Summary
The dynamic regulation of epigenetic states relies on complex macromolecular interactions. PRC2, the methyltransferase complex depositing H3K27me3, interacts with distinct accessory proteins to form the mutually exclusive subcomplexes PHF1-PRC2.1, MTF2-PRC2.1, PHF19-PRC2.1, and PRC2.2. The functions of these subcomplexes are thought to be largely redundant. Here we show that PRC2 subcomplexes have distinct roles in epigenetic repression of lineage-specific genes and stem cell differentiation. Using human pluripotent stem cells, we engineered a comprehensive set of separation-of-function mutants to dissect the roles of individual protein-protein and DNA-protein interactions. Our results show that PRC2.1 and PRC2.2 deposit H3K27me3 locus-specifically, resulting in opposing outcomes in cardiomyocyte differentiation. We find that MTF2 stimulates PRC2.1-mediated repression in stem cells and cardiac differentiation through its interaction with DNA and H3K36me3, while PHF19 antagonizes it. Together, these results reveal the importance and specificity of individual macromolecular interactions in Polycomb-mediated epigenetic repression in human stem cells and differentiation.
Keywords: Epigenetics, Polycomb, PRC2, H3K27me3, human pluripotent stem cells, cardiac differentiation
Introduction
The dynamic regulation of the epigenetic landscape is essential for development.1 Trimethylation of lysine 27 on histone H3 (H3K27me3) is the hallmark of facultative heterochromatin and controls epigenetic repression throughout development.2–4 The sole writer of H3K27me3 is Polycomb Repressive Complex 2 (PRC2), which catalyzes the mono-, di-, and trimethylation of H3K27.3 The PRC2 core complex consists of four subunits: EZH1/2, SUZ12, EED, and RBBP4/7. Dysregulation of PRC2 frequently leads to human diseases such as cancer and developmental defects.5–7 PRC2 inhibitors have proven effective in treating human diseases such as cancer.8–10
The spatiotemporal control of PRC2 recruitment and methylation specificity remains to be investigated. Among the PRC2 core subunits, the N-terminal SUZ12ΔVEFS has been demonstrated to be essential for PRC2 recruitment to CpG-rich promoters through its mutually exclusive interactions with PRC2 accessory subunits.11 PRC2’s accessory proteins play key roles in the regulation of PRC2.4,12–15 Two major PRC2 subcomplexes exist in the cell: PRC2.1, which contains one of the three Polycomb-like (PCL) proteins PHF1, MTF2, or PHF19 and occasionally EPOP and PALI1/2; and PRC2.2, which contains AEBP2 and JARID2.16 These accessory proteins contain functional domains that interact with DNA, RNA, the PRC2 core complex, and other epigenetic modifications such as H2AK119ub (AEBP2 and JARID217) and H3K36me3 (PCL proteins18–20). It remains unclear whether these dynamic macromolecular interactions have distinct roles in epigenetic repression.
Here we show that the specificity of PRC2-mediated epigenetic repression is regulated by a set of macromolecular interactions between the PRC2 core complex and different PRC2 accessory proteins, and between individual PRC2 accessory proteins and chromatin features such as histone modifications and DNA sequences. Using the separation-of-function mutants previously designed by Youmans et al.14, we identified distinct roles of the two PRC2 subcomplexes PRC2.1 and PRC2.2 in H3K27me3 deposition and opposing roles in stem cell cardiac differentiation. We determined the distinct roles of the three PRC2.1 subcomplexes containing each of the three PCL proteins using a combination of gene depletion and separation-of-function approaches. Furthermore, we identified distinct roles of PCL protein interactions with the PRC2 core complex, chromatin DNA, and H3K36me3 histone modification in epigenetic repression and cardiac differentiation. We propose that all these dynamic macromolecular interactions together contribute to fine-tuning the epigenetic state of developmental genes during stem cell differentiation.
Results
Distinct roles of the PRC2.1 and PRC2.2 subcomplexes in H3K27me3 deposition
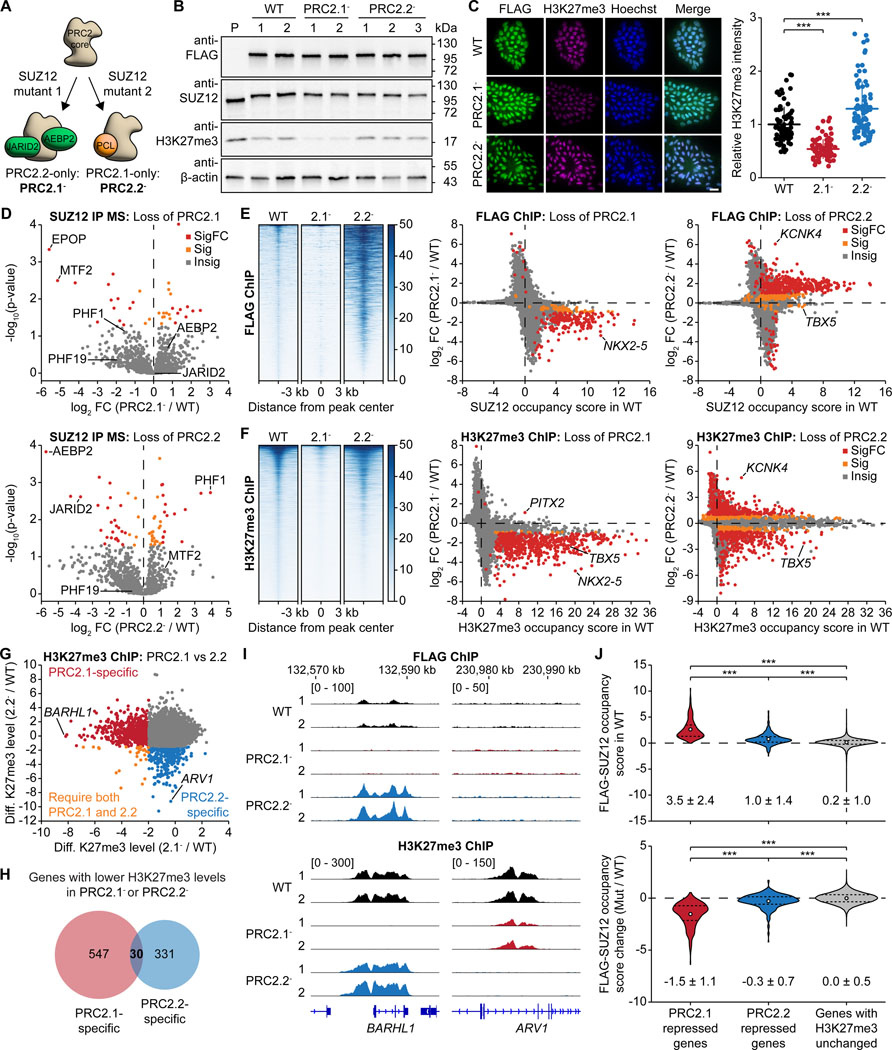
The SUZ12 subunit of the PRC2 core complex serves as an interaction hub with PRC2 accessory proteins.14,17,21 Two separation-of-function mutants of SUZ12 have been validated in human stem cells to separate the PRC2.1 and PRC2.2 subcomplexes: loss-of-PRC2.1 mutant ((338–353)-to-GSGSGS) and loss-of-PRC2.2 mutant (F99A, R103A, L105A, I106A) of SUZ12 to yield PRC2.2-only and PRC2.1-only complexes, respectively (Figure 1A).14 The identification of these two SUZ12 mutants allowed us to study the roles of the two subcomplexes, which in polyclonal cell lines were found to have opposite changes in SUZ12 occupancy.14 To further explore changes in H3K27me3 distribution, transcriptome and cell differentiation, we derived homozygous WT control, loss-of-PRC2.1 mutant SUZ12, and loss-of-PRC2.2 mutant SUZ12 clones from the human induced pluripotent stem cell (hiPSC) line WTC-11 using a dual antibiotic selection strategy for biallelic genome editing (Figures S1A-1C). The edited WT and mutant SUZ12 proteins contain a 3XFLAG tag and were expressed at similar levels as validated by western blotting (Figures 1B and S1D) and immunofluorescence staining (Figures 1C and S1E). Pluripotency factor genes and markers of all three germ layers were expressed at similar levels, confirming that all three lines remained pluripotent and were not pre-differentiated (Figures S1E and S1F).
Figure 1. Distinct roles of PRC2.1 and PRC2.2 subcomplexes in H3K27me3 deposition.
(A) Schematic showing the separation-of-function mutants to split the PRC2.1 and PRC2.2 subcomplexes.
(B) Western blot on whole-cell lysates of CRISPR-edited 3XFLAG-tagged WT and mutant SUZ12 hiPSCs PRC2.1- and PRC2.2-. 1–3, independent clones for each line; P, untagged parental line.
(C) Immunofluorescence micrographs of WT and mutant SUZ12 hiPSC lines stained with anti-FLAG and anti-H3K27me3 antibody. Scale bar represents 20 μm. Quantification of H3K27me3 signal is shown on the right. *** P < 0.001, two-tailed Student’s t-test.
(D) Mass spectrometry analysis of proteins co-immunoprecipitating with SUZ12 in WT and mutant SUZ12 hiPSCs. PRC2 accessory proteins are labelled. Insig: P ≥ 0.05, Sig: P < 0.05 and |log2FC| < 1, SigFC: P < 0.05 and |log2FC| ≥ 1.
(E and F) ChIP-seq analysis comparing genome-wide chromatin occupancy changes of FLAG-tagged SUZ12 (E) and H3K27me3 (F) upon disruption of PRC2.1 or PRC2.2. Heatmaps (left) and gene scatter plots (right) are shown. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC P < 0.1 and |log2FC| ≥ 1, empirical Wald test, FDR-adjusted.
(G) ChIP-seq scatter plot comparing the genome-wide differential H3K27me3 levels upon disruption of PRC2.1 and PRC2.2 compared to the WT, identifying PRC2.1 and PRC2.2-specific genes and those genes that require both subcomplexes. Cutoff: P < 0.1 and log2FC < 0.
(H) Venn diagram showing the overlap between PRC2.1 and PRC2.2-repressed genes as defined by their loss of H3K27me3 upon PRC2.1 or PRC2.2 disruption.
(I) FLAG and H3K27me3 ChIP-seq genome tracks of WT and mutant SUZ12 hiPSC lines at the BARHL1 and ARV1 gene loci.
(J) Violin plots showing the occupancy of FLAG-SUZ12 on PRC2.1 and PRC2.2-repressed genes in the WT (top) and its change upon loss of PRC2.1 or PRC2.2 (bottom). Numbers indicate the mean and standard deviation of each group of genes. *** P < 0.001, Welch’s t-test.
We next performed immunoprecipitation of SUZ12 followed by mass spectrometry analysis (IP MS) to confirm the specific loss of PRC2.1 or PRC2.2-associated proteins in each respective mutant. As expected, we found that PRC2.1-associated MTF2 and EPOP were the most dissociated proteins in the loss-of-PRC2.1 mutant, while PRC2.2-associated AEBP2 and JARID2 were among the most dissociated proteins in the loss-of-PRC2.2 mutant (Figures 1D, S2A, and S2B). We also identified previously unreported PRC2.1 and PRC2.2-associated proteins (Figure S2C, and Table S1). PHF1, one of the three PCL proteins in PRC2.1, was 15-fold more enriched with SUZ12 upon disruption of PRC2.2 (Figures 1D and S2B), implicating that loss of PRC2.2 may result in the formation of more PHF1-containing PRC2.1 subcomplexes.
We next assessed H3K27me3 levels in the SUZ12 variant lines. Western blot and immunofluorescence analyses showed that while SUZ12 levels were unchanged, H3K27me3 levels were decreased in the loss-of-PRC2.1 line but increased in the loss-of-PRC2.2 line compared to WT levels, indicating different effects of PRC2.1 and PRC2.2 in H3K27me3 deposition (Figures 1B, 1C, S1D, and S1E). We then asked whether the perturbation of PRC2 occupancy and H3K27me3 deposition was locus specific. FLAG ChIP-seq results showed that perturbation of PRC2.1 evicted SUZ12 from chromatin while perturbation of PRC2.2 increased SUZ12 chromatin occupancy (Figures 1E and S3A-S3C), consistent with previous results (Figure S3D).14 The increased SUZ12 chromatin occupancy in the absence of PRC2.2 was observed mostly on genes that were already significantly occupied by SUZ12 in the WT line (483 out of 619 genes, 78%), potentially driven by an increase in PHF1-containing PRC2.1 subcomplexes (Figures 1D and S2B). Beyond that, H3K27me3 ChIP-seq analysis revealed that perturbation of PRC2.1 abolished H3K27me3 peaks globally and on most PRC2 target genes (Figures 1F, S3B, and S3C, and Table S2), consistent with the global loss of SUZ12 occupancy (Figure 1E). Interestingly, perturbation of PRC2.2 led to a bidirectional change in H3K27me3 levels (Figure 1F and Table S2). On one hand, genes with a reduction of H3K27me3 in the loss-of-PRC2.2 line specifically depend on PRC2.2 for H3K27me3 deposition. On the other hand, a gain of H3K27me3 may be caused by elevated levels of PHF1-containing PRC2.1 (IP MS data in Figures 1D and S2B).
We furthermore observed distinct H3K27 methylation specificity by PRC2.1 and PRC2.2. For example, the adjacent ROPN1B and SLC41A3 genes exhibit comparable H3K27me3 levels in WT hiPSCs, but specifically require PRC2.1 and PRC2.2 for H3K27me3 deposition, respectively (Figure S3E). This gene-specific requirement for either PRC2.1 or PRC2.2 was also observed globally: loss of PRC2.1 led to a significant loss of H3K27me3 on 577 genes, while loss of PRC2.2 caused a significant loss of H3K27me3 on 361 genes (Figure 1G, 1H and S3F). Interestingly, 30 genes require both PRC2.1 and PRC2.2 to be present for H3K27me3 deposition. For the remaining 798 H3K27me3-decorated genes, PRC2.1 and PRC2.2 can compensate each other upon loss of the other subcomplex (Figures S3G-S3H), agreeing with the previously demonstrated overlapping function of the two subcomplexes.11,14 Gene ontology analysis revealed that PRC2.1-specific H3K27me3 deposition mainly occurred on developmental genes, especially transcription factor genes (Figure S3I). Genes with PRC2.1-specific H3K27me3 deposition had a significantly higher GC content compared to genes with PRC2.2-specific H3K27me3 deposition or all genes in the genome (Figure S3J), consistent with PRC2.1’s role in interacting with CpG islands.21–23 Interestingly, PRC2.2-specific genes (such as ARV1) had no FLAG-SUZ12 occupancy, in contrast to the highly SUZ12-occupied PRC2.1-specific genes (such as BARHL1) (Figure 1I). This phenomenon was observed globally: PRC2.2-specific genes had low SUZ12 occupancy in the WT and limited change of SUZ12 occupancy upon loss of PRC2.2 (Figure 1J), while PRC2.1-specific genes had high SUZ12 occupancy in the WT and significantly lower SUZ12 occupancy upon loss of PRC2.1. These observations suggest that PRC2.2 has low chromatin residence time and could “methylate and run”, while PRC2.1 stays at its target genes as a “reader” after H3K27me3 deposition.
Association with accessory proteins enhances histone methylation activity of PRC2 in a DNA-dependent manner
To biochemically evaluate how the binding of accessory proteins affects the catalytic activity of PRC2, we tested the histone methylation activities of a series of purified recombinant PRC2 complexes on reconstituted mononucleosomes and DNA-free histone octamers (Figure S4A). The PRC2 core complex exhibited almost identical methylation activity on mononucleosome and histone octamer substrates (Figure S4B). In contrast, association with PHF19, MTF2, AEBP2, or JARID2 significantly enhanced the catalytic activity of PRC2 specifically on the mononucleosome but not on the DNA-free histone octamer substrate (Figures S4C-S4F). PRC2.1 complexes exhibited higher methylation activities on the mononucleosome substrate than PRC2.2 complexes did, especially at high enzyme concentrations. The stimulation of PRC2 catalytic activity on mononucleosomes is potentially caused by an increase of nucleosome-PRC2 binding affinity (Figure S4G). These results suggest that accessory proteins help to engage the PRC2 enzyme with the histone H3 substrate in a DNA-dependent manner.
