Abstract
We have developed yeast artificial chromosome (YAC) transgenic mice expressing normal (YAC18) and mutant (YAC46 or YAC72) human huntingtin (htt), in a developmental- and tissue-specific manner, that is identical to endogenous htt. YAC72 mice develop selective degeneration of medium spiny projection neurons in the lateral striatum, similar to what is observed in Huntington disease. Mutant human htt expressed by YAC transgenes can compensate for the absence of endogenous htt and can rescue the embryonic lethality that characterizes mice homozygous for targeted disruption of the endogenous Hdh gene (−/−). YAC72 mice lacking endogenous htt (YAC72 −/−) manifest a novel phenotype characterized by infertility, testicular atrophy, aspermia, and massive apoptotic cell death in the testes. The testicular cell death in YAC72 −/− mice can be markedly reduced by increasing endogenous htt levels. YAC72 mice with equivalent levels of both wild-type and mutant htt (YAC72 +/+) breed normally and have no evidence of increased testicular cell death. Similar findings are seen in YAC46 −/− mice compared with YAC46 +/+ mice, in which wild-type htt can completely counteract the proapoptotic effects of mutant htt. YAC18 −/− mice display no evidence of increased cellular apoptosis, even in the complete absence of endogenous htt, demonstrating that the massive cellular apoptosis observed in YAC46 −/− mice and YAC72 −/− mice is polyglutamine-mediated toxicity from the mutant transgene. These data provide the first direct in vivo evidence of a role for wild-type htt in decreasing the cellular toxicity of mutant htt.
Introduction
The mutation in Huntington disease (HD [MIM 143100; also see The Genome Database [accession number 119307]) is the expansion of a CAG-trinucleotide repeat in the first exon of the HD gene (Huntington Disease Collaborative Research Group 1993). Alleles containing expansions >35 CAG repeats are associated with the clinical phenotype of HD, with an earlier age at onset occurring with higher CAG-repeat sizes (Andrew et al. 1993). The mutation in the HD gene produces a protein, huntingtin (htt), with an expanded polyglutamine tract. Proteolytic cleavage of htt, possibly by caspases, produces N-terminal htt fragments containing the expanded polyglutamine tract (Goldberg et al. 1996; Wellington et al. 1998, 2000). N-terminal fragments of mutant expanded htt have altered cellular interactions (Li et al. 1995; Bao et al. 1996; Burke et al. 1996; Kalchman et al. 1996, 1997; Wanker et al. 1997) and nuclear localization (Davies et al. 1997; DiFiglia et al. 1997; Becher et al. 1998; Hackam et al. 1998; Gutekunst et al. 1999; Hodgson et al. 1999; Schilling et al. 1999; Li et al. 2000; Wheeler et al. 2000) and, in a variety of in vitro model systems, are directly toxic to neuronal cells (Hackam et al. 1998; Martindale et al. 1998; Sandou et al. 1998). These htt fragments are also prone to intracellular aggregation and inclusion formation (Cooper et al. 1998; Hackam et al. 1998; Martindale et al. 1998), although the relevance of htt aggregation to the pathogenesis of HD remains unclear (reviewed in Sisoda 1998). The expansion of polyglutamine residues in htt has been proposed to result in a novel toxic gain of function of the mutant protein (MacDonald and Gusella 1996), but alterations in normal endogenous htt levels and loss of htt function may also play a role in the pathogenesis of HD.
htt is a large protein of uncertain function that is ubiquitously expressed in many tissues of the body but that is at its highest levels in brain and testis (Sharp and Ross 1996). Mice homozygous for targeted disruption of Hdh (−/−), the murine homologue of the HD gene, die at embryonic day 7.5 (Duyao et al. 1995; Nasir et al. 1995; Zeitlin et al. 1995). Mice with decreased levels of htt after targeted insertion of a neo construct into the Hdh gene have aberrant brain development and perinatal lethality (White et al. 1997). Mice heterozygous for targeted disruption of the Hdh gene (+/−) express half the normal levels of endogenous htt and develop neuronal degeneration in the basal ganglia during adulthood (O'Kusky et al. 1999). Together, these results suggest that htt may normally be involved both in embryogenesis and in neuronal survival in the adult.
Recently, we demonstrated that wild-type htt has antiapoptotic properties in vitro (Rigamonti et al. 2000). To assess the ability of wild-type htt to modulate the toxicity of mutant htt in vivo, we developed a transgenic mouse model in which the phenotypic effects that varying endogenous htt levels have on mice expressing transgenic htt can be assessed.
Our laboratory has recently produced YAC mice transgenic for the entire genomic region of the human HD gene, including all its regulatory sequences (Hodgson et al. 1999). Human htt is expressed appropriately during development in YAC transgenic mice, as demonstrated by the ability of the human transgene to rescue the embryonic lethality of Hdh-nullizygous mice (−/−) (Hodgson et al. 1996). Appropriate control of tissue specificity was confirmed by the identical expression patterns of endogenous and human transgenic htt, by western blot analysis and subcellular localization studies (Hodgson et al. 1996, 1999).
YAC transgenic mice have been generated that express human htt with 18 polyglutamines (YAC18), corresponding to a CAG-repeat length observed in unaffected persons; 46 polyglutamines (YAC46), corresponding to a CAG-repeat length observed in patients with adult-onset HD; and 72 polyglutamines (YAC72), corresponding to a repeat length causing juvenile-onset HD (Hodgson et al. 1999). These mice express similar levels of transgenic human htt, which differ only in polyglutamine-expansion length. Up to 24 mo of age, YAC18 mice have no observable phenotype, indicating that human htt with a polyglutamine tract of normal length is not pathogenic in mice. However, mice transgenic for mutant htt with an expanded polyglutamine develop a progressive phenotype characterized by behavioral, cellular, and neuropathological abnormalities similar to those observed in HD (Hodgson et al. 1999).
