Abstract
In searching for a putative third gene for autosomal dominant polycystic kidney disease (ADPKD), we studied the genetic inheritance of a large family (NFL10) previously excluded from linkage to both the PKD1 locus and the PKD2 locus. We screened 48 members of the NFL10 pedigree, by ultrasonography, and genotyped them, with informative markers, at both the PKD1 locus and the PKD2 locus. Twenty-eight of 48 individuals assessed were affected with ADPKD. Inspection of the haplotypes of these individuals suggested the possibility of bilineal disease from independently segregating PKD1 and PKD2 mutations. Using single-stranded conformational analysis, we screened for and found a PKD2 mutation (i.e., 2152delA; L736X) in 12 affected pedigree members. Additionally, when the disease status of these individuals was coded as “unknown” in linkage analysis, we also found, with markers at the PKD1 locus, significant LOD scores (i.e., >3.0). These findings strongly support the presence of a PKD1 mutation in 15 other affected pedigree members, who lack the PKD2 mutation. Two additional affected individuals had trans-heterozygous mutations involving both genes, and they had renal disease that was more severe than that in affected individuals who had either mutation alone. This is the first documentation of bilineal disease in ADPKD. In humans, trans-heterozygous mutations involving both PKD1 and PKD2 are not necessarily embryonically lethal. However, the disease associated with the presence of both mutations appears to be more severe than the disease associated with either mutation alone. The presence of bilineal disease as a confounder needs to be considered seriously in the search for the elusive PKD3 locus.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a common Mendelian disorder (PKD [MIM 173900]) that affects ∼1/500 live births. Clinically, it is characterized by progressive formation and enlargement of renal and extrarenal cysts, typically leading to chronic renal failure in late middle age. Overall, it accounts for 5%–10% of all cases of end-stage renal disease (Fick and Gabow 1994; Harris 1999). ADPKD is genetically heterogeneous. Linkage studies have shown that PKD1 (MIM 601313) on chromosome 16p is responsible for 80%–85% of all cases of ADPKD, whereas PKD2 (MIM 173910) on chromosome 4q is thought to account for most of the remaining cases (Parfrey et al. 1990; Fick and Gabow 1994; Harris 1999). Recent documentation of several families that do not show linkage to either of the known loci has led to the suggestion that there is at least one more locus (PKD3 [MIM 600666]) for ADPKD (Bogdanova et al. 1995; Daoust et al. 1995; De Almeida et al. 1995; Turco et al. 1996; Ariza et al. 1997; Paterson and Pei 1998). In searching for this putative locus, we studied the genetic inheritance of a large multigeneration family (NFL10) with ADPKD, which we previously had excluded from linkage to the known loci. Further analysis of this pedigree, however, revealed a complex pattern of inheritance, with bilineal disease arising from independently segregating PKD1 and PKD2 mutations.
Subjects and Methods
Study Subjects
Part of the NFL10 pedigree, consisting of 16 affected and 14 unaffected individuals, had been studied previously and excluded from linkage (i.e., LOD scores <−2.0) to either the PKD1 locus or the PKD2 locus (Parfrey et al. 1990). In the present study, we ascertained a more complete structure of this pedigree and traced the genealogy over six generations. Forty-four at-risk individuals and four spouses, all of whom gave informed consent, were studied. The research protocols used for this study were approved by the Human Subject Review Committees of both the University of Toronto and Memorial University in Newfoundland.
Clinical Outcome
All the at-risk individuals and spouses were screened with at least one abdominal ultrasound. All the scans were performed under the supervision of one ultrasonographer and were interpreted by a radiologist (Dr. Benvon Cramer, Department of Radiology, Memorial University, St. John’s, Newfoundland), who had no knowledge of the clinical status of the subjects. The following criteria were used for the diagnosis of ADPKD: (i) the presence of at least two renal cysts (unilateral or bilateral) in an at-risk individual aged <30 years; (ii) the presence of at least two cysts in each kidney in an at-risk individual aged 30–59 years; or (iii) the presence of at least four cysts in each kidney in an at-risk individual aged ⩾60 years (Ravine et al. 1992). The number of cysts present in the liver was graded semiquantitatively as (a) <5 (with the actual number reported); (b) 5–10; and (c) >10. Serum creatinine was measured with an autoanalyzer. Creatinine clearance, adjusted for age, gender, and body weight, was calculated using the formula of Cockcroft and Gault (1976).