PRC2.1 and PRC2.2 subcomplexes distinctly regulate PRC1 recruitment and the transcriptome
We next investigated the roles of the PRC2.1 and PRC2.2 subcomplexes in regulating the epigenome beyond H3K27me3. H3K27me3 recruits PRC1 to chromatin,24,25 while JARID2 and AEBP2 interact with the PRC1-catalyzed H2AK119ub mark.17 ChIP-seq for PRC1’s catalytic subunit RING1B showed a distinct alteration of RING1B chromatin occupancy upon perturbation of PRC2.1 or PRC2.2 (Figure S5A). CBX7, another PRC1 component, has been shown to mediate crosstalk between JARID2-PRC2.2 and PRC1.12 Indeed, perturbation of PRC2.2 caused a loss of CBX7 chromatin occupancy on 13 genes (Figure S5B). Surprisingly, perturbation of PRC2.1 evicted CBX7 from even more genes, which may be caused by the loss of H3K27me3 rather than JARID2-mediated CBX7 recruitment.12 In contrast to the drastic alterations of RING1B occupancy, the genome-wide changes in H2AK119ub levels were limited (Figure S5C). It is expected that changes in RING1B occupancy and H2AK119ub levels do not correlate, because the canonical PRC1 (cPRC1) that specifically recognizes H3K27me3 has a lower catalytic activity compared to the variant PRC1 (vPRC1) that does not recognize H3K27me3.26–28 Interestingly, PRC2.1 and PRC2.2 played opposite roles in recruiting RING1B to 15 genes, including PITX2 (Figure S5D), while both PRC2.1 and PRC2.2 were required at the TBX5 gene (Figure S5D).
For H3K4me3 and H3K36me3, both of which antagonize PRC2 activity,29–31 we found that loss of either PRC2.1 or PRC2.2 resulted in limited changes of either modification, with the loss of PRC2.2 impacting more genes than PRC2.1 did (Figures S5F and S5G).
RNA-seq showed that loss of PRC2.1 resulted in an upregulation of PRC2.1-repressed genes, most of which are developmental transcription factor genes and key signaling protein genes (Figures S6A-S6C and Table S3). Only some, but not all, of the PRC2.1-repressed genes were upregulated in the undifferentiated state, likely because the lack of lineage-specific transcription signals left most genes in an epigenetically primed but not yet transcriptionally activated state. The change in RNA transcript abundance anti-correlated with the change in H3K27me3 levels for each H3K27me3-decorated gene (Figure S6D). In contrast, we observed much larger transcriptomic changes in the loss-of-PRC2.2 lines (Figures S6A and S6B, and Table S3). In summary, we show that loss of PRC2.1 and PRC2.2 causes distinct changes to the epigenetic landscape such as PRC1 recruitment and transcriptomic responses in undifferentiated human stem cells.
PRC2.2 promotes, while PRC2.1 antagonizes cardiomyocyte differentiation
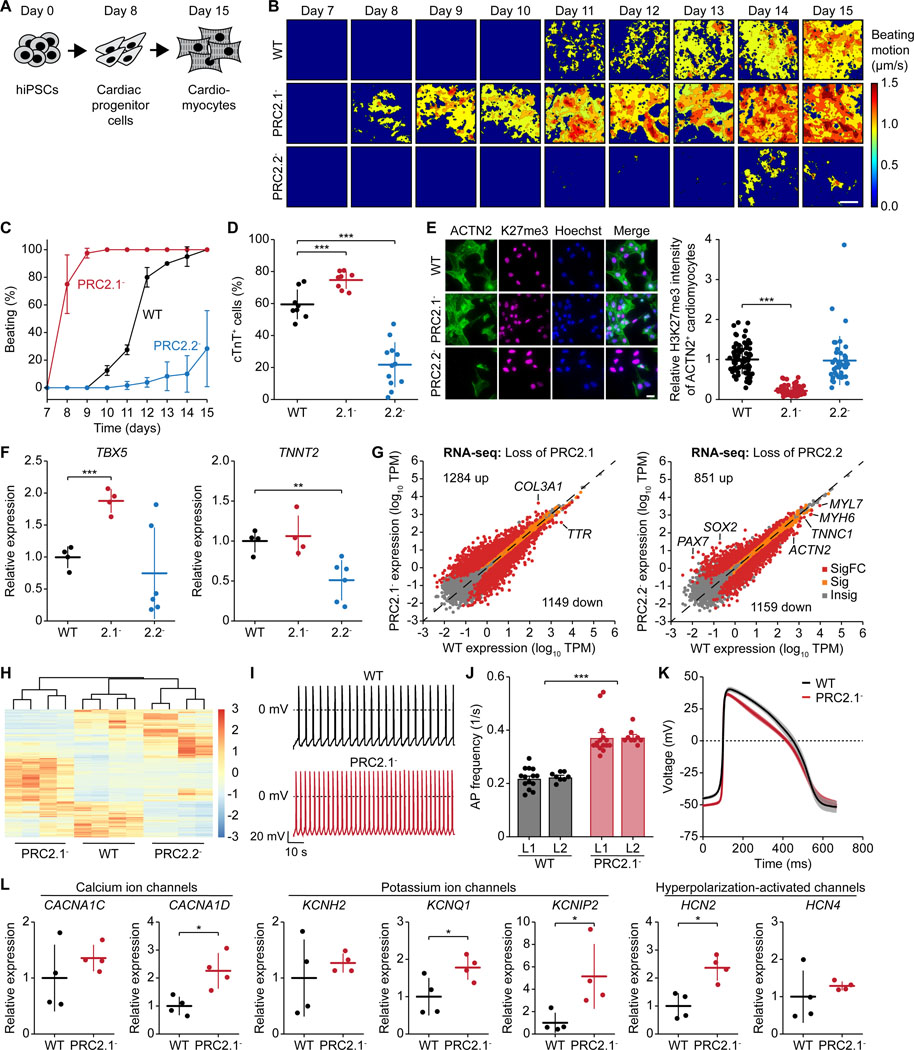
We next investigated the importance of PRC2.1 and PRC2.2 subcomplexes in stem cell differentiation. Notably, PRC2 subcomplexes are important for neural differentiation.13 As loss of PRC2.1 or PRC2.2 impacted the chromatin features of two master regulators of cardiac differentiation (NKX2.5 and TBX5, Figures 1E and 1F), we induced the differentiation of the WT and mutant SUZ12 hiPSC lines to cardiomyocytes (Figure 2A).32,33 Perturbation of PRC2.1 and PRC2.2 led to opposing effects on cardiac differentiation: The loss-of-PRC2.1 line exhibited accelerated differentiation as robust spontaneous contractions were observed by day 8 (Figures 2B and 2C). In contrast, the loss-of-PRC2.2 line showed delayed and weaker spontaneous contractions. These opposing roles in cardiac differentiation were further evident as perturbation of PRC2.1 increased, and perturbation of PRC2.2 decreased, the percentage of cTnT-expressing cells compared to the WT (Figures 2D and S7A). Immunofluorescence imaging of day 15 cardiomyocytes additionally showed that the loss-of-PRC2.2 line exhibited a lower percentage of cells expressing the cardiac marker genes ACTN2 and TBX5 (Figures 2E and S7B). ACTN2-expressing cardiomyocytes also featured decreased H3K27me3 levels in the loss-of-PRC2.1 line (Figure 2E), similar to the H3K27me3 dysregulation observed in the undifferentiated hiPSCs (Figures 1B, 1C, and 1F).
Figure 2. Distinct functions of PRC2.1 and PRC2.2 subcomplexes in cardiomyocyte differentiation.
(A) Schematic showing the induced cardiac differentiation of hiPSCs.
(B) Heatmaps showing the time-averaged magnitude of spontaneous contractions of WT, PRC2.1-, and PRC2.2- cardiomyocytes along the differentiation process.
(C) Percentage of cell culture wells with spontaneous contractions of WT and mutant SUZ12 cardiomyocytes over time.
(D) Percentage of WT and mutant SUZ12 cells expressing cTnT after 15 days of differentiation. *** P < 0.001, two-tailed Student’s t-test.
(E) Immunofluorescence micrographs of WT and mutant SUZ12 day 15 cardiomyocytes. Scale bar represents 20 μm. Quantification of the H3K27me3 signal of ACTN2+ day 15 cardiomyocytes is shown on the right. *** P < 0.001, two-tailed Student’s t-test.
(F) Relative expression of cardiomyocyte marker genes in day 15 cardiomyocytes as determined by qRT-PCR. * P < 0.05, ** P < 0.01, *** P < 0.001, two-tailed Student’s t-test.
(G) RNA-seq scatter plots showing gene expression changes upon loss of PRC2.1 or PRC2.2 in day 15 cardiomyocytes. Insig: P ≥ 0.05, Sig: P < 0.05 and |log2FC| < 1, SigFC: P < 0.05 and |log2FC| ≥ 1, two-sided Wald test.
(H) Gene cluster heatmap showing SigFC gene expression changes among WT, PRC2.1-, and PRC2.2- day 15 cardiomyocytes.
(I-K) Electrophysiological analysis of WT and PRC2.1- day 24 cardiomyocytes. Representative action potential (AP) traces (I), bar plots of average AP frequency (J), and average single AP trace of all measured cells over time (K) of spontaneous contractions are shown. *** P < 0.001, two-way ANOVA.
(L) Relative expression of ion channel genes in day 24 cardiomyocytes as determined by qRT-PCR. * P < 0.05, two-tailed Student’s t-test.
Regarding gene expression, the loss-of-PRC2.1 line featured significantly higher TBX5 expression levels, while the loss-of-PRC2.2 line expressed significantly less TNNT2 (encoding cTnT, Figure 2F). RNA-seq further revealed a massive dysregulation of gene expression in both lines (Figures 2G and 2H, and Table S4), with most dysregulated genes having key roles in development (Figure S7C). The drastic increase of transcriptomic changes in the loss-of-PRC2.1 line from undifferentiated to differentiated state has been observed previously13 and could be caused by the presence of lineage-specific signals.
Next, we aimed to determine if the loss of PRC2.1 and PRC2.2 affected the electrical properties of the cardiomyocytes. We were unable to record any spontaneous action potential (AP) in the loss-of-PRC2.2 line due to its cardiac differentiation defect. In contrast, we found that the loss-of-PRC2.1 cardiomyocytes featured an AP frequency that was almost doubled compared to the WT (Figures 2I and 2J). There was a trend for faster repolarization in the loss-of-PRC2.1 line (Figure 2K), which is also reflected by the significantly lower AP duration at 30% repolarization level (APD30) (Figures S7D and S7E). The significantly increased AP frequency points to an ion transport abnormality, potentially caused by a de-repression of ion channel genes (Figure 2L). The observed upregulation of HCN2 channels would increase spontaneous pacing rates and upregulation of KCNQ1 channels or the KCNIP2-encoded KChIP2 auxiliary subunit could accelerate repolarization. Together, we conclude that the PRC2 subcomplexes exert distinct functions during cardiac differentiation, with PRC2.2 promoting and PRC2.1 antagonizing the process.
Distinct and overlapping roles of PCL proteins in PRC2.1 function
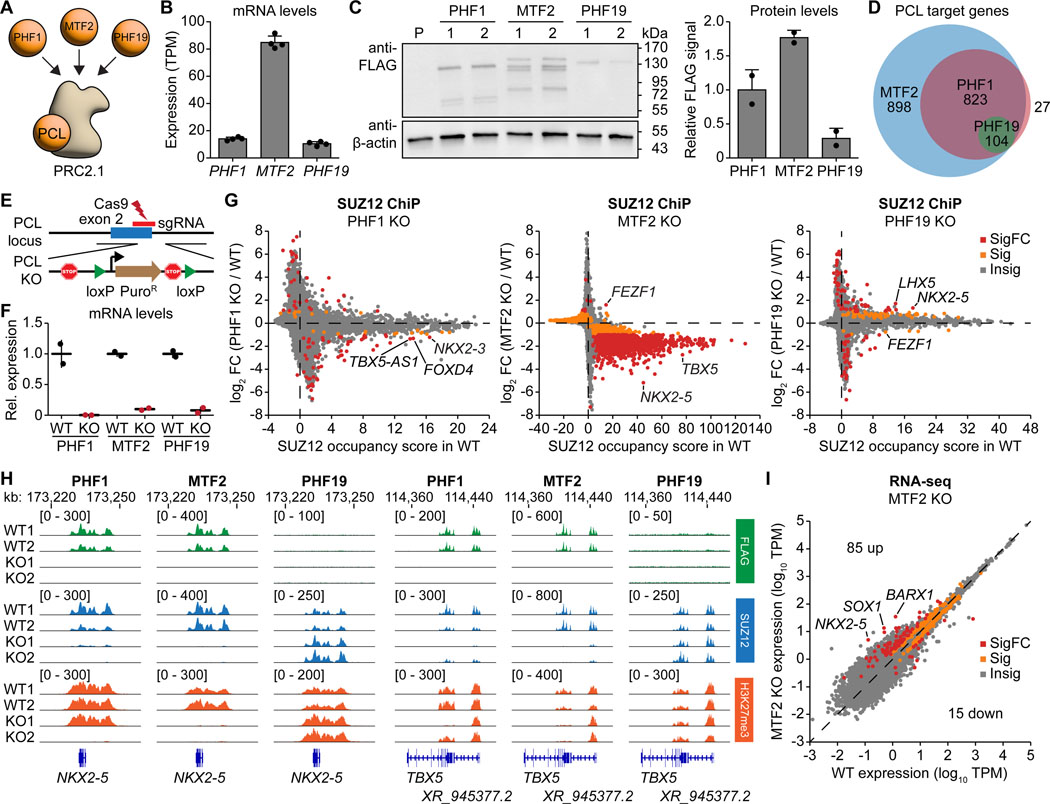
PRC2.1 can be further divided into three mutually exclusive subcomplexes (Figure 3A), and it remains unclear whether the three PCL proteins have overlapping or distinct functions. Among the PCL proteins, MTF2 showed the highest expression at the RNA level in hiPSCs (Figure 3B), although PCL protein expression patterns can change upon differentiation.34 We generated three hiPSC lines by attaching a 3XFLAG-HALO tag to the C-terminus of each PCL protein. Anti-FLAG western blotting confirmed that MTF2 is the most abundant PCL protein in hiPSCs (Figure 3C). FLAG ChIP-seq showed that the number of target genes positively correlates with the protein expression level of each PCL protein (Figure 3D). Interestingly, their target genes are highly overlapping (Figure 3D and Table S5), including similar pathway genes in cell differentiation and transcriptional regulation (Figure S8A).
Figure 3. Distinct and overlapping roles of PCL proteins in PRC2.1 function.
(A) Schematic illustrating the three PCL proteins PHF1, MTF2, and PHF19 associating with the PRC2 core to form three mutually exclusive PRC2.1 subcomplexes.
(B) Expression of the three PCL genes in hiPSCs as measured by RNA-seq.
(C) Western blot on whole-cell lysates of CRISPR-edited 3XFLAG-HALO-tagged PHF1, MTF2, and PHF19 hiPSCs. Quantification of the FLAG-tagged protein signal of each PCL protein normalized to the β-actin signal is shown on the right. P, untagged parental line.
(D) Venn diagram showing the number of distinct and overlapping target genes of the three PCL proteins as defined by FLAG-tagged PCL protein occupancy in the ChIP-seq experiments.
(E) Schematic illustrating the PCL gene depletion strategy by inserting poly(A) sites together with a loxP-flanked puromycin resistance gene expression cassette immediately downstream of the translation start site.
(F) Relative expression of each PCL gene in WT and KO hiPSC lines as determined by qRT-PCR.
(G) ChIP-seq gene scatter plot comparing genome-wide SUZ12 chromatin occupancy changes upon loss of PHF1, MTF2, or PHF19. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
(H) FLAG, SUZ12, and H3K27me3 ChIP-seq genome tracks of the PCL protein WT and KO hiPSC lines at the NKX2–5 and TBX5 gene loci.
(I) RNA-seq scatter plots showing gene expression changes upon MTF2 depletion. Insig: P ≥ 0.05, Sig: P < 0.05 and |log2FC| < 1, SigFC: P < 0.05 and |log2FC| ≥ 1, two-sided Wald test.
We next asked whether such high degree of shared target genes also translates to high functional redundancy. We generated knockout hiPSC lines for each PCL protein (Figure 3E) and confirmed their depletion at the mRNA and protein level (Figures 3F, S8B, and S8C). Depletion of MTF2 led to a global loss of SUZ12 chromatin occupancy, while depletion of PHF1 or PHF19 resulted in milder changes (Figures 3G and S8D). Two of the genes with the largest loss of SUZ12 occupancy upon depletion of MTF2 and PHF1 were the cardiac transcription factor genes NKX2–5 and TBX5 (Figure 3H). Surprisingly, depletion of PHF19 led to a gain of SUZ12 occupancy on NKX2–5 but not TBX5 (Figure 3H), suggesting that PHF19 inhibits PRC2 recruitment at selected gene loci. The changes in H3K27me3 levels were more modest and gene-specific, indicating that PRC2 catalytic activity may not always positively correlate with its chromatin occupancy and compensatory mechanisms may potentially rescue the H3K27me3 dysregulation (Figures S8E and S8F). Genes with altered expression in the MTF2 KO hiPSCs were associated with cell differentiation and transcriptional regulation (Figures 3I and S8G). These results suggest that the three PCL proteins have overlapping yet distinct functions in PRC2 recruitment, with MTF2 playing the most dominant role in undifferentiated hiPSCs.