When crossed to the Hdh-nullizygous background, YAC transgenic mice survive normally into adulthood (designation “YAC −/−”). These crosses provide a system in which one can examine the role of wild-type htt in the modulation of the cellular toxicity of mutant htt in vivo. In this study, we demonstrate that mutant human htt causes increased apoptotic cell death in the testes of transgenic mice expressing no endogenous htt. This proapoptotic effect of mutant htt can be completely inhibited by increased levels of murine wild-type htt, providing the first in vivo evidence that wild-type htt can reduce the toxicity of mutant htt.
Material and Methods
Generation of Experimental Mice
YAC transgenic mice (FVB/NJ strain) from lines 29 (YAC18), 668 (YAC46), and 2511 (YAC72) were bred with mice heterozygous for targeted disruption of the endogenous mouse Hdh gene (C57BL6 strain), to produce F1-generation hybrid mice. F1 hybrid mice positive for the YAC transgene and heterozygous for the Hdh gene were then bred to produce litters of F2 experimental mice, as described elsewhere (Hodgson et al. 1996). All the F2 offspring of these matings were genotyped and used to generate experimental data. We also bred selected F2 mice, to examine mating behavior, breeding success rates, and to obtain postcoital sperm counts.
Genotyping was performed, by standard PCR-based techniques, on genomic DNA from tail clippings prepared by phenol-chloroform extraction (Hodgson et al. 1996). Protein expression was determined by western blot in which 200 μg of total protein from homogenized testes was loaded onto a low-bis acrylamide gel, run at 100 V for 2 h and at 200 V for 3 h before being transferred to polyvinylidene fluoride membranes. Blots were probed with anti-htt antibody (HD3 at a diltution of 1/1,000; Gutekunst et al. 1999) and were detected by enhanced chemiluminescence immunodetection (Amersham).
Fertility, Mating Behavior, and Sperm Analysis
To assess breeding success, adult male mice of each genotype were placed in breeding cages with single FVB/NJ female mice for ⩾4 mo (a minimum of three male mice were tested per genotype). The total duration of time spent in breeding cages and the total number of live-born litters was recorded for each mouse. Failure to produce any litters after a cumulative breeding duration of ⩾4 mo was considered to represent male infertility. New wild-type females with previous successful breeding experience were placed in breeding cages containing infertile males, to control for any contribution of the female partner. YAC72 −/− male mice (i.e., those expressing human htt with 72 polyglutamines and no endogenous murine htt), YAC72 +/− male mice (i.e., those expressing human htt with 72 polyglutamines on a background of half the normal levels of endogenous murine htt), and YAC72 +/+ male mice (i.e., those expressing human htt with 72 polyglutamines on a background of normal levels of endogenous murine htt) were placed in breeding cages with pseudopregnant FVB/NJ females, to assess male sexual behavior and postcoital sperm counts. These wild-type female mice were injected with 0.1 ml of pregnant mare serum (Sigma) 48 h prior to stimulation with 10 IU of human chorionic gonadotropin (Sigma) and placement in breeding cages with the transgenic males. Mounting behavior of male mice was scored for 2–3 h after placement in breeding cages, and plug formation was determined by manual inspection of the female mice the following morning.
Postcoital sperm counts were determined on the basis of the extracted uterus of all female mice that had evidence of plug formation after breeding with male transgenic mice. After breeding, the plugged female mice were anesthetized, and the uterus and oviducts were removed in toto and gently were opened in a sterile 12-well tissue-culture plate; 0.5 ml of sterile saline was used to flush the uterus, and the resultant solution was collected and examined microscopically for the presence of sperm. To obtain quantitative sperm counts, the testes and epididymides of YAC72 +/+ mice, YAC72 +/− mice, and YAC72 −/− mice (n=6 mice per genotype) were removed and immediately weighed. The tissues were then sectioned and placed in tubes containing 0.5 ml of sterile saline. Total numbers of morphologically normal sperm were manually counted, by a hemacytometer (Hausser), for three samples from each tissue. The hemacytometer counts for each tissue were averaged, and the total counts per milliliter were calculated. Results are expressed as average ± SEM sperm count per milliliter, and significance was determined by Students t-test.
Histological and Ultrastructural Analysis
Testes were removed from anesthetized animals and immediately were placed in fixative (1.5% paraformaldehyde, 1.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.3). The capsules were nicked with a scalpel, and then the organs were left to fix for ∼1 h. The testes were cut into small pieces and were fixed for an additional hour. The pieces were washed with buffer, postfixed in buffered 1% OsO4 on ice for 1 h, washed with dH20, and stained en bloc in 1% aqueous uranyl acetate. The samples were washed with dH20, dehydrated through a graded concentration series of ethyl alcohols, and then embedded in JEMBED 812 (J. B. EM Services).
For histological analysis, thick (1 μm) sections were stained with toluidine blue and were evaluated by a Zeiss Axiophot microscope. For ultrastructural analysis, thin sections were cut on an ultramicrotome, stained with lead citrate and uranyl acetate, and then viewed and photographed by a Philips 300 electron microscope operated at 60 kV.
TUNEL labeling (Boehringer Mannheim) was performed, by standard techniques, on frozen sections of testes that were lightly immersion fixed in 3% paraformaldehyde. After removal of the testes, mice were injected with heparin and were perfused transcardially with 3% paraformaldehyde and 0.15% glutaraldehyde in phosphate buffer (pH 7.4). Brains were then removed and postfixed in 3% paraformaldehyde overnight.