Genotyping and Haplotype Construction
We genotyped all the study subjects, with six polymorphic simple-sequence repeat (SSR) markers at the PKD1 locus and five polymorphic SSR markers at the PKD2 locus. The locations of these markers relative to the PKD1 locus are as follows (the number between markers denotes intermarker distance [cM]): D16S521-2.0-HBAP1-2.0-KG8/PKD1-0.1-D16S665-0.1-D16S291-0.6-D16S2618 (Germino et al. 1992). KG8 is an intragenic marker located within the 3′ end of PKD1. The locations of these markers relative to the PKD2 locus are as follows: D4S231-2.0-D5S1534-2.3-SPP1-0.2-PKD2-0.5-D4S1563-2.0-D4S423 (San Millán et al. 1995). Genotyping was performed by [32P]α-dCTP–labeling of the PCR products and was analyzed by PAGE. All genotypings were performed at least twice and were scored independently by two of us (A.D.P. and K.R.W.), who had no knowledge of the clinical status of the study subjects. Any ambiguity in the allele scoring was resolved by retyping of the marker. Additionally, all intermarker recombinants were restudied and confirmed by repeated DNA sampling and genotyping. Haplotypes were constructed using the program SIMWALK 2 (Sobel and Lange 1996).
PKD2 Mutation Analysis
In two selected individuals (OP120 and OP14), we screened for mutations in the entire PKD2 coding sequence, by single-stranded conformational analysis (SSCA). All 15 exons, including their splice junctions, were amplified from genomic DNA and were screened at three or more different temperatures, through use of a thermoflow electrophoresis temperature control system (Novex). All PCR products were separated by electrophoresis on a 20% polyacrylamide gel and were visualized by silver staining (Pei et al. 1998). An SSCA variant was identified in exon 11 of individual OP120. To define this mutation, both strands of the PCR fragment were sequenced directly through use of the fluorescent dideoxy terminator method and were analyzed with an ABI 373 DNA sequencer (PE Biosystems). Allele-specific oligonucleotide (ASO) hybridization was performed to analyze the segregation of this mutation (2152delA) in other members of the pedigree. Two oligonucleotide probes (wild-type [WT], 5′-CTGAAAAAAAATACCGTG-3′; and mutant [MT], 5′-AACTGAAAAAAATACCGTG-3′) were used for the analysis (Pei et al. 1998).
PKD1 Linkage Studies
Two-point linkage analysis using the “affected-only analysis” subroutine was performed using the MLINK program of the FASTLINK package, version 4.0 (Lathrop and Lalouel 1984; Terwilliger and Ott 1994). An autosomal dominant model from a single locus with a disease-allele frequency of .001 and a phenocopy rate of .001 was assumed. The disease status of all individuals with the PKD2 mutation (2152delA) and of individual OP14 was coded as unknown. Marker-allele frequencies were obtained from married-in individuals and from reconstruction of the founder genotypes. All inbreeding loops were broken.
Results
Genealogy and Clinical Ascertainment
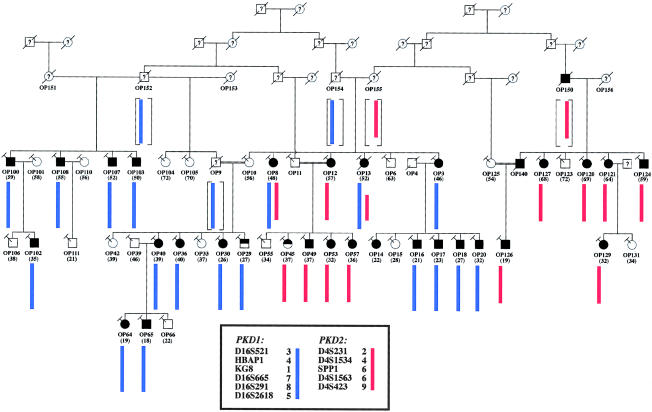
On the basis of the genotype data for 16 affected and 14 unaffected individuals, and assuming an autosomal dominant model from a single disease locus, we previously had excluded NFL10 from linkage to either the PKD1 locus or the PKD2 locus (data not shown) (Parfrey et al. 1990). In the present study, we extended the genealogy of NFL10 to construct a more complete pedigree structure. Further ascertainment revealed a large and complex pedigree consisting of ⩾130 members over six generations. Additionally, we identified three previously unknown consanguineous relationships (i.e., between individuals OP9 and OP10, between individuals OP11 and OP12, and between individuals OP125 and OP140) and two additional branches of the family (represented by individuals numbered “OP100”–”OP111” and “OP120”–”OP131”). Figure 1 shows a simplified and updated pedigree depicting the relationships of several key founders and of the 48 members who participated in the present study. The polycystic kidney disease in individual OP150 was documented, during abdominal surgery, as an incidental finding. The diagnosis of all other affected individuals was confirmed by ultrasonography. The diagnoses of individuals OP29 and OP45, who each have only one renal cyst, at ages 27 years and 37 years, respectively, were considered indeterminate.
Figure 1.