Disrupting the interaction of individual PCL proteins with the PRC2.1 subcomplex results in different epigenetic consequences
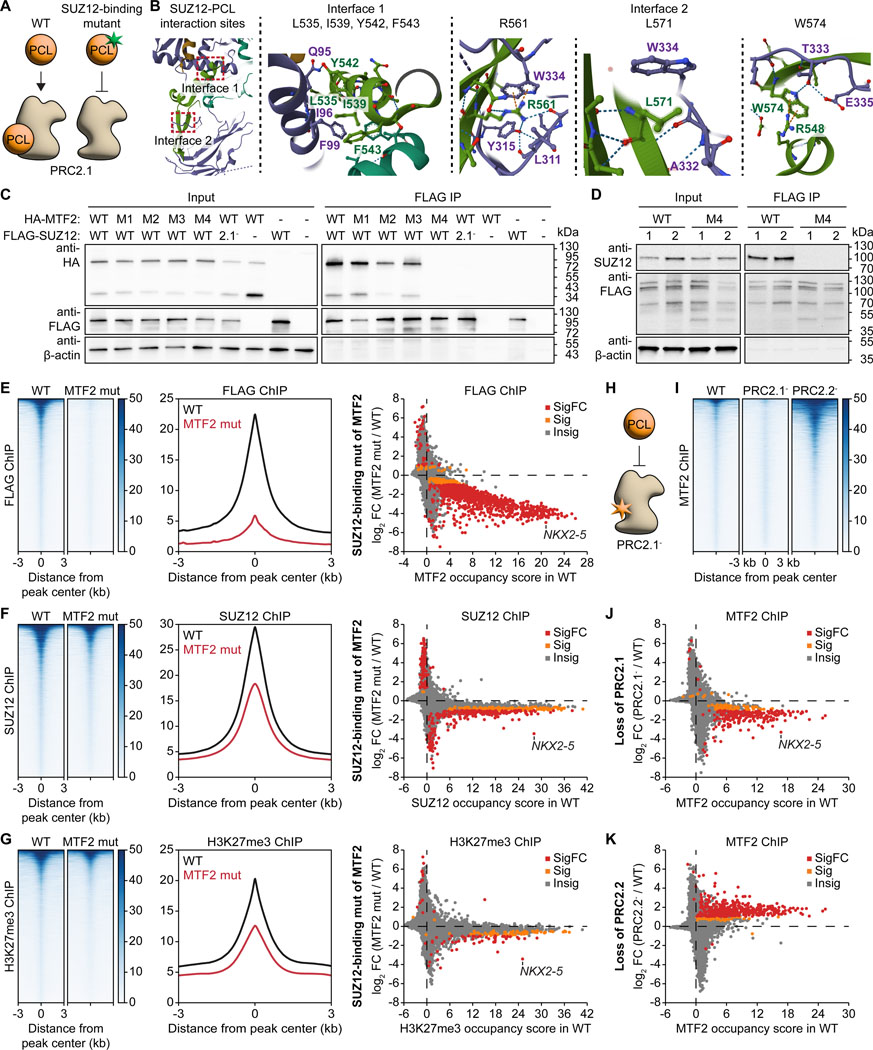
As the complete loss of PCL proteins could lead to secondary effects independent of PRC2 function, we next aimed to engineer separation-of-function mutants to disrupt the interaction between each of the three PCL proteins and PRC2 (Figure 4A). A crystal structure captured two interfaces between SUZ12 and PHF19 21 (Figure 4B). Mutation of all four residues (L535, I539, Y542, and F543) of interface 1 on MTF2 did not affect the interaction with SUZ12 (Figure 4C). However, mutation of the three residues of interface 2 (M4 mutant: R574A, L584A, and W587A) completely abolished SUZ12 interaction. These three conserved PCL residues of interface 2 are also essential for PHF1 and PHF19 to interact with SUZ12 (Figures S9A and S9B).
Figure 4. Splitting each PCL protein from the PRC2.1 subcomplex results in different epigenetic consequences.
(A) Schematic showing the engineering of PCL protein separation-of-function mutants to disrupt their interaction with SUZ12 and the PRC2 core.
(B) Crystal structure (PDB: 6NQ3) highlighting the two interaction sites between SUZ12 (purple) and PHF19 (green) including critical amino acid residues.
(C) Co-immunoprecipitation assay of transiently expressed ectopic HA-tagged MTF2 and FLAG-tagged SUZ12 in HEK293T cells. Input samples represent the whole cell lysate and the FLAG-IP samples the fraction eluted off the anti-FLAG beads. Mutants of MTF2: M1: L548A, I552A, Y555A, F556A; M2: L584A, W587A; M3: R574A; M4: R574A, L584A, W587A. PRC2.1- mutant of SUZ12: (338–353)GSGSGS.
(D) Co-immunoprecipitation assay performed in WT and SUZ12-binding mutant MTF2 hiPSCs. Whole cell lysate (input) was incubated with anti-FLAG beads to pull down the FLAG-tagged MTF2. M4 mutant as in (C): R574A, L584A, W587A.
(E-G) ChIP-seq analysis comparing genome-wide chromatin occupancy changes of FLAG-tagged MTF2 (E), SUZ12 (F), and H3K27me3 (G) between WT and the SUZ12-binding mutant of MTF2. Heatmaps (left), metaplots of the peak signals (middle), and gene scatter plots (right) are shown. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
(H) Schematic showing the disruption of the PCL protein-PRC2 core interaction using the PRC2.1- mutant.
(I-K) ChIP-seq analysis comparing genome-wide chromatin occupancy changes of MTF2 upon loss of PRC2.1 and PRC2.2. Heatmaps (I) and gene scatter plots for PRC2.1- (J) and PRC2.2- (K) are shown. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
We then introduced the identified SUZ12-binding mutations at the respective endogenous PCL gene locus in hiPSCs. Immunoprecipitation of FLAG-MTF2 confirmed that the SUZ12 interaction was abolished in the mutant (Figure 4D). ChIP-seq showed that loss of the MTF2-SUZ12 interaction decreased the occupancy of both MTF2 and SUZ12 on the majority of PRC2 target genes and was concomitant with decreased H3K27me3 levels (Figures 4E–4G and S9G), consistent with previous depletion studies.4,11–13,15 Disruption of the PHF1-SUZ12 interaction led to a reduction of SUZ12 chromatin occupancy on fewer genes (Figures S9C and S9E). Surprisingly, perturbation of the PHF19-SUZ12 interaction resulted in a global increase in SUZ12 chromatin occupancy (Figures S9D and S9F), suggesting that PHF19 antagonizes PRC2 recruitment. Our H3K27me3 ChIP-seq results further support the distinct roles of the PCL proteins (Figures 4G, S9E, and S9F).
We next found that the PCL proteins depend on the PRC2 core complex to stably associate with chromatin, as FLAG ChIP-seq results demonstrated that the PCL proteins collectively exerted lower genome-wide chromatin occupancy in the SUZ12-binding mutant PCL protein hiPSC lines (Figures 4E and S9C-S9F). This confirms the previous finding that deletion of SUZ12 leads to the loss of MTF2 from its target sites.11 We also performed MTF2 ChIP-seq in the loss-of-PRC2.1 and loss-of-PRC2.2 hiPSC lines (Figure 4H). Indeed, perturbation of PRC2.1 led to a loss of MTF2 chromatin occupancy genome-wide (Figures 4I and 4J). Conversely, perturbation of PRC2.2 led to increased MTF2 chromatin occupancy (Figures 4I and 4K). These data further support the distinct chromatin affinity of PRC2.1 and PRC2.2 (Figure 1E).
The DNA-PCL protein interaction is the main driver of Polycomb-mediated repression
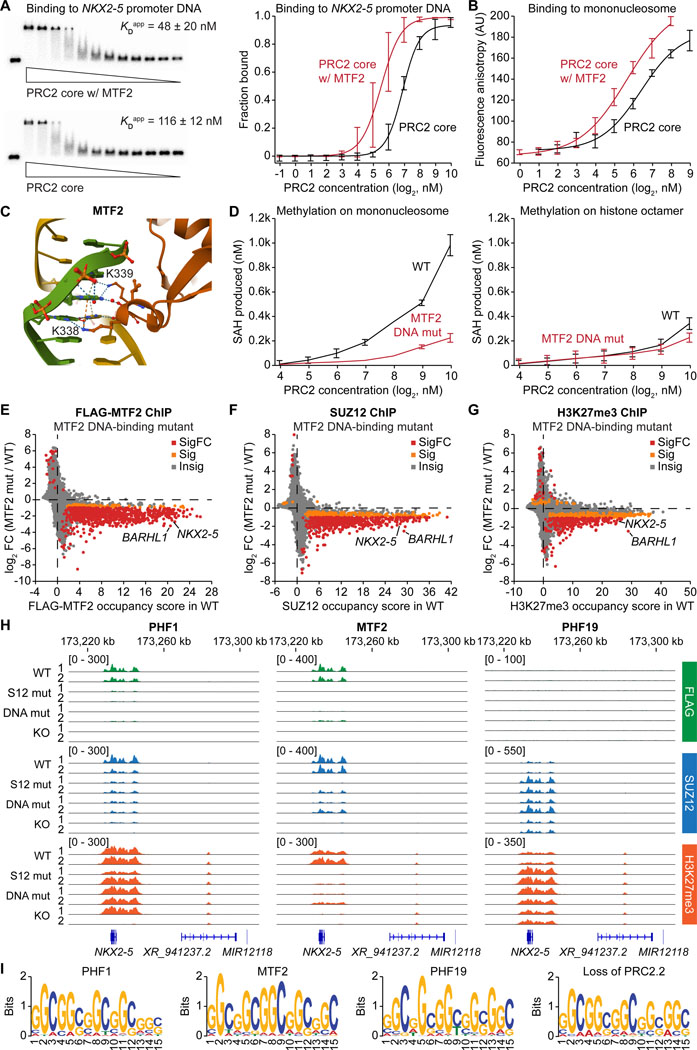
Mammalian PRC2 preferentially binds CpG-rich DNA sequences,35–38 and these DNA interactions depend on the winged-helix domain of the PCL proteins.21–23 We compared the DNA and nucleosome binding affinity of recombinant PRC2 core and MTF2-bound PRC2.1 complexes. Electrophoretic mobility shift assay (EMSA) showed that the MTF2-PRC2.1 complex exhibited a higher binding affinity to a dsDNA from the NKX2–5 promoter region than the PRC2 core complex alone did (Figure 5A). Significantly increased binding affinity was also observed on HOXA9 promoter DNA, but the difference was smaller on a non-PRC2 target SSTR4 promoter DNA (Figure S10A), indicating that MTF2-DNA binding is specific to PRC2 target genes. Fluorescence polarization assay further confirmed that binding to a reconstituted mononucleosome was significantly enhanced with the addition of MTF2 to the PRC2 core complex (Figure 5B).
Figure 5. The DNA-PCL protein interaction is a main driver of PRC2-chromatin interaction.
(A) EMSA comparing the binding affinity between an NKX2–5 promoter DNA and the recombinant PRC2 core or the MTF2-containing PRC2.1 complex. Representative EMSA gels (left) and quantification of the fraction of DNA bound (right) are shown.
(B) Fluorescence polarization assay measuring the binding affinity between the PRC2 core or the MTF2-containing PRC2.1 complex and fluorescently labeled mononucleosome.
(C) Crystal structure (PDB: 5XFR) highlighting the two lysine residues of MTF2 (K338 and K339) interacting with the major groove of double-stranded DNA.
(D) Histone H3 methylation assay using recombinant WT or DNA-binding mutant (K338A, K339A) MTF2-containing PRC2.1 complexes on either reconstituted mononucleosome or histone octamer.
(E-G) ChIP-seq gene scatter plots comparing genome-wide chromatin occupancy changes of FLAG-MTF2 (E), SUZ12 (F), and H3K27me3 (G) between WT and DNA-binding mutant MTF2 hiPSC lines. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
(H) FLAG, SUZ12, and H3K27me3 ChIP-seq genome tracks of WT, SUZ12-binding mutant, DNA-binding mutant, and KO of each PCL protein around the NKX2–5 gene locus.
(I) Sequence logos of the most significant DNA motif for PHF1, MTF2, and PHF19 identified in the FLAG ChIP-seq of the respective FLAG-tagged PCL protein hiPSC lines.
DNA-interacting lysine residues have been reported in the PCL protein winged-helix domain (Figure 5C).22 We purified a mutant MTF2-PRC2.1 complex carrying K338A and K339A mutations in MTF2. Compared to the WT, the mutant PRC2.1 exhibited a significant methylation activity defect on a DNA-containing mononucleosome, while no changes in activity were observed on a DNA-free histone octamer substrate (Figure 5D), suggesting that the two conserved lysine residues in MTF2 are critical for PRC2 engagement with the nucleosome substrate.
We then generated homozygous DNA-binding mutant PCL protein hiPSC lines with the double lysine mutations. FLAG ChIP-seq analysis revealed that both the DNA-binding mutants of PHF1 and MTF2 lost their association with chromatin (Figures 5E and S10B). Consequently, the DNA-binding mutant MTF2 lines exhibited a global loss of SUZ12 chromatin occupancy and decreased H3K27me3 levels on most PRC2 target genes (Figures 5F and 5G), while only a few genes were affected in the DNA-binding mutant of PHF1 (Figure S10B). Again, the PHF19 mutant showed a gain of SUZ12 occupancy and H3K27me3 levels on many PRC2 target genes (Figure S10C). Specifically, MTF2 increased both PRC2 recruitment and H3K27me3 levels at the cardiac transcription factor gene NKX2–5, while PHF19 decreased them (Figure 5H). Of note, the distinct functions of the PCL proteins are likely not based on different DNA binding preferences, as they recognize the same CGG-rich binding motif (Figure 5I). Moreover, this motif was also enriched in the loss-of-PRC2.2 but not loss-of-PRC2.1 hiPSCs (Figure 5I). Together, our data suggest that all three PCL proteins specifically recognize CGG-rich DNA sequences but exert distinct roles in PRC2 recruitment and H3K27me3 deposition.
The H3K36me3-MTF2 interaction provides an alternative recruitment mechanism for PRC2 and stimulates H3K36me3 demethylation at TBX5
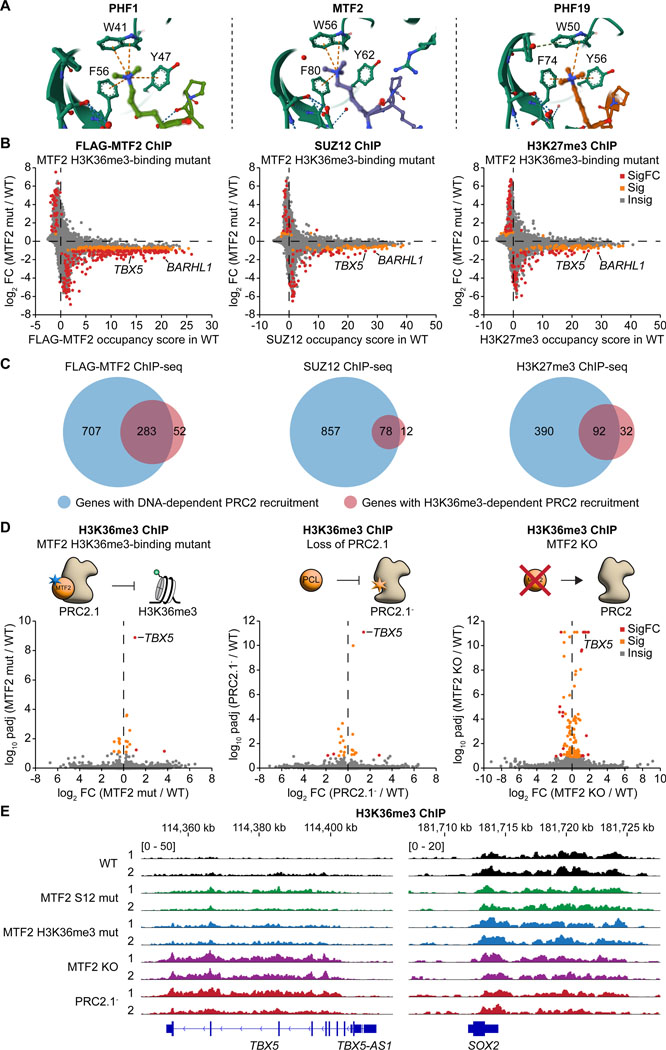
The N-terminal Tudor domain of PCL proteins specifically interacts with H3K36me3, which decorates the gene body of actively transcribed genes.22,39 The interaction is important for PHF1 and PHF19 to recruit PRC2 to H3K36me3-marked genes.18,19 In contrast, the Tudor domain of MTF2 has a lower affinity for H3K36me3,22 and its function remains unclear. Based on the solved structures of PHF1,40 MTF2,22 and PHF19,19 three residues within the Tudor domain form an aromatic cage to lock the H3K36me3 in place (Figure 6A). We generated an MTF2 H3K36me3-binding mutant hiPSC line by introducing the Y62A mutation. Mutation of this residue in PCL proteins (Y47A of PHF1 or Y56A of PHF19) has been validated to disrupt H3K36me3 recognition.18,19 ChIP-seq on FLAG-tagged MTF2, SUZ12, and H3K27me3 revealed that the Y62A mutation on MTF2 led to a predominant decrease in MTF2 and SUZ12 chromatin occupancy and H3K27me3 levels on PRC2 target genes, including TBX5 and BARHL1 (Figure 6B). These results indicate that the H3K36me3-MTF2 interaction plays a positive role in depositing H3K27me3 at genes that may have escaped Polycomb-mediated repression.