Immunocytochemistry
Testes were excised from anesthetized animals, the capsules were cut open with a scalpel, and the organs were immersion fixed (3% paraformaldehyde, 150 mM NaCl, 5 mM KCl, 3.2 mM Na2HPO4, 0.8 mM KH2PO4, pH 7.3) for 1–2 h. After fixation, the testes were washed three times (10 min each wash) with PBS (150 mM NaCl, 5 mM KCl, 3.2 mM Na2HPO4, 0.8 mM KH2PO4, pH 7.3) and then were frozen in Tissue-Tek embedding medium (Sakura) compound and sectioned on a cryostat. Sections (10 μm thick) were collected on polylysine-coated slides, and then the slides were immediately plunged into cold (−20°C) acetone for 5 min; then the slides were air-dried.
Sections were rehydrated for 30 min in TPBS (PBS, 0.05% Tween-20, 0.1% BSA) containing 5% normal goat serum (NGS) and then were incubated for 1 h at 37°C with primary antiserum diluted 1:100 (HD3) with TPBS containing 1% NGS. Sections were washed three times (10 min each wash) with TPBS and then were incubated for 1 h at 37°C with secondary antibody (goat anti-rabbit conjugated to Texas red) diluted 1:100 in TPBS. Sections again were washed with TPBS and then were mounted in Vetashield (Vector Laboratories) and were viewed by a Zeiss Axiophot microscope fitted with the appropriate fluorescence filter sets. Controls consisted of replacement of (1) the primary antibody by the same concentration of normal rat IgG (as the control for primary antibody), (2) the primary antibody with buffer alone (as the control for secondary antibody), and (3) the primary and secondary antibodies with buffer alone (as the control for autofluorescence).
Results
Rescue of the Hdh −/−Lethal Phenotype by YAC Transgenes Expressing Mutant Human htt
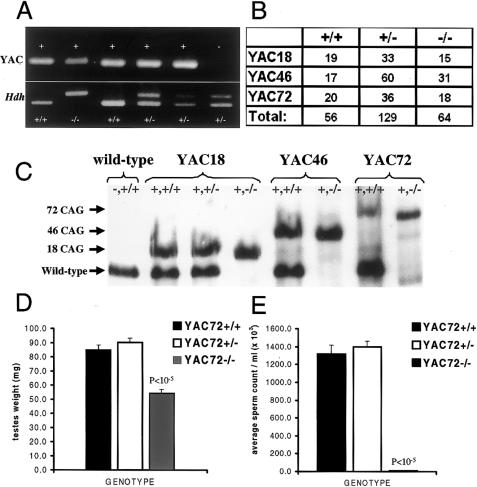
We generated YAC transgenic mice expressing normal (YAC18) or mutant (YAC46 or YAC72) human htt in the absence of endogenous mouse htt (Hdh −/−). Figure 1A demonstrates the genotypes of several offspring from a cross between two mice heterozygous for targeted disruption of the Hdh gene, one of which also carried the YAC72 transgene (YAC72 +/−). Mice with targeted disruption of both alleles of the Hdh gene can be rescued from the embryonic lethal phenotype by the YAC transgene expressing mutant htt with 72 CAG repeats (YAC72 −/−). In this litter, mice were generated with the YAC72 transgene and either 100% of the normal level of endogenous htt (YAC72 +/+), 50% of the normal level of endogenous htt (YAC72 +/−), or complete absence of endogenous htt (YAC72 −/−). A mouse lacking the YAC72 transgene but heterozygous for endogenous htt is also shown (fig. 1A, −, +/−). No Hdh −/− mice were generated in the absence of the YAC transgene, consistent with the previous finding that Hdh-nullizygous mice are not viable (Duyao et al. 1995; Nasir et al. 1995; Zeitlin et al. 1995). The F2 offspring of our experimental breedings had the expected 1:2:1 ratio of genotypes for all of the YAC transgenes examined (fig. 1B), demonstrating that both the normal (YAC18) and mutant (YAC46 or YAC72) human HD transgenes compensated for the lack of endogenous murine htt in Hdh −/− mice equally.
Figure 1.
Rescue of the Hdh-nullizygous lethal phenotype by YAC transgenes expressing mutant htt. Resultant genotypes for the F2 offspring of a cross between a YAC72 transgene–positive, Hdh-heterozygous mouse (+, +/− genotype) and a YAC72 transgene–negative, Hdh-heterozygous mouse (−, +/− genotype) are shown (A). The upper PCR bands represent the presence or absence of the YAC transgene, and the lower bands represent the state of the endogenous Hdh gene. The mouse represented in the second lane has the YAC72 transgene but lacks the endogenous Hdh gene (+, −/− genotype). This mouse demonstrates that mutant human htt expression from our YAC transgene rescued the Hdh-nullizygous state. Mice with targeted disruption of the Hdh gene were rescued from the embryonic lethal phenotype equally by all three of the YAC transgenes described in the present study. The F2 offspring of our experimental breedings had the expected 1:2:1 ratio of genotypes for all of the YAC transgenes examined (B). Western blot analysis of htt-protein expression (C) confirmed the absence of endogenous htt protein in Hdh-nullizygous mice (−/−), compared with wild-type mice (+/+), and demonstrated similar levels of human transgenic htt expression in YAC18-, YAC46-, and YAC72-rescued Hdh-nullizygous mice (+, −/−). Average testicular weight (D) and epididymal sperm counts (E) for YAC72 +/+ mice, YAC72 +/− mice, and YAC72 −/− mice at age 4 mo are shown. YAC72 −/− mice had significant testicular atrophy (P<10-5) and decreased sperm counts (P<10-5), compared with YAC72 +/+ mice and YAC72 +/− mice.
Levels of htt Expression in YAC Transgene–Rescued Hdh −/− Mice
Levels of the transgenic and wild-type htt protein for YAC18 (line 29), YAC46 (line 668), and YAC72 (line 2511) Hdh −/− mice were measured by western blot analysis using an antibody recognizing both human and mouse htt (Hodgson et al. 1996). The human transgenic protein is expressed at similar levels in the YAC18 mice, YAC46 mice, and YAC72 mice used in these experiments (fig. 1C). This result was replicated in three different western blots, and densitometric quantification of the transgenic protein level from these blots revealed a 1:1 ratio of transgenic versus endogenous htt levels in these mice. Transgenic protein levels from YAC18 mice averaged 102% of wild-type htt, YAC46 averaged 99% of wild-type htt, and YAC72 averaged 100% of wild-type htt. These three lines of YAC transgenic mice were selected for these experiments because they expressed identical levels of transgenic protein, differing only in the length of the CAG repeat.