Simplified pedigree structure of NFL10, and haplotype analysis at the PKD1 and PKD2 loci. In the present study, we have identified three previously unknown consanguineous relationships (between individuals OP9 and OP10, between OP11 and OP12, and between OP125 and OP140; denoted by double lines between the individuals) and two additional branches of the family (represented by individuals numbered “OP100”–”OP111” and “OP120”–”OP131”). The combination of the PKD1 haplotype 3-4-1-7-8-5 (blue bar) and the PKD2 haplotype 2-4-6-6-9 (red bar) accounted for the disease in all affected individuals except OP14; OP14, who had two renal cysts at age 22 years, was considered to be affected with ADPKD. These findings suggest the possibility of bilineal disease from independently segregating PKD1 and PKD2 mutations. Individuals with indeterminate disease status are denoted by the half-blackened symbols. The age at the last ultrasound test is shown in parentheses. All individuals with the slanted-T symbols have been genotyped.
Genetic Analysis
Haplotype construction was performed at both the PKD1 locus and the PKD2 locus. Four intermarker recombinants were identified at the PKD1 locus (in individuals OP8, OP10, OP14, and OP111), and three were identified at the PKD2 locus (in individuals OP13, OP36, and OP124) (data not shown). Consistent with our previous linkage results, no single haplotype at either locus segregated with the disease. Furthermore, no combination of two different haplotypes at either locus could explain the disease-segregation pattern. These latter observations provided strong evidence against bilineal disease from homozygous or compound heterozygous mutations at a single PKD locus. However, we did find that the combination of the PKD1 haplotype 3-4-1-7-8-5 and the PKD2 haplotype 2-4-6-6-9 accounted for the disease in all the affected individuals except OP14. This finding strongly suggests the possibility of bilineal disease from trans-heterozygous mutations of both PKD1 and PKD2. Individual OP14, who had two renal cysts at age 22 years, was considered to be affected with ADPKD. Her father, who had died at age 57 of renal-cell carcinoma, did not have renal cysts on autopsy.
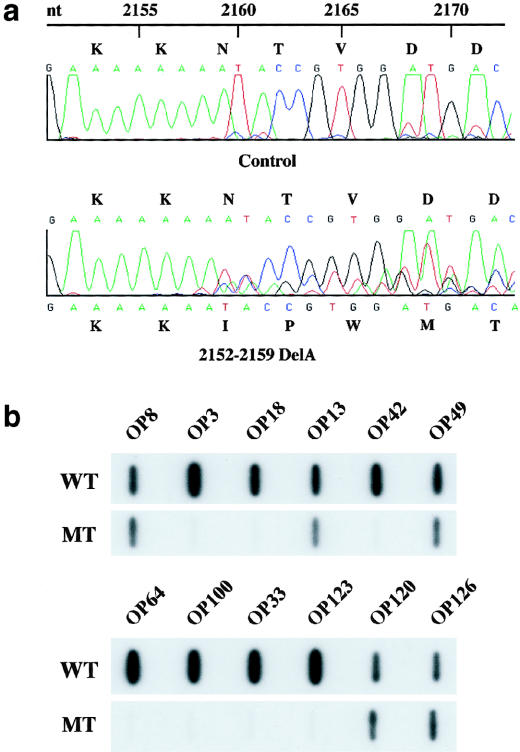
To further address the possibility of bilineal disease in this pedigree, we screened individual OP120, whose disease cosegregated with the PKD2 haplotype 2-4-6-6-9, for PKD2 mutations. We found a frameshift mutation (2152delA; L736X) on exon 11 in this individual (fig. 2a). ASO hybridization analysis further showed cosegregation of this mutation with only the affected individuals with the PKD2 haplotype 2-4-6-6-9 and with none of the unaffected individuals (figs. 1 and 2b). We screened individual OP14 for PKD2 mutations but did not find any SSCA variant. The disease in this individual was not explained by our haplotype analysis.
Figure 2.
PKD2 mutation analysis in NFL10. a, Sequence tracings of exon 11 PCR products from a control subject (top panel) and individual OP120 (bottom panel). A heterozygous PKD2 frameshift mutation (i.e., 2152delA) is detected in OP120. This mutation is expected to truncate the mutant protein product at codon 736 (i.e., L736X). b, Representative data from ASO hybridization analysis. Cosegregation of the mutation depicted in panel a occurs only with the affected individuals with the PKD2 haplotype 2-4-6-6-9 and not with the unaffected individuals.
