based study in Denmark. PLoS One. 2019;14:e0214183. doi: 10.1371/journal.pone.0214183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koller D, Schön G, Schäfer I, et al. Multimorbidity and long-term care dependency--a five-year follow-up. BMC Geriatr. 2014;14:70. doi: 10.1186/1471-2318-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemor A, Hernandez GA, Patel N, et al. Predictors and etiologies of 30‐day readmissions in patients with non‐ST‐elevation acute coronary syndrome. Cathet Cardio Intervent . 2019;93:373–9. doi: 10.1002/ccd.27838. [DOI] [PubMed] [Google Scholar]
- 37.Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]