Figure 6. The H3K36me3-MTF2 interaction provides an alternative recruitment mechanism for PRC2 and stimulates H3K36me3 demethylation at TBX5.
(A) Crystal structures showing the recognition of the tri-methylated lysine of H3K36me3 by the aromatic cage of the Tudor domain of PHF1 (PDB: 6WAV), MTF2 (PDB: 5XFR), and PHF19 (PDB: 4BD3).
(B) ChIP-seq gene scatter plots comparing genome-wide chromatin occupancy changes of FLAG-MTF2, SUZ12, and H3K27me3 between WT and H3K36me3-binding mutant (Y62A) MTF2 hiPSC lines. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
(C) Venn diagrams showing the number of genes with DNA- and H3K36me3-specific recruitment of MTF2 and SUZ12 and deposition of H3K27me3.
(D) ChIP-seq volcano plots comparing the gene-specific changes of H3K36me3 levels upon mutating the aromatic cage (Y62A) of MTF2, perturbing SUZ12’s interface with all three PCL proteins, and depleting MTF2. Insig: P ≥ 0.1, Sig: P < 0.1 and |log2FC| < 1, SigFC: P < 0.1 and |log2FC| ≥ 1, empirical Wald test.
(E) H3K36me3 ChIP-seq genome tracks of WT, MTF2 SUZ12-binding mutant, MTF2 H3K36me3-binding mutant, MTF2 KO, and PRC2.1- lines at the TBX5 and SOX2 loci.
DNA-mediated recruitment of MTF2 and PRC2.1 appears to play a more dominant role compared to the H3K36me3-mediated recruitment (Figure 6C). A majority of H3K36me3-mediated MTF2 recruitment also relied on DNA-MTF2 interaction. Gene ontology analysis indicates that these overlapping genes are mostly transcription factor genes, while the genes regulated specifically by only DNA-MTF2 or H3K36me3-MTF2 interaction are involved in developmental pathways (Figure S11A).
We next asked how the H3K36me3-MTF2 interaction regulates H3K36me3 itself. H3K36me3 ChIP-seq showed that a small subset of genes exhibited altered H3K36me3 levels upon disruption of the H3K36me3-MTF2 interaction, with the cardiac transcription factor gene TBX5 being the most significantly affected (Figures 6D and 6E). Indeed, of the 66 genes that were co-occupied by H3K36me3 and MTF2-containing PRC2.1, TBX5 had the highest MTF2 occupancy (Figure S11B). The increase in H3K36me3 levels on TBX5 was consistently observed in the loss-of-PRC2.1 SUZ12 mutant, the SUZ12-binding mutant of MTF2, a second H3K36me3-binding mutant of MTF2 (F80A), and the MTF2 KO, but not in the DNA-binding mutant of MTF2 (Figures 6D, 6E, and S11C). Similarly, H3K36me3 levels on TBX5 were not dysregulated in the SUZ12- and DNA-binding mutants or the KO of PHF1 and PHF19 (Figures S11D and S11E), suggesting that MTF2 is the dominant PCL protein in regulating H3K36me3 levels at the TBX5 locus.
Transcriptomic analysis of the MTF2 H3K36me3-binding mutant revealed even larger gene expression changes than observed in the MTF2 KO (305 versus 100 dysregulated genes) (Figures S11F and S11G), suggesting that lacking the H3K36me3 recognition while preserving PRC2 association is more detrimental to the cell than complete depletion of MTF2. Downregulated genes were enriched in prostate gland and urogenital development, while upregulated genes were enriched in cellular respiration pathways (Figure S11H). All these results support a PRC2-dependent role of MTF2 in the gene-specific removal of H3K36me3, either by an indirect mechanism through the loss of H3K27me3 or by recruiting an H3K36me3 demethylase, a function that has so far only been proposed for PHF19 among the three PCL proteins.20
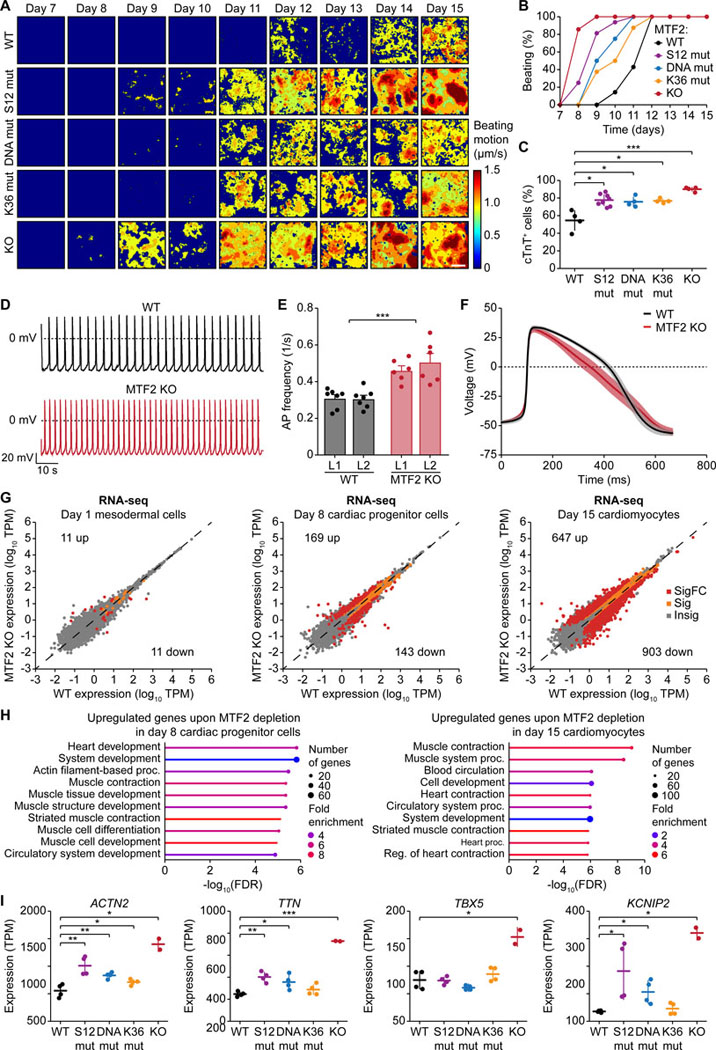
MTF2 loss-of-function accelerates cardiac differentiation
The important role of MTF2 in the epigenetic repression of the NKX2–5 and TBX5 genes implicates an involvement of MTF2 in early cardiac lineage commitment and differentiation. Indeed, the MTF2 KO line exhibited a much-accelerated spontaneous contraction phenotype, followed by the separation-of-function mutant lines (Figures 7A and 7B). Flow cytometry analysis showed a significantly higher percentage of cTnT-expressing cells for all MTF2 loss-of-function lines compared to the WT (Figures 7C and S12A). This difference could already be observed on day 8 but was more pronounced on day 15 (Figures S12A and S12B). Of note, differentiation of the PHF1 KO line similarly yielded more cTnT-expressing cells in comparison to the WT, while no significant difference could be observed between PHF19 WT and KO (Figure S12C).
Figure 7. MTF2 loss-of-function accelerates cardiac differentiation.
(A) Heatmaps showing the time-averaged magnitude of spontaneous contractions of WT and mutant MTF2 cardiomyocytes along the differentiation process.
(B) Percentage of cell culture wells with spontaneous contractions of WT and mutant MTF2 cardiomyocytes over time.
(C) Percentage of WT and mutant MTF2 cells expressing cTnT after 15 days of differentiation. * P < 0.05, *** P < 0.001, two-tailed Student’s t-test.
(D-F) Electrophysiological analysis of WT and MTF2 KO day 24 cardiomyocytes. Representative action potential (AP) traces (D), bar plots of average AP frequency (E), and average single AP trace of all measured cells over time (F) of spontaneous contractions are shown. *** P < 0.001, two-way ANOVA.
(G) RNA-seq scatter plots showing gene expression changes upon loss of MTF2 at day 1, 8, and 15 of the cardiac differentiation process. Insig: P ≥ 0.05, Sig: P < 0.05 and |log2FC| < 1, SigFC P < 0.05 and |log2FC| ≥ 1, two-sided Wald test.
(H) Gene ontology analysis of the upregulated genes upon depletion of MTF2 in day 8 cardiac progenitor cells and day 15 cardiomyocytes.
(I) Gene expression of ACTN2, TTN, TBX5, and KCNIP2 in WT and MTF2 mutant day 15 cardiomyocytes as determined by RNA-seq. * P < 0.05, ** P < 0.01, *** P < 0.001, two-tailed Student’s t-test.
Immunofluorescence staining revealed that the MTF2 KO cardiomyocytes exhibited significantly lower H3K27me3 levels compared to the WT (Figures S12D and S12E), similar to what we observed in the undifferentiated hiPSCs (Figures 1C and S1E). Interestingly, the PHF19 KO cardiomyocytes also exhibited lower H3K27me3 levels, inferring a positive role of PHF19 in H3K27me3 deposition in cardiomyocytes (Figures S12D and S12E). Importantly, patch clamp recordings showed that the electrophysical properties of the MTF2 KO cardiomyocytes phenocopied those of the loss-of-PRC2.1 line. Depletion of MTF2 led to significantly higher AP frequency (Figures 7D and 7E) and faster repolarization (Figure 7F), with unperturbed AP peak amplitude and duration (Figures S12F and S12G).
We lastly asked what transcriptomic changes ensued during the cardiac differentiation time course. RNA-seq analysis comparing MTF2 WT and KO lines collected on day 0, 1, 8, and 15 of cardiac differentiation suggests more pronounced global gene expression changes in later stages of the differentiation process (Figure 7G). Gene ontology analysis of the upregulated genes in day 8 cardiac progenitor cells and day 15 cardiomyocytes suggests an increased expression of cardiac genes (Figure 7H), such as ACTN2, TTN, TBX5, and KCNIP2 (Figure 7I). Interestingly, the three separation-of-function mutants of MTF2 exhibited unique gene expression profiles (Figure S12H), implicating specified roles of these macromolecular interactions during cell differentiation. In summary, we demonstrate that MTF2 sets the epigenetic barrier for cardiomyocyte differentiation, as MTF2 loss-of-function accelerates cardiac differentiation and increases AP frequency.
Discussion
The molecular mechanisms to specifically tune epigenetic repression during cell differentiation remain one of the conundrums in biology. PRC2 accessory proteins are involved in versatile macromolecular interactions, and complete depletion may limit an understanding of their molecular mechanism.4,11,13,15,23 In this study, we used a series of separation-of-function mutations as previously designed for SUZ1214 and engineered here for the PCL proteins, some of which are similarly found in cancer (Table S6). This approach allowed us to disrupt protein-protein, protein-DNA, and protein-histone modification interactions that mediate the recruitment process of PRC2.2 and the three mutually exclusive PCL protein-containing PRC2.1 complexes. Besides the overlapping roles of these subcomplexes, our study demonstrates the distinct specificity and role of each subcomplex in epigenetic repression.
Distinct versus overlapping roles for PRC2.1 and PRC2.2
The PRC2.1 and PRC2.2 subcomplexes were previously proposed to function redundantly.11,15 The previously reported overlapping roles have been observed mainly on the chromatin occupancy of the PRC2 core or its accessory subunits, and this overlapping occupancy has been confirmed in our study. Importantly, we identified gene-specific roles for PRC2.1 and PRC2.2 in depositing H3K27me3. The identification of PRC2.2-specific H3K27me3 peaks helps to explain the lack of strong correlation between PRC2.1 chromatin occupancy and H3K27me3 levels observed previously.11 The specificity could potentially be driven by their specific DNA motif recognition or differential sub-localization in chromatin compartments given that AEBP2 and JARID2 in PRC2.2 have also been shown to recognize CG-rich DNA motifs.41,42 PRC2.2-repressed genes are rarely occupied by the complex itself, suggesting a “methylate and run” model that is consistent with low affinity of PRC2.2-chromatin interaction.14 In contrast, PRC2.1-repressed genes are heavily occupied by the complex, consistent with its role as a H3K27me3 reader.43
Unidirectional competition between of PRC2.1 and PRC2.2
The competition between the two sets of accessory subunits in PRC2.1 and PRC2.2 appears to be unidirectional. Loss of PRC2.2 reinforced the interaction between SUZ12 and PHF1/MTF2 and led to a gain in SUZ12 chromatin occupancy, which can be explained by the higher chromatin affinity of PRC2.1 compared to PRC2.2.14 In contrast, loss of PRC2.1 did not lead to any significant change in PRC2.2 complex composition or chromatin occupancy. A reasonable hypothesis is that there are free/unbound PCL proteins (especially PHF1) available to bind to the exposed PRC2 core when PRC2.2 is disrupted. In contrast, no unbound JARID2 or AEBP2 proteins are available to form more PRC2.2 complexes upon disruption of PRC2.1.
Distinct and overlapping roles of the three PCL proteins
We identified distinct roles for the three PCL proteins. While it was previously thought that the PCL proteins have redundant functions in regulating PRC2,11 we showed in this study that the three PCL proteins employ distinct regulatory mechanisms and specificities. PHF19, the most lowly expressed PCL protein in stem cells, has an antagonistic role by competing for target genes with the MTF2-PRC2.1 subcomplex. This is potentially a self-regulatory mechanism to prevent hyperactivity of the enzyme complex. The PHF19-PRC2.1 complex may interact with additional inhibitor proteins to keep the enzyme in a poised and chromatin-unbound state. We also identified a gene-specific role of MTF2 in maintaining low H3K36me3 levels on the TBX5 gene to prevent premature activation before cardiac lineage commitment. The mechanism could be similar to what was reported for PHF19, which recruits an H3K36 demethylase to enhance epigenetic repression.20
Polycomb-mediated epigenetic regulation of cardiac differentiation
PRC2 is essential for heart development and functions, but previous studies primarily focused on its core subunits.33,44,45 We show here that the PRC2 subcomplexes play important roles in regulating cardiac differentiation by either promoting (PRC2.2) or repressing (PRC2.1) cardiac lineage commitment. PRC2.1 sets the epigenetic barrier through repression of key cardiac transcription factor genes such as NKX2–5 and TBX5 as well as ion channel genes, consistent with the identified roles of PRC2 during differentiation and normal heart function.33,44–46 In contrast, the requirement of PRC2.2 in cardiac differentiation could be mediated by the specific repression of genes that inhibit cardiac lineage commitment such as BMP antagonist gene CER1 and ectopic lineage transcription factor genes FOXA2, LHX5, and OLIG3 (Table S2). This suggests that the PRC2 subcomplexes provide a balance in lineage commitment that could facilitate the desired cellular output in development and regeneration after injury. This study opens the door to future investigations of PRC2 accessory proteins in the heart, especially since JARID2,47–49 PHF1,50 MTF2,4,51 and PHF1952 have been implicated in cardiac development and function. PRC1 has also been shown to regulate cardiomyocyte differentiation,53–55 and how PRC1 and PRC2 interplay in the process remains to be studied.