We also sought evidence for an additional PKD1 mutation in the other affected members who lack the PKD2 mutation. When the disease status of all the individuals with the PKD2 mutation and of individual OP14 was coded as unknown in linkage analysis, we found significant LOD scores (i.e., >3.0) in 4/6 informative markers at the PKD1 locus. KG8, which is an intragenic marker of PKD1, yielded a LOD score of 5.0 at θ=.00. These findings strongly support the presence of an independently segregating PKD1 mutation in the 15 other affected members, who lack the PKD2 mutation (table 1). Two additional affected individuals (i.e., OP8 and OP13) were found to have the PKD1 disease haplotype (i.e., 3-4-1-7-8-5) and the PKD2 mutation.
Table 1.
Two-Point Linkage Analysis Data at the PKD1 Locus (Affected-Only Analysis)[Note]
|
LOD Score at θ = |
|||||||
| Marker | .00 | .01 | .05 | .10 | .20 | .30 | .40 |
| D16S521 | 2.90 | 2.83 | 2.57 | 2.24 | 1.58 | .95 | .40 |
| HBAP1 | 2.69 | 2.63 | 2.40 | 2.11 | 1.53 | .97 | .46 |
| KG8 | 5.03 | 4.94 | 4.57 | 4.10 | 3.10 | 2.03 | .94 |
| D16S655 | 4.25 | 4.17 | 3.85 | 3.43 | 2.56 | 1.64 | .72 |
| D16S291 | 4.25 | 4.17 | 3.85 | 3.43 | 2.57 | 1.67 | .76 |
| D16S2618 | 3.54 | 3.49 | 3.26 | 2.94 | 2.21 | 1.40 | .59 |
Note.— Marker-allele frequencies estimated from founders and married-in subjects. The disease-segregation pattern with the PKD1 haplotype 3-4-1-7-8-5 (see fig. 1) is consistent with the LOD-score results.
Genotype-Phenotype Correlation
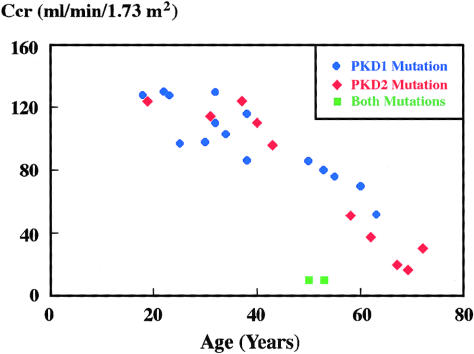
Figure 3 shows the distribution of creatinine clearances in all the affected individuals, by their age at their last serum creatinine measurement; prior to the creatinine measurement, none had either any concomitant renal disease or a nephrectomy. In this family, it is evident that the age-adjusted disease severity associated with the PKD1 germline mutation is similar to that associated with the PKD2 mutation. At the last follow-up, four affected individuals (i.e., OP8, OP13, OP120, and OP127) were diagnosed as having end-stage renal disease (ESRD). Individuals OP8 and OP13, who had trans-heterozygous mutations of both PKD1 and PKD2, developed ESRD at ages 48 years and 52 years, respectively. In contrast, individuals OP120 and OP127, who carried only the PKD2 mutation, developed ESRD at ages 69 years and 68 years, respectively. Additionally, at similar ages, the creatinine clearance of individuals OP8 and OP13 was severely reduced when compared with that of OP103 and OP107, who had only the PKD1 mutation (10 ml/min/1.73m2 vs. 80 and 86 ml/min/1.73 m2, respectively; normal reference range 95–130 ml/min/1.73 m2). Thus, the renal disease in individuals with the trans-heterozygous mutations was more severe than that in the other affected family members, who had either the PKD1 mutation or the PKD2 mutation alone.
Figure 3.
Genotype-phenotype correlation in NFL10. Age-adjusted creatinine clearances are plotted against individuals with a mutation in either PKD1 (blue symbol), PKD2 (red symbol), or both mutations (green symbol). The renal-disease severity is similar in individuals with either the PKD1 or PKD2 mutation but is more severe in the two individuals (i.e., OP8 and OP13) with the compound heterozygous mutations. The normal reference range is 95–130 ml/min/1.73 m2.
The liver is the second-most-common organ to undergo cystic changes in ADPKD. When a semiquantitative scoring system for number of cysts was used, individuals OP8 and OP13 appeared to have more liver cysts than did the other affected family members, who had either the PKD1 mutation or the PKD2 mutation alone (table 2). However, neither OP8 nor OP13 had massive polycystic liver resulting in either portal hypertension or hepatic failure. Moreover, cystic liver disease in females with ADPKD tends to be more severe than in males with ADPKD (Chauveau et al. 1997). Thus, we could not conclude definitively that the cystic liver disease in trans-heterozygous individuals was more severe than that in other affected members, who had the PKD1 mutation or the PKD2 mutation alone. None of the affected members of NFL10 had a history of intracranial arterial aneurysms.
Table 2.