Infertility of Mice Expressing Mutant htt
Expression of mutant (YAC46 and YAC72)—but not of wild-type (YAC18)—transgenic htt in the absence of endogenous htt leads to a novel phenotype that was initially identified by the observation that male Hdh −/− mice were unable to breed. We attempted to breed YAC46 −/− males and YAC72 −/− males with wild-type females for extended periods, with no success (i.e., no offspring were generated). Female littermates with the same genotype (YAC46 −/− or YAC72 −/−) had normal fertility when bred with wild-type mice. Male YAC72 −/− mice had normal secondary sexual characteristics and, when placed in cages with female wild-type mice, displayed identical sexual behavior (mounting) and libido as were observed in YAC72 +/− mice and YAC72 +/+ mice. Similar plug-formation rates were obtained for males of all three genotypes. Plugs were recovered in 8 of 12 breeding trials for YAC72 −/− mice, in 7 of 12 breeding trials for YAC72 +/− mice, and in 8 of 16 breeding trials for YAC72 +/+ mice. In sharp contrast to both YAC72 +/+ mice and YAC72 +/− mice, no postcoital sperm was ever recovered from plugged females after mating with YAC72 −/− mice. These results suggested that a defect in spermatogenesis—and not in breeding behavior—was responsible for the observed lack of fertility of male YAC72 −/− mice.
Decreased Fertility in Mice Expressing Mutant htt: A Result of Decreased Sperm Production
To assess spermatogenesis directly in YAC72 −/− mice, we performed sperm counts and examined the testes of these mice. At age 4 mo, YAC72 −/− mice had significantly decreased epididymal sperm counts compared with YAC72 +/+ mice (11 × 103/ml ± 2.9/ml vs. 13 × 105/ml ± 100/ml ; P<.00001) or YAC72 +/− mice (11 × 103/ml ± 2.9/ml vs. 14 × 105/ml ± 65/ml; P<.00001) littermates (fig. 1D).
Testicular Atrophy and Spermatid Degeneration in Mice Expressing Mutant htt
At age 4 mo, YAC72 −/− mice had significant testicular atrophy compared with YAC72 +/+ mice (average testes weight 54.0 mg ± 2.9 vs. 84.7 mg ± 3.6.9; P<.00001) and YAC72 +/− mice (average testes weight 54.0 mg ± 2.9 vs. 90.1 mg ± 3.0; P<.00001) littermates (fig. 1E).
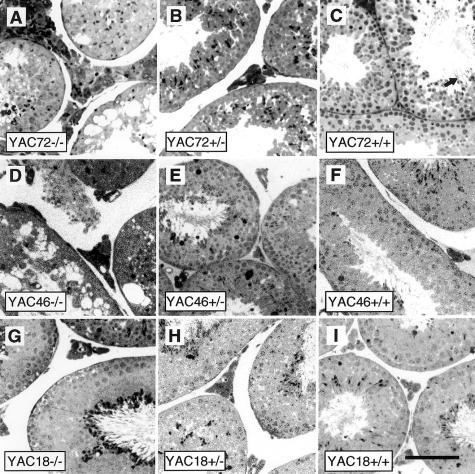
Histological examination of sections from the testes of adult mice expressing expanded mutant htt (YAC46 −/− or YAC72 −/−) stained with toluidine blue revealed massive disruption of spermatogenesis in the seminiferous tubules. The seminiferous tubules from these mice were full of large vacuoles and dying cells (fig. 2A and D), but the spermatagonial stem cells and Sertoli cells close to the basement lamina appeared relatively normal in appearance and number. Depletion—but not absence—of cells at later stages of spermatogenesis (spermatocytes and spermatids) was evident in all layers of degenerating tubules. Degenerating cells at various stages of development were identified, suggesting that the spermatogenic defect caused by mutant htt is not either limited to a single stage of development or due to defective maturation of spermatocytes. The normal stratified organization of cells within these seminiferous tubules was completely disrupted. Rarely, late spermatids were identified in the outer cell layer, but mature spermatozoa were not found in the lumen of these tubules, which were often filled with cellular debris (fig. 2A and D). Leydig cells appeared to be unaffected in the stromal interstitial tissue between degenerating tubules.
Figure 2.
Testicular morphology of YAC transgene–rescued Hdh-nullizygous mice. Semithin sections of testes stained with toluidine blue that are from 8-mo-old mice reveal the gross testicular morphology of mice with the YAC72 (A–C), YAC46 (D–F), and YAC18 (G–I) HD transgenes and either complete absence of endogenous htt (−/−), 50% of endogenous htt levels (+/−), or 100% of endogenous htt levels (+/+). Massive degeneration of spermatogenic cells occurs in the seminiferous tubules of mice expressing mutant htt with either 46 or 72 polyglutamine repeats (A and D). The cell death is most pronounced in YAC72 −/− (A), intermediate in YAC46 −/− (D), and not present in YAC18 −/− (G) Hdh-nullizygous mice. The human HD transgene in each of these lines of mice is identical except for the length of the CAG repeat, and these results suggest that this novel cell-death phenotype is CAG-repeat-length dependent. Increasing levels of endogenous htt markedly reduced the amount of spermatogenic-cell degeneration (B, C, E, and F) observed in YAC46 and YAC72 mice. (Scale bar = 100 μm)
Cellular Degeneration in Mice Expressing Mutant htt: Blockage by Expression of Endogenous htt and Dependence on CAG Length
This testicular degeneration was most striking in the testes of YAC72 −/− mice (fig. 2A). Occasionally, vacuolization and cellular degeneration were seen in the testes of YAC72 +/− mice expressing 50% of endogenous htt levels (fig. 2B), but these mice were able to produce mature sperm (fig. 1E). Increasing the endogenous htt expression to 100% of normal levels in YAC72 mice completely rescued the degenerative testicular phenotype (fig. 2C). Normal stratified organization was restored to the seminiferous tubules, and neither vacuolization nor an increased number of degenerating cells was present. Mature spermatozoa were found in the lumen of seminiferous tubules of YAC72 +/+ mice (fig. 2C, arrow), and these mice had normal fertility. These results suggest that the testicular cell death caused by the expression of polyglutamine-expanded htt in transgenic mice could be completely blocked by an increase in the level of endogenous htt.