Concluding remarks
Our study employed separation-of-function mutants to perturb macromolecular interactions in stem cells and differentiation. These findings not only uncovered the distinct roles of PRC2 subcomplexes in controlling epigenetic specificity, but also set a paradigm for the mechanistic study of complex epigenetic regulation.
Limitations of the study
Our genome editing approach enabled us to introduce a WT or mutant cDNA copy at the endogenous gene locus, which may alter the regulation of mRNA isoforms. Moreover, our male hiPSC line may miss a sex-specific function of PRC2 subcomplexes.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yicheng Long (yil4011@med.cornell.edu).
Materials availability
All cell lines and plasmids generated in this study are available from the lead contact with a completed material transfer agreement.
Data and code availability
ChIP-seq and RNA-seq data have been deposited at GEO under accession numbers GSE271752 and GSE271648 and are publicly available as of the date of publication.
Original western blot and microscopy images have been deposited at Mendeley Data (DOI: http://dx.doi.org/10.17632/xngr4x532x.1) and are publicly available as of the date of publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
STAR★Methods
Experimental model and study participant details
hiPSC culture
Male hiPSCs were maintained on vitronectin (Thermo Fisher Scientific, #A14700) in StemFlex medium (Thermo Fisher Scientific, #A3349401) supplemented with 25 U/mL penicillin/streptomycin (Thermo Fisher Scientific, #15140122) at 37°C and 5% CO2. Cells were not authenticated. Cells were routinely tested for mycoplasma contamination by PCR.
HEK293T cell culture
Female HEK293T cells were maintained in DMEM (Thermo Fisher Scientific, #11965092) supplemented with 10% fetal bovine serum (Cytiva, #SH30396), 1X GlutaMAX supplement (Thermo Fisher Scientific, #35050061), 1X non-essential amino acids (Thermo Fisher Scientific, #11140050), 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific, #15140122), 100 μM 2-mercaptoethanol (Thermo Fisher Scientific, # AC125472500) at 37°C and 5% CO2. Cells were not authenticated. Cells were routinely tested for mycoplasma contamination by PCR.
Method details
CRISPR genome editing
The CRISPR plasmid encoding Cas9 and guide RNA was generated by inserting the guide RNA sequence targeting the junction between exon 2 and intron 2 of the gene to be edited into pX330. The donor plasmids carrying either the WT or mutated complementary DNA were constructed by assembling the following fragments into a previously described donor plasmid:56 left homology arm, target gene cDNA, tag, 3′ UTR, 3x SV40 polyadenylation sites, 1X bGH polyadenylation site, hPGK promoter, puromycin or blasticidin resistance ORF, 1X bGH polyadenylation site, and right homology arm. KOs were generated with similar donor plasmids but lacking the cDNA, tag, and 3’UTR portion. A total of 2.5 μg of equal amounts of CRISPR plasmid and two instances of donor plasmids carrying either the puromycin or blasticidin resistance ORF were transfected into WTC-11 hiPSCs (Coriell Institute, #GM25256) in a 6-well plate using Lipofectamine Stem Transfection Reagent (Thermo Fisher Scientific, #STEM00015) following the manufacturer’s instructions. After two to three days, cells were passaged to a 10-cm plate coated with Geltrex (Thermo Fisher Scientific, #A1413301). Once cells reached 60–80% confluency, 1 μg/ml puromycin was added to the culture for four days. Cells were then selected in the presence of both 1 μg/ml puromycin and 5 μg/ml blasticidin for one week. At least 24 surviving colonies were manually picked, transferred to 24-well plates, and expanded. Successfully genome-edited colonies were verified by PCR, Sanger sequencing, and western blotting. Two or three independent clones of genome-edited hiPSC lines were used in subsequent experiments.
Western blotting
Cells were collected by Accutase treatment, washed with cold PBS, and incubated in lysis buffer (25 mM Tris pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Igepal, 5% glycerol, 2 mM TCEP, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989), 1 μL/mL benzonase (Millipore Sigma, #E1014)) for 10 min on ice. Protein extracts were collected as supernatant after centrifugation at 16,000 x g for 10 min at 4°C, incubated in Laemmli buffer for 10 min at 95°C, resolved on a 4–15% Mini-PROTEAN TGX Stain-Free Protein Gel (Bio-Rad, Cat#4568084), and transferred to a nitrocellulose membrane via wet transfer. Membranes were blocked in StartingBlock Blocking Buffer (Thermo Fisher Scientific, #37539) and probed with anti-β-actin (Thermo Fisher Scientific, #MA5–15739), anti-FLAG (Millipore Sigma, #F3165), anti-HA (Cell Signaling Technology, #3724), anti-H3K27me3 (Cell Signaling Technology, #9733), anti-SUZ12 (Cell Signaling Technology, #3737), or anti-MTF2 (Proteintech, #16208–1-AP) at a dilution of 1:1000 in blocking buffer overnight at 4°C or for 2 h at room temperature. The membrane was washed in PBS-T (0.1% Tween-20 in PBS), incubated with an anti-mouse (Cell Signaling Technology, #7076) or anti-rabbit (Cell Signaling Technology, #7074) HRP-coupled secondary antibody in PBS-T for 1 h, and washed again in PBS-T. Proteins were detected with the Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, #32106) and acquired using a ChemiDoc Imaging System (Bio-Rad). Data were analyzed using Image Lab (Bio-Rad, v6.1).
Immunofluorescence staining
Cells grown on cover slips were fixed with 4% formaldehyde in PBS for 10 min and then permeabilized in extraction buffer (0.5% Triton X-100, 20 mM HEPES-KOH pH 7.5, 50 mM NaCl, 3 mM MgCl2, 300 mM sucrose) for 10 min. Cover slips were then washed with PBS + 0.1% Triton X-100 twice and blocked in ABDIL buffer (3% BSA and 0.1% Triton X-100 in PBS) for 30 min. Samples were subsequently incubated in anti-α-actinin (Abcam, #ab9465, 1:200), anti-cTnT-Alexa Fluor 647 (BD Biosciences, #565744, 1:200), anti-FLAG (Millipore Sigma, #F1804, 1:400), anti-H3K27me3 (Cell Signaling Technology, #9733, 1:200), anti-NKX2–5 (Cell Signaling Technology, #8792, 1:400), anti-OCT-4 (Cell Signaling Technology, #2840, 1:200), or anti-TBX5 (Thermo Fisher Scientific, #42–6500, 1:200) primary antibody diluted in ABDIL buffer for a 1 h, washed three times in PBS, and incubated in anti-mouse Alexa Fluor 488 (Cell Signaling Technology, #4408) or anti-rabbit Alexa Fluor 647-conjugated (Cell Signaling Technology, #4414) secondary antibody diluted 1:500 in ABDIL buffer containing 10 μg/mL Hoechst 33342 (Thermo Fisher Scientific, #H3570) for 30 min. After another three PBS washes, cover slips were mounted onto a glass slide using ProLong Glass Antifade Mountant (Thermo Fisher Scientific, #P36984). Images were acquired as Z-stacks of 200-nm step size on a Leica DMi8 microscope with a Hamamatsu ORCA-Fusion BT digital camera and were equally processed using ImageJ/Fiji (NIH, v2.14.0) and Photoshop (Adobe, v25.9.1).
Immunoprecipitation followed by mass spectrometry
hiPSCs at 70–80% confluency were collected by Accutase treatment, washed with PBS, and extracted with 1 mL IP MS lysis buffer (50 mM Tris pH 8, 100 mM KCl, 5 mM MgCl2, 10 % glycerol, 0.5 % NP-40, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989), 1 μL/mL benzonase (Millipore Sigma, #E1014)) on ice for 10 min. Protein extracts were collected as supernatant after centrifugation at 16,000 x g for 10 min at 4°C. Pierce Protein A Magnetic Beads (Thermo Fisher Scientific, #88845) crosslinked to anti-SUZ12 antibody (Cell Signaling Technology, #3737) were added to the lysate and incubated at 4°C for 30 min. Beads were washed three times with IP MS lysis buffer without glycerol and twice with IP MS wash buffer (50 mM Tris pH 8, 0.5 % NP-40) at 4°C. Samples were eluted by incubating the beads in IP MS elution buffer (50 mM Tris pH 8, 0.5 % SDS) at 50°C for 15 min and magnetically removing the beads. Quality of the IP samples was confirmed by silver staining and western blotting.
For mass spectrometry, protein samples were acetone precipitated and resuspended in 0.1% RapiGest SF Surfactant (Waters Corporation, #186002123) and 25 mM ammonium bicarbonate. The samples were then reduced with DTT, alkylated with iodoacetamide, and digested with trypsin overnight at 37°C. The digests were desalted by C18-StageTip columns. The digests were analyzed using an EASY-nLC 1200 (Thermo Fisher Scientific) coupled online to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific). Buffer A (0.1% FA in water) and buffer B (0.1% FA in 80% ACN) were used as mobile phases for gradient separation. A 75 μm x 15 cm chromatography column (ReproSil-Pur C18-AQ, 3 μm, Dr. Maisch HPLC) was packed in-house for peptide separation. Peptides were separated with a gradient of 5–40% buffer B over 30 min and 40%−100% buffer B over 10 min at a flow rate of 400 nL/min. The mass spectrometer was operated in a data-independent acquisition (DIA) mode. MS1 scans were collected in the Orbitrap mass analyzer over 350–1400 m/z at a resolution of 120K. The instrument was set to select precursors in 45 × 14 m/z wide windows with 1 m/z overlap from 350–975 m/z for HCD fragmentation. The MS/MS scans were collected in the orbitrap at 15K resolution. Data were searched against the human Uniprot database (8/7/2021) using DIA-NN (v1.8)57 and filtered for 1% false discovery rate for both protein and peptide identifications. For the data analysis, the intensity values were log transformed, and the data was filtered to keep only those with at least 2 valid values in at least one experimental group. Differential analysis was performed using t-test. Multiple hypothesis correction of p-values was performed using the BH method.
ChIP-seq
hiPSCs at 70–80% confluency were collected by Accutase treatment and crosslinked with 1% formaldehyde in PBS at room temperature for 10 min. The crosslinking reaction was quenched by adding one volume of 1.25 M glycine to 10 volumes of PBS for 2 min. Cells were washed in ice-cold PBS and cell pellets stored at −80°C. Aliquots of 10 million cells were lysed in 1 mL ChIP lysis buffer (800 mM NaCl, 25 mM Tris pH 7.5, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989)) on ice for 30 min. Lysates were sonicated in MilliTubes (Covaris, #520135) with the Covaris S220 focused-ultrasonicator at 4°C for 10 min at a PIP of 140 W, a duty factor of 5%, and 200 cycles/burst. Lysate was cleared by centrifugation at 16,000xg for 10 min at 4°C, and supernatant was collected. For each IP, 1 mL chromatin dilution buffer (25 mM Tris pH 7.5, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989)) together with 20 ng spike-in chromatin from Drosophila melanogaster S2 cells (Active Motif, #53083) was added to the cleared lysate from 2.5–5.0 million cells. Chromatin samples were incubated with 2–4 μg of anti-CBX7 (Abcam, #ab21873, 4 μL), anti-FLAG (Millipore Sigma, #F3165, 2 μL), anti-H2AK119ub (Cell Signaling Technology, #8240, 5 μL), anti-H3K27me3 (Cell Signaling Technology, #9733, 6 μL), anti-H3K36me3 (Thermo Fisher Scientific, #MA5–24687, 3 μL), anti-H3K4me3 (Thermo Fisher Scientific, #MA5–33382, 4 μL), anti-JARID2 (Cell Signaling Technology, #13594, 4 μL), anti-MTF2 (Proteintech, #16208–1-AP, 5 μL), anti-RING1B (Cell Signaling Technology, #5694, 7 μL), or anti-SUZ12 (Cell Signaling Technology, #3737, 4 μL) antibody at 4°C overnight. A spike-in antibody against the Drosophila-specific histone variant H2Av (Active Motif, #61686) was added to all chromatin samples. Then, 25 μl of washed Pierce Protein A/G Magnetic Beads (Thermo Fisher Scientific, #88803) were added to the IP solution to incubate at room temperature for 2 h. Beads were washed twice with 1 mL of LSB buffer (20 mM Tris pH 8.0, 2 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100), HSB buffer (20 mM Tris pH 8.0, 2 mM EDTA, 500 mM NaCl, 0.1% SDS, 1% Triton X-100), LiCl buffer (10 mM Tris pH 8.0, 1 mM EDTA, 250 mM LiCl, 1% sodium deoxycholate, 1% Igepal), and TE buffer (10 mM Tris pH 8.0, 1 mM EDTA). Chromatin was then eluted by incubating the beads with 120 μl of elution buffer (100 mM sodium bicarbonate, 1% SDS) for 20 min at room temperature. Formaldehyde crosslinking was reversed by adding 5 μL of 5 M NaCl and incubating at 65°C for 3 h. Protein and RNA were removed by adding 14 μL of 1 M Tris pH 8, 3 μL of 500 mM EDTA, 3 μL of proteinase K (Thermo Fisher Scientific, #25530049), and 1 μL of 1 mg/mL RNase A (Thermo Fisher Scientific, #EN0531) and incubating at 37°C for 1 h. DNA was extracted by phenol-chloroform-isoamyl extraction followed by ethanol precipitation. DNA samples were resuspended in 30 μl of 10 mM Tris pH 8.0 and quantified using a Qubit fluorometer (Invitrogen). Between 2 ng and 100 ng of each sample was used for library preparation using the KAPA HyperPlus Kit (Roche, #7962428001) according to the manufacturer’s instructions, except for skipping the fragmentation step. DNA libraries were pooled and sequenced on a NovaSeq 6000 or NovaSeq X Plus using a 150-cycle kit (paired end, 2 × 150 bp).
ChIP-seq analysis was performed similar to what was described previously.33 Adapter sequences were trimmed from the read pairs using Cutadapt (v2.10).58 Trimmed reads were aligned to the human reference genome (hg38) using BWA-MEM (v0.7.17-r1188).59 Properly paired read alignments with mapping scores greater than 30 were used for downstream analysis. PCR duplicates were removed using Picard MarkDuplicates. Peak calling was performed with MACS260 with the options of ‘macs2 callpeak -g hs -f BAMPE --keep-dup all --bdg’. Bigwig files were generated using bedGraphToBigWig tool (v385).61 Peak metaplots and heatmaps were generated on pooled replicates using deepTools (v3.5.5).62 Gene-specific ChIP enrichment analysis was performed by comparing the number of reads mapped to gene bodies (defined by the featureCounts tool (Subread v2.0.0)63 using the GENCODE v42 annotation) in mutant versus WT cell lines. For each biological replicate (individual clone), the read counts of input and pulldown were normalized by total number of aligned reads for the genes whose counts were great than one. Similar to what was previously described,33 a generalized linear model was constructed to estimate log2Foldchange of the normalized read counts in pulldown relative to input while adjusting for cell line variability. Shrunken fold change was estimated using the R package DESeq2 (v1.44.0).64 The statistical significance of the log2Foldchange was calculated empirically by the R package fdrtool (v1.2.17)65, with the input of Wald statistics calculated using DESeq2 (v1.44.0).64 The differential ChIP enrichment between the mutant and WT lines was calculated with an interaction term of cell line (mutant versus WT) and library (pulldown versus input) in a regression model. Plots were generated using ggplot2 (v3.5.1)66 and Tableau software. Genome tracks were visualized using IGV (v2.4.10).67 Gene ontology analysis was performed using ShinyGO (v0.80).68
ChIP-qPCR
ChIP samples were processed as described in the ChIP-seq section. ChIP-qPCR samples were prepared using the SYBR Select Master Mix (Thermo Fisher Scientific, #4472908) according to the manufacturer’s instructions and run on a QuantStudio 3 Real-Time PCR System (Applied Biosystems). ChIP enrichment was calculated as percent input using the 2-ΔCt method.
RNA isolation, quantification, and quality control
Total RNA was extracted using TRIzol (Thermo Fisher Scientific, #15596018) and purified using the Direct-zol RNA MiniPrep kit (Zymo Research, #R2052) according to the manufacturer’s instructions. RNA concentration was measured with a Qubit fluorometer (Invitrogen), and RNA quality was determined using a TapeStation (Agilent Technologies).
qRT-PCR
Total RNA was reverse-transcribed using the SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific, #18091050) with random hexamers according to the manufacturer’s instructions. qRT-PCR samples were prepared using the SYBR Select Master Mix (Thermo Fisher Scientific, #4472908) according to the manufacturer’s instructions. Samples were run in technical duplicates on a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Ct values were normalized against the internal control GAPDH. Fold differences in expression levels were calculated according to the 2-ΔΔCt method.69
RNA-seq
RNA-seq libraries were prepared from 500 ng of total RNA using the KAPA RNA HyperPrep Kit with RiboErase (Roche, #8098131702) according to the manufacturer’s instructions. Libraries were pooled and sequenced on a NovaSeq 6000 or NovaSeq X Plus using a 150-cycle kit (paired end, 2 × 150 bp).