Comparison of Liver Cystic Disease in NFL10
| Patienta | Age at Time of Ultrasound(years) | No. of Liver Cysts |
| OP100 | 59 | 3 |
| OP108 | 55 | 2 |
| OP107 | 52 | 1 |
| OP103 | 50 | 0 |
| OP8a | 48 | >10 |
| OP12a | 57 | >10 |
| OP13a | 52 | >10 |
| OP3a | 46 | 5–10 |
| OP127a | 68 | 5–10 |
| OP120 | 69 | 0 |
| OP121 | 64 | 0 |
| OP124 | 59 | 0 |
Ultrasonographic film records were not available for the more precise estimation of cyst number.
Discussion
In searching for a putative novel locus for ADPKD, we unraveled the complex inheritance of a large, multigeneration family. Careful analysis of the NFL10 pedigree suggested the possibility of two independently segregating PKD1 and PKD2 mutations. The combination of the PKD1 haplotype 3-4-1-7-8-5 and the PKD2 haplotype 2-4-6-6-9 accounted for 27/28 definitively affected individuals, and neither haplotype was present in any clearly unaffected individuals (fig. 1). The disease in individual OP14, who carried neither of the aforementioned haplotypes but who had two renal cysts at age 22 years, remains unexplained. It is possible that she may represent a phenocopy, since simple renal cysts can occur in the normal population, albeit rarely in people <30 years of age (Laucks and McLachlan 1981; Tada et al. 1983). Alternatively, her disease could be due to a de novo mutation in PKD1.
To further address the possibility of bilineal disease in this pedigree, we screened for and found a frameshift PKD2 mutation in individual OP120. ASO hybridization analysis showed that this mutation cosegregated with the PKD2 haplotype 2-4-6-6-9 and that it appeared to have originated from two founders, OP150 and OP155, in the third generation (fig. 1). This finding thus provided definitive proof for at least two independently segregating mutations in this family. Presently, mutation screening of PKD1 remains a challenge because of both its size (i.e., there is a ∼13-kb open reading frame within its mRNA transcript) and its complexity (i.e., ∼75% of the gene is duplicated in at least three homologous genes, and the GC content is ∼65%) (Peral et al. 1996, 1997; Thomas et al. 1999; Watnick et al. 1999). Therefore, we did not attempt to prove the presence of a PKD1 disease allele by direct mutation screening. Instead, using linkage analysis, we found strong evidence supporting the presence of an additional PKD1 mutation in 15 other affected members, who lack the PKD2 mutation (table 1 and fig. 1). Although the actual PKD1 mutation in this family is presently unknown, it appears to have originated from two founders, OP152 and OP154. The marriage of individuals OP154 and OP155, in turn, introduced both the PKD1 mutation and the PKD2 mutation into the core family (fig. 1). Interestingly, in this pedigree, the renal disease severity in the individuals with the PKD1 haplotype is very similar to that in individuals with the PKD2 mutation. Although the renal disease is generally more severe in type 1 ADPKD than in type 2 ADPKD, there is a spectrum of phenotypic variability, and families with mild disease have been documented as having ADPKD type 1 (Hateboer et al. 1999). The basis for the interfamilial phenotypic variability in ADPKD is presently unknown, but it has been attributed to genetic and/or environmental factors (Peral et al. 1996; Hateboer et al.1999). Alternatively, this variability could be due to an allele-specific effect (Watnick et al. 1999).
Given that ADPKD affects 1 in 500–1,000 live births, bilineal disease is predicted to occur in ∼1 in 250,000–1,000,000 marriages in the general population (Paterson and Pei 1998). Two scenarios may result. First, in families in which the germline mutations involve both copies of either PKD1 or PKD2, homozygous or compound-heterozygous mutations in one of these genes are expected in 25% of the offspring; second, in families in which the germline mutations involve one copy each of PKD1 and PKD2, trans-heterozygous mutations in both genes are expected in 25% of the offspring. Despite these predictions, however, no definitive case of bilineal ADPKD had been documented prior to the present study. This raises the possibility that these mutation combinations may be lethal in humans (Wilkie 1994). Indeed, recent studies of both PKD1- and PKD2- “knockout” mice support this contention (Lu et al. 1997, 1999; Wu et al. 1998, 2000). Mice with heterozygous inactivation of either PKD1 or PKD2 develop focal cysts later in life. In contrast, mice with homozygous inactivation of either PKD1 or PKD2 die, either in utero or perinatally, with massive polycystic kidneys (Lu et al. 1997, 1999; Wu et al. 1998, 2000). Our documentation of two individuals with trans-heterozygous mutations of PKD1 and PKD2 suggests that this mutation combination is not necessarily lethal.