The testicular cell death caused by the expression of polyglutamine-expanded htt in transgenic mice (YAC46 −/− or YAC72 −/−) does not occur in mice expressing the same human transgene without the CAG-repeat expansion (YAC18 −/− mice; fig. 2G). YAC18 mice lacking endogenous htt had normal morphology of the seminiferous tubules, no evidence of increased testicular cell death, and normal fertility. No significant effect on YAC18 mice was seen when levels of endogenous htt were increased to 50% or 100% of the normal htt levels (fig. 2H and I).
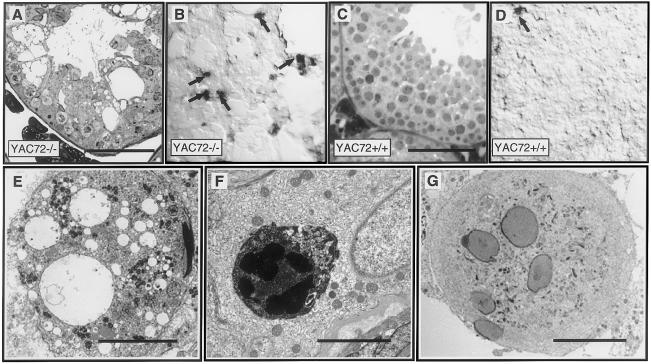
Histological examination of toluidine blue–stained sections from the testes of adult mice expressing mutant htt revealed massive cellular death in multiple layers of the seminiferous tubules (fig. 3A). TUNEL labeling (fig. 3B, arrows) confirmed the apoptotic nature of the widespread cell death in the testes of YAC72 −/− mice. Despite the drastically reduced numbers of cells within seminiferous tubules of testes of YAC72 −/− mice, the average number of TUNEL-positive cells (3.2 per low-power [20×] field) was ∼10-fold higher in these sections than in sections of testes of YAC72 +/+ mice containing normal numbers of spermatogenic cells (0.3 per 20× field). No increased testicular cell death was observed in YAC72 mice expressing 100% of normal levels of endogenous htt (YAC72 +/+), either by toluidine blue staining (fig. 3C) or by TUNEL labeling (fig. 3D).
Figure 3.
Morphological, biochemical, and ultrastructural evidence for apoptotic cell death in the testes of YAC72 mice lacking endogenous htt. Toluidine blue staining revealed massive death of spermatogenic cells in the testes of YAC72 −/− mice. Decreased numbers of spermatogenic cells and a disordered epithelium filled with vacuoles are present in these testes, compared with the very large numbers of spermatocytes in the well-ordered stratified epithelium of testes of YAC72 +/+ mice. Increased apoptosis was evident in the testes of YAC72 −/− mice, on the basis of increased TUNEL labeling of spermatogenic cells (B, arrows), compared with YAC72 mice that had normal levels of endogenous htt (D). EM analysis of degenerating testicular cells from YAC72 −/− mice also provided evidence of apoptosis. Ultrastructural analysis of testes from Hdh- nullizygous YAC72 mice reveals massive cell death of spermatids, phagocytosis of degenerating cells, and formation of multinucleated giant cells. The epithelium of YAC72 mice lacking endogenous htt was characterized by degenerating spermatids filled with cytoplasmic vacuoles (E), phagosomes containing shrunken electron-dense spermatids engulfed within Sertoli cells (F), and spermatogenic giant cells (G). (Scale bars in A–D = 100 μm; scale bar in E = 10 μm; scale bar in F = 5 μm; scale bar in G = 10 μm)
Ultrastructural analysis (by electron microscopy) of the testes in YAC72 mice nullizygous for endogenous htt (−/−) revealed large numbers of degenerating spermatids with diffuse cytoplasmic vacuolization (fig. 3E). Shrunken degenerating spermatids, with condensed nuclei and electron-dense cytoplasm, were phagocytosed and degraded by Sertoli cells (fig. 3F), suggesting ongoing apoptosis and confirming our TUNEL findings. Multinucleated giant cells were found throughout the testes of YAC72 −/− mice. These cells result from the opening of intercellular bridges between clones of spermatogenic cells. Importantly, no such degenerative phenotype was found by ultrastructural analysis of the testes of YAC18 mice (data not shown).