RNA-seq analysis was performed similar to what was described previously.33 Adapter sequences were trimmed from the read pairs using Cutadapt (v2.10).58 Trimmed reads were aligned to the human reference genome (hg38) using STAR (v2.7.10a)70 with GENCODE basic gene annotation (v42). Read counts were calculated as transcripts per million (TPM) using uniquely mapped reads counted using featureCounts (Subread v2.0.0)63 in a strand-specific way. Differential expression analysis between the mutant and WT lines was performed by modeling read counts in a generalized linear model accounting for cell lines. Fold change and p-values were calculated using DESeq2 (v1.44.0),64 in which two-sided Wald tests for regression coefficients of interest were performed. Differential expression heatmaps were generated using the R package pheatmap (v1.0.12). Scatter plots were generated using the Tableau software. Gene ontology analysis was performed using ShinyGO (v0.80).68
Recombinant protein expression and purification
Recombinant 4-subunit and 5-subunit PRC2 complexes were expressed and purified similarly to what was previously described.71 The 4-subunit PRC2 core complex contained EZH2, SUZ12, EED, and RBBP4 proteins, all of which included a PreScission-protease cleavable N-terminal 6XHis-MBP tag.33,38,72 The 5-subunit AEBP2-PRC2 complex contained an additional PreScission cleavable Strep-II tagged AEBP2 protein. The 5-subunit MTF2-PRC2 and PHF19-PRC2 complexes were reconstituted using the previously generated MTF2 and PHF19 insect cell expression plasmids.73 The 6-subunit AEBP2-JARID2-PRC2 complex was made using a single plasmid baculoviral expression system (gift by Dr. Vignesh Kasinath, University of Colorado Boulder).17
Expression vectors based on the pFastBac plasmid system were transformed into DH10Bac competent cells (Thermo Fisher Scientific, #10361012).14,33,71,73 Following manufacturer guidelines, baculovirus stocks (P0, P1, and P2) were generated in Sf9 insect cells (Thermo Fisher Scientific, #12659017) grown in Sf-900 III SFM medium (Thermo Fisher Scientific, #12658019). P2 titer was determined by a commercially available service based on gp64 expression (Expression Systems). 1 L of Tni insect cells (Expression Systems, #94–002) were seeded at 2×106 cells/mL in ESF-921 protein-free medium (Expression Systems, #96–001-01-CS) and infected at a MOI of 1.0 with each baculovirus. Cultures were grown at 27ºC and 130 rpm for 66 h before cells were harvested, snap-frozen, and stored at −80ºC until purification.
For complex purification, frozen Tni cells were lysed in lysis buffer (10 mM Tris pH 7.5, 250 mM NaCl, 0.5% Igepal, 1mM TCEP, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989)). Cell debris was removed by centrifugation at 29,000xg for 40 min. Clarified lysate was incubated with amylose resin (NEB, #E8021L) equilibrated in lysis buffer for 2 h under gentle rotation. Lysate-bead mixture was added to a glass 2.5 cm x 20 cm Econo-Pac chromatography column. The column was washed with 10 column value (cv) of lysis buffer, followed by 16 cv of high salt buffer (10 mM Tris pH 7.5, 500 mM NaCl, 1 mM TCEP), and 16 cv of low salt buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM TCEP). Protein was eluted off the column in 3 cv of elution buffer (low salt buffer, 10 mM maltose). Elution was collected across five fractions. Relevant fractions were combined and concentrated in an Amicon Ultra-15 centrifugal filter unit, 30 kDa MWCO (Millipore Sigma, #UFC903024). The NaCl concentration was increased to ~250 mM and PreScission protease (in house) was added at a mass ratio of 1 part in 50 for overnight cleavage of MBP tags. The protein was then loaded onto a HiTrap Heparin HP 5 × 5 mL affinity column (Cytiva, #17040703) equilibrated in start buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM TCEP). The protein was eluted in a linearly increasing gradient of elution buffer (10 mM Tris pH 7.5, 2 M NaCl, 1 mM TCEP) across 35 cv at a flow rate of 1.5 mL/min. Relevant fractions were combined and concentrated as above, then subsequently loaded onto a HiLoad 16/600 Superdex 200 pg preparative SEC column (Cytiva, #28989335) equilibrated in sizing buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 1 mM TCEP). Protein was eluted in sizing buffer at a flow rate of 0.5 mL/min. Relevant fractions were combined and concentrated as above. The protein was divided into single-use aliquots, which were then snap-frozen and stored at −80 ºC until use.
Nucleosome reconstitution
Nucleosomes were reconstituted using assembled human histone octamers (Histone Source, Colorado State University, SKU: HOCT_H3C96SC110A_500ug) and a 200-bp 5’-FAM-labeled mononucleosomal DNA containing the 147-bp Widom sequence. Nucleosome reconstitution was performed similarly to what was previously described.33 DNA was mixed with each histone octamer in three separate ratios (1:0.8, 1:1, and 1:1.2) in histone refolding buffer (2 M NaCl, 6 mM Tris pH 7.5, 0.3 mM EDTA, 0.3 mM TCEP). The mixture was first incubated at 37 °C for 30 min, and then the following volumes of reconstitution buffer (20 mM Tris pH 7.5, 1 mM EDTA, 1 mM DTT) were added in 30-min intervals: 10.8 μL, 12 μL, 28 μL, and 64 μL. Nucleosome assembly quality was assessed by gel electrophoresis in a native 6% polyacrylamide 1X TBE gel stained with RedSafe (Bulldog Bio, #21141), and the DNA/histone octamer ratio with no unbound DNA was used for downstream binding assays.
Methyltransferase activity
Endpoint PRC2 activity was determined using the MTase-Glo Methyltransferase Assay kit (Promega, #V7602). Serial dilutions of PRC2 were prepared in reaction buffer (20 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA, 3 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT) with a reaction concentration starting at 1 μM. S-adenosylmethionine (SAM) and biotinylated mononucleosome were added to each tube to final concentrations of 20 μM and 450 nM, respectively. The reactions were incubated at room temperature for 2 h before being quenched by trifluoroacetic acid to a final concentration of 0.1%. After quenching, MTase-Glo reagent was added to each reaction mixture to a final 1X concentration. After a 30-min incubation, an equal volume of MTase-Glo detection solution was added to each reaction. After another 30-min incubation, luminescence was measured with a TECAN Spark microplate reader. The luminescence of serially diluted S-adenosylhomocysteine (SAH) was run in parallel and a standard curve was generated by linear regression. Net luminescence was determined by subtracting the luminescence from a control containing no protein, and SAH produced by the reaction was determined by using the best-fit equation generated by the SAH standard curve.
Fluorescence polarization
Affinities between PRC2 and reconstituted mononucleosomes were determined by fluorescence polarization assays. Various concentrations (4–1000 nM) of the PRC2 complex were prepared by serial dilutions with a final reaction concentration starting at 1 μM in binding buffer (50 mM Tris pH 7.5, 10 mM KCl, 0.1 mM ZnCl2, 2mM BME, 0.1 mg/mL BSA, 5% glycerol). An equal volume of FAM-mononucleosome in the same binding buffer was added to a final concentration of 5 nM per reaction. The binding reactions were incubated at room temperature for 30 min before fluorescence anisotropy was measured with a TECAN Spark microplate reader. The FAM fluorophore was excited at 485 nM with a bandwidth of 20 nM, and emission was measured at 535 nM with a bandwidth of 25 nM. A standard binding curve was generated for each nucleosome with the baseline adjusted to the anisotropy value of the control sample without protein. Apparent dissociation constant KDapp and Hill coefficient were calculated from the mean value of three independent replicates.
Cardiomyocyte differentiation of hiPSCs
hiPSCs were differentiated into cardiomyocytes using the GSK3 inhibitor CHIR99021 and Wnt inhibitor IWP-2.32 Three days before induction of differentiation (day −3), hiPSCs were seeded at 250,000 cells per well of a Geltrex-coated (Thermo Fisher Scientific, #A1413301) 12-well plate in StemFlex medium (Thermo Fisher Scientific, #A3349401) with 5 μM ROCK inhibitor Y27632 (Apexbio Technology, #B129310). Medium was replaced daily with StemFlex medium until day 0. On day 0, when cells were around 90% confluent, medium was replaced with RPMI1640 medium (Thermo Fisher Scientific, #11875093) containing B-27 supplement, minus insulin (Thermo Fisher Scientific, #A1895601) and 10 μM CHIR99021 (Cayman Chemical, #13122). On day 1, medium was replaced with RPMI1640 medium containing B-27 supplement, minus insulin. On day 3, half of the existing medium was collected from each well and mixed with an equal amount of fresh RPMI1640 medium containing B-27 supplement, minus insulin and a final concentration of 5 μM IWP-2 (Cayman Chemical, #13951) to replace the remaining media in each well. On day 5, medium was replaced with RPMI1640 medium containing B-27 supplement, minus insulin. On day 7 and every 3 days afterwards, medium was replaced with RPMI1640 medium containing B-27 supplement (Thermo Fisher Scientific, #17504044).
From day 15, cardiomyocytes were further purified using metabolic selection with lactate.74 Medium was replaced with lactate selection medium (RPMI1640 medium, no glucose (Thermo Fisher Scientific, #11879020), 5 mM sodium DL-lactate, 213 μg/mL L-ascorbic acid, 500 μg/mL human albumin (Millipore Sigma, #A9731)) on day 15 and 17. On day 19, medium was replaced with RPMI1640 medium containing B-27 supplement. On day 22, cells were trypsinized and reseeded at a density of 200,000 cells per well of a Geltrex-coated 12-well plate in RPMI1640 medium containing 10% fetal bovine serum (Cytiva, #SH30396) and 5 μM ROCK inhibitor Y27632. From day 23, cardiomyocytes were maintained in RPMI1640 medium containing B-27 supplement with media changes every other day.
Time-lapse images of spontaneous contractions were captured using a Leica DMi8 microscope with a Hamamatsu ORCA-Fusion BT digital camera. Contractions were quantified using the MATLAB-based MotionGUI75 program.
Flow cytometry
Day 8 cardiac progenitor cells and day 15 cardiomyocytes were collected by trypsinization, fixed in 1% formaldehyde in PBS, and washed three times with PBS. Cells were then permeabilized with 90% methanol and washed three times with 0.5% BSA in PBS. Each sample was incubated with anti-cTnT Alexa Fluor 647 (BD Biosciences, #565744) or IgG1 κ Alexa Fluor 647 isotype control (BD Biosciences, #557732) diluted 1:200 in 0.5% BSA and 0.1% Triton X-100 in PBS for 1 h. Cells were washed twice in 0.5% BSA and 0.1% Triton X-100 in PBS, resuspended in 0.5% BSA in PBS, and filtered through a 70-μm cell strainer. Samples were acquired using a Sony MA900 Cell Sorter. Data were analyzed with FlowJo (BD Biosciences, v10.10.0).
Electrophysiological recordings
Spontaneous action potentials in cardiomyocytes were recorded in whole-cell current clamp mode at resting membrane potential. These recordings were made in an extracellular solution (140 mM NaCl, 5.4 mM KCl, 1.8 CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES pH 7.4). Borosilicate patch pipettes with 1.5–2 MΩ resistance were used for recording and filled with internal solution (125 mM K-gluconate, 20 mM KCl, 5 mM NaCl, 1 mM MgCl2, 5 mM MgATP, 10 mM HEPES pH 7.2). A HEKA EPC 10 amplifier was used for acquiring data and PatchMaster (HEKA Elektronik, v1.4.1) was used to export recorded data. Data were acquired at a sampling rate of 2.4 kHz. Action potential parameters were analyzed using Clampfit (Molecular Devices, v10.6.2).
Immunoprecipitation in HEK293T cells
HEK293T cells (ATCC, #CRL-11268) at around 50% confluency were forward transfected with equal amounts of pcDNA3.1 plasmids expressing either a 3XHA-tagged or a 3XFLAG-tagged protein using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, #11668019) according to the manufacturer’s instructions. Two days after transfection, cells were harvested by trypsinization, washed with cold PBS, and incubated in lysis buffer (25 mM Tris pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Igepal, 5% glycerol, 2 mM TCEP, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989), 1 μL/mL benzonase (Millipore Sigma, #E1014)) for 30 min on ice. Protein extracts were collected as supernatant after centrifugation at 16,000 x g for 10 min at 4°C. Clarified protein extracts were incubated with ANTI-FLAG M2 Affinity Gel (Millipore Sigma, #A2220) for 2 h at 4°C. Beads were washed five times with wash buffer (25 mM Tris pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Igepal, 5% glycerol, 2 mM TCEP). Proteins were eluted from the beads in Laemmli buffer for 10 min at 95°C and detected by western blotting.
Immunoprecipitation in hiPSCs
hiPSCs at around 70–80% confluency were collected by Accutase treatment, washed with cold PBS, and incubated in lysis buffer (25 mM Tris pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Igepal, 5% glycerol, 2 mM TCEP, 1X protease inhibitor cocktail (Thermo Fisher Scientific, #A37989), 1 μL/mL benzonase (Millipore Sigma, #E1014)) on end-over-end rotation for 30 min at 4°C. Cell lysates were collected as supernatant after centrifugation at 16,000 x g for 10 min at 4°C. Clarified protein extracts were incubated with pre-washed ANTI-FLAG M2 Affinity Gel (Millipore Sigma, #A2220) for 2 h at 4°C. Beads were washed five times with wash buffer (25 mM Tris pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Igepal, 5% glycerol, 2 mM TCEP). Proteins were eluted by incubating the beads in elution buffer (lysis buffer with 150 ng/μL 3XFLAG peptide (Apexbio Technology, #A60014)) for 30 min at room temperature and collected via centrifugation in Spin-X tubes (Corning, #8161) at 1,000xg for 2 min at room temperature. The elution was mixed with 1X Laemmli buffer for 10 min at 95°C and proteins were detected by western blotting.
Quantification and statistical analysis
All statistical analyses and definition of replicates are described in the corresponding figure legends. Statistical tests were performed using Microsoft Excel or R. Statistical significance was defined as: * P < 0.05, ** P < 0.01, and *** P < 0.001.
Supplementary Material
Document S1. Figures S1-S12 and supplemental reference
Table S2. List of genes with increasing or decreasing H3K27me3 levels upon loss of PRC2.1 and PRC2.2 in hiPSCs, related to Figure 1
Table S3. List of significantly up- and downregulated genes levels upon loss of PRC2.1 and PRC2.2 in hiPSCs, related to Figure 1
Table S4. List of significantly up- and downregulated genes levels upon loss of PRC2.1 and PRC2.2 in day 15 cardiomyocytes, related to Figure 2
Table S7. Primer sequences, related to the STAR methods.