At least two potential mechanisms may explain the increased disease severity in the individuals with the trans-heterozygous mutations, and they need not be mutually exclusive. First, recent human and knockout-mouse studies have shown that ADPKD is a clonal disorder of epithelial cell growth, triggered by somatic mutagenic events. Specifically, inactivation of both copies of either PKD1 or PKD2, through germline and somatic mutations within an epithelial cell, confers growth advantages for it to expand into a cyst (Qian et al. 1996; Brasier and Henske 1997; Lu et al. 1997, 1999; Wu et al. 1998, 2000; Koptides et al. 1999; Pei et al. 1999; Torra et al. 1999). This mechanism for cyst formation is analogous to Knudson's “two-hit” model of carcinogenesis (Reeders 1992). Since the somatic polycystic kidney disease mutations constitute the rate-limiting step for individual cyst formation, the frequency of such mutations is predicted to determine both the number of cysts eventually formed and the disease severity (Pei et al. 1999). In the individuals with the trans-heterozygous mutations, all cells have already suffered a “first hit” in both PKD1 and PKD2. The increased disease severity may simply reflect the fact that all the cells in these individuals have two loci that could be involved in the somatic “second hits.”
The second mechanism involves a “threshold” effect (Wilkie 1994). Digenic mutations have been documented in several Mendelian diseases, including retinitis pigmentosa (involving RDS and ROM1), Waardenburg syndrome (involving MITF and TYR), and junctional epidemolysis bullosa (involving COL17A1 and LAMB3) (Kajiwara et al. 1994; Morrell et al. 1997; Floeth and Bruckner-Tuderman 1999). In these disorders, individuals with heterozygous mutations of both genes were affected, whereas other family members, who had heterozygous mutations involving one of the two genes, were phenotypically normal. A threshold model has been proposed as the pathophysiological mechanism for these disorders, since in each case the disease results from the interactions of two mutant proteins that are components of a multimolecular complex or pathway. Recent studies have suggested that polycystin 1 and 2 (the gene products of PKD1 and PKD2, respectively) are components of a novel signaling pathway that is essential for tubulogenesis (Qian et al. 1997; Tsiokas et al.1997; Harris 1999). Polycystin 1 is thought to function as a receptor for cell-cell or cell-matrix interactions, whereas polycystin 2 is predicted to form a subunit of a channel complex. Both proteins, in turn, have been shown to interact with each other in vitro (Qian et al. 1997; Tsiokas et al.1997). Although the composition and stoichiometry of the polycystin signaling pathway remain to be fully elucidated, it is plausible that the interactions of the mutant proteins arising from the trans-heterozygous PKD1 and PKD2 mutations could lead to a reduction of the functional signaling complex below a “threshold,” which would predispose more cells to a cystic phenotype. Under this model, the type of the PKD1 and PKD2 mutations may be important for determination of the cystogenic threshold. However, additional modulating factors are required, since only a relatively small fraction of cells in the trans-heterozygous individuals acquired the cystic phenotype. It is of interest to note that both germline PKD1 and somatic PKD2 mutations—as well as the opposite—have been recently documented in a small subset (i.e., ∼10 %) of cysts from patients with ADPKD (Koptides et al. 2000; Watnick et al. 2000). With the recent availability of knockout-mouse models for PKD1 and PKD2, these two potential mechanisms now can be tested experimentally by crossing these animals to generate knockout mice with trans-heterozygous inactivation of PKD1 and PKD2 (S.S., unpublished data).
The recent application of molecular genetic analysis to clinical medicine has begun to unravel the complexity of pathogenic mechanisms in human diseases. The present study illustrates such complexity in an otherwise “simple” Mendelian disorder and provides insight into the genetic basis for intrafamilial disease variability in ADPKD. Such knowledge has the potential to improve the prediction of risk in individual patients and to provide the framework for the development of mechanism-based therapeutics. Although several families with ADPKD have now been reported to show no linkage to either of the known gene loci, the absence of linkage in these families does not necessarily imply the presence of another disease locus (Paterson and Pei 1998, 1999). A number of potential confounders, including genotyping errors, nonpaternity, misdiagnosis, and bilineal disease, could lead to false exclusion of linkage and will need to be vigorously excluded in these families. The existence of a third locus for ADPKD remains uncertain at this time.
Acknowledgments
We are indebted to all the participating members of the NFL10 family, to Dr. Benvon Cramer for her help with the ultrasound diagnosis, and to Dr. James Kennedy for providing access to computing resources. A.D.P. was a research fellow of the Medical Research Council of Canada. This research was supported by grants from the Polycystic Kidney Research Foundation, the Medical Research Council of Canada, and the Kidney Foundation of Canada (all to Y.P.).