Abnormal Protein Aggregates: Occurrence in the Testes of Mice Expressing Mutant htt
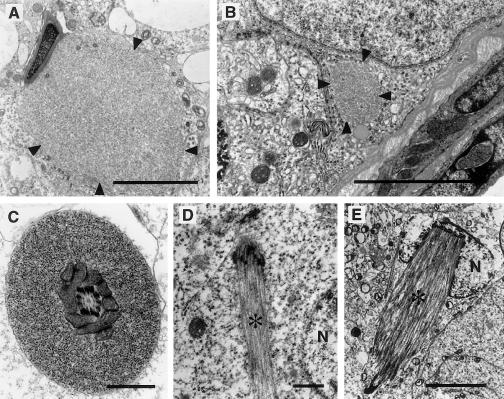
Ultrastructural analysis of the testes of YAC72 −/− mice revealed the presence of occasional abnormal aggregates of intracellular protein (fig. 4A–C, arrowheads) within spermatids (fig. 4A), Sertoli cells (fig. 4B), and sperm tails (fig. 4C). These aggregates were rare and were found at an incidence of much less than one per high-powered field. No protein aggregates were identified in YAC18 −/− mice (data not shown). Ectopic microtubule bundles (fig. 4D) and manchettes (fig. 4E) were also occasionally identified (fig. 4D and E, arrowheads) within spermatogonia and spermatids, respectively. Also interesting is the observation that actin-containing adhesion plaques (ectoplasmic specializations) that occur in Sertoli-cell cortical cytoplasm in regions of adhesion to spermatids often occurred in ectopic positions. Normally these structures occur only in regions of attachment to spermatid heads. In Sertoli cells of YAC72 −/− mice, ectoplasmic specializations were observed to completely surround elongate spermatids that had reacquired a circular form (data not shown).
Figure 4.
Protein aggregates in YAC72 −/− mice. Ultrastructural analysis of the testes of YAC72 mice lacking endogenous htt revealed the occasional presence of abnormal aggregates of intracellular protein (arrowheads) within elongate spermatids (A), Sertoli cells (B), and sperm tails (C). The composition of these protein aggregates is not entirely clear, but they resemble the ultrastructural appearance of htt aggregates found in human HD brain tissue. Ectopic microtubule bundles (D) and manchettes (E) also were identified (asterisks [*]). The bundle in panel E is in a spermatogonium. N = nucleus. (Scale bars in A and B = 5 μm; scale bar in C and D = 1 μm; scale bar in E = 5 μm)
Immunocytochemical analysis of htt localization in the testes of YAC72 −/− mice revealed that protein aggregates within a small number of degenerating spermatids (fig. 5A and B) contain htt. Similar htt immunoreactivity was identified in aggregates within Sertoli cells adjacent to the basal lamina of degenerating seminiferous tubules (fig. 5C and D). Labeling of filamentous actin by fluorescent phallotoxin revealed altered localization of filaments within the testes of YAC72 −/− mice (fig. 5I and J). In YAC72 +/+ mice (fig. 5E and F), actin filaments were concentrated in Sertoli-cell adhesion plaques (ectoplasmic specializations) found apically in association with spermatid heads and basally in association with junction complexes between neighboring Sertoli cells. In tissue of YAC72 −/− mice (fig. 5I and J), actin filaments occur in linear arrays perpendicular to the tubule wall and in areas not directly related to spermatid heads (fig. 5J, arrows with asterisks).
Figure 5.
Immunocytochemical analysis of protein aggregates and actin distribution in sections from the testes of YAC72 −/− mice. Abnormal protein aggregates within degenerating spermatogenic cells in the testes of YAC72 −/− mice contain htt (phase [A] and immunofluorescence [B]). In normal epithelium (phase [E] and fluorescence [F]), actin filaments in Sertoli cells are concentrated in unique adhesion plaques (ectoplasmic specializations) that occur at apical sites of attachment to spermatids (Apical) and at basal sites of attachment to neighboring Sertoli cells (Basal). In YAC72 −/− mice (G–J), filament bundles (asterisks [*]) in apical regions occur in areas not associated with spermatid heads, although filament bundles at basal sites occur in their normal position. (Scale bars in A–D = 10 μm; scale bars in E–J = 50 μm)
Discussion
In this article, we have provided evidence that wild-type htt can significantly reduce the cellular toxicity of mutant htt in vivo. Expression of human htt with an expanded polyglutamine tract (46 and 72 polyglutamines) in the absence of wild-type htt results in male infertility and in massive apoptotic cell death in the testes in all phases of spermatogenesis. The cell death can be modulated by the expression of normal htt. For example, mice expressing human htt with either 46 or 72 polyglutamines have no evidence of testicular atrophy or apoptosis in the testes when wild-type htt is expressed from both Hdh alleles. An intermediate phenotype is seen in these mice (YAC46 and YAC72), on the background of heterozygosity for targeted disruption in the mouse Hdh gene (YAC46 +/− or YAC72 +/−). The severity of the testicular atrophy and apoptotic cell death is also modulated by the length of the polyglutamine repeat. To prevent testicular degeneration, YAC72 transgenic mice require higher levels of wild-type htt (+/+) than do YAC46 transgenic mice (+/−). Abnormal protein aggregates, which contain htt, are occasionally found both in Sertoli cells and in spermatogenic cells in the testis of YAC72 −/− mice. Also, structures containing cytoskeletal elements form ectopically in these mice, suggesting that alterations either in the targeting of cytoskeletal elements to specific positions in the cells or of cytoskeletal function may be a mechanism promoting the massive apoptosis observed. These findings suggest that disruption of normal cytoskeletal organization may play a role in mediation of the toxic effect of mutant htt.
These findings may have relevance to the pathogenesis of HD. Interestingly, both mutant and wild-type htt proteins undergo cleavage (Wellington et al. 1998) and are recruited and sequestered into htt aggregates, leaving less-full-length wild-type htt available to counteract proapoptotic stimuli (Martindale et al. 1998). Polyglutamine expansion in one allele of the HD gene is associated with expression of half the cellular levels of wild-type htt compared with that in normal neurons, which, together with htt cleavage and sequestration of wild-type htt in aggregates, may further decrease functional htt levels. Mice heterozygous for targeted disruption of the Hdh gene express half the normal levels of wild-type htt and, elsewhere, have been shown to develop neuronal degeneration in the basal ganglia (Nasir et al. 1995; O'Kusky et al. 1999). One of the normal functions of htt in the brain may be to protect cells against proapoptotic stimuli, and partial loss of this function may underlie some of the selective vulnerability of striatal neurons to cell death in HD. These data support our previously published hypothesis that loss of function of wild-type htt may also contribute to the pathogenesis of HD (Nasir et al. 1995).