Table S1. List of proteins co-immunoprecipitating with SUZ12 in WT and mutant SUZ12 hiPSCs, related to Figure 1
Table S6. List of cancer-associated mutations of SUZ12 and PCL residues engineered for separation-of-function analyses in this study, related to Figure 1
Table S5. List of PCL target genes in hiPSCs, related to Figure 3
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-mouse IgG-Alexa Fluor 488 | Cell Signaling Technology | Cat#4408; RRID: AB_10694704 |
| Goat polyclonal anti-rabbit IgG-Alexa Fluor 647 | Cell Signaling Technology | Cat#4414; RRID: AB_10693544 |
| Goat polyclonal anti-rabbit IgG-HRP | Cell Signaling Technology | Cat#7074; RRID: AB_2099233 |
| Horse polyclonal anti-mouse IgG-HRP | Cell Signaling Technology | Cat#7076; RRID: AB_330924 |
| Mouse monoclonal anti-cTnT-Alexa Fluor 647 (clone 13–11) | BD Biosciences | Cat#565744; RRID: AB_2739341 |
| Mouse monoclonal anti-FLAG (clone M2) | Millipore Sigma | Cat#F1804; RRID: AB_262044 |
| Mouse monoclonal anti-FLAG (clone M2) | Millipore Sigma | Cat#F3165; RRID: AB_259529 |
| Mouse monoclonal anti-α-actinin (clone EA-53) | Abcam | Cat#ab9465; RRID: AB_307264 |
| Mouse monoclonal anti-β-actin (clone BA3R) | Thermo Fisher Scientific | Cat#MA5–15739; RRID: AB_10979409 |
| Mouse monoclonal IgG1 κ Alexa Fluor 647 (clone MOPC-21) | BD Biosciences | Cat#557732, RRID: AB_396840 |
| Rabbit monoclonal anti-H2AK119ub (clone D27C4) | Cell Signaling Technology | Cat#8240; RRID: AB_10891618 |
| Rabbit monoclonal anti-H3K27me3 (clone C36B11) | Cell Signaling Technology | Cat#9733; RRID: AB_2616029 |
| Rabbit monoclonal anti-H3K36me3 (clone RM155) | Thermo Fisher Scientific | Cat#MA5–24687; RRID: AB_2661912 |
| Rabbit monoclonal anti-H3K4me3 (clone RM340) | Thermo Fisher Scientific | Cat#MA5–33382; RRID: AB_2815520 |
| Rabbit monoclonal anti-HA (clone C29F4) | Cell Signaling Technology | Cat#3724; RRID: AB_1549585 |
| Rabbit monoclonal anti-JARID2 (clone D6M9X) | Cell Signaling Technology | Cat#13594; RRID: AB_2798269 |
| Rabbit monoclonal anti-NKX2–5 (clone E1Y8H) | Cell Signaling Technology | Cat#8792; RRID: AB_2797667 |
| Rabbit monoclonal anti-OCT-4A (clone C30A3) | Cell Signaling Technology | Cat#2840; RRID: AB_2167691 |
| Rabbit monoclonal anti-RING1B (clone D22F2) | Cell Signaling Technology | Cat#5694; RRID: AB_10705604 |
| Rabbit monoclonal anti-SUZ12 (clone D39F6) | Cell Signaling Technology | Cat#3737; RRID: AB_2196850 |
| Rabbit polyclonal anti-CBX7 | Abcam | Cat#ab21873; RRID: AB_726005 |
| Rabbit polyclonal anti-H2Av spike-in | Active Motif | Cat#61686; RRID: AB_2737370 |
| Rabbit polyclonal anti-MTF2 | Proteintech | Cat#16208–1-AP; RRID: AB_2147370 |
| Rabbit polyclonal anti-TBX5 | Thermo Fisher Scientific | Cat#42–6500; RRID: AB_2533533 |
| Bacterial and virus strains | ||
| MAX Efficiency DH10Bac Competent Cells | Thermo Fisher Scientific | Cat#10361012 |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| 2-Mercaptoethanol | Thermo Fisher Scientific | Cat#AC125472500 |
| 3XFLAG peptide | Apexbio Technology | Cat#A60014 |
| Albumin human | Millipore Sigma | Cat#A9731 |
| Amylose Resin | New England Biolabs | Cat#E8021L |
| ANTI-FLAG M2 Affinity Gel | Millipore Sigma | Cat#A2220 |
| B-27 Supplement | Thermo Fisher Scientific | Cat#17504044 |
| B-27 Supplement, minus insulin | Thermo Fisher Scientific | Cat#A1895601 |
| Benzonase Nuclease | Millipore Sigma | Cat#E1014 |
| CHIR99021 | Cayman Chemical | Cat#13122 |
| DMEM, high glucose | Thermo Fisher Scientific | Cat#11965092 |
| ESF 921 Insect Cell Culture Medium | Expression Systems | Cat#96–001-01-CS |
| Geltrex | Thermo Fisher Scientific | Cat#A1413301 |
| GlutaMAX Supplement | Thermo Fisher Scientific | Cat#35050061 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat#H3570 |
| HyClone Fetal Bovine Serum | Cytiva | Cat#SH30396 |
| IWP-2 | Cayman Chemical | Cat#13951 |
| Lipofectamine 2000 Transfection Reagent | Thermo Fisher Scientific | Cat#11668019 |
| Lipofectamine Stem Transfection Reagent | Thermo Fisher Scientific | Cat#STEM00015 |
| MEM Non-Essential Amino Acids Solution | Thermo Fisher Scientific | Cat#11140050 |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat#15140122 |
| Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat#32106 |
| Pierce Protease Inhibitor XL Capsules | Thermo Fisher Scientific | Cat#A37989 |
| Pierce Protein A Magnetic Beads | Thermo Fisher Scientific | Cat#88845 |
| Pierce Protein A/G Magnetic Beads | Thermo Fisher Scientific | Cat#88803 |
| PreScission protease | In house | N/A |
| ProLong Glass Antifade Mountant | Thermo Fisher Scientific | Cat#P36984 |
| Proteinase K Solution | Thermo Fisher Scientific | Cat#25530049 |
| RapiGest SF Surfactant | Waters Corporation | Cat#186002123 |
| RNase A | Thermo Fisher Scientific | Cat#EN0531 |
| RPMI1640 Medium | Thermo Fisher Scientific | Cat#11875093 |
| RPMI1640 Medium, no glucose | Thermo Fisher Scientific | Cat#11879020 |
| Sf-900 III SFM | Thermo Fisher Scientific | Cat#12658019 |
| StartingBlock Blocking Buffer | Thermo Fisher Scientific | Cat#37539 |
| StemFlex Medium | Thermo Fisher Scientific | Cat#A3349401 |
| TRIzol Reagent | Thermo Fisher Scientific | Cat#15596018 |
| Vitronectin | Thermo Fisher Scientific | Cat#A14700 |
| Y27632 | Apexbio Technology | Cat#B129310 |
| Critical commercial assays | ||
| Direct-zol RNA MiniPrep | Zymo Research | Cat#R2052 |
| KAPA HyperPlus Kit | Roche | Cat#7962428001 |
| KAPA RNA HyperPrep Kit with RiboErase | Roche | Cat#8098131702 |
| MTase-Glo Methyltransferase Assay | Promega | Cat#V7602 |
| SuperScript IV First-Strand Synthesis System | Thermo Fisher Scientific | Cat#18091050 |
| SYBR Select Master Mix | Thermo Fisher Scientific | Cat#4472908 |
| Deposited data | ||
| ChIP-seq data | This paper | GEO: GSE271752 |
| RNA-seq data | This paper | GEO: GSE271648 |
| Western blot and imaging data | This paper | http://dx.doi.org/10.17632/xngr4x532x.1 |
| Experimental models: Cell lines | ||
| HEK293T/17 cells | ATCC | Cat#CRL-11268; RRID: CVCL_1926 |
| S2 cells | Active Motif | Cat#53083 |
| Sf9 cells | Thermo Fisher Scientific | Cat#12659017 |
| Tni cells | Expression Systems | Cat#94–002 |
| WTC-11 hiPSCs | Coriell Institute for Medical Research | Cat#GM25256; RRID: CVCL_Y803 |
| MTF2-HaloTag-3XFLAG WT hiPSCs | This paper | N/A |
| MTF2-HaloTag-3XFLAG SUZ12 mut hiPSCs | This paper | N/A |
| MTF2-HaloTag-3XFLAG DNA mut hiPSCs | This paper | N/A |
| MTF2-HaloTag-3XFLAG H3K36me3 mut (Y62A) hiPSCs | This paper | N/A |
| MTF2-HaloTag-3XFLAG H3K36me3 mut (F80A) hiPSCs | This paper | N/A |
| MTF2 KO hiPSCs | This paper | N/A |
| PHF1-HaloTag-3XFLAG WT hiPSCs | This paper | N/A |
| PHF1-HaloTag-3XFLAG SUZ12 mut hiPSCs | This paper | N/A |
| PHF1-HaloTag-3XFLAG DNA mut hiPSCs | This paper | N/A |
| PHF1 KO hiPSCs | This paper | N/A |
| PHF19-HaloTag-3XFLAG WT hiPSCs | This paper | N/A |
| PHF19-HaloTag-3XFLAG SUZ12 mut hiPSCs | This paper | N/A |
| PHF19-HaloTag-3XFLAG DNA mut hiPSCs | This paper | N/A |
| PHF19 KO hiPSCs | This paper | N/A |
| SUZ12–3XFLAG WT hiPSCs | This paper | N/A |
| SUZ12–3XFLAG PRC2.1- hiPSCs | This paper | N/A |
| SUZ12–3XFLAG PRC2.2- hiPSCs | This paper | N/A |
| Experimental models: Organisms/strains | ||
| Oligonucleotides | ||
| See Table S7 for primer sequences | This paper | N/A |
| MTF2 sgRNA: TTATCTTGGCACTATCAAAA |
This paper | N/A |
| PHF1 sgRNA: AGAAGGTCTTACCTTTTTGA |
This paper | N/A |
| PHF19 sgRNA: GAGGGTAAACCCATCCTCTC |
This paper | N/A |
| SUZ12 sgRNA: TCTATAGATTTCTTCGAACT |
Youmans et al.14 | N/A |
| Mononucleosomal DNA for fluorescence polarization assays: /56- FAM/TAAAACGACGGCCAGTGAATTGCCGCATC GGAGAATCCCGGTGCCGAGGCCGCTCAATTG GTCGTAGACAGCTCTAGCACCGCTTAAACGCA CGTACGCGCTGTCCCCCGCGTTTTAACCGCCA AGGGGATTACTCCCTAGTCTCCAGGCACGTGT CAGATATATACATCGATTGCATGTAGCTTGGCG TAATCATGGTC |
This paper | N/A |
| Mononucleosomal DNA for methyltransferase assays: /5biotin/GACCATGATTACGCCAAGCTACCTGCA GTCGATGTATATATCTGACACGTGCCTGGAGA CTAGGGAGTAATCCCCTTGGCGGTTAAAACGC GGGGGACAGCGCGTACGTGCGTTTAAGCGGT GCTAGAGCTGTCTACGACCAATTGAGCGGCCT CGGCACCGGGATTCTCCGATGCGCGAATTCAC TGGCCGTCGTTTTA |
This paper | N/A |
| Recombinant DNA | ||
| 3XFLAG-SUZ12 WT expression plasmid | This paper | N/A |
| 3XFLAG-SUZ12 PRC2.1- expression plasmid | This paper | N/A |
| 3XHA-MTF2 WT expression plasmid | This paper | N/A |
| 3XHA-MTF2 SUZ12 mut1 expression plasmid | This paper | N/A |
| 3XHA-MTF2 SUZ12 mut2 expression plasmid | This paper | N/A |
| 3XHA-MTF2 SUZ12 mut3 expression plasmid | This paper | N/A |
| 3XHA-MTF2 SUZ12 mut4 expression plasmid | This paper | N/A |
| 3XHA-PRC2.1- expression plasmid | This paper | N/A |
| 3XHA-PHF1 WT expression plasmid | This paper | N/A |
| 3XHA-PHF1 SUZ12 mut1 expression plasmid | This paper | N/A |
| 3XHA-PHF1 SUZ12 mut2 expression plasmid | This paper | N/A |
| 3XHA-PHF19 WT expression plasmid | This paper | N/A |
| 3XHA-PHF19 SUZ12 mut1 expression plasmid | This paper | N/A |
| 3XHA-PHF19 SUZ12 mut2 expression plasmid | This paper | N/A |
| MTF2 sgRNA Cas9 plasmid | This paper | Based on RRID: Addgene_42230 |
| PHF1 sgRNA Cas9 plasmid | This paper | Based on RRID: Addgene_42230 |
| PHF19 sgRNA Cas9 plasmid | This paper | Based on RRID: Addgene_42230 |
| SUZ12 sgRNA Cas9 plasmid | This paper | Based on RRID: Addgene_42230 |
| MTF2-HaloTag-3XFLAG WT HDR plasmid | This paper | N/A |
| MTF2-HaloTag-3XFLAG SUZ12 mut HDR plasmid | This paper | N/A |
| MTF2-HaloTag-3XFLAG DNA mut HDR plasmid | This paper | N/A |
| MTF2-HaloTag-3XFLAG H3K36me3 mut (Y62A) HDR plasmid | This paper | N/A |
| MTF2-HaloTag-3XFLAG H3K36me3 mut (F80A) HDR plasmid | This paper | N/A |
| MTF2 KO HDR plasmid | This paper | N/A |
| PHF1-HaloTag-3XFLAG WT HDR plasmid | This paper | N/A |
| PHF1-HaloTag-3XFLAG SUZ12 mut HDR plasmid | This paper | N/A |
| PHF1-HaloTag-3XFLAG DNA mut HDR plasmid | This paper | N/A |
| PHF1 KO HDR plasmid | This paper | N/A |
| PHF19-HaloTag-3XFLAG WT HDR plasmid | This paper | N/A |
| PHF19-HaloTag-3XFLAG SUZ12 mut HDR plasmid | This paper | N/A |
| PHF19-HaloTag-3XFLAG DNA mut HDR plasmid | This paper | N/A |
| PHF19 KO HDR plasmid | This paper | N/A |
| SUZ12–3XFLAG WT HDR plasmid | Youmans et al.14 | N/A |
| SUZ12–3XFLAG PRC2.1- HDR plasmid | Youmans et al.14 | N/A |
| SUZ12–3XFLAG PRC2.2- HDR plasmid | Youmans et al.14 | N/A |
| MBP-6xHis-EZH2 baculovirus expression plasmid | Long et al.33 Wang et al.71 Davidovich et al.72 | N/A |
| MBP-6xHis-SUZ12 baculovirus expression plasmid | Long et al.33 Wang et al.71 Davidovich et al.72 | N/A |
| MBP-6xHis-EED baculovirus expression plasmid | Long et al.33 Wang et al.71 Davidovich et al.72 | N/A |
| MBP-6xHis-RBBP4 baculovirus expression plasmid | Long et al.33 Wang et al.71 Davidovich et al.72 | N/A |
| 6xHis-EED baculovirus expression plasmid | Zhang et al.73 | RRID: Addgene_125174 |
| 6xHis-RBBP4 baculovirus expression plasmid | Zhang et al.73 | RRID: Addgene_125173 |
| Strep II-ABEP2 baculovirus expression plasmid | This paper | N/A |
| 6-subunit JARID2-AEBP2-EZH2-SUZ12-EED-RBBP4 baculovirus expression plasmid | Kasinath et al.17 | N/A |
| MBP-6xHis-PHF19 baculovirus expression plasmid | Zhang et al.73 | RRID: Addgene_125168 |
| MBP-6xHis-MTF2 baculovirus expression plasmid | Zhang et al.73 | RRID: Addgene_125169 |
| MBP-6xHis-MTF2 K338A K339A mutant baculovirus expression plasmid | This paper | N/A |
| Software and algorithms | ||
| Adobe Photoshop v25.9.1 | Adobe | RRID: SCR_014199 |
| bedGraphToBigWig v385 | Kent et al.61 | https://github.com/BioQueue/ucsc-tools/tree/master/src/utils/bedGraphToBigWig |
| BWA-MEM v0.7.17-r1188 | Li et al.59 | N/A |
| Clampfit v10.6.2 | Molecular Devices | RRID: SCR_011323 |
| Cutadapt v2.10 | Martin58 | RRID: SCR_011841 |
| deepTools v3.5.5 | Ramirez et al.62 | RRID: SCR_016366 |
| DESeq2 v1.44.0 | Love et al.64 | RRID: SCR_015687 |
| DIA-NN v1.8 | Demichev et al.57 | RRID: SCR_022865 |
| fdrtool v1.2.17 | Strimmer65 | https://cran.r-project.org/web/packages/fdrtool/index.html |
| featureCounts (Subread v2.0.0) | Liao et al.63 | RRID: SCR_012919 |
| FlowJo v10.10.0 | BD Biosciences | RRID: SCR_008520 |
| ggplot2 v3.5.1 | Wickham66 | RRID: SCR_014601 |
| IGV v2.4.10 | Robinson et al.67 | RRID: SCR_011793 |
| Image Lab v6.1 | Bio-Rad | RRID: SCR_014210 |
| ImageJ v2.14.0 | NIH | RRID: SCR_003070 |
| MACS2 | Zhang et al.60 | RRID: SCR_013291 |
| MotionGUI | Huebsch et al.75 | https://huebschlab.wustl.edu/resources-2/ |
| Patchmaster v1.4.1 | HEKA Elektronik | RRID: SCR_000034 |
| Pheatmap v1.0.12 | Kolde | RRID: SCR_016418 |
| Picard | Broad Institute | RRID: SCR_006525 |
| ShinyGO v0.80 | Ge et al.68 | RRID: SCR_019213 |
| STAR v2.7.10a | Dobin et al.70 | RRID: SCR_004463 |
| Other | ||
Acknowledgements
We thank Vigneshwari Kumar, Roxanne Lamanna, and Cyril Gagnon (Weill Cornell) for their contributions at the early stages of the project. We thank Dr. Taeyoung Hwang (Johns Hopkins) for his assistance in our computational analysis. We are grateful for the helpful discussions and feedback from Dr. Jessica Tyler (Weill Cornell) and members of the Long and Pitt labs. We thank Drs. Daniel Youmans, Thomas Cech, Vignesh Kasinath (CU Boulder), and Chen Davidovich (Monash U) for kindly providing plasmids. This work is supported by the Weill Cornell Proteomics & Metabolomics, Epigenomics, and Genomics core facilities. Y.L. is supported by R35GM155228, R00GM132546 (NIGMS), AHA Career Development Award 935361, and Tri-SCI grant 2023–010. G.S.P. is supported by R01 HL146149, R01 HL151190, and R01 HL160089 (NHLBI).