Electronic-Database Information
Accession numbers and URL for data in this article are as follows:
- Online Mendelian Inheritance of Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PKD [MIM 173900], PKD1 [MIM 601313], PKD2 [MIM 173910] and PKD3 [MIM 600666])
References
- Ariza M, Alvarez V, Marin R, Aguado S, Lopez-Larrea C, Alvarez J, Menendez M, Coto E (1997) A family with a milder form of adult dominant polycystic kidney disease not linked to the PKD1 (16p) or PKD2 (4q) genes. J Med Genet 34:587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova N, Dworniczak B, Dragova D, Todorov V, Dimitrakov D, Kalinov K, Hallmayer J, Horst J, Kalaydjieva L (1995) Genetic heterogeneity of polycystic kidney disease in Bulgaria. Hum Genet 95:645–650 [DOI] [PubMed] [Google Scholar]
- Brasier J, Henske E (1997) Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13.3 in renal cysts supports a loss-of-function model for cyst pathogenesis. J Clin Invest 99:194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau D, Pirson Y, Le Moine A, Franco D, Belghiti J, Grunfeld JP (1997) Extrarenal manifestations in autosomal dominant polycystic kidney disease. Adv Nephrol Necker Hosp 26:265–289 [PubMed] [Google Scholar]
- Cockcroft D, Gault H (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- Daoust M, Reynolds D, Bichet D, Somlo S (1995) Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics 25:733–736 [DOI] [PubMed] [Google Scholar]
- De Almeida S, de Almeida E, Peters D, Pinto J, Tavora I, Lavinha J, Breuning M, Prata M (1995) Autosomal dominant polycystic kidney disease: evidence for the existence of a third locus in a Portuguese family. Hum Genet 96:83–88 [DOI] [PubMed] [Google Scholar]
- Fick G, Gabow P (1994) Hereditary and acquired cystic disease of kidney. Kidney Int 46:951–964 [DOI] [PubMed] [Google Scholar]
- Floeth M, Bruckner-Tuderman L (1999) Digenic junctional epidermolysis bullosa: mutations in COL17A1 and LAMB3 genes. Am J Hum Genet 65:1530–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino GG, Weinstat-Saslow D, Hammelbauer H, Gillespie G, Somlo S, Wirth B, Barton N, Harris K, Frischauf A, Reeders S (1992) The gene for autosomal dominant polycystic kidney disease lies in a 750-kb CpG-rich region. Genomics 13:144–151 [DOI] [PubMed] [Google Scholar]
- Harris PC (1999) Autosomal dominant polycystic kidney disease: clues to pathogenesis. Hum Mol Genet 8:1861–1869 [DOI] [PubMed] [Google Scholar]
- Hateboer N, Lazarou L, Williams A, Holmans P, Ravine D (1999) Familial phenotype differences in PKD1. Kidney Int 56:34–40 [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson E, Dryja T (1994) Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1. Science 264:1604–1608 [DOI] [PubMed] [Google Scholar]
- Koptides M, Hadjimichael C, Koupepidou P, Pierides A, Deltas C (1999) Germinal and somatic mutations in the PKD2 gene of renal cysts in autosomal dominant polycystic kidney disease. Hum Mol Genet 8:509–513 [DOI] [PubMed] [Google Scholar]
- Koptides M, Mean R, Demetriou K, Pierides A, Deltas C (2000) Genetic evidence for a trans-heterozygous model for cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet 9:447–452 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculations of Lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Laucks S, McLachlan M (1981) Aging and simple cysts of the kidney. Br J Radiol 54:12–14 [DOI] [PubMed] [Google Scholar]
- Lu W, Fan XH, Basora N, Babakhanlou H, Law T, Rifai N, Harris P, Perez-Atayde A, Rennke H, Zhou J (1999) Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat Genet 21:160–161 [DOI] [PubMed] [Google Scholar]
- Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J (1997) Perinatal lethality with kidney and pancreas defects in mice targeted Pkd1 mutation. Nat Genet 17:179–181 [DOI] [PubMed] [Google Scholar]
- Morell R, Spritz R, Ho L, Pierpont J, Guo W, Friedman T, Asher J (1997) Apparent digenic inheritance of Waardenburg syndrome type 2 and autosomal recessive ocular albinism. Hum Mol Genet 6:659–664 [DOI] [PubMed] [Google Scholar]
- Parfrey P, Bear J, Morgan J, Cramer B, McManamon P, Gault H, Churchill D, Singh M, Hewitt R, Somlo S, Reeders S (1990) The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med 323:1085–1090 [DOI] [PubMed] [Google Scholar]
- Paterson A, Pei Y (1998) A third gene for autosomal dominant polycystic kidney disease? Kidney Int 54:1759–1761 [DOI] [PubMed] [Google Scholar]