We have also recently demonstrated that wild-type human htt can protect hippocampal neurons from kainic acid–induced excitotoxicity (B. R. Leavitt, J. van Raamsdonk, J. G. Hodgson, C. L. Wellington, and M. R. Hayden, unpublished data). Furthermore, the very recent report that inactivation of Hdh expression in adult mice is associated with progressive apoptotic neurodegeneration provides further support for the antiapoptotic role of wild-type htt in the adult brain (Dragatsis et el. 2000).
A number of observations indicate that the ultimate outcome of htt toxicity in the testis is the apoptotic loss of spermatogenic cells. The dramatic vacuolization within the seminiferous tubules, the TUNEL staining of spermatogenic cells, and the ectopic positioning of manchettes (microtubule structures associated with spermatid nuclei) in these cells are consistent with this conclusion. The presence of giant cells in the epithelium; the occurrence, in Sertoli cells, of large phagosomes containing spermatids; and the obviously reduced numbers of spermatogenic cells in the epithelium, demonstrated by our EM analysis—all point to the conclusion that the primary testicular phenotype in YAC72 −/− mice is apoptotic death of spermatogenic cells, particularly of spermatids.
Although morphological changes and cell loss are most dramatic in the spermatogenic-cell population in the testes of YAC72 −/− mice, Sertoli cells also express abnormal features. Two of these features are the presence of protein aggregates in the cytoplasm and the ectopic positioning of actin filament–containing junction plaques normally found in regions adjacent to spermatid heads. Abnormal positioning of ectoplasmic specializations adjacent to spermatid cells may be a response to a primary defect in the associated spermatogenic cells. The presence of normally positioned junction plaques in basal regions of attachment to neighboring Sertoli cells is consistent with this conclusion. These findings suggest that mutant htt causes an intrinsic polyglutamine-mediated apoptotic cell death within spermatogenic cells, which can be blocked by wild-type htt.
Elsewhere, we have shown that human htt can compensate for the critical function of murine htt during gastrulation, by rescuing mice with targeted disruption on both Hdh alleles (Hodgson et al. 1996). These mice are rescued by both normal htt (YAC18) and mutant htt (YAC46 and YAC72), clearly indicating that expansion of the polyglutamine does not disturb this important role of htt in development. In the present study, we have provided further evidence for cross-species functional complementarity of htt. The presence of murine htt can completely protect cells against the proapoptotic effects of mutant human htt. Cross-species functional complementation between mouse htt and human htt, in both development and protection against apoptosis, must reflect their high degree of sequence conservation, with mouse htt and human htt sharing complete identity of nucleotides at a level of 90% similarity in amino acid structure (Lin et al. 1994).
The data in the present article provide strong in vivo evidence that wild-type htt can significantly modulate the apoptotic toxicity of mutant htt, and we suggest that wild-type htt may normally have an antiapoptotic function. Both mapping of the htt critical region responsible for this function and investigation of the mechanism by which this critical region influences cell-death pathways may identify novel therapeutic targets for HD and advance our understanding of htt's normal role in the delicate balance between life and death in cells.
Acknowledgments
We would like to thank our colleagues in our laboratories for useful comments and discussion, in particular Dr. Cheryl Wellington and Dr. Abigail Hackam. We would also like to thank Nagat Bissada, Krista McCutcheon, and Rosemary Oh, for excellent technical assistance. This work was supported by MRC/CIHR of Canada grants (to M.R.H., W.V., A.H., and B.R.L.), the Canadian Genetic Diseases Network, the Huntington Disease Society of America (support to M.R.H.). M.R.H. is an established investigator of the BC Children's Hospital.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Genome Database, The, http://www.gdb.org (for HD [accession number 119307])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HD [MIM 143100])
References
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR (1993) The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet 4:398–403 [DOI] [PubMed] [Google Scholar]
- Bao J, Sharp AH, Wagster MV, Becher M, Schilling G, Ross CA, Dawson VL, Dawson TM (1996) Expansion of polyglutamine repeat in huntingtin leads to abnormal protein interactions involving calmodulin. Proc Natl Acad Sci USA 93:5037–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher MW, Kotzuk JA, Sharp AH, Davies SW, Bates GP, Price DL, Ross CR (1998) Intranuclear neuronal inclusions in Huntington`s disease and dentatorubral and pallidoluysian atrophy: correlation between density of inclusions and IT15 triplet repeat length. Neurobiol Dis 4:387–397 [DOI] [PubMed] [Google Scholar]
- Burke JR, Enghild JJ, Martin ME, Jou Y-S, Myers RM, Roses AD, Vance JM, Strittmatter WJ (1996) Huntingtin and DRPLA proteins selectively interact with the enzyme GADPH. Nat Med 2:347–349 [DOI] [PubMed] [Google Scholar]
- Cooper JK, Schilling G, Peters MF, Herring WJ, Sharp AH, Kaminsky Z, Masone J, Khan FA, Delanoy M, Borschelt DR, Dawson VL, Dawson TM, Ross CA (1998) Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum Mol Genet 7:783–790 [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548 [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel J-P, Aronin NA (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993 [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Levine MS, Zeitlin S (2000) Inactivation of Hdh in the brain and testes results in progressive neurodegeneration and sterility in mice. Nat Genet 26:300–306 [DOI] [PubMed] [Google Scholar]