Footnotes
Declaration of interests
The authors declare no competing interests.
References
- 1.Atlasi Y, and Stunnenberg HG (2017). The interplay of epigenetic marks during stem cell differentiation and development. Nat Rev Genet 18, 643–658. 10.1038/nrg.2017.57. [DOI] [PubMed] [Google Scholar]
- 2.Trojer P, and Reinberg D (2007). Facultative heterochromatin: is there a distinctive molecular signature? Mol Cell 28, 1–13. 10.1016/j.molcel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Yu JR, Lee CH, Oksuz O, Stafford JM, and Reinberg D (2019). PRC2 is high maintenance. Gene Dev 33, 903–935. 10.1101/gad.325050.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh CH, van Genesen S, Perino M, Bark MR, and Veenstra GJC (2021). Loss of PRC2 subunits primes lineage choice during exit of pluripotency. Nat Commun 12, 6985. 10.1038/s41467-021-27314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer S, and Liefke R (2023). Polycomb-like Proteins in Gene Regulation and Cancer. Genes (Basel) 14. 10.3390/genes14040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, S CO-R, Dhiman A, Jiao G, Strohmier BP, Krusemark CJ, and Dykhuizen (2021). Polycomb group proteins in cancer: multifaceted functions and strategies for modulation. NAR Cancer 3, zcab039. 10.1093/narcan/zcab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laugesen A, Hojfeldt JW, and Helin K (2016). Role of the Polycomb Repressive Complex 2 (PRC2) in Transcriptional Regulation and Cancer. Cold Spring Harb Perspect Med 6. 10.1101/cshperspect.a026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straining R, and Eighmy W (2022). Tazemetostat: EZH2 Inhibitor. J Adv Pract Oncol 13, 158–163. 10.6004/jadpro.2022.13.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, Coindre JM, Blakemore SJ, Clawson A, Suttle B, et al. (2018). Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19, 649–659. 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 10.Kurmasheva RT, Sammons M, Favours E, Wu J, Kurmashev D, Cosmopoulos K, Keilhack H, Klaus CR, Houghton PJ, and Smith MA (2017). Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 64. 10.1002/pbc.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hojfeldt JW, Hedehus L, Laugesen A, Tatar T, Wiehle L, and Helin K (2019). Non-core Subunits of the PRC2 Complex Are Collectively Required for Its Target-Site Specificity. Mol Cell 76, 423–436 e423. 10.1016/j.molcel.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glancy E, Wang C, Tuck E, Healy E, Amato S, Neikes HK, Mariani A, Mucha M, Vermeulen M, Pasini D, and Bracken AP (2023). PRC2.1- and PRC2.2-specific accessory proteins drive recruitment of different forms of canonical PRC1. Mol Cell 83, 1393–1411 e1397. 10.1016/j.molcel.2023.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petracovici A, and Bonasio R (2021). Distinct PRC2 subunits regulate maintenance and establishment of Polycomb repression during differentiation. Mol Cell 81, 2625–2639 e2625. 10.1016/j.molcel.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youmans DT, Gooding AR, Dowell RD, and Cech TR (2021). Competition between PRC2.1 and 2.2 subcomplexes regulates PRC2 chromatin occupancy in human stem cells. Mol Cell 81, 488–501 e489. 10.1016/j.molcel.2020.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy E, Mucha M, Glancy E, Fitzpatrick DJ, Conway E, Neikes HK, Monger C, Van Mierlo G, Baltissen MP, Koseki Y, et al. (2019). PRC2.1 and PRC2.2 Synergize to Coordinate H3K27 Trimethylation. Mol Cell 76, 437–452 e436. 10.1016/j.molcel.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 16.van Mierlo G, Veenstra GJC, Vermeulen M, and Marks H (2019). The Complexity of PRC2 Subcomplexes. Trends Cell Biol 29, 660–671. 10.1016/j.tcb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kasinath V, Beck C, Sauer P, Poepsel S, Kosmatka J, Faini M, Toso D, Aebersold R, and Nogales E (2021). JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science 371. 10.1126/science.abc3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai L, Rothbart SB, Lu R, Xu B, Chen WY, Tripathy A, Rockowitz S, Zheng D, Patel DJ, Allis CD, et al. (2013). An H3K36 methylation-engaging Tudor motif of polycomb-like proteins mediates PRC2 complex targeting. Mol Cell 49, 571–582. 10.1016/j.molcel.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballare C, Lange M, Lapinaite A, Martin GM, Morey L, Pascual G, Liefke R, Simon B, Shi Y, Gozani O, et al. (2012). Phf19 links methylated Lys36 of histone H3 to regulation of Polycomb activity. Nat Struct Mol Biol 19, 1257–1265. 10.1038/nsmb.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brien GL, Gambero G, O’Connell DJ, Jerman E, Turner SA, Egan CM, Dunne EJ, Jurgens MC, Wynne K, Piao L, et al. (2012). Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol 19, 1273–1281. 10.1038/nsmb.2449. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Jiao L, Liu X, Yang X, and Liu X (2020). A Dimeric Structural Scaffold for PRC2-PCL Targeting to CpG Island Chromatin. Mol Cell 77, 1265–1278 e1267. 10.1016/j.molcel.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Liefke R, Jiang J, Kurland JV, Tian W, Deng P, Zhang W, He Q, Patel DJ, Bulyk ML, et al. (2017). Polycomb-like proteins link the PRC2 complex to CpG islands. Nature 549, 287–291. 10.1038/nature23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perino M, van Mierlo G, Karemaker ID, van Genesen S, Vermeulen M, Marks H, van Heeringen SJ, and Veenstra GJC (2018). MTF2 recruits Polycomb Repressive Complex 2 by helical-shape-selective DNA binding. Nat Genet 50, 1002–1010. 10.1038/s41588-018-0134-8. [DOI] [PubMed] [Google Scholar]
- 24.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, and Zhang Y (2002). Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043. 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 25.Min J, Zhang Y, and Xu RM (2003). Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev 17, 1823–1828. 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fursova NA, Blackledge NP, Nakayama M, Ito S, Koseki Y, Farcas AM, King HW, Koseki H, and Klose RJ (2019). Synergy between Variant PRC1 Complexes Defines Polycomb-Mediated Gene Repression. Molecular Cell 74, 1020-+. 10.1016/j.molcel.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose NR, King HW, Blckledge NP, Fursova NA, Ember KJI, Fischer R, Kessler BM, and Klose RJ (2016). RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. Elife 5. ARTN e18591 10.7554/elife.18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taherbhoy AM, Huang OW, and Cochran AG (2015). BMI1-RING1B is an autoinhibited RING E3 ubiquitin ligase. Nature Communications 6. ARTN 7621 10.1038/ncomms8621. [DOI] [PubMed] [Google Scholar]
- 29.Schmitges FW, Prusty AB, Faty M, Stützer A, Lingaraju GM, Aiwazian J, Sack R, Hess D, Li L, Zhou SL, et al. (2011). Histone Methylation by PRC2 Is Inhibited by Active Chromatin Marks. Molecular Cell 42, 330–341. 10.1016/j.molcel.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Finogenova K, Bonnet J, Poepsel S, Schafer IB, Finkl K, Schmid K, Litz C, Strauss M, Benda C, and Muller J (2020). Structural basis for PRC2 decoding of active histone methylation marks H3K36me2/3. Elife 9. 10.7554/eLife.61964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cookis T, Lydecker A, Sauer P, Kasinath V, and Nogales E (2024). Structural basis for the inhibition of PRC2 by active transcription histone posttranslational modifications. bioRxiv. 10.1101/2024.02.09.579730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, and Palecek SP (2013). Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc 8, 162–175. 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long Y, Hwang T, Gooding AR, Goodrich KJ, Rinn JL, and Cech TR (2020). RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem cells. Nature Genetics 52, 931–938. 10.1038/s41588-020-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharti H, Han S, Chang HW, and Reinberg D (2024). Polycomb repressive complex 2 accessory factors: rheostats for cell fate decision? Curr Opin Genet Dev 84, 102137. 10.1016/j.gde.2023.102137. [DOI] [PubMed] [Google Scholar]
- 35.Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, et al. (2008). Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet 4, e1000242. 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sing A, Pannell D, Karaiskakis A, Sturgeon K, Djabali M, Ellis J, Lipshitz HD, and Cordes SP (2009). A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell 138, 885–897. 10.1016/j.cell.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Woo CJ, Kharchenko PV, Daheron L, Park PJ, and Kingston RE (2010). A Region of the Human HOXD Cluster that Confers Polycomb-Group Responsiveness. Cell 140, 99–110. DOI 10.1016/j.cell.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Paucek RD, Gooding AR, Brown ZZ, Ge EJ, Muir TW, and Cech TR (2017). Molecular analysis of PRC2 recruitment to DNA in chromatin and its inhibition by RNA. Nat Struct Mol Biol 24, 1028–1038. 10.1038/nsmb.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner EJ, and Carpenter PB (2012). Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol 13, 115–126. 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong C, Nakagawa R, Oyama K, Yamamoto Y, Zhang W, Dong A, Li Y, Yoshimura Y, Kamiya H, Nakayama JI, et al. (2020). Structural basis for histone variant H3tK27me3 recognition by PHF1 and PHF19. Elife 9. 10.7554/eLife.58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, and Wysocka J (2009). Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139, 1290–1302. 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Kang K, and Kim J (2009). AEBP2 as a potential targeting protein for Polycomb Repression Complex PRC2. Nucleic Acids Research 37, 2940–2950. 10.1093/nar/gkp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uckelmann M, and Davidovich C (2021). Not just a writer: PRC2 as a chromatin reader. Biochem Soc T 49, 1159–1170. 10.1042/Bst20200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou DC, Cahan P, et al. (2012). Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 110, 406–415. 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Olguin P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A, and Bruneau BG (2012). Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nature Genetics 44, 343–347. 10.1038/ng.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, You T, Lu Y, Lin S, Li F, and Xu H (2021). Elevated EZH2 in ischemic heart disease epigenetically mediates suppression of Na(V)1.5 expression. J Mol Cell Cardiol 153, 95–103. 10.1016/j.yjmcc.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu M, Zhu JY, LaHaye S, Majumdar U, Jiao K, Han Z, and Garg V (2017). Epigenetic mechanisms underlying maternal diabetes-associated risk of congenital heart disease. JCI Insight 2. 10.1172/jci.insight.95085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mysliwiec MR, Bresnick EH, and Lee Y (2011). Endothelial Jarid2/Jumonji is required for normal cardiac development and proper Notch1 expression. J Biol Chem 286, 17193–17204. 10.1074/jbc.M110.205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho E, Mysliwiec MR, Carlson CD, Ansari A, Schwartz RJ, and Lee Y (2018). Cardiac-specific developmental and epigenetic functions of Jarid2 during embryonic development. J Biol Chem 293, 11659–11673. 10.1074/jbc.RA118.002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoolmeester JK, Sukov WR, Maleszewski JJ, Bedroske PP, Folpe AL, and Hodge JC (2013). JAZF1 rearrangement in a mesenchymal tumor of nonendometrial stromal origin: report of an unusual ossifying sarcoma of the heart demonstrating JAZF1/PHF1 fusion. Am J Surg Pathol 37, 938–942. 10.1097/PAS.0b013e318282da9d. [DOI] [PubMed] [Google Scholar]
- 51.Hachim MY, Al Heialy S, Senok A, Hamid Q, and Alsheikh-Ali A (2020). Molecular Basis of Cardiac and Vascular Injuries Associated With COVID-19. Front Cardiovasc Med 7, 582399. 10.3389/fcvm.2020.582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu W, Cheng Y, Wang S, Sun T, and Li Z (2021). PHD Finger Protein 19 Promotes Cardiac Hypertrophy via Epigenetically Regulating SIRT2. Cardiovasc Toxicol 21, 451–461. 10.1007/s12012-021-09639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Q, Wang S, Zhou X, Li Y, Xing S, Sha Y, Yang F, Huang W, Liu N, Li Z, et al. (2022). Essential role of MESP1-RING1A complex in cardiac differentiation. Dev Cell 57, 2533–2549 e2537. 10.1016/j.devcel.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Chrispijn ND, Elurbe DM, Mickoleit M, Aben M, de Bakker DEM, Andralojc KM, Huisken J, Bakkers J, and Kamminga LM (2019). Loss of the Polycomb group protein Rnf2 results in derepression of tbx-transcription factors and defects in embryonic and cardiac development. Sci Rep 9, 4327. 10.1038/s41598-019-40867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng X, Feng G, Zhang Y, and Sun Y (2021). PRC1 Stabilizes Cardiac Contraction by Regulating Cardiac Sarcomere Assembly and Cardiac Conduction System Construction. Int J Mol Sci 22. 10.3390/ijms222111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xi L, Schmidt JC, Zaug AJ, Ascarrunz DR, and Cech TR (2015). A novel two-step genome editing strategy with CRISPR-Cas9 provides new insights into telomerase action and TERT gene expression. Genome Biol 16, 231. 10.1186/s13059-015-0791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demichev V, Messner CB, Vernardis SI, Lilley KS, and Ralser M (2020). DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat Methods 17, 41–44. 10.1038/s41592-019-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin M (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011 17, 3. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 59.Li H, and Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137. 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kent WJ, Zweig AS, Barber G, Hinrichs AS, and Karolchik D (2010). BigWig and BigBed: enabling browsing of large distributed datasets. Bioinformatics 26, 2204–2207. 10.1093/bioinformatics/btq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dundar F, and Manke T (2016). deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44, W160–165. 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 64.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strimmer K (2008). fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 24, 1461–1462. 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 66.Wickham H (2009). Ggplot2 : elegant graphics for data analysis (Springer; ). [Google Scholar]
- 67.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, and Mesirov JP (2011). Integrative genomics viewer. Nat Biotechnol 29, 24–26. 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ge SX, Jung D, and Yao R (2020). ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629. 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Livak KJ, and Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 70.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Long Y, Paucek RD, Gooding AR, Lee T, Burdorf RM, and Cech TR (2019). Regulation of histone methylation by automethylation of PRC2. Genes Dev 33, 1416–1427. 10.1101/gad.328849.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidovich C, Zheng L, Goodrich KJ, and Cech TR (2013). Promiscuous RNA binding by Polycomb repressive complex 2. Nature Structural & Molecular Biology 20, 1250–1273. Doi 10.1038/Nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q, McKenzie NJ, Warneford-Thomson R, Gail EH, Flanigan SF, Owen BM, Lauman R, Levina V, Garcia BA, Schittenhelm RB, et al. (2019). RNA exploits an exposed regulatory site to inhibit the enzymatic activity of PRC2. Nat Struct Mol Biol 26, 237–247. 10.1038/s41594-019-0197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, et al. (2013). Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137. 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, et al. (2015). Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng Part C Methods 21, 467–479. 10.1089/ten.TEC.2014.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Figures S1-S12 and supplemental reference
Table S2. List of genes with increasing or decreasing H3K27me3 levels upon loss of PRC2.1 and PRC2.2 in hiPSCs, related to Figure 1
Table S3. List of significantly up- and downregulated genes levels upon loss of PRC2.1 and PRC2.2 in hiPSCs, related to Figure 1
Table S4. List of significantly up- and downregulated genes levels upon loss of PRC2.1 and PRC2.2 in day 15 cardiomyocytes, related to Figure 2
Table S7. Primer sequences, related to the STAR methods.
Table S1. List of proteins co-immunoprecipitating with SUZ12 in WT and mutant SUZ12 hiPSCs, related to Figure 1
Table S6. List of cancer-associated mutations of SUZ12 and PCL residues engineered for separation-of-function analyses in this study, related to Figure 1
Table S5. List of PCL target genes in hiPSCs, related to Figure 3
Data Availability Statement
ChIP-seq and RNA-seq data have been deposited at GEO under accession numbers GSE271752 and GSE271648 and are publicly available as of the date of publication.
Original western blot and microscopy images have been deposited at Mendeley Data (DOI: http://dx.doi.org/10.17632/xngr4x532x.1) and are publicly available as of the date of publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.