- ——— (1999) PKD3—to be or not to be? Nephrol Dial Transplant 14:2965 [DOI] [PubMed] [Google Scholar]
- Pei Y, He N, Wang K, Kasenda M, Paterson AD, Chan G, Liang Y, Roscoe J, Brissenden J, Hefferton D, Parfrey P, Somlo S, St. George-Hyslop P (1998) A spectrum of mutations in the polycystic kidney disease-2 (PKD2) gene from eight Canadian kindreds. J Am Soc Nephrol 9:1853–1860 [DOI] [PubMed] [Google Scholar]
- Pei Y, Watnick T, He N, Wang KW, Parfrey P, Germino G, St. George-Hyslop P (1999) Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model for cystogenesis in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 10:1524–1529 [DOI] [PubMed] [Google Scholar]
- Peral B, Gamble V, Strong C, Ong ACM, Sloane-Stanley J, Zerres K, Winearls CG, Harris PC (1997) Identification of mutations in the duplicated region of the polycystic kidney disease 1 gene (PKD1) by a novel approach. Am J Hum Genet 60:1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peral B, Ong A, San Millan J, Gamble V, Rees L, Harris P (1996) A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1). Hum Mol Genet 5:539–542 [DOI] [PubMed] [Google Scholar]
- Qian F, Germino J, Cai Y, Zhang XB, Somlo S, Germino G (1997) PKD1 interacts with PKD2 through a probable coiled-coiled domain. Nat Genet 16:179–183 [DOI] [PubMed] [Google Scholar]
- Qian F, Watnick T, Onuchic L, Germino G (1996) The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type 1. Cell 87:979–987 [DOI] [PubMed] [Google Scholar]
- Ravine D, Gibson R, Walker R, Sheffield L, Kincaid-Smith P, Danks D (1992) Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease. Lancet 340:1330–1333 [DOI] [PubMed] [Google Scholar]
- Reeders ST (1992) Multilocus polycystic disease. Nat Genet 1:235–237 [DOI] [PubMed] [Google Scholar]
- San Millán JL, Viribay M. Peral B, Martínez I, Weissenbach J, Moreno F (1995) Refining the localization of the PKD2 locus on chromosome 4q by linkage analysis in Spanish families with autosomal dominant polycystic kidney disease type 2. Am J Hum Genet 56:248–253 [PMC free article] [PubMed] [Google Scholar]
- Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping location scores, and marker-sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T (1983) The incidence of simple renal cyst by computer tomography. Clin Radiol 34:437–439 [DOI] [PubMed] [Google Scholar]
- Terwilliger J, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Thomas R, McConnell R, Whittacker J, Kirkpatrick P, Bradley J, Sandford R (1999) Identification of mutations in the repeated part of the autosomal dominant polycystic kidney disease type 1 gene, PKD1, by long-range PCR. Am J Hum Genet 65:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torra R, Badenas C, San Millán JL, Pérez-Oller L, Estivill X, Darnell A (1999) A loss-of-function model for cystogenesis in human autosomal dominant polycystic kidney disease type 2. Am J Hum Genet 65:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L, Kim E, Arnould T, Sukhatme V, Walz G (1997) Homo- and hetero-dimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco AE, Clementi M, Rossetti S, Tenconi R, Pignatti PF (1996) An Italian family with autosomal dominant polycystic kidney disease unlinked to either the PKD1 or PKD2 gene. Am J Kidney Dis 28:759–761 [DOI] [PubMed] [Google Scholar]
- Watnick T, He N, Wang KW, Liang Y, Parfrey P, Hefferton D, St George-Hyslop P, Germino G, Pei Y (2000) Somatic mutations in PKD1 in ADPKD2 tissue suggest a possible pathogenic role of trans-heterozygous mutations. Nat Genet 25:143–144 [DOI] [PubMed] [Google Scholar]
- Watnick T, Phakdeekitcharoen B, Johnson A, Gandolph M, Wang M, Briefel G, Klinger KW, Kimberling W, Gabow P, Germino GG (1999) Mutation detection of PKD1 identifies a novel mutation common to three families with aneurysms and/or very-early-onset disease. Am J Hum Genet 65:1561–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie A (1994) The molecular basis of genetic dominance. J Med Genet 31:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GQ, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds D, Maeda Y, Le TC, Hou H, Kucherlapati R, Edelmann W, Somlo S (1998) Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93:177–188 [DOI] [PubMed] [Google Scholar]
- Wu GQ, Markowitz G, Li L, D'Agati V, Factor S, Geng L, Tibara S, Tuchman J, Cai YQ, Park JH, van Adelsberg J, Hou H, Kucherlapati R, Edelmann W, Somlo S (2000) Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24:75–78 [DOI] [PubMed] [Google Scholar]