- Duyao MP, Auerbach AB, Ryan A, Persichetti F, Barnes GT, McNeil SM, Kowell NW, Ge P, Vonsattel J-P, Gusella JF, Joyner AL, MacDonald ME (1995) Inactivation of the mouse Huntingtin's disease gene homologue Hdh. Science 269:407–410 [DOI] [PubMed] [Google Scholar]
- Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, Graham RK, Bromm M, Kazemi-Esfajani P, Thornberry NA, Vaillancourt JP, Hayden MR (1996) Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet 13:442–449 [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ (1999) Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J Neurosci 19:2522–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam, AS, Singaraja R, Wellington CL, Metzler M, McCutcheon K, Zhang T, Kalchman MA, Hayden MR (1998) The influence of huntingtin protein size on nuclear localization and cellular toxicity. J Cell Biol 141:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens MJ, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR (1999) A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23:181–192 [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Smith DJ, McCutcheon K, Koide HB, Nishiyama K, Dinulos MB, Stevens ME, Bissada N, Nasir J, Kanazawa I, Disteche CM, Rubin EM, Hayden MR (1996) Human huntingtin derived from YAC transgenes compensates for loss of murine huntingtin by rescue of the embryonic lethal phenotype. Hum Mol Genet 5:1875–1885 [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72:971–983 [DOI] [PubMed] [Google Scholar]
- Kalchman MA, Graham RK, Xia G, Koide HB, Hodgson JG, Goldberg YP, Gietz RD, Pickart CM, Hayden MR (1996) Huntingtin is ubiquitinated and interacts with a specific ubiquitin-conjugating enzyme. J Biol Chem 271:19385–19394 [DOI] [PubMed] [Google Scholar]
- Kalchman MA, Koide HB, McCutcheon K, Graham RK, Nichol K, Nishiyama K, Kasemi-Esfarjani P, Lynn FC, Wellington CL, Metzler M, Goldberg YP, Kanazawa I, Gietz RD, Hayden MR (1997) HIP1, a human homologue of S. cerevesiae Sla2p, interacts with membrane associated huntingtin in brain. Nat Genet 16:44–53 [DOI] [PubMed] [Google Scholar]
- Li H, Li S-H, Johnston H, Shelbourne PF, Li X-J (2000) Amino-terminal fragments of mutant huntingtin show selective accumulation in striatal neurons and synaptic toxicity. Nat Genet 25:385–389 [DOI] [PubMed] [Google Scholar]
- Li X-J, Sharp AH, Nucifora FC, Schilling G, Lanahan A, Worely P, Snyder SH, Ross CA (1995) A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378:398–402 [DOI] [PubMed] [Google Scholar]
- Lin B, Nasir J, MacDonald H, Hutchison G, Graham R, Rommens JM, Hayden MR (1994) Sequence of the murine Huntington disease gene: evidence for conservation, alternate splicing and polymorphism in a triplet (CCG) repeat. Hum Mol Genet 3:85–92 [DOI] [PubMed] [Google Scholar]
- MacDonald ME and Gusella J (1996) Huntington's disease: translating a CAG repeat into a pathogenic mechanism. Curr Opin Neurobiol 6:638–643 [DOI] [PubMed] [Google Scholar]
- Martindale D, Hackam A, Weiczorek A, Ellerby L, Wellington CL, McCutcheon K, Singaraja R, Kazemi-Esfarjani P, Devon R, Bredesen D, Tufaro F, Hayden MR (1998) Length of the protein and polyglutamine tract influence localization and frequency of intracellular huntingtin aggregates. Nat Genet 18:150–154 [DOI] [PubMed] [Google Scholar]
- Nasir J, Floresco SB, O'Kusky JR, Diewert VM, Richman JM, Zeisler J, Borowski A, Marth JD, Phillips AG, Hayden MR (1995) Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell 81:811–823 [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, Nasir J, Cicchetti F, Parent A, Hayden MR (1999) Neuronal degeneration in the basal ganglia and loss of pallido-subthalamic synapses in mice with targeted disruption of the Huntington's disease gene. Brain Res 818:468–479 [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Bauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, Clementi E, Hackam A, Hayden MR, Li Y, Cooper, JK, Ross CA, Govoni S, Vincenz C, Cattaneo E (2000) Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci 20:3705–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandou F, Finkbinder S, Devys D, Greenberg ME (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95:55–66 [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borschelt DR (1999) Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet 8:397–407 [DOI] [PubMed] [Google Scholar]
- Sharp AH, Ross CA (1996) Neurobiology of Huntington's disease. Neurobiol Dis 3:3–15 [DOI] [PubMed] [Google Scholar]
- Sisoda SS (1998) Nuclear inclusions in glutamine repeat disorders: are they pernicious, coincidental or beneficial? Cell 95:1–4 [DOI] [PubMed] [Google Scholar]
- Wanker EE, Rovira C, Scherzinger E, Hasenbank R, Walter S, Tait D, Colicelli J, Lehrach H (1997) HIP-1: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum Mol Genet 6:487–495 [DOI] [PubMed] [Google Scholar]
- Wellington CL, Ellerby LM, Hackam A, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thornberry N, Pinsky L, Kakisuka A, Ross CA, Nicholson DW, Bredesen DE, Hayden MR (1998) Caspase cleavage of gene products associated with triplet repeat expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem 273:9158–9167 [DOI] [PubMed] [Google Scholar]
- Wellington CL, Singaraja R, Ellerby L, Savill J, Roy S, Leavitt BR, Cattaneo E, Hackam A, Sharp A, Thornberry N, Nicholson DW, Bredesen DE, Hayden MR (2000) Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J Biol Chem 275:19831–19838 [DOI] [PubMed] [Google Scholar]
- Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li X-J, Li S-H, Yi H, Vonsattel J-P, Gusella JE, Hersch S, Auerbach W, Joyner AL, MacDonald ME (2000) Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet 9:503–513 [DOI] [PubMed] [Google Scholar]
- White JK, Auerbach W, Duyao MP, Vonsattel J-P, Gusella JF, Joyner AL, MacDonald ME, (1997) Huntingtin is required for neurogenesis and is not impaired by Huntington's disease CAG expansion. Nat Genet 17:404–410 [DOI] [PubMed] [Google Scholar]
- Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Esfratiadis A (1995) Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington's disease homologue. Nat Genet 11:155–162 [DOI] [PubMed] [Google Scholar]